Abstract
Three-dimensional (3D) in vitro models have been established to study the physiology and pathophysiology of the endometrium. With emerging evidence that the native extracellular matrix (ECM) provides appropriate cues and growth factors essential for tissue homeostasis, we describe, a novel 3D endometrium in vitro model developed from decellularized human endometrial tissue repopulated with primary endometrial cells. Analysis of the decellularized endometrium using mass spectrometry revealed an enrichment of cell adhesion molecules, cytoskeletal proteins, and ECM proteins such as collagen IV and laminin. Primary endometrial cells within the recellularized scaffolds proliferated and remained viable for an extended period of time in vitro. In order to evaluate the hormonal response of cells within the scaffolds, the recellularized scaffolds were treated with a modified 28-day hormone regimen to mimic the human menstrual cycle. At the end of 28 days, the cells within the endometrial scaffold expressed both estrogen and progesterone receptors. In addition, decidualization markers, IGFBP-1 and prolactin, were secreted upon addition of dibutyryl cyclic AMP indicative of a decidualization response. This 3D model of the endometrium provides a new experimental tool to study endometrial biology and drug testing.
Keywords: decellularization, recellularization, ECM, endometrium, primary cells
Summary Sentence
Primary endometrial cells within a recellularized scaffold respond to a 28-day menstrual cycle.
Introduction
The human endometrium responds dynamically to the ovarian steroid hormones, estrogen, and progesterone during a 28-day menstrual cycle. Despite the dynamic nature of the tissue function, few studies evaluate endometrial biology in response to transitional hormonal cues. The first 14 days of the reproductive cycle are dominated by the presence of rising levels of estrogen from the follicle. During this period, the cells within the endometrium (stromal, epithelial, and endothelial cells) proliferate [1]. Postovulation, the corpus luteum synthesizes and secretes increasing levels of progesterone which leads to cell differentiation and acquisition of a secretory phenotype in the endometrium. In the absence of fertilization, the corpus luteum regresses and progesterone levels fall leading to menstruation. This study provides characteristics of a 28-day hormonally responsive endometrial model which phenocopies these critical hallmarks of a healthy tissue.
Conventional two-dimensional (2D) cultures have been used extensively to study endometrial biology; however, they differ from 3D cultures in terms of structure, gene expression, and in vivo physiological responses [2–4]. Several 3D endometrial cell culture models have been developed to study different aspects of endometrial biology including tissue structure, cell–cell interactions, decidualization, implantation, menstruation, cancer and drug testing [5–14]. Three-dimensional cultures retain the structural polarity and differentiated function of cells, thus resembling in vivo morphology. Current 3D models of the endometrium vary from simple single-cell-type units to complex structures containing different cell types using an array of 3D scaffolds depending on the process being investigated. The types of extracellular matrix (ECM) scaffolds used for 3D cell cultures have included purified ECM molecules such as collagen and fibronectin as well as mixtures of ECM components such as matrigel. Purified ECM proteins do not recapitulate the complex mixture of proteins in the native tissue, and the undefined nature of matrigel derived from proteins secreted by mouse sarcoma cells indicates there is room for improvement for the development of a scaffold that is closer to normal tissue physiology [15, 16]. Acellular scaffolds have been used widely in the area of whole organ engineering and transplantation [17]. Several decellularization and recellularization techniques have been established in tissue engineering [18–21]. Different techniques have been comparatively used to decellularize murine uterus [22, 23]. Recently, successful decellularization, recellularization, and transplantation of uterine patches which led to successful pregnancies were shown in rats [24, 25]. However, acellular scaffolds have not been used in the construction of 3D models of the human endometrium. The aim of this study was to establish a novel 3D model of the human endometrium constructed from native ECM and primary cells that is responsive to ovarian steroid hormones.
Materials and methods
Study participants and tissue collection
Endometrial tissue samples (n = 29) were collected from premenopausal (18–40 years) women undergoing hysterectomy for benign uterine leiomyoma at Northwestern University. Human tissue acquisition was approved by the Northwestern Institutional review board for human research. Written informed consent was obtained from women included in the study. Endometrial tissues used were histologically classified as normal endometrium from either the proliferative or secretory phase by a pathologist (Table 1). Endometrial samples from women undergoing hormone therapy were excluded in this study.
Table 1.
Menstrual phase of patients.
| Experiment | Number of patients | Menstrual phase |
|---|---|---|
| Characterization of decellularized scaffolds | 8 | 3 proliferative |
| 5 secretory | ||
| DNA quantification | 3 | 1 proliferative |
| 2 secretory | ||
| Electron microscopy | 5 | Proliferative |
| Proteomics | 5 | Proliferative |
| Recellularization | ||
| Cells | 1 | Proliferative |
| Scaffolds | 1 | Proliferative |
| 28-day experiment | ||
| Cells | 3 | Proliferative |
| Scaffolds | 3 | 1 basal |
| 1 secretory | ||
| 1 proliferative | ||
Decellularization of endometrial tissue
Pieces of uterine tissue (∼1–2 cm2), obtained from the pathologist, were transported on ice to the laboratory. With the endometrial side up, tissue was sliced to 0.5-mm thin sections using a Thomas stadie riggs tissue slicer (Thermos Scientific). Only the top layer of the tissue was considered endometrium as some adjoining myometrial tissue was present. Decellularization was performed as described for the heart model with slight modifications [26]. Endometrial tissue pieces were incubated in equal parts 0.25% Triton X-100 (Fisher Scientific) and 0.25% sodium deoxycholate (Fisher scientific) in 50 ml tubes (Falcon) at 37°C for 48 h in a shaker (100rpm) followed by rinsing with Dubelcco modified Eagle medium (DMEM/F12 Gibco, NY) for 72 h at 4°C. Media was changed during the rinse cycle every day. Residual nucleic materials were subsequently removed by nuclease digestion using 100 μg/ml ribonuclease (Roche) and 150 IU/ml deoxyribonuclease (DNase I) (Sigma-Aldrich) at 37°C for 48 h. Decellularized scaffolds were washed in DMEMF/12 for additional 24 h at 4°C and stored in phosphate buffered saline (PBS) containing 1% penicillin/streptomycin (P/S).
DNA isolation and quantification
Double-stranded (ds) DNA content of three patient-matched native endometrium and decellularized scaffolds were isolated using DNeasy blood & tissue kit (QIAGEN, Germany) following the manufacturer's protocol. Isolated dsDNA was quantified using Quant-iT Picogreen dsDNA assay kit (Invitrogen, Eugene, OR, USA) according to manufacturer's instructions. Fluorescence was measured at 535 nm with excitation at 485 nm, and DNA content was quantified using a standard curve.
Electron microscopy
For Transmission electron microscopy (TEM) preparation, decellularized samples were fixed in 2% paraformaldehyde and 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer pH 7.3 and postfixed with unbuffered 2% osmium tetroxide, rinsed with distilled water, en bloc stained with 3% uranyl acetate, rinsed with distilled water, dehydrated in ascending grades of 50%, 70%, 80% 90%, and 100% ethanol, embedded in resin mixture from Embed 812 kit, and cured in a 60°C oven. Samples were sectioned on a Leica Ultracut UC6 ultramicrotome. Seventy nanometer sections were collected on 200 mesh copper grids; thin sections were stained with 3% uranyl acetate and Reynolds lead citrate. Samples were processed by the Center of Advanced Microscopy at Northwestern University Feinberg School of Medicine.
For SEM preparation, endometrial biopsies and decellularized endometrial scaffolds were fixed for at least 24 h in 10% buffered formalin at 4°C and postfixed using 1% OsO4 in 0.1 M phosphate buffer (pH 7.4) for 2 h. The specimens were then dehydrated in a graded series of ethanol (50%, 70%, 90%, and 100%), critical-point-dried with carbon dioxide (Samdri-790, Tousimis, Rockville, USA), mounted, and coated with 10 nm gold in a sputter coater. Finally, the specimens were observed under a scanning electron microscope (JCM- 6000PLUS, JEOL, Japan).
Endometrial cell isolation and expansion
The endometrium was separated from the adjoining myometrium using a dissecting microscope. The endometrial tissue was minced into 1- to 2-mm fragments and subjected to enzymatic digestion using 1.51-mg/ml collagenase I (Invitrogen) and 5-mg/ml DNase I (Sigma-Aldrich) in Hanks balanced salt solution (HBSS) (Invitrogen) in a 37°C shaker (100 rpm) for 1–2 h. After digestion, total endometrial cells (∼5 × 106) were washed and resuspended in DMEM/F12 containing 10% FBS and 1% P/S (10% DMEMF/12) plated on cell culture plates (tissue culture dish 150, TPP) and cultured at 37°C in a humidified atmosphere containing 5% CO2. Endometrial cells were allowed to expand for 5–7 days without passaging. Media was changed every second day.
Recellularization of endometrial scaffolds
Decellularized endometrial scaffolds (0.5 mm thin) were cut into 8 mm diameter pieces using a biopsy punch (Integra Miltex). These endometrial scaffolds were rinsed in culture media 10% DMEMF/12 and set up in a 24-well plate (Denville) containing 0.4-μm cell culture inserts (Millicell). One scaffold per insert was placed in each well. A minimum of three recellularized scaffolds were generated from each patient (one scaffold per treatment group). Expanded endometrial cells were trypsinized, washed and resuspended in 10% DMEMF/12. A dense suspension (∼1.0 × 106 live cells in 50 μl of culture media) of the expanded endometrial cells was seeded on the endometrial scaffold within the cell culture inserts. Cells were allowed to attach to the endometrial scaffold for 20 min before culture media supplemented with 50 μg/ml L-ascorbic acid (Sigma) was added to the 24-well plates. Ascorbic acid promotes collagen accumulation and stabilization [27, 28]. Media was changed every second day.
Immunohistochemistry and trichrome staining
Native endometrium, decellularized scaffolds and recellularized scaffolds were fixed in 10% neutral buffered formalin at 4°C overnight. After serial dehydration, the native endometrium, decellularized scaffolds and recellularized scaffolds were separately paraffin-embedded in an upright position and cut longitudinally into 5-μm sections. Histological evaluation was done with hematoxylin and eosin (H&E) using a Leica Autostainer XL (Leica Microsystems).
Immunohistochemistry was used to confirm the presence of collagen IV (1:500, Abcam), pan-laminin (1:50, Abcam) and Elastin (1:100, Abcam) and fibronectin (1:100) in native, decellularized scaffolds and recellularized scaffolds. All antibodies used for immunohistochemical (IHC) staining of ECM protein were of IgG isotype. Rabbit IgG (1:100) was used as negative control. In brief, the fixed native endometrium, decellularized or recellularized scaffold sections were deparaffinized and rehydrated by a gradient of ethanol solutions. Antigen retrieval was performed using target retrieval solution (DAKO) in a water bath (95°C–99°C) for 10 min. After peroxidase and protein blocking, antibodies were added and incubated overnight at 4°C. Secondary antibodies (DAKO) were added, and the staining was developed using the 3,3΄-diaminobenidine chromogen that was supplied in the DAKO kit for less than 30 s at room temperature. Sections were counterstained by hematoxylin for 30 s, washed until clear and then treated with lithium carbonate for 6 s. Sections were subsequently dehydrated in ethanol and xylene. Mounting media was added to each section and coverslips were placed on top. Sections were allowed to harden at room temperature. IHC staining was conducted to detect vimentin (1:1000, Dako), cytokeratin (1:500, Dako), Ki-67 (1:75, Abcam), cleaved caspase-3 (1:300, Cell Signaling), estrogen receptor (1:200, Thermo scientific), progesterone receptor (1:1600, Dako), using the Leica Bond-Max protocols. The details of primary antibodies are available in table 2.
Table 2.
Antibodies used for immunohistochemical stainings.
| Name of antibody | Manufacturer, catalog, and/or clone number | Species raised in; monoclonal or polyclonal | Titer |
|---|---|---|---|
| ER | Thermo scientific, (SP1) Rm-9101-S | Rabbit monoclonal | 1:200 |
| PR | Dako, PgR636 | Mouse monoclonal | 1:1600 |
| Ki-67 | Dako, MIB-1 | Mouse monoclonal | 1:100 |
| Cleaved Caspase-3 | Cell Signaling, 9661 | Rabbit polyclonal | 1:300 |
| Vimentin | Dako, V9 | Mouse monoclonal | 1:1000 |
| Cytokeratin | Dako, AE1/AE3 | Mouse monoclonal | 1:500 |
| Collagen IV | Abcam6586 | Rabbit polyclonal | 1:500 |
| Laminin | Abcam 11 575 | Rabbit polyclonal | 1:50 |
| Elastin | Abcam23747 | Rabbit polyclonal | 1:100 |
| Fibronectin | Abcam2413 | Rabbit monoclonal | 1:100 |
Trichrome staining (Abcam) was performed on native tissue and decellularized scaffolds according to manufacturer's instruction.
Twenty-eight-day hormone protocol
A stepwise hormone protocol was used to treat cells within the recellularized scaffolds to mimic the human 28-day menstrual cycle as previously described with some modifications [29]. Recellularized scaffolds were divided into three treatment groups. The first group received 0.1 nM E2 (day 0–7), 1 nM E2 (day 7–21), and 0.1 nM E2 (day 21–28). The second group received 0.1 nM E2 (day 0–7), 1 nM E2 (day 7–14), 1 nM E2 + 10 nM P4 (day 14–21), and 0.1 nM E2 + 50 nM P4 (day 21–28). The third group received 0.1 nM E2 (day 0–7), 1 nM E2 (day 7–14), 1 nM E2 + 10 nM P4 + 0.5 mM dibutyryl cyclic Adenosine monophosphate (AMP) (dbcAMP) (day 14–21), and 0.1n M E2 + 50 nM P4 + 0.5 mM dbcAMP (day 21–28). One recellularized scaffold per patient (n = 3) was used for each treatment. Two-thirds of media was collected and replenished every second day and stored at –20°C until use.
Proteomic analysis
Native endometrial tissues and corresponding decellularized scaffolds (containing no cells) from 3 patients in the proliferative phase were collected for proteomic analysis. Tissues were digested in lysis buffer (8 M Urea, 50 mM Tris pH 8.2, 75 mM NaCl, Halt Protease Inhibitor Cocktail) using MACS Tissue dissociator, and protein concentration was measured by BCA protein assay. Proteins were then digested in solution with trypsin and desalted using Pierce C18 Spin columns prior to analysis. Peptides were analyzed by LC-MS/MS using an EASY nanoLC and a linear ion trap—Orbitrap hybrid mass spectrometer (ThermoFisher Scientific). Proteins were identified from the MS raw files using Mascot search engine (Matrix science). MS/MS spectra were searched against the SwissProt human database. Gene ontology analysis was conducted using GeneGO.
ELISA for decidualization markers
Supernatants from the 28-day hormone treatment of recellularized scaffolds were assayed for IGFBP-1 and prolactin. Culture media was collected three times a week from each treatment group as described above and supernatants were kept at –20°C until tested. Secreted factors were measured using human insulin-like growth factor binding protein-1 ELISA kit (Alpha Diagnostics #900) and Human Prolactin ELISA kit (Alpha Diagnostics #300).
Statistical analysis
Student t-test was performed using Prism software (v5; GraphPad, San Diego, CA) analysis. P-values < 0.05 were considered significant.
Results
Characterization of decellularized endometrial scaffolds
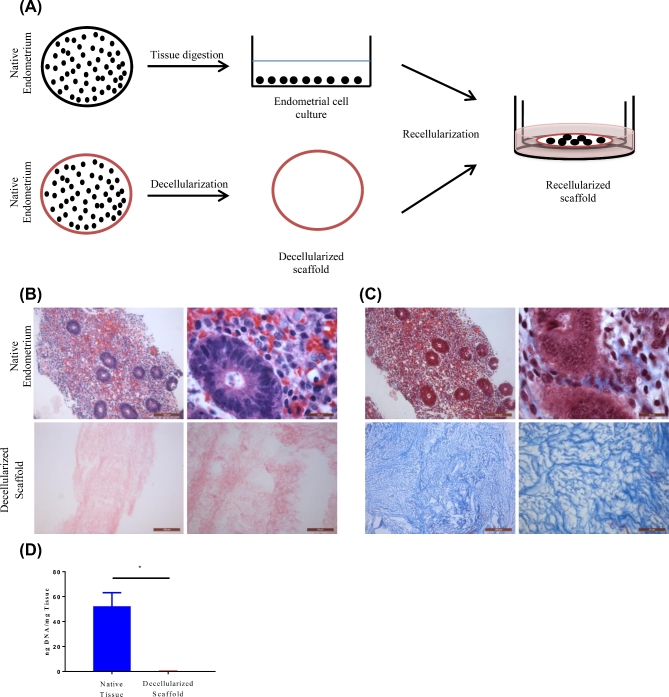
We developed a 3D model of the endometrium using decellularized endometrial scaffold and cells (see schematic diagram, Figure 1A). To generate decellularized endometrial scaffolds with high seeding susceptibility, we adapted a method first described by Rieder et al. with modifications [26]. Decellularization efficiency was determined by evaluating H&E staining, trichrome staining and quantifying dsDNA content. Compared to native endometrial tissue, decellularized scaffolds were devoid of nuclear staining as shown by H&E staining (Figure 1B). Trichrome staining which is used for the visualization of collagenous connective tissue fibers demonstrated the maintenance of these fibers despite negligible presence of cellular material (Figure 1C). Compared to the native endometrial tissue (45.4 ng DNA/mg tissue), the dsDNA content of the decellularized endometrial scaffold (0.162 ngDNA/mg tissue) was drastically reduced (Figure 1D).
Figure 1.
Diagrammatic representation of the development of the 3D recellularized endometrial culture system. Native endometrial tissue were either digested for cell isolation or prepared for decellularization. Isolated cells were allowed to expand for 5–7 days on 2D plates. Expanded endometrial cells were trypsinized and reseeded on decellularized scaffolds placed inside 12-mm-diameter inserts and cultured in a 24-well plate (A). Representative microscopic images (10× and 100×) of native human endometrium (top row) and decellularized scaffolds (bottom row). Cellular components were stained by H&E (B). ECM of native endometrium and decellularized scaffolds was stained with trichrome staining (C). DNA was isolated from matched native endometrium and decellularized scaffolds and measured n = 3 (D). Data are shown as mean ± standard deviation. *P < 0.05 error bar indicates statistically significant difference between native endometrium and decellularized scaffold. Scale bars represent 100 and 20 μm, respectively.
Native endometrium and decellularized endometrial scaffold were investigated using electron microscopy. Scanning electron microscopy (SEM) showed the luminal surface with the epithelial surface in the native endometrium (Figure 2A). SEM of the decellularized endometrial scaffolds revealed tangled collagen fibrils and transmission electron microscopy showed parallel arrays of collagen fibrils (Figure 2; Supplementary Figure S1). Both scanning and transmission electron microscopy revealed cell-free regions consisting mainly of collagen fibers (Figure 2; Supplementary Figure S1). These observations suggest an efficient decellularization of the tissue, without compromising the ECM component of the tissue.
Figure 2.
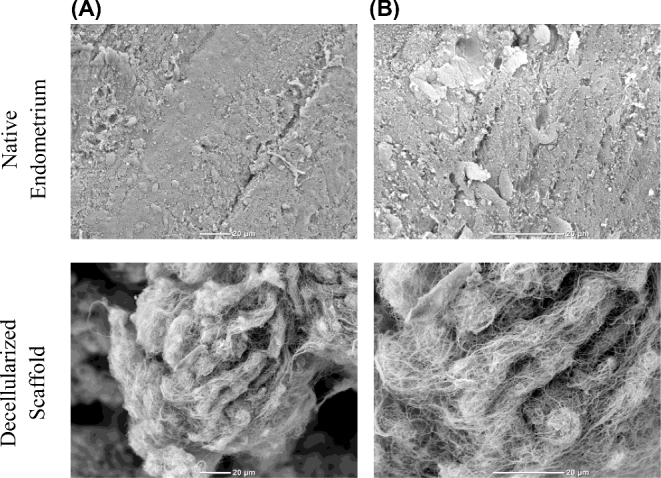
SEM images of normal endometrium and decellularized scaffolds. Native endometrial tissue or decellularized scaffold were fixed in 10% buffered formalin and processed for SEM at (A) 650× (B) 1500×. Scale bars represent 20 μm.
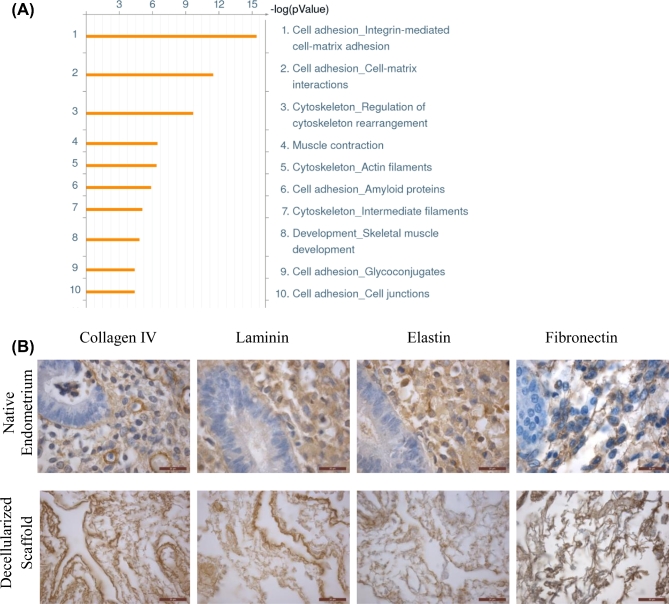
Proteomic analysis of native endometrial tissue and their corresponding decellularized endometrial scaffolds from 3 patients (proliferative phase) was performed to investigate the retention of essential ECM proteins. A total of 107 proteins were detected by mass spectrometry in the decellularized scaffolds (Supplementary Table S1). The top 10 processes identified by gene ontology analysis of the 107 proteins were those associated with cell adhesion, cell matrix, and cytoskeletal proteins (Figure 3A). The presence of collagen IV, laminin, elastin, and fibronectin was confirmed by IHC staining and found mostly localized to the stroma (Figure 3B).
Figure 3.
Proteomics analysis and immunohistochemical staining of retained ECM proteins in decellularized scaffolds. Gene ontology analysis was performed to determine the top processes enriched in the decellularized matrix from three patients (A). IHC staining IV, pan-laminin, elastin and fibronectin in native endometrium (top panel) and decellularized scaffolds (bottom panel) are shown (B). Scale bars represent 20 μm.
Recellularization of endometrial scaffolds
Endometrial cells were isolated from the tissue without separating stromal from epithelial cells. After expansion on 2D plates, cells were seeded onto decellularized scaffolds from another patient and recellularization was allowed to occur for up to 4 weeks. Recellularization of the scaffolds by endometrial cells was monitored using H&E staining (Supplementary Figure S2A). Immunohistochemical staining revealed that the cells populating the scaffolds were proliferating with minimal cell death as shown by Ki-67 and cleaved caspase-3 staining cells (Supplementary Figure S2B and C). Cells within the scaffold also expressed Estrogen receptor (ER) and Progesterone receptor (PR) (Supplementary Figure S2D and E). These data suggest that endometrial cells are capable of repopulating endometrial scaffolds and remain functional over an extended period of time in vitro.
Response to 28-day hormone treatment
To demonstrate viability and physiological response of the recellularized endometrial scaffolds to hormones, the 3D cultures were subjected to a 28-day hormone regimen (Supplementary Figure S4). Cells were allowed to recellularize the scaffolds for 2 weeks before hormone treatment. There were three hormone treatment groups: E2 only, E2+P4, and E2+P4+dbcAMP. H&E staining confirmed the presence of cells at the end of the 28-day treatment (Supplementary Figure S5). Immunohistochemical staining revealed that the cells were mostly vimentin positive with very few cytokeratin positive cells (Figure 4A and B). No endothelial or immune cells were detected as shown by the lack of CD31 and CD45 staining (Supplementary Figure S6). Next, the cells within the endometrial scaffolds were evaluated for expression of estrogen and progesterone receptors. Cells from all treatment groups expressed both ER and PR (Figure 5A and B).
Figure 4.
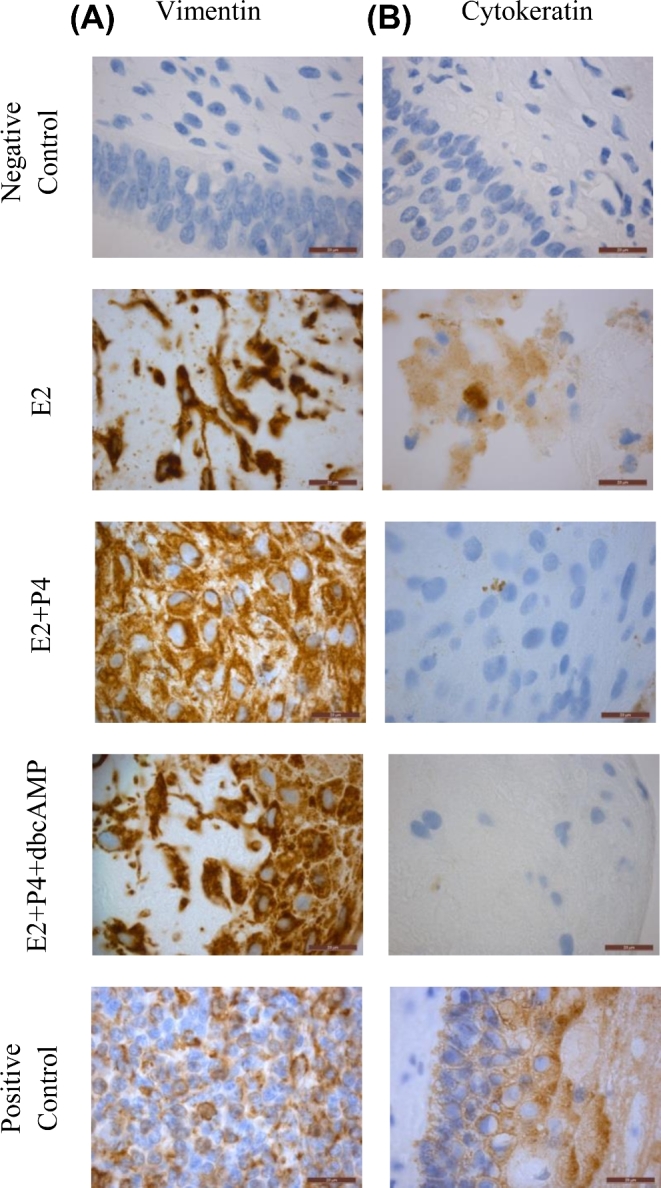
Recellularization of endometrial scaffold and treatment with a 28-day stepwise hormone protocol. At the end of the experiment, immunohistochemical staining of vimentin (A) and cytokeratin (B) was performed to confirm the presence of stromal and epithelial cells respectively. Representative images from recellularized scaffolds using cells from patients in the proliferative phase are shown (n=3). One recellularized scaffold per patient was used in each treatment group. Scale bars represent 20μm.
Figure 5.
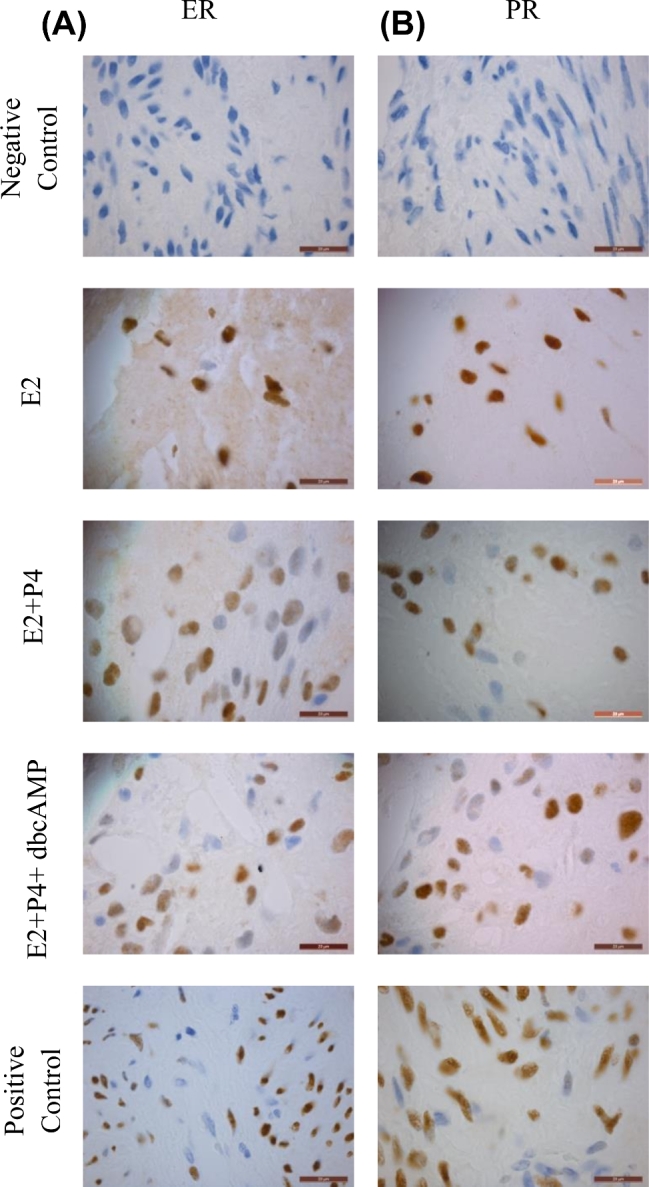
Hormone receptor expression of cells within the recellularized scaffolds after 28-day hormone treatment. Decellularized scaffold were seeded with primary cells and allowed to recellularize for 2 weeks before hormone treatment. At the end of the experiment, IHC staining was also used to determine the expression of estrogen receptor (A) and progesterone receptor (B) in the different treatment groups E2 (top row), E2+P4 (middle row), and E2+P4+dbcAMP (bottom row). Representative images from recellularized scaffolds using cells from patients in the proliferative phase are shown (n = 3). One recellularized scaffold per patient was used in each treatment group. Scale bars represent 20 μm.
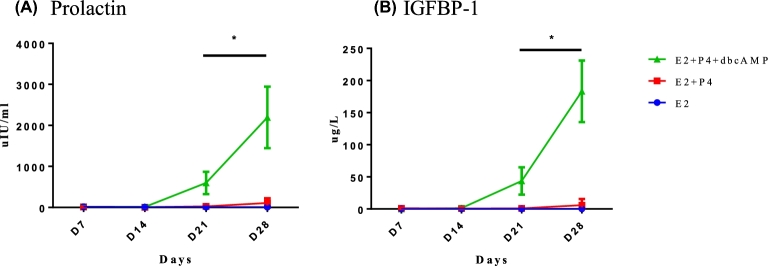
To determine if the cells within the recellularized scaffolds were able to respond to the hormone treatment, we measured levels of prolactin and IGFBP-1, which are synthesized and secreted by decidualizing stromal cells in response to long-term progesterone and enhanced upon addition of cAMP agonists. Media supernatants were measured for the presence of secreted IGFBP-1 and prolactin by ELISA. No IGFBP-1 or prolactin was detected in the supernatants from E2-only treated cultures (Figure 6A and B). Cells within the recellularized scaffold produced both prolactin (108.36 ± 114.52 μIU/ml) and IGFBP-1 (5.92 ± 9.62 μg/l) in response to E2+P4 at day 28 (Figure 6A and B). A statistically significant increase in levels of prolactin (2194.31 ± 750 μIU/ml) and IGFBP-1 (183.27 ± 47.90μg/l) was observed in supernatants from recellularized endometrium treated with E2+P4+dbcAMP at day 28 (Figure 6A and B). These data indicate that endometrial cells within the scaffold differentiate in response to hormonal stimuli.
Figure 6.
Production of decidualization markers. Supernatants of treated recellularized scaffolds were collected and measured for prolactin (A) and IGFBP-1 (B). Each data point on the graph represents the mean ± standard deviation of measurements from three different patients. One recellularized scaffold per patient (n = 3) was used in each treatment group. *P < 0.05 error bar indicates statistically significant difference between E2+P4 and E2+P4+dbcAMP treatment.
Discussion
The use of human culture systems has become fundamental to the study of the uterus as animal models and existing systems are not entirely representative of the physiologic events occurring during the human female menstrual cycle. Two-dimensional cultures of endometrial cells have generated important information but are limited to growth as monolayers and are not applicable in uterine repair or transplantation. In this study, we established a physiologically relevant 3D model of the human endometrium in which decellularized endometrial scaffold was used to support endometrial cell proliferation, hormone receptor expression, and production of secretory factors upon hormone treatment that mimics a 28-day human menstrual cycle.
The ECM provides the physical scaffold, cell adhesion molecules, and appropriate growth factors necessary for the unique biological function of the tissue [30, 31]. In the uterus, the ECM plays a fundamental role during menstruation, implantation, and parturition [32]. The decellularized endometrial ECM would provide the optimal scaffold for the construction of a 3D endometrium. We initially used sodium dodecyl sulfate to decellularize endometrial tissue; however, we were unable to recellularize these scaffolds as previously found by Rieder et al. [26]. We therefore adapted a method of decellularization previously described in porcine heart valves [26]. This method involves the combination of SDC and Triton X-100, adequate rinsing as well as ribonuclease digestion getting rid of possible viral contamination. We extended the timing of ribonuclease digestion from 24 to 48 h because we observed DAPI staining after 24 h of decellularization. After decellularization, the scaffolds were evaluated for optimal removal of cellular and nuclear content and the preservation of important ECM proteins in the endometrium. H&E staining and quantification of DNA content revealed that decellularized scaffolds had minimal cellular and nuclear content according to the guidelines established by Crapo et al. [30]. In addition, SEM of decellularized endometrial scaffolds revealed arrangement of collagen fibrils in the absence of cells.
Proteomic analysis of the decellularized scaffolds from three patients at the proliferative stage identified 107 proteins comprised of ECM proteins such as collagens and laminin, cytoskeletal proteins, cell adhesion proteins, kinases, and even histones. Limited tissue availability prevented the comparative evaluation of scaffolds from patients in the secretory phase. These proteins provide a rich scaffold for cells to settle and grow into compared to 2D cultures or the standard collagen or fibronectin gels that are used for 3D cultures. However, it is unclear how the presence of cellular proteins, including histones and kinases that were left behind from the decellularization process influence cell behavior. It would be worthwhile to compare endometrial scaffold proteins from normal versus diseased tissue as it has been shown that abnormal expression of laminin and collagen IV in the endometrium is associated with unexplained infertility [33, 34].
Recellularization of decellularized scaffold can be achieved by static or dynamic seeding [35, 36]. Dynamic seeding requires a vascular tree through which cells can be perfused into the decellularized organ. In this study using pieces of endometrial tissues, we were restricted to static seeding. Static seeding has an efficiency of about 10%–25% depending on factors such as tissue density [37]. We were able to overcome this drawback by using a tissue slicer to cut 0.5-mm thin slices of endometrial tissues before decellularization. Before recellularization, scaffolds were also cut into 8-mm (diameter) pieces using a biopsy punch to further optimize the recellularization process. We observed that upon recellularization, the scaffolds decreased in size and the initial dimensions were not maintained, potentially due to the stromal cells remodeling the tissue scaffolds. We also experienced the drawbacks of limited tissue availability, as we were unable to consistently match the scaffolds with the cells for recellularization from the same patient. Explants from proliferative phase survive longer in culture than those from the secretory phase in vitro [38]. As a result, we used mostly proliferative phase tissues for the recellularization of scaffolds. However, whether the ECM from different phases of the menstrual cycle affects the growth and hormone response of the recellularized cells requires additional investigation. Despite these limitations, IHC staining revealed that the scaffolds contained viable and proliferating stromal cells. In an attempt to optimize the growth of epithelial cells, we expanded endometrial stromal and epithelial cells separately in different media. Stromal cells were cultured in DMEMF/12 and epithelial cells in keratinocyte growth media (KGM) (data not shown). Although the KGM favored growth of epithelial cells in 2D, growth was slow and the media used for recellularization (DMEMF/12) did not support their survival or expansion within the scaffolds. It is possible that direct seeding of freshly isolated endometrial cells may encourage the survival of other cell types; however, this would severely limit the number of scaffolds that could be reseeded and the appropriate media to support growth of all cell types would have to be optimized. While stromal cells are easily expanded in culture, possess migratory and invasive behavior [39–41], primary epithelial cells cultured on plastic lose their polarity and function, and do not propagate well [42, 43]. The difficulty associated with growing primary endometrial epithelial cells has drawn researchers to use immortalized or cancer cell lines. While the use of cell lines eliminates donor variability and passage limitations of primary cells, ultimately, cell lines do not entirely represent the benign epithelial cell behavior. Self-renewing stem cells which have high proliferative and differentiative capacity would be ideal for recellularization purposes. Current attempts to reconstruct the endometrium using stem cells from different sources have been reviewed [44]. The human endometrium contains a small proportion of both stromal and epithelial progenitor cells [45]. Epithelial and stromal side populations that have characteristics of mesenchymal stem cells have also been identified in the human endometrium [46–49]. Menstrual blood also contains a clonogenic, multipotent mesenchymal stem cell population [50, 51]. Stem cells may be required to study epithelial cells in 3D models of the endometrium.
The 28-day hormone regimen that mimics the human menstrual cycle is one that is not conventionally used in vitro to study hormone responses. Recently, a 28-day model of human perivascular stroma and endothelial cells was established using fixed concentrations of oestradiol, medroxyprogesterone acetate, and cAMP [52]. The concentration of hormones used in our 28-day protocol was based on clinical data of serum levels of hormones in women during the menstrual cycle [53, 54]. Our group has previously used this treatment protocol on endocervical cells grown in a 3D polystyrene (Alvatex) scaffold [29] and primary fallopian tube explants [55] showing viability and hormone response in both tissues. One of the hallmarks of hormone response of endometrial cells is the decidualization response that becomes evident during the late secretory phase in endometrial stromal cells surrounding the spiral arteries and involves their transformation into differentiated, secretory decidual cells [56]. This process continues once there is a pregnancy until the entire endometrium becomes the decidua of pregnancy. Treatment of recellularized endometrial cells with E2 + P4 did promote production and secretion of the decidual markers, IGFBP1 and prolactin during the latter half of the hormone treatment protocol. The addition of dbcAMP amplified the production similar to cells grown in 2D [57–60] demonstrating the viability and ability to respond physiologically to hormones in the 3D system. The most widely used hormone treatment for inducing decidualization in vitro is high-dose (1 μM) medroxyprogesterone acetate for up to 10 days, at times with no estradiol priming [60, 61]. Given the increase in decidual markers in our system using our serum level based 28-day hormone protocol, which involves the natural progesterone at 10 and 50 nM, our model would be useful to study menstrual cycle changes of the endometrium in a more physiological setting compared to 2D cultures.
We describe here a 3D model of the human endometrium constructed with native ECM in which primary cells respond to a 28-day menstrual cycle hormone treatment. We have recently demonstrated similar but amplified responses of our recellularized endometrial scaffolds to a 28-day cycle driven by ovarian follicles in a microfluidic system [62]. Improvement of the 3D recellularized endometrial cultures would entail adding other cell types, including epithelial, endothelial and immune cells, to create a more complete endometrium. These cell types act together in response to ovarian hormones and play a significant role in remodeling the tissue. This would be important to understand processes such as menstruation and embryo implantation that are unique to the human species. As pathologies associated with these processes such as heavy uterine bleeding and infertility remain untreatable, it is essential that we have the appropriate model systems with which we can identify the mechanisms that drive the development of such pathologies. Our 3D recellularized endometrial constructs provide a novel system with which we can further develop to study hormone action on endometrial angiogenesis, endometrial receptivity and implantation.
Supplementary data
Supplementary data are available at BIOLRE online.
Supplementary Figure S1. Transmission electron microscopy revealed cell-free endometrial scaffolds with some cytoplasmic debris (A) 1900×. (B) Collagen fibers in decellularized endometrial scaffold (9300×).
Supplementary Figure S2. Recellularization of endometrial scaffolds at 4 weeks. (A) H&E. Immunohistochemical staining of (B) Ki-67, (C) cleaved caspase-3, (D) ER, (E) PR. Scale bar represent 50 μm.
Supplementary Figure S3. Immunohistochemical staining of ECM proteins in recellularized scaffolds. (A) control isotype, (B) collagen, (C) laminin, (D) elastin, (E) fibronectin.
Supplementary Figure S4. A diagrammatic depiction of the 28-day hormone treatment is shown. Decellularized scaffolds were seeded with primary cells and allowed to recellularize for 2 weeks before hormone treatment.
Supplementary Figure S5. H&E of all recellularized scaffolds from three patients used in the 28-day hormone treatment. Scale bar 100 μm.
Supplementary Figure S6. Immunohistochemical staining for CD31 endothelial cells and CD45 immune cells in hormone-treated recellularized endometrial scaffolds. Scale bars represent 20 μm.
Supplementary Table S1. (a) Protein list in native endometrium only (139 proteins). (b) Common protein list between native endometrium and decellularized scaffold (56 proteins). (c) Protein list in decellularized scaffold only (51 proteins).
Supplementary data are available at BIOLRE online.
Supplementary Figure S1. Transmission electron microscopy revealed cell-free endometrial scaffolds with some cytoplasmic debris (A) 1900×. (B) Collagen fibers in decellularized endometrial scaffold (9300×).
Supplementary Figure S2. Recellularization of endometrial scaffolds at 4 weeks. (A) H&E. Immunohistochemical staining of (B) Ki-67, (C) cleaved caspase-3, (D) ER, (E) PR. Scale bar represent 50 μm.
Supplementary Figure S3. Immunohistochemical staining of ECM proteins in recellularized scaffolds. (A) control isotype, (B) collagen, (C) laminin, (D) elastin, (E) fibronectin.
Supplementary Figure S4. A diagrammatic depiction of the 28-day hormone treatment is shown. Decellularized scaffolds were seeded with primary cells and allowed to recellularize for 2 weeks before hormone treatment.
Supplementary Figure S5. H&E of all recellularized scaffolds from three patients used in the 28-day hormone treatment. Scale bar 100 μm.
Supplementary Figure S6. Immunohistochemical staining for CD31 endothelial cells and CD45 immune cells in hormone-treated recellularized endometrial scaffolds. Scale bars represent 20 μm.
Supplementary Table S1. (a) Protein list in native endometrium only (139 proteins). (b) Common protein list between native endometrium and decellularized scaffold (56 proteins). (c) Protein list in decellularized scaffold only (51 proteins).
Acknowledgments
We thank Saurabh S. Malpani and Stacy Kujawa for their efforts in consenting patients, collecting tissue, and ensuring that our study remains within regulatory compliance. We would also like to thank Chanel Arnold-Murray and Keisha Barreto from the Ovarian Histology Core at Northwestern University for processing and sectioning the tissue samples.
Proteomics services were performed by the Northwestern Proteomics Core Facility, generously supported by National cancer institute (NCI) CCSG P30 CA060553 awarded to the Robert H Lurie Comprehensive Cancer Center and the National Resource for Translational and Developmental Proteomics supported by P41 GM108569.
We would like to thank Bella Shmaltsuyeva from the Human Pathology Core Facility at Northwestern University.
We appreciate the help of Paul Hoover from the Skin and Tissue Engineering Core of the NU-SDRC funded by the National Institutes of Health (NIH)/National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) (P30-AR057216–01).
We would like to thank Dr Farida Korobova and Lennell Reynolds from the Center of Advanced Microscopy at Northwestern University Feinberg School of Medicine. Imaging work was performed at the Northwestern University Center for Advanced Microscopy generously supported by NCI CCSG P30 CA060553 awarded to the Robert H Lurie Comprehensive Cancer Center.
We would like to thank Dr Carolyn Jones for her help with the interpretation of the electron microscopy images.
References
- 1. Jabbour HN, Kelly RW, Fraser HM, Critchley HO. Endocrine regulation of menstruation. Endocr Rev 2006; 27:17–46. [DOI] [PubMed] [Google Scholar]
- 2. Harma V, Virtanen J, Makela R, Happonen A, Mpindi JP, Knuuttila M, Kohonen P, Lotjonen J, Kallioniemi O, Nees M. A comprehensive panel of three-dimensional models for studies of prostate cancer growth, invasion and drug responses. PLoS One 2010; 5:e10431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chambers KF, Pearson JF, Aziz N, O’Toole P, Garrod D, Lang SH. Stroma regulates increased epithelial lateral cell adhesion in 3D culture: a role for actin/cadherin dynamics. PLoS One 2011; 6:e18796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Parrish EM, Siletz A, Xu M, Woodruff TK, Shea LD. Gene expression in mouse ovarian follicle development in vivo versus an ex vivo alginate culture system. Reproduction 2011; 142:309–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bentin-Ley U, Pedersen B, Lindenberg S, Larsen JF, Hamberger L, Horn T. Isolation and culture of human endometrial cells in a three-dimensional culture system. J Reprod Fertil 1994; 101:327–332. [DOI] [PubMed] [Google Scholar]
- 6. Akoum A, Doillon CJ, Koutsilieris M, Dompierre L, Maheux R, Villeneuve M, Bergeron J, Lemay A. Human endometrial cells cultured in a type I collagen gel. J Reprod Med 1996; 41:555–561. [PubMed] [Google Scholar]
- 7. Meng CX, Andersson KL, Bentin-Ley U, Gemzell-Danielsson K, Lalitkumar PG. Effect of levonorgestrel and mifepristone on endometrial receptivity markers in a three-dimensional human endometrial cell culture model. Fertil Steril 2009; 91:256–264. [DOI] [PubMed] [Google Scholar]
- 8. Kim MR, Park DW, Lee JH, Choi DS, Hwang KJ, Ryu HS, Min CK. Progesterone-dependent release of transforming growth factor-beta1 from epithelial cells enhances the endometrial decidualization by turning on the Smad signalling in stromal cells. Mol Hum Reprod 2005; 11:801–808. [DOI] [PubMed] [Google Scholar]
- 9. Mardon H, Grewal S, Mills K. Experimental models for investigating implantation of the human embryo. Semin Reprod Med 2007; 25:410–417. [DOI] [PubMed] [Google Scholar]
- 10. Lalitkumar PG, Lalitkumar S, Meng CX, Stavreus-Evers A, Hambiliki F, Bentin-Ley U, Gemzell-Danielsson K. Mifepristone, but not levonorgestrel, inhibits human blastocyst attachment to an in vitro endometrial three-dimensional cell culture model. Hum Reprod 2007; 22:3031–3037. [DOI] [PubMed] [Google Scholar]
- 11. Arnold JT, Kaufman DG, Seppala M, Lessey BA. Endometrial stromal cells regulate epithelial cell growth in vitro: a new co-culture model. Hum Reprod 2001; 16:836–845. [DOI] [PubMed] [Google Scholar]
- 12. Arnold JT, Lessey BA, Seppala M, Kaufman DG. Effect of normal endometrial stroma on growth and differentiation in Ishikawa endometrial adenocarcinoma cells. Cancer Res 2002; 62:79–88. [PubMed] [Google Scholar]
- 13. Park DW, Choi DS, Ryu HS, Kwon HC, Joo H, Min CK. A well-defined in vitro three-dimensional culture of human endometrium and its applicability to endometrial cancer invasion. Cancer Lett 2003; 195:185–192. [DOI] [PubMed] [Google Scholar]
- 14. Schutte SC, Taylor RN. A tissue-engineered human endometrial stroma that responds to cues for secretory differentiation, decidualization, and menstruation. Fertil Steril 2012; 97:997–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kleinman HK, Martin GR. Matrigel: basement membrane matrix with biological activity. Semin Cancer Biol 2005; 15:378–386. [DOI] [PubMed] [Google Scholar]
- 16. Burdett E, Kasper FK, Mikos AG, Ludwig JA. Engineering tumors: a tissue engineering perspective in cancer biology. Tissue Eng Part B Rev 2010; 16:351–359. [DOI] [PubMed] [Google Scholar]
- 17. Soto-Gutierrez A, Yagi H, Uygun BE, Navarro-Alvarez N, Uygun K, Kobayashi N, Yang YG, Yarmush ML. Cell delivery: from cell transplantation to organ engineering. Cell Transplant 2010; 19:655–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Keane TJ, Swinehart IT, Badylak SF. Methods of tissue decellularization used for preparation of biologic scaffolds and in vivo relevance. Methods 2015; 84:25–34. [DOI] [PubMed] [Google Scholar]
- 19. Fu RH, Wang YC, Liu SP, Shih TR, Lin HL, Chen YM, Sung JH, Lu CH, Wei JR, Wang ZW, Huang SJ, Tsai CH et al. Decellularization and recellularization technologies in tissue engineering. Cell Transplant 2014; 23:621–630. [DOI] [PubMed] [Google Scholar]
- 20. Scarritt ME, Pashos NC, Bunnell BA. A review of cellularization strategies for tissue engineering of whole organs. Front Bioeng Biotechnol 2015; 3:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wolf MT, Daly KA, Reing JE, Badylak SF. Biologic scaffold composed of skeletal muscle extracellular matrix. Biomaterials 2012; 33:2916–2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hellstrom M, El-Akouri RR, Sihlbom C, Olsson BM, Lengqvist J, Backdahl H, Johansson BR, Olausson M, Sumitran-Holgersson S, Brannstrom M. Towards the development of a bioengineered uterus: comparison of different protocols for rat uterus decellularization. Acta Biomater 2014; 10:5034–5042. [DOI] [PubMed] [Google Scholar]
- 23. Santoso EG, Yoshida K, Hirota Y, Aizawa M, Yoshino O, Kishida A, Osuga Y, Saito S, Ushida T, Furukawa KS. Application of detergents or high hydrostatic pressure as decellularization processes in uterine tissues and their subsequent effects on in vivo uterine regeneration in murine models. PLoS One 2014; 9:e103201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Miyazaki K, Maruyama T. Partial regeneration and reconstruction of the rat uterus through recellularization of a decellularized uterine matrix. Biomaterials 2014; 35:8791–8800. [DOI] [PubMed] [Google Scholar]
- 25. Hellstrom M, Moreno-Moya JM, Bandstein S, Bom E, Akouri RR, Miyazaki K, Maruyama T, Brannstrom M. Bioengineered uterine tissue supports pregnancy in a rat model. Fertil Steril 2016; 106:487–496. [DOI] [PubMed] [Google Scholar]
- 26. Rieder E, Kasimir MT, Silberhumer G, Seebacher G, Wolner E, Simon P, Weigel G. Decellularization protocols of porcine heart valves differ importantly in efficiency of cell removal and susceptibility of the matrix to recellularization with human vascular cells. J Thorac Cardiovasc Surg 2004; 127:399–405. [DOI] [PubMed] [Google Scholar]
- 27. Hata R, Senoo H. L-ascorbic acid 2-phosphate stimulates collagen accumulation, cell proliferation, and formation of a three-dimensional tissuelike substance by skin fibroblasts. J Cell Physiol 1989; 138:8–16. [DOI] [PubMed] [Google Scholar]
- 28. Anderson SM, McLean WH, Elliott RJ. The effects of ascorbic acid on collagen synthesis by cultured human skin fibroblasts. Biochem Soc Trans 1991; 19:48S. [DOI] [PubMed] [Google Scholar]
- 29. Arslan SY, Yu Y, Burdette JE, Pavone ME, Hope TJ, Woodruff TK, Kim JJ. Novel three dimensional human endocervix cultures respond to 28-day hormone treatment. Endocrinology 2015; 156:1602–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Crapo PM, Gilbert TW, Badylak SF. An overview of tissue and whole organ decellularization processes. Biomaterials 2011; 32:3233–3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Boudreau NJ. Organized living: from cell surfaces to basement membranes. Sci STKE 2003; 2003:pe34. [DOI] [PubMed] [Google Scholar]
- 32. Salamonsen LA. Tissue injury and repair in the female human reproductive tract. Reproduction 2003; 125:301–311. [DOI] [PubMed] [Google Scholar]
- 33. Bilalis DA, Klentzeris LD, Fleming S. Immunohistochemical localization of extracellular matrix proteins in luteal phase endometrium of fertile and infertile patients. Hum Reprod 1996; 11:2713–2718. [DOI] [PubMed] [Google Scholar]
- 34. Tanaka T, Wang C, Umesaki N. Remodeling of the human endometrial epithelium is regulated by laminin and type IV collagen. Int J Mol Med 2009; 23:173–180. [PubMed] [Google Scholar]
- 35. Villalona GA, Udelsman B, Duncan DR, McGillicuddy E, Sawh-Martinez RF, Hibino N, Painter C, Mirensky T, Erickson B, Shinoka T, Breuer CK. Cell-seeding techniques in vascular tissue engineering. Tissue Eng Part B Rev 2010; 16:341–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Weinand C, Xu JW, Peretti GM, Bonassar LJ, Gill TJ. Conditions affecting cell seeding onto three-dimensional scaffolds for cellular-based biodegradable implants. J Biomed Mater Res B Appl Biomater 2009; 91:80–87. [DOI] [PubMed] [Google Scholar]
- 37. Aunins JG, Woodson BA Jr., Hale TK, Wang DI. Effects of paddle impeller geometry on power input and mass transfer in small-scale animal cell culture vessels. Biotechnol Bioeng 1989; 34:1127–1132. [DOI] [PubMed] [Google Scholar]
- 38. Schafer WR, Fischer L, Roth K, Jullig AK, Stuckenschneider JE, Schwartz P, Weimer M, Orlowska-Volk M, Hanjalic-Beck A, Kranz I, Deppert WR, Zahradnik HP. Critical evaluation of human endometrial explants as an ex vivo model system: a molecular approach. Mol Hum Reprod 2011; 17:255–265. [DOI] [PubMed] [Google Scholar]
- 39. Gentilini D, Busacca M, Di Francesco S, Vignali M, Vigano P, Di Blasio AM. PI3K/Akt and ERK1/2 signalling pathways are involved in endometrial cell migration induced by 17beta-estradiol and growth factors. Mol Hum Reprod 2007; 13:317–322. [DOI] [PubMed] [Google Scholar]
- 40. Schwenke M, Knofler M, Velicky P, Weimar CH, Kruse M, Samalecos A, Wolf A, Macklon NS, Bamberger AM, Gellersen B. Control of human endometrial stromal cell motility by PDGF-BB, HB-EGF and trophoblast-secreted factors. PLoS One 2013; 8:e54336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Weimar CH, Macklon NS, Post Uiterweer ED, Brosens JJ, Gellersen B. The motile and invasive capacity of human endometrial stromal cells: implications for normal and impaired reproductive function. Hum Reprod Update 2013; 19:542–557. [DOI] [PubMed] [Google Scholar]
- 42. Mulholland J, Winterhager E, Beier HM. Changes in proteins synthesized by rabbit endometrial epithelial cells following primary culture. Cell Tissue Res 1988; 252:123–132. [DOI] [PubMed] [Google Scholar]
- 43. Li ML, Aggeler J, Farson DA, Hatier C, Hassell J, Bissell MJ. Influence of a reconstituted basement membrane and its components on casein gene expression and secretion in mouse mammary epithelial cells. Proc Natl Acad Sci USA 1987; 84:136–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gargett CE, Ye L. Endometrial reconstruction from stem cells. Fertil Steril 2012; 98:11–20. [DOI] [PubMed] [Google Scholar]
- 45. Gargett CE, Schwab KE, Zillwood RM, Nguyen HP, Wu D. Isolation and culture of epithelial progenitors and mesenchymal stem cells from human endometrium. Biol Reprod 2009; 80:1136–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Masuda H, Matsuzaki Y, Hiratsu E, Ono M, Nagashima T, Kajitani T, Arase T, Oda H, Uchida H, Asada H, Ito M, Yoshimura Y et al. Stem cell-like properties of the endometrial side population: implication in endometrial regeneration. PLoS One 2010; 5:e10387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cervello I, Gil-Sanchis C, Mas A, Delgado-Rosas F, Martinez-Conejero JA, Galan A, Martinez-Romero A, Martinez S, Navarro I, Ferro J, Horcajadas JA, Esteban FJ et al. Human endometrial side population cells exhibit genotypic, phenotypic and functional features of somatic stem cells. PLoS One 2010; 5:e10964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tsuji S, Yoshimoto M, Takahashi K, Noda Y, Nakahata T, Heike T. Side population cells contribute to the genesis of human endometrium. Fertil Steril 2008; 90:1528–1537. [DOI] [PubMed] [Google Scholar]
- 49. Kato K, Yoshimoto M, Kato K, Adachi S, Yamayoshi A, Arima T, Asanoma K, Kyo S, Nakahata T, Wake N. Characterization of side-population cells in human normal endometrium. Hum Reprod 2007; 22:1214–1223. [DOI] [PubMed] [Google Scholar]
- 50. Hida N, Nishiyama N, Miyoshi S, Kira S, Segawa K, Uyama T, Mori T, Miyado K, Ikegami Y, Cui C, Kiyono T, Kyo S et al. Novel cardiac precursor-like cells from human menstrual blood-derived mesenchymal cells. Stem Cells 2008; 26:1695–1704. [DOI] [PubMed] [Google Scholar]
- 51. Patel AN, Park E, Kuzman M, Benetti F, Silva FJ, Allickson JG. Multipotent menstrual blood stromal stem cells: isolation, characterization, and differentiation. Cell Transplant 2008; 17:303–311. [DOI] [PubMed] [Google Scholar]
- 52. Gnecco JS, Pensabene V, Li DJ, Ding T, Hui EE, Bruner-Tran KL, Osteen KG. Compartmentalized culture of perivascular stroma and endothelial cells in a microfluidic model of the human endometrium. Ann Biomed Eng 2017, DOI:10.1007/S10439-017-1795-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Martin VT, Behbehani M. Ovarian hormones and migraine headache: understanding mechanisms and pathogenesis–part 2. Headache 2006; 46:365–386. [DOI] [PubMed] [Google Scholar]
- 54. Moghissi KS, Syner FN, Evans TN. A composite picture of the menstrual cycle. Am J Obstet Gynecol 1972; 114:405–418. [DOI] [PubMed] [Google Scholar]
- 55. Zhu J, Xu Y, Rashedi AS, Pavone ME, Kim JJ, Woodruff TK, Burdette JE. Human fallopian tube epithelium co-culture with murine ovarian follicles reveals crosstalk in the reproductive cycle. Mol Hum Reprod 2016; 22:756–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Maruyama T, Yoshimura Y. Molecular and cellular mechanisms for differentiation and regeneration of the uterine endometrium. Endocr J 2008; 55:795–810. [DOI] [PubMed] [Google Scholar]
- 57. Kajihara T, Brosens JJ, Ishihara O. The role of FOXO1 in the decidual transformation of the endometrium and early pregnancy. Med Mol Morphol 2013; 46:61–68. [DOI] [PubMed] [Google Scholar]
- 58. Tanaka N, Miyazaki K, Tashiro H, Mizutani H, Okamura H. Changes in adenylyl cyclase activity in human endometrium during the menstrual cycle and in human decidua during pregnancy. J Reprod Fertil 1993; 98:33–39. [DOI] [PubMed] [Google Scholar]
- 59. Telgmann R, Gellersen B. Marker genes of decidualization: activation of the decidual prolactin gene. Hum Reprod Update 1998; 4:472–479. [DOI] [PubMed] [Google Scholar]
- 60. Gellersen B, Brosens J. Cyclic AMP and progesterone receptor cross-talk in human endometrium: a decidualizing affair. J Endocrinol 2003; 178:357–372. [DOI] [PubMed] [Google Scholar]
- 61. Takano M, Lu Z, Goto T, Fusi L, Higham J, Francis J, Withey A, Hardt J, Cloke B, Stavropoulou AV, Ishihara O, Lam EW et al. Transcriptional cross talk between the forkhead transcription factor forkhead box O1A and the progesterone receptor coordinates cell cycle regulation and differentiation in human endometrial stromal cells. Mol Endocrinol 2007; 21:2334–2349. [DOI] [PubMed] [Google Scholar]
- 62. Xiao S, Coppeta JR, Rogers HB, Isenberg BC, Zhu J, Olalekan SA, McKinnon KE, Dokic D, Rashedi AS, Haisenleder DJ, Malpani SS, Arnold-Murray CA et al. A microfluidic culture model of the human reproductive tract and 28-day menstrual cycle. Nat Comm 2017; 8:14584. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data are available at BIOLRE online.
Supplementary Figure S1. Transmission electron microscopy revealed cell-free endometrial scaffolds with some cytoplasmic debris (A) 1900×. (B) Collagen fibers in decellularized endometrial scaffold (9300×).
Supplementary Figure S2. Recellularization of endometrial scaffolds at 4 weeks. (A) H&E. Immunohistochemical staining of (B) Ki-67, (C) cleaved caspase-3, (D) ER, (E) PR. Scale bar represent 50 μm.
Supplementary Figure S3. Immunohistochemical staining of ECM proteins in recellularized scaffolds. (A) control isotype, (B) collagen, (C) laminin, (D) elastin, (E) fibronectin.
Supplementary Figure S4. A diagrammatic depiction of the 28-day hormone treatment is shown. Decellularized scaffolds were seeded with primary cells and allowed to recellularize for 2 weeks before hormone treatment.
Supplementary Figure S5. H&E of all recellularized scaffolds from three patients used in the 28-day hormone treatment. Scale bar 100 μm.
Supplementary Figure S6. Immunohistochemical staining for CD31 endothelial cells and CD45 immune cells in hormone-treated recellularized endometrial scaffolds. Scale bars represent 20 μm.
Supplementary Table S1. (a) Protein list in native endometrium only (139 proteins). (b) Common protein list between native endometrium and decellularized scaffold (56 proteins). (c) Protein list in decellularized scaffold only (51 proteins).