Abstract
Hepatitis C virus (HCV) infection is frequently associated with various extrahepatic manifestations, such as autoimmune features and immune complex deposit diseases. Oral lichen planus (OLP) is one such extrahepatic manifestation of HCV infection. Recently, direct-acting antivirals (DAA) have proved to be highly effective and safe for the eradication of HCV. Herein, we report a case of OLP accompanied by HCV-related hepatocellular carcinoma (HCC) that disappeared after liver transplantation and achievement of sustained virological response following interferon (IFN)-free treatment with ledipasvir (LDV) and sofosbuvir (SOF). The 50-year-old patient developed erosive OLP during IFN therapy, with hyperthyroidism at 53 years of age and HCC at 55 years. He received immunosuppressive drugs and IFN-free DAA treatment after liver transplantation at 60 years of age, which led to disappearance of the symptoms of OLP. The patient was treated safely and effectively with LDV/SOF, although it is not known whether the disappearance of OLP resulted from the eradication of HCV or the immunosuppressive therapy.
Keywords: Oral lichen planus, Extrahepatic manifestations, Hepatitis C virus, Interferon, Direct-acting antivirals, Liver transplantation
Introduction
Hepatitis C virus (HCV) infection is a major health problem with 185 million people chronically infected worldwide [1]. In Japan, about 1.5 million people are chronically infected with HCV [2]. HCV and hepatitis B virus infection are the leading causes of cirrhosis, hepatocellular carcinoma (HCC), and liver transplantation worldwide. Until recently, interferon (IFN) treatment was the standard therapy for the eradication of HCV [3, 4]. Lately, IFN-free direct-acting antivirals (DAA) with high sustained virological response (SVR) rates and few side effects have changed the treatment of hepatitis C markedly [5, 6, 7].
HCV causes not only liver disease but also disorders of other organs and tissues [8]. Several extrahepatic manifestations have been reported. Lichen planus (LP) is one such extrahepatic manifestation of HCV infection [9, 10]. LP is a chronic inflammatory disease that can affect the skin and any lining mucosa. Oral lichen planus (OLP) mostly affects middle-aged and older females. Epidemiologic studies have shown that OLP develops in approximately 10–20% of Japanese individuals with HCV infection in northern Kyushu [11, 12, 13] and Hiroshima prefecture [14, 15]. While the mechanism of HCV-related LP is not known, we identified SNPs for LP in a genome-wide association study (GWAS) of Japanese patients with HCV infection [16].
We have previously reported the successful use of IFN-free DAAs in patients with HCV-associated OLP [17, 18]. Herein, we report a case of OLP accompanied by HCV-related HCC that disappeared after liver transplantation and achievement of SVR, following IFN-free DAA.
Case Presentation
In June 2008, a 53-year-old Japanese male presented to the Kurume University Hospital (Fukuoka, Japan) with a burning pain in the lower lip when eating and drinking (Fig. 1a). The oral lesion was diagnosed pathologically as OLP.
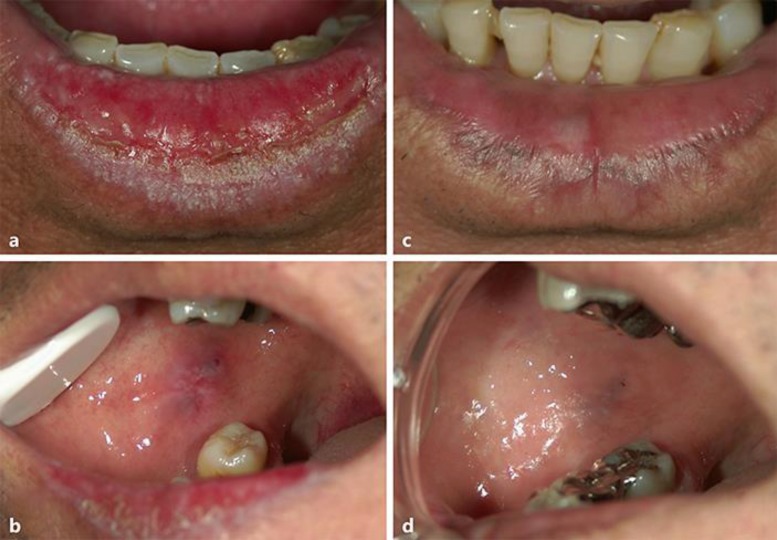
Fig. 1.
a Erosive oral lichen planus (OLP) lesions affecting the lower lip of the 53-year-old male at his first visit to Kurume University Hospital (June 2008). b Bilateral buccal mucosa OLP onset in the 55-year-old male (July 2010). c Disappearance of OLP showing postinflammatory melanin pigmentation in the lower lip (December 2016). d Disappearance of OLP showing postinflammatory melanin pigmentation in the buccal mucosa (December 2016).
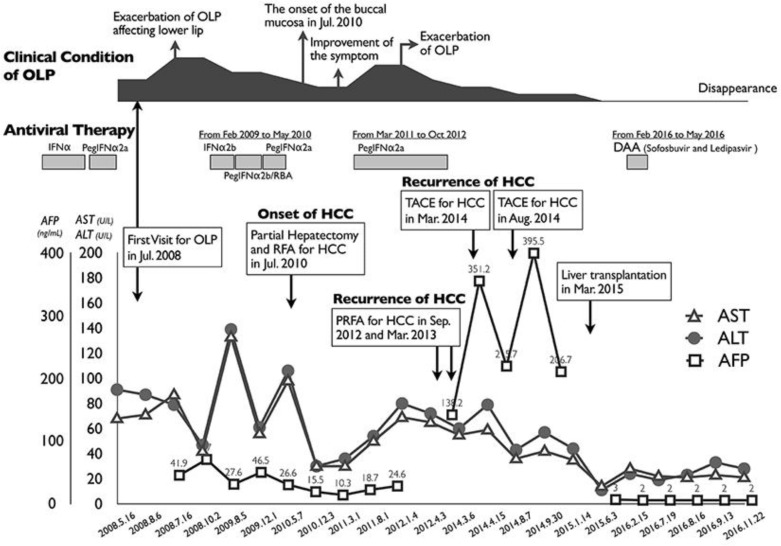
Concerning his history according to the medical record of Nakasonekazu Medical Clinic (Okinawa, Japan), which is a family doctors’ practice, he was diagnosed with chronic hepatitis C at the age of 42 years (in 1996) and received IFNα2b at a dose of 6 million units/day 3 times a week for 24 weeks. However, he did not achieve SVR. In April 2002, the 47-year-old patient was treated with IFNα2b and ribavirin (RBV) at a dose of 800 mg/day. In April 2005, the 50-year-old patient was treated with pegylated interferon (Peg-IFN)α2b, 120 μg/week, and RBV at a dose of 1,000 mg/day. In July 2005, he developed a refractory lesion (considered to be OLP) of the lower lip as a side effect of IFN therapy. The schedule of his treatment for chronic hepatitis C was as follows: self-administration of IFN alfacon-1 at 51 years of age, Peg-IFNα2b and RBV at 51 years, and Peg-IFNα2a at 53 years (Fig. 2). In June 2006, he developed hyperthyroidism as a side effect of IFN therapy. The lesion of the lower lip worsened each time he received IFN therapy. Therefore, the IFN treatment was stopped because of pain and bleeding from the exacerbation of refractory cheilitis and, consequently, he did not achieve SVR.
Fig. 2.
Clinical course of the patient. The black illustration on the top shows the severity of the symptoms of oral lichen planus (OLP). RFA, radiofrequency ablation; PRFA, percutaneous radiofrequency ablation; TACE, transcatheter arterial chemoembolization.
There was no history of blood transfusion or tattooing and his family history was not contributory. The laboratory data at his first visit for examination of oral membrane disease in 2008 were AST 71 U/L, ALT 87 U/L, HCV RNA concentration 6.8 logIU/mL, genotype 1b, IL28B (also known as IFNL3) nonTT, wild-type aa 70 and non-wild-type aa 91 in the HCV core region, number of ISDR mutations zero, and negative results for HBsAg (Table 1). We performed a GWAS to identify genetic variants associated with HCV-related OLP [16]. In our previous report, we identified novel associations of rs884000 in neuropilin-2 (NRP2), rs538399 on insulin-like growth binding proteins factor 4 (IGFBP4), and supported the association of the HLA-DR/DQ genes, with HCV-positive LP in the Japanese and Italian population. This patient had no risk allele (TT) at rs884000 in NRP2 and no resistance allele (TT) at rs538399 in IGFBP4. The patient underwent ultrasonographic examination and computed tomography. Alternative potential predictors of progression of liver cirrhosis were applied. The patient was diagnosed with liver cirrhosis. The erosive OLP lesion was not widely aggravated by the application of steroids (Dexaltin Oral Ointment®; Nippon Kayaku Co. Ltd., Tokyo, Japan) and glycyrrhizin (Stronger Neo-Minophagen C®; Minophagen Pharmaceutical Co. Ltd., Tokyo, Japan).
Table 1.
Summary of the clinical condition of oral lesion and laboratory data
| Normal range | First visit to examine oral lesions | Follow-up of oral lesions | Admission for recurrent HCC | Before liver transplantation | Liver transplantation | After liver transplantation | Before DAA treatment | Start of DAA treatment | SVR12 | SVR24 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Date | Jul. 17, 2008 | Jul. 10, 2010 | Aug. 7, 2014 | Jan. 14, 2015 | Mar. 10, 2015 | Jun. 3, 2015 | Feb. 15, 2016 | Feb. 29, 2016 | Aug. 16, 2016 | Nov. 7, 2016 | |
| Age, years | 53 | 55 | 59 | 60 | 60 | 60 | 61 | 61 | 61 | 61 | |
| Clinical condition of OLP | Presence | Exacerbation | Presence | Presence | Presence | Improvement | Improvement | Disappearance | Disappearance | ||
| Sites of OLP | Lower lip | Lower lip and buccal mucosa | Lower lip and buccal mucosa | Lower lip and buccal mucosa | Lower lip and buccal mucosa | Lower lip and buccal mucosa | Lower lip and buccal mucosa | OLP disappearance | OLP disappearance | ||
| Laboratory data | |||||||||||
| AST, U/L | 13–33 | 71 | 50 | 36 | 35 | 14 | 28 | 21 | 21 | ||
| ALT, U/L | 6–30 | 87 | 58 | 43 | 44 | 11 | 24 | 23 | 28 | ||
| T. protein, g/dL | 6.70–8.30 | 6.5 | 7 | 6.99 | 6.5 | 6 | 6.8 | 6.6 | 6.6 | ||
| Albumin, g/dL | 4.00–5.00 | 3.22 | ND | 3.23 | 3.3 | 3.5 | 4.4 | 4 | 4.1 | ||
| Gamma-GTP, U/I | 10–47 | 457 | 351 | 181 | 57 | 109 | 199 | 187 | 209 | ||
| ALP, U/L | 115–359 | 1,086 | 973 | 1,532 | 793 | 463 | 469 | 388 | 367 | ||
| T. bilirubin, mg/dL | 0.30–1.20 | 0.92 | ND | 0.88 | 1.20 | 0.5 | 0.7 | 0.8 | 1 | ||
| D. bilirubin, mg/dL | ≤0.60 | 0.27 | ND | 0.21 | 0.30 | 0.1 | 0.2 | 0.2 | 0.2 | ||
| T. cholesterol, mg/dL | 128–219 | 110 | 124 | 214 | 150 | ND | ND | ND | ND | ||
| Hb, g/dL | 11.0–15.0 | 15.4 | ND | 14.7 | 14.9 | 11.4 | 15.6 | 16.1 | 15.1 | ||
| PLT, ×10−4/µL | 13.0–36.0 | 9.9 | ND | 5.3 | 6.0 | 12.1 | 9.4 | 13.7 | 11.4 | ||
| AFP, ng/mL | ≤8.7 | 41.9 | 29.6 | 215.7 | 206.7 | ND | 3.0 | 2 | 2 | ||
| FT4, ng/dL | 0.88–1.56 | ND | ND | ND | ND | ND | 1.44 | 0.64 | 1.01 | ||
| TSH, µIU/L | 0.210–3.850 | ND | ND | ND | ND | ND | 3.4 | 30.36 | 10 | ||
| FBS, mg/dL | 80–109 | 128 | 102 | 82 | 107 | ND | ND | ND | ND | ||
| HbA1c, % | 4.9–6.0 | 5 | ND | 4.9 | ND | ND | 5.8 | 5.9 | 5.9 | ||
| HCV viral load, logIU/mL | 6.8 | 6.9 | ND | ND | ND | 7.24 | Negative | Negative | |||
T., total; D., direct; ND, not determined.
In July 2010, the 55-year-old patient developed OLP at the bilateral buccal mucosas other than the lower lip (Fig. 1b) and received surgical hepatic resection and percutaneous radiofrequency ablation for the treatment of HCC. The schedule of his treatment for recurrent HCC was as follows: percutaneous radiofrequency ablation in September 2012, radiofrequency ablation in March 2013, transcatheter arterial chemoembolization in March 2014, and liver transplantation from a living donor in March 2015. The symptom of OLP did not become aggravated. Treatment with immunosuppressive agents, tacrolimus (Prograf®), mycophenolate mofetil (CellCept®), and prednisolone, and antiviral agents, entecavir (Baraclude®) as prophylaxis therapy against hepatitis B virus reactivation [19], was started. Table 1 and Figure 2 show the results of the clinical examinations.
He received DAA treatment involving a 12-week course of ledipasvir (LDV) 90 mg and sofosbuvir (SOF) 400 mg (Harvoni®; Gilead Sciences Inc., Foster City, CA, USA) from February 29, 2016, until May 23, 2016, at the Ryukyu University Hospital (Okinawa, Japan) and SVR was achieved. The OLP lesion was not aggravated during the DAA therapy. After DAA therapy (SVR24), the OLP of the lower lip and buccal mucosal had disappeared (Fig. 1c, d). Clinical examination after 8 years and 5 months (December 3, 2016) revealed the presence of an asymptomatic pigmented lesion on the lower lip that was not present at the first evaluation.
Discussion
Several studies have confirmed that HCV infection is an important correlate in patients with OLP, especially in Japan and Italy. Recently, we identified SNPs for LP in a GWAS of Japanese patients with HCV infection [16]. The GWAS has become a powerful tool for investigating the genetic basis of various diseases. Our GWAS supports the association of previously reported HLA-DR/DQ genes (rs9461799) and newly suggests that the NRP2 and IGFBP4 loci are associated with HCV-related LP susceptibility. We found 2 SNPs (rs884000 in the NRP2 locus and rs538399 in the IGFBP4 locus). The odds ratios of minor alleles (95% confidence interval) of rs884000, rs538399, and rs9461799 were 3.25 (1.95–5.41), 0.40 (0.25–0.63), and 2.15 (1.41–3.28), respectively. This case did not have the risk allele at rs884000 in NRP2 or no resistance allele at rs538399 in IGFBP4.
Erosive OLP is painful and interferes with the normal daily activities of the patient, including eating, drinking, and speaking. The symptoms of HCV-associated OLP are often exacerbated following IFN therapy [20, 21, 22]. Therefore, until now, it has been important to predict the onset and exacerbation of OLP and determine an appropriate period of therapeutic intervention. This case was not able to complete treatment because of exacerbation of OLP, regardless of many types and doses of IFN therapy.
The glycyrrhizin used for this patient was useful as anti-inflammatory treatment of OLP, as we reported previously [23, 24]. However, the use of glycyrrhizin is not a radical treatment of OLP, because OLP relapses when administration of glycyrrhizin is stopped.
The recently developed series of DAAs has greatly improved the treatment outcome of patients with HCV infection. A Japanese phase 3 trial included 341 treatment-naïve and treatment-experienced patients who were randomized to receive LDV/SOF for 12 weeks (group I, 171 patients) or LDV/SOF plus RBV for 12 weeks (group II, 170 patients) [7]. SVR12 was achieved by 171 (100%) patients (83/83 treatment-naïve and 88/88 treatment-experienced patients) who received LDV/SOF and 167/170 (98%) patients (80/83 treatment-naïve and 87/87 treatment-experienced patients) who received LDV/SOF plus RBV. Antiviral treatment resulting in viral eradication is the only way to improve liver transplant patient and graft survival. The use of second-generation DAAs in transplant patients was reported to achieve better efficacy [25].
There are a few reports about IFN-free DAA treatment of HCV-associated extrahepatic manifestations, including OLP, mixed cryoglobulinemia, and rheumatic diseases [17, 18, 26, 27]. We have previously reported the outcomes of HCV-associated OLP in patients who received successful treatment with IFN-free DAAs, using the daclatasvir plus asunaprevir combination therapy [17, 18]. The symptoms of OLP subsided in all patients. Yoshikawa et al. [28] reported successful SOF and RBV treatment for genotype 2 HCV infection with compensated cirrhosis and OLP.
This is a case report of HCV-related OLP after liver transplantation, with successful IFN-free treatment with LDV/SOF. A follow-up visit in December 2016, 8 years and 5 months after the patient's first visit, disclosed the presence of a dark brown-pigmented alteration of his oral mucosa. The pathogenesis of oral postinflammatory pigmentation has rarely been investigated. Mergoni et al. [29] reported a clinicopathological evaluation of oral postinflammatory pigmentation, with a discussion of its possible etiopathogenesis and diagnosis. Melanocytes are cells that produce melanin, a dark brown pigment responsible for the color of the skin, and also participate in the inflammatory response. Melanocytes have been found to be the source of and responsive to a variety of inflammatory mediators [30].
The effects of immunosuppressive agents, such as tacrolimus and mycophenolate mofetil, on the severity and progression of OLP have been reported [31, 32]. Tacrolimus inhibits the activation and proliferation of T-lymphocytes by inhibiting the phosphatase activity of calcineurin and mycophenolate mofetil inhibits lymphocyte proliferation and activation. Immunosuppressive therapy following liver transplantation for HCC may have led to the disappearance of the OLP lesion in our patient, as in previous reports [33, 34]. We reported a case of OLP accompanied by primary biliary cirrhosis that disappeared after liver transplantation and immunosuppressive drug therapy [34].
In conclusion, the patient with HCV-associated OLP in this case study was treated using IFN-free DAAs, which led to disappearance of the symptoms of OLP. This is the first report of LDV/SOF administered for genotype 1 HCV-associated OLP. Long-term follow-up is needed to elucidate the therapeutic effects of transplantation.
Statement of Ethics
The study protocol was approved by the Ethics Committee of Saga University (reference number: 27-2, 27-10, 27-26, and 27–85) (27–36 and 28–47) and the Ethics Committee of Kurume Medical School (reference number: 36 and 36-2) in accordance with the Declaration of Helsinki. Written informed consent for participation in the study was obtained from the patient.
Disclosure Statement
Y.N., K.K., and Y.K. belong to a department funded by Nishinihon Hospital. Y.T. has received research grants from Bristol-Myers Squibb Company, MSD K.K., Chugai Pharmaceutical Co., Ltd., Janssen Pharmaceutical K.K., Gilead Sciences, and AbbVie Inc. The remaining authors disclose no conflicts of interest.
Acknowledgements
This study was supported by Grant-in-Aid for Scientific Research (C) (No.25463274 and 17K12012) from the Ministry of Education, Culture, Sports, Science and Technology of Japan and a grant-in-aid from the Japan Agency for Medical Research and Development, AMED (H25-kanen-ippan-005 and H28-kanen-16668373).
References
- 1.Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology. 2013;57:1333–1342. doi: 10.1002/hep.26141. [DOI] [PubMed] [Google Scholar]
- 2.Lavanchy D. The global burden of hepatitis C. Liver Int. 2009;29((suppl 1)):74–81. doi: 10.1111/j.1478-3231.2008.01934.x. [DOI] [PubMed] [Google Scholar]
- 3.Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, Goodman ZD, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958–965. doi: 10.1016/s0140-6736(01)06102-5. [DOI] [PubMed] [Google Scholar]
- 4.Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Goncales FL, Jr, Haussinger D, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–982. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 5.Kumada H, Suzuki Y, Ikeda K, Toyota J, Karino Y, Chayama K, Kawakami Y, et al. Daclatasvir plus asunaprevir for chronic HCV genotype 1b infection. Hepatology. 2014;59:2083–2091. doi: 10.1002/hep.27113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Omata M, Nishiguchi S, Ueno Y, Mochizuki H, Izumi N, Ikeda F, Toyoda H, et al. Sofosbuvir plus ribavirin in Japanese patients with chronic genotype 2 HCV infection: an open-label, phase 3 trial. J Viral Hepat. 2014;21:762–768. doi: 10.1111/jvh.12312. [DOI] [PubMed] [Google Scholar]
- 7.Mizokami M, Yokosuka O, Takehara T, Sakamoto N, Korenaga M, Mochizuki H, Nakane K, et al. Ledipasvir and sofosbuvir fixed-dose combination with and without ribavirin for 12 weeks in treatment-naive and previously treated Japanese patients with genotype 1 hepatitis C: an open-label, randomised, phase 3 trial. Lancet Infect Dis. 2015;15:645–653. doi: 10.1016/S1473-3099(15)70099-X. [DOI] [PubMed] [Google Scholar]
- 8.Gumber SC, Chopra S. Hepatitis C: a multifaceted disease. Review of extrahepatic manifestations. Ann Intern Med. 1995;123:615–620. doi: 10.7326/0003-4819-123-8-199510150-00008. [DOI] [PubMed] [Google Scholar]
- 9.Nagao Y, Sata M, Tanikawa K, Itoh K, Kameyama T. Lichen planus and hepatitis C virus in the northern Kyushu region of Japan. Eur J Clin Invest. 1995;25:910–914. doi: 10.1111/j.1365-2362.1995.tb01966.x. [DOI] [PubMed] [Google Scholar]
- 10.Lodi G, Pellicano R, Carrozzo M. Hepatitis C virus infection and lichen planus: a systematic review with meta-analysis. Oral Dis. 2010;16:601–612. doi: 10.1111/j.1601-0825.2010.01670.x. [DOI] [PubMed] [Google Scholar]
- 11.Nagao Y, Sata M, Fukuizumi K, Tanikawa K, Kameyama T. High incidence of oral precancerous lesions in a hyperendemic area of hepatitis C virus infection. Hepatol Res. 1997;8:173–177. [Google Scholar]
- 12.Nagao Y, Sata M, Fukuizumi K, Ryu F, Ueno T. High incidence of oral lichen planus in an HCV hyperendemic area. Gastroenterology. 2000;119:882–883. doi: 10.1053/gast.2000.17936. [DOI] [PubMed] [Google Scholar]
- 13.Nagao Y, Kawaguchi T, Tanaka K, Kumashiro R, Sata M. Extrahepatic manifestations and insulin resistance in an HCV hyperendemic area. Int J Mol Med. 2005;16:291–296. [PubMed] [Google Scholar]
- 14.Nagao Y, Tanaka J, Nakanishi T, Moriya T, Katayama K, Kumagai J, Komiya Y, et al. High incidence of extrahepatic manifestations in an HCV hyperendemic area. Hepatol Res. 2002;22:27–36. doi: 10.1016/s1386-6346(01)00114-0. [DOI] [PubMed] [Google Scholar]
- 15.Nagao Y, Myoken Y, Katayama K, Tanaka J, Yoshizawa H, Sata M. Epidemiological survey of oral lichen planus among HCV-infected inhabitants in a town in Hiroshima Prefecture in Japan from 2000 to 2003. Oncol Rep. 2007;18:1177–1181. [PubMed] [Google Scholar]
- 16.Nagao Y, Nishida N, Toyo-Oka L, Kawaguchi A, Amoroso A, Carrozzo M, Sata M, et al. Genome-wide association study identifies risk variants for lichen planus in patients with hepatitis C virus infection. Clin Gastroenterol Hepatol. 2017;15:937–944. doi: 10.1016/j.cgh.2016.12.029. e935. [DOI] [PubMed] [Google Scholar]
- 17.Nagao Y, Kimura K, Kawahigashi Y, Sata M. Successful treatment of hepatitis C virus-associated oral lichen planus by interferon-free therapy with direct-acting antivirals. Clin Transl Gastroenterol. 2016;7:e179. doi: 10.1038/ctg.2016.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Misaka K, Kishimoto T, Kawahigashi Y, Sata M, Nagao Y. Use of direct-acting antivirals for the treatment of hepatitis C virus-associated oral lichen planus: a case report. Case Rep Gastroenterol. 2016;10:617–622. doi: 10.1159/000450679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsubouchi H, Kumada H, Kiyosawa K, Mochida S, Sakaida I, Tanaka E, Ichida T, et al. Prevention of immunosuppressive therapy or chemotherapy-induced reactivation of hepatitis B virus infection – joint report of the Intractable Liver Diseases Study Group of Japan and the Japanese Study Group of the Standard Antiviral Therapy for Viral Hepatitis (In Japanese). Kanzo. 2009;50:38–42. [Google Scholar]
- 20.Nagao Y, Sata M, Ide T, Suzuki H, Tanikawa K, Itoh K, Kameyama T. Development and exacerbation of oral lichen planus during and after interferon therapy for hepatitis C. Eur J Clin Invest. 1996;26:1171–1174. doi: 10.1046/j.1365-2362.1996.610607.x. [DOI] [PubMed] [Google Scholar]
- 21.Nagao Y, Kawaguchi T, Ide T, Kumashiro R, Sata M. Exacerbation of oral erosive lichen planus by combination of interferon and ribavirin therapy for chronic hepatitis C. Int J Mol Med. 2005;15:237–241. [PubMed] [Google Scholar]
- 22.Grossmann Sde M, Teixeira R, de Aguiar MC, do Carmo MA. Exacerbation of oral lichen planus lesions during treatment of chronic hepatitis C with pegylated interferon and ribavirin. Eur J Gastroenterol Hepatol. 2008;20:702–706. doi: 10.1097/MEG.0b013e3282f1cc5d. [DOI] [PubMed] [Google Scholar]
- 23.Nagao Y, Sata M, Tanikawa K, Kameyama T. A case of oral lichen planus with chronic hepatitis C successfully treated by glycyrrhizin. Kansenshogaku Zasshi. 1995;69:940–944. doi: 10.11150/kansenshogakuzasshi1970.69.940. [DOI] [PubMed] [Google Scholar]
- 24.Nagao Y, Sata M, Suzuki H, Tanikawa K, Itoh K, Kameyama T. Effectiveness of glycyrrhizin for oral lichen planus in patients with chronic HCV infection. J Gastroenterol. 1996;31:691–695. doi: 10.1007/BF02347618. [DOI] [PubMed] [Google Scholar]
- 25.Coilly A, Roche B, Duclos-Vallee JC, Samuel D. Optimal therapy in hepatitis C virus liver transplant patients with direct acting antivirals. Liver Int. 2015;35((suppl 1)):44–50. doi: 10.1111/liv.12728. [DOI] [PubMed] [Google Scholar]
- 26.Makara M, Sulyok M, Csacsovszki O, Sulyok Z, Valyi-Nagy I. Successful treatment of HCV-associated cryoglobulinemia with ombitasvir/paritaprevir/ritonavir, dasabuvir and ribavirin: a case report. J Clin Virol. 2015;72:66–68. doi: 10.1016/j.jcv.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 27.Cacoub P, Commarmond C, Sadoun D, Desbois AC. Hepatitis C virus infection and rheumatic diseases: the impact of direct-acting antiviral agents. Rheum Dis Clin North Am. 2017;43:123–132. doi: 10.1016/j.rdc.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 28.Yoshikawa A, Terashita K, Morikawa K, Matsuda S, Yamamura T, Sarashina K, Nakano S, et al. Interferon-free therapy with sofosbuvir plus ribavirin for successful treatment of genotype 2 hepatitis C virus with lichen planus: a case report. Clin J Gastroenterol. 2017;10:270–273. doi: 10.1007/s12328-017-0742-3. [DOI] [PubMed] [Google Scholar]
- 29.Mergoni G, Ergun S, Vescovi P, Mete O, Tanyeri H, Meleti M. Oral postinflammatory pigmentation: an analysis of 7 cases. Med Oral Patol Oral Cir Bucal. 2011;16:e11–e14. doi: 10.4317/medoral.16.e11. [DOI] [PubMed] [Google Scholar]
- 30.Morelli JG, Norris DA. Influence of inflammatory mediators and cytokines on human melanocyte function. J Invest Dermatol. 1993;100:191s–195s. [PubMed] [Google Scholar]
- 31.Rozycki TW, Rogers RS, 3rd, Pittelkow MR, McEvoy MT, el-Azhary RA, Bruce AJ, Fiore JP, et al. Topical tacrolimus in the treatment of symptomatic oral lichen planus: a series of 13 patients. J Am Acad Dermatol. 2002;46:27–34. doi: 10.1067/mjd.2002.119648. [DOI] [PubMed] [Google Scholar]
- 32.Wee JS, Shirlaw PJ, Challacombe SJ, Setterfield JF. Efficacy of mycophenolate mofetil in severe mucocutaneous lichen planus: a retrospective review of 10 patients. Br J Dermatol. 2012;167:36–43. doi: 10.1111/j.1365-2133.2012.10882.x. [DOI] [PubMed] [Google Scholar]
- 33.Oleaga JM, Gardeazabal J, Sanz de Galdeano C, Diaz PJ. Generalized lichen planus associated with primary biliar cirrhosis which resolved after liver transplantation. Acta Derm Venereol. 1995;75:87. doi: 10.2340/000155557587. [DOI] [PubMed] [Google Scholar]
- 34.Nagao Y, Sata M. Disappearance of oral lichen planus after liver transplantation for primary biliary cirrhosis and immunosuppressive therapy in a 63-year-old Japanese woman. Hepat Mon. 2014;14:e16310. doi: 10.5812/hepatmon.16310. [DOI] [PMC free article] [PubMed] [Google Scholar]