Abstract
CYP24A1 is an enzyme that inactivates vitamin D. Loss-of-function mutations in this enzyme are rare but have been linked with idiopathic infantile hypercalcemia as well as adult-onset nephrocalcinosis and nephrolithiasis. Genetic testing for this mutation should be considered in the presence of calciuria, elevated serum calcium, elevated 1,25-dihydroxyvitamin D, and suppressed parathyroid hormone. We present a case with these lab findings as well as an elevated 25-hydroxyvitamin D/24,25-dihydroxyvitamin D ratio in whom compound heterozygous CYP24A1 mutations were found. His hypercalciuria resolved and 1,25-vitamin D level improved with ketoconazole treatment. We suggest that it is clinically important to identify patients with this phenotype as testing and treatment options are available which could reduce progression to chronic kidney disease in this population.
Keywords: CYP24A1; 1,25-Dihydroxyvitamin D; Nephrolithiasis; Hypercalciuria
Background
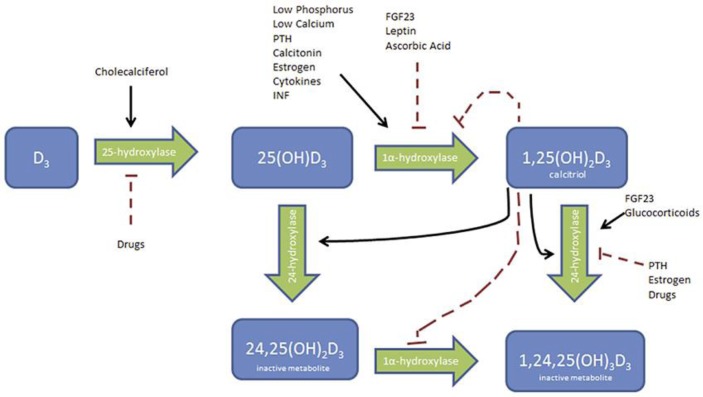
CYP24A1 is an enzyme that encodes part of the vitamin D 24-hydroxylase enzyme which results in the formation of an inactivated form of vitamin D, 24,25-dihydroxyvitamin D (Fig. 1). Homozygous or compound heterozygous loss-of-function mutations in this enzyme can cause an array of clinical presentations caused by increased 1,25-dihydroxyvitamin D, including idiopathic infantile hypercalcemia as well as nephrocalcinosis and nephrolithiasis in children and adults, which can lead to renal insufficiency [1]. This type of mutation, although rare, should be considered in the presence of calciuria, elevated serum calcium (may only be the upper limit of normal), elevated 1,25-dihydroxyvitamin D, and suppressed parathyroid hormone.
Fig. 1.
Vitamin D metabolism. PTH, parathyroid hormone; INF, interferon; OH, hydroxyl; FGF, fibroblast growth factor.
Case Report
We present the case of an otherwise healthy 53-year-old Caucasian male with recurrent calcium phosphate apatite kidney stones. He started having stones at age 24 years and during that period had 12 lithotripsy treatments and 1 percutaneous nephrolithotomy. He also had abdominal imaging that demonstrated numerous stones but no nephrocalcinosis. He was found to have significant hypercalciuria at 524 mg/day, elevated 1,25-dihydroxyvitamin D to 88 pg/mL, hypercalcemia to 2.84 mmol/L in the setting of hydrochlorothiazide use, and unremarkable angiotensin-converting enzyme activity level, parathyroid hormone, parathyroid hormone-related peptide, serum protein electrophoresis, and normal chest radiograph done to evaluate for granulomatous disease (Table 1). His 25-hydroxyvitamin D/24,25-dihydroxyvitamin D ratio was 132. This constellation of findings prompted genetic testing which was notable for 2 CYP24A1 mutations. He was started on therapy with ketoconazole utilizing its effect as a cytochrome P450 inhibitor (including 25-hydroxylases and 1α-hydroxylase) to decrease production of 1,25-dihydroxyvitamin D (Fig. 1). He showed response with a decrease in 1,25-dihydroxyvitamin D levels (40 pg/mL from 88 pg/mL) and resolution of hypercalciuria (124 mg/day) (Table 1) within 2 months of therapy. He is getting quarterly evaluation of his liver function tests given the known toxicity with ketoconazole. He did have increasing fatigue and was noted to have a decreased testosterone level and is currently being started in testosterone injections.
Table 1.
Laboratory data
| Before treatment (normal value) | On thiazide | On ketoconazole | |
|---|---|---|---|
| 1,25-OH vitamin D | 88 pg/mL (19.9–79.3) | ||
| 25-OH vitamin D | 97 ng/mL (20–50) | 47.9 ng/ml | |
| 25-OH vitamin D2 | <4 ng/mL | ||
| 25-OH vitamin D3 | 97 ng/mL | ||
| Serum calcium | 2.59 mmol/L (2.12–2.62) | 2.84 mmol/L | 2.15 mmol/L |
| Ionized calcium | 1.39 mmol/L (1.19–1.3) | ||
| 24-h urine calcium | 524 mg/day (40–320) | 131 mg/day | 142 mg/day |
| PTH | <3 pg/mL (8–68) | ||
| PTHrP | <2 pmol/L | ||
| Creatinine | 118 µmol/L | 132 µmol/L | 111 µmol/L |
| Angiotensin-converting enzyme activity | 34 nmol/mL/min | ||
| Serum protein electrophoresis | normal |
OH, hydroxy; PTH, parathyroid hormone; PTHrP, parathyroid hormone-related peptide.
Discussion
In this patient, significant hydrochlorothiazide-induced hypercalcemia was an indication of an inability to normally compensate for increased calcium resorption. Also, his 25-hydroxyvitamin D/24,25-dihydroxyvitamin D ratio of 132 (normal <25) indicated that defective 24-hydroxylase enzyme function could be contributing to his pathology [2]. These findings prompted genetic testing to evaluate for CYP24A1 mutation with analysis showing a compound heterozygous mutation leading to a nonfunctional 24-hydroxylase enzyme.
We suggest that it is clinically important to identify patients with this phenotype as testing and treatment options are available which could reduce progression to chronic kidney disease in this population. The population frequency of CYP24A1 mutations is not known and testing is not yet widely available, but utilization of the 25-dihydroxyvitamin D/24,25-dihydroxyvitamin D ratio (after ruling out other causes of hypercalcemia) has been shown to be an effective tool to identify patients who are good candidates for genetic testing [3, 4, 5].
Ketoconazole was an effective treatment in this patient and has been used to treat hypercalcemia related to sarcoidosis and hypercortisolism in Cushing disease, but as it impacts multiple steroidogenic pathways, further study is warranted to evaluate long-term safety in this population [6, 7]. It has been used for up to 2 years in patients with hypercalcemia and sarcoidosis with periodic liver function testing, but, as with this patient, they also suffered from decreased testosterone levels [8]. In addition to treatment targeted at 1,25-dihydroxyvitamin D, patients should have bone mineral density testing and receive education regarding limiting the intake of dietary calcium and judicious use of sunscreen [7].
Statement of Ethics
We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us. We confirm that we have given due consideration to the protection of intellectual property associated with this work and that there are no impediments to publication, including the timing of publication, with respect to intellectual property. In so doing we confirm that we have followed the regulations of our institutions concerning intellectual property.
Disclosure Statement
The authors have no financial or other conflict of interest to disclose.
Acknowledgements
The authors thank the physicians Dr. Asplin from Litholink and Dr. Singh from Mayo Clinic.
References
- 1.Jones G, Prosser D, Kauffman M. 25-Hydroxyvitamin D-24-hydroxylase (CYP24A1): its important role in the degradation of vitamin D. Arch Biochem Biophys. 2012;523:9–18. doi: 10.1016/j.abb.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 2.Ketha H, Kumar R, Singh R. LC-MS/MS for identifying patients with CYP24A1 mutations. Clin Chem. 2016:236–242. doi: 10.1373/clinchem.2015.244459. [DOI] [PubMed] [Google Scholar]
- 3.Cools M, Goemaere S, Baetens D, Raes A, Desloovere A, Kauffman JM, De Schepper J, Jans I, Vanderschueren D, Billen J, De Baere E, Fiers T, Bouillon R. Calcium and bone homeostasis in heterozygous carriers of CYP24A1 mutations: a cross sectional study. Bone. 2015;81:89–96. doi: 10.1016/j.bone.2015.06.018. [DOI] [PubMed] [Google Scholar]
- 4.Pasquali M, Tartaglione L, Rotondi S, Muci ML, Mandanici G, Farcomeni A, Marangella M, Mazzaferro S. Calcitriol/calcifediol ratio: an indicator of vitamin D hydroxylation efficiency? BBA Clin. 2015;3:251–256. doi: 10.1016/j.bbacli.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nesterova G, Malicdan MC, Yasuda K, Sakaki T, Vilboux T, Ciccone C, Horst R, Huang Y, Golas G, Introne W, Huizing M, Adams D, Boerkoel CF, Collins MT, Gahl WA. 1,25-(OH)2D-24 hydroxylase (CYP24A1) deficiency as a cause of nephrolithiasis. Clin J Am Soc Nephrol. 2013;8:649–657. doi: 10.2215/CJN.05360512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tebben PJ, Miller DS, Horst RL, Harris PC, Singh RJ, Wu Y, Foreman JW, Chelminski PR, Kumar R. Hypercalcemia, hypercalciuria, and elevated calcitriol concentrations with autosomal dominant transmission due to CYP24A1 mutations: effects of ketoconazole therapy. J Clin Endocrinol Metab. 2012;97:E423–E427. doi: 10.1210/jc.2011-1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jacobs TP, Kaufman M, Jones G, Kumar R, Schlingmann KP, Shapses S, Bilezikian JP. A lifetime of hypercalcemia and hypercalciuria, finally explained. J Clin Endocrinol Metab. 2014;99:708–712. doi: 10.1210/jc.2013-3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bia MJ, Insogna K. Treatment of sarcoidosis-associated hypercalcemia with ketoconazole. Am J Kidney Dis. 1991;18:702–705. doi: 10.1016/s0272-6386(12)80613-5. [DOI] [PubMed] [Google Scholar]