Accurately diagnosing fibrotic interstitial lung disease (ILD) is a challenge even for expert clinicians. It requires multidisciplinary integration of clinical, radiological, and pathological features that are then compared against a series of formal and informal diagnostic criteria for different conditions (1). Diagnostic criteria for idiopathic pulmonary fibrosis (IPF) (2) and the remaining idiopathic interstitial pneumonias (1, 3) have helped standardize this process, but many conditions remain loosely and inconsistently defined (4–6). The current approach therefore results in significant diagnostic heterogeneity (5), which has major implications for patients whose treatment plan and prognosis depend on an accurate diagnosis.
Two distinct approaches to the classification of fibrotic ILD have evolved in clinical practice. In the first approach, assignment of a diagnosis is based on strict adherence to diagnostic criteria, resulting in a large number of unclassifiable cases. In the second, assignment of a diagnosis is based on clinical judgment (i.e., what the provider believes is the likely diagnosis regardless of whether all diagnostic guideline criteria are met), generally resulting in a smaller number of unclassifiable cases. Both approaches are defensible: one maximizes diagnostic certainty at the expense of clinical utility, and the other maximizes clinical utility at the expense of diagnostic certainty (7). The lack of consistency in diagnostic approach is problematic, and we suspect is a major reason for the observed diagnostic discordance among expert centers (5).
In recognition of this issue, the authors organized an international working group, which met face-to-face, by conference call, and via email over the course of a year. The objectives of this international working group perspective are to describe limitations of the current diagnostic approach to fibrotic ILD and to propose an ontological framework for standardizing the diagnostic classification of patients with fibrotic ILD. It is anticipated that this framework will help standardize fibrotic ILD classification and that future research will provide objective data to further refine this approach.
Diagnostic Limitations in IPF
A diagnostic pathway for IPF was most recently defined in 2011 by an evidence-based international guideline committee (2) that provided a clear description of how to incorporate clinical, radiological, and (when available) pathological data within a multidisciplinary discussion. Patients with no identifiable alternative etiology for fibrotic ILD who had usual interstitial pneumonia (UIP) pattern on high-resolution computed tomography (HRCT) or who had specified combinations of HRCT pattern and surgical lung biopsy pattern were considered to have IPF. In a minority of cases, the guidelines are unable to categorically assign a diagnosis (e.g., a possible UIP HRCT and a possible UIP surgical biopsy), and the ultimate diagnosis is left to the discretion of a multidisciplinary discussion. There is ongoing debate and uncertainty regarding how best to categorize these patients.
Strict adherence to IPF guideline criteria likely improves reproducibility across centers by providing a clear definition of IPF (2); however, there are still important areas of variability. For example, interobserver agreement for radiological and histopathological UIP pattern is only moderate, even among experienced observers (4). In addition, there is no objective guidance on how to incorporate clinical features (e.g., age, disease behavior) that may impact diagnostic confidence (1, 8, 9). Finally, there is frequent reluctance to perform surgical lung biopsy in patients with clinically unclassifiable ILD due to safety concerns. The challenges and limitations of strict adherence to guideline criteria have led some clinicians to diagnose IPF on the basis of clinical judgment (i.e., a strong belief that the patient has IPF on the basis of multidisciplinary review); however, this approach has not been compared with the stricter guideline-based approach, and it may be less accurate in less-experienced centers (10).
Diagnostic Limitations in Chronic Hypersensitivity Pneumonitis
The term chronic hypersensitivity pneumonitis (HP) is commonly used to describe a form of ILD that shares many characteristics with other fibrotic ILDs (1). Radiological findings have been described as highly suggestive or even diagnostic in the right clinical scenario, but there remains no consensus on diagnostic criteria (11–14). Histopathological findings from surgical lung biopsy in HP can be definitive, but they often share significant overlap with IPF, idiopathic nonspecific interstitial pneumonia (NSIP), and connective tissue disease–associated ILD (CTD-ILD) (15–20). Previous studies have suggested a role for serum precipitins and bronchoalveolar lavage cellular differential; however, their positive predictive value remains controversial (21–33). Despite the multiple suggestive features, the absence of consensus guidelines for the diagnosis of chronic HP has resulted in substantial variability and lack of reproducibility in the assignment of an HP diagnosis (5, 18, 34).
Diagnostic Limitations in Idiopathic NSIP
The pathological pattern of NSIP is characterized by varying amounts of interstitial inflammation and fibrosis with a uniform appearance and distribution (1, 35). Typical features of NSIP on HRCT include bilateral lower lung–predominant reticulation, ground glass, and traction bronchiectasis in a peripheral distribution and often with subpleural sparing (1, 35). Idiopathic NSIP is currently considered a distinct clinical entity among the idiopathic interstitial pneumonias (1, 35), although many other diagnoses can have an NSIP pattern on surgical lung biopsy (e.g., connective tissue disease, drug toxicity, chronic HP, IPF) (35).
Previous consensus statements from the American Thoracic Society and European Respiratory Society suggest that a multidisciplinary discussion including surgical lung biopsy should be the primary method for establishing a diagnosis of idiopathic NSIP (1, 3); however, there are important limitations to this approach. Many centers reserve a label of idiopathic NSIP for patients with a confirmatory surgical lung biopsy, as previously suggested by these consensus statements (1, 3), citing an inability to confidently exclude alternative diagnoses without histopathological sampling. Other centers have diagnosed idiopathic NSIP on the basis of compatible clinical and imaging features without a surgical lung biopsy, although this approach is not consistent with previous consensus statements (1, 3). Many centers find the prevalence of biopsy-proven idiopathic NSIP to be decreasing, with increasing evidence that many patients historically diagnosed with idiopathic NSIP have evidence of an underlying autoimmune disease that can be identified with a comprehensive and systematic evaluation (36). It is unclear whether these patients should instead be considered to have a subtle form of CTD-ILD (37), although previous consensus statements currently classify such patients as having idiopathic NSIP if they do not meet established criteria for a defined CTD (1, 3). It can also be challenging to distinguish idiopathic NSIP from chronic HP and IPF, as all of these conditions can present with a radiological or pathological pattern suggestive of NSIP (38–40). These issues are reflected in the poor interobserver agreement for the diagnosis of idiopathic NSIP, a limitation that exists even among experienced multidisciplinary teams (5).
Diagnostic Limitations in Unclassifiable ILD
The inability to achieve a multidisciplinary diagnosis occurs in approximately 10 to 25% of all patients with ILD (41–48), with a higher prevalence suggested in an elderly population (49). These patients are categorized as “unclassifiable ILD” (1, 3); however, this population includes a diverse collection of patients with no standard definition. Definitions used in previous studies include the absence of a clear diagnosis on the basis of available data and the absence of a clear diagnosis after a complete evaluation with a surgical lung biopsy. There are limitations to each approach. The first definition is strongly influenced by the thoroughness of the diagnostic evaluation, raising concern that the introduction of the term “unclassifiable ILD” into the medical parlance could be used to justify an incomplete evaluation in some patients (43). The second definition is likely more reproducible and rigorous but excludes patients who are unable or unwilling to undergo surgical lung biopsy, leaving these patients in yet another category of diagnostic uncertainty. The high prevalence and heterogeneity of unclassifiable ILD highlight the need for a broader consensus on how to diagnose fibrotic ILDs and how to recognize and classify the heterogeneous collection of patients with undiagnosable disease who have variable clinical courses and prognoses (41, 42).
Proposed Ontological Framework for Classification of Fibrotic ILD
The above discussions demonstrate the need for clarity and consensus around the definitions of and diagnostic criteria for the common forms of fibrotic ILD. This is the task of clinical researchers and experts going forward and is beyond the scope of this article. A second, more immediate need, however, is a consistently applied ontological (i.e., formalized naming and relational) framework for the diagnosis of fibrotic ILDs. This second need was the focus of our working group.
Key considerations for a fibrotic ILD ontological framework include whether to adhere to strict guideline criteria when available, whether and how to indicate the level of diagnostic confidence, whether to report a differential diagnosis, how to approach the definition and terminology of unclassifiable ILD, and whether a single strategy can and should be applied in both clinical and research settings. There are several desirable features of this framework. First, it should use concise, accurate, and unambiguous terminology. Second, it should accommodate all forms of fibrotic ILD and different types of diagnostic criteria (e.g., criteria based vs. consensus based). Third, it should be agnostic to changes in existing diagnostic criteria or the creation of new diagnostic criteria. Finally, it should balance diagnostic certainty with clinical practicality, a balance that may differ in clinical vs. research settings.
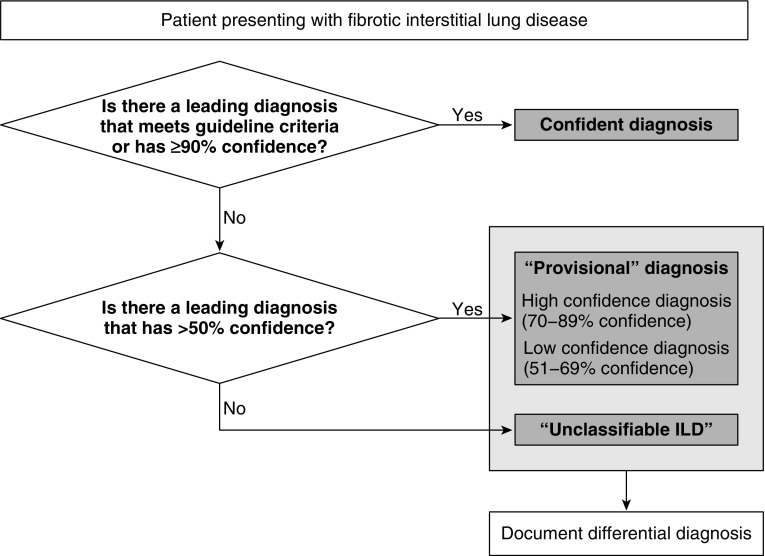
We propose the following ontological framework for the classification of fibrotic ILD (Figure 1). Patients who meet guideline criteria for a specific ILD or who have a 90% or greater likelihood of a diagnosis on the basis of clinical judgment would be provided a confident diagnosis. Patients with a confident diagnosis would not need any preceding descriptor such as “definite,” “confident,” or “confirmed” and would just be called by the diagnostic name (e.g., “idiopathic pulmonary fibrosis,” “hypersensitivity pneumonitis”). Such descriptors were believed to be redundant and potentially create confusion. Patients who do not have a confident diagnosis but who have a leading diagnosis that is considered by the clinician more likely than not (i.e., >50% and <90% likely) on the basis of clinical judgment would be called “provisional” diagnoses. The working group preferred this term to other options (e.g., “probable,” “possible,” “suspected”) as it highlights the diagnostic uncertainty that exists and the need to reassess the diagnosis over time. It also avoids terms that have been used in describing radiological and histopathological patterns. Provisional diagnoses could be further subcategorized as “high confidence” (if diagnostic likelihood was believed to be 70–89%) or “low confidence” (if diagnostic likelihood was believed to be 51–69%). Patients without a leading diagnosis that is considered more likely than not should be categorized as “unclassifiable ILD,” recognizing that this is a diverse collection of patients and not a specific entity. Providing a differential diagnosis for patients without a confident diagnosis (i.e., patients with a provisional diagnosis or who are unclassifiable) was endorsed, as it was believed to aid management decisions and prognostication.
Figure 1.
Proposed approach to the classification of fibrotic interstitial lung disease (ILD).
We believe that “unclassifiable ILD” should remain the primary label for patients who cannot be given a precise diagnosis (i.e., those without a confident or “provisional” diagnosis). There was general support for subcategorization of unclassifiable ILD, in particular by the presence or absence of adequate histopathological sampling (50). “Unclassifiable ILD with adequate biopsy” and “unclassifiable ILD without adequate biopsy” were identified as the most simple and objective terms for these subgroups; however, there was no clear consensus on this terminology or what is considered an adequate biopsy. Further research is specifically needed on the role of transbronchial lung cryobiopsy in patients with fibrotic ILD (51–54). Additional subcategorization approaches were discussed (e.g., stratification by whether IPF is considered a possible diagnosis [41], whether patients possess specific morphological features [41, 42], on the basis of anticipated disease behavior and response to therapy [1, 42]). It was recognized that these and other approaches may apply differently in clinical versus research settings and that additional studies on unclassifiable ILD subgroups are needed to inform an evidence-based approach to addressing the heterogeneity of this population.
A more precise quantification of diagnostic likelihood for the primary diagnosis was not included in this ontology; we believe that this would require greater precision than can be achieved. The proposed four-tiered ontology with confident, provisional (high and low confidence), and unclassifiable ILD seemed the most appropriate and balanced from a clinical perspective, and similar approaches have been successfully adopted in other diseases (55). Future studies are needed to determine the reproducibility of these categories across multidisciplinary groups, identify specific reasons for variability, and evaluate strategies to improve reproducibility. The working group also highlighted the distinction between this framework and the concept of a working diagnosis that could be used to guide management in all patients with ILD, including those with unclassifiable ILD (56).
Conclusions
The absence of a standardized ontological framework for fibrotic ILD has contributed to diagnostic heterogeneity that has significant consequences for clinical care and research. In this perspective, we have proposed a standardized ontological framework for the classification of fibrotic ILD that incorporates strict adherence to guidelines and clinical judgment and uses a disease-agnostic approach to documenting diagnostic certainty that can be applied to all fibrotic ILDs and in a variety of clinical and research settings. This perspective is not intended to replace existing or future diagnostic criteria but rather to provide an ontological framework into which these criteria can be incorporated. We further recognize that this framework is predominantly opinion based and that research is needed to refine this approach. We hope that this document will help standardize fibrotic ILD classification and terminology, leading to the generation of more reliable and generalizable evidence and facilitating future diagnostic guideline development.
Footnotes
Originally Published in Press as DOI: 10.1164/rccm.201702-0400PP on April 17, 2017
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Travis WD, Costabel U, Hansell DM, King TE, Jr, Lynch DA, Nicholson AG, Ryerson CJ, Ryu JH, Selman M, Wells AU, et al. ATS/ERS Committee on Idiopathic Interstitial Pneumonias. An official American Thoracic Society/European Respiratory Society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2013;188:733–748. doi: 10.1164/rccm.201308-1483ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, Colby TV, Cordier JF, Flaherty KR, Lasky JA, et al. ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183:788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Thoracic Society; European Respiratory Society. American Thoracic Society/European Respiratory Society international multidisciplinary consensus classification of the idiopathic interstitial pneumonias: this joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med. 2002;165:277–304. doi: 10.1164/ajrccm.165.2.ats01. [DOI] [PubMed] [Google Scholar]
- 4.Walsh SL, Calandriello L, Sverzellati N, Wells AU, Hansell DM UIP Observer Consort. Interobserver agreement for the ATS/ERS/JRS/ALAT criteria for a UIP pattern on CT. Thorax. 2016;71:45–51. doi: 10.1136/thoraxjnl-2015-207252. [DOI] [PubMed] [Google Scholar]
- 5.Walsh SL, Wells AU, Desai SR, Poletti V, Piciucchi S, Dubini A, Nunes H, Valeyre D, Brillet PY, Kambouchner M, et al. Multicentre evaluation of multidisciplinary team meeting agreement on diagnosis in diffuse parenchymal lung disease: a case-cohort study. Lancet Respir Med. 2016;4:557–565. doi: 10.1016/S2213-2600(16)30033-9. [DOI] [PubMed] [Google Scholar]
- 6.de Andrade J, Schwarz M, Collard HR, Gentry-Bumpass T, Colby T, Lynch D, Kaner RJ IPFnet Investigators. The Idiopathic Pulmonary Fibrosis Clinical Research Network (IPFnet): diagnostic and adjudication processes. Chest. 2015;148:1034–1042. doi: 10.1378/chest.14-2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cottin V. Lung biopsy in interstitial lung disease: balancing the risk of surgery and diagnostic uncertainty. Eur Respir J. 2016;48:1274–1277. doi: 10.1183/13993003.01633-2016. [DOI] [PubMed] [Google Scholar]
- 8.Fell CD, Martinez FJ, Liu LX, Murray S, Han MK, Kazerooni EA, Gross BH, Myers J, Travis WD, Colby TV, et al. Clinical predictors of a diagnosis of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2010;181:832–837. doi: 10.1164/rccm.200906-0959OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brownell R, Moua T, Henry TS, Elicker BM, White D, Vittinghoff E, Jones KD, Urisman A, Aravena C, Johannson KA, et al. The use of pretest probability increases the value of high-resolution CT in diagnosing usual interstitial pneumonia. Thorax. 2017;72:424–429. doi: 10.1136/thoraxjnl-2016-209671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flaherty KR, Andrei AC, King TE, Jr, Raghu G, Colby TV, Wells A, Bassily N, Brown K, du Bois R, Flint A, et al. Idiopathic interstitial pneumonia: do community and academic physicians agree on diagnosis? Am J Respir Crit Care Med. 2007;175:1054–1060. doi: 10.1164/rccm.200606-833OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lynch DA, Newell JD, Logan PM, King TE, Jr, Müller NL. Can CT distinguish hypersensitivity pneumonitis from idiopathic pulmonary fibrosis? AJR Am J Roentgenol. 1995;165:807–811. doi: 10.2214/ajr.165.4.7676971. [DOI] [PubMed] [Google Scholar]
- 12.Adler BD, Padley SP, Müller NL, Remy-Jardin M, Remy J. Chronic hypersensitivity pneumonitis: high-resolution CT and radiographic features in 16 patients. Radiology. 1992;185:91–95. doi: 10.1148/radiology.185.1.1523340. [DOI] [PubMed] [Google Scholar]
- 13.Hansell DM, Wells AU, Padley SP, Müller NL. Hypersensitivity pneumonitis: correlation of individual CT patterns with functional abnormalities. Radiology. 1996;199:123–128. doi: 10.1148/radiology.199.1.8633133. [DOI] [PubMed] [Google Scholar]
- 14.Silva CI, Müller NL, Lynch DA, Curran-Everett D, Brown KK, Lee KS, Chung MP, Churg A. Chronic hypersensitivity pneumonitis: differentiation from idiopathic pulmonary fibrosis and nonspecific interstitial pneumonia by using thin-section CT. Radiology. 2008;246:288–297. doi: 10.1148/radiol.2453061881. [DOI] [PubMed] [Google Scholar]
- 15.Takemura T, Akashi T, Kamiya H, Ikushima S, Ando T, Oritsu M, Sawahata M, Ogura T. Pathological differentiation of chronic hypersensitivity pneumonitis from idiopathic pulmonary fibrosis/usual interstitial pneumonia. Histopathology. 2012;61:1026–1035. doi: 10.1111/j.1365-2559.2012.04322.x. [DOI] [PubMed] [Google Scholar]
- 16.Lima MS, Coletta EN, Ferreira RG, Jasinowodolinski D, Arakaki JS, Rodrigues SC, Rocha NA, Pereira CA. Subacute and chronic hypersensitivity pneumonitis: histopathological patterns and survival. Respir Med. 2009;103:508–515. doi: 10.1016/j.rmed.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 17.Churg A, Sin DD, Everett D, Brown K, Cool C. Pathologic patterns and survival in chronic hypersensitivity pneumonitis. Am J Surg Pathol. 2009;33:1765–1770. doi: 10.1097/PAS.0b013e3181bb2538. [DOI] [PubMed] [Google Scholar]
- 18.Morell F, Villar A, Montero MA, Muñoz X, Colby TV, Pipvath S, Cruz MJ, Raghu G. Chronic hypersensitivity pneumonitis in patients diagnosed with idiopathic pulmonary fibrosis: a prospective case-cohort study. Lancet Respir Med. 2013;1:685–694. doi: 10.1016/S2213-2600(13)70191-7. [DOI] [PubMed] [Google Scholar]
- 19.Churg A, Muller NL, Flint J, Wright JL. Chronic hypersensitivity pneumonitis. Am J Surg Pathol. 2006;30:201–208. doi: 10.1097/01.pas.0000184806.38037.3c. [DOI] [PubMed] [Google Scholar]
- 20.Akashi T, Takemura T, Ando N, Eishi Y, Kitagawa M, Takizawa T, Koike M, Ohtani Y, Miyazaki Y, Inase N, et al. Histopathologic analysis of sixteen autopsy cases of chronic hypersensitivity pneumonitis and comparison with idiopathic pulmonary fibrosis/usual interstitial pneumonia. Am J Clin Pathol. 2009;131:405–415. doi: 10.1309/AJCPNWX4SLZRP9SW. [DOI] [PubMed] [Google Scholar]
- 21.Yoshizawa Y, Ohtani Y, Hayakawa H, Sato A, Suga M, Ando M. Chronic hypersensitivity pneumonitis in Japan: a nationwide epidemiologic survey. J Allergy Clin Immunol. 1999;103:315–320. doi: 10.1016/s0091-6749(99)70507-5. [DOI] [PubMed] [Google Scholar]
- 22.Fenoglio CM, Reboux G, Sudre B, Mercier M, Roussel S, Cordier JF, Piarroux R, Dalphin JC. Diagnostic value of serum precipitins to mould antigens in active hypersensitivity pneumonitis. Eur Respir J. 2007;29:706–712. doi: 10.1183/09031936.00001006. [DOI] [PubMed] [Google Scholar]
- 23.Cormier Y, Bélanger J, Durand P. Factors influencing the development of serum precipitins to farmer’s lung antigen in Quebec dairy farmers. Thorax. 1985;40:138–142. doi: 10.1136/thx.40.2.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cormier Y, Létourneau L, Racine G. Significance of precipitins and asymptomatic lymphocytic alveolitis: a 20-yr follow-up. Eur Respir J. 2004;23:523–525. doi: 10.1183/09031936.04.00021104. [DOI] [PubMed] [Google Scholar]
- 25.Chmelik F, Flaherty DK, Reed CE. Precipitating antibodies in office workers and hospitalized patients directed toward antigens causing hypersensitivity pneumonitis. Am Rev Respir Dis. 1975;111:201–205. doi: 10.1164/arrd.1975.111.2.201. [DOI] [PubMed] [Google Scholar]
- 26.Moore VL, Fink JN. Immunologic studies in hypersensitivity pneumonitis: quantitative precipitins and complement-fixing antibodies in symptomatic and asymptomatic pigeon breeders. J Lab Clin Med. 1975;85:540–545. [PubMed] [Google Scholar]
- 27.Fink JN, Schlueter DP, Sosman AJ, Unger GF, Barboriak JJ, Rimm AA, Arkins JA, Dhaliwal KS. Clinical survey of pigeon breeders. Chest. 1972;62:277–281. doi: 10.1378/chest.62.3.277. [DOI] [PubMed] [Google Scholar]
- 28.doPico GA, Reddan WG, Chmelik F, Peters ME, Reed CE, Rankin J. The value of precipitating antibodies in screening for hypersensitivity pneumonitis. Am Rev Respir Dis. 1976;113:451–455. doi: 10.1164/arrd.1976.113.4.451. [DOI] [PubMed] [Google Scholar]
- 29.Dalphin JC, Toson B, Monnet E, Pernet D, Dubiez A, Laplante JJ, Aiache JM, Depierre A. Farmer’s lung precipitins in Doubs (a department of France): prevalence and diagnostic value. Allergy. 1994;49:744–750. doi: 10.1111/j.1398-9995.1994.tb02097.x. [DOI] [PubMed] [Google Scholar]
- 30.Cantin A, Bégin R, Boileau R, Drapeau G, Rola-Pleszczynski M. Features of bronchoalveolar lavage differentiating hypersensitivity pneumonitis and pulmonary sarcoidosis at time of initial presentation. Clin Invest Med. 1984;7:89–94. [PubMed] [Google Scholar]
- 31.Godard P, Clot J, Jonquet O, Bousquet J, Michel FB. Lymphocyte subpopulations in bronchoalveolar lavages of patients with sarcoidosis and hypersensitivity pneumonitis. Chest. 1981;80:447–452. doi: 10.1378/chest.80.4.447. [DOI] [PubMed] [Google Scholar]
- 32.Weinberger SE, Kelman JA, Elson NA, Young RC, Jr, Reynolds HY, Fulmer JD, Crystal RG. Bronchoalveolar lavage in interstitial lung disease. Ann Intern Med. 1978;89:459–466. doi: 10.7326/0003-4819-89-4-459. [DOI] [PubMed] [Google Scholar]
- 33.Meyer KC, Raghu G, Baughman RP, Brown KK, Costabel U, du Bois RM, Drent M, Haslam PL, Kim DS, Nagai S, et al. American Thoracic Society Committee on BAL in Interstitial Lung Disease. An official American Thoracic Society clinical practice guideline: the clinical utility of bronchoalveolar lavage cellular analysis in interstitial lung disease. Am J Respir Crit Care Med. 2012;185:1004–1014. doi: 10.1164/rccm.201202-0320ST. [DOI] [PubMed] [Google Scholar]
- 34.Singh S, Collins BF, Sharma BB, Joshi JM, Talwar D, Katiyar S, Singh N, Ho L, Samaria JK, Bhattacharya P, et al. Interstitial lung disease (ILD) in India: results of a prospective registry. Am J Respir Crit Care Med. 2017;195:801–813. doi: 10.1164/rccm.201607-1484OC. [DOI] [PubMed] [Google Scholar]
- 35.Travis WD, Hunninghake G, King TE, Jr, Lynch DA, Colby TV, Galvin JR, Brown KK, Chung MP, Cordier JF, du Bois RM, et al. Idiopathic nonspecific interstitial pneumonia: report of an American Thoracic Society project. Am J Respir Crit Care Med. 2008;177:1338–1347. doi: 10.1164/rccm.200611-1685OC. [DOI] [PubMed] [Google Scholar]
- 36.Kinder BW, Collard HR, Koth L, Daikh DI, Wolters PJ, Elicker B, Jones KD, King TE., Jr Idiopathic nonspecific interstitial pneumonia: lung manifestation of undifferentiated connective tissue disease? Am J Respir Crit Care Med. 2007;176:691–697. doi: 10.1164/rccm.200702-220OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fischer A, Antoniou KM, Brown KK, Cadranel J, Corte TJ, du Bois RM, Lee JS, Leslie KO, Lynch DA, Matteson EL, et al. “ERS/ATS Task Force on Undifferentiated Forms of CTD-ILD”. An official European Respiratory Society/American Thoracic Society research statement: interstitial pneumonia with autoimmune features. Eur Respir J. 2015;46:976–987. doi: 10.1183/13993003.00150-2015. [DOI] [PubMed] [Google Scholar]
- 38.Hanak V, Golbin JM, Ryu JH. Causes and presenting features in 85 consecutive patients with hypersensitivity pneumonitis. Mayo Clin Proc. 2007;82:812–816. doi: 10.4065/82.7.812. [DOI] [PubMed] [Google Scholar]
- 39.Fernández Pérez ER, Swigris JJ, Forssén AV, Tourin O, Solomon JJ, Huie TJ, Olson AL, Brown KK. Identifying an inciting antigen is associated with improved survival in patients with chronic hypersensitivity pneumonitis. Chest. 2013;144:1644–1651. doi: 10.1378/chest.12-2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mooney JJ, Elicker BM, Urbania TH, Agarwal MR, Ryerson CJ, Nguyen ML, Woodruff PG, Jones KD, Collard HR, King TE, et al. Radiographic fibrosis score predicts survival in hypersensitivity pneumonitis. Chest. 2013;144:586–592. doi: 10.1378/chest.12-2623. [DOI] [PubMed] [Google Scholar]
- 41.Ryerson CJ, Urbania TH, Richeldi L, Mooney JJ, Lee JS, Jones KD, Elicker BM, Koth LL, King TE, Jr, Wolters PJ, et al. Prevalence and prognosis of unclassifiable interstitial lung disease. Eur Respir J. 2013;42:750–757. doi: 10.1183/09031936.00131912. [DOI] [PubMed] [Google Scholar]
- 42.Hyldgaard C, Bendstrup E, Wells AU, Hilberg O. Unclassifiable interstitial lung diseases: clinical characteristics and survival. Respirology. 2017;22:494–500. doi: 10.1111/resp.12931. [DOI] [PubMed] [Google Scholar]
- 43.Troy L, Glaspole I, Goh N, Zappala C, Hopkins P, Wilsher M, Moodley Y, Corte T. Prevalence and prognosis of unclassifiable interstitial lung disease. Eur Respir J. 2014;43:1529–1530. doi: 10.1183/09031936.00003414. [DOI] [PubMed] [Google Scholar]
- 44.Ooi A, Iyenger S, Ferguson J, Ritchie AJ. VATS lung biopsy in suspected, diffuse interstitial lung disease provides diagnosis, and alters management strategies. Heart Lung Circ. 2005;14:90–92. doi: 10.1016/j.hlc.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 45.Sigurdsson MI, Isaksson HJ, Gudmundsson G, Gudbjartsson T. Diagnostic surgical lung biopsies for suspected interstitial lung diseases: a retrospective study. Ann Thorac Surg. 2009;88:227–232. doi: 10.1016/j.athoracsur.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 46.Zhang D, Liu Y. Surgical lung biopsies in 418 patients with suspected interstitial lung disease in China. Intern Med. 2010;49:1097–1102. doi: 10.2169/internalmedicine.49.3225. [DOI] [PubMed] [Google Scholar]
- 47.Casoni GL, Tomassetti S, Cavazza A, Colby TV, Dubini A, Ryu JH, Carretta E, Tantalocco P, Piciucchi S, Ravaglia C, et al. Transbronchial lung cryobiopsy in the diagnosis of fibrotic interstitial lung diseases. PLoS One. 2014;9:e86716. doi: 10.1371/journal.pone.0086716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Karakatsani A, Papakosta D, Rapti A, Antoniou KM, Dimadi M, Markopoulou A, Latsi P, Polychronopoulos V, Birba G, Ch L, et al. Hellenic Interstitial Lung Diseases Group. Epidemiology of interstitial lung diseases in Greece. Respir Med. 2009;103:1122–1129. doi: 10.1016/j.rmed.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 49.Patterson KC, Shah RJ, Porteous MK, Christie JD, D’Errico CA, Chadwick M, Triano MJ, Deshpande C, Rossman MD, Litzky LA, et al. Interstitial lung disease in the elderly. Chest. 2017;151:838–844. doi: 10.1016/j.chest.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 50.Cottin V, Wells A. Unclassified or unclassifiable interstitial lung disease: confusing or helpful disease category? Eur Respir J. 2013;42:576–579. doi: 10.1183/09031936.00107713. [DOI] [PubMed] [Google Scholar]
- 51.Ganganah O, Guo SL, Chiniah M, Li YS. Efficacy and safety of cryobiopsy versus forceps biopsy for interstitial lung diseases and lung tumours: a systematic review and meta-analysis. Respirology. 2016;21:834–841. doi: 10.1111/resp.12770. [DOI] [PubMed] [Google Scholar]
- 52.Johannson KA, Marcoux VS, Ronksley PE, Ryerson CJ. Diagnostic yield and complications of transbronchial lung cryobiopsy for interstitial lung disease: a systematic review and metaanalysis. Ann Am Thorac Soc. 2016;13:1828–1838. doi: 10.1513/AnnalsATS.201606-461SR. [DOI] [PubMed] [Google Scholar]
- 53.Sharp C, McCabe M, Adamali H, Medford AR. Use of transbronchial cryobiopsy in the diagnosis of interstitial lung disease-a systematic review and cost analysis. QJM. 2017;110:207–214. doi: 10.1093/qjmed/hcw142. [DOI] [PubMed] [Google Scholar]
- 54.Tomassetti S, Wells AU, Costabel U, Cavazza A, Colby TV, Rossi G, Sverzellati N, Carloni A, Carretta E, Buccioli M, et al. Bronchoscopic lung cryobiopsy increases diagnostic confidence in the multidisciplinary diagnosis of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2016;193:745–752. doi: 10.1164/rccm.201504-0711OC. [DOI] [PubMed] [Google Scholar]
- 55.PIOPED Investigators. Value of the ventilation/perfusion scan in acute pulmonary embolism: results of the prospective investigation of pulmonary embolism diagnosis (PIOPED) JAMA. 1990;263:2753–2759. doi: 10.1001/jama.1990.03440200057023. [DOI] [PubMed] [Google Scholar]
- 56.Wells AU. “Any fool can make a rule and any fool will mind it”. BMC Med. 2016;14:23. doi: 10.1186/s12916-016-0562-1. [DOI] [PMC free article] [PubMed] [Google Scholar]