Abstract
Rationale: The findings of the NLST (National Lung Screening Trial) are the basis for screening high-risk individuals according to age and smoking history. Although screening is covered for eligible Medicare beneficiaries, the generalizability of the NLST in the elderly population has been questioned.
Objectives: Compare outcomes of patients diagnosed with stage 1 non–small cell lung cancer in the NLST to a nationally representative cohort of elderly patients
Methods: Analysis of Surveillance, Epidemiology, and End Results (SEER)-Medicare and NLST datasets for patients with stage 1 disease aged 65 to 74 years.
Measurements and Main Results: Lung cancer–specific mortality, all-cause mortality, and 30-, 60-, and 90-day treatment mortality were measured. When compared with the NLST group undergoing surgery for stage 1 non–small cell lung cancer, those in the SEER-Medicare NLST eligible cohort had no difference in adjusted odds ratios for 30-, 60-, and 90-day surgical mortality (P values = 0.97, 0.65, and 0.46, respectively). Although the 5-year cancer-specific survival did not differ between cohorts (hazard ratio [HR], 0.84 NLST vs. SEER-Medicare NLST eligible; P = 0.21), the adjusted HR estimate for all-cause mortality was better in the NLST cohort (HR, 0.71; P < 0.01). For patients who did not receive surgery for early-stage disease (presumably for curative intent), the outcomes were far worse (13.1, 18.9, 23.9%, for 30-, 60-, and 90-day treatment mortality, respectively).
Conclusions: Elderly patients with minimal comorbid conditions meeting the inclusion criteria of the NLST who underwent surgery had excellent postoperative outcomes and similar lung cancer–specific 5-year survivorship. In those with significant comorbidities or those not undergoing surgery, competing causes of death may diminish the benefit, and there is no evidence to recommend screening in this group.
Keywords: lung cancer screening, stage 1 lung cancer outcomes, comorbidities
At a Glance Commentary
Scientific Knowledge on the Subject
The NLST (National Lung Screening Trial) demonstrated a mortality benefit from screening high-risk patients with low-dose computed tomography scan. How these results will translate in populations underrepresented in the trial, such as the elderly with comorbid conditions, has been an open question as broad-based screening is implemented.
What This Study Adds to the Field
This study supports screening elderly patients with minimal comorbid conditions meeting the inclusion criteria of the NLST. It suggests that the benefit of screening is diminished by competing causes of death in those with significant comorbidity or those not undergoing surgery for a screen-detected cancer.
In a population at high risk for lung cancer as defined by age and smoking history, the NLST (National Lung Screening Trial) demonstrated a 20% reduction in lung cancer–associated mortality and a 7% reduction in overall mortality by screening with low-dose computed tomography (LDCT) of the chest (1). The number needed to screen with annual LDCT over 3 years to prevent one death from lung cancer is 320, similar to that of mammography for detecting breast cancer in women aged 50–69 years (1, 2). This breakthrough has the potential to lead to a significant public health benefit, considering that in the absence of screening only 17% of patients are diagnosed with a disease stage that has potential for cure by surgical resection, whereas the 5-year survival for those with stage 1 non–small cell lung cancer (NSCLC) is 58 to 73% after surgery (3).
Approximately 8 million Americans meet screening criteria (1). However, compared with the U.S. population meeting these criteria, the participants in the NLST are demographically different (4). Trial participants were younger, better educated, less ethnically diverse, and less likely to be active smokers (4). Generalizability of the results to the U.S. population may also be problematic for a number of additional reasons. First, participants in the NLST were enrolled in urban, tertiary care hospitals with expertise in all aspects of cancer care, which may not be the case for all sites where patients with lung cancer receive care. Second, the mortality rate from lung cancer surgery was 1% in NLST, whereas the national average is between 1 and 5% (5, 6, 7). Third, although the NLST protocol allowed for patients to choose where they had their evaluation and management for screen-detected nodules, it is likely that many were managed at NLST sites with high volume and dedicated thoracic surgeons, both of which have been shown to improve outcomes (8, 9).
It is unclear how LDCT screening will perform in subgroups of the U.S. population that were not well represented in the NLST. Although 70 years is the average age for lung cancer diagnosis, only 9% of the NLST study population was older than 70 years. In an analysis of the NLST that compared Medicare-eligible participants to those younger than 65 years, the false-positive rate was higher in the 65 and older age group. In addition, invasive procedures after false positives were higher and 5-year overall survival was lower in the older age group (10).
We undertook this study to more fully characterize the potential benefits and risks of screening elderly patients, an underrepresented subpopulation of the NLST. Because a randomized trial similar to the NLST is not likely to be repeated solely in the elderly population, we more closely evaluated the surgical outcomes of the elderly participants in the NLST. In addition, we developed a cohort of patients older than age 65 years with stage 1 lung cancer from the Surveillance, Epidemiology, and End Results program (SEER)-Medicare databases and compared their outcomes to the participants of the NLST.
Methods
This study was approved by the Medical University of South Carolina institutional review board (Pro00024909).
Description of Datasets
NLST
The NLST study design has been described in detail previously (11). Briefly, eligible participants were 55 to 74 years of age at time of randomization, had a history of cigarette smoking of at least 30 pack-years, and, if former smokers, had quit within the previous 15 years. Patients had to be asymptomatic and deemed good operative candidates to be included in that trial. A total of 53,452 persons were enrolled and randomly assigned to three rounds of annual screening with LDCT or chest radiography. The median duration of follow-up was 6.5 years, with a maximum duration of 7.4 years in each group. The primary endpoint was lung cancer–specific mortality in the two arms. This study included patients from the original NLST cohort aged 65 years and older diagnosed with stage 1A and 1B lung cancers. Self-reported demographic characteristics of the participants in terms of age, sex, race, and smoking status were collected along with diagnosis, treatment, and outcomes.
SEER-Medicare
The SEER-Medicare data are linked data from two separate sources: the SEER program of cancer registries and the Medicare claims covered for health care services received from the time of Medicare eligibility until death (12). This cohort included those patients aged 65 years and older diagnosed with stage 1A and 1B NSCLC from 1998 to 2010.
Definition of Variables
Surgery was identified using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9) procedure, Current Procedure Terminology, and Common Procedure Coding System codes. Radiation therapy was identified using data from Medicare claims files Part A or B.
Smoking status was categorized as current, former, or never smokers. Current smokers were defined by ICD-9 code 305.1—Tobacco abuse disorder, and former smokers were defined by by ICD-9 code V15.82—History of tobacco use. Never smokers were identified as having no record of either of these codes (13). Never smokers were not included in the analysis.
The Charlson Comorbidity Index (CCI) is a method of weighting patient comorbidities on the basis of 19 comorbid medical conditions that was designed for use with medical records (14). We used the Charlson-Deyo method, which is a modification that is based on 17 of the 19 weighted comorbid conditions that allow the CCI to be calculated using ICD diagnostic codes in administrative data (15). Although other comorbidity indexes are available, the CCI is the most widely used and consists of a numbered scoring system based on comorbidity category. Each is assigned a weight (from 1 to 6), and the sum of all weights results in a single patient comorbidity score, which can be used for comparison and risk evaluation. For example, a score of 1 would be assigned for cardiovascular disease or chronic pulmonary disease, and a score of 2 assigned for severe renal disease. A person with no comorbid conditions would have a CCI of 0, and someone with chronic pulmonary disease (1 point) and congestive heart failure (1 point) would have a CCI of 2.
Statistical Analysis
This is a secondary analysis of a pooled dataset from a randomized trial (NCT00047385) and an elderly subset of patients in the SEER-Medicare database with stage 1A and 1B lung cancer. Sample characteristics were summarized using means and proportions, and appropriate group comparison was made using either t test or chi-square tests.
Cox regression was used to estimate hazard ratios (HRs) and corresponding 95% confidence intervals (CIs) for the association between lung cancer–specific mortality and the variables of interest, including study cohort, age at the time of diagnosis, cancer stage, CCI, sex, and smoking status. In this analysis, subjects who did not die of lung cancer, who dropped out, or who survived to the end of the study were considered censored. We also considered a competing risk analysis where the outcomes of interest include time to each of these three events (censored, deceased from lung cancer, and deceased from other causes). This was done via Cox proportional hazard regression, using the Fine and Gray competing risk model for censored or deceased from other causes to estimate the cumulative mortality function (16). Model assumptions of proportionality of hazard over time and linearity in the logit were first tested and confirmed, followed by residual assessments to ensure we used the most appropriate data fit and to identify any potential outliers or influential observations. The assumption of proportional hazards was tested by testing the interaction between covariates and log(time), and additional model checking was made via residual analysis. Kaplan-Meier survival curves were examined for differences in survival on the basis of study cohort, CCI, lung cancer stage, and age at the time of diagnosis using log-rank test.
Unadjusted 30-, 60-, and 90-day treatment mortality rates were generated for each cohort stratified by CCI. Logistic regression was used to model the association between these binary outcomes and covariates of interest (age at the time of diagnosis, sex, lung cancer stage, smoking status, and CCI). The models were adjusted for additional patient demographics to determine confounding and effect modification of these variables. Assumptions of linearity in log odds and model diagnostics were made using residual plots. Estimates of odds ratios and corresponding 95% CIs and P values are displayed. All statistical analyses were performed with SAS 9.4 software (SAS Inc., Cary, NC).
Results
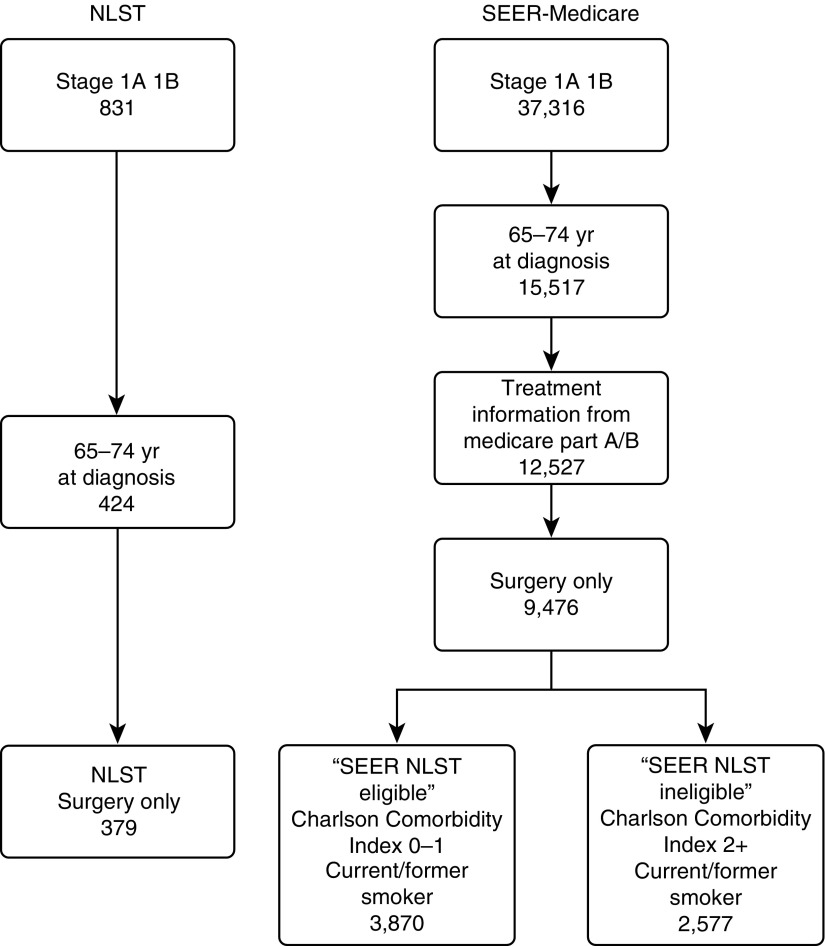
The SEER-Medicare dataset had 37,316 patients diagnosed with stage 1A and 1B NSCLC between 1998 and 2010. After selecting for those aged 65 to 74 years with treatment information available from Medicare Claims, data from 9,476 patients treated with surgical resection were available. Of these, 3,870 were current or former smokers with a CCI of 0 or 1 (denoted as “SEER NLST eligible” cohort). There were 2,577 current or former smokers undergoing surgery with CCI of 2 or more (denoted as “SEER NLST ineligible” cohort). (Figure 1). An additional 2,864 received radiation therapy in lieu of surgery, and 187 received other forms of treatment, which were less well defined. Of the 831 patients diagnosed with stage 1A and 1B lung cancer in the NLST dataset and 379 between the ages of 65 and 74 years, all who had CCI of 0 or 1 underwent surgical resection (denoted as “NLST” cohort).
Figure 1.
Cohort inclusion diagram. NLST = National Lung Screening Trial; SEER = Surveillance, Epidemiology, and End Results.
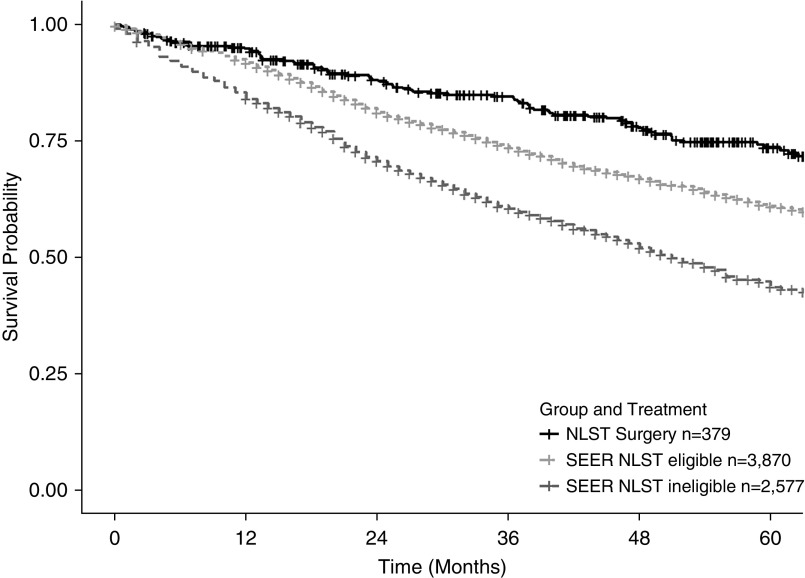
The demographics for the three cohorts of elderly patients undergoing surgical resection for stage 1 lung cancer are reported in Table 1. The majority of patients were white, with an even distribution of current and former smokers. The 30-, 60-, and 90-day surgical mortality was comparable in the NLST and SEER NLST eligible cohorts, but nearly twofold higher in the SEER NLST ineligible cohort. Similarly, the 5-year all-cause survivorship was better in the NLST and SEER NLST eligible cohort compared with SEER NLST ineligible patients: 73.6, 63.8, and 47.1%, respectively (P < 0.001) (Figure 2).
Table 1.
Characteristics of Those with Stage 1 Lung Cancer Undergoing Surgery
| NLST |
SEER NLST Eligible |
SEER NLST Ineligible |
||||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| All | 379 | 100 | 3,870 | 100 | 2,577 | 100 |
| Stage | ||||||
| 1A | 273 | 72.03 | 2,268 | 58.60 | 1,537 | 59.64 |
| 1B | 106 | 27.97 | 1,602 | 41.40 | 1,040 | 40.36 |
| Age, yr | ||||||
| 65–69 | 226 | 59.63 | 2,042 | 52.76 | 1,006 | 39.04 |
| 70–74 | 153 | 40.37 | 1,828 | 47.24 | 1,571 | 60.96 |
| Race | ||||||
| Other | 33 | 8.71 | 352 | 9.10 | 275 | 10.67 |
| White | 346 | 91.29 | 3,518 | 90.90 | 2,302 | 89.33 |
| Smoking status | ||||||
| Former | 184 | 48.55 | 2,080 | 53.75 | 1,142 | 44.32 |
| Current | 195 | 51.45 | 1,790 | 46.25 | 1,435 | 55.68 |
| CCI | ||||||
| 0 | 247 | 65.17 | 2,069 | 53.46 | ||
| 1 | 132 | 34.83 | 1,801 | 46.54 | ||
| ≥2 | 2,755 | 100 | ||||
| Mortality | ||||||
| 30 d | 6 | 1.58 | 56 | 1.45 | 69 | 2.68 |
| 60 d | 8 | 2.11 | 90 | 2.33 | 112 | 4.35 |
| 90 d | 9 | 2.37 | 113 | 2.92 | 152 | 5.90 |
| 5-yr all-cause survivorship* | 73.60 | 62.80 | 47.14 | |||
Definition of abbreviations: CCI = Charlson Comorbidity Index; NLST = National Lung Screening Trial; SEER = Surveillance, Epidemiology, and End Results.
Calculated using Kaplan-Meier method.
Figure 2.
Kaplan-Meier survival curves for current and former smokers undergoing surgery for stage 1 lung cancer. NLST = National Lung Screening Trial; SEER = Surveillance, Epidemiology, and End Results.
A comparison of the NLST and SEER NLST eligible cohorts demonstrated no difference between the age at diagnosis (P = 0.86) and CCI (P = 0.61); however, those in the SEER NLST eligible cohort had more stage 1B disease (P < 0.01), had a higher proportion of men (P < 0.01), and were more likely to be current smokers (P = 0.04) (see Table E1 in the online supplement). After adjustment for age, stage, CCI, sex, and smoking, in addition to accounting for censoring due to other competing risks (mortality due to other causes), the SEER NLST eligible group had no significant difference in 30-, 60-, and 90-day surgical mortality compared with the NLST group (Table 2). Thus, once matched for all relevant confounding factors that influence mortality, elderly patients similar to those enrolled in the NLST had similar surgical outcomes. In addition, there was similar 5-year lung cancer–specific mortality (HR, 0.84; 95% CI, 0.64–1.10); however, those in the NLST group had a better 5-year all-cause mortality than those in the SEER NLST eligible cohort (HR, 0.71; 95% CI, 0.57–0.88) (Table 3).
Table 2.
Unadjusted and Adjusted Odds Ratio for 30-, 60-, and 90-Day All-Cause Mortality in National Lung Screening Trial (n = 379) and Surveillance, Epidemiology, and End Results National Lung Screening Trial–Eligible Cohorts (n = 3,870)
| Effect | OR | 95% CI | P Value | |
|---|---|---|---|---|
| 30-d all-cause mortality |
|
|
|
|
| Unadjusted |
Group (NLST) |
1.10 |
0.47–2.56 |
0.83 |
| Adjusted |
Group (NLST) |
1.02 |
0.43–2.40 |
0.97 |
| |
Age at diagnosis |
1.09 |
0.99–1.19 |
0.08 |
| |
Stage (1B) |
1.09 |
0.66–1.82 |
0.74 |
| |
Charlson Index (1) |
1.70 |
1.01–2.85 |
0.05 |
| |
Sex (female) |
0.59 |
0.35–1.00 |
0.05 |
| |
Smoking status (former) |
0.87 |
0.53–1.44 |
0.59 |
| 60-d all-cause mortality |
|
|
|
|
| Unadjusted |
Group (NLST) |
0.91 |
0.44–1.88 |
0.79 |
| Adjusted |
Group (NLST) |
0.84 |
0.40–1.76 |
0.65 |
| |
Age at diagnosis |
1.03 |
0.96–1.11 |
0.37 |
| |
Stage (1B) |
1.15 |
0.77–1.73 |
0.49 |
| |
Charlson Index (1) |
1.40 |
0.93–2.11 |
0.10 |
| |
Sex (female) |
0.58 |
0.38–0.87 |
0.01 |
| |
Smoking status (former) |
0.84 |
0.56–1.25 |
0.38 |
| 90-d all-cause mortality |
|
|
|
|
| Unadjusted |
Group (NLST) |
0.81 |
0.41–1.61 |
0.55 |
| Adjusted |
Group (NLST) |
0.77 |
0.39–1.54 |
0.46 |
| |
Age at diagnosis |
1.02 |
0.96–1.09 |
0.54 |
| |
Stage (1B) |
1.38 |
0.96–1.99 |
0.08 |
| |
Charlson Index (1) |
1.30 |
0.90–1.87 |
0.16 |
| |
Sex (female) |
0.58 |
0.40–0.85 |
0.00 |
| Smoking status (former) | 0.78 | 0.54–1.12 | 0.18 |
Definition of abbreviations: CI = confidence interval; NLST = National Lung Screening Trial; OR = odds ratio; SEER = Surveillance, Epidemiology, and End Results.
Reference groups: group: SEER-Medicare; stage: 1A; Charlson Index: 0; sex: male; smoking status: current.
Table 3.
Unadjusted and Adjusted Hazard Ratio Estimates for 5-Year Lung Cancer–Specific and All-Cause Mortality in National Lung Screening Trial (n = 379) and Surveillance, Epidemiology, and End Results National Lung Screening Trial–Eligible Cohorts (n = 3,870)
| Effect | HR | 95% CI | P Value | |
|---|---|---|---|---|
| Lung cancer–specific mortality | ||||
| Unadjusted | Group (NLST) | 0.81 | 0.62–1.06 | 0.12 |
| Adjusted | Group (NLST) | 0.84 | 0.64–1.10 | 0.21 |
| Age at diagnosis | 1.01 | 0.99–1.04 | 0.31 | |
| Stage (1B) | 1.72 | 1.48–1.98 | <0.01 | |
| Charlson Index (1) | 1.24 | 1.07–1.44 | <0.01 | |
| Sex (female) | 0.80 | 0.69–0.93 | <0.01 | |
| Smoking status (former) | 0.89 | 0.77–1.03 | 0.12 | |
| All-cause mortality | ||||
| Unadjusted | Group (NLST) | 0.70 | 0.57–0.87 | <0.01 |
| Adjusted | Group (NLST) | 0.71 | 0.57–0.88 | <0.01 |
| Age at diagnosis | 1.02 | 1.00–1.04 | 0.02 | |
| Stage (1B) | 1.53 | 1.37–1.71 | <0.01 | |
| Charlson Index (1) | 1.25 | 1.12–1.40 | <0.01 | |
| Sex (female) | 0.77 | 0.69–0.86 | <0.01 | |
| Smoking status (former) | 0.77 | 0.69–0.86 | <0.01 |
Definition of abbreviations: CI = confidence interval; HR = hazard ratio; NLST = National Lung Screening Trial; SEER = Surveillance, Epidemiology, and End Results.
Reference groups: group: SEER-Medicare; stage: 1A; Charlson Index: 0; sex: male; smoking status: current.
When compared with other treatments, those who underwent surgery had a better 5-year survivorship. In addition, as age, comorbidity index, and cancer stage increased, survivorship was worse (Figures E1A and E1B). There were 2,864 patients identified in the SEER-Medicare dataset who did not undergo surgery but received radiation therapy for early-stage disease (presumably with curative intent). In the NLST cohort, there were 25 patients (6%) who underwent radiation therapy for their screen-detected cancer (Table E2). The 5-year survivorship was significantly worse; those who underwent radiation in the NLST and the SEER-Medicare groups had a 5-year survivorship of 26 and 25%, respectively, compared with the patients who underwent surgery and had a 74 and 60% survivorship (P value for intragroup comparison < 0.001) (Figure E1A). The type of radiation delivered is not captured; however, it is likely, given the time frame, that treatment was with conventional radiotherapy as opposed to stereotactic body radiotherapy (SBRT).
Discussion
There have been questions raised about the generalizability of the findings of the NLST, particularly as it relates to the outcomes from surgery and the applicability to the elderly (17). On the one hand, the fact that NLST participants were healthy before screening would argue that their outcomes should be better. Conversely, heavy smokers, elderly patients, and those cared for outside of high-volume centers irrespective of their baseline health status might have worse outcomes. This study informs this question in three important ways. First, surgical mortality and 5-year lung cancer–specific survivorship in a large, nationally representative cohort of elderly patients with stage 1 lung cancer and minimal comorbidities matching those enrolled in the NLST cohort had similar outcomes, although 5-year all-cause mortality was better in the NLST cohort. This suggests the results of the NLST are generalizable to those elderly Americans eligible for screening. Second, elderly patients with significant comorbidities (NLST ineligible) have worse 30-, 60-, and 90-day surgical mortality and, more importantly, significantly worse 5-year survivorship. This suggests that competing causes of death limit the value of screening this population. Third, patients with stage 1 lung cancer treated by nonsurgical approaches do poorly in both NLST and SEER-Medicare cohorts. This study affirms that there is no evidence to support screening patients who do not meet the asymptomatic and healthy criteria of the NLST.
As surgery is the treatment of choice for a screen-detected stage 1 lung cancer, the importance of surgical mortality cannot be overemphasized, because higher surgical mortality compromises the survival benefit of lung cancer screening. It has been difficult to extrapolate surgical outcomes from the literature to the findings of the NLST because of the selective nature of those included in that trial. Sixty-day surgical mortality for all undergoing resection for lung cancer was 1% in the NLST (18), which has not been reported elsewhere. There are number of possibilities for this, including a very healthy and asymptomatic population, the majority of whom underwent surgery at high-volume centers by dedicated thoracic surgeons, both of which have been shown to improve outcomes (8, 9). Analyses of lung cancer surgeries spanning 1999 to 2006 reported 30-day mortalities of 2.5 to 5.2% (5, 19, 20). Database analyses from patients undergoing lobectomy from 2010 to 2013 demonstrate 30-day mortalities between 1.4 and 2.6% (7).
Similarly, in one study, 30-day mortality after lobectomy in healthy elderly patients (aged 65–80 years) who were eligible for lung cancer screening was low at 2.34%; however, long-term survivorship, which would account for competing causes of death, was not reported (21). In another study, lung cancer surgical outcomes in elderly patients found that although 30-day mortality was similar, 5-year survivorship decreased as age increased (22). In addition, in elderly patients with worse lung function and a heavy smoking history, the 30-day surgical mortality was acceptably low at 2%; however, the 3-year survivorship was significantly worse (59 vs. 76%) when compared with the standard-risk patients (23). Our study confirms these findings by demonstrating a low near-term surgical mortality mirroring the NLST findings in the elderly with minimal comorbidities but worse surgical mortality and 5-year overall survivorship in sicker patients. When deciding who should be offered screening, a balance should be struck between short-term surgical outcomes and longer-term competing causes of death. In one recent study of patients undergoing curative-intent surgery for stage 1 lung cancer, as age increased past 65 years, all-cause mortality was higher than lung cancer–specific mortality (24). The patients in the SEER NLST eligible cohort in this study had similar 5-year lung cancer–specific mortality to the NLST patients but worse overall survival at 5 years. The reasons for this are unclear. It is possible that confounding factors that influence overall mortality were not adequately captured in the SEER-Medicare cohort.
In addition, our study supports the U.S. Preventive Services Task Force recommendation that screening only be offered to those healthy enough and willing to undergo surgical resection; those treated with radiation therapy in either cohort had significantly worse 5-year survivorship, although this was likely with older forms of radiotherapy, and no conclusions can be reliably drawn about what survivorship would be with SBRT. Patients enrolled in the NLST were meant to be excluded if they were not healthy enough to undergo surgery, and yet some were treated with radiation therapy, begging the question of whether or not they underreported their health status at the time of screening or, once diagnosed with a cancer, decided on a nonsurgical treatment approach.
Attempts have been made to compare the efficacy of SBRT and lobectomy for early-stage lung cancers; however, prospective double-arm randomized trials have failed to recruit. (Randomized Study to Compare Cyberknife to Surgical Resection in Stage I Non-small Cell Lung Cancer [STARS]: NCT00840749; Trial of Either Surgery or Sterotactic Radiotherapy for Early Stage [IA] Lung Cancer [ROSEL]: NCT00687986). Although a pooled analysis of two of these trials suggested that SBRT was superior to lobectomy, the study was limited by small numbers (58 total patients) (25). Large database studies examining the same question have suggested that lobectomy is superior to SBRT; however, these did not exclude patients with comorbidities, which confer a health-related bias toward lobectomy (26, 27). One study, which excluded patients receiving SBRT unfit to undergo surgery, demonstrated a benefit to lobectomy (5-year survivorship, 58 vs. 40%; P < 0.001) (28). These findings mirror our own; those in the NLST cohort, although it was small, had worse outcomes with radiation therapy; however, it may be that there is a selection bias, in that these patients may not have undergone SBRT.
Our study has limitations. First, this is a secondary data analysis with all the traditional limitations associated with large database review. However, a prospective randomized trial such as the NLST is unlikely to be repeated in the elderly population. Second, the SEER-Medicare cohort of patients is not a screen-detected population, although patients with stage 1 lung cancers are often asymptomatic and most closely represent a screen-detected population. Third, the smoking histories collected through the SEER-Medicare database are not often complete, as evidenced by one-third of the larger cohort being labeled as never smokers. Similarly, we were unable to adjust for amount of smoking, as pack years are not reported. It is also possible that by only including those who had a smoking-related claim that a selection bias may have been introduced, as those identified as smokers may differ from those falsely labeled as nonsmokers (e.g., they may have been heavier smokers). Last, although the CCI is the most widely used comorbidity index when dealing with administrative data, it is not the ideal way to assess preoperative fitness in this population.
The NLST showed that lung cancer screening using low-dose chest CT was both safe and efficacious. Our analysis of a large, nationally representative cohort of elderly patients with similar comorbidities appears to confirm the safety of surgery in this group and suggest the results of the NLST can be generalized; these findings should be confirmed in future research of elderly screened patients. Our results suggest a lack of evidence to support offering screening outside this well-selected group, as the benefit is reduced, with higher postoperative mortality and, importantly, significantly lower 5-year survivorship, likely due to comorbid disease. Although a higher short-term surgical mortality may seem acceptable, it is the overall reduction in long-term survivorship that diminishes the benefit of screening in those with competing causes of death. It is important for clinicians and patients to consider both aspects when deciding on whether or not to be screened. Similarly, screening those with multiple comorbidities with the intent to treat a screen-detected cancer with radiation is not supported by our data. There is a complicated balance in patient selection for lung screening. Secondary analysis of NLST participants demonstrated that those screened in the highest quintile of risk had fewer false-positive results and a lower number needed to screen (29); however, these patients were also asymptomatic and healthy. As screening is applied to the general eligible population, it is possible that many of those falling into the highest-risk quintile who stand to benefit the most will not be as healthy as NLST participants in terms of number of comorbid conditions. Future research and development of risk-prediction models to better assist clinicians and their patients in understanding the tradeoffs between short-term morbidity and long-term quality of life and survivorship when deciding patient eligibility to be screened are needed.
In conclusion, elderly patients should only be considered for screening for lung cancer if they match the inclusion criteria of the NLST, particularly as it relates to their comorbid status. If they cannot undergo surgery for any reason, competing causes of death drastically diminish benefit garnered from screening. It is reassuring to confirm that elderly patients who fit the criteria for screening can be safely cared for and are likely to experience excellent outcomes, and future research is needed to confirm this as broad-based screening is implemented.
Acknowledgments
Acknowledgment
The authors thank the National Cancer Institute; the Office of Research, Development, and Information, Centers for Medicare and Medicaid Services; Information Management Services, Inc.; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries for the creation of the SEER-Medicare database.
Footnotes
Supported by the American Cancer Society (N.T.T.). This study used the linked Surveillance, Epidemiology, and End Results–Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors.
Author Contributions: Conception and design: N.T.T., M.G., and G.A.S.; analysis and interpretation: N.T.T., L.D., B.C.B., M.G., and G.A.S.; drafting the manuscript: N.T.T., L.D., B.C.B., M.G., and G.A.S.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201705-0914OC on July 19, 2017
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, Fagerstrom RM, Gareen IF, Gatsonis C, Marcus PM, Sicks JD National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hendrick RE, Helvie MA. Mammography screening: a new estimate of number needed to screen to prevent one breast cancer death. AJR Am J Roentgenol. 2012;198:723–728. doi: 10.2214/AJR.11.7146. [DOI] [PubMed] [Google Scholar]
- 3.Goldstraw P, Crowley J, Chansky K, Giroux DJ, Groome PA, Rami-Porta R, Postmus PE, Rusch V, Sobin L International Association for the Study of Lung Cancer International Staging Committee; Participating Institutions. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol. 2007;2:706–714. doi: 10.1097/JTO.0b013e31812f3c1a. [DOI] [PubMed] [Google Scholar]
- 4.Aberle DR, Adams AM, Berg CD, Clapp JD, Clingan KL, Gareen IF, Lynch DA, Marcus PM, Pinsky PF National Lung Screening Trial Research Team. Baseline characteristics of participants in the randomized national lung screening trial. J Natl Cancer Inst. 2010;102:1771–1779. doi: 10.1093/jnci/djq434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boffa DJ, Allen MS, Grab JD, Gaissert HA, Harpole DH, Wright CD. Data from The Society of Thoracic Surgeons General Thoracic Surgery database: the surgical management of primary lung tumors. J Thorac Cardiovasc Surg. 2008;135:247–254. doi: 10.1016/j.jtcvs.2007.07.060. [DOI] [PubMed] [Google Scholar]
- 6.El-Sherif A, Gooding WE, Santos R, Pettiford B, Ferson PF, Fernando HC, Urda SJ, Luketich JD, Landreneau RJ. Outcomes of sublobar resection versus lobectomy for stage I non-small cell lung cancer: a 13-year analysis. Ann Thorac Surg. 2006;82:408–415. [Discussion, pp. 415-416.]. doi: 10.1016/j.athoracsur.2006.02.029. [DOI] [PubMed] [Google Scholar]
- 7.Seder CW, Salati M, Kozower BD, Wright CD, Falcoz PE, Brunelli A, Fernandez FG. Variation in pulmonary resection practices between the Society of Thoracic Surgeons and the European Society of Thoracic Surgeons general thoracic surgery databases. Ann Thorac Surg. 2016;101:2077–2084. doi: 10.1016/j.athoracsur.2015.12.073. [DOI] [PubMed] [Google Scholar]
- 8.Bach PB, Cramer LD, Schrag D, Downey RJ, Gelfand SE, Begg CB. The influence of hospital volume on survival after resection for lung cancer. N Engl J Med. 2001;345:181–188. doi: 10.1056/NEJM200107193450306. [DOI] [PubMed] [Google Scholar]
- 9.Silvestri GA, Handy J, Lackland D, Corley E, Reed CE. Specialists achieve better outcomes than generalists for lung cancer surgery. Chest. 1998;114:675–680. doi: 10.1378/chest.114.3.675. [DOI] [PubMed] [Google Scholar]
- 10.Pinsky PF, Gierada DS, Hocking W, Patz EF, Jr, Kramer BS. National Lung Screening Trial findings by age: Medicare-eligible versus under-65 population. Ann Intern Med. 2014;161:627–633. doi: 10.7326/M14-1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aberle DR, Berg CD, Black WC, Church TR, Fagerstrom RM, Galen B, Gareen IF, Gatsonis C, Goldin J, Gohagan JK, et al. National Lung Screening Trial Research Team. The National Lung Screening Trial: overview and study design. Radiology. 2011;258:243–253. doi: 10.1148/radiol.10091808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Cancer Institute. SEER-Medicare: brief description of the SEER-Medicare Database. 2016 [accessed 2016 Sept 13]. Available from: http://healthcaredelivery.cancer.gov/seermedicare/overview/
- 13.Wiley LK, Shah A, Xu H, Bush WS. ICD-9 tobacco use codes are effective identifiers of smoking status. J Am Med Inform Assoc. 2013;20:652–658. doi: 10.1136/amiajnl-2012-001557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 15.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 16.Fine JP, Gray RJ. A proportional hazards model for the sub distribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 17.Silvestri GA. Screening for lung cancer: it works, but does it really work? Ann Intern Med. 2011;155:537–539. doi: 10.7326/0003-4819-155-8-201110180-00364. [DOI] [PubMed] [Google Scholar]
- 18.Church TR, Black WC, Aberle DR, Berg CD, Clingan KL, Duan F, Fagerstrom RM, Gareen IF, Gierada DS, Jones GC, et al. National Lung Screening Trial Research Team. Results of initial low-dose computed tomographic screening for lung cancer. N Engl J Med. 2013;368:1980–1991. doi: 10.1056/NEJMoa1209120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meguid RA, Brooke BS, Chang DC, Sherwood JT, Brock MV, Yang SC. Are surgical outcomes for lung cancer resections improved at teaching hospitals? Ann Thorac Surg. 2008;85:1015–1024. [Discussion, pp. 1024–1025.]. doi: 10.1016/j.athoracsur.2007.09.046. [DOI] [PubMed] [Google Scholar]
- 20.Little AG, Rusch VW, Bonner JA, Gaspar LE, Green MR, Webb WR, Stewart AK.Patterns of surgical care of lung cancer patients Ann Thorac Surg 2005802051–2056. [Discussion, p. 2056.] [DOI] [PubMed] [Google Scholar]
- 21.Bravo Iñiguez CE, Armstrong KW, Cooper Z, Weissman JS, Ducko CT, Wee JO, Martinez MP, Bueno R, Jaklitsch MT, Wiener DC. Thirty-day mortality after lobectomy in elderly patients eligible for lung cancer screening. Ann Thorac Surg. 2016;101:541–546. doi: 10.1016/j.athoracsur.2015.08.067. [DOI] [PubMed] [Google Scholar]
- 22.Fernandez FG, Furnary AP, Kosinski AS, Onaitis MW, Kim S, Boffa D, Cowper P, Jacobs JP, Wright CD, Putnam JB., Jr Longitudinal follow-up of lung cancer resection from the Society of Thoracic Surgeons general thoracic surgery database in patients 65 years and older. Ann Thorac Surg. 2016;101:2067–2076. doi: 10.1016/j.athoracsur.2016.03.034. [DOI] [PubMed] [Google Scholar]
- 23.Sancheti MS, Melvan JN, Medbery RL, Fernandez FG, Gillespie TW, Li Q, Binongo JN, Pickens A, Force SD.outcomes after surgery in high-risk patients with early stage lung cancer Ann Thorac Surg 20161011043–1050. [Discussion, p. 1051.] [DOI] [PubMed] [Google Scholar]
- 24.Eguchi T, Bains S, Lee MC, Tan KS, Hristov B, Buitrago DH, Bains MS, Downey RJ, Huang J, Isbell JM, et al. Impact of increasing age on cause-specific mortality and morbidity in patients with stage I non-small-cell lung cancer: a competing risks analysis. J Clin Oncol. 2017;35:281–290. doi: 10.1200/JCO.2016.69.0834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang JY, Senan S, Paul MA, Mehran RJ, Louie AV, Balter P, Groen HJ, McRae SE, Widder J, Feng L, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol. 2015;16:630–637. doi: 10.1016/S1470-2045(15)70168-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Puri V, Crabtree TD, Bell JM, Broderick SR, Morgensztern D, Colditz GA, Kreisel D, Krupnick AS, Patterson GA, Meyers BF, et al. Treatment outcomes in stage I lung cancer: a comparison of surgery and stereotactic body radiation therapy. J Thorac Oncol. 2015;10:1776–1784. doi: 10.1097/JTO.0000000000000680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu JB, Soulos PR, Cramer LD, Decker RH, Kim AW, Gross CP. Comparative effectiveness of surgery and radiosurgery for stage I non-small cell lung cancer. Cancer. 2015;121:2341–2349. doi: 10.1002/cncr.29359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosen JE, Salazar MC, Wang Z, Yu JB, Decker RH, Kim AW, Detterbeck FC, Boffa DJ. Lobectomy versus stereotactic body radiotherapy in healthy patients with stage I lung cancer. J Thorac Cardiovasc Surg. 2016;152:44–54.e9. doi: 10.1016/j.jtcvs.2016.03.060. [DOI] [PubMed] [Google Scholar]
- 29.Kovalchik SA, Tammemagi M, Berg CD, Caporaso NE, Riley TL, Korch M, Silvestri GA, Chaturvedi AK, Katki HA. Targeting of low-dose CT screening according to the risk of lung-cancer death. N Engl J Med. 2013;369:245–254. doi: 10.1056/NEJMoa1301851. [DOI] [PMC free article] [PubMed] [Google Scholar]