Pulmonary arterial hypertension (PAH) has been frequently compared with a type of malignant disease and has an incredible number of pathogenic mechanisms similar to cancer (1–3). A cancer model for PAH was first proposed in 1998 by Voelkel and colleagues (4) and had been confirmed and expanded by later studies from many research groups. A critical role for a cancer-like metabolic shift from oxidative phosphorylation to glycolysis despite adequate oxygen supply (the Warburg effect) in PAH pathogenesis had been demonstrated; multiple cancer-shared abnormalities in mitochondrial metabolism and dynamics have been reported as key modulators of pathogenic changes in PAH pulmonary vasculature and right ventricle (RV), including mitochondrial hyperpolarization, altered activity of mitochondrial pyruvate dehydrogenase, superoxide dismutase 2 deficiency, fragmentation and/or hyperpolarization of the mitochondrial reticulum, and dysregulated mitochondrial dynamics due to down-regulation of the fusion protein mitofusin 2 and up-regulation of the fission protein dynamin-related protein 1. The benefits of mitochondria-targeting strategies (i.e., pyruvate dehydrogenase kinase inhibitor, dichloroacetate) have been demonstrated for both experimental pulmonary hypertension (PH) and human cancer, suggesting applicability of cancer-targeting therapies to human PAH (5–8).
Another line of evidence supporting similarities between PAH and cancer came from the microRNA (miR) field. Dysregulation of numerous microRNAs, reported in human cancers, appeared to play an important role in multiple features of PAH pathogenesis, including pulmonary vascular remodeling, inflammation, impaired angiogenesis, and RV hypertrophy (reviewed in depth by Courboulin and colleagues [9]).
The existence of such fundamental similarities with cancer not only dramatically changed our current view on the mechanisms of PAH pathogenesis, but also triggered the development of novel treatment strategies for patients with PAH.
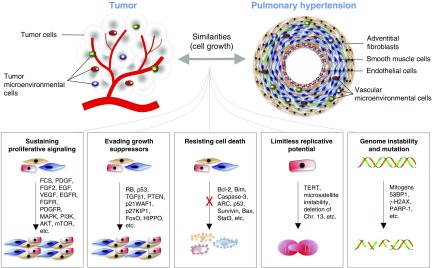
In this context, a specific cluster of tumor hallmarks certainly plays a more prominent role compared with the others; therefore the application of the Hanahan and Weinberg approach (10, 11) could be useful to effectively summarize the state of the art in PAH research, emphasizing the most promising areas of investigation. Various cellular processes that characterize the pathogenesis of both PAH and cancer have been identified: sustained proliferative signaling, evasion of growth suppressors, resistance to apoptosis, deregulation of cellular energetics, and limitless replicative potential and DNA instability due to epigenetic and genetic alterations, along with the activation of specific signal transduction. Similarly, chronic inflammation, pathological angiogenesis, and immune system evasion are also features that characterize the pathogenesis of both PAH and cancer (1–3).
The aim of this article is to review the scientific reasons that support the intriguing vision of PAH as a disease with a cancer-like nature (Figure 1) and to understand whether this point of view may have fruitful consequences for the overall management of PAH. For these reasons, we attempt to review various signal transduction pathways that act as “central signaling hubs” in PAH and cancer, particularly those that have a crucial role in driving pulmonary vascular cell proliferation and survival, and discuss new opportunities for the cross-development of anticancer agents that can be used to improve PAH care; the current cellular, preclinical, and clinical status of these so-called “oncological compounds”; and future perspectives for the treatment of PAH.
Figure 1.
Shared pathological mechanisms respective to cell growth between cancer and pulmonary arterial hypertension. 53BP1 = p53-binding protein 1; AKT = v-akt murine thymoma viral oncogene homolog; ARC = apoptosis repressor with caspase recruitment domain; Bax = Bcl-2–associated X protein; Bcl-2 = B-cell lymphoma 2; Bim = Bcl-2–interacting mediator of cell death; Chr. = chromosome; EGF = epidermal growth factor; EGFR = epidermal growth factor receptor; FCS = fetal calf serum; FGF-2 = fibroblast growth factor 2; FGFR = fibroblast growth factor receptor; FoxO = forkhead-box class O; γ-H2AX = histone H2A variant H2AX phosphorylated at Ser-139; MAPK = mitogen-activated protein kinase; mTOR = mechanistic target of rapamycin; p21WAF1 = cyclin-dependent kinase inhibitor 1A; p27KIP1 = cyclin-dependent kinase inhibitor 1B; p53 = tumor protein 53; PARP-1 = poly(ADP-ribose) polymerase 1; PDGF = platelet-derived growth factor; PDGFR = platelet-derived growth factor receptor; PI3K = phosphatidylinositol 3-kinase; PTEN = phosphatase and tensin homolog; RB = retinoblastoma; Stat3 = signal transducer and activator of transcription 3; TERT = telomerase reverse transcriptase; TGF-β1 = transforming growth factor-β1; VEGF = vascular endothelial growth factor.
Pathogenesis of Pulmonary Arterial Hypertension
PAH (group I PH) is a severe and progressive disease, characterized by increased pulmonary vascular resistance (PVR) culminating in right heart failure and premature death. The pathogenesis of PAH is multifactorial, and includes remodeling of pulmonary vascular walls, concentric disintegration of the vessel lumen, varying degrees of inflammation, as well as thrombosis in situ (12). A hallmark of vascular remodeling in PAH is medial and adventitial hypertrophy due to increased proliferation and resistance to apoptosis of pulmonary artery smooth muscle cells (PASMCs) and accumulation of pulmonary artery adventitial fibroblasts (PAAFs) and myofibroblasts (12, 13), neomuscularization of small peripheral PAs, and intimal thickening and vessel occlusion associated with pulmonary artery endothelial cell (PAEC) dysfunction (14). Data suggest a significant role for inflammation in driving vascular remodeling (15). As a functional consequence of these structural/functional changes, the PVR drastically increases, causing an increase in RV afterload, RV hypertrophy, and failure.
Analogous Features with Carcinogenesis
Sustaining Proliferative Signaling
In contrast to nondiseased cells, cancer cells do not require external growth signals and are able to sustain chronic proliferation (10, 11) via at least five different mechanisms: constitutive activation of cell surface receptors, overexpression of cell surface receptors, constitutive activation of signaling proteins downstream from the receptors, release of its own growth signal, and stimulation of nearby normal (stromal) cells to produce growth factors (16).
At present, there is no evidence of structurally abnormal receptors in PAH pulmonary vascular cells that can be constitutively active in the absence of growth factors. However, PAECs and PASMCs derived from patients with PAH have increased proliferative response to mitogenic stimuli, including fetal calf serum, fibroblast growth factor (FGF)-2, epidermal growth factor (EGF), vascular endothelial growth factor (VEGF), and platelet-derived growth factor (PDGF), and are less sensitive to apoptosis induction by serum deprivation (17–22). Such hyperproliferative potential could be explained by overexpression and/or activation of receptor tyrosine kinases (RTKs), including EGF, FGF, and PDGF receptors (EGFR, FGFR, and PDGFR, respectively) (23), up-regulation of which is also found in many cancers. EGFR is widely up-regulated and mediates cell proliferation, protection from apoptosis, and motility in solid tumors (16), and its activation by serine elastases is implicated in the pathobiology of PAH (24). Moreover, the expression of both PDGF and PDGFR is significantly increased in lung tissue from the lungs of patients with PAH compared with healthy donor lungs (25). PDGF and EGF stimulate PASMC proliferation and may be involved in the vascular changes observed in PAH (26, 27).
The VEGF receptor (VEGFR) is another RTK that frequently contributes to tumor progression (28) and PAEC proliferation in severe PAH (29). To note, PAECs in plexiform lesions overexpress both VEGF and VEGFR-2, suggesting that PAECs are self-stimulated by their own growth signals (30).
Pulmonary vascular cells may also be supplied with an excessive amount of growth factors by neighboring cells. PAECs from patients with idiopathic pulmonary arterial hypertension (IPAH) release excessive amounts of soluble growth factors and cytokines, including endothelin-1, serotonin, and FGF-2, which, in addition to their ability to act on PAECs in an autocrine manner (19), also may contribute to the increased SMC proliferation in a paracrine manner (21, 31).
Not surprisingly, both cancer and PAH pulmonary vascular resident cells demonstrate constitutive activation of RTK effector pathways, including mitogen-activated protein kinases (MAPKs), phosphatidylinositol 3-kinase (PI3K)–Akt, and mechanistic target of rapamycin (mTOR) (32, 33), suggesting major contribution of growth factor–induced signaling to the PAH pathology (22, 26, 34). Interestingly, the Akt–mTOR pathway negatively regulates expression of mitochondrial fusion protein mitofusin 2 (35, 36), a deficiency of which had been reported in PAH PASMCs (8). Of note, mitofusin 2 also acts like a Ras effector molecule and suppresses Raf–MAPK signaling and cell proliferation via binding with Ras at the effector binding domain (37, 38), providing a molecular link from PI3K–Akt–mTOR to Raf–MAPK activation and increased PASMC proliferation in PAH.
Of note, constitutive hyperactivation of Ras–Raf–MAPK and PI3K–Akt–mTOR in cancer cells can also be driven by activating (e.g., mutations in Ras, Raf isoforms, and PI3K catalytic subunit p110α) or loss-of-function mutations (e.g., deletion of PTEN [phosphatase and tensin homolog] on chromosome 10), and amplification of Akt isoforms (39, 40). At present, there is no evidence of such alterations in human PAH. However, PASMCs and PAAFs from human PAH lungs demonstrate unstimulated hyperactivation of Akt–mTOR and inactivation of forkhead box O (FoxO), which support elevated proliferation and apoptosis resistance (15, 17, 22, 41, 42). Reports indicate that activation of the Akt–mTOR axis and the self-sustained proliferation/apoptosis resistance of PASMCs and PAAFs in PAH may also be driven by self-supporting signaling circuits, for example, the HIPPO–Yap/Taz–fibronectin–integrin-linked kinase 1 (ILK1) feed-forward loop (PASMCs) (42) and the YAP/TAZ–miR-130/301 feedback circuit (PAAFs) (43), which are initiated by changes in extracellular matrix (ECM) composition and/or stiffness and amplify PH via further modulation of ECM and/or secretion of vasoactive effectors.
Evading Growth Suppressors
In addition to sustaining proliferative signaling, cancer cells circumvent powerful programs that negatively regulate cell proliferation and become insensitive to antigrowth signals. Well-described growth suppressors are retinoblastoma (RB)-associated and p53 proteins (11). Other examples include transforming growth factor (TGF)-β, PTEN, FoxO, p21WAF1, p27KIP1 (44), and HIPPO (45).
p53 (also known as tumor protein 53) is one of the best known tumor suppressor proteins, genetic defects and/or reduced activity of which is linked to human cancers (46). Although p53 mutations were never described in PH, p53 knockout mice develop more severe PH under chronic hypoxia compared with wild-type mice (47). Interestingly, direct pharmacological inactivation of p53 (pifithrin-α) induces pulmonary vascular remodeling and/or aggravates monocrotaline-induced PH (MCT-PH) in rats (48).
The RB pathway consists of five families of proteins: CDKN (e.g., Ink4a), D-type cyclins, cyclin-dependent protein kinases (cdk4, cdk6), the RB family of pocket proteins (RB, p107, p130), and the E2F family of transcription factors. Several components of this pathway, that is, p16Ink4a, cyclin D1, and RB, are frequently altered in cancer cells (49). Although no reports have evaluated the RB pathway in detail, up-regulation of cyclin D1 and p16 (plexiform lesions) (3, 17) and reduced expression of cyclin-dependent kinase inhibitors (CDKIs) p21CIP1 and p27KIP1 (plexiform lesions) (50, 51), are reported in the pulmonary vasculature from patients with PAH and in animal models of PH. Importantly, the modulation of CDKIs is suggested to have a therapeutic benefit, for example, nebulization of nitrite was shown to exert therapeutic benefit in limiting PAH via up-regulation of p21CIP1 (52).
The PTEN tumor suppressor inhibits cell growth, proliferation, and survival via inactivating PI3K-dependent signaling. PTEN is one of the most commonly lost tumor suppressors in human cancer (53), and its inactivation is also reported in the pulmonary vasculature of patients with PH/animal models of PH (54, 55). Further, PASMC-specific chronic inactivation of PTEN represents a critical mediator of PH progression, leading to cell-autonomous events and increased production of growth factors and cytokines (55).
Several epithelial malignancies are characterized by insensitivity of cells to the homeostatic effect of TGF-β. TGF-β inhibits growth of many cell types by blocking cyclin–CDK complexes that inactivate pRB (10). Many inactivating mutations in TGF-β receptors (TGF-βRs) and Smad genes have been found to be an underlying cause for human cancer (56).
Along a similar line, reduced expression or function of bone morphogenic protein receptor II (BMPRII), a member of the TGF-βR superfamily, is an essential characteristic of PASMCs from patients with PAH (57). An association between mutations in BMPRII (BMPR2), ALK1 (ACVRL1), and SMAD8 (SMAD8) and PAH has been described (58–60). Indeed, there is a growing literature that associates BMPs and their receptors with cell growth control in both cancer (61) and PAH (62). PASMCs from patients with PAH show an altered growth response to BMPs (63), implying that mutations in BMPRII could lead to BMP resistance. Moreover, reduced expression or function of BMPRII may lead to exaggerated TGF-β signaling, which was strongly supported by enhanced activity of the TGF-β pathway in human PAH lungs (57, 64).
The HIPPO tumor suppressor cassette (catalytic core includes protein kinases MST1/2 and LATS1/2) controls organ size by inhibiting proliferation and inducing differentiation and/or apoptosis. HIPPO is inactivated in a broad range of human carcinomas (45), and in PASMCs in human PAH lungs and two PH rodent models (42). It appeared to be required for up-regulating transcriptional coactivators Yap and Taz; concomitant activation of several pro-proliferative/prosurvival pathways, including Akt–mTOR; and maintenance of the PASMC proliferative/apoptosis-resistant phenotype (42).
Resisting Cell Death
Apoptosis resistance, one of the main features of cancer (10), has been implicated in the pathogenesis of PAH. Compared with healthy cells, IPAH-PAECs and IPAH-PASMCs display enhanced responses to growth factors ex vivo due to, in part, decreased apoptosis (17, 18). The absence of apoptotic cells in plexiform vascular lesions in human PAH (65) and experimental Sugen/hypoxia PH (66) further supports the concept that apoptosis-resistant endothelial cells (ECs) contribute to PH pathogenesis. Similarly, lack of apoptosis in PASMCs has been considered one of the culprits leading to uncontrolled PASMC proliferation (17, 20, 22).
One of the mechanisms of apoptosis resistance in vascular cells is an imbalance of proapoptotic and antiapoptotic proteins. Among those, the B-cell lymphoma-2 (Bcl-2) family, the caspase family, p53, and the inhibitors of apoptosis protein (IAP) were shown to be dysregulated in PAH (63, 67–69).
The Bcl-2 protein is overexpressed in many cancers and prevents apoptosis by inhibiting mitochondrial release of cytochrome c (70). Similarly, overexpression of Bcl-2 and deficiency of proapoptotic members of the Bcl-2 gene family, Bax and Bim, is observed in plexiform lesions, PAECs in severely damaged PAs (67), and microvascular PASMCs from IPAH lungs (22). As with cancers, antisense Bcl-2 strategies proved to be effective in the treatment of experimental PH (71).
In a rodent model of angioproliferative PH, caspase inhibition prevents the growth of intravascular PAECs and protects against development of severe PH (68). Further, increased expression of apoptosis repressor with caspase recruitment domain (ARC), an endogenous inhibitor of cell death, had been reported to increase in lumen-occluding lesions of patients with PAH (69).
Survivin, a member of the IAP family, is expressed in essentially all cancers but not in most nondiseased adult cell types (72). However, survivin is markedly expressed in PASMCs of patients with PAH, and inhalative adenoviral gene therapy, employing a dominant negative mutant of survivin, reversed MCT-PH (63).
Further molecular abnormalities contributing to the apoptosis resistance in PAH include dysregulation or increased expression of signal transducer and activator of transcription 3 (STAT3) and changes in the transcription factors (TFs) nuclear factor of activated T cells (NFAT) and FoxO (17, 73, 74).
Limitless Replicative Potential
Apart from the features described previously, tumor cells attain limitless replicative potential to ensure expansive tumor growth (11) and possess two main abnormalities, namely immortality and monoclonality (10).
The molecular mechanism determining replicative potential seems to be controlled by a single process, that of telomere shortening. Telomerase that serves to maintain telomere length is markedly up-regulated in 90% of human tumors (75). Similarly, telomerase reverse transcriptase (TERT), the protein component of telomerase, is up-regulated in PASMCs from remodeled PAs in both patients with IPAH and mice with experimentally induced PH (76).
Monoclonality represents the earliest event involved in the generation of neoplasms (77). Notably, PAECs within plexiform lesions of patients with IPAH expand in a monoclonal fashion, whereas secondary PH lesions develop via polyclonal EC expansion (78). The monoclonal expansion of PAECs in IPAH might be due to the disruption of a cell-autonomous limitation in replicative potential. In support, these proliferative PAECs demonstrated somatic genomic abnormalities such as microsatellite instability and deletion of chromosome 13 with concomitant perturbation of growth- and apoptosis-related gene expression (SMAD9, TGF-βRII, RB1, BRCA2 [breast cancer type 2 susceptibility protein], and Bax), akin to neoplasia (79–81).
Genome Instability and Mutations
Genomic instability is a hallmark of cancer that leads to an increase in genetic alterations, thus enabling the acquisition of additional capabilities for carcinogenesis (11, 82). Interestingly, levels of baseline and mutagen-induced DNA damage are intrinsically higher in heritable, idiopathic, or associated PAH PAECs. In addition, increased genomic instability (instability of short DNA microsatellite sequences), vulnerability to DNA double-strand breaks, and dysregulation of several DNA repair–associated genes (e.g., TopBP1) have been identified in PAH, similar to cancer (83, 84).
As in cancer, DNA double-strand breaks and microsatellite instability could manifest as exaggerated DNA damage that, while leading to decreased survival of resident vascular cells, also may give rise to clonally derived vascular cell populations bearing a PAH-specific hyperproliferative and apoptosis-resistant phenotype (79). Vulnerability to DNA double-strand breaks and dysregulation of several DNA repair–associated genes due to ongoing inflammation or induced by the PAH environment provides further selective advantage by promoting exaggerated contractility and proliferation of vascular cells (85). In that way, clonal selection may be continued through PAH development.
Furthermore, genomic instability could also be attributable to failure of DNA repair mechanisms, as Li and colleagues demonstrated that PAH-associated EC dysfunction and genomic instability are mediated through BMPR2 deficiency–associated loss of DNA damage control (86). Interestingly, in human PAH PASMCs, increased DNA damage was associated with up-regulation of poly(ADP-ribose) polymerase (PARP)-1, a critical enzyme implicated in DNA repair, which caused down-regulation of miR-204 and the subsequent activation of NFAT and hypoxia-inducible factor 1α (HIF1α), supporting cell proliferation and apoptosis resistance (87).
Pulmonary Vascular Cell Proliferation and Cell Cycle Dysregulation
Advances in PAH research, discussed previously, have allowed us to gain a more detailed view of the “molecular circuitry” of PAH. Given that available therapies, although providing symptomatic improvement, largely fail to reverse established pulmonary vascular remodeling (88), effective antiproliferative/proapoptotic strategies are needed. Emerging similarities between the mechanisms driving proliferation/apoptosis imbalance in the PAH pulmonary vasculature and cancers provide the exciting opportunity to employ certain cancer-specific strategies and/or to repurpose anticancer agents for the treatment of PAH (summarized in Table 1). To date, substantial progress had been made in preclinical and clinical testing of RTK inhibitors, developed for the treatment of cancers.
Table 1.
Targeting Signaling Pathways Involved in Cancer Biology for Controlling/Regressing Enhanced Proliferation, Survival, and Apoptosis Resistance of Lung Vascular Cells in Pulmonary Hypertension
| Molecule/Pathway | Cell Type | Status in PAH/Experimental PH | Function | Inhibitor(s)/Activator(s) Tested | Effect of Inhibitor(s)/Activator(s) |
|---|---|---|---|---|---|
| RTKs | |||||
| PDGFR | PASMCs | PASMCs: | PASMCs: | Imatinib | Inhibits proliferation of human IPAH and rat MCT-PH PASMCs (25) |
| PAECs | ↑ Human IPAH | ↑ Proliferation | Reverses MCT-PH in rats (25, 94) | ||
| ↑ Rat HPH | ↓ Apoptosis | ||||
| ↑ Rat MCT-PH | |||||
| Showed efficacy in phase II (121) and III (123) trials | |||||
| Improves PVR and 6MWD in patients with severe PAH (123) | |||||
| Nilotinib | Reverses pulmonary vascular remodeling in rat MCT-PH and HPH (94) | ||||
| EGFR | PASMCs | No significant alterations in experimental/clinical PH | PASMCs: | PKI166 | Mediates apoptosis in PA organ culture (24) |
| ↑ Proliferation | Attenuates rat MCT-PH (24) | ||||
| ↓ Apoptosis | Gefitinib | Attenuates rat MCT-PH (95) | |||
| No significant benefits in mouse HPH (95) | |||||
| Erlotinib | Attenuates rat MCT-PH (95) | ||||
| No significant benefits in mouse HPH (95) | |||||
| Lapatinib | No therapeutic benefit in experimental PH (95) | ||||
| FGFR | PAECs | ↑ Human IPAH (FGF-2) | PAECs: | SU5402 | Reverses rat MCT-PH (21) |
| PASMCs | ↑ Rat MCT-PH (FGF-2, FGFR1) | ↑ Proliferation | |||
| PASMCs: | |||||
| ↑ Proliferation | |||||
| Ras/Raf/MAPK | PAECs | ↑ Rat MCT-PH (p-Raf-1, p-ERK) | PAECs: | Sorafenib | Attenuates rat MCT-PH (98) |
| ↑ Rat HPH | ↑ Proliferation | ||||
| ↑ Rat SuHx-PH (p-ERK, p-MEK1/2) | Cardiomyocytes: | Improves RV function in PAB rats (99) | |||
| ↓ Vasopressin-induced hypertrophy | Attenuates rat HPH and SuHx-PH (34) | ||||
| PI3K | PASMCs PAECs | Unknown | PASMCs: | LY294002 | Inhibits growth factor–induced PASMC proliferation and migration (27) |
| ↑ Mitogen-induced proliferation and migration | |||||
| PAECs: | Attenuates development of HPH in rats (109) | ||||
| ↑ Proliferation | |||||
| ↓ Apoptosis | |||||
| ↑ NO production and vasodilation | |||||
| Akt | PASMCs PAAFs PAECs | PASMCs: | PASMCs: | Triciribine | Attenuates development of HPH in rats (109) |
| ↑ Human IPAH | ↑ Proliferation | ||||
| ↑ Rat HPH | ↓ Apoptosis | ||||
| PAAFs: | PAAFs: | ||||
| ↑ Human IPAH | ↑ Proliferation | ||||
| ↑ Rat HPH | PAECs: | ||||
| PAECs: | ↑ Proliferation | ||||
| Unknown | ↓ Apoptosis | ||||
| ↑ NO production and vasodilation | |||||
| mTORC1 | PASMCs | ↑ Human IPAH | ↑ Proliferation | Rapamycin | Inhibits proliferation of human IPAH PASMCs (22) |
| ↑ Rat HPH | Inhibits proliferation of rat MCT-PH PASMCs (113) | ||||
| ↑ Rat MCT-PH | Everolimus | Improves PVR and 6MWD in patients with severe PAH (117) | |||
| mTORC2 | PASMCs | ↑ Human IPAH | ↑ Proliferation | PP242 (dual) | Inhibits proliferation, induces apoptosis in human IPAH PASMCs (22) |
| ↑ Rat HPH | ↓ Apoptosis | ||||
| ↑ Rat MCT-PH | Reverses pulmonary vascular remodeling in rat HPH (22) | ||||
| Notch3/HES | PASMCs | ↑ Human PAH | ↓ Differentiation | DAPT | Inhibits human IPAH growth (20) |
| ↑ Mouse HPH | ↑ Proliferation | Reverses mouse HPH (20) | |||
| FoxOs | PASMCs | ↓ Human PAH | ↑ Proliferation | Paclitaxel | Inhibits human IPAH growth (17) |
| ↓ Rat MCT-PH | ↓ Apoptosis | Abraxane | Reverses rat MCT-PH (17) | ||
| ↓ Rat SuHx-PH | Reverses rat SuHx-PH (17, 74) |
Definition of abbreviations: 6MWD = 6-minute-walk distance; Akt = v-akt murine thymoma viral oncogene homolog; DAPT = N-[N-(3,5-difluorophenacetyl)-l-alanyl]-S-phenylglycine t-butyl ester; EGFR = epidermal growth factor receptor; ERK = extracellular signal–regulated kinase; FGF-2 = fibroblast growth factor 2; FGFR = fibroblast growth factor receptor; FoxO = forkhead-box class O; HES = hairy/enhancer of split; HPH = hypoxia-induced pulmonary hypertension; IPAH = idiopathic pulmonary arterial hypertension; MAPK = mitogen-activated protein kinase; MCT-PH = monocrotaline-induced PH; MEK = MAPK/ERK kinase; mTOR = mechanistic target of rapamycin; mTORC1 = mTOR complex 1; mTORC2 = mTOR complex 2; PA = pulmonary artery; PAB = pulmonary artery banding; PAAFs = pulmonary artery adventitial fibroblasts; PAECs = pulmonary artery endothelial cells; PAH = pulmonary arterial hypertension; PASMCs = pulmonary artery smooth muscle cells; PDGFR = platelet-derived growth factor receptor; PH = pulmonary hypertension; PI3K = phosphatidylinositol 3-kinase; PVR = pulmonary vascular resistance; RTK = receptor tyrosine kinase; RV = right ventricular; SuHx = Sugen/hypoxia.
However, the abnormal proliferative response in PAH is a highly complex process that occurs in all three layers of the vessel wall and involves all three types of resident microvascular cells: PAECs, PASMCs, and PAAFs. It is induced by multiple stimuli, including elevated levels of growth factors and proinflammatory mediators, a hypoxic environment, disturbed ECM composition, and increased matrix stiffness (13, 41, 43, 89). Not surprisingly, in addition to RTKs, pulmonary vascular cells in PAH show activation of other cell surface receptors (23, 41) and undergo complex signaling reprograming that supports a metabolically active, proliferative, apoptosis-resistant phenotype. Further, PASMCs and PAAFs in established PAH demonstrate an ability for unstimulated proliferation (15, 22) that is not necessarily associated with known mutations and is driven, at least in part, by self-supporting signaling loops (42, 43).
Given the redundancy of exogenous pro-proliferative stimuli and the existence of pro-proliferative/antiapoptotic endogenous signaling circuits, the strategies targeting single promitogenic factors or their respective receptors might have limited efficacy and/or only transient effects. The understanding that major signaling pathways cross-talk and inter-control each other via shared signaling molecules at points below receptor levels led to the emerging concept to target central downstream effector molecules that integrate the signals from multiple receptors, induce cell cycle entry and progression, cell proliferation, and survival (i.e., “signaling hubs”). Although more studies are needed, targeting such hubs, selectively dysregulated in PAH, may represent a potentially attractive therapeutic strategy to reverse pulmonary vascular remodeling by targeting elimination of “reprogrammed” cell populations, while reducing harmful effects on nonmodified pulmonary vascular cells.
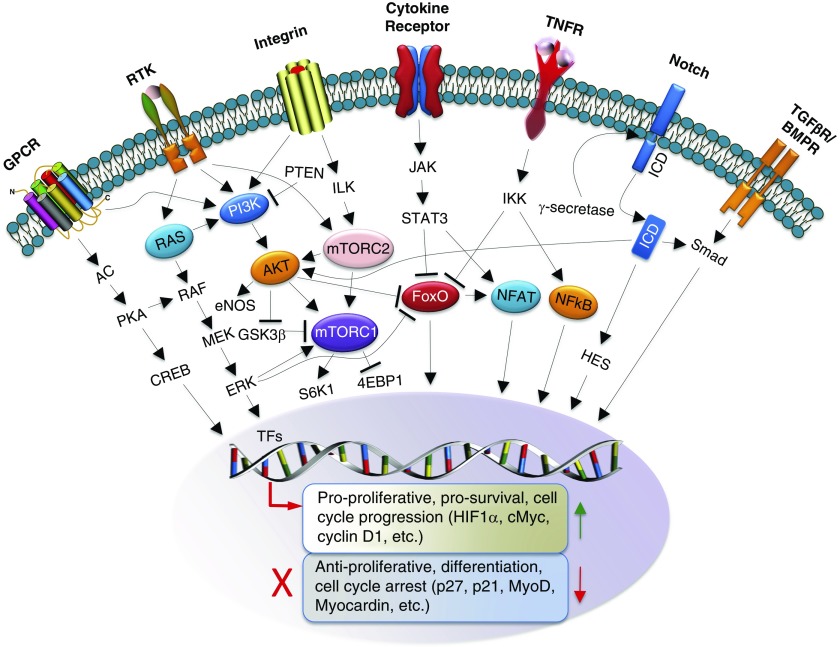
There are several promising candidate hub pathways shared between PAH and cancer, including, but not limited to, Ras/Raf/MAPK, PI3K/Akt/mTOR, Notch3, and Wnt (90) signaling (Figure 2). Those pathways are consistently dysregulated in PAH, act as positive regulators of the G1–S cell cycle checkpoint, and provide potent survival, proliferation, and apoptosis resistance signals via multiple mechanisms, including regulation of various TFs (HIF [91], NF-κB, FoxOs, NFAT, HES), some of which are also under consideration as attractive molecular targets. Current progress in mechanistic, clinical, and preclinical studies of PAH that relate to RTKs, Ras/Raf/MAPK, PI3K/Akt/mTOR, Notch3/HES, and TFs is summarized below. For other advances in understanding PAH-specific molecular abnormalities and in emerging therapeutic strategies we refer our readers to References 13, 23, and 92.
Figure 2.
Overview of major proliferative/prosurvival signaling pathways and drug targets in pulmonary arterial hypertension and cancer. Signaling nodes are highlighted. 4EBP1 = 4R-binding protein 1; AC = adenylyl cyclase; AKT = v-akt murine thymoma viral oncogene homolog; BMPR = bone morphogenetic protein receptor; CREB = cyclic AMP–responsive element binding protein; eNOS = endothelial nitric oxide synthase; ERK = extracellular signal–regulated kinase; FoxO = forkhead-box class O; GPCR = G protein–coupled receptor; GSK3β = glycogen synthase-kinase 3β; HES = hairy/enhancer of split; HIF1 = hypoxia-inducible factor 1; ICD = intracellular domain; IKK = IκBα kinase complexes; ILK = integrin-linked kinase; JAK = Janus kinase; MEK = mitogen-activated protein kinase kinase; mTOR = mechanistic target of rapamycin; mTORC1 = mTOR complex 1; mTORC2 = mTOR complex 2; MyoD = myogenic differentiation 1; NFAT = nuclear factor of activated T cells; NF-κB = nuclear factor κ-light-chain-enhancer of activated B cells; PI3K = phosphatidylinositol 3-kinase; PKA = protein kinase A; PTEN = phosphatase and tensin homolog; RAF = rapidly accelerated fibrosarcoma; RAS = rat sarcoma; RTK = receptor tyrosine kinase; S6K1 = p70 S6 kinase 1; STAT3 = signal transducer and activator of transcription 3; TF = transcription factor; TGF-βR = transforming growth factor-β receptor; TNFR = tumor necrosis factor receptor.
Targeting Growth and Proliferation Signaling Hubs
RTKs
On the basis of compelling evidence of the pathological role of RTKs and their ligands in PAH (23), several small-molecule tyrosine kinase inhibitors (TKIs), developed for the treatment of cancer (93), have been tested in animal models of PH and have largely provided promising results. EGFR and PDGFR inhibition demonstrated positive effects on hemodynamics, remodeling, and survival in experimental PH (24, 25), and the PDGFR antagonist imatinib (STI571) reversed pulmonary vascular remodeling in two PH animal models (25), prevented PDGFR-β phosphorylation, and downstream signaling. Importantly, imatinib is the only TKI that has completed a phase III clinical trial in PAH (discussed below). Nilotinib, a second-generation TKI 30-fold more potent than imatinib, caused a nearly complete reversal of pulmonary vascular remodeling in experimental PH models (94). Further, FGF-2 small interfering RNA or pharmacological FGFR1 inhibition (SU5402) reversed established experimental PH (21).
Similarly, EGFR TKIs, PKI166 (24), gefitinib, erlotinib, and lapatinib significantly reduced right ventricular systolic pressure (RVSP) in rats with MCT-PH (95). However, even highest tolerable doses of these EGFR antagonists showed little benefits in mice with hypoxia-induced PH. On the other hand, VEGFR blockade (SU5416) resulted in the potentiation of PAH and worsening of pathological vascular remodeling, reproducing some of the “angioproliferative” features typical of patients with advanced PAH, in several PH animal models (96).
Despite these highly encouraging results, strategies targeting single growth factors or RTKs might be afflicted with limited efficacy due to the redundancy of the multiple stimuli that activate vascular cell proliferation via parallel mechanisms (i.e., up-regulation of other RTKs), as is often seen in malignant diseases (97). Likewise, the VEGFR inhibitor SU5416 up-regulates several growth-signaling molecules (e.g., VEGFR2, p-MAPK), and may contribute to the emergence of apoptosis-resistant cells with increased growth potential (96).
One strategy to overcome such limitations might be to broaden this approach by using nonspecific RTK inhibitors. In support of this notion, sorafenib, an antineoplastic agent and inhibitor of multiple RTKs (PDGFR, VEGFR, Raf, Kit [stem cell factor receptor], and Flt-3 [FMS-like tyrosine kinase 3]), attenuated pulmonary remodeling and improved cardiac and pulmonary function in experimental PH (34, 98, 99). Another multikinase inhibitor, sunitinib (VEGFR, PDGFR, Kit, Flt-3, CSF-1R [colony-stimulating factor-1 receptor], and Kit), demonstrated potent antiremodeling effects in experimental PH models (99, 100).
Ras/Raf/MEK/ERK Signaling
The Ras proteins are small guanosine triphosphatases associated with a number of signal transduction pathways involved in cell cycle progression, cell motility, apoptosis, senescence, and other vital functions. Numerous cell surface molecules activate Ras proteins, which, in turn, transduce the signals through Raf, MEK (MAPK/ERK [extracellular signal–regulated kinase] kinase), and MAPK1/3 (also called ERK1/2) to the TFs (including FOS, MYC, ELK, and c-JUN) that modulate gene expression required for cell proliferation and survival. Raf/MEK/MAPK—a major effector pathway of Ras—has a well-described role in cancer. Hyperactivation of Raf, a serine/threonine kinase, leads to dysregulated proliferation, differentiation, and apoptosis, and oncogenic Ras mutations have been identified in a diverse array of human cancers (101).
In PAECs, Ras/Raf/MEK/MAPK/AP1 signaling was demonstrated as an important consequence of BMPR2 silencing. Notably, Raf family members and MAPK1/2 were constitutively activated after BMPR2 knockdown, leading to a proliferative and mitogenic cellular phenotype (102). Furthermore, aberrant MAPK is described in the pulmonary vasculature of patients with advanced PAH (103). More recently, a gain-of-function RAF1 mutation has been associated with the development of rapidly fatal PAH in two infants (104). While exploring the importance of Raf/MAPK signaling in PAH, Raf-1 kinase inhibitor protein knockout mice were found to exhibit exaggerated hypoxia-induced PH (105). Collectively, sustained Ras/Raf/MEK/MAPK signaling, downstream from growth factors and their associated RTKs, may represent a promising platform for new therapeutic approaches to pathological vascular remodeling in PAH. Accordingly, sorafenib (originally identified as a Raf-1 inhibitor and subsequently as an inhibitor of PDGFR, VEGFR, Kit, and Flt-3) has been shown to be effective in experimental models of PH and RV hypertrophy (34, 98, 99).
PI3K–Akt–mTOR Signaling
The PI3K–Akt–mTOR signaling network is a critical regulator of cell growth, proliferation, survival, and motility (101). Class I PI3Ks are activated by RTKs, G protein–coupled receptors, β-integrins, and Ras and, in turn, induce PDK1 (3-phosphoinositide-dependent protein kinase 1)-dependent activation of Akt. Akt promotes cell growth, proliferation, and survival via multiple mechanisms (reviewed in depth by Manning and Cantley [106]). Akt up-regulates HIF1α, inhibits FoxOs, and activates mTOR complex 1 (mTORC1; mTOR-raptor)–S6K1/4EBP1 signaling, a master regulator of cell growth and proliferation and a major target of the allosteric inhibitor rapamycin. Interestingly, mTOR is also a member of functionally distinct mTORC2 (mTOR-rictor), which is rapamycin insensitive in many cell types; is regulated by growth factors, ILK, Nox4 and its association with ribosomes; and acts as a direct upstream activator of Akt by phosphorylating it at Ser-473 (Figure 2).
The PI3K–Akt–mTOR pathway is one of the most frequently dysregulated networks in human cancers and is under active investigation as an anticancer therapeutic target (107). Although the status of PI3K in human PAH pulmonary vasculature is not known, in vitro studies demonstrated the key role for class I PI3K in growth factor–induced proliferation and migration of PASMCs (27, 108), and pharmacological inhibition of PI3K or Akt attenuated the development of hypoxia-induced pulmonary vascular remodeling in rats (109). Mice with VSMC-specific depletion of Akt1 showed attenuated PH under hypoxia (110), while VSMC-specific knockdown of either PTEN or tuberous sclerosis complex 1, respective inhibitors of PI3K and mTORC1, led to PH development (111, 112), supporting the role for PI3K cascade in VSMC remodeling. In PAECs, the PI3K–Akt pathway is activated by VEGF and contributes to cell growth, proliferation, survival, and migration. It should be noted, however, that Akt directly phosphorylates and activates endothelial NO synthase leading to increased NO synthesis and vasodilation (106), suggesting that it possesses both pathological and protective roles in PH.
PASMC-specific activation of both mTORC1–S6K1 and mTORC2–Akt has been reported in small remodeled PAs from subjects with IPAH and rats with HPH, MCT- and SU5416/hypoxia-induced PH (22, 113, 114). Interestingly, hypoxia-induced proliferation of PASMCs, while requiring activation of mTORC1 and mTORC2, was not associated with changes in PI3K activity and/or MAPK signaling (115), suggesting alternative mechanisms of mTOR activation. In human IPAH PASMCs, mTORC2 was required for activation of both Akt and mTORC1, and supported increased cell proliferation and apoptosis resistance (22). Further, the dual mTORC1/mTORC2 inhibitor PP242 has been shown to selectively reduce proliferation and induce apoptosis in human IPAH PASMCs and to reverse established pulmonary vascular remodeling in experimental PH (22), suggesting potential benefits of mTOR kinase inhibitors to reverse established PAH.
Importantly, the mTORC1 inhibitor rapamycin potently reduced human PAH and rat PH PASMC proliferation in clinically relevant doses and attenuated, although not reversed, pulmonary vascular remodeling in experimental PH (112, 113, 115, 116). In a safety and efficacy pilot trial, the rapamycin derivative everolimus suggested therapeutic benefit of rapalogs for the treatment of PAH (Seyfarth and colleagues [117], discussed below). It should be noted, however, that rapalogs in the doses used for clinical applications have therapeutically proven cytostatic function with no appreciable proapoptotic effect (22, 112, 115, 118). Indeed, even high doses of rapamycin did not induce apoptosis in human IPAH and mouse PH PASMCs (22, 112), which may limit the benefits of rapamycin use as a single agent and calls for rapamycin-based combinational strategies.
Notch/HES Signaling
Notch/hairy/enhancer of split (HES) controls organ development and tissue homeostasis. Notch1–4 are transmembrane receptors that are activated by binding with extracellular ligands secreted by neighboring cells (Jagged, Delta-like families) and undergo series of proteolytic cleavages by the ADAM/TACE proteases and γ-secretase complex, leading to release of intracellular domain (ICD). The ICD cross-talks with PI3K and TGF-β pathways at the Akt and Smad levels, and promotes transcription of HES and HRT (HES-related transcription factor), which, in turn, down-regulate Mash, Myogenic differentiation 1 (myoD), myocardin, and the cell cycle regulators p27kip1 and p21waf/cip1, inducing cell cycle progression and cell dedifferentiation (119). Notch signaling plays a pro-oncogenic role in several solid tumors, and γ-secretase inhibitors are under investigation as anticancer drugs (119).
Notch3, expressed solely in vascular smooth muscle cells (VSMCs), is required for maintenance of PASMC proliferative capacity during postnatal vessel maturation. Importantly, VSM-specific Notch3 overexpression has been detected in small remodeled PAs from patients with PAH and rodents with experimental PH and was correlated with disease severity. Activation of Notch3–HES5 was responsible for the maintenance of PAH PASMCs in a dedifferentiated state, thereby promoting cell proliferation, VSM remodeling, and PH (20). The γ-secretase inhibitor N-[N-(3,5-difluorophenacetyl)-l-alanyl]-S-phenylglycine t-butyl ester (DAPT) blocked Notch3 activation and reversed hypoxia-induced PH in mice (20), suggesting the attractiveness of Notch3–HES signaling as a potential target pathway for therapeutic intervention.
Transcription Factor Signaling Hubs: FoxOs and Beyond
FoxO TFs are involved in multiple signaling pathways and play critical roles in a number of physiological and pathological processes including cancer. Mounting evidence suggests that FoxOs function as tumor suppressors in a variety of cancers. FoxOs are actively involved in promoting apoptosis in a mitochondria-independent and -dependent manner by inducing the expression of death receptor ligands, including Fas ligand and tumor necrosis factor–related apoptosis-inducing ligand, and Bcl-2 family members. Thus, restoration of FoxO activity is being explored as a potential cancer therapy (120).
FoxO1 was noted to be down-regulated (expression, phosphorylation, and nuclear exclusion) in PASMCs in human IPAH and in two experimental PH models. Importantly, PASMC FoxO1 was demonstrated as a critical integrator of multiple signaling pathways (cytokines and growth factors) driving PH. Genetic ablation of FoxO1 in VSM reproduced PH features in vitro and in vivo. Either pharmacological reconstitution of FoxO1 activity with intravenous or inhaled paclitaxel, or reconstitution of the transcriptional activity of FoxO1 by gene therapy, restored the physiologically quiescent PASMC phenotype in vitro, linked to changes in cell cycle control (cyclin D1, p27kip1, BCL6, GADD45a) and BMPR2 signaling, and reversed vascular remodeling and right heart hypertrophy in vivo, suggesting reconstitution of FoxO1 activity as a potential therapeutic option for PH (17, 74).
Experiences and Perspective from the Clinic
To date, imatinib, a TKI targeting PDGFR signaling, is the only antiproliferative approach that successfully passed to a phase III clinical trial in PH. In a phase II study, imatinib demonstrated safety, tolerability, and efficacy in patients with functional class II–IV PAH (121, 122). Subsequently, a 24-week, multicenter, double-blind, placebo-controlled phase III trial on imatinib revealed encouraging data (123). Patients with advanced PAH who were symptomatic and displayed PVR equal to or greater than 800 dyn⋅s⋅cm–5 despite treatment with at least two PAH drugs were included in this study. Analysis of the placebo-corrected treatment effects at week 24 versus baseline showed that imatinib substantially improved PVR, cardiac output, and 6-minute-walk distance (6MWD). The side effect profile of imatinib was comparable to previous experience in oncological studies, resulting in an increased dropout rate, and there was an unexpected enhanced appearance of subdural hematomas while receiving imatinib treatment. This occurred, however, only when concomitant chronic oral anticoagulation was applied, the rationale of which in patients with PH is currently questioned (124, 125). Moreover, there was a disadvantageous effect on the time to clinical worsening, which may also be related to the side effect profile of this drug. For this reason, the pharmaceutical company stopped the drug approval process both at the U.S. Food and Drug Administration and the European Medicines Agency. Nevertheless, the impressive effects of imatinib on hemodynamics and exercise capacity in many patients in the IMPRES trial (Imatinib [QTI571] in Pulmonary Arterial Hypertension, a Randomized, Efficacy Study) and in an additional observational study (126) show that even on top of maximized vasodilator therapy, novel antiproliferative approaches may have additional (potentially life-saving) effects.
Importantly, these studies revealed remarkable heterogeneity in the individual response to imatinib, and provide compelling evidence that a subgroup of patients with beneficial responsiveness to this TKI does exist. The pathomechanisms underlying “response” or “nonresponse” of patients with PAH to imatinib have not yet been decrypted. However, this observation supports the notion that individualization of therapy, including dosage regimen, in patients with PAH is an important step forward in imatinib or any other TKI therapy. It could be possible by combining “omics” technologies (RNA-seq, proteomics, etc.) with local cell harvesting, that is, PAECs from the PA vessel wall attached to the balloon tip of the flow-directed PA (Swan-Ganz) catheter used for routine right heart catheterization (127). Moreover, peripheral blood mononuclear cells, easily accessible by venous puncture and known to intimately interact with diseased endothelial surfaces during lung passage, might be employed as early/predictive indicators of individual TKI responsiveness in PAH, as shown for oncological indications (128). The same might hold true for microvesicles shed from the surface of the lung vasculature. However, the proof of concept for these approaches in clinical PH is still pending.
On the other hand, the risk of systemic side effects of imatinib therapy, as encountered in the IMPRES trial, may be overcome by local drug delivery employing aerosolization technologies. Such lung-selective delivery may be combined with packaging of the drug into nanoparticles or liposomes for retarded/controlled release. From various prostanoid and NO inhalation studies it is known that the diseased precapillary resistance vessels in PAH may be directly targeted from the alveolar surface, to which they are directly adjacent. This approach can be expected to increase the local over the systemic drug concentration by at least two orders of magnitude, enabling strong pulmonary efficacy even at a low overall drug dosage.
Further extending the TK inhibition approach in PAH, the multikinase inhibitor sorafenib was found to improve exercise capacity in a monocentric open-label trial (129). However, the substantial side effect profile brings into question the suitability of such broad multikinase inhibition for PAH treatment.
Beyond the TKI-based approaches, targeting “signaling hubs” offers new options for therapeutic intervention. Two of these compounds have already been assessed in pilot trials. Tacrolimus (FK506), a drug targeting calcineurin–NFATc activity and activating BMPR2 signaling (130), was found to exert a clinical benefit at low dosage in end-stage PAH (131), and a phase IIb clinical study of GS-4997, an inhibitor of apoptosis signal-regulating kinase 1 (ASK1) in adults with PAH (ARROW), is ongoing (NCT02234141).
Along the same lines, in a safety and efficacy pilot trial, the rapamycin derivative everolimus was well tolerated in patients with severe PAH and showed improvements in PVR and 6MWD (117); mTORC1 inhibitors may thus offer a promise for the treatment of PAH, but the definite clinical proof is still pending. Importantly, as discussed for imatinib, local delivery of such inhibitors by aerosolization or by developing albumin-bound formulations with improved penetration in lung tissue should be exploited to limit the probability of unforeseen systemic side effects. For example, the clinical trial for ABI-009, an albumin-bound mTOR inhibitor for patients with severe PAH (NCT02587325), is ongoing, and the development of new nanoparticle formulations is underway for paclitaxel to achieve alveolar retardation and controlled release of this compound, which has shown strong preclinical efficacy in PAH (17).
Footnotes
Originally Published in Press as DOI: 10.1164/rccm.201606-1226PP on September 14, 2016
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Guignabert C, Tu L, Le Hiress M, Ricard N, Sattler C, Seferian A, Huertas A, Humbert M, Montani D. Pathogenesis of pulmonary arterial hypertension: lessons from cancer. Eur Respir Rev. 2013;22:543–551. doi: 10.1183/09059180.00007513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sakao S, Tatsumi K. Vascular remodeling in pulmonary arterial hypertension: multiple cancer-like pathways and possible treatment modalities. Int J Cardiol. 2011;147:4–12. doi: 10.1016/j.ijcard.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 3.Rai PR, Cool CD, King JA, Stevens T, Burns N, Winn RA, Kasper M, Voelkel NF. The cancer paradigm of severe pulmonary arterial hypertension. Am J Respir Crit Care Med. 2008;178:558–564. doi: 10.1164/rccm.200709-1369PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Voelkel NF, Cool C, Lee SD, Wright L, Geraci MW, Tuder RM. Primary pulmonary hypertension between inflammation and cancer. Chest. 1998;114(3) Suppl:225S–230S. doi: 10.1378/chest.114.3_supplement.225s. [DOI] [PubMed] [Google Scholar]
- 5.Ryan JJ, Archer SL. The right ventricle in pulmonary arterial hypertension: disorders of metabolism, angiogenesis and adrenergic signaling in right ventricular failure. Circ Res. 2014;115:176–188. doi: 10.1161/CIRCRESAHA.113.301129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Archer SL, Gomberg-Maitland M, Maitland ML, Rich S, Garcia JGN, Weir EK. Mitochondrial metabolism, redox signaling, and fusion: a mitochondria–ROS–HIF-1α–Kv1.5 O2-sensing pathway at the intersection of pulmonary hypertension and cancer. Am J Physiol Heart Circ Physiol. 2008;294:H570–H578. doi: 10.1152/ajpheart.01324.2007. [DOI] [PubMed] [Google Scholar]
- 7.Sutendra G, Michelakis ED. The metabolic basis of pulmonary arterial hypertension. Cell Metab. 2014;19:558–573. doi: 10.1016/j.cmet.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 8.Ryan J, Dasgupta A, Huston J, Chen K-H, Archer SL. Mitochondrial dynamics in pulmonary arterial hypertension. J Mol Med (Berl) 2015;93:229–242. doi: 10.1007/s00109-015-1263-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Courboulin A, Ranchoux B, Cohen-Kaminsky S, Perros F, Bonnet S. MicroRNA networks in pulmonary arterial hypertension: share mechanisms with cancer? Curr Opin Oncol. 2016;28:72–82. doi: 10.1097/CCO.0000000000000253. [DOI] [PubMed] [Google Scholar]
- 10.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 11.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 12.Tuder RM, Archer SL, Dorfmüller P, Erzurum SC, Guignabert C, Michelakis E, Rabinovitch M, Schermuly R, Stenmark KR, Morrell NW. Relevant issues in the pathology and pathobiology of pulmonary hypertension. J Am Coll Cardiol. 2013;62(25) Suppl:D4–D12. doi: 10.1016/j.jacc.2013.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rabinovitch M. Molecular pathogenesis of pulmonary arterial hypertension. J Clin Invest. 2012;122:4306–4313. doi: 10.1172/JCI60658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Voelkel NF, Gomez-Arroyo J, Abbate A, Bogaard HJ, Nicolls MR. Pathobiology of pulmonary arterial hypertension and right ventricular failure. Eur Respir J. 2012;40:1555–1565. doi: 10.1183/09031936.00046612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stenmark KR, Tuder RM, El Kasmi KC. Metabolic reprogramming and inflammation act in concert to control vascular remodeling in hypoxic pulmonary hypertension. J Appl Physiol (1985) 2015;119:1164–1172. doi: 10.1152/japplphysiol.00283.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Witsch E, Sela M, Yarden Y. Roles for growth factors in cancer progression. Physiology (Bethesda) 2010;25:85–101. doi: 10.1152/physiol.00045.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Savai R, Al-Tamari HM, Sedding D, Kojonazarov B, Muecke C, Teske R, Capecchi MR, Weissmann N, Grimminger F, Seeger W, et al. Pro-proliferative and inflammatory signaling converge on FoxO1 transcription factor in pulmonary hypertension. Nat Med. 2014;20:1289–1300. doi: 10.1038/nm.3695. [DOI] [PubMed] [Google Scholar]
- 18.Masri FA, Xu W, Comhair SA, Asosingh K, Koo M, Vasanji A, Drazba J, Anand-Apte B, Erzurum SC. Hyperproliferative apoptosis-resistant endothelial cells in idiopathic pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol. 2007;293:L548–L554. doi: 10.1152/ajplung.00428.2006. [DOI] [PubMed] [Google Scholar]
- 19.Tu L, Dewachter L, Gore B, Fadel E, Dartevelle P, Simonneau G, Humbert M, Eddahibi S, Guignabert C. Autocrine fibroblast growth factor-2 signaling contributes to altered endothelial phenotype in pulmonary hypertension. Am J Respir Cell Mol Biol. 2011;45:311–322. doi: 10.1165/rcmb.2010-0317OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li X, Zhang X, Leathers R, Makino A, Huang C, Parsa P, Macias J, Yuan JX, Jamieson SW, Thistlethwaite PA. Notch3 signaling promotes the development of pulmonary arterial hypertension. Nat Med. 2009;15:1289–1297. doi: 10.1038/nm.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Izikki M, Guignabert C, Fadel E, Humbert M, Tu L, Zadigue P, Dartevelle P, Simonneau G, Adnot S, Maitre B, et al. Endothelial-derived FGF2 contributes to the progression of pulmonary hypertension in humans and rodents. J Clin Invest. 2009;119:512–523. doi: 10.1172/JCI35070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goncharov DA, Kudryashova TV, Ziai H, Ihida-Stansbury K, DeLisser H, Krymskaya VP, Tuder RM, Kawut SM, Goncharova EA. Mammalian target of rapamycin complex 2 (mTORC2) coordinates pulmonary artery smooth muscle cell metabolism, proliferation, and survival in pulmonary arterial hypertension. Circulation. 2014;129:864–874. doi: 10.1161/CIRCULATIONAHA.113.004581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schermuly RT, Ghofrani HA, Wilkins MR, Grimminger F. Mechanisms of disease: pulmonary arterial hypertension. Nat Rev Cardiol. 2011;8:443–455. doi: 10.1038/nrcardio.2011.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Merklinger SL, Jones PL, Martinez EC, Rabinovitch M. Epidermal growth factor receptor blockade mediates smooth muscle cell apoptosis and improves survival in rats with pulmonary hypertension. Circulation. 2005;112:423–431. doi: 10.1161/CIRCULATIONAHA.105.540542. [DOI] [PubMed] [Google Scholar]
- 25.Schermuly RT, Dony E, Ghofrani HA, Pullamsetti S, Savai R, Roth M, Sydykov A, Lai YJ, Weissmann N, Seeger W, et al. Reversal of experimental pulmonary hypertension by PDGF inhibition. J Clin Invest. 2005;115:2811–2821. doi: 10.1172/JCI24838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rajkumar R, Konishi K, Richards TJ, Ishizawar DC, Wiechert AC, Kaminski N, Ahmad F. Genomewide RNA expression profiling in lung identifies distinct signatures in idiopathic pulmonary arterial hypertension and secondary pulmonary hypertension. Am J Physiol Heart Circ Physiol. 2010;298:H1235–H1248. doi: 10.1152/ajpheart.00254.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goncharova EA, Ammit AJ, Irani C, Carroll RG, Eszterhas AJ, Panettieri RA, Krymskaya VP. PI3K is required for proliferation and migration of human pulmonary vascular smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2002;283:L354–L363. doi: 10.1152/ajplung.00010.2002. [DOI] [PubMed] [Google Scholar]
- 28.Niu G, Chen X. Vascular endothelial growth factor as an anti-angiogenic target for cancer therapy. Curr Drug Targets. 2010;11:1000–1017. doi: 10.2174/138945010791591395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tuder RM, Cool CD, Yeager M, Taraseviciene-Stewart L, Bull TM, Voelkel NF. The pathobiology of pulmonary hypertension: endothelium. Clin Chest Med. 2001;22:405–418. doi: 10.1016/s0272-5231(05)70280-x. [DOI] [PubMed] [Google Scholar]
- 30.Tuder RM, Chacon M, Alger L, Wang J, Taraseviciene-Stewart L, Kasahara Y, Cool CD, Bishop AE, Geraci M, Semenza GL, et al. Expression of angiogenesis-related molecules in plexiform lesions in severe pulmonary hypertension: evidence for a process of disordered angiogenesis. J Pathol. 2001;195:367–374. doi: 10.1002/path.953. [DOI] [PubMed] [Google Scholar]
- 31.Shao D, Park JE, Wort SJ. The role of endothelin-1 in the pathogenesis of pulmonary arterial hypertension. Pharmacol Res. 2011;63:504–511. doi: 10.1016/j.phrs.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 32.Fruman DA, Rommel C. PI3K and cancer: lessons, challenges and opportunities. Nat Rev Drug Discov. 2014;13:140–156. doi: 10.1038/nrd4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moreno-Vinasco L, Gomberg-Maitland M, Maitland ML, Desai AA, Singleton PA, Sammani S, Sam L, Liu Y, Husain AN, Lang RM, et al. Genomic assessment of a multikinase inhibitor, sorafenib, in a rodent model of pulmonary hypertension. Physiol Genomics. 2008;33:278–291. doi: 10.1152/physiolgenomics.00169.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ma LI, Chang Y, Yu L, He W, Liu Y. Pro-apoptotic and anti-proliferative effects of mitofusin-2 via PI3K/Akt signaling in breast cancer cells. Oncol Lett. 2015;10:3816–3822. doi: 10.3892/ol.2015.3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dasgupta A, Chen K-H, Munk RB, Sasaki CY, Curtis J, Longo DL, Ghosh P. Mechanism of activation-induced downregulation of mitofusin 2 in human peripheral blood T cells. J Immunol. 2015;195:5780–5786. doi: 10.4049/jimmunol.1501023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen K-H, Dasgupta A, Ding J, Indig FE, Ghosh P, Longo DL. Role of mitofusin 2 (Mfn2) in controlling cellular proliferation. FASEB J. 2014;28:382–394. doi: 10.1096/fj.13-230037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang Z, Niu Q, Peng X, Li M, Liu Y, Liu J, Wen S, Wei Y. Mitofusin 2 ameliorates aortic remodeling by suppressing ras/raf/ERK pathway and regulating mitochondrial function in vascular smooth muscle cells. Int J Cardiol. 2015;178:165–167. doi: 10.1016/j.ijcard.2014.10.122. [DOI] [PubMed] [Google Scholar]
- 39.Yuan TL, Cantley LC. PI3K pathway alterations in cancer: variations on a theme. Oncogene. 2008;27:5497–5510. doi: 10.1038/onc.2008.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dhillon AS, Hagan S, Rath O, Kolch W. MAP kinase signalling pathways in cancer. Oncogene. 2007;26:3279–3290. doi: 10.1038/sj.onc.1210421. [DOI] [PubMed] [Google Scholar]
- 41.de Jesus Perez VA. Molecular pathogenesis and current pathology of pulmonary hypertension. Heart Fail Rev. 2016;21:239–257. doi: 10.1007/s10741-015-9519-2. [DOI] [PubMed] [Google Scholar]
- 42.Kudryashova TV, Goncharov DA, Pena A, Kelly N, Vanderpool R, Baust J, Kobir A, Shufesky W, Mora AL, Morelli AE, et al. HIPPO–integrin linked kinase crosstalk controls self-sustaining proliferation and survival in pulmonary hypertension. Am J Respir Crit Care Med. 2016;194:866–877. doi: 10.1164/rccm.201510-2003OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bertero T, Cottrill KA, Lu Y, Haeger CM, Dieffenbach P, Annis S, Hale A, Bhat B, Kaimal V, Zhang Y-Y, et al. Matrix remodeling promotes pulmonary hypertension through feedback mechanoactivation of the YAP/TAZ–miR-130/301 circuit. Cell Rep. 2015;13:1016–1032. doi: 10.1016/j.celrep.2015.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sherr CJ. Principles of tumor suppression. Cell. 2004;116:235–246. doi: 10.1016/s0092-8674(03)01075-4. [DOI] [PubMed] [Google Scholar]
- 45.Harvey KF, Zhang X, Thomas DM. The Hippo pathway and human cancer. Nat Rev Cancer. 2013;13:246–257. doi: 10.1038/nrc3458. [DOI] [PubMed] [Google Scholar]
- 46.Joerger AC, Fersht AR. The p53 pathway: origins, inactivation in cancer, and emerging therapeutic approaches. Annu Rev Biochem. 2016;85:375–404. doi: 10.1146/annurev-biochem-060815-014710. [DOI] [PubMed] [Google Scholar]
- 47.Mizuno S, Bogaard HJ, Kraskauskas D, Alhussaini A, Gomez-Arroyo J, Voelkel NF, Ishizaki T. p53 gene deficiency promotes hypoxia-induced pulmonary hypertension and vascular remodeling in mice. Am J Physiol Lung Cell Mol Physiol. 2011;300:L753–L761. doi: 10.1152/ajplung.00286.2010. [DOI] [PubMed] [Google Scholar]
- 48.Jacquin S, Rincheval V, Mignotte B, Richard S, Humbert M, Mercier O, Londoño-Vallejo A, Fadel E, Eddahibi S. Inactivation of p53 is sufficient to induce development of pulmonary hypertension in rats. PLoS One. 2015;10:e0131940. doi: 10.1371/journal.pone.0131940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cancer Genome Atlas Research Network. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455:1061–1068. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cool CD, Stewart JS, Werahera P, Miller GJ, Williams RL, Voelkel NF, Tuder RM. Three-dimensional reconstruction of pulmonary arteries in plexiform pulmonary hypertension using cell-specific markers: evidence for a dynamic and heterogeneous process of pulmonary endothelial cell growth. Am J Pathol. 1999;155:411–419. doi: 10.1016/S0002-9440(10)65137-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yu L, Quinn DA, Garg HG, Hales CA. Gene expression of cyclin-dependent kinase inhibitors and effect of heparin on their expression in mice with hypoxia-induced pulmonary hypertension. Biochem Biophys Res Commun. 2006;345:1565–1572. doi: 10.1016/j.bbrc.2006.05.060. [DOI] [PubMed] [Google Scholar]
- 52.Zuckerbraun BS, Shiva S, Ifedigbo E, Mathier MA, Mollen KP, Rao J, Bauer PM, Choi JJ, Curtis E, Choi AM, et al. Nitrite potently inhibits hypoxic and inflammatory pulmonary arterial hypertension and smooth muscle proliferation via xanthine oxidoreductase–dependent nitric oxide generation. Circulation. 2010;121:98–109. doi: 10.1161/CIRCULATIONAHA.109.891077. [DOI] [PubMed] [Google Scholar]
- 53.Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang SI, Puc J, Miliaresis C, Rodgers L, McCombie R, et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275:1943–1947. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- 54.Ravi Y, Selvendiran K, Meduru S, Citro L, Naidu S, Khan M, Rivera BK, Sai-Sudhakar CB, Kuppusamy P. Dysregulation of PTEN in cardiopulmonary vascular remodeling induced by pulmonary hypertension. Cell Biochem Biophys. 2013;67:363–372. doi: 10.1007/s12013-011-9332-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Horita H, Furgeson SB, Ostriker A, Olszewski KA, Sullivan T, Villegas LR, Levine M, Parr JE, Cool CD, Nemenoff RA, et al. Selective inactivation of PTEN in smooth muscle cells synergizes with hypoxia to induce severe pulmonary hypertension. J Am Heart Assoc. 2013;2:e000188. doi: 10.1161/JAHA.113.000188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Massagué J. TGFβ in cancer. Cell. 2008;134:215–230. doi: 10.1016/j.cell.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Morrell NW. Pulmonary hypertension due to BMPR2 mutation: a new paradigm for tissue remodeling? Proc Am Thorac Soc. 2006;3:680–686. doi: 10.1513/pats.200605-118SF. [DOI] [PubMed] [Google Scholar]
- 58.Aldred MA, Vijayakrishnan J, James V, Soubrier F, Gomez-Sanchez MA, Martensson G, Galie N, Manes A, Corris P, Simonneau G, et al. BMPR2 gene rearrangements account for a significant proportion of mutations in familial and idiopathic pulmonary arterial hypertension. Hum Mutat. 2006;27:212–213. doi: 10.1002/humu.9398. [DOI] [PubMed] [Google Scholar]
- 59.Harrison RE, Flanagan JA, Sankelo M, Abdalla SA, Rowell J, Machado RD, Elliott CG, Robbins IM, Olschewski H, McLaughlin V, et al. Molecular and functional analysis identifies ALK-1 as the predominant cause of pulmonary hypertension related to hereditary haemorrhagic telangiectasia. J Med Genet. 2003;40:865–871. doi: 10.1136/jmg.40.12.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shintani M, Yagi H, Nakayama T, Saji T, Matsuoka R. A new nonsense mutation of SMAD8 associated with pulmonary arterial hypertension. J Med Genet. 2009;46:331–337. doi: 10.1136/jmg.2008.062703. [DOI] [PubMed] [Google Scholar]
- 61.Beck SE, Jung BH, Del Rosario E, Gomez J, Carethers JM. BMP-induced growth suppression in colon cancer cells is mediated by p21WAF1 stabilization and modulated by RAS/ERK. Cell Signal. 2007;19:1465–1472. doi: 10.1016/j.cellsig.2007.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rudarakanchana N, Flanagan JA, Chen H, Upton PD, Machado R, Patel D, Trembath RC, Morrell NW. Functional analysis of bone morphogenetic protein type II receptor mutations underlying primary pulmonary hypertension. Hum Mol Genet. 2002;11:1517–1525. doi: 10.1093/hmg/11.13.1517. [DOI] [PubMed] [Google Scholar]
- 63.McMurtry MS, Archer SL, Altieri DC, Bonnet S, Haromy A, Harry G, Bonnet S, Puttagunta L, Michelakis ED. Gene therapy targeting survivin selectively induces pulmonary vascular apoptosis and reverses pulmonary arterial hypertension. J Clin Invest. 2005;115:1479–1491. doi: 10.1172/JCI23203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Botney MD, Bahadori L, Gold LI. Vascular remodeling in primary pulmonary hypertension. Potential role for transforming growth factor-beta. Am J Pathol. 1994;144:286–295. [PMC free article] [PubMed] [Google Scholar]
- 65.Ameshima S, Golpon H, Cool CD, Chan D, Vandivier RW, Gardai SJ, Wick M, Nemenoff RA, Geraci MW, Voelkel NF. Peroxisome proliferator-activated receptor gamma (PPARgamma) expression is decreased in pulmonary hypertension and affects endothelial cell growth. Circ Res. 2003;92:1162–1169. doi: 10.1161/01.RES.0000073585.50092.14. [DOI] [PubMed] [Google Scholar]
- 66.Sakao S, Taraseviciene-Stewart L, Lee JD, Wood K, Cool CD, Voelkel NF. Initial apoptosis is followed by increased proliferation of apoptosis-resistant endothelial cells. FASEB J. 2005;19:1178–1180. doi: 10.1096/fj.04-3261fje. [DOI] [PubMed] [Google Scholar]
- 67.Geraci MW, Moore M, Gesell T, Yeager ME, Alger L, Golpon H, Gao B, Loyd JE, Tuder RM, Voelkel NF. Gene expression patterns in the lungs of patients with primary pulmonary hypertension: a gene microarray analysis. Circ Res. 2001;88:555–562. doi: 10.1161/01.res.88.6.555. [DOI] [PubMed] [Google Scholar]
- 68.Taraseviciene-Stewart L, Kasahara Y, Alger L, Hirth P, Mc Mahon G, Waltenberger J, Voelkel NF, Tuder RM. Inhibition of the VEGF receptor 2 combined with chronic hypoxia causes cell death–dependent pulmonary endothelial cell proliferation and severe pulmonary hypertension. FASEB J. 2001;15:427–438. doi: 10.1096/fj.00-0343com. [DOI] [PubMed] [Google Scholar]
- 69.Zaiman AL, Damico R, Thoms-Chesley A, Files DC, Kesari P, Johnston L, Swaim M, Mozammel S, Myers AC, Halushka M, et al. A critical role for the protein apoptosis repressor with caspase recruitment domain in hypoxia-induced pulmonary hypertension. Circulation. 2011;124:2533–2542. doi: 10.1161/CIRCULATIONAHA.111.034512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yip KW, Reed JC. Bcl-2 family proteins and cancer. Oncogene. 2008;27:6398–6406. doi: 10.1038/onc.2008.307. [DOI] [PubMed] [Google Scholar]
- 71.Pozeg ZI, Michelakis ED, McMurtry MS, Thébaud B, Wu XC, Dyck JR, Hashimoto K, Wang S, Moudgil R, Harry G, et al. In vivo gene transfer of the O2-sensitive potassium channel Kv1.5 reduces pulmonary hypertension and restores hypoxic pulmonary vasoconstriction in chronically hypoxic rats. Circulation. 2003;107:2037–2044. doi: 10.1161/01.CIR.0000062688.76508.B3. [DOI] [PubMed] [Google Scholar]
- 72.Chen X, Duan N, Zhang C, Zhang W. Survivin and tumorigenesis: molecular mechanisms and therapeutic strategies. J Cancer. 2016;7:314–323. doi: 10.7150/jca.13332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Paulin R, Courboulin A, Meloche J, Mainguy V, Dumas de la Roque E, Saksouk N, Côté J, Provencher S, Sussman MA, Bonnet S. Signal transducers and activators of transcription-3/Pim1 axis plays a critical role in the pathogenesis of human pulmonary arterial hypertension. Circulation. 2011;123:1205–1215. doi: 10.1161/CIRCULATIONAHA.110.963314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bonnet S, Rochefort G, Sutendra G, Archer SL, Haromy A, Webster L, Hashimoto K, Bonnet SN, Michelakis ED. The nuclear factor of activated T cells in pulmonary arterial hypertension can be therapeutically targeted. Proc Natl Acad Sci USA. 2007;104:11418–11423. doi: 10.1073/pnas.0610467104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PL, Coviello GM, Wright WE, Weinrich SL, Shay JW. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266:2011–2015. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- 76.Mouraret N, Houssaïni A, Abid S, Quarck R, Marcos E, Parpaleix A, Gary-Bobo G, Dubois-Randé JL, Derumeaux G, Boczkowski J, et al. Role for telomerase in pulmonary hypertension. Circulation. 2015;131:742–755. doi: 10.1161/CIRCULATIONAHA.114.013258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fearon ER, Hamilton SR, Vogelstein B. Clonal analysis of human colorectal tumors. Science. 1987;238:193–197. doi: 10.1126/science.2889267. [DOI] [PubMed] [Google Scholar]
- 78.Lee SD, Shroyer KR, Markham NE, Cool CD, Voelkel NF, Tuder RM. Monoclonal endothelial cell proliferation is present in primary but not secondary pulmonary hypertension. J Clin Invest. 1998;101:927–934. doi: 10.1172/JCI1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yeager ME, Halley GR, Golpon HA, Voelkel NF, Tuder RM. Microsatellite instability of endothelial cell growth and apoptosis genes within plexiform lesions in primary pulmonary hypertension. Circ Res. 2001;88:E2–E11. doi: 10.1161/01.res.88.1.e2. [DOI] [PubMed] [Google Scholar]
- 80.Aldred MA, Comhair SA, Varella-Garcia M, Asosingh K, Xu W, Noon GP, Thistlethwaite PA, Tuder RM, Erzurum SC, Geraci MW, et al. Somatic chromosome abnormalities in the lungs of patients with pulmonary arterial hypertension. Am J Respir Crit Care Med. 2010;182:1153–1160. doi: 10.1164/rccm.201003-0491OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Drake KM, Comhair SA, Erzurum SC, Tuder RM, Aldred MA. Endothelial chromosome 13 deletion in congenital heart disease–associated pulmonary arterial hypertension dysregulates SMAD9 signaling. Am J Respir Crit Care Med. 2015;191:850–854. doi: 10.1164/rccm.201411-1985LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pikor L, Thu K, Vucic E, Lam W. The detection and implication of genome instability in cancer. Cancer Metastasis Rev. 2013;32:341–352. doi: 10.1007/s10555-013-9429-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Federici C, Drake KM, Rigelsky CM, McNelly LN, Meade SL, Comhair SA, Erzurum SC, Aldred MA. Increased mutagen sensitivity and DNA damage in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2015;192:219–228. doi: 10.1164/rccm.201411-2128OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.de Jesus Perez VA, Yuan K, Lyuksyutova MA, Dewey F, Orcholski ME, Shuffle EM, Mathur M, Yancy L, Jr, Rojas V, Li CG, et al. Whole-exome sequencing reveals TopBP1 as a novel gene in idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med. 2014;189:1260–1272. doi: 10.1164/rccm.201310-1749OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ranchoux B, Meloche J, Paulin R, Boucherat O, Provencher S, Bonnet S. DNA damage and pulmonary hypertension. Int J Mol Sci. 2016;17:990. doi: 10.3390/ijms17060990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li M, Vattulainen S, Aho J, Orcholski M, Rojas V, Yuan K, Helenius M, Taimen P, Myllykangas S, De Jesus Perez V, et al. Loss of bone morphogenetic protein receptor 2 is associated with abnormal DNA repair in pulmonary arterial hypertension. Am J Respir Cell Mol Biol. 2014;50:1118–1128. doi: 10.1165/rcmb.2013-0349OC. [DOI] [PubMed] [Google Scholar]
- 87.Meloche J, Pflieger A, Vaillancourt M, Paulin R, Potus F, Zervopoulos S, Graydon C, Courboulin A, Breuils-Bonnet S, Tremblay E, et al. Role for DNA damage signaling in pulmonary arterial hypertension. Circulation. 2014;129:786–797. doi: 10.1161/CIRCULATIONAHA.113.006167. [DOI] [PubMed] [Google Scholar]
- 88.Macchia A, Marchioli R, Tognoni G, Scarano M, Marfisi R, Tavazzi L, Rich S. Systematic review of trials using vasodilators in pulmonary arterial hypertension: why a new approach is needed. Am Heart J. 2010;159:245–257. doi: 10.1016/j.ahj.2009.11.028. [DOI] [PubMed] [Google Scholar]
- 89.Rabinovitch M, Guignabert C, Humbert M, Nicolls MR. Inflammation and immunity in the pathogenesis of pulmonary arterial hypertension. Circ Res. 2014;115:165–175. doi: 10.1161/CIRCRESAHA.113.301141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hemnes AR, Zhao M, West J, Newman JH, Rich S, Archer SL, Robbins IM, Blackwell TS, Cogan J, Loyd JE, et al. Critical genomic networks and vasoreactive variants in idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med. 2016;194:464–475. doi: 10.1164/rccm.201508-1678OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ball MK, Waypa GB, Mungai PT, Nielsen JM, Czech L, Dudley VJ, Beussink L, Dettman RW, Berkelhamer SK, Steinhorn RH, et al. Regulation of hypoxia-induced pulmonary hypertension by vascular smooth muscle hypoxia-inducible factor-1α. Am J Respir Crit Care Med. 2014;189:314–324. doi: 10.1164/rccm.201302-0302OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pullamsetti SS, Schermuly R, Ghofrani A, Weissmann N, Grimminger F, Seeger W. Novel and emerging therapies for pulmonary hypertension. Am J Respir Crit Care Med. 2014;189:394–400. doi: 10.1164/rccm.201308-1543PP. [DOI] [PubMed] [Google Scholar]
- 93.Hojjat-Farsangi M. Small-molecule inhibitors of the receptor tyrosine kinases: promising tools for targeted cancer therapies. Int J Mol Sci. 2014;15:13768–13801. doi: 10.3390/ijms150813768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pullamsetti SS, Berghausen EM, Dabral S, Tretyn A, Butrous E, Savai R, Butrous G, Dahal BK, Brandes RP, Ghofrani HA, et al. Role of Src tyrosine kinases in experimental pulmonary hypertension. Arterioscler Thromb Vasc Biol. 2012;32:1354–1365. doi: 10.1161/ATVBAHA.112.248500. [DOI] [PubMed] [Google Scholar]
- 95.Dahal BK, Cornitescu T, Tretyn A, Pullamsetti SS, Kosanovic D, Dumitrascu R, Ghofrani HA, Weissmann N, Voswinckel R, Banat GA, et al. Role of epidermal growth factor inhibition in experimental pulmonary hypertension. Am J Respir Crit Care Med. 2010;181:158–167. doi: 10.1164/rccm.200811-1682OC. [DOI] [PubMed] [Google Scholar]
- 96.Nicolls MR, Mizuno S, Taraseviciene-Stewart L, Farkas L, Drake JI, Al Husseini A, Gomez-Arroyo JG, Voelkel NF, Bogaard HJ. New models of pulmonary hypertension based on VEGF receptor blockade–induced endothelial cell apoptosis. Pulm Circ. 2012;2:434–442. doi: 10.4103/2045-8932.105031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Alexander PB, Wang XF. Resistance to receptor tyrosine kinase inhibition in cancer: molecular mechanisms and therapeutic strategies. Front Med. 2015;9:134–138. doi: 10.1007/s11684-015-0396-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Klein M, Schermuly RT, Ellinghaus P, Milting H, Riedl B, Nikolova S, Pullamsetti SS, Weissmann N, Dony E, Savai R, et al. Combined tyrosine and serine/threonine kinase inhibition by sorafenib prevents progression of experimental pulmonary hypertension and myocardial remodeling. Circulation. 2008;118:2081–2090. doi: 10.1161/CIRCULATIONAHA.108.779751. [DOI] [PubMed] [Google Scholar]
- 99.Kojonazarov B, Sydykov A, Pullamsetti SS, Luitel H, Dahal BK, Kosanovic D, Tian X, Majewski M, Baumann C, Evans S, et al. Effects of multikinase inhibitors on pressure overload–induced right ventricular remodeling. Int J Cardiol. 2013;167:2630–2637. doi: 10.1016/j.ijcard.2012.06.129. [DOI] [PubMed] [Google Scholar]
- 100.Zhao L, Ashek A, Wang L, Fang W, Dabral S, Dubois O, Cupitt J, Pullamsetti SS, Cotroneo E, Jones H, et al. Heterogeneity in lung 18FDG uptake in PAH: potential of dynamic 18FDG positron emission tomography with kinetic analysis as a bridging biomarker for pulmonary vascular remodeling targeted treatments. Circulation. 2013;128:1214–1224. doi: 10.1161/CIRCULATIONAHA.113.004136. [DOI] [PubMed] [Google Scholar]
- 101.De Luca A, Maiello MR, D’Alessio A, Pergameno M, Normanno N. The RAS/RAF/MEK/ERK and the PI3K/AKT signalling pathways: role in cancer pathogenesis and implications for therapeutic approaches. Expert Opin Ther Targets. 2012;16:S17–S27. doi: 10.1517/14728222.2011.639361. [DOI] [PubMed] [Google Scholar]
- 102.Awad KS, Elinoff JM, Wang S, Gairhe S, Ferreyra GA, Cai R, Sun J, Solomon MA, Danner RL. Raf/ERK drives the proliferative and invasive phenotype of BMPR2-silenced pulmonary artery endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2016;310:L187–L201. doi: 10.1152/ajplung.00303.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lane KB, Blackwell TR, Runo J, Wheeler L, Phillips JA, III, Loyd JE. Aberrant signal transduction in pulmonary hypertension. Chest. 2005;128(6) Suppl:564S–565S. doi: 10.1378/chest.128.6_suppl.564S-a. [DOI] [PubMed] [Google Scholar]
- 104.Hopper RK, Feinstein JA, Manning MA, Benitz W, Hudgins L. Neonatal pulmonary arterial hypertension and Noonan syndrome: two fatal cases with a specific RAF1 mutation. Am J Med Genet A. 2015;167A:882–885. doi: 10.1002/ajmg.a.37024. [DOI] [PubMed] [Google Scholar]
- 105.Morecroft I, Doyle B, Nilsen M, Kolch W, Mair K, Maclean MR. Mice lacking the Raf-1 kinase inhibitor protein exhibit exaggerated hypoxia-induced pulmonary hypertension. Br J Pharmacol. 2011;163:948–963. doi: 10.1111/j.1476-5381.2011.01305.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 106.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dienstmann R, Rodon J, Serra V, Tabernero J. Picking the point of inhibition: a comparative review of PI3K/AKT/mTOR pathway inhibitors. Mol Cancer Ther. 2014;13:1021–1031. doi: 10.1158/1535-7163.MCT-13-0639. [DOI] [PubMed] [Google Scholar]
- 108.Teng X, Li D, Champion HC, Johns RA. FIZZ1/RELMα, a novel hypoxia-induced mitogenic factor in lung with vasoconstrictive and angiogenic properties. Circ Res. 2003;92:1065–1067. doi: 10.1161/01.RES.0000073999.07698.33. [DOI] [PubMed] [Google Scholar]
- 109.Garat CV, Crossno JT, Jr, Sullivan TM, Reusch JEB, Klemm DJ. Inhibition of phosphatidylinositol 3-kinase/Akt signaling attenuates hypoxia-induced pulmonary artery remodeling and suppresses CREB depletion in arterial smooth muscle cells. J Cardiovasc Pharmacol. 2013;62:539–548. doi: 10.1097/FJC.0000000000000014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tang H, Chen J, Fraidenburg DR, Song S, Sysol JR, Drennan AR, Offermanns S, Ye RD, Bonini MG, Minshall RD, et al. Deficiency of Akt1, but not Akt2, attenuates the development of pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2015;308:L208–L220. doi: 10.1152/ajplung.00242.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nemenoff RA, Simpson PA, Furgeson SB, Kaplan-Albuquerque N, Crossno J, Garl PJ, Cooper J, Weiser-Evans MCM. Targeted deletion of PTEN in smooth muscle cells results in vascular remodeling and recruitment of progenitor cells through induction of stromal cell–derived factor-1α. Circ Res. 2008;102:1036–1045. doi: 10.1161/CIRCRESAHA.107.169896. [DOI] [PubMed] [Google Scholar]
- 112.Houssaini A, Abid S, Derumeaux G, Wan F, Parpaleix A, Rideau D, Marcos E, Kebe K, Czibik G, Sawaki D, et al. Selective tuberous sclerosis complex 1 gene deletion in smooth muscle activates mammalian target of rapamycin signaling and induces pulmonary hypertension. Am J Respir Cell Mol Biol. 2016;55:352–367. doi: 10.1165/rcmb.2015-0339OC. [DOI] [PubMed] [Google Scholar]
- 113.Houssaini A, Abid S, Mouraret N, Wan F, Rideau D, Saker M, Marcos E, Tissot C-M, Dubois-Randé J-L, Amsellem V, et al. Rapamycin reverses pulmonary artery smooth muscle cell proliferation in pulmonary hypertension. Am J Respir Cell Mol Biol. 2013;48:568–577. doi: 10.1165/rcmb.2012-0429OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Aghamohammadzadeh R, Zhang Y-Y, Stephens TE, Arons E, Zaman P, Polach KJ, Matar M, Yung L-M, Yu PB, Bowman FP, et al. Up-regulation of the mammalian target of rapamycin complex 1 subunit Raptor by aldosterone induces abnormal pulmonary artery smooth muscle cell survival patterns to promote pulmonary arterial hypertension. FASEB J. 2016;30:2511–2527. doi: 10.1096/fj.201500042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Krymskaya VP, Snow J, Cesarone G, Khavin I, Goncharov DA, Lim PN, Veasey SC, Ihida-Stansbury K, Jones PL, Goncharova EA. mTOR is required for pulmonary arterial vascular smooth muscle cell proliferation under chronic hypoxia. FASEB J. 2011;25:1922–1933. doi: 10.1096/fj.10-175018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.McMurtry MS, Bonnet S, Michelakis ED, Bonnet S, Haromy A, Archer SL. Statin therapy, alone or with rapamycin, does not reverse monocrotaline pulmonary arterial hypertension: the rapamcyin–atorvastatin–simvastatin study. Am J Physiol Lung Cell Mol Physiol. 2007;293:L933–L940. doi: 10.1152/ajplung.00310.2006. [DOI] [PubMed] [Google Scholar]
- 117.Seyfarth H-J, Hammerschmidt S, Halank M, Neuhaus P, Wirtz HR. Everolimus in patients with severe pulmonary hypertension: a safety and efficacy pilot trial. Pulm Circ. 2013;3:632–638. doi: 10.1086/674311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rizzo P, Osipo C, Foreman K, Golde T, Osborne B, Miele L. Rational targeting of Notch signaling in cancer. Oncogene. 2008;27:5124–5131. doi: 10.1038/onc.2008.226. [DOI] [PubMed] [Google Scholar]
- 120.Lam EW, Brosens JJ, Gomes AR, Koo CY. Forkhead box proteins: tuning forks for transcriptional harmony. Nat Rev Cancer. 2013;13:482–495. doi: 10.1038/nrc3539. [DOI] [PubMed] [Google Scholar]
- 121.Ghofrani HA, Morrell NW, Hoeper MM, Olschewski H, Peacock AJ, Barst RJ, Shapiro S, Golpon H, Toshner M, Grimminger F, et al. Imatinib in pulmonary arterial hypertension patients with inadequate response to established therapy. Am J Respir Crit Care Med. 2010;182:1171–1177. doi: 10.1164/rccm.201001-0123OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ghofrani HA, Seeger W, Grimminger F. Imatinib for the treatment of pulmonary arterial hypertension. N Engl J Med. 2005;353:1412–1413. doi: 10.1056/NEJMc051946. [DOI] [PubMed] [Google Scholar]
- 123.Hoeper MM, Barst RJ, Bourge RC, Feldman J, Frost AE, Galié N, Gómez-Sánchez MA, Grimminger F, Grünig E, Hassoun PM, et al. Imatinib mesylate as add-on therapy for pulmonary arterial hypertension: results of the randomized IMPRES study. Circulation. 2013;127:1128–1138. doi: 10.1161/CIRCULATIONAHA.112.000765. [DOI] [PubMed] [Google Scholar]
- 124.Preston IR, Roberts KE, Miller DP, Sen GP, Selej M, Benton WW, Hill NS, Farber HW. Effect of warfarin treatment on survival of patients with pulmonary arterial hypertension (PAH) in the Registry to Evaluate Early and Long-Term PAH Disease Management (REVEAL) Circulation. 2015;132:2403–2411. doi: 10.1161/CIRCULATIONAHA.115.018435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Olsson KM, Delcroix M, Ghofrani HA, Tiede H, Huscher D, Speich R, Grünig E, Staehler G, Rosenkranz S, Halank M, et al. Anticoagulation and survival in pulmonary arterial hypertension: results from the Comparative, Prospective Registry of Newly Initiated Therapies for Pulmonary Hypertension (COMPERA) Circulation. 2014;129:57–65. doi: 10.1161/CIRCULATIONAHA.113.004526. [DOI] [PubMed] [Google Scholar]
- 126.Speich R, Ulrich S, Domenighetti G, Huber LC, Fischler M, Treder U, Breitenstein A. Efficacy and safety of long-term imatinib therapy for pulmonary arterial hypertension. Respiration. 2015;89:515–524. doi: 10.1159/000381923. [DOI] [PubMed] [Google Scholar]
- 127.Pollett JB, Benza RL, Murali S, Shields KJ, Passineau MJ. Harvest of pulmonary artery endothelial cells from patients undergoing right heart catheterization. J Heart Lung Transplant. 2013;32:746–749. doi: 10.1016/j.healun.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 128.Kudo K, Arao T, Tanaka K, Nagai T, Furuta K, Sakai K, Kaneda H, Matsumoto K, Tamura D, Aomatsu K, et al. Antitumor activity of BIBF 1120, a triple angiokinase inhibitor, and use of VEGFR2+pTyr+ peripheral blood leukocytes as a pharmacodynamic biomarker in vivo. Clin Cancer Res. 2011;17:1373–1381. doi: 10.1158/1078-0432.CCR-09-2755. [DOI] [PubMed] [Google Scholar]
- 129.Gomberg-Maitland M, Maitland ML, Barst RJ, Sugeng L, Coslet S, Perrino TJ, Bond L, Lacouture ME, Archer SL, Ratain MJ. A dosing/cross-development study of the multikinase inhibitor sorafenib in patients with pulmonary arterial hypertension. Clin Pharmacol Ther. 2010;87:303–310. doi: 10.1038/clpt.2009.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Spiekerkoetter E, Tian X, Cai J, Hopper RK, Sudheendra D, Li CG, El-Bizri N, Sawada H, Haghighat R, Chan R, et al. FK506 activates BMPR2, rescues endothelial dysfunction, and reverses pulmonary hypertension. J Clin Invest. 2013;123:3600–3613. doi: 10.1172/JCI65592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Spiekerkoetter E, Sung YK, Sudheendra D, Bill M, Aldred MA, van de Veerdonk MC, Vonk Noordegraaf A, Long-Boyle J, Dash R, Yang PC, et al. Low-dose FK506 (tacrolimus) in end-stage pulmonary arterial hypertension. Am J Respir Crit Care Med. 2015;192:254–257. doi: 10.1164/rccm.201411-2061LE. [DOI] [PMC free article] [PubMed] [Google Scholar]