Abstract
The antiproliferative treatment options for neuroendocrine tumors (NET)/neuroendocrine carcinomas of the gastrointestinal tract critically depend on the proliferation rate, evaluated by immunohistochemical staining for Ki-67. According to their grading, tumors are treated with somatostatin analogs, mTOR inhibitors, or cytotoxic substances. This case illustrates downgrading of a primarily highly proliferative NET achieved by a variation of cytotoxic chemotherapy regimens, followed by a combination therapy using everolimus together with lanreotide. The latter medication might lead to a good clinical response as far as tumor growth is concerned.
Keywords: Neuroendocrine tumor, Chemotherapy, Liver biopsy, mTor, Somatostatin
Introduction
In recent years, the increasing number of randomized controlled trials in the field of neuroendocrine tumors (NETs) resulted in more treatment options of this disease entity, which is only rarely encountered. Gastrointestinal NETs are usually treated according to their grade. Therapeutic regimens include somatostatin analogs (G1 and G2 with Ki-67 <10%) and inhibitors of the mammalian target of rapamycin (mTOR) (G1 and G2 with Ki-67 <20%). According to international guidelines (ENETS), G3 tumors (Ki-67 >20%) should be treated with cytotoxic drugs [1, 2, 3, 4, 5, 5]. However, the G3 fraction represents a heterogeneous group of neoplasms, as patients with well-differentiated tumors and lower Ki-67 (20–55%) show a poorer response to platinum-based chemotherapy than patients with Ki-67 >55%. Therefore, neuroendocrine neoplasms currently assigned to the WHO G3 category have been suggested to be subdivided into two subtypes: G3 NET (Ki-67 20–55%) and G3 neuroendocrine carcinoma (NEC) (Ki-67 >55%) [6, 7, 8, 9, 10].
Case Report
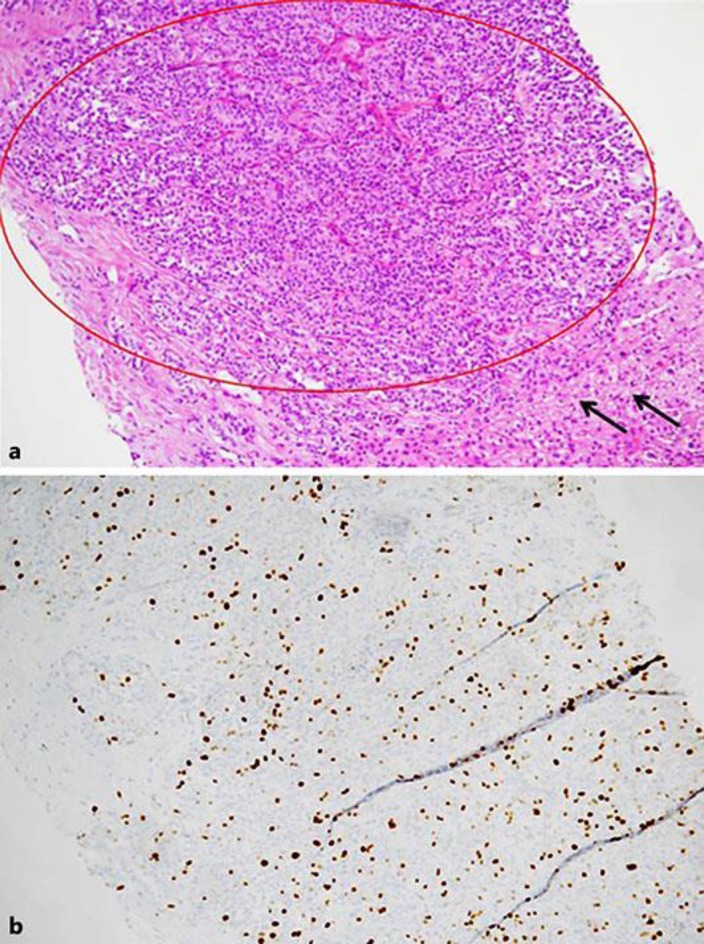
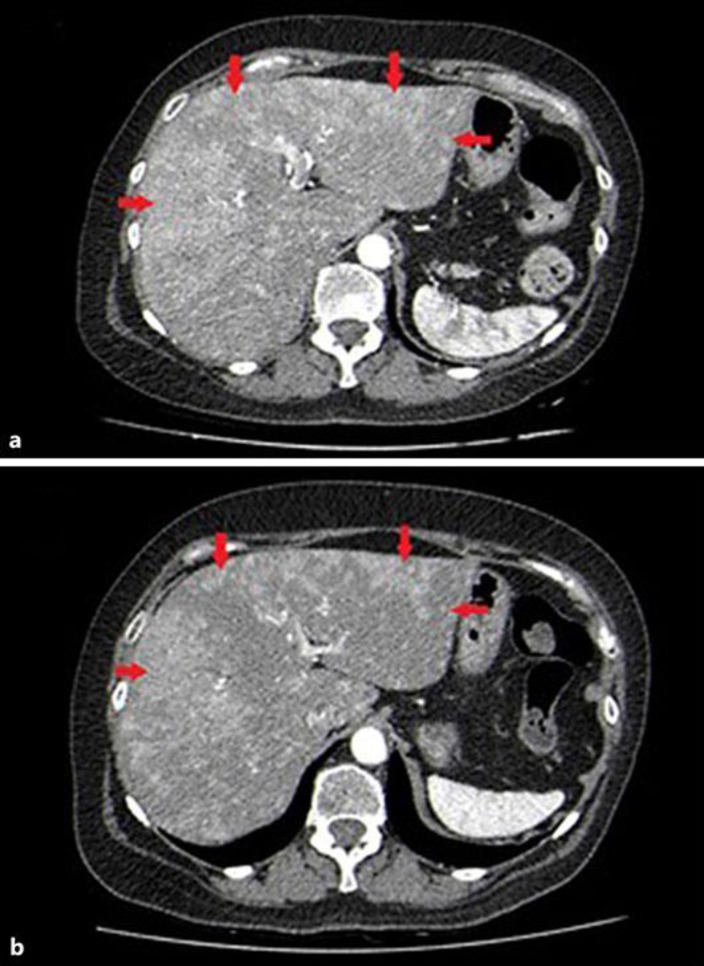
A 61-year-old female patient was diagnosed with a histologically proven NET of the small bowel with metastases to the local lymph nodes (pT1, N1 [2/3], G3, Ki-67 25%). Apart from hypertension, a mild chronic kidney insufficiency, and a successfully treated ductal carcinoma of the left breast 10 years ago, her medical record was inconspicuous. The primary tumor and local lymph node stations were resected in a curative attempt, followed by a first-line adjuvant chemotherapy with 4 cycles of cisplatin in combination with etoposide. Two years later, the patient was found to be free of recurrence. However, a CT scan revealed tumor progression manifesting as retroperitoneal lymphadenopathy. Subsequently, further chemotherapy with topotecan was initiated, but liver metastases developed despite ongoing therapy. Application of trofosfamide as well as a combination of temozolomide and capecitabine failed to stabilize the disease. To find further treatment options, the patient underwent restaging, including a 68Ga-DOTA-NOC PET-CT scan, which revealed strong expression of somatostatin receptors. For further clarification, the patient's liver metastases were investigated by liver biopsy, revealing a proliferation index (Ki-67) of 15% (Fig. 1). This surprising result was interpreted as downgrading from a G3 to a G2 NET. Consequently, a guideline-consented therapy for G2 NETs with everolimus in combination with lanreotide was started according to the receptor expression. After a 3-month treatment period, the CT scan indicated a partial response (Fig. 2), and her chromogranin A levels in the blood sample normalized. CT scans performed 12 months after the first application of everolimus and lanreotide showed that the disease had stabilized.
Fig. 1.
a Liver biopsy with formation of a solid-trabecular growing tumor (red circle), non-neoplastic liver parenchyma (black arrows). b A Ki-67 proliferation fraction of approximately 15% (10×).
Fig. 2.
a October 2015. Contrast-enhanced axial CT scan. The arterial phase image reveals multiple liver metastases with confluent, hypervascular lesions in both liver lobes (red arrows). b January 2016. Follow-up CT scan 3 months later after initiation of everolimus and lanreotide shows gradual reduction in number and size of the liver lesions.
Discussion
G3 NETs of the small bowel are a rare finding with an estimated survival time of 30 months only [5, 11]. Only little is known about treatment options for these tumors, and many recommendations are based on findings achieved by investigating high proliferative NECs of the pancreas.
The G3 NET subgroup with a Ki-67 index of 20–55% commonly develops early lymph node and liver metastasis and often insufficiently responds to chemotherapy. Various response rates ranging from 17 to 67% in first-line chemotherapy with cisplatin and etoposide have been reported in all G3 NETs and NECs [5]. Locoregional therapies, such as chemoembolization (TACE), radiofrequency ablation (RFA), laser-induced thermotherapy or selective hepatic transcatheter arterial embolization (TAE), selective internal radiotherapy (SIRT), as well as palliative liver surgery, can be used for treating liver metastases, but may have serious side effects. No other medical treatment than cytotoxic chemotherapy is recommended for G3 tumors of the neuroendocrine system. It remains uncertain whether somatostatin analogs and mTOR inhibitors are able to induce response or disease stabilization in the initial G3 tumor. This raises the question if even G3 NETs with a Ki-67 over 20% and less than 55% will benefit from the use of mTOR inhibitors and somatostatin analogs. In a small cohort of 27 heterogeneous patients with poorly differentiated NET, a combination of chemotherapy with slow-release lanreotide showed response rates of up to 37% [3, 12]. Gilabert et al. [13] recently published promising results on the use of mTOR inhibitors in combination with somatostatin analogs in a small cohort of patients with NECs of the pancreas (median Ki-67 45%), who refused cytotoxic chemotherapy. Another recent study, including 15 patients with pancreatic G3 NET (average Ki-67 30%), reported disease stabilization in 40% of patients over 12 months [14]. This case raises the question about the possibility of down-staging a NET/NEC with cytotoxic chemotherapy to open up alternative treatment strategies. The biopsy of a liver metastasis revealed a better differentiated G2 tumor and enabled a combination therapy with lanreotide and everolimus, which led at least to a partial response not achieved by guideline-conformed cytotoxic chemotherapy. We suggest that the change in the proliferation index from G3 to G2 NET was due to the chemotherapies mentioned above. The accuracy of Ki-67 quantification techniques, such as digital image analysis and manual counting, was demonstrated by Tang et al. [15]. The risk of an initial misinterpretation of the Ki-67 status in this case was minimized by consultation of two independent pathologists using manual counting. Heterogeneity in the proliferation index among primary tumor and metastasis, as well as metastasis among each other, is well known. However, tumor biology aggravates with increasing duration of disease, and metastasis is supposed to be less differentiated than the primary tumor. Two independent studies showed that the Ki-67 index was higher in metastases than in the primary tumor [16, 17]. In our case, the metastases were better differentiated than the primary tumor, which underlines the impact of downgrading as a future treatment strategy for this challenging G3 NET subtype.
To the best of our knowledge, this is the first case in the literature to describe the downgrading of a G3 NET of the small bowel to a G2 NET.
Conclusion
Based on this case, we conclude that G3 NETs with insufficient treatment response to cytotoxic chemotherapy could be considered for restaging as downgrading of the tumor may occur, which potentially opens the chance for additional treatment options.
Statement of Ethics
The authors have no ethical conflicts to disclose.
Disclosure Statement
This publication is sponsored by Ipsen Pharma.
References
- 1.Yoo C, Cho H, Song MJ, Hong SM, Kim KP, Chang HM, Chae H, Kim TW, Hong YS, Ryu MH, Kang YK, Kim SC, Ryoo BY. Efficacy and safety of everolimus and sunitinib in patients with gastroenteropancreatic neuroendocrine tumor. Cancer Chemother Pharmacol. 2017;79:139–146. doi: 10.1007/s00280-016-3215-3. [DOI] [PubMed] [Google Scholar]
- 2.Kos-Kudła B. Treatment of neuroendocrine tumors: new recommendations based on the CLARINET study. Contemp Oncol (Pozn) 2015;19:345–349. doi: 10.5114/wo.2015.56006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oeberg K, Knigge U, Kwekkeboom D, Perren A. Neuroendocrine gastroenteropancreatic tumours: ESMO Clinical Practice Guidelines. Ann Oncol. 2012 Oct;23(Suppl 7):vii124–30. doi: 10.1093/annonc/mds295. [DOI] [PubMed] [Google Scholar]
- 4.Pavel ME, Hainsworth JD, Baudin E, Peeters M, Hörsch D, Winkler RE, Klimovsky J, Lebwohl D, Jehl V, Wolin EM, Oberg K, Van Cutsem E, Yao JC, RADIANT-2 Study Group Everolimus plus octreotide long-acting repeatable for the treatment of advanced neuroendocrine tumors associated with carcinoid syndrome (RADIANT-2): a randomised, placebo-controlled, phase 3 study. Lancet. 2011;378:2005–2012. doi: 10.1016/S0140-6736(11)61742-X. [DOI] [PubMed] [Google Scholar]
- 5.Ilett E, Langer S, Olsen I, Federspiel B, Kjær A, Knigge U. Neuroendocrine Carcinomas of the Gastroenteropancreatic System: A Comprehensive Review. Diagnostics (Basel) 2015;5:119–176. doi: 10.3390/diagnostics5020119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sorbye H, Welin S, Langer SW, Vestermark LW, Holt N, Osterlund P, Dueland S, Hofsli E, Guren MG, Ohrling K, Birkemeyer E, Thiis-Evensen E, Biagini M, Gronbaek H, Soveri LM, Olsen IH, Federspiel B, Assmus J, Janson ET, Knigge U. Predictive and prognostic factors for treatment and survival in 305 patients with advanced gastrointestinal neuroendocrine carcinoma (WHO G3): the NORDIC NEC study. Ann Oncol. 2013;24:152–160. doi: 10.1093/annonc/mds276. [DOI] [PubMed] [Google Scholar]
- 7.Vélayoudom-Céphise FL, Duvillard P, Foucan L, Hadoux J, Chougnet CN, Leboulleux S, Malka D, Guigay J, Goere D, Debaere T, Caramella C, Schlumberger M, Planchard D, Elias D, Ducreux M, Scoazec JY, Baudin E. Are G3 ENETS neuroendocrine neoplasms heterogeneous? Endocr Relat Cancer. 2013;20:649–657. doi: 10.1530/ERC-13-0027. [DOI] [PubMed] [Google Scholar]
- 8.Basturk O, Tang L, Hruban RH, Adsay V, Yang Z, Krasinskas AM, Vakiani E, La Rosa S, Jang KT, Frankel WL, Liu X, Zhang L, Giordano TJ, Bellizzi AM, Chen JH, Shi C, Allen P, Reidy DL, Wolfgang CL, Saka B, Rezaee N, Deshpande V, Klimstra DS. Poorly differentiated neuroendocrine carcinomas of the pancreas: a clinicopathologic analysis of 44 cases. Am J Surg Pathol. 2014;38:437–447. doi: 10.1097/PAS.0000000000000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fazio N, Milione M. Heterogeneity of grade 3 gastroenteropancreatic neuroendocrine carcinomas: new insights and treatment implications. Cancer Treat Rev. 2016;50:61–67. doi: 10.1016/j.ctrv.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 10.Tang LH, Basturk O, Sue JJ, Klimstra DS. A Practical Approach to the Classification of WHO Grade 3 (G3) Well-differentiated Neuroendocrine Tumor (WD-NET) and Poorly Differentiated Neuroendocrine Carcinoma (PD-NEC) of the Pancreas. Am J Surg Pathol. 2016;40:1192–1202. doi: 10.1097/PAS.0000000000000662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamaguchi T, Machida N, Morizane C, Kasuga A, Takahashi H, Sudo K, Nishina T, Tobimatsu K, Ishido K, Furuse J, et al. Multicenter retrospective analysis of systemic chemotherapy for advanced neuroendocrine carcinoma of the digestive system. Cancer Sci. 2014;105:1176–1181. doi: 10.1111/cas.12473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Correale P, Sciandivasci A, Intrivici C, Pascucci A, del Vecchio MT, Marsili S, Savelli V, Voltolini L, di Bisceglie M, Guarnieri A, et al. Chemo-hormone therapy of non-well-differentiated endocrine tumours from different anatomic sites with cisplatinum, etoposide and slow release lanreotide formulation. Br J Cancer. 2007;96:1343–1347. doi: 10.1038/sj.bjc.6603734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gilabert M, Rho YS, Kavan P. Targeted Therapies Provide Treatment Options for Poorly Differentiated Pancreatic Neuroendocrine Carcinomas. Oncology. 2017;92:170–172. doi: 10.1159/000452279. [DOI] [PubMed] [Google Scholar]
- 14.Panzuto F, Rinzivillo M, Spada F, Antonuzzo L, Ibrahim T, Campana D, Fazio N, Delle Fave G. Everolimus in Pancreatic Neuroendocrine Carcinomas G3. Pancreas. 2017;46:302–305. doi: 10.1097/MPA.0000000000000762. [DOI] [PubMed] [Google Scholar]
- 15.Tang LH, Gonen M, Hedvat C, Modlin IM, Klimstra DS. Objective quantification of the Ki67 proliferative index in neuroendocrine tumors of the gastroenteropancreatic system: a comparison of digital image analysis with manual methods. Am J Surg Pathol. 2012;36:1761–1770. doi: 10.1097/PAS.0b013e318263207c. [DOI] [PubMed] [Google Scholar]
- 16.Hentic O, Couvelard A, Rebours V, Zappa M, Dokmak S, Hammel P, Maire F, O’Toole D, Levy P, Sauvanet A, et al. Ki-67 index, tumor differentiation, and extent of liver involvement are independent prognostic factors in patients with liver metastases of digestive endocrine carcinomas. Endocr Relat Cancer. 2011;18:51–59. doi: 10.1677/ERC-09-0319. [DOI] [PubMed] [Google Scholar]
- 17.Delektorskaya VV, Kozlov NA, Chemeris GY. Clinico-morphological analysis of the neuroendocrine neoplasms of the gastroenteropancreatic system. Klin Lab Diagn. 2013;48–50:10–13. [PubMed] [Google Scholar]