Abstract
Primary pleomorphic sarcoma of the left atrium is a rare tumor. There is no actual evidence of the management of this pathological entity, so the main treatment is individualized, surgical management being the cornerstone of the treatment. We present a 78-year-old female who had a clinical picture of heart failure, documenting an atrial mass of the left atrium, with high-grade pleomorphic sarcoma revealed in histopathology. The tumor was surgical removed, with no clinical evidence of residual mass. The tumor recurred again within 3 years, to which the patient succumbed.
Keywords: Sarcoma, Neoplasms, Heart failure, Heart atria
Case Presentation
A female patient aged 78 years with a clinical record of arterial hypertension consulted the emergency department for appearance of 15 days of worsening in her functional class before the event (NYHA II/IV, now III/IV, with orthopnea, paroxysmal nocturnal dyspnea, and bendopnea). At the first evaluation the patient had normal vital signs according to her age, with positive findings in the physical exam of bilateral rales, abdominojugular test, and edema of lower limbs. Because of the above it was considered that the patient had a new onset heart failure, and treatment with neurohumoral blockage was started. At the same time a transthoracic echocardiography as done.
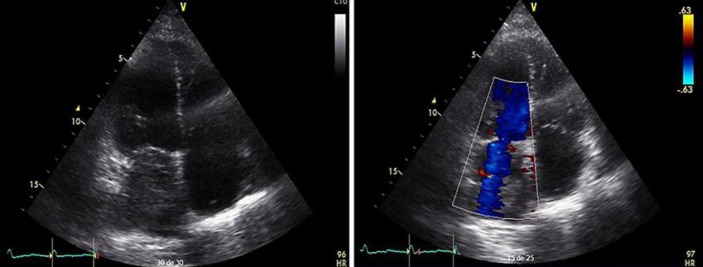
The transthoracic echocardiography finding (Fig. 1) was consistent with an atrial mass inside the left atrium, with an area of 6.4 × 3.2 × 6.9 cm, occupying 60% of the size, without involvement of the mitral valve. The cardiovascular surgery department indicated magnetic resonance imaging of the heart that revealed a mass in the left atrium previously described, dependent on the posterolateral wall, in relation with the pulmonary veins, compressing them without complete obstruction. The mass was of lobulated contours, lightly hyperintense, with respect to the myocardium in T1 and T2 in direct contact with the valvular plane obstructing the output tract in 60–70% and protruding discreetly into the left ventricular cavity (Fig. 2).
Fig. 1.
Transthoracic echocardiography, apical projection four chambers, 2D mode, and Doppler color, showing a mass in the left atrium that occupies 60% without mitral regurgitation.
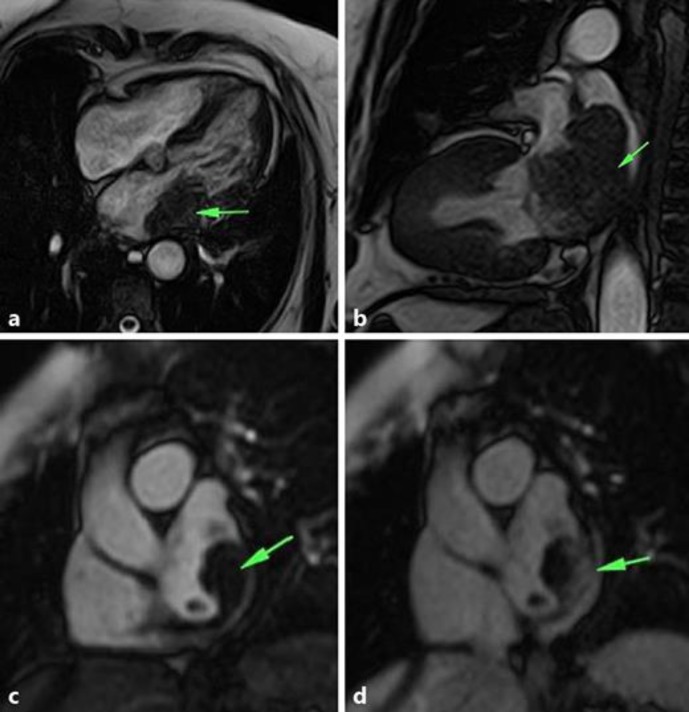
Fig. 2.
Magnetic resonance imaging of the heart showing a mass dependent on the wall of the left atrium, which extended to the mitral valve plane. a SSFP sequence in a four-camera plane. b SSFP sequence in a long two-chamber plane showing extension of the mass to the mitral valve plane and to the pulmonary veins. c, d Planes of short height at the height of the left atrium during the first step of contrast medium, which shows the rapid capture of contrast in the mass.
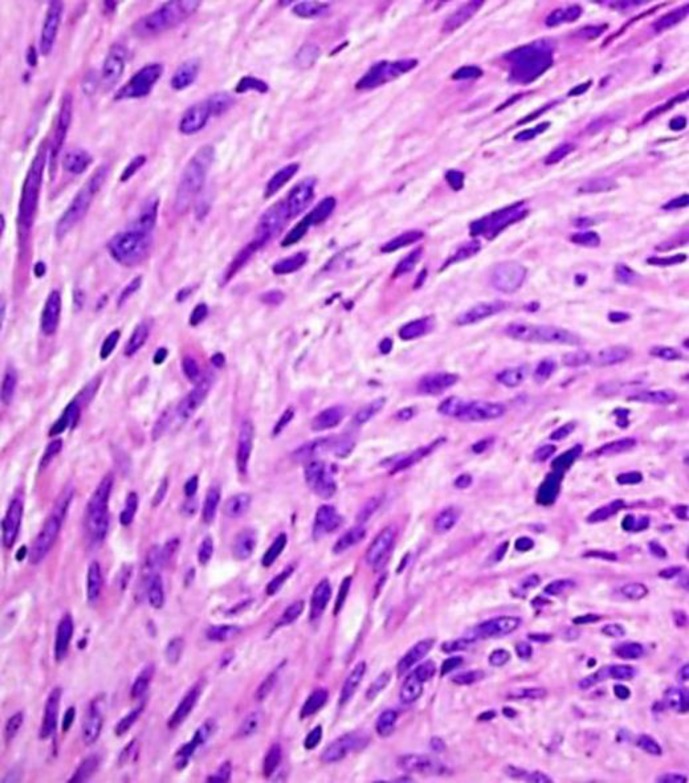
The cardiovascular surgeon decides to completely resect this mass, with was done without complications. The sample was sent to pathology (Fig. 3), which reported a malignant, poorly differentiated tumor with epithelioid cells with atypical mitotic figures distributed in sheets accompanied by branched vascular spaces, with areas of hyalinization and necrosis, without recognition of healthy organ. Immunochemistry found focal reactivity to CD68 and actin of the smooth muscle, without reactivity to CD34, FLI-1, or S100. The measured proliferation index with Ki-67 was 40%, conclusive of high-grade pleomorphic sarcoma.
Fig. 3.
Fusiform cells with atypia, pleomorphism, and frequent mitosis. Hematoxylin and eosin stain, ×40.
The patient was proposed adjuvant therapy with chemotherapy, which she refuses. She showed symptomatic complete improvement, and ambulatory follow-up was decided.
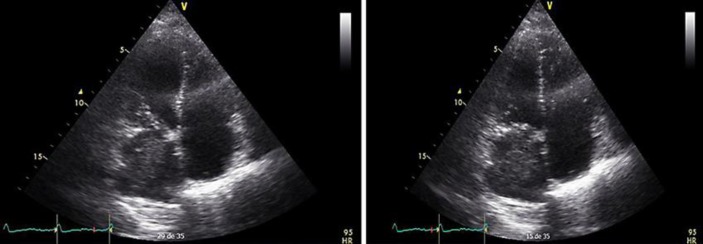
The patient came back 3 years later with a clinical picture of decompensated heart failure. Echocardiographic follow-up (Fig. 4) finding tumor recurrence, with pathological fractures and progression of the disease to the woven bone (right humerus, left hip, and lumbosacral region), so it was decide to do conservative and palliative management. A week after hospitalization the patient died.
Fig. 4.
Transthoracic echocardiography, apical projection four chambers, 2D mode, and Doppler color in different phases of the cardiac cycle showing relapse of the initial lesion in the left atrium.
Discussion
Cardiac tumors are a rare, with an estimated frequency of 0.0017 to 0.33% [1]. Of these tumors, the benign ones correspond to 75% and the malignant ones to 25% [2]. The frequency is similar in males and females, being higher in the right atrium, later left atrium, and both ventricles [3]. The difference with benign tumors, because of the similarity in the figures, lies in the clinical course and the histology [4]. Metastatic tumors are 20–40 times more common than primary malignant cardiac neoplasms [5]; 20% of malignant tumors of the heart present with metastasis. The order of frequency is lung, liver, bones, thoracic lymph nodes, adrenal, and brain [4, 6].
The locoregional recurrence or the appearance of metastasis happens during the first year [7]. Patients with malignant heart tumors of the left side have more frequent distant metastases, while the malignant heart tumors of the right side have more locally advanced disease [7].
In the largest study carried out on cardiac masses, it was found that the average age at appearance of the disease was 47.1 ± 16.1 years [8]. The median follow-up was 51.2 months; until the time to analysis, 69.7% had died, 43% had metastasis, 44.9% of the patients with metastasis had pulmonary metastasis, and 20.9% metastasis to the brain [8].
The clinical presentation of these tumors is very heterogeneous, and the symptoms are due to compromise in ventricular afterload, arrhythmias, embolic events, and metastasis [9, 10]. The patients also can present dyspnea (48%), chest pain (22%), heart failure (13%), and pericarditis (5%). In some cases there have been reports of neurological symptoms, neurovascular syndrome [8, 11], and in some cases cardiogenic shock [12].
Early and immediate detection to start a therapeutic strategy are important to improve the survival of patients [13]. As an initial diagnostic approach, echocardiography could be a useful tool. Magnetic resonance imaging of heart could be utilized in the evaluation of cardiac masses, as it provides contrast resolution to soft tissues and can evaluate extension to the myocardium [14]. Transesophageal echocardiography has proven to be a noninvasive and relatively effective tool [15]. The combination of echocardiography with magnetic resonance imaging of the heart could be the used to differentiate tumor or thrombus [15].
The forecasting characteristics are unspecific; nevertheless, a worse prognosis in patients with high mitotic index, extensive tumor necrosis, infiltration to myocardium, and high grade has been documented [2]. The survival in those patients is less than 1 year with a diagnosis of rhabdomyosarcoma [2]. The main factor related to the survival in patients with these types of malignancies is related to the total resection of the mass, reaching a survival twice as high when the mass is resected totally. Despite of advances in treatment, the prognosis of those patients is bad [16]. The common cause of death after microscopic resection is local recurrence of the tumor, which appears in half of the patients, with survival until 16.5 months [17, 18]. Another factors related to a better prognosis is the anatomical localization, more than the histological type [19].
The use of chemotherapy and radiotherapy is limited [20] to adjuvant therapy in cases where resections are not totally achieved, or in cases where the pathology demonstrates aggressive behavior. The limit of radiotherapy is also well known in cases where sarcoma is shown in pathology, as the radiobiology of these tumors tends to have resistance to it [20].
The use of chemotherapy its related to better survival in patients without metastasis [19]. Recent data suggest that taxanes tend to provide a better survival than standard therapy (ifosfamide + doxorubicin) [8].
Statement of Ethics
The authors have no ethical conflicts to declare.
Disclosure Statement
The authors have no conflicts of interest to disclose.
References
- 1.Corradi D, Contini GA, Gherli T, Nicolini F. A left atrial myxomalike rhabdomyosarcoma. J Thorac Cardiovasc Surg. 2012;144:e7–e10. doi: 10.1016/j.jtcvs.2012.03.073. [DOI] [PubMed] [Google Scholar]
- 2.Burke A, Tazelaar H, Butany J, El-Demellawy D, Loire R, Geva T, et al. Cardiac sarcomas. In: Travis W, Brambilla E, Muller-Hermelink H, Harris C, editors. Pathology and Genetics of Tumours of the Lung, Pleura, Thymus and Heart. Lyon: IARC Press; 2004. pp. 273–281. [Google Scholar]
- 3.Ma J, Sun JP, Chen M, Zhang L, Xu N, Wang J. Left atrial rhabdomyosarcoma. Circulation. 2014;129:e503–e505. doi: 10.1161/CIRCULATIONAHA.113.003478. [DOI] [PubMed] [Google Scholar]
- 4.McAllister HA., Jr Primary tumors and cysts of the heart and pericardium. Curr Probl Cardiol. 1979;4:350–352. doi: 10.1016/0146-2806(79)90008-2. [DOI] [PubMed] [Google Scholar]
- 5.Fernandes F, Soufen HN, Ianni BM, Arteaga E, Ramires FJ, Mady C. Primary neoplasms of the heart. Clinical and histological presentation of 50 cases. Arq Bras Cardiol. 2001;76:231–237. doi: 10.1590/s0066-782x2001000300006. [DOI] [PubMed] [Google Scholar]
- 6.McManus B, Lee CH. Chapter 69: Primary tumors of the heart. In: Libby P, Bonnow RO, Mann DL, et al., editors. Braunwald's Heart Disease. ed 8. Philadelphia: Elsevier Science; 2008. pp. 1815–1828. [Google Scholar]
- 7.Ibrahim A, Luk A, Singhal P, Wan B, Zavodni A, et al. Primary intimal (spindle cell) sarcoma of the heart: a case report and review of the literature. Case Rep Med. 2013;2013:461815. doi: 10.1155/2013/461815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Isambert N, Ray-Coquard I, Italiano A, Rios M, Kerbrat P, et al. Primary cardiac sarcomas: a retrospective study of the French Sarcoma Group. Eur J Cancer. 2014;50:128–136. doi: 10.1016/j.ejca.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 9.Modi A, Lipnevicius A, Moorjani N, Haw M. Prolonged survival with left atrial spindle cell sarcoma. Interact Cardiovasc Thorac Surg. 2009;8:703–704. doi: 10.1510/icvts.2009.203562. [DOI] [PubMed] [Google Scholar]
- 10.Shanmugam G. Primary cardiac sarcoma. Eur J Cardiothorac Surg. 2006;29:925–932. doi: 10.1016/j.ejcts.2006.03.034. [DOI] [PubMed] [Google Scholar]
- 11.Caballero PE. Left atrial sarcoma presenting as cerebral infarction. Neurologist. 2008;14:131–133. doi: 10.1097/NRL.0b013e31816606dd. [DOI] [PubMed] [Google Scholar]
- 12.Hsieh CH, Seak CJ, Chiu TF, Chen JC, Li CH. An uncommon cause of heart failure: cardiac sarcomas in the left atrium. J Emerg Med. 2011;40:e123–e124. doi: 10.1016/j.jemermed.2008.11.025. [DOI] [PubMed] [Google Scholar]
- 13.Canadyova J, Setina M, Smetanova S, Mokracek A. Leiomyosarcoma of the left atrium. Asian Cardiovasc Thorac Ann. 2008;16:e7–e9. doi: 10.1177/021849230801600128. [DOI] [PubMed] [Google Scholar]
- 14.Eswaran P, Devadoss P, Narasimhan LS, Kannan K. Synovial sarcoma of the heart: a case report and literature review. J Cancer Res Ther. 2015;11:659. doi: 10.4103/0973-1482.139391. [DOI] [PubMed] [Google Scholar]
- 15.Patil HR, Singh D, Hajdu M. Cardiac sarcoma presenting as heart failure and diagnosed as recurrent myxoma by echocardiogram. Eur J Echocardiogr. 2010;11:E12. doi: 10.1093/ejechocard/jep201. [DOI] [PubMed] [Google Scholar]
- 16.Mehta N, Desai A, Shivdasani B, Suryawanshi S, Mehta AB, et al. Left atrial spindle cell sarcoma – case report. Indian Heart J. 2012;64:416–419. doi: 10.1016/j.ihj.2012.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burke AP, Cowan D, Virmani R. Primary sarcomas of the heart. Cancer. 1992;69:387–395. doi: 10.1002/1097-0142(19920115)69:2<387::aid-cncr2820690219>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 18.Donsbeck AV, Ranchere D, Coindre JM, Le Gall F, Cordier JF, Loire R. Primary cardiac sarcomas: an immunohistochemical and grading study with long-term follow-up of 24 cases. Histopathology. 1999;34:295–304. doi: 10.1046/j.1365-2559.1999.00636.x. [DOI] [PubMed] [Google Scholar]
- 19.Kumar N, Agarwal S, Ahuja A, Das P, Airon B, Ray R. Spectrum of cardiac tumors excluding myxoma: experience in a tertiary center with review of the literature. Pathol Res Pract. 2011;207:769–774. doi: 10.1016/j.prp.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 20.Gupta A. Primary cardiac sarcomas. Expert Rev Cardiovasc Ther. 2008;6:1295–1297. doi: 10.1586/14779072.6.10.1295. [DOI] [PubMed] [Google Scholar]