Abstract
Although much research has been done on repetitive transcranial magnetic stimulation (rTMS) in chronic stroke, only sparse research has been done in acute stroke despite the particularly rich potential for neuroplasticity in this stage. We attempted a preliminary clinical trial in one active, high-quality inpatient rehabilitation facility (IRF) in the -United States. But after enrolling only 4 patients in the grant period, the study was stopped because of low enrollment. The purpose of this paper is to offer a perspective describing the important physiologic rationale for including rTMS in the early phase of stroke, the reasons for our poor patient enrollment in our attempted study, and recommendations to help future studies succeed. We conclude that, if scientists and clinicians hope to enhance stroke outcomes, more attention must be directed to leveraging conventional rehabilitation with neuromodulation in the acute phase of stroke when the capacity for neuroplasticity is optimal. Difficulties with patient enrollment must be addressed by reassessing traditional inclusion and exclusion criteria. Factors that shorten patients’ length of stay in the IRF must also be reassessed at all policy-making levels to make ethical decisions that promote higher functional outcomes while retaining cost consciousness.
Stroke is a devastating disorder that affects approximately 795,000 people each year in the United States.1 One of the many possible sequelae of stroke is loss of motor function, causing stroke to be the leading cause of long-term disability.1 The motor impairment following stroke stems not only from neurons killed by the stroke but also from neurons that survive the stroke but become downregulated in excitability and dysfunctional through vasogenic edema,2 deafferentation,3 exaggerated interhemispheric inhibition,4 and learned non-use.5 In this way, people with stroke become “doubly disabled,”6 not only by the stroke itself but by secondary processes. Clinicians and scientists are then challenged to reverse these processes in patients and restore cortical excitability in remaining motor networks so that they can be recruited voluntarily to execute functional activities as best as possible.
Physical therapy and occupational therapy are known to be valuable in restoring movement following stroke but the possibility remains for further optimizing rehabilitation through the coupling of transcranial magnetic stimulation (TMS) with conventional therapy early on. It is impressive in a person with stroke that a single pulse of TMS applied externally to the head over the ipsilesional motor cortex can induce intracellular ionic currents that, depending on cortical excitability and pathway integrity, activate the corticospinal tract to elicit a muscle contraction in the paretic hand. But it is much more impressive, with strong implications for rehabilitation, that repetitive (r)TMS, coupled with hand motor training can alter cortical excitability, leading to improved voluntary recruitment of surviving but dormant primary motor cortex (M1) neurons with aftereffects that outlast the stimulation.7 Two different rTMS approaches can be used. One involves low-frequency (1 Hz) rTMS, thought to induce long-term depression (LTD)–like mechanisms8 that, when applied to contralesional M1, can reduce interhemispheric inhibition of contralesional M1 transcallosally acting on ipsilesional M1, thereby disinhibiting the latter.6 The other involves high-frequency (≥3 Hz) rTMS, which is thought to induce long-term potentiation (LTP)–like mechanisms9 that, when applied to ipsilesional M1, can facilitate excitability.6
Meta-analyses of rTMS to promote motor recovery in chronic stroke have shown mixed results, including benefits10 and no benefits.11 Consistent with these mixed results, we found hand function benefits in children with chronic stroke12 but not in adults with chronic stroke13 after receiving 6-Hz primed, 1-Hz rTMS to contralesional M1. In the adults, we did observe that there were distinct responders and nonresponders, with responders characterized by greater preservation of the posterior limb of the internal capsule and less clinical depression. But beyond differences in patient characteristics, another determinant affecting responsiveness is the acuteness of the stroke with its accompanying time-dependent endogenous processes that likely contribute to the criticality of early compared with delayed initiation of rehabilitation in promoting recovery.14
Early-Stage Recovery Processes
Cerebral Edema
Cerebral edema within the brain parenchyma early after stroke can lead to increased intracranial pressure and secondary neuronal dysfunction.2 Plasier et al15 showed in control animals experiencing 1 hour of middle cerebral artery occlusion followed by reperfusion that cerebral edema was elevated at 24 hours and 3 days post-reperfusion but then became significantly reduced at 7 days post-reperfusion. They observed a corresponding increase in behavioral function at 7 days. Although infarct size also reduced at 7 days, confounding the definitiveness of the mechanism for the improved behavioral function, the prevailing thought is that early resolution of cerebral edema contributes to early functional recovery.16
Angiogenesis
Angiogenesis is known to contribute to the restoration process following stroke. Although new vessel formation, with its obvious conveyance of energy and nutrients to cells, is too delayed to prevent the initial ischemia-induced neuronal cell death,17 it also has the less recognized but important function of conveying neuroblasts from the embryonic-rich subventricular zone to the stroke margins. New vessels spawned by neural injury produce vascular endothelial growth factor, brain-derived neurotrophic factor, and other growth factors that promote the adoption of neuroblasts into the cerebral tissue and synaptogenesis.18 Thus, angiogenesis is intimately linked to neurogenesis to form a “neurovascular niche” that sustains neuronal circuits in the first weeks after stroke.19 Indeed, Moisan et al20 studied microvascular plasticity at 3, 7, 16, and 25 days after a 90-minute middle cerebral artery occlusion in rats and, consistent with potent changes occurring early after stroke, they found increasing vascular density throughout the time course but then a slowdown at day 25. As a caution, although stroke-induced neurogenesis in laboratory animals18,21 and its favorable relationship to sensorimotor recovery are tempting to project to humans, it must be understood that knowledge of adult neurogenesis in humans with stroke is still in its infancy.22
Axonal Sprouting
Axonal sprouting is a key process in forming new synapses as a restorative process following stroke in animals and likely in humans. Growth associated protein (GAP)-43 is increased in the peri-infarct cortex and plays an important role in inducing axonal sprouting, which leads to remapping of connections of the somatosensory peri-infarct cortex23 and is correlated with functional recovery following stroke in rats.24 Notably, related to the importance of time course, GAP-43 levels in rats increase in this region from 3 to 14 days after stroke, but then levels decline.25
Gamma-aminobutyric Acid Type A Inhibition
Gamma-aminobutyric acid type A (GABAA) is an inhibitory neurotransmitter known to have a suppressive effect on both cortical excitability and neuroplasticity.26 But soon after stroke onset, GABAA expression decreases, which creates an environment conducive to neuroplasticity.27 GABAA, which mediates chloride transport into neurons causing hyperpolarization, has 2 sources: (i) intracellular processes involving vesicular release from GABAergic cells and (ii) extracellular processes involving reversal of GABA uptake in postsynaptic cells.28 Particularly the latter is important in creating a circulating extracellular tonic inhibitory current that affects extrasynaptic GABAA receptors, thereby lowering the overall membrane potential of the neuron and its propensity to fire. The greater the tonic GABA current, the more hyperpolarized the cell becomes and less likely to fire in response to an excitatory stimulus; the smaller the tonic GABA current, the more depolarized the cell becomes and more likely to fire.29
Modulation of GABA levels has been shown to be important for motor learning in healthy humans and for motor recovery from stroke in both animals and humans. Floyer-Lea et al30 used MR spectroscopy to quantify changes in GABA concentration in healthy humans in the sensorimotor cortex contralateral to the hand performing a motor learning task. They found that 30 minutes of learning significantly reduced GABA levels. Combined with other evidence, they emphasized that short-term modulation of cortical inhibition may be an important facilitatory mechanism for long-term potentiation-like activity critical to motor cortex plasticity during motor skill learning.
In rats with induced stroke, Schiene et al31 found increased excitability in the ipsilesional hemisphere compared with controls during paired-pulse TMS, which is the definitive test for intracortical inhibition (ICI)32 and reflective of GABAergic inhibitory activity.33 This finding was in accord with their autoradiography finding of reduced GABAA receptor expression. Importantly, they found that the increased excitability peaked at 3–7 days poststroke, after which it receded.
In humans with acute stroke, Liepert et al34 demonstrated reduced ICI in the ipsilesional hemisphere compared with healthy controls, which they attributed to reduced GABAergic inhibitory activity early on, but they did not study the subsequent time course. Manganotti et al35 evaluated ICI in people 5–7, 30, and 90 days poststroke. Consistent with decreased GABAergic activity early on, they found in patients with good recovery reduced ICI in the ipsilesional hemisphere at 5–7 and 30 days, but by 90 days it had normalized. In contrast, for patients with poor motor recovery, ICI remained low throughout the 90-day assessment. They suggested that reduced inhibition (ie, disinhibition) recorded over the ipsilesional hemisphere may have an immediate role in facilitating motor outputs and functional reorganization of the cortex. Cicinelli et al36 found similar results for the ipsilesional hemisphere in patients 20–42 days poststroke and speculated that reduced GABA activity occurred to promote cortical plasticity and higher motor recovery. The importance of GABA levels as a factor in stroke recovery is further underlined by Lazar et al,37,38 who showed that administration of the GABA agonist midazolam in people with stroke whose clinical deficits had substantially improved caused their deficits to transiently reemerge.
To be clear, the critical time frames mentioned in the stroke recovery processes above pertain predominantly to animal models, and it is impossible to extrapolate with precision the corresponding critical time frames in humans. But despite this extrapolation inexactness, there appears to be a uniqueness in the biology of the very early stage of stroke recovery that deserves special consideration and action in humans.
Need for Acute-Stage rTMS Research
All of the above endogenous processes likely contribute to the phenomenon of spontaneous recovery following stroke. But so far, there is no evidence indicating that rTMS is more efficacious directly because of the presence of these processes. Nonetheless, the evidence of an altered environment in the early stage of stroke that is particularly conducive to promoting neuroplasticity and recovery is undeniable.19,26,39-42 Importantly then, it is not only logical but imperative to explore rTMS, with its LTD- and LTP-like capabilities,8,9 specifically during this crucial time of heightened plasticity to capitalize on possible synergistic interactions that could lead to amplified aftereffects43 and improved quality of life. Heretofore, stroke rehabilitation research in this critical window of opportunity has been inordinately small, and rehabilitation science has been justifiably criticized for this neglect.44-47
But thus far, 6 studies have explored rTMS as an add-on to customary physical therapy in acute stroke and all have shown beneficial effects. Khedr et al48 showed in 52 patients (5–10 days poststroke) that 3-Hz ipsilesional rTMS produced significantly greater improvements in stroke disability scales compared with sham rTMS. Similarly, Khedr et al49 demonstrated in 48 patients (5–15 days poststroke) that those receiving either 3- or 10-Hz ipsilesional rTMS experienced significantly greater recovery in motor function on the hemiplegic side compared with sham rTMS. Conforto et al50 showed in 30 patients (5–45 days poststroke) that 1-Hz contralesional rTMS produced significantly greater improvements on the Jebsen-Taylor test and in pinch force than sham rTMS. Khedr et al6 found in 36 patients (7–20 days poststroke) that both 1-Hz contralesional rTMS and 3-Hz ipsilesional rTMS, applied separately to different groups, produced significantly greater improvements in grip strength and in keyboard and pegboard tasks than sham rTMS; further, those receiving 1-Hz outperformed those receiving 3 Hz. However, Sasaki et al51 found in 29 patients (6–29 days poststroke) that only 10-Hz ipsilesional rTMS produced significantly greater gains in grip strength and tapping frequency compared with sham rTMS, whereas 1-Hz contralesional rTMS did not. Sasaki et al52 compared 10-Hz contralesional rTMS with the combination of 10-Hz ipsilesional rTMS alternated with 1-Hz contralesional rTMS (ie, bilateral rTMS) in 58 patients (<15 days poststroke) and found that those receiving bilateral rTMS had significantly greater improvement in Brunnstrom Recovery Stage than those receiving the 10-Hz rTMS alone.
These studies are encouraging for adopting rTMS as an adjunct to conventional therapy in the acute phase of stroke, but much still remains unknown on the safety, optimal dosing parameters, long-term effects, characteristics of responders versus nonresponders, effectiveness of rTMS given by clinicians rather than researchers, etc. Accordingly, we attempted to explore the efficacy of 6-Hz primed low-frequency rTMS applied by trained clinicians to contralesional M1 in adults with acute stroke. But at the end of the 18-month grant period, which included 9 months of patient recruitment following the 9 months needed to gain all regulatory approvals, we stopped the trial because sufficient patient enrollment was not feasible with our inclusion and exclusion criteria. Others have also reported this difficulty in acute stroke research.53,54 So, rather than dismiss this as a failed effort, the purpose of this paper is to present a perspective on our patient enrollment problems and suggest strategies for future rTMS studies.
Study Overview
The intent was to use this preliminary trial to acquire data that would lead to a more largely funded multicenter trial. We sought to enroll 34 patients with acute stroke, operationally defined to be within 30 days post-ictus,44 and randomize them to receive either active- or sham-primed rTMS13 combined with conventional therapy. We conducted the study at a single site because of the limited budget with our small grant and the availability of only one rTMS device. The site we chose was Courage Kenny Rehabilitation Institute in Minneapolis, Minnesota because of its strong clinical reputation as an inpatient rehabilitation facility (IRF), commitment to research, and high volume of stroke admissions. Admission statistics for the preceding year indicated that 210 people with stroke were admitted, which we considered to be a satisfactory admission rate to conduct the trial.
Part of our feasibility question was whether clinicians, as opposed to researchers, could apply rTMS safely in the real-world acute rehabilitation setting following training. Thus, 2 physical therapists were trained by university investigators (J.C. and T.K.). An experienced physical therapist reviewed the medical records for each newly admitted patient with stroke and physically screened patients passing the records-review phase. Inclusion criteria were: >18 years of age, single unilateral stroke of 5–30 days duration post-onset, inability to voluntarily extend fingers of the paretic hand with the same range and speed as the nonparetic hand, and length of stay (LOS) >7 days. Exclusion criteria were hemorrhagic stroke, seizure within 2 years, metal in head (dental permitted), pregnancy, clinical depression, receiving tricyclic antidepressants or neuroleptics, global aphasia, neglect, Mini-Mental State Examination55 score <22, and any indwelling medical device. Eligible patients were then examined by a physician who gave final approval for participation.
Nonproblematic Factors
Regulatory bodies
We acknowledge that approval from regulatory bodies was labor intensive. Reapproval of our existing Investigational Device Exemption from the FDA was needed. Also, approvals were needed from 2 separate internal review boards, one for the university spearheading the study and one for the IRF where the study was conducted. Ultimately, however, these approvals were obtained and the processes, although time-consuming, were not the main problem.
Patient volume
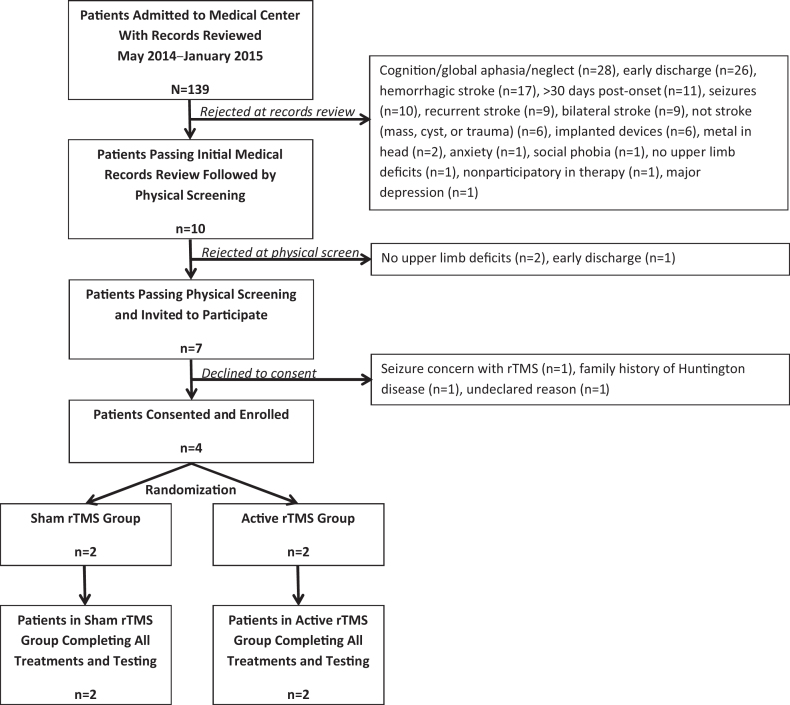
From May 2014 to January 2015, 139 patients were considered but 132 patients were rejected for not meeting enrollment criteria (Figure). With this rate of IRF admission, we deemed that overall patient volume was not a problem. Three patients declined participation, with reasons cited in the figure, but only one expressed concern about seizure as a possible adverse event, and so we did not consider patient or family reaction to the declared risks of rTMS as a problem. Physician approval was also not a problem, as the physician approved all patients who were recommended by the therapist who reviewed the records and screened the patients. Ultimately, at the conclusion of the 18-month study, only 4 patients were enrolled. All 4 finished the study, so we do not consider patient aversion to rTMS following their first exposure to it to be a problem.
Figure.
Consort flow diagram showing numbers of patients recruited and excluded and reasons for exclusion. rTMS=repetitive transcranial magnetic stimulation.
Safety
There were no adverse events, and the performance of the trained clinical therapists who administered the rTMS was proficient and posed no problem. The study was reviewed at the end by the institution's Data and Safety Monitoring Board, which deemed that there were no safety concerns. Ultimately, we consider the main problem to be the overly conservative inclusion/exclusion criteria that we set, as detailed next.
Inclusion/Exclusion Problems and Recommendations
Cognition and depression
Twenty-eight (20.1%) patients were excluded due to medical record review revealing impaired cognition, global aphasia, or neglect. We set these as exclusion criteria for 2 reasons: (i) to protect the patients’ right to understand the procedures they would receive, and (ii) to prevent cognitive impairments from confounding behavioral test results. In this category, exclusion could occur by the screening therapist at the medical record review or by the testing therapist at the physical examination. The screening therapist was authorized to exclude patients who, in her judgment upon reading the physician admission/referral report, considerably lacked the mental capacity to understand commands or their body image. No objective criteria were used, only narrative comments in the medical record based on the experience of a physician or a therapist who had recently seen the patient. Those approved for participation by the screening therapist were then screened at the physical examination by the testing therapist for cognition using the Mini-Mental State Examination,55 and all passed. But, in retrospect, these criteria should not be absolute contraindications, as rTMS has been used safely on patients with these conditions.56-61 We could have had an authorized surrogate consent on these patients’ behalf,62 thereby allowing them the right to possibly benefit from the study. Regarding confounding test results, these conditions have ranges of impairment, and medical record review alone could have excluded some patients with moderate cognitive impairment who possibly could have performed our behavioral tests with validity and possibly benefited from the rTMS. Further, the narrative comment could have been written earlier in time or in a strongly medicated state that did not represent the patient's true cognitive state at the time of possible entry into the study. Thus, we recommend that all patients receive a physical screen, and not just a medical record screen, to determine the magnitude of cognitive impairments as they specifically relate to the planned behavioral tests to avert the loss of possibly acceptable patients who could conceivably benefit from the intervention.
Clinical depression, although a common problem following stroke63 that can hinder recovery,64 was not a recruitment problem in this study. Only one patient was excluded at the medical record review because of a prior physician diagnosis of major medical depression. We did further assess depression in those passing the medical record screen using the Beck Depression Inventory65 and all achieved a score of ≤10, signifying no major medical depression.66
Length of stay
Twenty-seven (19.4%) patients were excluded because their LOS was less than the required 7 days. Seven days were needed for the combined pretest day, 5 treatment days, and a posttest day. It is relevant that LOS in early IRFs in the United States is declining for patients with stroke, attributable to the US prospective payment system.67 Of the 6 studies on rTMS for acute stroke motor recovery mentioned earlier,6,48-52 none was done in the United States. Indeed, Stuart et al68 found that the average LOS was 40 days longer in a high reputation rehabilitation hospital in Switzerland compared with a high reputation rehabilitation hospital in the United States, leading them to suggest that Europe offers greater rehabilitation research opportunities than the United States. Although shorter LOS following stroke has been debated from cost containment,69 functional outcomes,70 and ethical principles,71 its impact on curtailing innovative rehabilitation research in acute stroke must also be recognized.
An important recommendation here, as emphasized earlier by Stein,71 is to make institutional, regional, and national policymakers aware of the growing imperfections in the current payment system and the need for change, specifically with relation to LOS. We do not contend that longer LOS should be allowed simply to conduct more research trials. Rather, longer LOS should be justified by evidence. Importantly, substantial evidence already exists. As discussed above, there is growing and compelling animal research on early-stage stroke biology that enriches the capacity for neuroplasticity. Also, there are numerous studies showing the importance of beginning stroke rehabilitation in the acute stage compared with the chronic stage to achieve higher recovery.14,72-77 And specific to LOS, studies have shown that shortening LOS is positively associated with declining Functional Independence Measurement scores at discharge.69,70,78 Furthermore, Camicia et al79 showed that after stratifying patients with stroke into mild, moderate, and severely impaired groups, a longer LOS was positively associated with discharge to the community, as opposed to an institution, but only in the severely impaired group. They expressed that this reinforces the importance of providing more intensive rehabilitation to those who need it most and that longer LOS may not have a significant impact on higher functioning patients. Thus, we do not advocate for longer LOS categorically for all patients with stroke but for those who show high need and corresponding potential.
Although increasing LOS should be tied to existing evidence, research cannot be totally dismissed from this argument. Without future research in acute stroke, rehabilitation will be destined to be non-innovative and suboptimal. Therefore, a secondary benefit of increasing LOS for appropriate patients is that valuable research could be done leading to new interventions that hopefully would yield higher quality of life in patients and possibly reduced long-term care costs. Realistically, even with successful advocacy, the complicated sociopolitical forces that influence the health care payment system move slowly and so we recommend that research grant agencies should also be approached and encouraged to allow budgeting to compensate IRFs for allowing longer LOS in experimental trials. This could include funding to allow for stays in General Clinical Research Centers.
Lastly, we do not consider it to be reasonable to conduct acute stroke clinical trials at transitional or long-term care centers, to where acute patients are frequently discharged, as such centers are widely distributed and do not hold the concentrated numbers of research-eligible patients that are needed.
Recurrent or bilateral stroke
Eighteen (13.0%) patients were excluded because of recurrent or bilateral stroke. In the purest scientific sense, excluding such patients is logical to promote sample homogeneity and avoid possible confounding results from different lesions in the same person. But, in the practical sense, we now consider these exclusions overly restrictive and nonrealistic if it is agreed that research in acute stroke is needed, especially since the incidence of new lesions after the initial stroke occurs as commonly as 26.4% in the first week.80 Rather than categorically rejecting these patients, we contend that it would be legitimate to include them in pretest/posttest designs and evaluate their unique characteristics (eg, bilateral stroke) a posteriori for possible outlier status and responder versus nonresponder correlates.
Hemorrhagic stroke
Seventeen (12.2%) patients were excluded because of hemorrhagic stroke. Hemorrhagic stroke was excluded because of its increased risk for seizure. In a multicenter study that included 2021 patients with stroke, Bladin et al81 reported a 1-year actuarial seizure risk of 20% for hemorrhagic stroke and 14% for ischemic stroke (P = .002). However, in hindsight, we contend that hemorrhagic stroke should not be an absolute contraindication for rTMS, as there is no evidence indicating that hemorrhagic stroke shows a predilection for an rTMS-induced seizure. A series of rTMS studies82-85 have been conducted in chronic stroke that included a total 141 people of the hemorrhagic type and no seizures were reported.
Whether it is safe to use rTMS for acute hemorrhagic stroke is still not known. However, in a meta-analysis review on post-stroke seizures, Zhang et al86 reported that for those with hemorrhagic stroke who experienced a seizure, 50%–70% occurred within 24 hours of stroke onset and 90% occurred within the first 3 days. For our study, we required that patients be no less than 5 days post-onset to increase medical stability for all patients. This same waiting period doubly serves to postpone rTMS until the most critical time for seizure in hemorrhagic stroke is passed. Wei et al87 found that despite patients with hemorrhagic stroke recovering faster initially compared with patients with ischemic stroke, at 12 months patients with ischemic stroke were twice as likely to have a more favorable outcome compared with hemorrhagic stroke. Thus, despite the greater risk of seizure in hemorrhagic stroke, these patients have early advantages for recovery and deserve the same opportunity to benefit from rTMS as those with ischemic stroke to promote higher long-term outcomes. Still, for acute patients who have had a seizure soon after their stroke, we believe that because of their high risk for having recurrent seizures,81 the risk/benefit ratio becomes shifted to unacceptable risk and they should be excluded until long-term stability with no seizures has been established.
Admission >30 days poststroke
Eleven (8.0%) patients were excluded because they were transferred to our IRF from another facility more than 30 days poststroke. We originally considered expanding the stroke duration allowance to 60 days, which would have increased enrollment. However, we defend an upper limit of 30 days to specifically study the possible advantage of rTMS intervention early on when the endogenous processes cited above are most operative.
Implanted cardiac devices
Six (4.3%) patients were excluded because of implanted cardiac devices, but it is not certain that rTMS applied to the top of the head interferes with these devices. Rossi et al88 consider TMS to be safe with cardiac pacemakers if the TMS coil is not near the neck or chest. As the trend for pacemaker implantations continues to ramp upward,89 the likelihood for people with stroke already having such a device will also increase. Consequently, research is needed specifically to confirm the safety of rTMS in people with cardiac pacemakers so as to not unnecessarily deny them the possible benefit of rTMS in their recovery from stroke.
Overall Assessment
We concur with the assessment by Stinear et al44 that more clinical trials need to be done in the early phase of stroke to (i) capitalize on the unique physiological conditions in that phase, (ii) facilitate the eventual translation of such research into mainstream clinical use, and (iii) add evidence as to which rehabilitation interventions should be implemented early on after stroke, as not necessarily all interventions may be appropriate at this phase.
We advocate for judicious loosening of the inclusion/exclusion criteria in future acute stroke clinical trials so that more patients can be studied (Table), but we recognize that there are potential pitfalls as well. We acknowledge that by including more patients who traditionally would be excluded from clinical studies, less homogeneity across patients would be introduced, possibly triggering greater variability in results and lower statistical power in achieving significant findings. Still, for acute stroke, with all its uniqueness yet minimal researching, we contend that this is important to do in the early exploration of innovative interventions to get a preliminary perspective on safety, feasibility, and efficacy. Thereafter, as data emerge and scientific questions become more focused, enrollment criteria may need to become more restrictive.
Table.
Recommended Criteria for Enrolling Adults With Acute Stroke in Trials with Repetitive Transcranial Magnetic Stimulation (rTMS) to Promote Motor Recovery
| Criteria | Recommendation | Rationale |
|---|---|---|
| Age | Include ≥ 18 y | Inclusivity |
| Stroke type | Include ischemic and hemorrhagic. | Hemorrhagic has higher risk for seizure81 but not observed with rTMS.82-85 |
| Include single and recurrent. | Recurrent silent strokes occur more frequently than recognized80 and are difficult to avoid. | |
| Stroke location | Include unilateral and bilateral. | Bilateral confounds general analysis but can be managed with introspective analysis. |
| Include cortical, subcortical, or brainstem. | Variation in location unavoidably confounds general analysis but can be managed with introspective analysis. | |
| Stroke duration | Exclude poststroke onset <5 days and >30 days. | Immediately post stroke could possibly impinge on excitotoxicity cascade. Waiting 5 days avoids the period when seizures are most common in hemorrhagic stroke.86 Waiting >30 days could exclude possible advantages of early-stage recovery mechanisms. |
| Motor function | Exclude no visible impairment. Include both no and partial active movement. | No impairment cannot benefit. |
| Both no and partial active movement could benefit and be stratified for analysis. | ||
| Cognition | Include impaired cognition, all aphasias, and neglect. | Inclusivity. Consent can be obtained from surrogate. These patients have received rTMS in past.56-61 |
| Length of stay | Exclude if anticipated stay is less than total days for treatment and testing. | Early discharge would eliminate patient's data. Efforts to -increase length of stay must occur. |
| Seizure history | Exclude seizures within past 2 y (even if on antiseizure -medication). | Cortical stability needs to be affirmed before introducing -intervention with seizure risk. |
| Metal in head | Exclude most metals. | Risk of disrupting metal with magnetic stimulation. |
| Include dental metal. | ||
| Indwelling medical device | Exclude until specific indwelling device is tested and shown to be unaffected. | Possible disruption of device. Risk of rTMS in presence of cardiac pacemaker and other devices must be determined rather than assumed. |
| Pregnancy | Exclude. | Unknown risks to fetus. |
| Depression | Exclude score >10 on Beck Depression Inventory.66 | Depression associated with poor recovery from stroke.64 |
| Medications | Exclude tricyclic antidepressants or neuroleptics. | Increased risk of seizures or other brain dysfunction. |
As noted earlier, it is concerning that no rTMS trials in acute stroke have been done in the United States. Regardless of whether this void stems from its unique prospective payment system, federal grant priorities, or other factors, the United States has responsibility for a leadership role in optimizing stroke rehabilitation outcomes, beginning in the potently plastic acute phase, to advance the science of stroke recovery worldwide. All stakeholders, including grant agencies, legislators, patient advocacy organizations, clinicians, researchers, and the general public, must take notice and work synergistically to correct this void.
We conclude that if scientists and clinicians hope to enhance stroke outcomes, more attention must be directed to leveraging conventional rehabilitation with neuromodulation in the acute phase of stroke when the capacity for neuroplasticity is optimal. Patient enrollment must be expanded by reassessing traditional inclusion and exclusion criteria. Factors influencing LOS in the IRF must also be reassessed at all policymaking levels to make ethical decisions that maximize functional rehabilitation outcomes while retaining cost consciousness.
Author Contributions
Concept/idea/research design: J.R. Carey, D.M. Chappuis, M.J. Finkelstein, L.K. Leuty, L.I.E. Oddsson, T.J. Kimberley
Writing: J.R. Carey, M.J. Finkelstein, T.J. Kimberley
Data collection: J.R. Carey, D.M. Chappuis, K.L. Frost, L.K. Leuty, A.L. McNulty, E.M. Seifert, T.J. Kimberley
Data analysis: J.R. Carey, M.J. Finkelstein
Project management: J.R. Carey, D.M. Chappuis, L.I.E. Oddsson
Fund procurement: J.R. Carey
Participants: D.M. Chappuis, E.M. Seifert
Facilities/equipment: D.M. Chappuis, L.I.E. Oddsson
Institutional liaisons: D.M. Chappuis, L.I.E. Oddsson
Clerical/secretarial support: E.M. Seifert
Consultation (including review of manuscript before submitting): D.M. Chappuis, M.J. Finkelstein, K.L. Frost, L.K. Leuty, L.I.E. Oddsson, E.M. Seifert, T.J. Kimberley
Funding
This research was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health Award Number UL1TR000114. The funding source had no involvement in the design, data collection, analysis, interpretation, or writing in this study.
References
- 1. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Després J-P, Fullerton HJ. Heart disease and stroke statistics—2016 update a report from the american heart association. Circulation. 2015:CIR. 0000000000000350. [DOI] [PubMed] [Google Scholar]
- 2. Brouns R, De Deyn P. The complexity of neurobiological processes in acute ischemic stroke. Clin Neurol Neurosurg. 2009, 111:483–495. [DOI] [PubMed] [Google Scholar]
- 3. Classen J, Schnitzler A, Binkofski F, Werhahn KJ, Kim YS, Kessler KR, Benecke R. The motor syndrome associated with exaggerated inhibition within the primary motor cortex of patients with hemiparetic. Brain. 1997, 120:605–619. [DOI] [PubMed] [Google Scholar]
- 4. Murase N, Duque J, Mazzocchio R, Cohen LG. Influence of interhemispheric interactions on motor function in chronic stroke. Ann Neurol. 2004, 55:400–409. [DOI] [PubMed] [Google Scholar]
- 5. Taub E, Crago J, Burgio L, Groomes T, Cook EI, DeLuca S. An operant approach to rehabilitation medicine: overcoming learned nonuse by shaping. J Exp Anal Behav. 1994, 61:281–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Khedr EM, Abdel-Fadeil MR, Farghali A, Qaid M. Role of 1 and 3 Hz repetitive transcranial magnetic stimulation on motor function recovery after acute ischaemic stroke. Euro J Neurol. 2009, 16:1323–1330. [DOI] [PubMed] [Google Scholar]
- 7. Chang WH, Kim Y-H, Yoo W-K, Goo K-H, Park C-H, Kim ST, Pascual-Leone A. rTMS with motor training modulates cortico-basal ganglia-thalamocortical circuits in stroke patients. Restor Neurol Neurosci. 2012, 30:179–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen R, Classen J, Gerloff C, Celnik P, Wassermann EM, Hallett M, Cohen LG. Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology. 1997, 48:1398–1403. [DOI] [PubMed] [Google Scholar]
- 9. Pascual-Leone A, Valls-Sole J, Wassermann E, Hallett M. Responses to rapid-rate transcranial magnetic stimulation of the human motor cortex. Brain. 1994, 117:847–858. [DOI] [PubMed] [Google Scholar]
- 10. Hsu W-Y, Cheng C-H, Liao K-K, Lee IH, Lin Y-Y. Effects of repetitive transcranial magnetic stimulation on motor functions in patients with stroke: a meta-analysis. Stroke. 2012, 43:1849–1857. [DOI] [PubMed] [Google Scholar]
- 11. Hao Z, Wang D, Zeng Y, Liu M. Repetitive transcranial magnetic stimulation for improving function after stroke. Cochrane Database Syst Rev. 2013, 5:CD008862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gillick BT, Krach LE, Feyma T, Rich TL, Moberg K, Thomas W, Cassidy JM, Menk J, Carey JR. Primed low-frequency repetitive transcranial magnetic stimulation and constraint-induced movement therapy in pediatric hemiparesis: a randomized controlled trial. Dev Med Child Neurol. 2013, 56:44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Carey JR, Deng H, Gillick BT, Cassidy JM, Anderson DC, Zhang L, Al e Serial treatments of primed low-frequency rtms in stroke: characteristics of responders vs. Nonresponders. Restor Neurol Neurosci. 2014, 32:323–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Maulden SA, Gassaway J, Horn SD, Smout RJ, DeJong G. Timing of initiation of rehabilitation after stroke. Arch Phys Med Rehabil. 2005, 86:34–40. [DOI] [PubMed] [Google Scholar]
- 15. Plaisier F, Bastide M, Ouk T, Pétrault O, Laprais M, Stolc S, Bordet R. Stobadine-induced hastening of sensorimotor recovery after focal ischemia/reperfusion is associated with cerebrovascular protection. Brain Res. 2008, 1208:240–249. [DOI] [PubMed] [Google Scholar]
- 16. Jolkkonen J, Jokivarsi K, Laitinen T, Grohn O. Subacute hemorrhage and resolution of edema in rose bengal stroke model in rats coincides with improved sensorimotor functions. Neurosci Lett. 2007, 428:99–102. [DOI] [PubMed] [Google Scholar]
- 17. Hermann DM, Zechariah A. Implications of vascular endothelial growth factor for postischemic neurovascular remodeling. J Cereb Blood Flow Metab. 2009, 29:1620–1643. [DOI] [PubMed] [Google Scholar]
- 18. Chopp M, Zhang ZG, Jiang Q. Neurogenesis, angiogenesis, and MRI indices of functional recovery from stroke. Stroke. 2007, 38:827–831. [DOI] [PubMed] [Google Scholar]
- 19. Ohab JJ, Fleming S, Blesch A, Carmichael ST. A neurovascular niche for neurogenesis after stroke. Journal of Neuroscience. 2006, 26:13007–13016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Moisan A, Favre IM, Rome C, Grillon E, Naegele B, Barbieux M, De Fraipont F, Richard M-J, Barbier EL, Remy C, Detante O. Microvascular plasticity after experimental stroke: a molecular and mri study. Cerebrovas Dis. 2014, 38:344–353. [DOI] [PubMed] [Google Scholar]
- 21. Ohab JJ, Carmichael ST. Poststroke neurogenesis: emerging principles of migration and localization of immature neurons. Neuroscientist. 2008, 14:369–380. [DOI] [PubMed] [Google Scholar]
- 22. Merson TD, Bourne JA. Endogenous neurogenesis following ischaemic brain injury: insights for therapeutic strategies. Int J Biochem Cell Biol. 2014, 56:4–19. [DOI] [PubMed] [Google Scholar]
- 23. Carmichael ST. Plasticity of cortical projections after stroke. Neuroscientist. 2003, 9:64–75. [DOI] [PubMed] [Google Scholar]
- 24. Dijkhuizen RM, Singhal AB, Mandeville JB, Wu O, Halpern EF, Finklestein SP, Rosen BR, Lo EH. Correlation between brain reorganization, ischemic damage, and neurologic status after transient focal cerebral ischemia in rats: a functional magnetic resonance imaging study. J Neurosci. 2003, 23:510–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stroemer RP, Kent TA, Hulsebosch CE. Neocortical neural sprouting, synaptogenesis, and behavioral recovery after neocortical infarction in rats. Stroke. 1995, 26:2135–2144. [DOI] [PubMed] [Google Scholar]
- 26. Clarkson AN, Huang BS, Macisaac SE, Mody I, Carmichael ST. Reducing excessive gaba-mediated tonic inhibition promotes functional recovery after stroke. Nature. 2010, 468:305–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Huynh W, Vucic S, Krishnan AV, Lin CSY, Hornberger M, Kiernan MC. Longitudinal plasticity across the neural axis in acute stroke. Neurorehabil Neural Repair. 2013, 27:219–229. [DOI] [PubMed] [Google Scholar]
- 28. Glykys J, Mody I. Activation of GABA a receptors: views from outside the synaptic cleft. Neuron. 2007, 56:763–770. [DOI] [PubMed] [Google Scholar]
- 29. Carmichael ST. Brain excitability in stroke: the yin and yang of stroke progression. Arch Neurol. 2012, 69:161–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Floyer-Lea A, Wylezinska M, Kincses T, Matthews PM. Rapid modulation of gaba concentration in human sensorimotor cortex during motor learning. J Neurophysiol. 2006, 95:1639–1644. [DOI] [PubMed] [Google Scholar]
- 31. Schiene K, Bruehl C, Zilles K, Qü M, Hagemann G, Kraemer M, Witte OW. Neuronal hyperexcitability and reduction of gabaa-receptor expression in the surround of cerebral photothrombosis. J Cereb Blood Flow Metab. 1996, 16:906–914. [DOI] [PubMed] [Google Scholar]
- 32. Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Wroe S, Asselman P, Marsden CD. Corticocortical inhibition in human motor cortex. J Physiol. 1993, 471:501–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ziemann U, Rothwell JC, Ridding MC. Interaction between intracortical inhibition and facilitation in human motor cortex. J Physiol. 1996, 496:873–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Liepert J, Storch P, Fritsch A, Weiller C. Motor cortex disinhibition in acute stroke. Clin Neurophysiol. 2000, 111:671–676. [DOI] [PubMed] [Google Scholar]
- 35. Manganotti P, Acler M, Zanette G, Smania N, Fiaschi A. Motor cortical disinhibition during early and late recovery after stroke. Neurorehabil Neural Repair. 2008, 22:396–403. [DOI] [PubMed] [Google Scholar]
- 36. Cicinelli P, Pasqualetti P, Zaccagnini M, Traversa R, Oliveri M, Rossini PM. Interhemispheric asymmetries of motor cortex excitability in the postacute stroke stage - a paired-pulse transcranial magnetic stimulation study. Stroke. 2003, 34:2653–2658. [DOI] [PubMed] [Google Scholar]
- 37. Lazar RM, Fitzsimmons B-F, Marshall RS, Berman MF, Bustillo MA, Young WL, Mohr J, Shah J, Robinson JV. Reemergence of stroke deficits with midazolam challenge. Stroke. 2002, 33:283–285. [DOI] [PubMed] [Google Scholar]
- 38. Lazar RM, Berman MF, Festa JR, Geller AE, Matejovsky TG, Marshall RS. GABAergic but not anti-cholinergic agents re-induce clinical deficits after stroke. J Neurol Sci. 2010, 292:72–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Carmichael ST. Cellular and molecular mechanisms of neural repair after stroke: making waves. Ann Neurol. 2006, 59:735–742. [DOI] [PubMed] [Google Scholar]
- 40. Murphy TH, Corbett D. Plasticity during stroke recovery: from synapse to behaviour. Nat Rev Neurosci. 2009, 10:861–872. [DOI] [PubMed] [Google Scholar]
- 41. Cramer SC, Chopp M. Recovery recapitulates ontogeny. Trends Neurosci. 2000, 23:265–271. [DOI] [PubMed] [Google Scholar]
- 42. Felling RJ, Song H. Epigenetic mechanisms of neuroplasticity and the implications for stroke recovery. Exp Neurol. 2015, 268:37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Stinear CM, Byblow WD. Predicting and accelerating motor recovery after stroke. Curr Opin Neurol. 2014, 27:624–630. [DOI] [PubMed] [Google Scholar]
- 44. Stinear C, Ackerley S, Byblow W. Rehabilitation is initiated early after stroke, but most motor rehabilitation trials are not: a systematic review. Stroke. 2013, 44:2039–2045. [DOI] [PubMed] [Google Scholar]
- 45. Krakauer JW, Carmichael ST, Corbett D, Wittenberg GF. Getting neurorehabilitation right what can be learned from animal models? Neurorehabil Neural Repair. 2012, 26:923–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kwakkel G, Kollen B, Lindeman E. Understanding the pattern of functional recovery after stroke: facts and theories. Restor Neurol Neurosci. 2004, 22:281–299. [PubMed] [Google Scholar]
- 47. Gresham GE. Stroke outcome research. Stroke. 1986, 17:358–360. [DOI] [PubMed] [Google Scholar]
- 48. Khedr EM, Ahmed MA, Fathy N, Rothwell JC. Therapeutic trial of repetitive transcranial magnetic stimulation after acute ischemic stroke. Neurology. 2005, 65:466–468. [DOI] [PubMed] [Google Scholar]
- 49. Khedr EM, Etraby AE, Hemeda M, Nasef AM, Razek AAE. Long-term effect of repetitive transcranial magnetic stimulation on motor function recovery after acute ischemic stroke. Acta Neurol Scand. 2010, 121:30–37. [DOI] [PubMed] [Google Scholar]
- 50. Conforto A, Anjos S, Saposnik G, Mello E, Nagaya E, Santos W, Ferreiro K, Melo E, Reis F, Scaff M, Cohen L. Transcranial magnetic stimulation in mild to severe hemiparesis early after stroke: a proof of principle and novel approach to improve motor function. J Neurol. 2012, 259:1399–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sasaki N, Mizutani S, Kakuda W, Abo M. Comparison of the effects of high- and low-frequency repetitive transcranial magnetic stimulation on upper limb hemiparesis in the early phase of stroke. J Stroke Cerebrovasc Dis. 2013, 22:413–418. [DOI] [PubMed] [Google Scholar]
- 52. Sasaki N, Kakuda W, Abo M. Bilateral high- and low-frequency rtms in acute stroke patients with hemiparesis: a comparative study with unilateral high-frequency rtms. Brain Injury. 2014, 28:1682–1686. [DOI] [PubMed] [Google Scholar]
- 53. Anjos SM, Cohen LG, Sterr A, de Andrade KN, Conforto AB. Translational neurorehabilitation research in the third world what barriers to trial participation can teach us. Stroke. 2014, 45:1495–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Elkins JS, Khatabi T, Fung L, Rootenberg J, Johnston SC. Recruiting subjects for acute stroke trials a meta-analysis. Stroke. 2006, 37:123–128. [DOI] [PubMed] [Google Scholar]
- 55. Folstein M, Folstein S, McHugh P. “Mini-mental state:” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975, 12:189–198. [DOI] [PubMed] [Google Scholar]
- 56. Heiss W-D, Hartmann A, Rubi-Fessen I, Anglade C, Kracht L, Kessler J, Weiduschat N, Rommel T, Thiel A. Noninvasive brain stimulation for treatment of right- and left-handed poststroke aphasics. Cerebrovasc Dis. 2013, 36:363–372. [DOI] [PubMed] [Google Scholar]
- 57. Naeser MA, Martin PI, Nicholas M, Baker EH, Seekins H, Helm-Estabrooks N, Cayer-Meade C, Kobayashi M, Theoret H, Fregni F, Tormos JM, Kurland J, Doron KW, Pascual-Leone A. Improved naming after tms treatments in a chronic, global aphasia patient–case report. Neurocase. 2005, 11:182–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kim BR, Chun MH, Kim D-Y, Lee SJ. Effect of high- and low-frequency repetitive transcranial magnetic stimulation on visuospatial neglect in patients with acute stroke: a double-blind, sham-controlled trial. Arch Phys Med Rehabil. 2013, 94:803–807. [DOI] [PubMed] [Google Scholar]
- 59. Cazzoli D, Muri RM, Hess CW, Nyffeler T. Treatment of hemispatial neglect by means of rTMS–a review. Restor Neurol Neurosci. 2010, 28:499–510. [DOI] [PubMed] [Google Scholar]
- 60. Devi G, Voss HU, Levine D, Abrassart D, Heier L, Halper J, Martin L, Lowe S. Open-label, short-term, repetitive transcranial magnetic stimulation in patients with Alzheimer's disease with functional imaging correlates and literature review. Am J Alzheimers Dis Other Demen. 2014, 29:248–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Rabey JM, Dobronevsky E, Aichenbaum S, Gonen O, Marton RG, Khaigrekht M. Repetitive transcranial magnetic stimulation combined with cognitive training is a safe and effective modality for the treatment of alzheimer's disease: a randomized, double-blind study. J Neural Transm (Vienna). 2013, 120:813–819. [DOI] [PubMed] [Google Scholar]
- 62. Rincon F, Lee K. Ethical considerations in consenting critically ill patients for bedside clinical care and research. J Intensive Care Med. 2015, 30:141–150. [DOI] [PubMed] [Google Scholar]
- 63. Fei K, Benn EKT, Negron R, Arniella G, Tuhrim S, Horowitz CR. Prevalence of depression among stroke survivors: racial-ethnic differences. Stroke. 2016, 47:512–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Gainotti G, Antonucci G, Marra C, Paolucci S. Relation between depression after stroke, antidepressant therapy, and functional recovery. J Neurol Neurosurg Psychiatry. 2001, 71:258–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Beck A, Steer R, Brown G. Beck Depression Inventory: Second Edition Manual. San Antonio, TX: The Psychological Corporation; 1996. [Google Scholar]
- 66. Berg A, Palomaki H, Lehtihalmes M, Lonnqvist J, Kaste M. Poststroke depression: an 18-month follow-up. Stroke. 2003, 34:138–143. [DOI] [PubMed] [Google Scholar]
- 67. O’Brien S. Trends in inpatient rehabilitation stroke outcomes before and after advent of the prospective payment system: a systematic review. J Neurol Phys Ther. 2010, 34:17–23. [DOI] [PubMed] [Google Scholar]
- 68. Stuart M, Ryser C, Levitt A, Beer S, Kesselring J, Chard S, Weinrich M. Stroke rehabilitation in Switzerland versus the United States: a preliminary comparison. Neurorehabil Neural Repair. 2005, 19:139–147. [DOI] [PubMed] [Google Scholar]
- 69. Dobrez D, Heinemann AW, Deutsch A, Manheim L, Mallinson T. Impact of medicare's prospective payment system for inpatient rehabilitation facilities on stroke patient outcomes. Am J Phys Med Rehabil. 2010, 89:198–204. [DOI] [PubMed] [Google Scholar]
- 70. O’Brien SR, Xue Y, Ingersoll G, Kelly A. Shorter length of stay is associated with worse functional outcomes for medicare beneficiaries with stroke. Phys Ther. 2013, 93:1592–1602. [DOI] [PubMed] [Google Scholar]
- 71. Stein J. Ethical issues in inpatient rehabilitation length of stay determination. Top Stroke Rehabil. 2012, 19:86–92. [DOI] [PubMed] [Google Scholar]
- 72. Cifu DX, Stewart DG. Factors affecting functional outcome after stroke: a critical review of rehabilitation interventions. Arch Phys Med Rehabil. 1999, 80:S35–S39. [DOI] [PubMed] [Google Scholar]
- 73. Novack TA, Satterfield WT, Lyons K, Kolski G, Hackmeyer L, Connor M. Stroke onset and rehabilitation: time lag as a factor in treatment outcome. Arch Phys Med Rehabil. 1984, 65:316–319. [PubMed] [Google Scholar]
- 74. Ottenbacher KJ, Jannell S. The results of clinical trials in stroke rehabilitation research. Arch Neurol. 1993, 50:37–44. [DOI] [PubMed] [Google Scholar]
- 75. Paolucci S, Antonucci G, Grasso MG, Morelli D, Troisi E, Coiro P, Bragoni M. Early versus delayed inpatient stroke rehabilitation: a matched comparison conducted in Italy. Arch Phys Med Rehabil. 2000, 81:695–700. [DOI] [PubMed] [Google Scholar]
- 76. Smith ME, Garraway WM, Smith DL, Akhtar AJ. Therapy impact on functional outcome in a controlled trial of stroke rehabilitation. Arch Phys Med Rehabil. 1982, 63:21–24. [PubMed] [Google Scholar]
- 77. Speach DP, Dombovy ML. Recovery from stroke: rehabilitation. Baillieres Clin Neurol. 1995, 4:317–338. [PubMed] [Google Scholar]
- 78. Gillen R, Tennen H, McKee T. The impact of the inpatient rehabilitation facility prospective payment system on stroke program outcomes. Am J Phys Med Rehabil. 2007, 86:356–363. [DOI] [PubMed] [Google Scholar]
- 79. Camicia M, Wang H, DiVita M, Mix J, Niewczyk P. Length of stay at inpatient rehabilitation facility and stroke patient outcomes. Rehab Nurs. 2015, 41:78–90. [DOI] [PubMed] [Google Scholar]
- 80. Nolte CH, Albach FN, Heuschmann PU, Brunecker P, Villringer K, Endres M, Fiebach JB. Silent new DWI lesions within the first week after stroke. Cerebrovasc Dis. 2012, 33:248–254. [DOI] [PubMed] [Google Scholar]
- 81. Bladin CF, Alexandrov AV, Bellavance A, Bornstein N, Chambers B, Coté R, Lebrun L, Pirisi A, Norris JW. Seizures after stroke: a prospective multicenter study. Arch Neurol. 2000, 57:1617–1622. [DOI] [PubMed] [Google Scholar]
- 82. Kakuda W, Abo M, Shimizu M et al. ; NEURO Investigators A multi-center study on low-frequency rTMS combined with intensive occupational therapy for upper limb hemiparesis in post-stroke patients. J Neuroeng Rehabil. 2012, 9:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Kakuda W, Abo M, Kobayashi K, Momosaki R, Yokoi A, Fukuda A, Ito H, Tominaga A. Combination treatment of low-frequency rTMS and occupational therapy with levodopa administration: an intensive neurorehabilitative approach for upper limb hemiparesis after stroke. Int J Neurosci. 2011, 121:373–378. [DOI] [PubMed] [Google Scholar]
- 84. Kakuda W, Abo M, Kobayashi K, Momosaki R, Yokoi A, Fukuda A, Ito H, Tominaga A, Umemori T, Kameda Y. Anti-spastic effect of low-frequency rTMS applied with occupational therapy in post-stroke patients with upper limb hemiparesis. Brain Inj. 2011, 25:496–502. [DOI] [PubMed] [Google Scholar]
- 85. Kakuda W, Abo M, Kobayashi K, Momosaki R, Yokoi A, Fukuda A, Umemori T. Application of combined 6-Hz primed low-frequency rTMS and intensive occupational therapy for upper limb hemiparesis after stroke. Neurorehabilitation. 2011, 29:365–371. [DOI] [PubMed] [Google Scholar]
- 86. Zhang C, Wang X, Wang Y, J-g Zhang, Hu W, Ge M, Zhang K, Shao X. Risk factors for post-stroke seizures: a systematic review and meta-analysis. Epilepsy Res. 2014, 108:1806–1816. [DOI] [PubMed] [Google Scholar]
- 87. Wei JW, Heeley EL, Wang J-G, Huang Y, Wong LKS, Li Z, Heritier S, Arima H, Anderson CS, China QI. Comparison of recovery patterns and prognostic indicators for ischemic and hemorrhagic stroke in China: the ChinaQUEST (Quality Evaluation of Stroke Care and Treatment) registry study. Stroke. 2010, 41:1877–1883. [DOI] [PubMed] [Google Scholar]
- 88. Rossi S, Hallett M, Rossini PM, Pascual-Leone A; Safety of TMS Consensus Group Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009, 120:2008–2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Boriani G, Berti E, Belotti LMB, Biffi M, Carboni A, Bandini A, Casali E, Tomasi C, Toselli T, Baraldi P. Cardiac resynchronization therapy: implant rates, temporal trends and relationships with heart failure epidemiology. J Cardiovasc Med. 2014, 15:147–154. [DOI] [PubMed] [Google Scholar]