Abstract
Rationale: Cystatin A and SPINK5 are endogenous protease inhibitors (EPIs) that may play key roles in epithelial barrier function.
Objectives: To investigate the roles of EPIs in the pathogenesis of chronic rhinosinusitis (CRS).
Methods: We examined the expression of cystatin A and SPINK5 in the nasal epithelial cells of patients with CRS. Additionally, the in vitro effects of recombinant EPIs on the secretion of the epithelial-derived cytokines IL-25, IL-33, and thymic stromal lymphopoietin in airway epithelial cells, and the in vivo effects of recombinant EPIs in the nasal epithelium of mice exposed to multiple airborne allergens (MAA) were examined.
Measurements and Main Results: Compared with control subjects and patients with noneosinophilic CRS, patients with eosinophilic CRS showed significantly lower protein and mRNA expression of cystatin A and SPINK5 in the nasal epithelium. Allergen-induced production of IL-25, IL-33, and thymic stromal lymphopoietin in normal human bronchial epithelial cells was inhibited by treatment with recombinant cystatin A or SPINK5. Conversely, the production of these cytokines was increased when cystatin A or SPINK5 were knocked down with small interfering RNA. Chronic MAA exposure induced goblet cell metaplasia and epithelial disruption in mouse nasal epithelium and decreased the tissue expression and nasal lavage levels of cystatin A and SPINK5. Intranasal instillations of recombinant EPIs attenuated this MAA-induced pathology.
Conclusions: Cystatin A and SPINK5 play an important role in protecting the airway epithelium from exogenous proteases. The preservation of EPIs may have a therapeutic benefit in intractable airway inflammation, such as eosinophilic CRS.
Keywords: protease, cytokine, chronic rhinosinusitis, epithelial cell, endogenous protease inhibitor
At a Glance Commentary
Scientific Knowledge on the Subject
Airborne allergen–derived proteases may be involved in the pathogenesis of chronic rhinosinusitis through breakdown of the barrier function of epithelial cells. Endogenous protease inhibitors play a key role in protecting the airway epithelium from airborne allergen–derived exogenous proteases, and their preservation may have a therapeutic benefit in intractable allergic airway inflammation.
What This Study Adds to the Field
Analysis of the interactions between airborne allergen–derived proteases and endogenous protease inhibitors, which are aggressive and protective factors, may improve the efficacy of treatments for eosinophilic chronic sinusitis.
Chronic rhinosinusitis (CRS) is generally believed to be caused by a complex interaction of local, systemic, microbial, genetic, environmental, and iatrogenic factors (1). Most patients with CRS without nasal polyps (CRSwNP) have prominent tissue eosinophilia, edema formation, and T-helper cell type 2 (Th2)-dominant inflammation, and CRSwNP is often associated with asthma and aspirin-sensitivity in Europe and the United States (2–4). However, more than half of patients with CRSwNP in East Asian countries including Japan, Korea, and China have noneosinophilic inflammation with purulent rhinorrhea and Th1/Th17-dominant inflammation (5–7). In Japan, CRSwNP is classified into two subtypes: eosinophilic CRS (ECRS) and noneosinophilic CRS (NECRS). ECRS is comparable with CRSwNP in Europe and the United States (8).
Proteases can be classified as endogenous or exogenous. Endogenous proteases including elastase in neutrophils or tryptase and chymase in mast cells have key roles in allergic inflammation (9, 10). In contrast, airborne allergen, such as fungi, pollen, and mites, and microorganisms, such as bacteria, rhinovirus, and influenza virus, are major sources of exogenous proteases (11–14). Recent reports have indicated that the innate response to exogenous proteases from inhaled allergens plays a key role in the development of Th2 immunity (15–17). Allergen proteases have been demonstrated to cause epithelial barrier breakdown, consequently extending the portal of entry for allergens, and to stimulate various types of cells through IgE-independent mechanisms (12). These findings imply that environmental proteases are key contributors to primary sensitization to allergens and the exacerbation of allergic diseases via the abrogation of the epidermal/epithelial barrier and the induction of innate responses.
Humans are equipped with several endogenous protease inhibitors (EPIs) of endogenous and exogenous protease activity. EPIs may decrease the risk of allergen sensitization, inhibit IgE antibody production, and prevent the exacerbation of allergic diseases (18–21). Indeed, genetic deficiencies in EPIs, including cystatin A and SPINK5, a cysteine and serine protease inhibitor, respectively, which help regulate epithelial barrier maintenance, have been associated with atopic dermatitis (22, 23). Furthermore, heterogeneous single-nucleotide polymorphisms and low expression of SPINK5 might contribute to the development of CRS (24). Kato and colleagues (25) have reported that the allergenicity of the major house dust mite (HDM) allergens, Der f 1 and Der p 1, is suppressed by the high-affinity binding of cystatin A. Thus, EPIs may play critical roles in the pathogenesis of allergic diseases; however, knowledge about the roles of EPIs in chronic airway inflammation and about CRS pathogenesis is currently limited.
This study aimed to fill this knowledge gap and to elucidate the roles of EPIs in the pathophysiology of ECRS. To this end, we investigated the expression of cystatin A and SPINK5, for which a preliminary study had indicated the strongest down-regulation in patients with ECRS of all EPIs, in nasal epithelial cells of patients with CRS. Then, we examined the in vitro effects of recombinant EPIs on the production of the epithelial-derived cytokines, IL-25, IL-33, and thymic stromal lymphopoietin (TSLP) in cultured airway epithelial cells, and the in vivo effects of recombinant EPIs in the nasal epithelium of mice exposed to multiple airborne allergens (MAA).
Methods
Patients
Sinonasal tissues were obtained from patients with paranasal sinus disease during endoscopic sinus surgery. CRSwNP was diagnosed based on the criteria of the European Academy of Allergology and Clinical Immunology position paper (1). None of the patients included had been treated with systemic or topical corticosteroids for at least 4 weeks before surgery, although some patients had received antihistaminic agents or macrolide antibiotics. The patients were classified into ECRS and NECRS groups. ECRS was histologically defined as having an eosinophil count of greater than or equal to 70 per microscopic field (×400 magnification) when five fields in the subepithelial area of nasal polyps (NPs) were counted (8). The atopic status of patients with CRS was evaluated by skin prick test or Phadia CAP-RAST test. Allergic rhinitis was defined as the presence of typical nasal symptoms and a serum CAP-RAST score for allergens of greater than or equal to 0.70 UA/ml. As control subjects, uncinate tissues were obtained from patients with frontal sinus cysts or maxillary sinus tumors during surgery, and inferior turbinates (ITs) were harvested during septoplasty. None of these patients had CRS or nasal allergy. Eosinophils and neutrophils in tissues were quantified by two of the authors independently. The eosinophils and neutrophils were counted in five fields of hematoxylin and eosin–stained tissues using light microscopy (×400 magnification). All patients gave written informed consent before sample collection, and the study was approved by the institutional review board of Shiga University of Medical Science.
Reagents and Antibodies
The materials are detailed in the online supplement.
Cell Culture
The culture methods for normal human bronchial epithelial (NHBE) cells are detailed in the online supplement.
Intranasal Instillation with MAA in Mice
Six-week-old female BALB/c mice were purchased from Shimizu Laboratory Supplies Co., Ltd. (Kyoto, Japan). All animal experiments in this study were approved by the Institutional Animal Care and Use Committee of the Shiga University of Medical Science. The method for intranasal instillation of the mice is provided in the online supplement.
RNA Isolation and Real-Time Quantitative Reverse-Transcriptase Polymerase Chain Reaction
Total RNA was purified from cultured primary nasal epithelial cells using a PureLink RNA Mini Kit (Invitrogen, Grand Island, NY). Further details are provided in the online supplement.
Mouse Nasal Specimens
After stripping of the facial skins, the mouse heads were severed between the upper and lower jaws, and the noses were removed. Samples were immediately fixed in 10% formalin at 4°C for 2 days and decalcified in 0.12 mol/L ethylenediaminetetraacetic acid solution (pH 6.5) for 10 days at room temperature. The ethylenediaminetetraacetic acid solution was changed daily. The nasal tissues (NT) were fixed in 10% formalin, embedded in paraffin, cut into 10-μm sections, and stained with hematoxylin and eosin or periodic acid–Schiff.
Immunofluorescence Staining
Immunofluorescence staining was performed as described previously (26). Detailed methods are provided in the online supplement.
Statistical Analysis
All data are reported as the mean ± SEM from the indicated number of samples. Two-sided differences between two samples were analyzed using the Mann-Whitney U test. For comparisons of three groups, the Kruskal-Wallis test (nonparametric one-way analysis of variance) was used. P values less than 0.05 were considered significant. Although the data may ideally be presented as medians and interquartile ranges, we presented as means and SEMs for clarity.
Results
Patient Characteristics
The clinical and demographic characteristics of the patients included in this study are shown in Table 1. Patients with ECRS had more eosinophils in their ITs and NPs than the control subjects and patients with NECRS, whereas patients with NECRS had more neutrophils in their ITs and NPs than the control subjects and patients with ECRS.
Table 1.
Patient Characteristics
| IT |
UT | NP |
||||
|---|---|---|---|---|---|---|
| Control | NECRS | ECRS | Control | NECRS | ECRS | |
| Total | 13 (9M/4F) | 14 (9M/5F) | 12 (7M/5F) | 13 (8M/5F) | 20 (13M/7F) | 19 (12M/7F) |
| Average age (range) | 47.9 (32–66) | 51.2 (30–65) | 48.7 (26–69) | 54.1 (40–67) | 51.6 (20–76) | 50.4 (26–69) |
| Asthma | 0 | 0 | 6 | 0 | 0 | 9 |
| Atopy | 0 | 4 | 8 | 0 | 5 | 13 |
| Eos, number/HPF | 1.8 ± 1.4 | 3.5 ± 2.6 | 98.8 ± 20.3* | 1.9 ± 1.5 | 3.0 ± 2.1 | 120.1 ± 34.9 |
| Neu, number/HPF | 1.8 ± 0.5 | 6.5 ± 2.0† | 2.7 ± 1.4 | 1.7 ± 0.6 | 7.5 ± 2.4† | 3.8 ± 1.6 |
Definition of abbreviations: ECRS = eosinophilic chronic rhinosinusitis; Eos = eosinophils; F = female; HPF = high-power field; IT = inferior turbinate; M = male; NECRS = noneosinophilic chronic rhinosinusitis; Neu = neutrophils; NP = nasal polyp; UT = uncinate tissue.
Data for Eos and Neu are mean ± SEM.
P < 0.01 (Kruskal-Wallis test).
P < 0.05 (Kruskal-Wallis test).
Cystatin A and SPINK5 Expression in Nasal Epithelial Cells
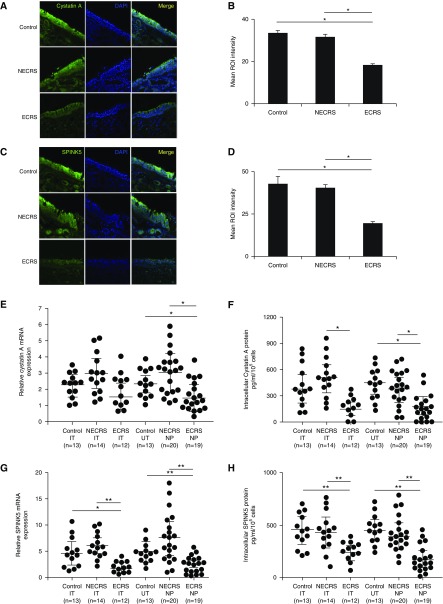
The results of immunohistochemical staining for cystatin A and SPINK5 in NTs are shown in Figure 1. Cystatin A and SPINK5 were mainly expressed in the cytoplasm of nasal epithelial cells in all three patient groups (Figures 1A and 1C), and the expression levels were significantly lower in patients with ECRS than in control subjects or patients with NECRS (Figures 1B and 1D). SPINK5 mRNA was significantly lower in the ITs and NPs of patients with ECRS than in those of control subjects and patients with NECRS, whereas the cystatin A mRNA level was significantly lower in the NPs of patients with ECRS (Figures 1E and 1G). To confirm this observation at the protein level, we measured the concentrations of cystatin A and SPINK5 in primary nasal epithelial cells of the ITs, uncinate tissues, and NPs by ELISA. In agreement with the mRNA data, the cystatin A and SPINK5 protein levels in the ITs and NPs of patients with ECRS were significantly lower, than in those of control subjects and patients with NECRS (Figures 1F and 1H).
Figure 1.
Immunofluorescence staining of nasal polyps (NPs; noneosinophilic chronic rhinosinusitis [NECRS] and eosinophilic chronic rhinosinusitis [ECRS]) and uncinate tissue (UT; control) with antihuman cystatin A and SPINK5 antibodies (green fluorescence). (A and C) Representative staining with cystatin A (A) and SPINK5 (C) antibodies in control (top), NECRS (middle), and ECRS (bottom) patients. Nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI; blue fluorescence). (B and D) Quantitative image analysis of samples stained with cystatin A (B) and SPINK5 (D) antibodies; n = 6. (E and G) The mRNA expression levels of cystatin A (E) and SPINK5 (G) in primary human nasal epithelial cells. Total RNA was extracted from inferior turbinates, UT, and NP, and the expression levels of cystatin A and SPINK5 were analyzed with quantitative reverse-transcriptase polymerase chain reaction. (F and H) Protein concentrations of cystatin A (F) and SPINK5 (H) in cell lysates of primary human nasal epithelial cells. The concentrations of cystatin A and SPINK5 were measured with ELISA. *P < 0.05 and **P < 0.01. IT = inferior turbinates; ROI = region of interest.
Role of EPIs in Allergen- or Protease-induced Secretion of IL-25, IL-33, and TSLP in Cultured Airway Epithelial Cells
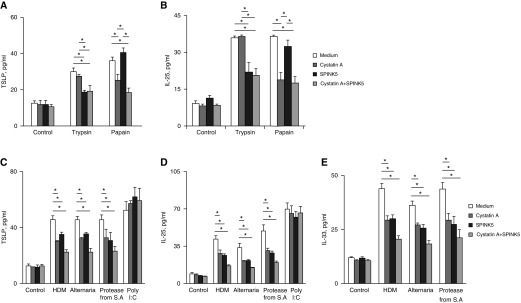
Previous reports have demonstrated that microbial allergens and proteases, such as trypsin and papain, induce the secretion of epithelial-derived cytokines, such as IL-25, IL-33, and TSLP (27–29). To investigate the effect of EPIs on the secretion of these cytokines, we pretreated microbial allergens and proteases with recombinant EPIs before adding them to NHBE cells. When the serine protease trypsin was pretreated with SPINK5, the trypsin-induced secretion of TSLP and IL-25 was significantly inhibited, and pretreatment of the cysteine protease, papain with cystatin A, inhibited the papain-induced secretion of TSLP and IL-25. Treatment with a mixture of cystatin A and SPINK5 showed the same inhibitory effect as that observed for each alone (Figures 2A and 2B). Trypsin or papain did not induce IL-33 release (data not shown). These results suggest that endogenous serine and cysteine protease inhibitors specifically inhibit serine and cysteine protease activities, respectively. When microbial allergens, such as HDM, Alternaria, and protease from Staphylococcus aureus were pretreated with recombinant cystatin A, SPINK5, or a mixture of both, the allergen-induced secretion of TSLP, IL-25, and IL-33 was significantly inhibited; however, poly (I:C)-induced secretion of IL-25 and TSLP was unaffected (Figures 2C–2E).
Figure 2.
Effects of recombinant endogenous protease inhibitors on allergen- or protease-induced secretion of thymic stromal lymphopoietin (TSLP; A and C), IL-25 (B and D), and IL-33 (E) from normal human bronchial epithelial cells. Normal human bronchial epithelial cells were incubated with house dust mite (100 μg/ml), Alternaria (400 μg/ml), protease from Staphylococcus aureus (1 μg/ml), trypsin (100 nM), papain (1 μM), or Poly(I:C) (10 μg/ml) for 2 hours (for IL-33) or 24 hours (for TSLP or IL-25), pretreated with or without recombinant cystatin A (0.5 µg/ml), SPINK5 (1 µg/ml), or a mixture of both for 30 minutes at 37°C. *P < 0.05, n = 5. HDM = house dust mite; S.A. = Staphylococcus aureus.
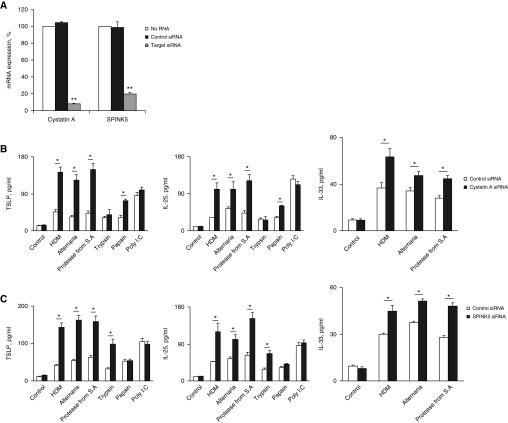
To examine whether diminished EPI expression affects the induction of epithelial-derived cytokines after exposure to microbial allergens, we knocked down cystatin A or SPINK5 in NHBE cells with small interfering RNA (siRNA) and then stimulated the cells with microbial allergens or proteases. Cystatin A and SPINK5 mRNA expression was significantly suppressed by the target siRNA, but not by control siRNA (Figure 3A). Allergen-induced secretion of IL-25, IL-33, and TSLP was increased after knockdown of cystatin A and SPINK5 (Figures 3B and 3C). Trypsin-induced and papain-induced secretion of IL-25 and TSLP was unaffected by knockdown of cystatin A and SPINK5, respectively (Figures 3B and 3C). Poly (I:C)-induced secretion of IL-25 and TSLP was unaffected. These results indicate that protease activities are important in the allergen-induced secretion of IL-25, IL-33, and TSLP, and that EPIs, such as cystatin A and SPINK5, mediate this secretion.
Figure 3.
(A) Normal human bronchial epithelial cells were transfected with small interfering RNA (siRNA) against cystatin A or SPINK5 or with control siRNA for 48 hours. The mRNA expression of the target molecules was examined with quantitative reverse-transcriptase polymerase chain reaction. Data are expressed as a percentage of mock-transfected cells without siRNA, for which expression was set as 100%. **P < 0.01 versus control siRNA; n = 4. (B and C) Transfected normal human bronchial epithelial cells were stimulated with medium alone (control), house dust mite (100 μg/ml), Alternaria (400 μg/ml), protease from Staphylococcus aureus (1 μg/ml), trypsin (100 nM), papain (1 μM), or Poly(I:C) (10 μg/ml) for 2 hours (for IL-33) or 24 hours (for thymic stromal lymphopoietin or IL-25). *P < 0.05 versus no siRNA; n = 5. HDM = house dust mite; S.A. = Staphylococcus aureus; TSLP = thymic stromal lymphopoietin.
Innate Cytokines Increase Rapidly after Allergen Exposure of Naive Mice
We investigated the role of EPIs in the secretion of IL-25, IL-33, and TSLP in the mouse airway via intranasal instillation of MAA, combination of HDM, Alternaria, and protease from S. aureus, as described previously with slight modification (30). After single MAA exposure, NT concentrations of IL-25, IL-33, and TSLP increased to a peak level at 6 hours, and then declined to their baseline levels at 12–24 hours (see Figure E1A in the online supplement). The concentration of IL-25, IL-33, and TSLP in nasal lavage (NL) fluid increased 3, 1, and 6 hours after MAA exposure, respectively, and then declined quickly (see Figure E1B). The increases in IL-25 and TSLP were lower than that in IL-33.
Next, we examined the effect of intranasal instillation of a combination of mouse recombinant cystatin A and SPINK5 on MAA-exposed mice. This combinatorial treatment suppressed MAA-induced production of IL-25, IL-33, and TSLP in NT 6 hours after MAA-exposure (see Figure E1C).
Chronic Exposure to MAA Induces Type-2 Immune Responses
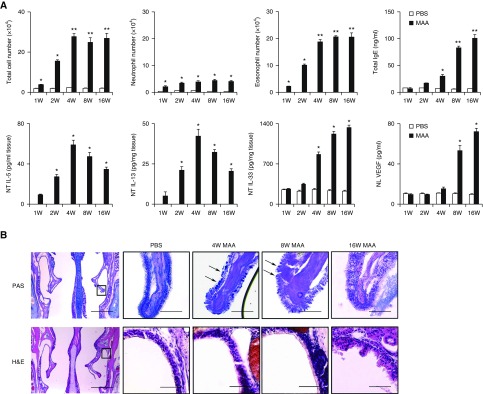
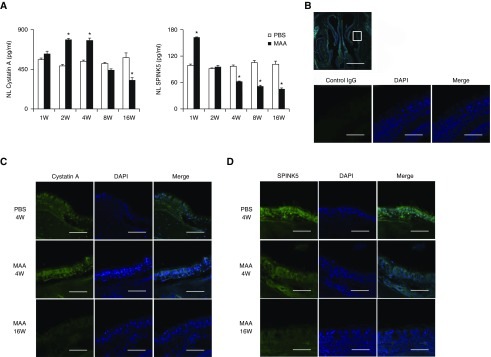
To examine the role of cystatin A and SPINK5 in the pathogenesis of inflammation after chronic MAA exposure, mice were exposed to MAA intranasally once every 2 days for up to 16 weeks, after which cystatin A and SPINK5 expression was assessed. Chronic MAA exposure induced eosinophil and neutrophil in NL fluid; production of IgE in plasma and of vascular endothelial growth factor (VEGF) in NL fluid; and enhanced levels of IL-5, IL-13, and IL-33 in the NT. TSLP was undetectable in the NT, and IL-25 was unaffected by MAA exposure at the time of evaluation (data not shown). Eosinophil infiltration and NT concentrations of IL-5 and IL-13 peaked at 4 weeks, and then declined, whereas plasma total IgE, NL concentration of VEGF, and NT concentration of IL-33 continued to increase at 8–16 weeks (Figure 4A). Morphologically, periodic acid–Schiff–positive epithelial goblet cells in the nasal turbinate tissue increased, peaked at 4 weeks, and then gradually declined. Epithelial disruption and mucosal undulation were found in the lateral wall of the nasal cavity at 16 weeks (Figure 4B). Cystatin A and SPINK5 concentrations in NL fluid increased and peaked at 1 and 4 weeks after MAA exposure, respectively. Interestingly, cystatin A and SPINK5 concentrations continued to decline for up to 16 weeks (Figure 5A). Immunohistochemical staining showed decreased expression of cystatin A and SPINK5 in the nasal epithelial mucosa at 16 weeks (Figures 5C and 5D). These findings demonstrated that decreased expression of cystatin A and SPINK5 occurs after chronic exposure to MAA.
Figure 4.
(A) Eosinophil and neutrophil counts, vascular endothelial growth factor in nasal lavage fluids, concentrations of IL-5, IL-13 in nasal tissues (NT), plasma IgE antibodies, and concentration of IL-33 in NT of mice intranasally exposed to multiple airborne allergens once every 2 days for up to 16 weeks. Results are given as mean ± SEM (n = 5 in each group). *P < 0.05, **P < 0.01. (B) Representative histochemical staining of mouse nasal epithelium with periodic acid–Schiff (PAS) and hematoxylin and eosin. PAS-positive goblet cells and epithelial disruption are found in the nasal epithelium (arrows). Scale bars: 1 mm (B, left) or 100 μm. H&E = hematoxylin and eosin; MAA = multiple airborne allergens; NL = nasal lavage; PBS = phosphate-buffered saline; VEGF = vascular endothelial growth factor.
Figure 5.
(A) Concentrations of cystatin A and SPINK5 in nasal lavage (NL) of mice intranasally exposed to multiple airborne allergens (MAA) once every 2 days for up to 16 weeks. NL fluids were analyzed for cystatin A and SPINK5 concentrations. Results are given as mean ± SEM (n = 5 in each group). *P < 0.05 versus mice exposed to phosphate-buffered saline (PBS). (B–D) Immunofluorescence assay in nasal specimens with normal control IgG (B) or antimouse cystatin A (C) and SPINK5 (D) antibodies (green fluorescence). (C and D) Representative immunostaining for cystatin A (C) and SPINK5 (D) after exposure to PBS for 4 weeks (top), MAA for 4 weeks (middle), and MAA for 16 weeks (bottom). Nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI; blue fluorescence). Scale bars: 1 mm (B, top) or 100 μm.
Role of Endogenous EPIs in the Airways of MAA-exposed Mice
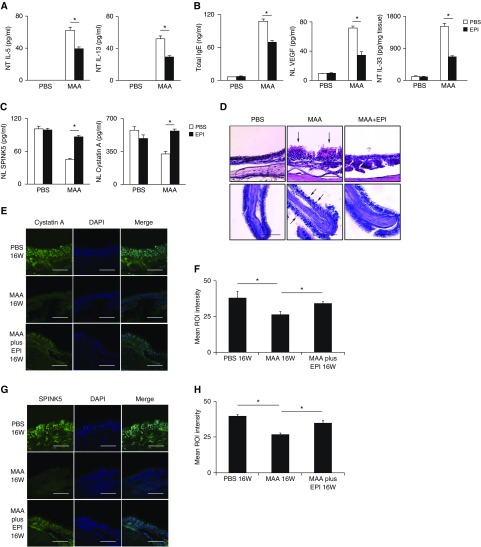
The effects of intranasal instillation of recombinant EPIs in MAA-exposed mice were examined at 4 and 16 weeks. NT concentrations of IL-5 and IL-13 were significantly suppressed at 4 weeks after instillation (Figure 6A). Plasma total IgE, NL VEGF, and NT IL-33 were significantly suppressed at 16 weeks (Figure 6B). Cystatin A and SPINK5 concentrations in NL fluid were not decreased at 16 weeks (Figure 6C). Histologic examination showed that periodic acid–Schiff–positive goblet cell metaplasia at 4 weeks and epithelial disruption and mucosal undulation at 16 weeks were attenuated in recombinant EPI-treated mice (Figure 6D). In mice treated with recombinant EPI, the decrease in expression of cystatin A and SPINK5 in the nasal mucosa at 16 weeks was inhibited as indicated by immunohistochemistry (Figures 6E and 6G). Quantitative examination revealed that the cystatin A and SPINK5 expression levels had also recovered (Figures 6F and 6H).
Figure 6.
(A–C) Effects of intranasal instillation of recombinant endogenous protease inhibitors in multiple airborne allergens (MAA)-exposed mice on nasal lavage (NL) concentrations of IL-5 and IL-13 at 4 weeks (A), plasma total IgE, NL concentration of vascular endothelial growth factor, nasal tissues concentration of IL-33 at 16 weeks (B), and NL concentration of cystatin A and SPINK5 at 16 weeks (C). Results are given as mean ± SEM (n = 5 in each group; *P < 0.05). (D) Representative photomicrographs of hematoxylin and eosin–stained nasal specimens. The periodic acid–Schiff–positive goblet cells and epithelial disruption are found in the nasal epithelium (arrows). (E and G) An immunofluorescence assay in nasal specimens was performed with antimouse cystatin A (E) or SPINK5 (G) antibodies (green fluorescence). Representative immunostaining is shown for cystatin A (E) and SPINK5 (G) after exposure to phosphate-buffered saline (top), MAA (middle), and MAA plus EPI (bottom) for 16 weeks. Nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI; blue fluorescence). (F and H) Quantitative image analysis of samples stained with cystatin A (F) and SPINK5 (H) antibodies; n = 6. Scale bars: 100 μm. EPI = endogenous protease inhibitors; NT = nasal tissues; PBS = phosphate-buffered saline; ROI = region of interest; VEGF = vascular endothelial growth factor.
Discussion
Previous reports have shown that the proteases contained in antigens induce the secretion of epithelial-derived cytokines, IL-25, IL-33, and TSLP, which are involved in the initiation and development of allergic diseases (27–32). All of the allergens used in this study (S. aureus, Alternaria, and HDM) have serine or cysteine protease activity (15, 33–36). Our results showed that endogenous cysteine and serine protease inhibitors, cystatin A, and SPINK5 decreased MAA-induced secretion of IL-25, IL-33, and TSLP in airway epithelial cells in vitro and in vivo (Figures 2A and 2B). In addition, the results of in vivo experiments demonstrated that EPI administration hinders the breakdown of barrier function or Th2-type inflammation caused by the allergen exposure. These results suggest that EPIs play key roles in the response to environmental and exogenous proteases that induce Th2-type inflammation.
The etiology of ECRS is highly debated. Multiple studies have proposed that abnormal immune responses may accompany exposure to microorganisms or their products, including fungi, S. aureus, and bacterial biofilms. This hypothesis has remained a main topic of research. Recently, intranasal or intraperitoneal injection of Aspergillus extract (37), a combination of ovalbumin and Aspergillus protease (38), and a combination of ovalbumin and staphylococcal enterotoxin B (39) have been used to develop mouse models of eosinophilic chronic sinusitis. In the present study, epithelial disruption and mucosal undulation were induced with chronic (16 wk) MAA exposure, and EPIs inhibited these MAA-induced morphologic changes. Chronic MAA exposure inhibited the tissue expression of the EPIs cystatin A and SPINK5, and EPI administration significantly reduced these changes. These results indicate that chronic exposure to the protease activities of allergens induces tissue remodeling and that EPIs protect the epithelium against allergen-derived protease activities. Homer and Elias (40) established that VEGF induces remodeling in asthma by enhancing Th2-mediated antigen sensitization and inflammation in the lungs and by increasing the number of activated dendritic cells. VEGF in NL fluid is elevated only in patients with CRS with nasal polyposis and is implicated in the development of NPs (41, 42).
In this study, VEGF levels in NL fluids were induced on 8 weeks of MAA exposure, and epithelial disruption occurred after 16 weeks, which suggests that VEGF may also be an inducer of remodeling in ECRS. However, although polypoid lesions in the paranasal mucosa were induced in the mouse model of eosinophilic chronic sinusitis, no large NPs occupying the paranasal sinus, which occur in patients with ECRS, were formed. Additional studies of the development of ECRS in mouse models will be needed to investigate the etiology, pathology, and treatment of ECRS. The question remains whether the complication with atopy influences the pathogenesis of ECRS or not. The atopic condition does not seem to differentiate ECRS and NECRS (Table 1). Thus, atopic condition by itself is unlikely sufficient to cause ECRS. Indeed, we found no difference in the expression levels of EPIs between patients with allergic rhinitis and normal subjects (data not shown). Therefore, both development of ECRS and decreased expression of endogenous proteases may likely have little to do with atopic status in patients.
Studies have shown that IL-33 is detected at higher levels in the airways of patients with chronic allergic disease and is correlated with disease severity (43, 44). IL-33 seems to be involved in both the innate and the adaptive phases of type-2 immunity via activity on a variety of cell types including but not limited to group 2 innate lymphoid cells, dendritic cells, CD4+ T cells, mast cells, and eosinophils (45). Iijima and colleagues (30) have reported that among various immunologic factors, IL-33 seems to be particularly important in mediating immunity because it is significantly increased during both the early and the chronic phases of responses to allergen exposure. In addition, the authors observed increased levels of immunoreactive IL-33 in lung epithelial cells and pneumocytes during chronic inflammation. In the present study, IL-33 levels increased rapidly after acute allergen exposure in vitro and in vivo. Intracellular IL-33 in NTs was the most abundant cytokine during the chronic phase of allergen exposure, and remained elevated for at least 8 weeks (Figure 4A). IL-33 is reportedly released spontaneously at picomolar concentrations from airway epithelial cells cultured from patients with severe asthma (44). Therefore, increased total tissue levels of IL-33 likely induce IL-33 protein secretion into the extracellular space.
Expression of the EPI cystatin SN is reportedly elevated in the epithelial cells of the nasal mucosa of patients with cedar pollen–induced seasonal allergic rhinitis (46). Thus, EPIs may be up-regulated periodically to play a protective role during exposure to large amounts of natural allergens. In contrast, decreases in EPI levels in NL fluid or epithelial cells after chronic MAA exposure were not suppressed by EPI, which suggests that protease activity of natural allergens directly down-regulates the EPIs. To counter prolonged exposure to allergen-derived proteases, EPIs (including those in epithelial cells) may be consumed and gradually depleted. Further studies are needed to investigate the factors regulating EPIs. The tight junction proteins of epithelial cells, such as occludin, claudin-1, and ZO-1, are reportedly impaired by allergen-derived proteases (47, 48). We found that when cystatin A or SPINK5 mRNA expression was suppressed by using siRNA, allergen-induced secretion of IL-25, IL-33, and TSLP increased (Figures 3B and 3C). Reduced barrier function might increase the susceptibility to sensitization and lower the threshold of antigen exposure required to drive local allergen-dependent inflammation. These findings imply that prolonged exposure to airborne proteases exacerbates Th2-type inflammation by abrogating the barrier function of epithelial cells and by weakening the antiprotease activity.
Allergic diseases are not isolated disorders but rather syndromes consisting of various phenotypes. All pathologic conditions are determined by interactions between genetic and environmental factors (1, 49). Some patients with atopic dermatitis have mutations in cystatin A- or SPINK5-encoding genes (22, 23). In particular, low expression of SPINK5 causes Netherton syndrome, a serious type of atopic dermatitis (23). With regard to ECRS, although there are no reports on cystatin A, SPINK5 gene polymorphism has been reported in patients with aspirin-intolerant CRS (24). In the present study, we found decreased expression of the cysteine protease inhibitor cystatin A and the serine protease inhibitor SPINK5 in patients with ECRS compared with control subjects. It is unclear whether this impairment is the result of genetics or environmental factors, including the effects of inhaled allergens, but decreases in cystatin A and SPINK5 may increase the risk for allergic sensitization through the epithelium. Thus, the elucidation of the regulatory mechanism of EPIs is critical.
Our findings revealed that airborne allergen–derived proteases have an important role in the pathogenesis of chronic allergic diseases by breaking down the barrier function of epithelial cells, including EPIs. Moreover, EPIs control the function of exogenous proteases, which induce the Th2 immune response. Analysis of the interactions between airborne allergen–derived proteases and EPIs, which are aggressive and protective factors, may improve the efficacy of treatments for allergic diseases.
Footnotes
Supported in part by grants from the Ministry of Education, Culture, Sports, Science and Technology, Japan (Grant-in-Aid for Scientific Research, #26462582).
Author Contributions: H. Kouzaki, study design and research. S.S., study design. H. Kikuoka, K.M., T.K., and I.T., surgeons. H. Kita and T.S., study design and the overall organization.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201603-0529OC on October 25, 2016
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Akdis CA, Bachert C, Cingi C, Dykewicz MS, Hellings PW, Naclerio RM, Schleimer RP, Ledford D. Endotypes and phenotypes of chronic rhinosinusitis: a PRACTALL document of the European Academy of Allergy and Clinical Immunology and the American Academy of Allergy, Asthma & Immunology. J Allergy Clin Immunol. 2013;131:1479–1490. doi: 10.1016/j.jaci.2013.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ediger D, Sin BA, Heper A, Anadolu Y, Misirligil Z. Airway inflammation in nasal polyposis: immunopathological aspects of relation to asthma. Clin Exp Allergy. 2005;35:319–326. doi: 10.1111/j.1365-2222.2005.02194.x. [DOI] [PubMed] [Google Scholar]
- 3.Van Zele T, Claeys S, Gevaert P, Van Maele G, Holtappels G, Van Cauwenberge P, Bachert C. Differentiation of chronic sinus diseases by measurement of inflammatory mediators. Allergy. 2006;61:1280–1289. doi: 10.1111/j.1398-9995.2006.01225.x. [DOI] [PubMed] [Google Scholar]
- 4.Van Crombruggen K, Zhang N, Gevaert P, Tomassen P, Bachert C. Pathogenesis of chronic rhinosinusitis: inflammation. J Allergy Clin Immunol. 2011;128:728–732. doi: 10.1016/j.jaci.2011.07.049. [DOI] [PubMed] [Google Scholar]
- 5.Zhang N, Van Zele T, Perez-Novo C, Van Bruaene N, Holtappels G, DeRuyck N, Van Cauwenberge P, Bachert C. Different types of T-effector cells orchestrate mucosal inflammation in chronic sinus disease. J Allergy Clin Immunol. 2008;122:961–968. doi: 10.1016/j.jaci.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 6.Baba S, Kagoya R, Kondo K, Suzukawa M, Ohta K, Yamasoba T. T-cell phenotypes in chronic rhinosinusitis with nasal polyps in Japanese patients. Allergy Asthma Clin Immunol. 2015;11:33. doi: 10.1186/s13223-015-0100-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim JW, Hong SL, Kim YK, Lee CH, Min YG, Rhee CS. Histological and immunological features of non-eosinophilic nasal polyps. Otolaryngol Head Neck Surg. 2007;137:925–930. doi: 10.1016/j.otohns.2007.07.036. [DOI] [PubMed] [Google Scholar]
- 8.Tokunaga T, Sakashita M, Haruna T, Asaka D, Takeno S, Ikeda H, Nakayama T, Seki N, Ito S, Murata J, et al. Novel scoring system and algorithm for classifying chronic rhinosinusitis: the JESREC Study. Allergy. 2015;70:995–1003. doi: 10.1111/all.12644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amitani R, Wilson R, Rutman A, Read R, Ward C, Burnett D, Stockley RA, Cole PJ. Effects of human neutrophil elastase and Pseudomonas aeruginosa proteinases on human respiratory epithelium. Am J Respir Cell Mol Biol. 1991;4:26–32. doi: 10.1165/ajrcmb/4.1.26. [DOI] [PubMed] [Google Scholar]
- 10.Amin K. The role of mast cells in allergic inflammation. Respir Med. 2012;106:9–14. doi: 10.1016/j.rmed.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 11.Reed CE, Kita H. The role of protease activation of inflammation in allergic respiratory diseases. J Allergy Clin Immunol. 2004;114:997–1008, quiz 1009. doi: 10.1016/j.jaci.2004.07.060. [DOI] [PubMed] [Google Scholar]
- 12.Takai T, Ikeda S. Barrier dysfunction caused by environmental proteases in the pathogenesis of allergic diseases. Allergol Int. 2011;60:25–35. doi: 10.2332/allergolint.10-RAI-0273. [DOI] [PubMed] [Google Scholar]
- 13.Kesic MJ, Hernandez M, Jaspers I. Airway protease/antiprotease imbalance in atopic asthmatics contributes to increased influenza A virus cleavage and replication. Respir Res. 2012;13:82. doi: 10.1186/1465-9921-13-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Costenaro L, Kaczmarska Z, Arnan C, Janowski R, Coutard B, Solà M, Gorbalenya AE, Norder H, Canard B, Coll M. Structural basis for antiviral inhibition of the main protease, 3C, from human enterovirus 93. J Virol. 2011;85:10764–10773. doi: 10.1128/JVI.05062-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Snelgrove RJ, Gregory LG, Peiró T, Akthar S, Campbell GA, Walker SA, Lloyd CM. Alternaria-derived serine protease activity drives IL-33-mediated asthma exacerbations. J Allergy Clin Immunol. 2014;134:583–592.e6. doi: 10.1016/j.jaci.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hara K, Iijima K, Elias MK, Seno S, Tojima I, Kobayashi T, Kephart GM, Kurabayashi M, Kita H. Airway uric acid is a sensor of inhaled protease allergens and initiates type 2 immune responses in respiratory mucosa. J Immunol. 2014;192:4032–4042. doi: 10.4049/jimmunol.1400110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kamijo S, Takeda H, Tokura T, Suzuki M, Inui K, Hara M, Matsuda H, Matsuda A, Oboki K, Ohno T, et al. IL-33-mediated innate response and adaptive immune cells contribute to maximum responses of protease allergen-induced allergic airway inflammation. J Immunol. 2013;190:4489–4499. doi: 10.4049/jimmunol.1201212. [DOI] [PubMed] [Google Scholar]
- 18.Sakata Y, Arima K, Takai T, Sakurai W, Masumoto K, Yuyama N, Suminami Y, Kishi F, Yamashita T, Kato T, et al. The squamous cell carcinoma antigen 2 inhibits the cysteine proteinase activity of a major mite allergen, Der p 1. J Biol Chem. 2004;279:5081–5087. doi: 10.1074/jbc.M311585200. [DOI] [PubMed] [Google Scholar]
- 19.Ogawa T, Takai T, Kato T, Kikuchi Y, Niyonsaba F, Ikeda S, Okumura K, Ogawa H. Upregulation of the release of granulocyte-macrophage colony-stimulating factor from keratinocytes stimulated with cysteine protease activity of recombinant major mite allergens, Der f 1 and Der p 1. Int Arch Allergy Immunol. 2008;146:27–35. doi: 10.1159/000112500. [DOI] [PubMed] [Google Scholar]
- 20.Seto T, Takai T, Ebihara N, Matsuoka H, Wang XL, Ishii A, Ogawa H, Murakami A, Okumura K. SLPI prevents cytokine release in mite protease-exposed conjunctival epithelial cells. Biochem Biophys Res Commun. 2009;379:681–685. doi: 10.1016/j.bbrc.2008.12.060. [DOI] [PubMed] [Google Scholar]
- 21.Deraison C, Bonnart C, Lopez F, Besson C, Robinson R, Jayakumar A, Wagberg F, Brattsand M, Hachem JP, Leonardsson G, et al. LEKTI fragments specifically inhibit KLK5, KLK7, and KLK14 and control desquamation through a pH-dependent interaction. Mol Biol Cell. 2007;18:3607–3619. doi: 10.1091/mbc.E07-02-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vasilopoulos Y, Cork MJ, Teare D, Marinou I, Ward SJ, Duff GW, Tazi-Ahnini R. A nonsynonymous substitution of cystatin A, a cysteine protease inhibitor of house dust mite protease, leads to decreased mRNA stability and shows a significant association with atopic dermatitis. Allergy. 2007;62:514–519. doi: 10.1111/j.1398-9995.2007.01350.x. [DOI] [PubMed] [Google Scholar]
- 23.Briot A, Deraison C, Lacroix M, Bonnart C, Robin A, Besson C, Dubus P, Hovnanian A. Kallikrein 5 induces atopic dermatitis-like lesions through PAR2-mediated thymic stromal lymphopoietin expression in Netherton syndrome. J Exp Med. 2009;206:1135–1147. doi: 10.1084/jem.20082242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fruth K, Goebel G, Koutsimpelas D, Gosepath J, Schmidtmann I, Mann WJ, Brieger J. Low SPINK5 expression in chronic rhinosinusitis. Laryngoscope. 2012;122:1198–1204. doi: 10.1002/lary.23300. [DOI] [PubMed] [Google Scholar]
- 25.Kato T, Takai T, Mitsuishi K, Okumura K, Ogawa H. Cystatin A inhibits IL-8 production by keratinocytes stimulated with Der p 1 and Der f 1: biochemical skin barrier against mite cysteine proteases. J Allergy Clin Immunol. 2005;116:169–176. doi: 10.1016/j.jaci.2005.03.044. [DOI] [PubMed] [Google Scholar]
- 26.Kouzaki H, Matsumoto K, Kato T, Tojima I, Shimizu S, Shimizu T. Epithelial cell-derived cytokines contribute to the pathophysiology of eosinophilic chronic rhinosinusitis. J Interferon Cytokine Res. 2016;36:169–179. doi: 10.1089/jir.2015.0058. [DOI] [PubMed] [Google Scholar]
- 27.Kouzaki H, O’Grady SM, Lawrence CB, Kita H. Proteases induce production of thymic stromal lymphopoietin by airway epithelial cells through protease-activated receptor-2. J Immunol. 2009;183:1427–1434. doi: 10.4049/jimmunol.0900904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kouzaki H, Tojima I, Kita H, Shimizu T. Transcription of interleukin-25 and extracellular release of the protein is regulated by allergen proteases in airway epithelial cells. Am J Respir Cell Mol Biol. 2013;49:741–750. doi: 10.1165/rcmb.2012-0304OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kouzaki H, Iijima K, Kobayashi T, O’Grady SM, Kita H. The danger signal, extracellular ATP, is a sensor for an airborne allergen and triggers IL-33 release and innate Th2-type responses. J Immunol. 2011;186:4375–4387. doi: 10.4049/jimmunol.1003020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iijima K, Kobayashi T, Hara K, Kephart GM, Ziegler SF, McKenzie AN, Kita H. IL-33 and thymic stromal lymphopoietin mediate immune pathology in response to chronic airborne allergen exposure. J Immunol. 2014;193:1549–1559. doi: 10.4049/jimmunol.1302984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hammad H, Lambrecht BN. Dendritic cells and epithelial cells: linking innate and adaptive immunity in asthma. Nat Rev Immunol. 2008;8:193–204. doi: 10.1038/nri2275. [DOI] [PubMed] [Google Scholar]
- 32.Tang H, Cao W, Kasturi SP, Ravindran R, Nakaya HI, Kundu K, Murthy N, Kepler TB, Malissen B, Pulendran B. The T helper type 2 response to cysteine proteases requires dendritic cell-basophil cooperation via ROS-mediated signaling. Nat Immunol. 2010;11:608–617. doi: 10.1038/ni.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hewitt CR, Brown AP, Hart BJ, Pritchard DI. A major house dust mite allergen disrupts the immunoglobulin E network by selectively cleaving CD23: innate protection by antiproteases. J Exp Med. 1995;182:1537–1544. doi: 10.1084/jem.182.5.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thomas WR, Smith WA, Hales BJ, Mills KL, O’Brien RM. Characterization and immunobiology of house dust mite allergens. Int Arch Allergy Immunol. 2002;129:1–18. doi: 10.1159/000065179. [DOI] [PubMed] [Google Scholar]
- 35.Takai T, Kato T, Sakata Y, Yasueda H, Izuhara K, Okumura K, Ogawa H. Recombinant Der p 1 and Der f 1 exhibit cysteine protease activity but no serine protease activity. Biochem Biophys Res Commun. 2005;328:944–952. doi: 10.1016/j.bbrc.2005.01.051. [DOI] [PubMed] [Google Scholar]
- 36.Dubin G. Extracellular proteases of Staphylococcus spp. Biol Chem. 2002;383:1075–1086. doi: 10.1515/BC.2002.116. [DOI] [PubMed] [Google Scholar]
- 37.Lindsay R, Slaughter T, Britton-Webb J, Mog SR, Conran R, Tadros M, Earl N, Fox D, Roberts J, Bolger WE. Development of a murine model of chronic rhinosinusitis. Otolaryngol Head Neck Surg. 2006;134:724–730, discussion 731–732. doi: 10.1016/j.otohns.2005.11.048. [DOI] [PubMed] [Google Scholar]
- 38.Kim JH, Yi JS, Gong CH, Jang YJ. Development of Aspergillus protease with ovalbumin-induced allergic chronic rhinosinusitis model in the mouse. Am J Rhinol Allergy. 2014;28:465–470. doi: 10.2500/ajra.2014.28.4100. [DOI] [PubMed] [Google Scholar]
- 39.Kim DW, Khalmuratova R, Hur DG, Jeon SY, Kim SW, Shin HW, Lee CH, Rhee CS. Staphylococcus aureus enterotoxin B contributes to induction of nasal polypoid lesions in an allergic rhinosinusitis murine model. Am J Rhinol Allergy. 2011;25:e255–e261. doi: 10.2500/ajra.2011.25.3727. [DOI] [PubMed] [Google Scholar]
- 40.Homer RJ, Elias JA. Airway remodeling in asthma: therapeutic implications of mechanisms. Physiology (Bethesda) 2005;20:28–35. doi: 10.1152/physiol.00035.2004. [DOI] [PubMed] [Google Scholar]
- 41.Lee CG, Link H, Baluk P, Homer RJ, Chapoval S, Bhandari V, Kang MJ, Cohn L, Kim YK, McDonald DM, et al. Vascular endothelial growth factor (VEGF) induces remodeling and enhances TH2-mediated sensitization and inflammation in the lung. Nat Med. 2004;10:1095–1103. doi: 10.1038/nm1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee HS, Myers A, Kim J. Vascular endothelial growth factor drives autocrine epithelial cell proliferation and survival in chronic rhinosinusitis with nasal polyposis. Am J Respir Crit Care Med. 2009;180:1056–1067. doi: 10.1164/rccm.200905-0740OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baba S, Kondo K, Kanaya K, Suzukawa K, Ushio M, Urata S, Asakage T, Kakigi A, Suzukawa M, Ohta K, et al. Expression of IL-33 and its receptor ST2 in chronic rhinosinusitis with nasal polyps. Laryngoscope. 2014;124:E115–E122. doi: 10.1002/lary.24462. [DOI] [PubMed] [Google Scholar]
- 44.Préfontaine D, Nadigel J, Chouiali F, Audusseau S, Semlali A, Chakir J, Martin JG, Hamid Q. Increased IL-33 expression by epithelial cells in bronchial asthma. J Allergy Clin Immunol. 2010;125:752–754. doi: 10.1016/j.jaci.2009.12.935. [DOI] [PubMed] [Google Scholar]
- 45.Bartemes KR, Kita H. Dynamic role of epithelium-derived cytokines in asthma. Clin Immunol. 2012;143:222–235. doi: 10.1016/j.clim.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Imoto Y, Tokunaga T, Matsumoto Y, Hamada Y, Ono M, Yamada T, Ito Y, Arinami T, Okano M, Noguchi E, et al. Cystatin SN upregulation in patients with seasonal allergic rhinitis. PLoS One. 2013;8:e67057. doi: 10.1371/journal.pone.0067057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Runswick S, Mitchell T, Davies P, Robinson C, Garrod DR. Pollen proteolytic enzymes degrade tight junctions. Respirology. 2007;12:834–842. doi: 10.1111/j.1440-1843.2007.01175.x. [DOI] [PubMed] [Google Scholar]
- 48.Tai HY, Tam MF, Chou H, Peng HJ, Su SN, Perng DW, Shen HD. Pen ch 13 allergen induces secretion of mediators and degradation of occludin protein of human lung epithelial cells. Allergy. 2006;61:382–388. doi: 10.1111/j.1398-9995.2005.00958.x. [DOI] [PubMed] [Google Scholar]
- 49.Lötvall J, Akdis CA, Bacharier LB, Bjermer L, Casale TB, Custovic A, Lemanske RF, Jr, Wardlaw AJ, Wenzel SE, Greenberger PA. Asthma endotypes: a new approach to classification of disease entities within the asthma syndrome. J Allergy Clin Immunol. 2011;127:355–360. doi: 10.1016/j.jaci.2010.11.037. [DOI] [PubMed] [Google Scholar]