Abstract
Objective
To estimate the crude prevalence of minor depressive disorder (MinD) in a clinic-based population of adults with type 2 diabetes.
Methods
We screened a clinical sample of 702 adults with type 2 diabetes for depressive symptoms using the Patient Health Questionnaire-2 and performed a structured diagnostic psychiatric interview on 52 screen-positive and a convenience sample of 51 screen-negative individuals. Depressive disorder diagnoses were made using Diagnostic and Statistical Manual of Mental Disorders IV (DSM-IV) Text Revised criteria and categorized as MinD, major depressive disorder (MDD), or no depressive disorder. We estimated prevalence of MinD and MDD and derived 95% CIs.
Results
The crude prevalence of current, past, and current or past MinD was 4.3% (95% CI: 0.9–9.2%), 9.6% (95% CI: 3.9–15.9%), and 13.9% (95% CI: 7.7–21.2%), respectively. The crude prevalence of current, past, and current or past MDD was slightly higher—5.0% (95% CI: 1.9–9.4%), 12.0% (95% CI: 6.1–19.5%), and 17.0% (95% CI: 10.1–24.8%), respectively. There was a high prevalence of coexisting anxiety disorders in individuals with MinD (42.2%) and MDD (8.1%). Hemoglobin A1c levels were not significantly different in individuals with MinD or MDD compared to those without a depressive disorder.
Conclusions
MinD is comparably prevalent to MDD in patients with type 2 diabetes; both disorders are associated with concomitant anxiety disorders. MinD is not included in the DSM-5; however, our data support continuing to examine patients with chronic medical conditions for MinD.
Keywords: type 2 diabetes mellitus, major depressive disorder, minor depression, anxiety disorders
It is estimated that worldwide 43 million people with diabetes have symptoms of depression.1 Major depressive disorder (MDD) is associated with higher risk of type 2 diabetes (T2DM), as well as with an increased risk of microvascular and macrovascular complications, worse metabolic control, and all-cause mortality. There is less evidence supporting similar associations between minor depression (MinD) and T2DM or its secondary complications. In adults with diabetes, the prevalence of MDD is estimated at 11.4%.2 However, the prevalence of “elevated depressive symptoms” based on self-reported questionnaires is much higher at 31%,2 suggesting that a large number of patients with diabetes have a milder depressive disorder. Prior studies assessing depression in T2DM using self-report questionnaires are unable to distinguish MDD from MinD and may conflate individuals with depressive and anxiety disorders. MinD is defined in the Diagnostic and Statistical Manual of Mental Disorders IV-Text Revision (DSM-IV-TR) as a distinct syndrome that does not meet criteria for MDD or other depressive disorders (i.e., depressive disorder not otherwise specified, adjustment disorder with depressed mood).3
Few studies have examined the prevalence of non–MDD depressive disorders in T2DM. One study found the prevalence of dysthymia in adults with diabetes to be 3.5% in the United States.4 In that study, however, the prevalence of MinD as defined by DSM-IV-TR was not reported, diabetes was assessed by self-report, and it is unclear whether participants had type 1 diabetes or T2DM or both. The low prevalence of dysthymia in this study suggests that most individuals with diabetes and depressive symptoms have MinD. Although 8% of individuals with diabetes were estimated to have MinD in the Pathways Study based on Patient Health Questionnaire-9 (PHQ-9) criteria, to our knowledge, the prevalence of MinD has not been estimated using DSM-IV-TR diagnostic criteria based on a structured psychiatric interview.5,6 In this study, we sought to estimate the specific, crude prevalence of MinD using a diagnostic interview in a population of adults with T2DM, and to compare it to the prevalence of MDD and other psychiatric disorders.
MATERIAL AND METHODS
Study Population and Depression Screening
Participants with physician-confirmed T2DM who were 18 years of age or older were recruited from the Diabetes Center clinics at Johns Hopkins Hospital, where 5 physicians, 3 nurse practitioners, and a nutritionist see patients daily. As part of routine practice, patients were screened for depressive symptoms using the PHQ-2. The PHQ-2 asks the following 2 questions, which were scored between “0” (not at all) and “3” (nearly every day): “Over the past 2 weeks how often have you been bothered by any of the following problems?”
Little interest or pleasure in doing things.
Feeling down, depressed, or hopeless.
Individuals were considered screen positive with a score of ≥3 (summing answers from both questions), which reflects a 75% probability of having any depressive disorder.7 We also asked participants if they had been told that they have MDD or were being treated with antidepressants or psychotherapy, to capture individuals with MDD not detected by the PHQ-2 because their symptoms were adequately controlled. We included these individuals among those who screened positive on the PHQ-2 to avoid misclassification and underestimation of the prevalence of MDD. Otherwise, individuals scoring <3 on the PHQ-2 were considered not to have clinically significant depressive symptoms.
Participant Recruitment for Psychiatric Diagnostic Interview
Between February 1, 2011 and June 30, 2013, all patients with T2DM seen in the clinic underwent PHQ-2 screening as a routine clinical practice and had psychiatric history obtained, providing the sample prevalence of those screening positive and negative for depression for our T2DM clinic population. Individuals who screened positive for depressive symptoms on the PHQ-2 (score ≥3), who carried a diagnosis of MDD, or who were taking antidepressant medications (collectively referred to as “screen positive” henceforth) were asked about their willingness to participate in our study. Patients with positive screening for depressive symptoms who provided written informed consent were enrolled, completed questionnaires, underwent a formal indepth structured diagnostic interview to further characterize their depressive disorder and to detect past, as well as current, depressive symptomology. At the time of study enrollment in 2011, we excluded individuals taking antipsychotic medications, as these individuals were likely to have a major mental illness such as bipolar disorder. We do acknowledge, however, that current practice incorporates antipsychotic use as augmentation strategies for MDD.8 We also excluded individuals using glucocorticoids because glucocorticoids can induce mood disorders including depression. Our goal was to identify 100 patients who screened positive for depressive symptoms or who had other evidence of depression. In a prior study, 13.6% of patients with diabetes and congestive heart failure were identified as depressed using the PHQ-2,9 so we anticipated a priori needing to screen approximately 750 individuals to identify 100 patients who screened positive and were eligible to be further evaluated with a structured diagnostic interview. This subsample of patients undergoing the structured diagnostic interview provided depression prevalence estimates within patients who screened positive for depressive symptoms.
To address the potential for screening bias of the PHQ-2, namely false negatives, we also invited a convenience sample of 50 consecutive individuals who screened negative (defined as PHQ-2 score <3 without self-reported MDD) and agreed to be contacted and consented for the structured diagnostic interview. This subsample of patients undergoing Structured Clinical Interview for DSM-IV (SCID) provided depression prevalence estimates for patients who were screened negative for depressive symptoms. Patients who were willing to participate provided written informed consent. This study was approved by a Johns Hopkins University School of Medicine Institutional Review Board.
SCID Axis I Disorders, Nonpatient Edition (SCID-I/NP)
Participants who screened positive and a sample of those who screened negative and agreed to be contacted underwent a diagnostic interview using the SCID-I/NP research version within 30–60 days of their clinic visit. The SCID is deemed a gold standard, structured interview for the diagnosis of mental health disorders.10,11 The SCID-I/NP is designed for use in studies in which the participants are not identified as psychiatric patients.10 The SCID-I/NP was administered by a physician research coordinator (M. N.) trained and supervised by a study psychiatrist with expertise in depressive disorders (J. P.). He administered the modules outlined in Table 1, which enabled us to specifically characterize depressive disorders and distinguish them from other Axis I disorders. Module J included diagnostic criteria for MinD. The SCID-I/NP was scored according to the User’s Guide.11 The interview questions on the SCID each represent criteria needed to make the diagnosis of specific disorders (i.e., MDD and MinD) and distinguish them from other disorders, as outlined in Table 1. Presence or absence of each criterion was determined by the rules provided for each criterion. The lifetime presence of a disorder was first determined followed by assessment of whether the criteria for the disorder were currently met.10
TABLE 1.
Diagnostic Coverage of Selected Structured Clinical Interview for Diagnostic and Statistical Manual (SCID) Axis I Disorders Modules
| SCID module | Disorders identified |
|---|---|
| Module A: mood episodes |
Major depressive episode (current/past) Manic episode (current/past) Hypomanic episode (current/past) Dysthymic disorder (current only) Mood disorder due to a general medical condition Substance-induced mood disorder |
| Module D: mood disorders | Bipolar I disorder Bipolar II disorder Other bipolar disorders (cyclothymic disorder, bipolar disorder not otherwise specified [NOS]) Major depressive disorder Depressive disorder NOS |
| Module F: anxiety disorders | Panic disorder with and without agoraphobia Agoraphobia without history of panic disorder Phobia (social and specific) Obsessive-compulsive disorder Posttraumatic stress disorder Generalized anxiety disorder (current only) Anxiety disorder due to a general medical condition Substance-induced anxiety disorder Anxiety disorder NOS |
| Module I: adjustment disorder | Adjustment disorder (current only) |
| Module J: optional module | Acute stress disorder Minor depressive disorder Mixed anxiety depressive disorder |
Depression diagnoses sought from various modules are bolded.
Statistical Analyses
Given that the SCID-I/NP was performed in 2 sub-samples, 1 from the clinic sample who screened positive and the other from those who screened negative, the prevalence of MinD, Pr(MinD) was estimated by the following formula:
where the following estimates were used:
Pr(MinD|Screen+): prevalence of MinD according to SCID-I/NP results from the subsample taken among those who screened positive in the T2DM clinic sample,
Pr(Screen+): prevalence of positive screening in the T2DM clinic sample,
Pr( MinD|Screen−): prevalence of MinD according to SCID-I/NP results from the subsample taken among those who screened negative in the T2DM clinic sample, and
Pr(Screen−): prevalence of negative screening in the T2DM clinic sample.
The prevalence of MDD was estimated in a similar fashion using the following formula:
By assuming that the SCID completed subsamples were representative of their corresponding screening samples, we derived 95% CIs for the estimated prevalence of MinD and MDD in the T2DM clinic population through bootstrapping. The representativeness assumption and the bootstrapping approach are needed because we only performed SCID-I/NP evaluations on subsamples of those who screened negative or positive in our clinic sample.
To compare selected participant characteristics by depression diagnosis (no depression, MinD [current or past or both], and MDD [current or pastor both]), we used generalized linear models to compare least square means, Kruskal-Wallis test to compare medians, and Chi-square tests to compare frequency distributions of categorical variables. For comparison of categorical variables with small observed or expected cell sizes, Fisher exact test was used. We used multivariable linear regression to determine the mean difference in HbA1c between individuals with MinD vs no depressive disorder, or between individuals with MDD vs no depressive disorder, adjusting for age, sex, race, and diabetes duration. A 2-sided p value of < 0.05 was used as the cutoff for statistical significance. Analyses were conducted using SAS version 9.4.
RESULTS
Screening
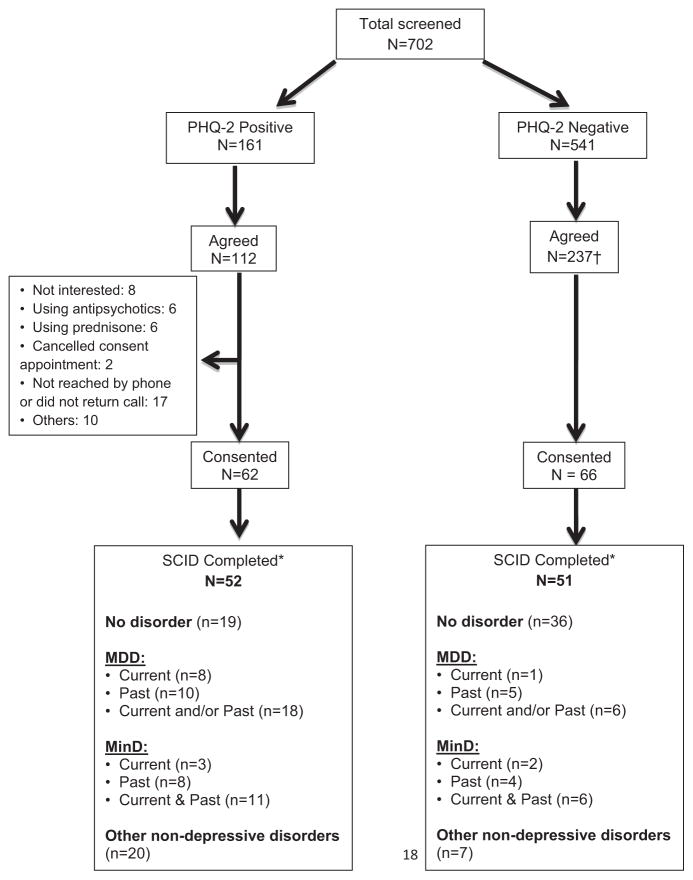
Between February 1, 2011 and June 30, 2013, 702 patients with T2DM completed the PHQ-2 as part of routine clinical care in our Diabetes Center, of whom 161 (23%) screened positive and 541 (77%) screened negative for depressive symptoms (Figure 1). Among the screen-positive patients, 112 (70%) agreed to be contacted to undergo informed consent for the SCID-I/NP interview. We were unable to determine the reasons why individuals who screened positive for depressive symptoms did not agree to be contacted for consent to undergo the SCID-I/NP. Of those who agreed to be contacted, 62 (55%) provided written informed consents, and 52 (46%) ultimately completed the SCID-I/NP interview. Among screen-negative patients, 237 (44%) agreed to be contacted for a SCID-I/NP interview, and from those we selected a convenience sample of 51 who provided written informed consents and completed the SCID-I/NP interview.
FIGURE 1.
Participant Flow Diagram. †We selected a sample of patients from the 237 who agreed to be contacted to be consented for the SCID-I/NP until we had interviewed a minimum of 50 screen-negative patients. *Some participants had more than one SCID Diagnosis (e.g., a mood disorder and an anxiety disorder).
Prevalence of Minor and Major Depression and Associated Characteristics
The number of screen-positive and screen-negative patients who met diagnostic criteria for current or past or both MDD, MinD, and other nonmood disorders (i.e., anxiety disorders) based on the SCID-I/NP are in the Figure 1. The estimated crude prevalence of current, past, and current or past MinD in this population are in Table 2, as are estimates of crude prevalence for MDD, which are slightly higher.
TABLE 2.
Crude Prevalence of Minor and Major Depression in a Clinic-Based Population of Patients With Type 2 Diabetes*
| Minor depression | Major depression | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| n | Prevalence (%) | Bootstrapped 95% CI (%) | n | Prevalence (%) | Bootstrapped 95% CI (%) | |
| Current | 5 | 4.3 | 0.9–9.2 | 9 | 5.0 | 1.9–9.4 |
| Past only | 12 | 9.6 | 3.9–15.9 | 15 | 12.0 | 6.1–19.5 |
| Current or past | 17 | 13.9 | 7.7–21.2 | 24 | 17.0 | 10.1–24.8 |
CI = confidence interval.
Based on 103 patients (52 PHQ-2 screen positives and 51 PHQ-2 screen negatives) completing SCID interview.
Table 3 summarizes characteristics of the patients based on their SCID-I/NP diagnosis, irrespective of their original depression screening status. Among the SCID-I/NP examined patients, those with MinD were younger compared to those without a depressive disorder, whereas those with MDD had a shorter duration of diabetes (Table 3). In our multivariable linear regression model adjusted for age, sex, race, and diabetes duration, there was no significant difference in HbA1c between individuals with MDD vs those without a depressive disorder, or between individuals with MinD vs those without a depressive disorder.
TABLE 3.
Selected Characteristics of Patients With Type 2 Diabetes by SCID Diagnosis
| Characteristic | disorder n = 62 |
MinD (current or past) n = 17 |
MDD (current or past) n = 24 |
p Value |
|---|---|---|---|---|
| Age in years, mean ± SD | 61.5 ± 12.1 | 55.4 ± 11.4 | 57.1 ± 10.2 | 0.08 |
| Sex (%) | 0.04 | |||
| Female | 50.0 | 64.7 | 79.2 | |
| Race/ethnicity (%) | 0.67* | |||
| White | 35.5 | 23.5 | 33.3 | |
| Black | 56.4 | 76.5 | 58.3 | |
| Other | 8.1 | 0 | 8.3 | |
| SSRI antidepressant medication use (%) | 9.7 | 11.8 | 16.7 | 0.63* |
| Diabetes duration (years), median (first, third quartile) | 13.5 (5, 20) | 10 (6.5, 14) | 8 (4, 13) | 0.047† |
| Diabetes treatment (%) | ||||
| Oral agents only | 27.4 | 23.5 | 33.3 | 0.77 |
| Noninsulin injectables | 4.8 | 0 | 0 | 0.74* |
| Insulin | 64.5 | 64.7 | 54.2 | 0.65 |
| HbA1c (%), median (First, third quartile) | 8.1 (6.9, 9.5) | 8.4 (7.2, 9.6) | 7.7 (6.5, 8.7) | 0.34 |
| HbA1c (mmol/mol) | 65 (52, 80) | 68 (55, 81) | 61 (48, 72) | |
HbA1c =hemoglobin A1c; MDD =major depressive disorder; SCID =Selected Structured Clinical Interview for DSM; SD =standard deviation; SSRI = selective serotonin reuptake inhibitor.
Note: χ2 tests were used to compare categorical variables (unless noted otherwise); linear models were used to compare least square means; and the Kruskal-Wallis test was used to compare medians.
Fisher exact test was used for group comparisons with small cell sizes.
Statistically significant difference between patients with no disorder vs those with MDD.
Prevalence of Coexisting Anxiety Disorders
During the SCID-I/NP interviews, we found that a significant number of participants (26%; n = 27) had anxiety disorders, which were most frequent among those with MDD and MinD (62.5% and 42.2%, respectively) compared to those with no depressive disorder (8.1%; Table 4). Participants with MDD were significantly more likely to also have generalized anxiety disorder and posttraumatic stress disorder compared to those with MinD or no depressive disorder. Those with MinD were significantly more likely to also have phobias compared to those with MDD or no depressive disorder (Table 4).
TABLE 4.
Prevalence of Coexisting Anxiety Diagnoses by SCID in Patients With Type 2 Diabetes by Depression Diagnosis
| Anxiety diagnosis | No disorder n = 62 |
MinD (current or past) n = 17 |
MDD (current or past) n = 24 |
p Value |
|---|---|---|---|---|
| Any anxiety disorder | 8.1% (n = 5) | 42.2% (n = 7) | 62.5% (n = 15)* | <0.0001 |
| Generalized anxiety disorder | 0% (n = 0) | 0% (n = 0) | 20.8% (n = 5) | 0.0002 |
| Posttraumatic stress disorder | 0% (n = 0) | 5.9% (n = 1) | 37.5% (n = 9) | <0.0001 |
| Phobias | 3.2% (n = 2) | 23.5% (n = 4) | 8.3% (n = 2) | 0.02 |
| Panic disorder | 3.2% (n = 2) | 11.8% (n = 2) | 12.5% (n = 3) | 0.21 |
| Other anxiety disorder | 0.97% (n = 1) | 0% (n = 0) | 0% (n = 0) | 0.72 |
Totally 4 participants with MDD had 2 anxiety diagnoses, where the total individuals with any anxiety diagnosis is n = 15 instead of n = 19 for number of anxiety diagnoses.
DISCUSSION
In this clinic-based population of individuals with T2DM, we estimated a lifetime MinD prevalence of 13.9% and a lifetime MDD prevalence of 17.0% based on the SCID-I/NP. The prevalence of coexisting anxiety disorders was high among individuals with MinD and MDD, with phobias being most common among those with MinD and generalized anxiety and posttraumatic stress disorders being most common among those with MDD.
The primary objective of this study was to estimate the prevalence of MinD in patients with T2DM. In general, rates of depressive disorders, assessed by psychiatric interview protocols, have ranged from 8–15% in adults with type 1 diabetes and T2DM.2,12 Only a few studies have specifically estimated the prevalence of MinD in patients with T2DM. In the Pathways Study of adults with T2DM enrolled in a health maintenance organization in western Washington State, the prevalence of MinD, based on PHQ-9 score approximating DSM-IV criteria for MinD, was 8.4%.13–15 The prevalence of MinD was similar (8.7%) when estimated using the PHQ-9 score in a Canadian population–based study of individuals with diabetes.16 Our study is the first to our knowledge to estimate MinD prevalence using a diagnostic interview. In our study, using the SCID-I/NP, we estimated a lifetime prevalence of MinD at 13.9%, similar to the prior questionnaire-based estimate. This suggests that MinD may be as prevalent as MDD in individuals with T2DM.
As seen in prior studies, we found both types of depression disorders to be more common among women. Although prior studies have shown glycemic control to be worse among those with MDD compared to no depression,17 in this clinic-based population, HbA1c was not higher in those with MDD or MinD. This may reflect the fact that compared to individuals with MinD, those with MDD have more frequent contact with the medical system and more opportunities for management of diabetes and other medical conditions. In the Pathways Study, HbA1c was highest in those with MDD, followed by MinD, then no depression.14 Depressive disorders are hypothesized to have a negative effect on glycemic control in those with diabetes by adversely affecting diabetes self-management behaviors or activating neuroendocrine and inflammatory pathways that induce insulin resistance and hyperglycemia.18 We also found that individuals with MDD had the shortest duration of diabetes. This is in contrast to other studies, which showed that MDD was not associated with diabetes duration13,19–23 or that it was associated with a longer diabetes duration.24–27 A recent study in elderly men with diabetes found a J-shaped association between self-reported diabetes duration and late-life depression, assessed by the Geriatric Depression Scale—the odds of depression was significantly higher in those with diabetes duration less than 10 years and greater than 20 and 30 years.28 This study supports 2 hypotheses regarding the association of both short and long diabetes duration with depression. First, depression may be associated with shorter duration of T2DM (<10 years) because patients may develop depression following their diagnosis due to the increased demand associated with disease management, as is seen in type 1 diabetes.29 Patients with depression being seen by a physician may be more likely to be screened for and diagnosed with T2DM. Second, depression may be associated with longer T2DM duration (>20 years) due to an increased likelihood of complications, which are known to be associated with diabetes,30,31 or due to adverse effects of hyperglycemia on brain regions that control mood.32
We found a high prevalence of coexisting anxiety disorders in individuals with MinD and MDD. Studies using questionnaires found a high prevalence of both depression and anxiety symptoms among individuals with T2DM.33–35 In a study of patients with type 1 diabetes and T2DM who underwent the Mini International Neuropsychiatric Interview, there was a high prevalence of both anxiety and depressive disorders,36 similar to this study. Our findings suggest that it may be beneficial to use evidence-based treatments for both MDD and anxiety disorders (e.g., SSRIs and psychotherapy) in individuals with T2DM.
In our study, 11.8% of individuals with MinD were taking SSRIs; however, studies have shown that antidepressant drugs are largely ineffective in improving symptoms in those with subthreshold and mild depression compared to placebo.37–40 Consequently, the National Institutes of Health Clinical Excellence (NICE) guidelines do not recommend routine use of antidepressants to treat subthreshold and mild depressive symptoms and instead recommend psychoeducation and low-intensive psychosocial interventions as first-line treatment.41 A meta-analysis of randomized trials examining the effects of psychologic treatments (e.g., cognitive behavior therapy, problem solving therapy, and psychoeducation) for subthreshold depression compared to placebo demonstrated short-term improvement in depressive symptoms with a trend toward reduced long-term incidence of MDD.42 None of these studies focused specifically on individuals with T2DM.
Our study has several strengths. This is the first published study that estimates the prevalence of MinD among individuals with T2DM based on a diagnostic interview. Prior studies have used the PHQ-9 or other symptom questionnaire that may not accurately identify individuals with MinD. Because we used a diagnostic interview, we were able to distinguish MinD from MDD and other non-MDD depressive disorders (i.e., dysthymia, adjustment disorder with depressed mood, and depressive disorder not otherwise specified) and estimate its crude prevalence in a clinical population of adults with T2DM.
Our study, however, has several limitations that should be considered in interpreting our findings. First, we did not administer all the SCID-I/NP modules (e.g., psychoses). As our study focused on distinguishing MinD from MDD and other disorders subsumed under “elevated depressive symptoms” (i.e., anxiety, adjustment, and mixed anxiety/depressive disorders), we chose the mood, anxiety, and adjustment disorder modules, as well as the module that included the diagnostic algorithm for MinD to make these distinctions without additional participant burden. We also excluded individuals treated with antipsychotic medications to reduce the likelihood of including individuals with psychoses or psychotic depression; however, current psychiatric practice use antipsychotics as augmentation agents for MDD treatment. Thus, our results are not generalizable to patients with MDD treated with antipsychotics, and we may have underestimated the prevalence of MDD. However, our prevalence estimates were similar to those previously reported for patients with T2DM. Second, while a population-based study would have been optimal, it would have been very costly to recruit a nonclinic-based cohort for this study where our financial resources were finite. The following were the advantages of a clinic-based sample: (1) a large volume diabetes clinical practice from which to accrue participants and (2) a mechanism in place as part of routine practice in this setting to screen for depressive symptoms. However, our findings are only generalizable to clinic-based and not population-based individuals with T2DM and we may have over-estimated the prevalence of depressive disorders. Third, we did not have resources clinically to perform the SCID-I/NP on all PHQ-2 screen-negative individuals. The screen-negative individuals that we interviewed were a convenience sample and may have been more likely to have depressive symptoms not identified by the PHQ-2, which may explain why the weighted prevalence of MinDD was higher in the screen-negative than the screen-positive group. However, because we interviewed a sample of individuals who screened negative on the PHQ-2, we were able to adjust for these screening biases in the PHQ-2 in our prevalence estimate. Future studies should interview a larger, more representative sample of screen-negative individuals to confirm our findings. Fourth, based on a priori sample size calculations, we estimated that we would need to screen 750 patients to identify 100 who screened positive for depression. We did in fact reach our target identifying 161 patients who were screened positive for depression and 112 who agreed to be consented. However, while we identified elevated depressive symptoms in nearly 23% of this clinic population, only about one-third were ultimately interviewed, reflecting the challenges in recruiting depressed individuals. The prevalence estimate relied on the assumption that the interviewed subsample was representative of the screening sample, and might thus be subject to selection bias. However, sex distributions were not different between the interviewed and not-interviewed subsamples, stratified by the screening results. Furthermore, mean PHQ-2 scores were identical among screen-positive individuals who were SCID-interviewed (4.2 ± 1.3) and those who were not SCID-interviewed (4.2 ± 1.1). Similarly, among screen-negative individuals, the mean PHQ-2 scores were very similar for those who were interviewed (0.6 ± 0.8) and those who were not (0.5 ± 0.8). Finally, while it appeared that Black patients were more likely to have MinD than White patients, the difference was not statistically significant. Thus, owing to our small sample size, we may have been underpowered to detect additional significant group differences in Table 3.
CONCLUSIONS
Despite its limitations, to our knowledge, this study provides a first estimate of the prevalence of MinD among adults with T2DM using a psychiatric interview. Given that the MDD prevalence estimates are similar to other studies of individuals with T2DM, it is likely that the MinD estimate may be generalizable as well to clinic-based settings. Like MDD, MinD is similarly prevalent in patients with T2DM, and both depressive disorders are also frequently associated with concomitant anxiety disorders in the clinical setting. Currently MinD is not included in the DSM-5; however, our data support continuing to examine patients with chronic medical conditions for MinD. Finally, our findings point to the importance of identifying effective psychotherapeutic interventions in individuals with diabetes and MinD in the clinical setting.
Acknowledgments
This study was supported by the National Institute of Diabetes, Digestive, and Kidney Diseases, United States (R03 DK088997 awarded to S.H.G.) and a Diabetes Research Center Grant, United States (P30DK079637). The funding agency did not have a role in study design; collection, analysis, or interpretation of data; writing the report; or the decision to submit the manuscript for publication.
Footnotes
Disclosure: S.H.G., N.S., M.N., F.H.B., G.S.W., N.W., S.L., and C.L. have no conflict of interest to disclose. J.L.P. does legal consulting for Eli Lilly, Pfizer, Astra Zeneca, and Johnson and Johnson and receives research support from SAGE therapeutics.
References
- 1.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes care. 2004;27(5):1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 2.Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes care. 2001;24(6):1069–1078. doi: 10.2337/diacare.24.6.1069. [DOI] [PubMed] [Google Scholar]
- 3.First MB, Frances A, Pincus HA. Diagnostic and Statistical Manual of Mental Disorders. Washington, DC: American Psychiatric Publishing, Inc; 2004. [Google Scholar]
- 4.Lin EH, Von Korff M, Alonso J, et al. Mental disorders among persons with diabetes—results from the World Mental Health Surveys. J Psychosom Res. 2008;65(6):571–580. doi: 10.1016/j.jpsychores.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nichols L, Barton PL, Glazner J, McCollum M. Diabetes, minor depression and health care utilization and expenditures: a retrospective database study. Cost Eff Resour Alloc. 2007;5:4. doi: 10.1186/1478-7547-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCollum M, Ellis SL, Regensteiner JG, Zhang W, Sullivan PW. Minor depression and health status among US adults with diabetes mellitus. Am J Manag Care. 2007;13(2):65–72. [PubMed] [Google Scholar]
- 7.Kroenke K, Spitzer RL, Williams JB. The Patient Health Questionnaire-2: validity of a two-item depression screener. Med Care. 2003;41(11):1284–1292. doi: 10.1097/01.MLR.0000093487.78664.3C. [DOI] [PubMed] [Google Scholar]
- 8.Han C, Wang SM, Kato M, et al. Second-generation antipsychotics in the treatment of major depressive disorder: current evidence. Expert Rev Neurother. 2013;13(7):851–870. doi: 10.1586/14737175.2013.811901. [DOI] [PubMed] [Google Scholar]
- 9.Taylor JK, Schoenbaum M, Katon WJ, Pincus HA, Hogan DM, Unutzer J. Strategies for identifying and channeling patients for depression care management. Am J Manag Care. 2008;14(8):497–504. [PMC free article] [PubMed] [Google Scholar]
- 10.First MB, Spitzer RL, Williams JBW, Gibbon M. Handbook of Psychiatric Measures. 1. Washington, DC: American Psychiatric Association; 2000. Structured clinical interview for DSM-IV axis I disorders (SCID-I) pp. 49–53. [Google Scholar]
- 11.First M, Gibbon M, Spitzer RL, Williams JB. User’s Guide for the Structured Clinical Interview for DSM-IV-TR Axis I disorDers—Research Version (SCID-I for DSM-IV-TR, November 2002 Revision) New York: Biometrics Research; 2002. [Google Scholar]
- 12.Pouwer F, Nefs G, Nouwen A. Adverse effects of depression on glycemic control and health outcomes in people with diabetes: a review. Endocrinol Metab Clin North Am. 2013;42(3):529–544. doi: 10.1016/j.ecl.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 13.Katon W, von Korff M, Ciechanowski P, et al. Behavioral and clinical factors associated with depression among individuals with diabetes. Diabetes Care. 2004;27(4):914–920. doi: 10.2337/diacare.27.4.914. [DOI] [PubMed] [Google Scholar]
- 14.Yu MK, Weiss NS, Ding X, Katon WJ, Zhou XH, Young BA. Associations between depressive symptoms and incident ESRD in a diabetic cohort. Clin J Am Soc Nephrol. 2014;9(5):920–928. doi: 10.2215/CJN.08670813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coleman SM, Katon W, Lin E, Von Korff M. Depression and death in diabetes; 10-year follow-up of all-cause and cause-specific mortality in a diabetic cohort. Psychosomatics. 2013;54(5):428–436. doi: 10.1016/j.psym.2013.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmitz N, Messier L, Nitka D, et al. Factors associated with disability and depressive symptoms among individuals with diabetes: a community study in Quebec. Psychosomatics. 2011;52(2):167–177. doi: 10.1016/j.psym.2010.11.020. [DOI] [PubMed] [Google Scholar]
- 17.Lustman PJ, Anderson RJ, Freedland KE, de GM, Carney RM, Clouse RE. Depression and poor glycemic control: a meta-analytic review of the literature. Diabetes Care. 2000;23(7):934–942. doi: 10.2337/diacare.23.7.934. [DOI] [PubMed] [Google Scholar]
- 18.Champaneri S, Wand GS, Malhotra SS, Casagrande SS, Golden SH. Biological basis of depression in adults with diabetes. Curr Diab Rep. 2010;10(6):396–405. doi: 10.1007/s11892-010-0148-9. [DOI] [PubMed] [Google Scholar]
- 19.Camara A, Balde NM, Enoru S, Bangoura JS, Sobngwi E, Bonnet F. Prevalence of anxiety and depression among diabetic African patients in Guinea: association with HbA1c levels. Diabetes Metab. 2015;41(1):62–68. doi: 10.1016/j.diabet.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 20.Davis TM, Hunt K, Bruce DG, et al. Prevalence of depression and its associations with cardio-metabolic control in Aboriginal and Anglo-Celt patients with type 2 diabetes: the Fremantle Diabetes Study Phase II. Diabetes Res Clin Pract. 2015;107(3):384–391. doi: 10.1016/j.diabres.2014.12.014. [DOI] [PubMed] [Google Scholar]
- 21.Raval A, Dhanaraj E, Bhansali A, Grover S, Tiwari P. Prevalence & determinants of depression in type 2 diabetes patients in a tertiary care centre. Indian J Med Res. 2010;132:195–200. [PubMed] [Google Scholar]
- 22.Gonzalez JS, Safren SA, Cagliero E, et al. Depression, self-care, and medication adherence in type 2 diabetes: relationships across the full range of symptom severity. Diabetes Care. 2007;30(9):2222–2227. doi: 10.2337/dc07-0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Katon W, Russo J, Lin EH, et al. Depression and diabetes: factors associated with major depression at five-year follow-up. Psychosomatics. 2009;50(6):570–579. doi: 10.1176/appi.psy.50.6.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ishizawa K, Babazono T, Horiba Y, et al. The relationship between depressive symptoms and diabetic complications in elderly patients with diabetes: analysis using the Diabetes Study from the Center of Tokyo Women’s Medical University (DIACET) J Diabetes Complications. 2016 doi: 10.1016/j.jdiacomp.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 25.Ascher-Svanum H, Zagar A, Jiang D, et al. Associations between glycemic control, depressed mood, clinical depression, and diabetes distress before and after insulin initiation: an exploratory, post hoc analysis. Diabetes Ther. 2015;6(3):303–316. doi: 10.1007/s13300-015-0118-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khuwaja AK, Lalani S, Dhanani R, Azam IS, Rafique G, White F. Anxiety and depression among outpatients with type 2 diabetes: a multi-centre study of prevalence and associated factors. Diabetol Metab Syndr. 2010;2:72. doi: 10.1186/1758-5996-2-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun JC, Xu M, Lu JL, et al. Associations of depression with impaired glucose regulation, newly diagnosed diabetes and previously diagnosed diabetes in Chinese adults. Diabet Med. 2015;32(7):935–943. doi: 10.1111/dme.12649. [DOI] [PubMed] [Google Scholar]
- 28.Almeida OP, McCaul K, Hankey GJ, et al. Duration of diabetes and its association with depression in later life: the Health In Men Study (HIMS) Maturitas. 2016;86:3–9. doi: 10.1016/j.maturitas.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 29.Grey M, Cameron ME, Lipman TH, Thurber FW. Psychosocial status of children with diabetes in the first 2 years after diagnosis. Diabetes Care. 1995;18(10):1330–1336. doi: 10.2337/diacare.18.10.1330. [DOI] [PubMed] [Google Scholar]
- 30.de Groot M, Anderson R, Freedland KE, Clouse RE, Lustman PJ. Association of depression and diabetes complications: a meta-analysis. Psychosom Med. 2001;63(4):619–630. doi: 10.1097/00006842-200107000-00015. [DOI] [PubMed] [Google Scholar]
- 31.Lin EH, Rutter CM, Katon W, et al. Depression and advanced complications of diabetes: a prospective cohort study. Diabetes Care. 2010;33(2):264–269. doi: 10.2337/dc09-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ho N, Sommers MS, Lucki I. Effects of diabetes on hippocampal neurogenesis: links to cognition and depression. Neurosci Biobehav Rev. 2013;37(8):1346–1362. doi: 10.1016/j.neubiorev.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu SF, Young LS, Yeh FC, Jian YM, Cheng KC, Lee MC. Correlations among social support, depression, and anxiety in patients with type-2 diabetes. J Nurs Res. 2013;21(2):129–138. doi: 10.1097/jnr.0b013e3182921fe1. [DOI] [PubMed] [Google Scholar]
- 34.Masmoudi J, Damak R, Zouari H, et al. Prevalence and impact of anxiety and depression on type 2 diabetes in tunisian patients over sixty years old. Depress Res Treat. 2013;2013:341782. doi: 10.1155/2013/341782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Collins MM, Corcoran P, Perry IJ. Anxiety and depression symptoms in patients with diabetes. Diabet Med. 2009;26(2):153–161. doi: 10.1111/j.1464-5491.2008.02648.x. [DOI] [PubMed] [Google Scholar]
- 36.Maia AC, de Braga AA, Brouwers A, Nardi AE, Oliveira e Silva AC. Prevalence of psychiatric disorders in patients with diabetes types 1 and 2. Compr Psychiatry. 2012;53(8):1169–1173. doi: 10.1016/j.comppsych.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 37.Baumeister H. Inappropriate prescriptions of antidepressant drugs in patients with subthreshold to mild depression: time for the evidence to become practice. J Affect Disord. 2012;139(3):240–243. doi: 10.1016/j.jad.2011.05.025. [DOI] [PubMed] [Google Scholar]
- 38.Barbui C, Cipriani A, Patel V, Ayuso-Mateos JL, van Ommeren M. Efficacy of antidepressants and benzodiazepines in minor depression: systematic review and meta-analysis. Br J Psychiatry. 2011;198(1):11–16. doi: 10.1192/bjp.bp.109.076448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fournier JC, DeRubeis RJ, Hollon SD, et al. Antidepressant drug effects and depression severity a patient-level meta-analysis. J Am Med Assoc. 2010;303(1):47–53. doi: 10.1001/jama.2009.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kirsch I, Deacon BJ, Huedo-Medina TB, Scoboria A, Moore TJ, Johnson BT. Initial severity and antidepressant benefits: a meta-analysis of data submitted to the food and drug administration. PLoS Med. 2008;5(2):260–268. doi: 10.1371/journal.pmed.0050045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.NICE. Depression: The Treatment and Management of Depression in Adults (NICE Clinical Guideline 90) 2009 Available from: http://guidance.nice.org.uk/CG90.
- 42.Cuijpers P, Smit F, van Straten A. Psychological treatments of subthreshold depression: a meta-analytic review. Acta Psychiatr Scand. 2007;115(6):434–441. doi: 10.1111/j.1600-0447.2007.00998.x. [DOI] [PubMed] [Google Scholar]