Abstract
An unruptured aneurysm was incidentally found in the right middle cerebral artery in a 67-year-old woman. During an attempt to turn the temporalis muscle for surgical clipping, systolic blood pressure suddenly increased. After opening the dura mater, we found a subarachnoid hemorrhage and severe brain swelling. We promptly expanded the craniotomy area to reach the aneurysm while pulling part of the frontal lobe to apply a clip. We retrospectively analyzed the aneurysm using computational fluid dynamics. Our analysis suggests that the rupture of the aneurysm occurred at a location with very low wall shear stress.
Keywords: Cerebral aneurysm, Intraoperative rupture, Clipping, Craniotomy
Introduction
Intraoperative rupture of an unruptured cerebral aneurysm is very rare. Nevertheless, it is a noteworthy complication in surgical clipping and can severely impact the prognosis for patient recovery [1]. While intraoperative re-ruptures of ruptured aneurysms have been reported in the literature, reports of intraoperative rupture of unruptured aneurysms are scarce [1, 2, 3, 4].
Case Report
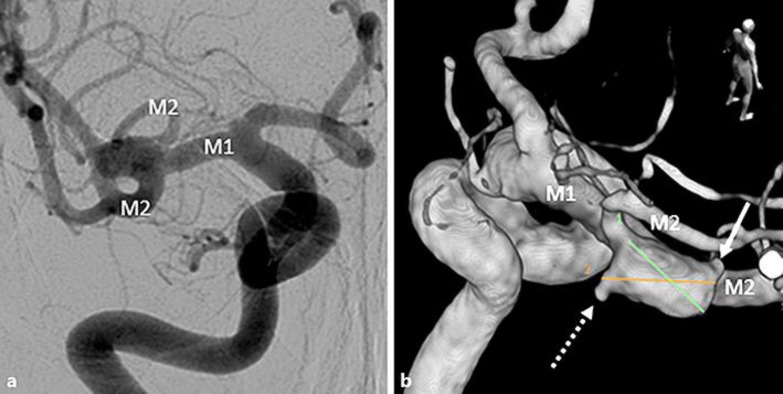
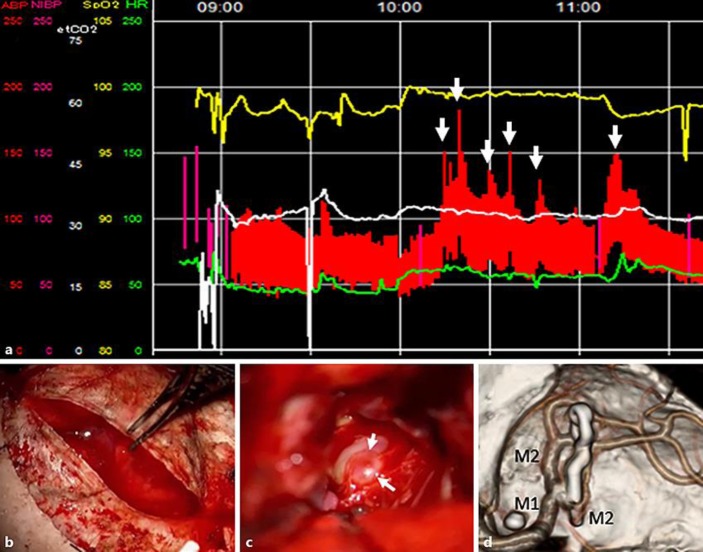
A 67-year-old woman consulted a neurosurgeon for dizziness. Magnetic resonance angiography showed an aneurysm at the bifurcation of M1 and M2 in the right middle cerebral artery (MCA). Digital subtraction angiography (DSA) revealed that the aneurysm had two blebs, largest diameter of 9.5 mm and a neck of 5.9 mm (Fig. 1). After skin incision for surgical clipping, during an attempt to turn the temporalis muscle together with the skin, systolic blood pressure suddenly increased from 80 mm Hg to 150 mm Hg (Fig. 2a). When we opened the dura mater, we found a subarachnoid hemorrhage (Fig. 2b). Because the brain was swelling up, we promptly expanded the craniotomy area towards the midline. We pulled part of the swollen frontal lobe, and found a thick hematoma and an aneurysm. No rupture was observed in the bleb within the surgical field (Fig. 2c). Therefore, the bleb on the opposite site of the surgical field must have been ruptured. Clipping was performed using a 12-mm bayonet titanium clip. Postoperative three-dimensional computerized tomography angiography confirmed the absence bloodstream in the aneurysm (Fig. 2d). Three months after surgery, the scant left hemiparesis remained with a final modified Ranking Scale score of 1.
Fig. 1.
a The aneurysm (largest diameter 9.5 mm and neck 5.9 mm) was found in the bifurcation of right middle cerebral artery by frontal right carotid angiography. b A three-dimensional rotational angiography from the diagonal rear was drawn. The aneurysm had two blebs, one (arrow) could be observed from the surgical field, but the other (dotted arrow) was difficult to see because it was located at the opposite site of the surgical field. Two lines were drawn to measure the size of aneurysm: line 1 was 8.6 mm long; line 2, 9.5 mm long.
Fig. 2.
a Intraoperative vital signs were recorded. The elevation of blood pressure occurred at the time points indicated by arrows. The first elevation was recorded at 10: 14 h when we finished turning the skin and cleaned blood of the skull using a cotton pad. We believe that the rupture of the aneurysm occurred at this time point. b Subarachnoid hemorrhage already existed when dura mater was cut. c The bleb was identified when we reached the aneurysm. However this bleb was intact (arrows). d A three-dimensional computerized tomography angiography was performed to image the facies of the blood vessel. The clip was applied appropriately.
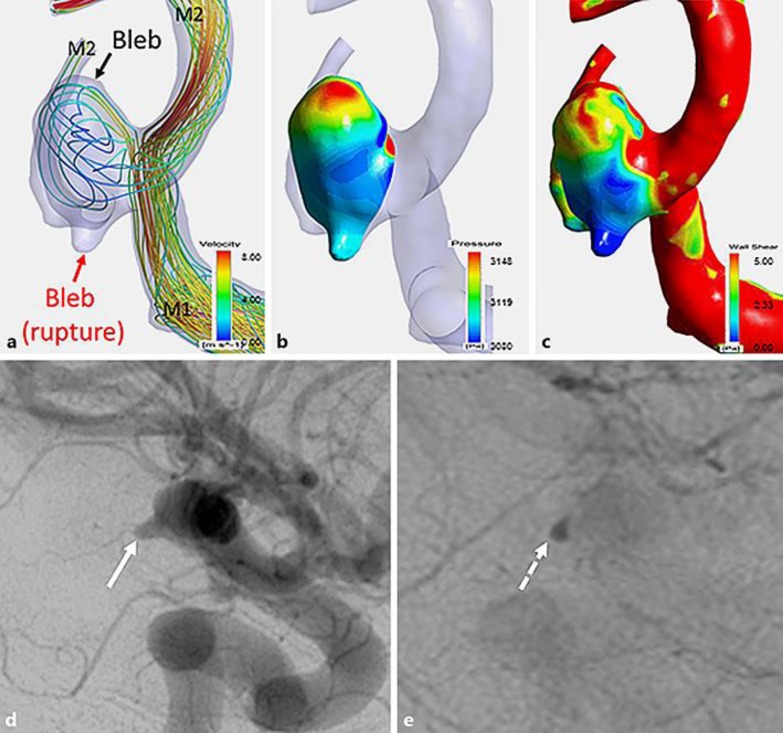
We performed computational fluid dynamics (CFD) simulations (Fig. 3a, b, c) similar to previous reports [5]. The fluid domains were extruded at the inlet to allow for fully developed flow and meshed using the ICEM CFD software (Version 16.2, Ansys Inc., Canonsburg, PA, USA) to create finite volume tetrahedral elements and 7 layers of wall prism elements. A rigid-wall no-slip boundary condition was implemented at the vessel walls. We performed a pulsatile-flow simulation with ANSYS CFX (version 16.2, ANSYS Inc.), with the average flow set to 0.254 L/min and pulse set to 70 beats/min. The bloodstream in the aneurysm was faint (Fig. 3a). The pressure in the bleb of the upper part (unruptured) was high (3,148 Pa) because blood streamed through. However, the pressure in the bleb of the lower part (ruptured) was relatively low (3,099 Pa; Fig. 3b). Wall shear stress (WSS) was very low (less than 0.3 Pa) in the lower bleb (ruptured; Fig. 3c). Flow stagnation in the lower bleb was confirmed by presurgical DSA (Fig. 3d, e).
Fig. 3.
The aneurysm was analyzed using computational fluid dynamics (CFD). a The aneurysm had two blebs. The upper bleb was identified in the surgical field. The lower bleb appears to have been ruptured. The bloodstream in the aneurysm was markedly low, as indicated by low intra-aneurysmal flow velocity. b The pressure was high (3,148 Pa) at the upper bleb because blood streamed into the bleb directly. Pressure was low at the lower bleb (3,099 Pa). c The wall shear stress was high (5 Pa) in the upper bleb, but low in the lower bleb (less than 0.3 Pa). d, e Preoperative digital subtraction angiography (lateral view) of arterial phase (d) and venous phase (e) shows that the contrast medium in the ruptured bleb was found both in the arterial phase and in the vein phase. This result is in line with flow velocity decline of bloodstream in CFD analysis.
Discussion
This is the first report describing intraoperative rupture of an unruptured aneurysm. The intraoperative rupture occurred during turning of the skin flap. Ruptures during craniotomy have been reported to be mainly caused by vibrations of power instruments and changes of the intracranial pressure upon opening the dura matter or first brain towing [2]. It is unlikely that the change of intracranial pressure triggered the intraoperative rupture because subarachnoid hemorrhage was already present when opening the dura mater. Gradually increasing pain due to shallow anesthetic depth might have caused the intraoperative rupture [2]. Propofol administration was continued at 2–3 µg/mL with 0.15–0.20 µg/kg/min of remifentanil, in accordance with proper use [6].
We analyzed the aneurysm using CFD (Fig. 3a, b, c): It appears that the rupture of the aneurysm occurred at a part showing very low WSS (<0.3 Pa). A previous study demonstrated that average WSS of the middle cerebral artery aneurysm and parent vessel was 1.64 ± 1.16 and 3.64 ± 1.25 Pa, respectively [7]. Ruptures have been reported in both parts with higher and parts with lower WSS [7]. Our findings support the hypothesis that parts with low WSS tend to rupture. If WSS is low, the resident time of proinflammatory blood cells at the endothelial cell increases, and inflammatory blood cells start to produce matrix metalloproteinases. As a result, matrix degradation and aneurysm wall fragility occur [5].
Intraoperative rupture during craniotomy of unruptured cerebral aneurysms is an extremely rare complication. However, surgeons should be aware that this complication may occur especially if very low WSS areas are present in the aneurysm.
Statement of Ethics
Informed consent was obtained from the family of the patient.
Disclosure Statement
The authors declare that they have no conflicts of interest.
References
- 1.Zhen Y, Yan K, Zhang H, Zhao S, Xu Y, Zhang H, He L, Shen L. Analysis of the relationship between different bleeding positions on intraoperative rupture anterior circulation aneurysm and surgical treatment outcome. Acta Neurochir (Wien) 2014;156:481–491. doi: 10.1007/s00701-013-1953-0. [DOI] [PubMed] [Google Scholar]
- 2.Batjer H, Samson D. Intraoperative aneurysmal rupture: incidence, outcome, and suggestions for surgical management. Neurosurgery. 1986;18:701–707. doi: 10.1227/00006123-198606000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Houkin K, Kuroda S, Takahashi A, Takikawa S, Ishikawa T, Yoshimoto T, Itamato K. Intra-operative premature rupture of the cerebral aneurysms. Analysis of the causes and management. Acta Neurochir (Wien) 1999;141:1255–1263. doi: 10.1007/s007010050428. [DOI] [PubMed] [Google Scholar]
- 4.Sandalcioglu IE, Schoch B, Regel JP, Wanke I, Gasser T, Forsting M, Stolke D, Wiedemayer H. Does intraoperative aneurysm rupture influence outcome? Analysis of 169 patients. Clin Neurol Neurosurg. 2004;106:88–92. doi: 10.1016/j.clineuro.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 5.Meng H, Tutino VM, Xiang J, Siddiqui A. High WSS or low WSS? Complex interactions of hemodynamics with intracranial aneurysm initiation, growth, and rupture: toward a unifying hypothesis. AJNR Am J Neuroradiol. 2014;35:1254–1262. doi: 10.3174/ajnr.A3558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Twersky RS, Jamerson B, Warner DS, Fleisher LA, Hogue S. Hemodynamics and emergence profile of remifentanil versus fentanyl prospectively compared in a large population of surgical patients. J Clin Anesth. 2001;13:407–416. doi: 10.1016/s0952-8180(01)00292-6. [DOI] [PubMed] [Google Scholar]
- 7.Shojima M, Oshima M, Takagi K, Torii R, Hayakawa M, Katada K, Morita A, Kirino T. Magnitude and role of wall shear stress on cerebral aneurysm: computational fluid dynamic study of 20 middle cerebral artery aneurysms. Stroke. 2004;35:2500–2505. doi: 10.1161/01.STR.0000144648.89172.0f. [DOI] [PubMed] [Google Scholar]