Abstract
Technology for preserving sperm is useful for disseminating valuable male genetic traits. Cold storage is suitable for easily transporting sperm as an alternative to the shipment of live animals. However, there is a technical limitation in that the fertility of cold-stored sperm declines within 3 days. To overcome this problem, we examined the protective effects of quercetin and dimethyl sulfoxide (DMSO). DMSO and quercetin maintained the fertility and motility of cold-stored sperm for 10 days. In addition, quercetin attenuated the reduction of mitochondrial membrane potential of cold-stored sperm during sperm preincubation, allowing the induction of capacitation, and it localized to the midpiece of sperm. Furthermore, DMSO and quercetin enhanced the level of tyrosine phosphorylation of cold-stored sperm. DMSO and quercetin have life-prolonging effects on sperm during cold storage. Cold storage using DMSO and quercetin will provide a robust system for internationally transporting valuable sperm samples.
Keywords: sperm, cold storage, quercetin, dimethyl sulfoxide, mitochondria, tyrosine phosphorylation
Summary Sentence
Cold storage of mouse sperm with dimethyl sulfoxide and quercetin achieved the longest period to produce two-cell embryos by in vitro fertilization.
Introduction
Assisted reproductive technology is a useful tool in the fields of medicine, veterinary, agriculture, and biotechnology. Improving techniques for preserving reproductive cells is an important way of enhancing their quality and applicability. In 1949, Polge discovered the cryoprotective effect of glycerol (a permeable cryoprotectant) on sperm [1]. An important discovery of a compound that can protect sperm at low temperatures established the modern theory of sperm cryobiology.
Successful reports have been published regarding mouse sperm cryopreservation using an optimized cryoprotectant composed of raffinose pentahydrate (a nonpermeable reagent) and skim milk with small amounts of l-glutamine or monothioglycerol [2–4]. Using sperm cryopreservation, mouse repositories have been established to enhance the availability of high-quality resources for genetically engineered mice for biomedical research in North America, Europe, Asia, and Oceania [5–7]. The archived resources are searchable via the website of International Mouse Strain Resource (http://www.findmice.org/) [5]. In the mouse repositories, the archived sperm of genetically modified mice can be efficiently reanimated via in vitro fertilization (IVF) and embryo transfer [8]. However, the transportation of cryopreserved sperm requires the maintenance of an ultra-low temperature in a special container with dry ice or a dry shipper, and the quality of sperm samples must be ensured to successfully reanimate the mice. The technical limitations of material shipment represent a critical barrier to establishing seamless access to mouse resources.
To overcome these problems, transporting unfrozen sperm at refrigerated temperatures is a useful technique for transferring the genetics of genetically engineered mice. In general, mouse sperm is vulnerable to hypothermic stress, under which its fertility quickly declines within 24 h [9]. Recently, we reported that the motility and fertility of sperm could be maintained for 3 days via the cold storage of the mouse cauda epididymis [10–12]. The shipment of cold-stored sperm of genetically engineered mice is available for short-distance transport of mice. However, to establish a global network of mouse resources using cold-stored sperm, a longer-term storage method for mouse sperm is needed.
The preservation solution is a critical factor that determines the motility and fertility of sperm stored at refrigerated temperatures. Lifor perfusion medium (Lifor), which is a cold storage solution for human organs, was more effective for prolonging the storage period of mouse sperm than paraffin oil, M2 medium, or CPS-1 [10, 13]. To enhance the protective ability of preservation medium, we focused on the natural compound quercetin, which is present in the xylem parenchyma cells of boreal hardwood species, which can survive at subfreezing temperatures [14]. Previous studies indicated that quercetin alleviated oxidative stress in horse, human, and rabbit sperm [15–17]. However, the protective effect of quercetin on cold-stored sperm has not been examined.
In this study, we aimed to prolong the storage period of mouse sperm at refrigerated temperatures by adding a protective agent to the preservation medium. First, we examined the effects of quercetin and solvent (dimethyl sulfoxide [DMSO]) on the fertility of cold-stored sperm. Second, we measured the storage period of sperm cold-stored with DMSO and quercetin and evaluated their motility and fertility. Then, we analyzed sperm cold-stored with DMSO and quercetin regarding parameters of sperm activity (mitochondrial membrane potential) and sperm capacitation (protein tyrosine phosphorylation) after preincubation in a capacitating condition. Finally, we examined the developmental ability of embryos derived from IVF using sperm cold-stored with DMSO and quercetin.
Materials and methods
Animals
C57BL/6JJcl mice were purchased from CLEA Japan (Tokyo, Japan) for use as sperm and oocyte donors. Sperm were obtained from mature male mice (12–18 weeks old), and oocytes were obtained from mature female mice (8–12 weeks old). All animals were kept under a 12-h/12-h dark/light cycle (lights on: 07:00–19:00) at a constant temperature of 22 ± 1°C with free access to food and water. All animal experiments were approved by the Animal Care and Use Committee at Kumamoto University.
Media
Epididymides were collected and stored in Lifor (Lifeblood Medical Inc., NJ, USA) supplemented as previously described [18]. Specifically, DMSO was dissolved in Lifor at a final concentration of 1%–10%, whereas quercetin was dissolved in DMSO (1.0 mg/mL), producing a final concentration of 1–100 μg/mL.
BSA-free Toyoda Yokoyama Hoshi medium (fTYH), a modified Krebs–Ringer bicarbonate solution, was used for sperm incubation [19, 20]. BSA-free TYH containing 0.75 mM MBCD (Sigma) and 1.0 mg/mL polyvinyl alcohol (cold water soluble; Sigma) (cTYH) was used for sperm preincubation to induce capacitation [21]. Modified human tubal fluid (mHTF) medium containing 1.0 mM GSH was used as a medium for IVF [22–24]. Potassium simplex optimized medium (KSOM/AA) was used to culture two-cell embryos [25].
Cold storage of cauda epididymides
Cold storage of mouse epididymides was performed as previously described [10]. For the cold storage experiments, male mice were euthanized via cervical dislocation, and their cauda epididymides were collected and transferred to 0.2-mL plastic tubes (three epididymides/tube) each containing 0.2 mL of preservation medium. The tubes were placed in a cardboard box with a tracker that also functions as a digital thermometer and data logger (Thermochron iButton; Maxim Integrated Products, Inc.), and a sheet of cotton wool. To reduce temperature fluctuations in the box caused by external temperatures, the box was placed in a vacuum bottle (JMK-500, Thermos Co., USA) with two cold packs (60 mm × 180 mm) that were precooled in a refrigerator at 4°C. The bottle was placed in a Styrofoam box (140 mm × 250 mm). The packed Styrofoam box was then stored in a refrigerator at 4°C for 0–11 days. The cold-transport kit is available via CosmoBio Co. Ltd (KYD-006-EX).
In vitro fertilization
After cold storage, the cauda epididymides were removed from the storage medium, and the medium was gently wiped off with a filter paper. The cauda epididymides were washed in mHTF and transferred to a sperm incubation dish containing paraffin oil. In the paraffin oil, the duct of each cauda epididymides was cut using microdissecting scissors (Supplemental Figure S1). Clots of sperm released from the cauda epididymides were transferred into a 100-μL drop of cTYH using a dissecting needle. To induce capacitation, sperm were incubated for 60 min at 37°C and 5% CO2.
Mature female mice were superovulated via intraperitoneal injections of 7.5 IU of equine chorionic gonadotropin (ASKA Pharmaceutical Co. Ltd, Tokyo, Japan) and 7.5 IU of human chorionic gonadotropin (ASKA Pharmaceutical Co. Ltd) administered 48 h apart. Mice were sacrificed 15–17 h later via cervical dislocation, and oviducts were quickly removed and transferred into a fertilization dish containing paraffin oil. Four to six cumulus–oocyte complexes (COCs) were obtained from the ampullae of the fallopian tubes of two to three females. COCs were transferred to a dish containing a 200-μL drop of IVF medium covered with paraffin oil.
An aliquot of sperm suspension was carefully collected from the edge of the sperm incubation drop, transferred to the IVF drop containing COCs, and then incubated at 37°C and 5% CO2. The concentration of inseminated sperm was 1000–2000 sperm/μL in an IVF drop. Three hours later, the inseminated oocytes were washed three times in a 100-μL drop of mHTF covered with paraffin oil and then cultured at 37°C with 5% CO2. After 5–6 h of insemination, the pronuclear formation was observed to confirm the monospermic, polyspermic, or parthenogenic oocyte by phase-contrast microscopy. Polyspermic or parthenogenic oocyte was not observed in this experiment. Twenty-four hours after insemination, fertilization rates were calculated as the total number of two-cell embryos divided by the total number of inseminated oocytes ×100.
Assessment of sperm motility
The motility of cold-stored sperm preserved in preservation medium at 4°C was evaluated using a computer-assisted sperm analyzer (CASA, IVOS Sperm Analyzer, Hamilton-Thorne Research, Beverly, MA, USA) [26]. Epididymal sperm were transferred and incubated in a 100-μL drop of cTYH for 60 min. The sperm suspension was applied to a cell counting chamber (Hamilton-Thorne Research). Sperm motility was denoted by movement at a velocity of 5 μm/s in any direction. Progressive motility denoted a path velocity >50 μm/s and a straightness ratio >50%. In addition, the average path velocity, progressive velocity, track speed, lateral amplitude, and beat cross frequency were evaluated. In each experiment, 500–1000 sperm were analyzed. The experiments were independently conducted four to eight times.
Analysis of quercetin levels in cold-stored sperm
To examine the localization of quercetin in sperm, we observed the localization of quercetin and measured the levels of quercetin by flow cytometry. Epididymal sperm were transferred into a 100-μL drop of fTYH. The sperm were observed via fluorescence microscopy (Biorevo; Keyence Co., Japan). The level of quercetin in sperm was quantified via the fluorescence intensity of quercetin using a flow cytometer (Becton Dickinson, San Jose, CA). The relative mean fluorescence intensity was determined by dividing the fluorescence intensity of quercetin-stained cells by that of untreated quercetin-stained cells. More than 10,000 sperm were analyzed in each experiment. The experiments were independently conducted four times.
Evaluation of mitochondrial activity in cold-stored sperm
The mitochondrial activity of sperm cold-stored for 7 days was examined using a MitoProbe JC-1 Assay Kit (Thermo Fisher Scientific Inc., MA, USA). Sperm were incubated in a 100-μL drop of cTYH for 0, 30, 60, 90, or 120 min. After incubation, aliquots of the sperm suspension were mixed with 2 × JC-1 in cTYH and incubated for 30 min at 37°C and 5% CO2 in the dark. Sperm with live mitochondria were stained with JC-1 and visualized via JC-1 fluorescence (excitation, 514/529 nm; emission, 590 nm). The sperm were centrifuged and washed twice in fTYH. JC-1-stained sperm were observed and counted via fluorescence microscopy. Fifty sperm were randomly observed in each experiment, and this experiment was independently repeated four times. Mitochondrial activity (%) was calculated as the total number of JC-1-stained sperm divided by the total number of sperm ×100. The retention rate of mitochondrial activity (%) was calculated as the mitochondrial activity of each experimental group divided by the mitochondrial activity of noncultured sperm ×100. In each experiment, 50 sperm were observed under fluorescence microscopy. The experiments were independently conducted four times.
Detection of sperm incubation-associated tyrosine phosphorylation
Sperm were incubated in a 100-μL drop of cTYH for 0 or 60 min. After incubation, the concentration of each sperm suspension was measured using a PiCOSCOPE® (USHIO, Japan) and equalized. Aliquots of the sperm suspension were collected by centrifugation at 14,000 × g for 5 min. Subsequent to washing with 1 mL of phosphate-buffered saline, the sperm pellet was resuspended in Laemmli sample buffer and 2-mercaptoethanol and boiled for 5 min. After disruption of sperm sample by ultrasonication for 1 min, SDS-PAGE was performed using Mini-PROTEAN® Tetra Vertical Electrophoresis Cell (Bio-Rad, USA), and proteins were transferred to a Trans-Blot® Turbo Mini PVDF Transfer Pack (Bio-Rad). Western blot analysis was performed using the SNAP i.d.® 2.0 Protein Detection System (Merck Millipore, Germany). The protein-transferred membrane was blocked with PO Noise Cancelling Reagents for Phosphoprotein Detection using chemiluminescence or fluorescence techniques (Bløk; Merck Millipore) at room temperature. After removing the blocking solution, the membrane was immunoblotted with a monoclonal antibody against phosphotyrosine (4G10® Platinum, Anti-Phosphotyrosine Antibody; Merck Millipore) for 10 min at room temperature and horseradish peroxidase-conjugated secondary antibodies for 10 min at room temperature. α-Tubulin was used as the internal control.
Embryo transfer
After IVF, two-cell embryos were washed three times in a 100-μL drop of KSOM/AA covered with paraffin oil and then transferred into the oviducts (6–12 embryos/oviduct) of pseudopregnant ICR (Institute of Cancer Research) female mice on the day a vaginal plug was found [27]. After 19 days, the number of offspring was recorded. Developmental ability was evaluated by the birth rate (number of live pups per number of transferred two-cell embryos ×100).
Statistical analysis
The results are expressed as the mean ±SD. The means for each treatment were compared by analysis of variance after arcsine transformation of the percentages. When more than one pair of means was to be compared, Tukey–Kramer's pairwise comparisons were performed on the means, with P < 0.05 or P < 0.01 indicating significance.
Results
Experiment 1: DMSO and quercetin enhanced the fertility of cold-stored sperm
Both quercetin and DMSO increased the fertilization rates of sperm cold-stored at 4°C for 7 days (Figure 1). Co-treatment with 10% DMSO and 100 μg/mL quercetin produced the highest fertilization rate. Based on this result, the subsequent experiments were performed using Lifor, Lifor containing 10% DMSO (Lifor-DMSO), or Lifor containing 10% DMSO and 100 μg/mL quercetin (Lifor-DMSO-Q).
Figure 1.
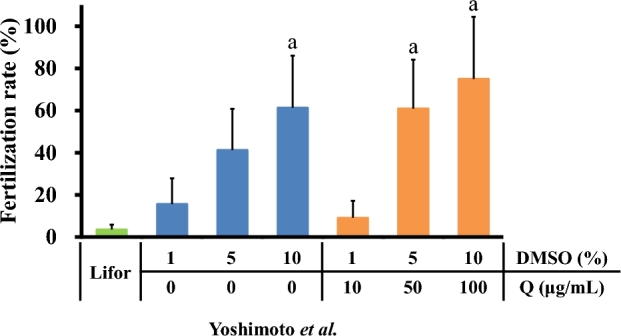
Concentration-dependent effects of DMSO or quercetin on the fertility of cold-stored sperm. Cauda epididymides were taken from sacrificed male mice and stored in Lifor containing various concentrations of DMSO (1%, 5%, or 10%) or quercetin (10, 50, or 100 μg/mL) at 4°C for 7 days. After storage, sperm were isolated and incubated in BSA-free Toyoda Yokoyama Hoshi medium containing 0.75 mM MBCD and 1.0 mg/mL polyvinyl alcohol for 60 min. Oocytes were incubated in IVF medium (modified human tubal fluid containing 1.0 mM GSH) for 30–60 min. Subsequently, incubated sperm were introduced into the IVF medium. The fertilization rate was calculated as the number of two-cell embryos divided by the number of inseminated oocytes ×100. Values are given as the mean ±SD (n = 4). aP < 0.05 compared between Lifor and Lifor-DMSO or Lifor-DMSO-Q. DMSO, dimethyl sulfoxide. Q, quercetin.
Experiment 2: DMSO and quercetin exerted protective effects on cold-stored sperm for up to 10 days
To examine the fertility of cold-stored sperm, we performed IVF using sperm in cold-stored Lifor, Lifor-DMSO, or Lifor-DMSO-Q for 0–11 days. Compared to the effects of Lifor alone, Lifor-DMSO maintained the fertility of cold-stored sperm for 8 days and Lifor-DMSO-Q for 10 days (Figure 2).
Figure 2.
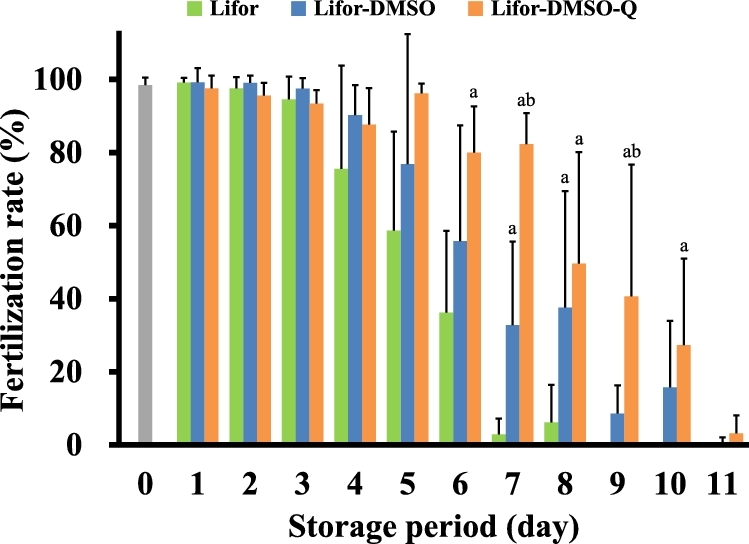
Effect of the storage period on the fertility of cold-stored sperm. Cauda epididymides were taken from sacrificed male mice and stored in Lifor, Lifor-DMSO, or Lifor-DMSO-Q at 4°C for 0–11 days. After storage, sperm were isolated and incubated in BSA-free Toyoda Yokoyama Hoshi medium containing 0.75 mM MBCD and 1.0 mg/mL polyvinyl alcohol for 60 min. Oocytes were incubated in IVF medium (modified human tubal fluid containing 1.0 mM GSH) for 30 min. Subsequently, incubated sperm were introduced into the IVF medium. The fertilization rate was calculated as the number of two-cell embryos divided by the number of inseminated oocytes ×100. Values are given as the mean ±SD (n = 4–8). aP < 0.05 compared between Lifor and Lifor-DMSO or Lifor-DMSO-Q. bP < 0.05 compared between Lifor-DMSO and Lifor-DMSO-Q. DMSO, dimethyl sulfoxide. Q, quercetin.
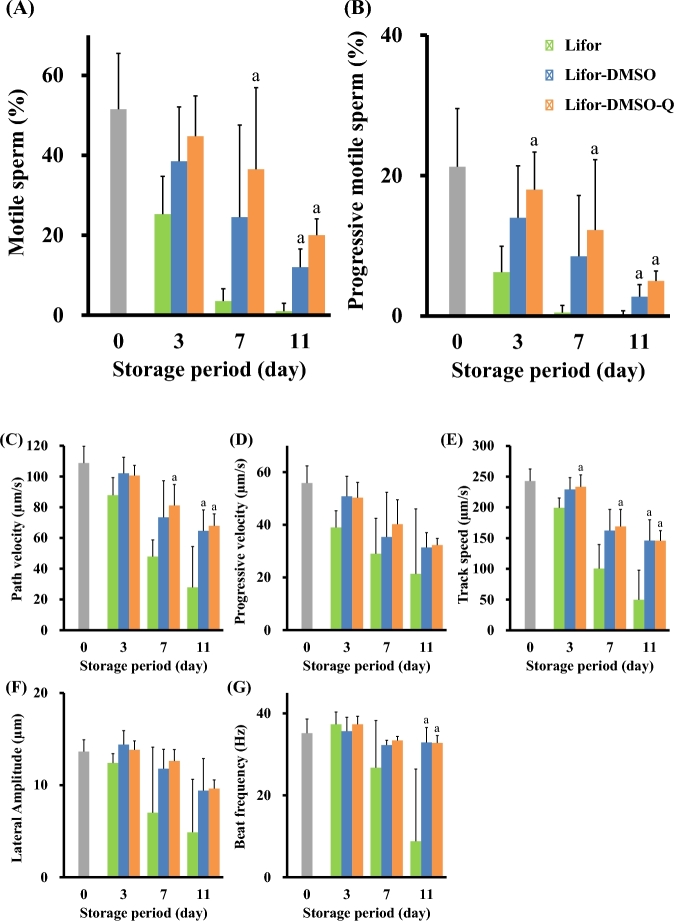
Experiment 3: DMSO and quercetin improved the motility of cold-stored sperm
To examine the quality of sperm cold-stored in Lifor, Lifor-DMSO, or Lifor-DMSO-Q for 0, 3, 7, or 11 days, sperm motility was analyzed using a CASA. Lifor-DMSO-Q produced the highest rates of motile sperm and progressive motile sperm after 7 and 11 days of storage (Figure 3A and B, Supplemental Videos S1–S3, Figures 1–3).
Figure 3.
Effect of the storage period on the motility of cold-stored sperm. Cauda epididymides were taken from sacrificed male mice and stored in Lifor, Lifor-DMSO, or Lifor-DMSO-Q at 4°C for 0, 3, 7, or 11 days. After storage, sperm were isolated and incubated in BSA-free Toyoda Yokoyama Hoshi medium containing 0.75 mM MBCD and 1.0 mg/mL polyvinyl alcohol for 60 min. Subsequently, the incubated sperm were diluted with modified human tubal fluid, and their motility was analyzed using an HTM-IVOS. The following motility parameters were measured: percentage of motile sperm (A), percentage of progressive motile sperm (B), path velocity (C), progressive velocity (D), track speed (E), lateral amplitude (F), and beat frequency (G). Values are given as the mean ±SD (n = 4–8). aP < 0.05 compared between Lifor and Lifor-DMSO or Lifor-DMSO-Q. DMSO, dimethyl sulfoxide. Q, quercetin.
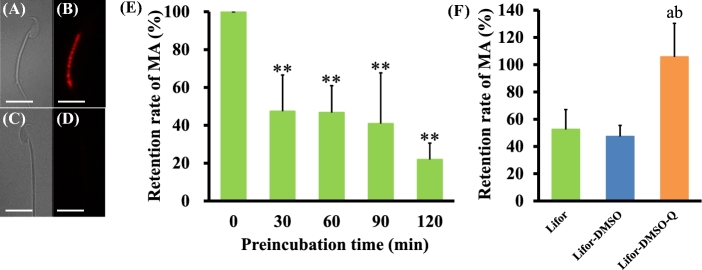
Experiment 4: DMSO and quercetin maintained the mitochondrial activity of cold-stored sperm
Because the motility and fertility of sperm were highly maintained during cold storage in Lifor-DMSO-Q, the physiological activity of cold-stored sperm was monitored on the basis of mitochondrial membrane potential (mitochondrial activity) using JC-1 staining. The mitochondrial activity of sperm quickly decreased after 30 min of incubation (Figure 4E). Next, we examined the mitochondrial activity of sperm cold-stored in Lifor, Lifor-DMSO, and Lifor-DMSO-Q, and Lifor-DMSO-Q was associated with higher mitochondrial activity than Lifor or Lifor-DMSO (Figure 4F).
Figure 4.
Effect of preincubation on the mitochondrial activity of cold-stored sperm. Cauda epididymides were taken from sacrificed male mice and stored in Lifor, Lifor-DMSO, or Lifor-DMSO-Q at 4°C for 7 days. After storage, sperm were isolated and incubated in BSA-free Toyoda Yokoyama Hoshi medium containing 0.75 mM MBCD and 1.0 mg/mL polyvinyl alcohol (cTYH). After incubation, sperm with live mitochondria were stained with JC-1 and visualized via JC-1 fluorescence. (A, B) Sperm with live mitochondria. (C, D) Sperm with dead mitochondria. The mitochondrial activity (MA) (%) was calculated as the total number of JC-1-stained sperm divided by the total number of sperm ×100. The retention rate of MA (%) was calculated as the MA of each experimental group divided by the MA of noncultured sperm × 100. (E) Cauda epididymides were stored in Lifor at 4°C for 7 days. After storage, sperm were incubated in cTYH for 0, 30, 60, 90, or 120 min. Values are given as the mean ±SD (n = 4). An asterisk indicates a significant difference (P < 0.01) compared with the control (0 min). (F) Cauda epididymides were stored in Lifor, Lifor-DMSO, or Lifor-DMSO-Q at 4°C for 7 days. After storage, sperm were incubated in cTYH for 0 or 60 min. Values are given as the mean ±SD (n = 4). **P < 0.01 compared with 0 min. aP < 0.05 compared between Lifor and Lifor-DMSO or Lifor-DMSO-Q. bP < 0.05 compared between Lifor-DMSO and Lifor-DMSO-Q. The bars indicate 10 μm. DMSO, dimethyl sulfoxide. Q, quercetin.
Experiment 5: quercetin accumulated in the midpiece of sperm
To examine the localization and amounts of quercetin in sperm after cold storage in Lifor, Lifor-DMSO, or Lifor-DMSO-Q, the fluorescence of quercetin was observed by fluorescence microscopy, and the fluorescence intensity of quercetin was measured by flow cytometry. The results illustrated that quercetin was highly localized in the midpiece of sperm after cold storage in Lifor-DMSO-Q (Figure 5).
Figure 5.
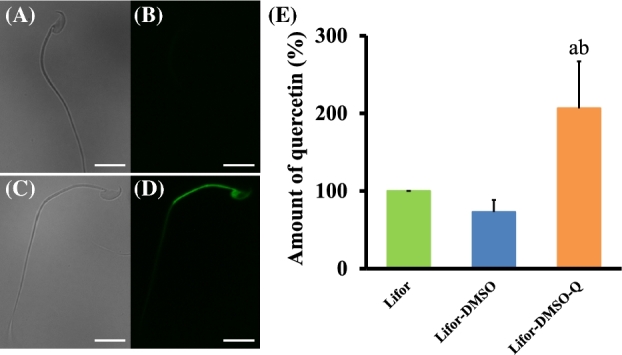
The localization of quercetin in cold-stored sperm. Cauda epididymides were taken from sacrificed male mice and stored in Lifor or Lifor-DMSO-Q at 4°C for 7 days. After storage, sperm were transferred in BSA-free Toyoda Yokoyama Hoshi medium and observed via fluorescence microscopy. Nonstained (A, B) or quercetin-stained sperm (C, D) were quantified on the basis of the fluorescence intensity of quercetin by fluorescence-activated cell sorting. The amount of quercetin (%) was calculated as follows: (mean fluorescent intensity [MFI] of each experimental group/MFI of sperm cold-stored in Lifor) × 100. Values are given as the mean ±SD (n = 4). aP < 0.05 compared between Lifor and Lifor-DMSO or Lifor-DMSO-Q. bP < 0.05 compared between Lifor-DMSO and Lifor-DMSO-Q. The bars indicate 10 μm. DMSO, dimethyl sulfoxide. Q, quercetin.
Experiment 6: DMSO and quercetin maintained the capacitating activity of sperm
To examine sperm capacitation after cold storage in Lifor, Lifor-DMSO, or Lifor-DMSO-Q, the occurrence of sperm capacitation was evaluated according to the status of protein tyrosine phosphorylation. Protein tyrosine phosphorylation increased after 60 min of incubation in Lifor, although the level was lower than that of fresh sperm (Figure 6A). Compared to the findings for cold storage in Lifor, protein tyrosine phosphorylation was elevated following storage in Lifor-DMSO and Lifor-DMSO-Q, as indicated by bands at 50 kDa and approximately 250 kDa.
Figure 6.
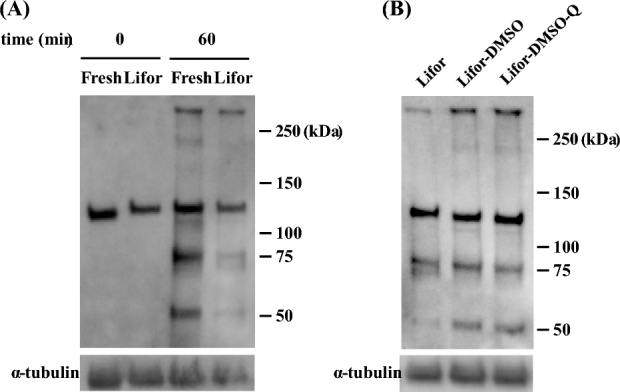
Effect of DMSO and quercetin on the protein tyrosine phosphorylation of cold-stored sperm during capacitation. Cauda epididymides were taken from sacrificed male mice and stored in Lifor, Lifor-DMSO, or Lifor-DMSO-Q at 4°C for 7 days. After storage, sperm were isolated and incubated in BSA-free Toyoda Yokoyama Hoshi medium containing 0.75 mM MBCD and 1.0 mg/mL polyvinyl alcohol. The protein tyrosine phosphorylation of cold-stored sperm was assessed by western blot analysis. α-Tubulin was used as the loading control. (A) Sperm stored in Lifor were incubated for 0 or 60 min. Fresh sperm was used as the control. (B) Sperm stored in Lifor, Lifor-DMSO, or Lifor-DMSO-Q were incubated for 60 min. DMSO, dimethyl sulfoxide. Q, quercetin.
Experiment 7: development of two-cell embryos derived from sperm cold-stored with DMSO and quercetin into live pups
To examine the developmental ability of two-cell embryos produced by IVF using fresh sperm or sperm cold-stored with DMSO and quercetin, birth rates were evaluated by embryo transfer. Birth rates were similar between fresh sperm and sperm cold-stored in Lifor-DMSO-Q (Table 1).
Table 1.
Birth rate derived from in vitro fertilization using cold-stored sperm.
| Storage period (day) | Sperm preservation medium | No. of transferred two-cell embryos (no. of recipients) | No. of pups (%) |
|---|---|---|---|
| 0 | - | 112 (6) | 34 (30.4 ± 35.6) |
| 7 | Lifor-DMSO-Q | 124 (6) | 39 (31.5 ± 22.5) |
Results are expressed as the mean ± SD (n = 6).
DMSO, dimethyl sulfoxide; Q, quercetin.
Discussion
In this study, we demonstrated that DMSO and quercetin could prolong the motility and fertility of cold-stored sperm. In addition, the reduction of mitochondrial activity associated with cold storage was alleviated by exposure to quercetin, and the agent localized to the midpiece of sperm. Furthermore, protein tyrosine phosphorylation, as a marker of capacitation, was observed in sperm cold-stored with DMSO and quercetin. These results suggest that DMSO and quercetin can prolong the vitality of sperm stored at refrigerated temperatures.
Genetically engineered mice are frequently shipped worldwide. However, shipping live mice has potential risks of expanding pathogens, losing animals by escape or death, and unintentionally violating regulations or rules of genetically modified organisms. In addition, based on the animal welfare policy, a suitable alternative transport system is required. Previously, many researchers addressed to the transport technique by preserving mouse sperm in various environments and producing embryos using the sperm. Cryopreserved, freeze-dried, or evaporative-dried technologies are possible means to store and transport mouse sperm. Sperm cryopreservation is the best way to preserve mouse resource because the sperm can permanently maintain motility and fertility and applicable to IVF after thawing [8]. More than 22,000 strains of cryopreserved sperm were archived in mouse repositories, and the sperm can be transported at −196°C in dryshipper or −79°C using dry ice [5, 28, 29]. However, the motility of frozen–thawed mouse sperm declined compared with that of fresh sperm, and the quality of cryopreserved sperm depends on protocol, skill, and mouse strains. Cold-stored sperm indicate similar motility with fresh sperm and can be helpful to reduce the variation of sperm quality due to simple procedure. In addition, in the case of keeping live mice, the cold transport of cauda epididymis collected from anesthetized male mice can skip the process of sperm cryopreservation for the shipment of mouse line. On the contrary, freeze-dried or evaporative-dried mouse sperm is possible to store for 2 years or more at refrigerated or ambient temperatures, which needs intracytoplasmic sperm injection (ICSI) technique to produce embryos from the sperm [30, 31]. The high portability of the freeze-dried or evaporative-dried sperm is very useful to handle the sample without special device for shipment and storage. Recently, freeze-dried mouse sperm traveled to space and yielded healthy mice after 9 months storage in space [32]. The application of the cold storage of sperm will transport the mouse lines between research institutes, whereas the freeze-dried and evaporative-dried techniques will be the choice to send the sample to the frontier place. There are some reports about the storage of sperm in whole body at refrigeration or freezing temperatures. In cold-stored male mice after euthanization, sperm of outbred and hybrid strains of ICR and B6D2F1 mice could fertilize oocytes by IVF using zona-free or partial zona dissected oocytes for 7 days or ICSI for 20 days [33, 34]. Ogonuki et al produced live pups using sperm and spermatid retrieved from frozen reproductive organs or whole body by ICSI or round spermatid injection (ROSI) [35]. The storage of organs or whole body at refrigeration or freezing temperatures is simple means to emergently store male germ cells of valuable mouse lines when the mice suddenly died. However, the advanced reproductive techniques of zona dissection, ICSI, and ROSI required producing embryos using the low-motile or immotile sperm. In this case, the cold storage of cauda epididymides collected from dead mice can rescue the mouse line by IVF using the sperm with high motility.
Cold storage of sperm is a reliable means of transferring haploid male genetics to share useful resources, manage efficient breeding, and avoid the transmission of pathogens. The technique of shipping cold-stored sperm is potentially applicable for transporting human sperm to assess its quality at diagnostic andrology laboratories and disseminate boar sperm for breeding livestock of excellent quality at farms [36, 37]. Practically, the cold-transport technique can be utilized to share and archive useful strains of genetically engineered mice. To establish a transport system for cold-stored mouse sperm, we have continuously improved the shipment box, preservation medium, sperm preincubation medium, and fertilization medium to maintain motility during cold storage and enhance fertility in IVF [10–12]. The shipment technique was applicable to the short-distance transport of mouse sperm (within 3 days), which is acceptable for domestic transportation. However, this duration is not sufficient to ensure international transportation. In this study, we prolonged the transportable period of cold-stored sperm to 7 days. This duration would permit the transport of sperm to research institutes around the world.
Previously, we identified Lifor as a suitable preservation medium for storing mouse sperm at refrigerated temperatures for 3 days [10]. In this study, we found that the addition of DMSO and quercetin to Lifor can prolong the survival period to 10 days. Specifically, the addition of high concentrations of DMSO (10%) and quercetin (100 μg/mL) to Lifor alleviated the reduction of motility associated with cold storage at 4°C. Similarly, a low concentration of DMSO (1%) in preservation medium (0.2% KCl and 0.7% NaCl) prolonged the survival period of rainbow trout sperm stored at 4°C [38]. DMSO is a polar aprotic solvent that has antioxidant activity based on its ability to react with the harmful reactive oxygen species hydroxyl radicals [39]. The excess production of hydroxyl radicals results in damage to plasma membranes and mitochondria, leading to reduced sperm motility [40]. In the cold storage of mouse sperm, DMSO may scavenge hydroxyl radicals produced at refrigerated temperatures.
Quercetin increased the protective effect of DMSO and produced the highest rates of fertility, motility, and mitochondrial activity (Figures 2, 3A, and 4F). Quercetin is a flavonoid produced in cold plants with cryoprotective ability, and it has strong ability to scavenge free radicals by inhibiting xanthine oxidase [14, 41, 42]. Treatment with quercetin protected against oxidative stress in precryopreserved human (50 μM, 15.1 μg/mL) and horse semen (150 μM, 45.3 μg/mL) and chilled rabbit semen (100–200 μM, 30.2–60.4 μg/mL) [15–17, 43]. The motility of quercetin-treated sperm was increased via its ability to suppress the production of intracellular H2O2 and lipid peroxidation. However, the ability of quercetin to maintain sperm motility and fertility was not examined using reproductive technology, and the detailed mechanism has not been fully investigated.
We first revealed that quercetin maintained the mitochondrial activity of cold-stored sperm during sperm preincubation, allowing them to undergo capacitation (Figure 4F). Additionally, quercetin localized to the midpiece of sperm (Figure 5D). We assume that quercetin protected the function of mitochondria in sperm during cold storage and sperm preincubation. The integrity of mitochondrial membrane potential is important for maintaining sperm motility and fertility [44, 45]. In addition to its antioxidant effects, quercetin was recently identified as a phytochemical that can modulate mitochondrial function [46]. In human cells, treatment with quercetin (0.3–30 μM, 0.9–9.1 μg/mL) restored mitochondrial membrane potential by improving ATP levels and complex I activity, suppressing caspase-3 activity, or reducing DNA fragmentation [47–50]. Further investigation is needed to elucidate the novel protective effects of quercetin on sperm.
Protein tyrosine phosphorylation is a robust marker of sperm capacitation [51, 52]. Our study illustrated that sperm cold-stored in Lifor displayed decreased protein tyrosine phosphorylation compared with the findings in fresh sperm (Figure 6A). Exposure to Lifor-DMSO and Lifor-DMSO-Q partially restored protein tyrosine phosphorylation in cold-stored sperm (Figure 6B). Proteins observed to undergo tyrosine phosphorylation are candidate moieties involved in the restoration of the capacitating ability of cold-stored sperm following exposure to DMSO and quercetin.
We confirmed the birth of offspring derived via IVF using sperm cold-stored with DMSO and quercetin for 7 days (Table 1). The data established that cold-stored sperm can be used to produce embryos and live pups in mice. Practically, we have already introduced a shipment system for cold-stored sperm in the mouse repository at the Center for Animal Resources and Development (CARD) in Kumamoto University, Japan [53]. Recently, almost all depositors have been sending us cauda epididymides to archive genetically modified mice. At CARD, we have been producing and archiving two-cell embryos by IVF using cold-stored sperm or cryopreserved sperm after cold storage [11]. Cryopreserved sperm can be permanently stored in liquid nitrogen, and they are available for colony expansion and rederivation. In some cases, we have performed IVF using cold-stored sperm and vitrified-warmed oocytes [54]. Cold-transport technology for mouse sperm can seamlessly connect geographically separated institutes and permit long-range projects, thus providing an efficient global platform for mouse research. We strongly believe that the rapid expansion of improved reproductive technology will accelerate innovative discoveries in medical science using genetically engineered mice.
This study demonstrated the life-prolonging effects of DMSO and quercetin on cold-stored sperm. The cold storage technology using DMSO and quercetin will be helpful for improving the transport system of clinical samples to assess male infertility, livestock and marine resources to breed efficiently, and valuable genetic material to preserve endangered species.
Supplementary data
Supplementary data are available at BIOLRE online.
Supplemental Figure S1. Sperm preparation for cold storage and IVF. Cauda epididymides were taken from sacrificed male mice and stored in storage medium at 4°C. After cold storage, the cauda epididymides were removed and were transferred to a sperm incubation dish containing paraffin oil. In the paraffin oil, the duct of each cauda epididymides was cut using microdissecting scissors. Clots of sperm released from the cauda epididymides were transferred into the preincubation medium using a dissecting needle. After incubation, an aliquot of sperm suspension was carefully collected from the edge of the sperm incubation drop and transferred to the IVF drop containing COCs.
Supplemental Video S1. Sperm stored in Lifor. Cauda epididymides were taken from sacrificed male mice and stored in Lifor at 4°C for 7 days. After storage, sperm were isolated and incubated in BSA-free Toyoda Yokoyama Hoshi medium containing 0.75 mM MBCD and 1.0 mg/mL polyvinyl alcohol for 60 min.
Supplemental Video S2. Sperm stored in Lifor-DMSO. Cauda epididymides were taken from sacrificed male mice and stored in Lifor-DMSO at 4°C for 7 days. After storage, sperm were isolated and incubated in BSA-free Toyoda Yokoyama Hoshi medium containing 0.75 mM MBCD and 1.0 mg/mL polyvinyl alcohol for 60 min. DMSO, dimethyl sulfoxide.
Supplemental Video S3. Sperm stored in Lifor-DMSO-Q. Cauda epididymides were taken from sacrificed male mice and stored in Lifor-DMSO-Q at 4°C for 7 days. After storage, sperm were isolated and incubated in BSA-free Toyoda Yokoyama Hoshi medium containing 0.75 mM MBCD and 1.0 mg/mL polyvinyl alcohol for 60 min. DMSO, dimethyl sulfoxide. Q, quercetin.
Supplementary data are available at BIOLRE online.
Acknowledgments
We are grateful to Prof. Seizo Fujikawa, Division of Bioresources and Product Science, Research Group of Forest Resource Science, Graduate School of Agriculture Research Faculty of Agriculture, Hokkaido University, for his useful advice and comments. We thank Ms Shiori Takeuji, Ms Koharu Kirikihira, and Dr Shota Tsuchida of our laboratory for their technical assistance and useful discussion with the experiments.
References
- 1. Polge C, Smith AU, Parkes AS. Revival of spermatozoa after vitrification and dehydration at low temperatures. Nature 1949; 164:666. [DOI] [PubMed] [Google Scholar]
- 2. Nakagata N. Cryopreservation of mouse spermatozoa. Mamm Genome 2000; 11:572–576. [DOI] [PubMed] [Google Scholar]
- 3. Takeo T, Nakagata N. Combination medium of cryoprotective agents containing L-glutamine and methyl-{beta}-cyclodextrin in a preincubation medium yields a high fertilization rate for cryopreserved C57BL/6J mouse sperm. Lab Anim 2010; 44:132–137. [DOI] [PubMed] [Google Scholar]
- 4. Ostermeier GC, Wiles MV, Farley JS, Taft RA, El-Shemy HA. Conserving, distributing and managing genetically modified mouse lines by sperm cryopreservation. PLoS One 2008; 3:e2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Eppig JT, Motenko H, Richardson JE, Richards-Smith B, Smith CL. The International Mouse Strain Resource (IMSR): cataloging worldwide mouse and ES cell line resources. Mamm Genome 2015; 26:448–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Raess M, De Castro AA, Gailus-Durner V, Fessele S, Hrabe de Angelis M. INFRAFRONTIER: a European resource for studying the functional basis of human disease. Mamm Genome 2016; 27:445–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Donahue LR, Hrabe De Angelis M, Hagn M, Franklin C, Lloyd KCK, Magnuson T, Mckerlie C, Nakagata N, Obata Y, Read S, Wurst W, Horlein A et al. . Centralized mouse repositories. Mamm Genome 2012; 23:559–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Takeo T, Nakagata N. Reduced glutathione enhances fertility of frozen/thawed C57BL/6 mouse sperm after exposure to methyl-beta-cyclodextrin1. Biol Reprod 2011; 85:1066–1072. [DOI] [PubMed] [Google Scholar]
- 9. Kaneko T, Fukumoto K, Haruguchi Y, Kondo T, Machida H, Koga M, Nakagawa Y, Tsuchiyama S, Saiki K, Noshiba S, Nakagata N. Fertilization of C57BL/6 mouse sperm collected from cauda epididymides after preservation or transportation at 4°C using laser-microdissected oocytes. Cryobiology 2009; 59:59–62. [DOI] [PubMed] [Google Scholar]
- 10. Takeo T, Tsutsumi A, Omaru T, Fukumoto K, Haruguchi Y, Kondo T, Nakamuta Y, Takeshita Y, Matsunaga H, Tsuchiyama S, Sakoh K, Nakao S et al. . Establishment of a transport system for mouse epididymal sperm at refrigerated temperatures. Cryobiology 2012; 65:163–168. [DOI] [PubMed] [Google Scholar]
- 11. Takeo T, Fukumoto K, Kondo T, Haruguchi Y, Takeshita Y, Nakamuta Y, Tsuchiyama S, Yoshimoto H, Shimizu N, Li M, Kinchen K, Vallelunga J et al. . Investigations of motility and fertilization potential in thawed cryopreserved mouse sperm from cold-stored epididymides. Cryobiology 2014; 68:12–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yoshimoto H, Takeo T, Irie T, Nakagata N. Fertility of cold-stored mouse sperm is recovered by promoting acrosome reaction and hyperactivation after cholesterol efflux by methyl-beta-cyclodextrin. Biol Reprod 2017; 96:446–455. [DOI] [PubMed] [Google Scholar]
- 13. Regner KR, Nilakantan V, Ryan RP, Mortensen J, White SM, Shames BD, Roman RJ. Protective effect of Lifor solution in experimental renal ischemia-reperfusion injury. J Surg Res 2010; 164:e291–e297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kasuga J, Hashidoko Y, Nishioka A, Yoshiba M, Arakawa K, Fujikawa S. Deep supercooling xylem parenchyma cells of katsura tree (Cercidiphyllum japonicum) contain flavonol glycosides exhibiting high anti-ice nucleation activity. Plant Cell Environ 2008; 31:1335–1348. [DOI] [PubMed] [Google Scholar]
- 15. Gibb Z, Butler TJ, Morris LHA, Maxwell WMC, Grupen CG. Quercetin improves the postthaw characteristics of cryopreserved sex-sorted and nonsorted stallion sperm. Theriogenology 2013; 79:1001–1009. [DOI] [PubMed] [Google Scholar]
- 16. Zribi N, Chakroun NF, Ben Abdallah F, Elleuch H, Sellami A, Gargouri J, Rebai T, Fakhfakh F, Keskes LA. Effect of freezing–thawing process and quercetin on human sperm survival and DNA integrity. Cryobiology 2012; 65:326–331. [DOI] [PubMed] [Google Scholar]
- 17. Johinke D, De Graaf SP, Bathgate R. Quercetin reduces the in vitro production of H2O2 during chilled storage of rabbit spermatozoa. Anim Reprod Sci 2014; 151:208–219. [DOI] [PubMed] [Google Scholar]
- 18. Stowe DF, Camara AKS, Heisner JS, Aldakkak M, Harder DR. Ten-hour preservation of guinea pig isolated hearts perfused at low flow with air-saturated Lifor solution at 26 C: comparison to ViaSpan solution. Am J Physiol Heart Circ Physiol 2007; 293:H895–H901. [DOI] [PubMed] [Google Scholar]
- 19. Toyoda Y, Yokoyama M, Hosi T. Studies on the fertilization of mouse eggs in vitro. Jpn J Anim Reprod 1971; 16:152–157. [Google Scholar]
- 20. Toyoda Y, Yokoyama M. The early history of the TYH medium for in vitro fertilization of mouse ova. J Mamm Ova Res 2016; 33:3–10. [Google Scholar]
- 21. Takeo T, Hoshii T, Kondo Y, Toyodome H, Arima H, Yamamura K, Irie T, Nakagata N. Methyl-beta-cyclodextrin improves fertilizing ability of C57BL/6 mouse sperm after freezing and thawing by facilitating cholesterol efflux from the cells. Biol Reprod 2008; 78:546–551. [DOI] [PubMed] [Google Scholar]
- 22. Takeo T, Nakagata N. Combination medium of cryoprotective agents containing L-glutamine and methyl-beta-cyclodextrin in a preincubation medium yields a high fertilization rate for cryopreserved C57BL/6J mouse sperm. Lab Anim 2010; 44:132–137. [DOI] [PubMed] [Google Scholar]
- 23. Quinn P, Kerin JF, Warnes GM. Improved pregnancy rate in human in vitro fertilization with the use of a medium based on the composition of human tubal fluid. Fertil Steril 1985; 44:493–498. [DOI] [PubMed] [Google Scholar]
- 24. Kito S, Hayao T, Noguchi-Kawasaki Y, Ohta Y, Uhara H, Tateno S. Improved in vitro fertilization and development by use of modified human tubal fluid and applicability of pronucleate embryos for cryopreservation by rapid freezing in inbred mice. Comp Med 2004; 54:564–570. [PubMed] [Google Scholar]
- 25. Lawitts JA, Biggers JD. Culture of preimplantation embryos. Methods Enzymol 1993; 225:153–164. [DOI] [PubMed] [Google Scholar]
- 26. Itach SB, Finklestein M, Etkovitz N, Breitbart H. Hyper-activated motility in sperm capacitation is mediated by Phospholipase D-dependent actin polymerization. Dev Biol 2012; 362:154–161. [DOI] [PubMed] [Google Scholar]
- 27. Nakagata N. Embryo transfer through the wall of the fallopian tube in mice. Jikken Dobutsu 1992; 41:387–388. [DOI] [PubMed] [Google Scholar]
- 28. Takahashi H, Liu C. Archiving and distributing mouse lines by sperm cryopreservation, IVF, and embryo transfer. Methods Enzymol 2010; 476:53–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Raspa M, Guan M, Paoletti R, Montoliu L, Ayadi A, Marschall S, Fray M, Scavizzi F. Dry ice is a reliable substrate for the distribution of frozen mouse spermatozoa: a multi-centric study. Theriogenology 2017; 96:49–57. [DOI] [PubMed] [Google Scholar]
- 30. Ward MA, Kaneko T, Kusakabe H, Biggers JD, Whittingham DG, Yanagimachi R. Long-term preservation of mouse spermatozoa after freeze-drying and freezing without cryoprotection. Biol Reprod 2003; 69:2100–2108. [DOI] [PubMed] [Google Scholar]
- 31. Liu J, Lee GY, Lawitts JA, Toner M, Biggers JD, Drevet JR. Live pups from evaporatively dried mouse sperm stored at ambient temperature for up to 2 years. PLoS One 2014; 9:e99809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wakayama S, Kamada Y, Yamanaka K, Kohda T, Suzuki H, Shimazu T, Tada MN, Osada I, Nagamatsu A, Kamimura S, Nagatomo H, Mizutani E et al. . Healthy offspring from freeze-dried mouse spermatozoa held on the International Space Station for 9 months. Proc Natl Acad Sci USA 2017; 114:5988–5993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. An TZ, Wada S, Edashige K, Sakurai T, Kasai M. Viable spermatozoa can be recovered from refrigerated mice up to 7 days after death. Cryobiology 1999; 38:27–34. [DOI] [PubMed] [Google Scholar]
- 34. Kishikawa H, Tateno H, Yanagimachi R. Fertility of mouse spermatozoa retrieved from cadavers and maintained at 4 C. Reproduction 1999; 116:217–222. [DOI] [PubMed] [Google Scholar]
- 35. Ogonuki N, Mochida K, Miki H, Inoue K, Fray M, Iwaki T, Moriwaki K, Obata Y, Morozumi K, Yanagimachi R, Ogura A. Spermatozoa and spermatids retrieved from frozen reproductive organs or frozen whole bodies of male mice can produce normal offspring. Proc Natl Acad Sci USA 2006; 103:13098–13103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Allan IW, Irvine DS, Macnamee M, Aitken RJ. Field trial of a diluent for the transportation of human semen at ambient temperatures. Fertil Steril 1997; 67:348–354. [DOI] [PubMed] [Google Scholar]
- 37. Johnson LA, Weitze KF, Fiser P, Maxwell WMC. Storage of boar semen. Anim Reprod Sci 2000; 62:143–172. [DOI] [PubMed] [Google Scholar]
- 38. Ubilla A, Fornari D, Figueroa E, Effer B, Valdebenito I. Short-term cold storage of the semen of rainbow trout Oncorhynchus mykiss (Walbaum, 1792) incorporating DMSO in the sperm diluent. Effects on motility and fertilizing capacity. Aquac Res 2015; 46:37–44. [Google Scholar]
- 39. Popham PL, Novacky A. Use of dimethyl sulfoxide to detect hydroxyl radical during bacteria-induced hypersensitive reaction. Plant Physiol 1991; 96:1157–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Aitken RJ, Baker MA. Oxidative stress, sperm survival and fertility control. Mol Cell Endocrinol 2006; 250:66–69. [DOI] [PubMed] [Google Scholar]
- 41. Formica JV, Regelson W. Review of the biology of Quercetin and related bioflavonoids. Food Chem Toxicol 1995; 33:1061–1080. [DOI] [PubMed] [Google Scholar]
- 42. Nijveldt RJ, van Nood E, van Hoorn DE, Boelens PG, van Norren K, van Leeuwen PA. Flavonoids: a review of probable mechanisms of action and potential applications. Am J Clin Nutr 2001; 74:418–425. [DOI] [PubMed] [Google Scholar]
- 43. Johinke D, De Graaf S, Bathgate R. The effect of sperm concentration and storage vessel on quercetin-supplemented rabbit semen during chilled storage. Reprod Domest Anim 2015; 50:567–573. [DOI] [PubMed] [Google Scholar]
- 44. Amaral A, Lourenco B, Marques M, Ramalho-Santos J. Mitochondria functionality and sperm quality. Reproduction 2013; 146:R163–R174. [DOI] [PubMed] [Google Scholar]
- 45. Piomboni P, Focarelli R, Stendardi A, Ferramosca A, Zara V. The role of mitochondria in energy production for human sperm motility. Int J Androl 2012; 35:109–124. [DOI] [PubMed] [Google Scholar]
- 46. De Oliveira MR, Nabavi SM, Braidy N, Setzer WN, Ahmed T, Nabavi SF. Quercetin and the mitochondria: a mechanistic view. Biotechnol Adv 2016; 34:532–549. [DOI] [PubMed] [Google Scholar]
- 47. Bali E, Ergin V, Rackova L, Bayraktar O, Kucukboyaci N, Karasu C. Olive leaf extracts protect cardiomyocytes against 4-hydroxynonenal-induced toxicity in vitro: comparison with oleuropein, hydroxytyrosol, and quercetin. Planta Med 2014; 80:984–992. [DOI] [PubMed] [Google Scholar]
- 48. Ben Salem I, Prola A, Boussabbeh M, Guilbert A, Bacha H, Lemaire C, Abid-Essefi S. Activation of ER stress and apoptosis by alpha- and beta-zearalenol in HCT116 cells, protective role of Quercetin. NeuroToxicology 2016; 53:334–342. [DOI] [PubMed] [Google Scholar]
- 49. Carrasco-Pozo C, Pastene E, Vergara C, Zapata M, Sandoval C, Gotteland M. Stimulation of cytosolic and mitochondrial calcium mobilization by indomethacin in Caco-2 cells: modulation by the polyphenols quercetin, resveratrol and rutin. Biochim Biophys Acta 2012; 1820:2052–2061. [DOI] [PubMed] [Google Scholar]
- 50. Carrasco-Pozo C, Mizgier ML, Speisky H, Gotteland M. Differential protective effects of quercetin, resveratrol, rutin and epigallocatechin gallate against mitochondrial dysfunction induced by indomethacin in Caco-2 cells. Chem Biol Interact 2012; 195:199–205. [DOI] [PubMed] [Google Scholar]
- 51. Visconti PE, Kopf GS. Regulation of protein phosphorylation during sperm capacitation. Biol Reprod 1998; 59:1–6. [DOI] [PubMed] [Google Scholar]
- 52. Visconti PE, Moore GD, Bailey JL, Leclerc P, Connors SA, Pan D, Olds-Clarke P, Kopf GS. Capacitation of mouse spermatozoa. II. Protein tyrosine phosphorylation and capacitation are regulated by a cAMP-dependent pathway. Development 1995; 121:1139–1150. [DOI] [PubMed] [Google Scholar]
- 53. Nakagata N, Yamamura K. Current activities of CARD as an international core center for mouse resources. Exp Anim 2009; 58:343–350. [DOI] [PubMed] [Google Scholar]
- 54. Nakagata N, Takeo T, Fukumoto K, Kondo T, Haruguchi Y, Takeshita Y, Nakamuta Y, Matsunaga H, Tsuchiyama S, Ishizuka Y, Araki K. Applications of cryopreserved unfertilized mouse oocytes for in vitro fertilization. Cryobiology 2013; 67:188–192. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data are available at BIOLRE online.