Abstract
Hybrid benign peripheral nerve sheath tumors are rare tumors exhibiting areas of more than one neural neoplasm such as schwannoma/neurofibroma, neurofibroma/perineurioma, or schwannoma/perineurioma. These tumors usually arise in the skin of the extremities and trunk, and less commonly affect extracutaneous sites. Here we report two cases of these tumors exhibiting hybrid features of schwannoma/perineurioma and schwannoma/neurofibroma located in the scalp and the ankle, respectively.
Keywords: Hybrid benign peripheral nerve sheath tumor, Schwannoma/perineurioma, Schwannoma/neurofibroma
Introduction
There are 3 common tumors of benign peripheral nerve sheath tumors (BPNST): perineurioma, schwannoma, and neurofibroma. Examples of hybrid BPNST have been previously described, such as schwannoma/neurofibroma, neurofibroma/perineurioma, schwannoma/perineurioma, or schwannoma/perineurioma/neurofibroma [1, 2, 3, 4]. Tumors with divergent neural differentiation including hybrid schwannoma/neurothekeoma [5] and melanocytic nevus with schwannoma and perineurioma have also been reported [6]. These tumors usually arise in the skin of the extremities and trunk, and less commonly affect extracutaneous sites [1, 7]. Here we report 2 rare cases of these tumors exhibiting hybrid features of schwannoma/perineurioma and schwannoma/neurofibroma located, respectively, in the scalp and the ankle.
Case Reports
Case 1
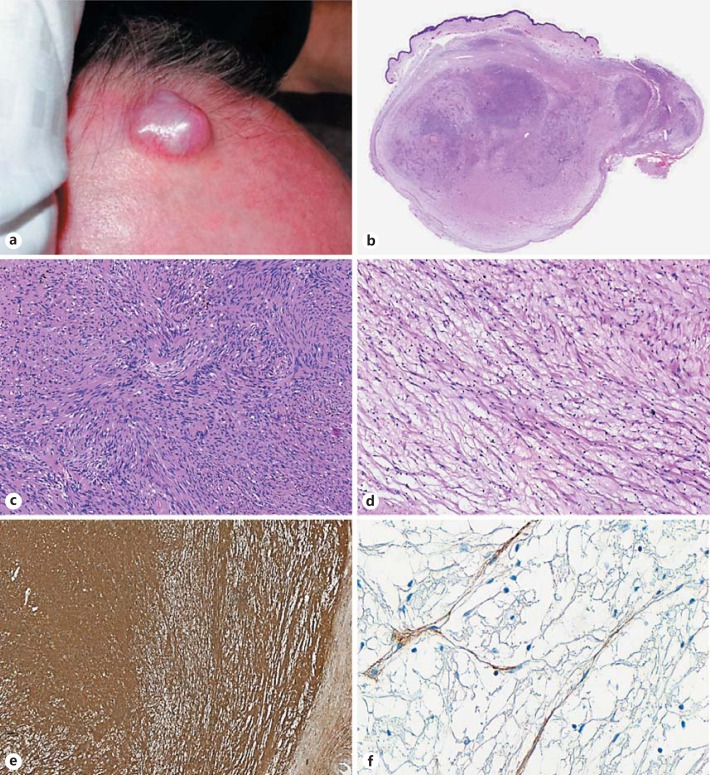
A 72-year-old man presented with a scalp lesion that was thought clinically to be a benign cyst. There was no familial history of neurofibromatosis or a previous radiation treatment. The patient underwent surgical excision. At gross examination, the lesion was a nodular mass measuring 2 cm in its largest diameter (Fig. 1a).
Fig. 1.
Hybrid schwannoma and perineurioma. Nodular lesion of the scalp (a). Whole-mount of the lesion: a multinodular dermal growth with heterogenous areas (b, scanning magnification), including a schwannomatous component with plump-spindled cells arranging in Antoni A and Antoni B areas (c, original magnification ×100), and hypocellular myxoid areas with slender-spindled cells corresponding to the perineurial component (d, original magnification ×100). Diffuse S100 protein expression in the schwannomatous component (e, original magnification ×50). Focal epithelial membrane antigen expression in the perineurial component (f, original magnification ×400).
Microscopic analysis revealed a well-circumscribed but unencapsulated dermal lesion covered by normal-appearing skin (Fig. 1b) and represented by a biphasic proliferation of cellular areas in the center (Fig. 1c) and hypocellular to myxoid areas at the periphery (Fig. 1d). Alternating hyper- and hypocellularity resembling Antoni A and Antoni B areas were observed in the center (Fig. 1c). The spindle cells in this area showed abundant fibrillary cytoplasm with plump nuclei and coarse chromatin. The myxoid areas at the periphery of the lesion exhibited slender spindle cells with delicate nuclei and elongated bipolar cytoplasm (Fig. 1d). There was no evidence of atypia or mitotic figures.
The first component was diffusely S100 positive but negative for epithelial membrane antigen (EMA), corresponding to a schwannoma (Fig. 1e). The second component exhibited the opposite immunophenotype and was consistent with a perineurioma (Fig. 1f).
The perineurial component was present in the margins. Postoperative evolution showed no evidence of recurrence (follow-up period of 36 months).
Case 2
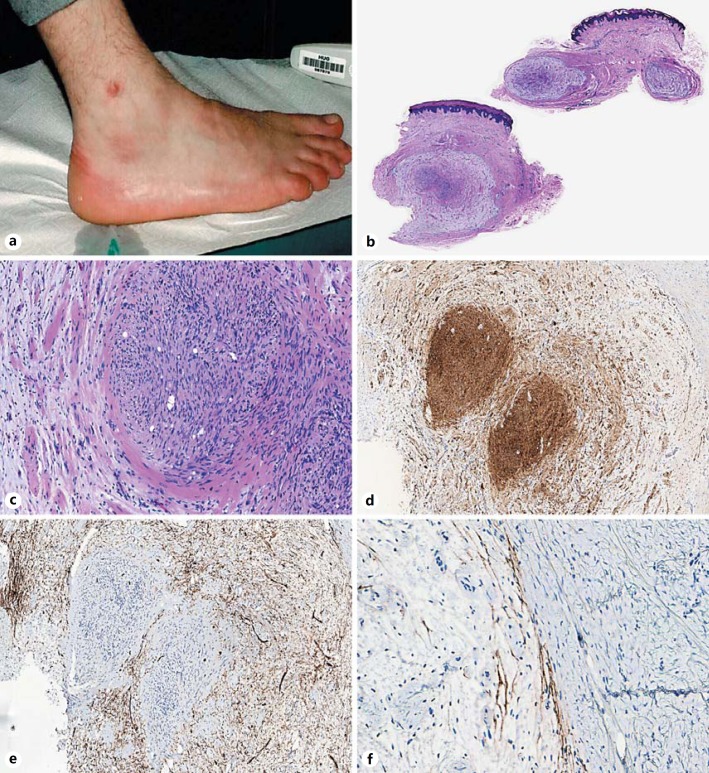
A 24-year-old man presented with a cutaneous lesion on the right ankle (Fig. 2a) diagnosed clinically as a lipoma versus cyst or benign fibrous histiocytoma. There was no familial history or any previous radiation therapy at this site, nor stigmata of neurofibromatosis. The lesion was excised and showed at gross examination a multinodular dermal tumor measuring 0.6 cm in its largest diameter.
Fig. 2.
Hybrid schwannoma and neurofibroma. Nodular lesion of the ankle (a). Scanning magnification showing a multinodular dermal lesion (b), with a schwannomatous component in the center of one nodule (right) surrounded by a neurofibromatous component (left) (c, original magnification ×100). Diffuse S100 protein expression in the schwannomatous component, with only partial expression in the neurofibroma (d, original magnification ×50). CD34 highlighting the neurofibromatous component but negative in the schwannomatous nodule (e, original magnification ×50). Epithelial membrane antigen highlighting the capsule at the periphery of the lesion (f, original magnification ×200).
Histological examination showed a multilobular well-circumscribed plexiform dermal lesion with a biphasic proliferation (Fig. 2b) mostly represented by lobules of myxoid hypocellular areas. A second distinct cellular nodular component was found in the center of one lobule (Fig. 2c). The two components were clearly separated with an abrupt transition (Fig. 2c). The peripheral areas were consistent with a neurofibroma composed of spindle cells with heterogenous cytology arranged in a myxoid stroma, intermingled with some collagen bundles and some mast cells (Fig. 2c). By immunohistochemistry, these cells expressed CD34 (Fig. 2e) and partially S100 protein (Fig. 2d). A slender capsule positive for EMA could be identified at the periphery of the lobules (Fig. 2f). The central nodular area was consistent with a schwannoma represented by a plump spindle cell population with a palisading disposition (Verocay bodies) and expressed diffusely S100 protein by immunohistochemistry (Fig. 2d), but was negative for EMA and CD34 (Fig. 2e).
The neurofibromatous component was present in the margins; however, postoperative evolution showed no evidence of recurrence (follow-up period of 8 months).
Discussion
Common benign nerve sheath tumors include neurofibromas, schwannomas, and perineuriomas. Schwannomas and perineuriomas are composed of a uniform population of a single cell type (Schwann cells and perineurial cells, respectively), whereas neurofibromas are composed of different cell types, including Schwann cells, fibroblasts, perineurial cells, and scattered axons [1, 7].
Benign nerve sheath tumors showing hybrid features of more than one tumor type have been reported in the recent last years. A biphasic neural proliferation showing an abrupt transition between the two components has been noted in most hybrid BPNST [1, 2].
Hornick et al. [1] discribed 42 cases of benign nerve sheath tumors showing hybrid features of perineurioma and schwannoma as in our first example. These tumors appeared to be architecturally typical of soft-tissue perineurioma, being well circumscribed but unencapsulated and showing storiform to whorled and lamellar growth patterns. The cytologic features, however, showed a dominant schwannian component, with spindled plump cells having tapering nuclei, and palely eosinophilic cytoplasm. The perineurial component was inconspicuous in most cases. These perineurial cells were recognized by their slender elongated bipolar cytoplasmic processes. The dual cell lineages were evident by immunohistochemistry, the predominant larger spindled cells being positive for S100 protein, whereas the less conspicuous slender cells being positive for EMA and usually also for claudin-1. Degenerative nuclear atypia, not observed in our case, was reported in 25% of the cases and seemed to be of no clinical consequence [1].
Hybrid BPNST with neurofibromatous and schwannomatous differentiation (hybrid neurofibroma/schwannoma) as in our second case has also been described. Feany et al. [8] reported the largest series of hybrid neurofibroma/schwannoma, including a total of 9 tumors arising in 9 patients. These tumors were reported to arise sometimes in the setting of a neurofibromatosis 1 (NF1) or schwannomatosis, but are not confined to this type of patients, as they were also reported in patients with no stigmata for these syndromes [8, 9]. The cases reported by Feany et al. [8] were described to arise in dermal, subcutaneous, or subfascial areas. They often showed a predominance of neurofibromatous loose myxoid areas in plexiform arrangement, delimited at the periphery by a perineurium and including in their center a well-defined nodule of a schwannomatous differentiation. Interestingly, plexiform neurofibromas are considered pathognomonic for NF1; however, in our case and in 5 out of the 9 patients with hybrid plexiform neurofibroma including schwannomatous nodules reported by Feany et al. [8], the clinical examination failed to demonstrate stigmata of neurofibromatosis. In this report, one patient with nonplexiform hybrid tumors was diagnosed with NF1 [8]. Mitoses were rarely found in the schwannomatous component but not in the neurofibromatous one. Recurrence was reported in only 1 out of the 9 cases reported by Feany et al. [8], and it occurred 44 years after the initial excision.
The main differential diagnosis for hybrid BPNST includes other BPNST and low-grade malignant peripheral nerve sheath tumors (MPNST). The composite nature of these lesions could be easily overlooked if only limited immunohistochemistry is performed (for example only S100 protein or EMA). As nuclear atypia could be seen in the hybrid tumors, a low-grade MPNST might be considered. However, MPNST show variation in cellularity and a mitotic activity that is generally either very low or absent in hybrid BPNST. The atypia seen in hybrid tumors are of a degenerative nature similar to that seen in other benign nerve sheath tumors (i.e., smudged chromatin, nuclear pseudoinclusions) [1].
Most hybrid BPNST seem to pursue a benign clinical course with only rare recurrence [1, 8, 10] and malignant transformation [11]. Rare cases have been reported to occur after radiation therapy [12]. In our cases no recurrence was noted, although clinical follow-up is limited.
In summary, hybrid BPNST exhibit cell differentiation profiles of more than one neural neoplasm. Here we report two cases of hybrid cutaneous schwannoma/perineurioma and schwanomma/neurofibroma arising, respectively, in the scalp and ankle of two adult men. Postoperative evolution showed no evidence of recurrence. However, neural tumors with hybrid features may exceptionally recur or undergo malignant transformation.
The pathogenetic basis of the biphasic differentiation in hybrid schwannoma/perineurioma and schwannoma/neurofibroma is uncertain. Awareness of its morphological characteristics and potential occurrence in diverse sites may help the recognition of these rare tumors.
Statement of Ethics
The patients gave their informed consent for this publication.
Disclosure Statement
The authors have no conflicts of interest to disclose.
References
- 1.Hornick JL, Bundock EA, Fletcher CD. Hybrid schwannoma/perineurioma: clinicopathologic analysis of 42 distinctive benign nerve sheath tumors. Am J Surg Pathol. 2009;33:1554–1561. doi: 10.1097/PAS.0b013e3181accc6c. [DOI] [PubMed] [Google Scholar]
- 2.Kacerovska D, Michal M, Kazakov DV. Hybrid epitheloid schwannoma/perineurioma. Am J Dermatopathol. 2016;38:e90–e92. doi: 10.1097/DAD.0000000000000532. [DOI] [PubMed] [Google Scholar]
- 3.Kacerovska D, Michal M, Kuroda N, Tanaka A, Sima R, Denisjuk N, Kreuzberg B, Ricarova R, Dmitry V. Hybrid peripheral nerve sheath tumors, including a malignant variant in type 1 neurofibromatosis. Am J Dermatopathol. 2013;35:641–649. doi: 10.1097/DAD.0b013e31827e2917. [DOI] [PubMed] [Google Scholar]
- 4.Shelekhova KV, Danilova AB, Michal M, Kazakov DV. Hybrid neurofibroma-perineurioma: an additional example of an extradigital tumor. Ann Diagn Pathol. 2008;12:233–234. doi: 10.1016/j.anndiagpath.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 5.Requena L, Sitthinamsuwan P, Fried I, Kaddu S, Schirren CG, Schärer L, Hantschke M, Cerroni L, McCalmont TH, Kutzner H. A benign cutaneous plexiform hybrid tumor of perineurioma and cellular neurothekeoma. Am J Surg Pathol. 2013;37:845–852. doi: 10.1097/PAS.0b013e31827edfda. [DOI] [PubMed] [Google Scholar]
- 6.Wang L, Wang G, Gao T. Congenital melanocytic nevus with features of hybrid schwannoma/perineurioma. J Cutan Pathol. 2013;40:497–502. doi: 10.1111/cup.12076. [DOI] [PubMed] [Google Scholar]
- 7.Weiss SW, Goldblum JR, editors. 5th ed. St. Louis: Mosby; 2008. Benign tumors of peripheral nerves; in Enzinger and Weiss's Soft Tissue Tumors; pp. 825–901. [Google Scholar]
- 8.Feany MB, Anthony DC, Fletcher CD. Nerve sheath tumours with hybrid features of neurofibroma and schwannoma: a conceptual challenge. Histopathology. 1998;32:405–410. doi: 10.1046/j.1365-2559.1998.00419.x. [DOI] [PubMed] [Google Scholar]
- 9.Lang S-S, Zager EL, Coyne TM, Nangunoori R, Kneeland JB, Nathanson KL. Hybrid peripheral nerve sheath tumor. J Neurosurg. 2012;117:897–901. doi: 10.3171/2012.8.JNS111841. [DOI] [PubMed] [Google Scholar]
- 10.Hayashi T, Hirose T, Nishimura Y, Fukuoka J, Kishikawa M. Hybrid schwannoma/perineurioma of the spinal nerve: multifocal occurrence, and recurrence as an intraneural perineurioma. Pathol Int. 2013;63:368–373. doi: 10.1111/pin.12073. [DOI] [PubMed] [Google Scholar]
- 11.Evans DG, Baser ME, McGaughran J, Scharif S, Howard E, Moran A. Malignant peripheral nerve sheath tumors in neurofibromatosis 1. J Med Genet. 2002;39:311–314. doi: 10.1136/jmg.39.5.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ohata C, Imai N, Hinogami H, Akamatsu K, Sumimura Y. Hybrid schwannoma/perineurioma: a report of two cases including a possible radiation-induced case. J Cutan Pathol. 2012;39:56–62. doi: 10.1111/j.1600-0560.2011.01805.x. [DOI] [PubMed] [Google Scholar]