Abstract
Uterine luminal epithelium (LE) is essential for establishing uterine receptivity. Previous microarray analysis revealed upregulation of Atp6v0d2 in gestation day 4.5 (D4.5) LE in mice. Realtime PCR showed upregulation of uterine Atp6v0d2 starting right before embryo attachment ∼D4.0. In situ hybridization demonstrated specific uterine localization of Atp6v0d2 in LE upon embryo implantation. Atp6v0d2 encodes one subunit for vacuolar-type H+-ATPase (V-ATPase), which regulates acidity of intracellular organelles and extracellular environment. LysoSensor Green DND-189 detected acidic signals in LE and glandular epithelium upon embryo implantation, correlating with Atp6v0d2 upregulation in early pregnant uterus. Atp6v0d2−/− females had significantly reduced implantation rate and marginally reduced delivery rate from first mating only, but comparable number of implantation sites and litter size compared to control and comparable fertility to control from subsequent matings, suggesting a nonessential role of Atp6v0d2 subunit in embryo implantation. Successful implantation in both control and Atp6v0d2−/− females was associated with uterine epithelial acidification. No significant compensatory upregulation of Atp6v0d1 mRNA was detected in D4.5 Atp6v0d2−/− uteri. To determine the role of V-ATPase instead of a single subunit in embryo implantation, a specific V-ATPase inhibitor bafilomycin A1 (2.5 μg/kg) was injected via uterine fat pad on D3 18:00 h. This treatment resulted in reduced uterine epithelial acidification, delayed implantation, and reduced number of implantation sites. It also suppressed oil-induced artificial decidualization. These data demonstrate uterine epithelial acidification as a novel phenomenon during embryo implantation and V-ATPase is involved in uterine epithelial acidification and uterine preparation for embryo implantation.
Keywords: embryo implantation, uterine epithelial acidification, Atp6v0d2, V-ATPase, bafilomycin A1, uterine fat pad injection
Our findings that mouse uterine epithelium becomes more acidic upon embryo implantation initiation and suppression of uterine epithelial acidification adversely affects embryo implantation provide a novel direction for understanding mechanisms in establishing uterine receptivity.
Introduction
A receptive uterus is indispensable for the success of embryo implantation, which is a mandatory step for the success of mammalian reproduction. Precise cellular and molecular mechanisms of how a uterus transforms into a receptive state is far from being well understood [1–3]. Uterine epithelium includes luminal epithelium (LE) that lines up the uterine lumen and glandular epithelium (GE) that extends from LE into the underlying stromal layer. Luminal epithelium is the first layer of cells that an embryo communicates with for embryo implantation and is also essential for uterine receptive sensitivity [4, 5]. Glandular epithelium secretes molecules critical for embryo development and uterine preparation for embryo implantation [6]. Embryo implantation is associated with dynamic morphological and molecular changes in the LE [7–9]. It is expected that the dynamic expression of certain genes in the peri-implantation LE will hold a key to understanding mechanisms for the establishment of uterine receptivity. Our microarray analysis reveals Atpase, H+ transporting, lysosomal V0 subunit D2 (Atp6v0d2) as one of the most highly expressed and most highly upregulated genes in the gestation day 4.5 (D4.5) LE compared to the preimplantation D3.5 LE [4].
Atp6v0d2 encodes the more tissue-specific d subunit of the vacuolar-type H+-ATPase (V-ATPase). The d2 subunit is potentially involved in assembling the integral V0 domain and the peripheral V1 domain of V-ATPase [10]. The transmembrane integral V0 domain is composed of six subunits (a, c, c΄, c΄΄, d, and e) and involved in proton translocation, while the cytoplasmic peripheral V1 domain contains eight subunits (A–H) and is responsible for ATP hydrolysis [11–13]. The ATP-dependent proton transport by V-ATPase is from the cytoplasmic compartment to the opposite side of the membrane, which can be either the lumen of intracellular organelles (e.g., lysosomes, endosomes, and secretory vesicles) or the lumen of extracellular environment (e.g., epididymal lumen), to acidify the intracellular organelles or the extracellular environment, respectively [11]. Besides different cellular localizations, different tissue distributions and different utilizations of specific subunit(s) may also contribute to the diverse functions of V-ATPase [14, 15].
V-ATPase is crucial for male reproduction [16, 17]. Most of the V-ATPase subunits are strongly expressed in the apical side of rodent epididymal and vas deferens epithelium to establish and maintain an acidic luminal environment for sperm maturation and storage [18]. The importance of this acidic luminal environment has also been demonstrated in c-ros and forkhead box i1 (Foxi1) deficient male mice, which lack several V-ATPase subunits leading to increased epididymal luminal pH and infertility, with the latter caused by the defective movement of spermatozoa through the female reproductive tract for fertilization [19, 20].
The potential roles of V-ATPase in female reproduction, especially uterine epithelial function during embryo implantation, are largely unknown. One study demonstrates that three V-ATPase subunits (A, B, and c) are highly expressed in bovine uterine LE at the beginning of embryo–maternal interaction, suggesting potential participation of the uterine V-ATPase in embryo implantation [21]. A histochemical study finds increased lysosomal numbers and activity in D5.5 (postimplantation initiation) rat uterine epithelium compared to that in the nonpregnant state (estrus or diestrus stages) [22]. Lysosome is the most acidic organelle in the cell, and V-ATPase is a key regulator of lysosomal pH [23]. The upregulation of a V-ATPase subunit Atp6v0d2 in D4.5 LE [4] would suggest a potential function of V-ATPase in LE acidification and uterine preparation for embryo implantation. It was therefore hypothesized that Atp6v0d2 upregulation was important for LE acidification, and LE acidification was critical for embryo implantation. This hypothesis was tested using both genetic approach (Atp6v0d2−/− females) and pharmacological approach (V-ATPase inhibitor bafilomycin A1) in mice.
Materials and methods
Reagents
TRIzol, Superscript III and 20 × Saline-Sodium Citrate, LysoSensor Green DND-189 (Invitrogen, Carlsbad, CA, USA); dNTPs (Biomiga, San Diego, CA, USA); Taq DNA polymerase (Lucigen, Middleton, WI, USA); power SYBR Green PCR master and 384-well plates (Applied Biosystems, Carlsbad, CA, USA); superfrost plus slides, triton X-100 and formamide (Fisher Scientific, Pittsburgh, PA, USA); bafilomycin A1 and sesame oil (Sigma, St. Louis, MO, USA); Evans blue dye (Alfa Aesar, Ward Hill, MA, USA); isoflurane (Webster Veterinary, Devens, MA, USA); pGEM-T vector (Promega, Madison, WI, USA); DIG RNA labeling mix, blocking reagent, antidigoxigenin antibody, and NBT/BCIP (Roche Diagnostics, Indianapolis, IN, USA); methyl green, levamisole hydrochloride, and dextran sulfate sodium salt (MP biomedicals, Solon, OH, USA); iSript Reverse Transcription Supermix for RT-qPCR, SsoAdvanced Universal SYBR Green Supermix (Bio-rad, Hercules, CA, USA); primers for realtime PCR (Integrated DNA Technologies, Coralville, IA, USA).
Animals
C57BL6/129 mixed background wild type (WT), Atp6v0d2+/−, and Atp6v0d2−/− mice were derived from Dr. Yongwon Choi's colony at University of Pennsylvania and genotyped as previously described [24]. C57BL6/129 mixed background WT mice that were not used as a control for Atp6v0d2−/− mice were derived from our Lpar3 mouse colony [25, 26]. Mice were housed in polypropylene cages with free access to regular food and water from water sip tubes in a reverse osmosis system. The animal facility is on a 12-h light/dark cycle (6:00 AM to 6:00 PM) at 23 ± 1°C with 30–50% relative humidity. All methods used in this study were approved by the University of Georgia IACUC Committee (Institutional Animal Care and Use Committee) and conform to National Institutes of Health guidelines and public law.
Luminal epithelium isolation and uterine tissue collection
Young virgin WT females or Atp6v0d2−/– females were mated naturally with WT stud males and checked for a vaginal plug the next morning. The day a vaginal plug identified was designated as gestation day 0.5 (D0.5, mating night as D0). D3.5 and D4.5 LE cells were isolated as previously described using 0.5% dispase and gentle scraping [4, 27]. Uterine tissues were collected from euthanized WT females between 11:00 h and 12:00 h on D0.5, D2.5, D3.5, D4.5, and D5.5, and midnight of D4.0. Uterine tissues from Atp6v0d2−/– females and their littermates were collected between 11:00 h and 12:00 h on D3.5 and D4.5. Both uterine horns from D0.5 and D2.5 females were quickly removed and snap-frozen on dry ice. Oviducts from these mice were flushed with 1 × PBS for the presence of embryos/oocytes to determine the pregnancy status. About one-third of a uterine horn from each euthanized D3.5 female was frozen on dry ice for tissue sectioning. The remaining D3.5 uterine horns were flushed with 1 × PBS (to determine the status of pregnancy and to remove embryos for uterine gene expression study) and frozen on dry ice for realtime PCR. On D4.5 and D5.5, mice were anesthetized with isoflurane by inhalation and intravenously (i.v.) injected with Evans blue dye to visualize the implantation sites as previously described [28]. Uterine horns from D4.5 and D5.5 were frozen on dry ice for RNA isolation and/or tissue sectioning. In addition, flushed uterine horns from pseudopregnant and pregnant mice on D3.5 at 22:00 h were also collected and frozen. At least three pregnant mice were included in each group.
RNA isolation and realtime PCR
For quantitative gene expression in WT females, total RNAs from LE and uterus were isolated using TRIzol, and cDNA was prepared using Superscript III reverse transcriptase with random primers. Realtime PCR was performed in 384-well plates using SYBR Green I intercalating dye on ABI 7900. For quantitative gene expression between Atp6v0d2−/− and WT females, total RNA from D3.5 and D4.5 uteri and D4.5 LE was isolated using TRIzol, and cDNA was prepared using iSript Reverse Transcription Supermix for Realtime PCR, which was performed in 384-well plates using SsoAdvanced Universal SYBR Green Supermix on CFX384 Real-Time System. Primer sequences were listed in Table 1. The mRNA expression levels of Atp6v0a1, Atp6v0a2, Atp6v0a3, Atp6v0a4, Atp6v0b, Atp6v0c, Atp6v0d1, Atp6v0d2, Atp6v0e1, Atp6v0e2, mucolipin-1 (Mcoln1), two pore segment channel 1 (Tpcn1), chloride channel, voltage sensitive 7 (Clcn7), cystic fibrosis transmembrane conductance regulator (Cftr), hypoxanthine phosphoribosyltransferase 1 (Hprt1), and glyceraldehyde 3-phosphate dehydrogenase (Gapdh) were determined by realtime PCR. Two housekeeping genes, Gapdh and Hprt1, were used as controls.
Table 1.
Gene-specific primer sequences used in realtime PCR and/or in situ hybridization.
| PCR product | ||
|---|---|---|
| Gene | Primer sequence | size (bp) |
| Atp6v0a1 | F: AGGCAGCTGCTAAGAACA | 395 |
| R: AAAATCTCCGAACATCACAG | ||
| Atp6v0a2 | F: CATCTACCACATGCTCAACA | 388 |
| R: AGTCTGGGGTGATTCTCATT | ||
| Atp6v0a3 | F: GCTACTGCTGGAGACCTTG | 380 |
| R: CTGTTCTCCAAGGTGGATG | ||
| Atp6v0a4 | F: AAACAGAGTCTCACCGACAG | 391 |
| R: CCAACCTGTTCTGTGGAGT | ||
| Atp6v0b | F: TACACAGTCACCATCAGCAG | 383 |
| R: GAAGGGGAAGGTGATGATAG | ||
| Atp6v0c | F: TGGTGGTGGCAGTACTTATC | 398 |
| R: GCACTAGGACACTGCACATT | ||
| Atp6v0d1 | F: TGAAGCTGC ACCTACAGAGT | 390 |
| R: TTCATCTCATCAAGGTCCTG | ||
| Atp6v0d2 | F: GAGATGGAAGCTGTCAACAT | 398 |
| R: TCTGCCACTCTCTTCATCTG | ||
| Atp6v0e1 | F: GGACCACAGTTGAAAAATGA | 384 |
| R: GTCTCGCAGCAATTCTTAAA | ||
| Atp6v0e2 | F: CACCCATCTGTATGACCATC | 390 |
| R: TATTTCAACACGGTGGAGAC | ||
| Mcoln1 | F: CCTCTTCATCTACATGGTGC | 241 |
| R: CTTGGATCAGTTCACCAGC | ||
| Tpcn1 | F: ATGCCTCTGATGGAACAGG | 368 |
| R: ACCTTCCTGGAGGTAGATTG | ||
| Clcn7 | F: CATGGCCAACGTCTCTAAG | 341 |
| R: GGTTGATTCGTCTTTCCTCC | ||
| Cftr | F: CGAGCCAAAAGCATTGACC | 307 |
| R: GTGGATAAGCTGGGGATTCT | ||
| Hprt1 | F: GCTGACCTGCTGGATTACAT | 172 |
| R: CAATCAAGACATTCTTTCCAGT | ||
| Gapdh | F: GCCGAGAATGGGAAGCTTGTCAT | 205 |
| R: GTGGTTCACACCCATCACAAACAT | ||
| Abp1 | F: TACCCTAATGGTGTGATGGA | 398 |
| R: TCAGCCATAGAGTGGATCTG |
F: forward primer; R: reverse primer, 5΄→3΄.
In situ hybridization
In situ hybridization was done as previously described [4, 29–31]. The primer sequences used for making sense and antisense probes were listed in Table 1.
LysoSensor Green DND-189 staining
LysoSensor Green DND-189 is an acidotropic fluorescence probe that becomes fluorescent only when it is inside acidic compartments (pKa ∼ 5.2) and its fluorescence intensity correlates with intracellular acidity thus serves as an intracellular pH indicator [32–35]. To investigate uterine epithelial acidification during estrous cycle (determined by vaginal smear [36–38]), early pregnancy, and artificial decidualization [39] in WT mice, as well as in D4.5 Atp6v0d2−/− females with or without detectable implantation sites, 2 μl of LysoSensor Green DND-189 (2 μM) was injected into both uterine horns ∼20 min before dissection ∼11:00–12:00 h. Uterine horns were frozen on dry ice. Frozen uterine sections were heated at 55°C for 20 min and detected for fluorescence under a fluorescent microscope with excitation at 488 nm and emission at 505 nm. At least three mice were examined at each time point except for estrous cycle, in which two mice each at proestrus, estrus, metestrus, and diestrus were examined. At least two sections from each uterus were evaluated; for those with implantation sites, at least one implantation site per female and two sections per implantation site were evaluated.
Fertility test in Atp6v0d2−/− females
Young virgin (2 months old) Atp6v0d2−/− females (N = 19) and their littermate control females (N = 18, Atp6v0d2+/+ or Atp6v0d2+/−) were cohabitated with the same stud males. Copulation plug was checked every morning. Cohabitation continued till late pregnancy. Some females had multiple plugs before a term pregnancy occurred. All the females delivered their first litters within 3 to 6 weeks from the first day of cohabitation. Gestation period and litter size were recorded.
Embryo implantation in Atp6v0d2−/− females
Another set of 2-month-old virgin Atp6v0d2−/− females (N = 27) and control females (N = 8, Atp6v0d2+/+ or Atp6v0d2+/−, littermates to some of the Atp6v0d2−/− females) were mated similarly as for fertility test. Embryo implantation was detected on D4.5 as previously described [28]. The reproductive tracts of eight Atp6v0d2−/− females without implantation sites were flushed with 1 × PBS to check the presence of embryos/oocytes. One ovary was fixed for histology.
Uterine fat pad injection
This method was established in rats by Dr. Koji Yoshinaga [40–45] and was adapted in mice by our group to deliver drugs into the uterus without disturbing the intrauterine environment [39]. Young virgin WT females were mated naturally with WT stud males. Plugged females were randomly distributed into control group and V-ATPase inhibitor bafilomycin A1-treated groups. Bafilomycin A1 specifically inhibits V-ATPase via binding to V0 domain [46]. On D3 at 18:00 h, a small incision was made in the dorsolateral region on the right side of flank under anesthesia with isoflurane inhalation. Five microliter vehicle (20% of ethanol in 1× PBS with blue dye to monitor the injection) or bafilomycin A1 (0.5 or 2.5 μg/kg in vehicle) was injected at 1–2 spots on the adipose tissue next to the right uterine horn only. Implantation sites were detected using blue dye injection [28] on D4.5, D5.5, and D7.5 for vehicle control and 2.5 μg/kg bafilomycin A1 treatment groups, and on D4.5 for 0.5 μg/kg bafilomycin A1 treatment group. If no implantation sites on D4.5 were detected, the uterine horns were flushed with 1× PBS to determine the presence and health status of blastocysts. Uterine tissues were snap-frozen and kept in –80°C for in situ hybridization and some were also processed for LysoSensor Green DND-189 staining as described above.
Artificial decidualization
Artificial decidualization was induced as previously described [47]. Briefly, sesame oil (20 μl) was infused intraluminally in the right uterine horn of pseudopregnant WT females on D3.5 at 10:00 h (mating night with vasectomized male mice was defined as D0). On pseudopregnant D3 at 18:00 h, 5 μl of vehicle or bafilomycin A1 (2.5 μg/kg) was injected into the uterine fat pad surrounding the arteries of the oil-infused uterine horn (right side) following the procedure described above. On pseudopregnant D7.5, the females were i.v. injected with Evans blue dye to visualize decidual response similar as to visualize implantation sites [28], and the weights of the infused (right) uterine horns were recorded. The right uterine horns were frozen for in situ hybridization to detect a molecular marker Abp1 for decidual response [48].
Statistical analyses
Two-tail unequal variance Student t-test was used to compare the mRNA expression levels, the numbers of implantation sites, and the weights of the right uterine horns. Two-tailed Fisher exact test was used to compare the rates (e.g., implantation rate and delivery rate). The significant level was set at P < 0.05.
Results
Spatiotemporal expression of Atp6v0d2 in early pregnant wild-type uterus
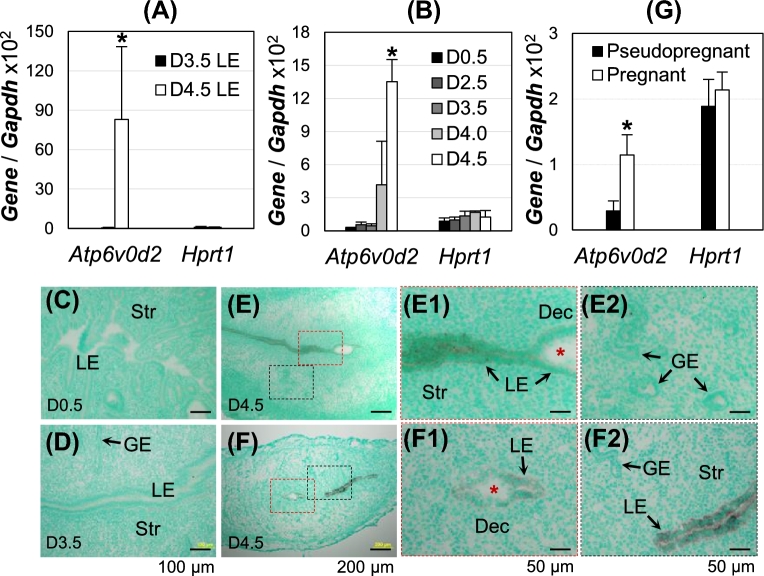
Our previous microarray analysis revealed upregulation of Atp6v0a4, Atp6v0c, and Atp6v0d2, and downregulation of Atp6v0a1 and Atp6v0d1 among all 23 V-ATPase subunits (Atp6v0a1, Atp6v0a2, Atp6v0a3, Atp6v0a4, Atp6v0b, Atp6v0c, Atp6v0d1, Atp6v0d2, Atp6v0e1, Atp6v0e2, Atp6v1A, Atp6v1B1, Atp6v1B2, Atp6v1C1, Atp6v1C2, Atp6v1D, Atp6v1E1, Atp6v1E2, Atp6v1F, Atp6v1G1, Atp6v1G2, Atp6v1G3, Atp6v1H) in gestation day 4.5 (D4.5) LE compared to D3.5 LE. The differential expression of these genes was modest (<2 fold) except for Atp6v0d2 [4], which was dramatically upregulated in D4.5 LE. Realtime PCR of isolated LE from D3.5 (preimplantation) and D4.5 (postimplantation initiation) pregnant mice confirmed the dramatic upregulation (>100 fold) of Atp6v0d2 in D4.5 LE compared to D3.5 LE (Figure 1A). Realtime PCR of early pregnant uteri demonstrated basal levels of expression in preimplantation uteri by D3.5; >8 fold increase in D4.0 uterus compared to D3.5 uterus and continued increase at D4.5 (Figure 1B). In situ hybridization verified the upregulation and LE-specific localization of Atp6v0d2 upon embryo implantation. Atp6v0d2 level was lower in the LE at the implantation site compared to nonimplantation site on D4.5 (Figure 1E–F2). No significant expression level of Atp6v0d2 mRNA was detected in other uterine compartments (Figure 1E2 and F2). Atp6v0d2 remained highly expressed in the LE on D5.5 (data not shown). The upregulation of Atp6v0d2 was related to pregnancy since a significantly higher level of Atp6v0d2 was detected in the pregnant uterus than in the pseudopregnant uterus on D3 at 22:00 h (Figure 1G), right before embryo attachment in pregnant uterus on ∼D4.0 [49]. These results demonstrated that Atp6v0d2 expression in LE was correlated with pregnancy and its upregulation started before implantation initiation and continued during initial stages of embryo implantation.
Figure 1.
Spatiotemporal expression of Atp6v0d2 in early pregnant WT mouse uterus. (A, B, G) Realtime PCR, normalized with Gapdh and Hprt1 as a second control; *P < 0.05; error bar: standard deviation; (C–F) in situ hybridization, counterstained with methyl green; D4.0, gestation day 4.0, about time for embryo attachment in mice. (A) Atp6v0d2 mRNA expression in D3.5 and D4.5 LE, N = 5–6. (B) Atp6v0d2 mRNA expression level in peri-implantation uterus, N = 4–6. (C–F) Localization of Atp6v0d2 mRNA in D0.5 (C), D3.5 (D), and D4.5 (E, F) uteri. E1 and E2, enlarged images from the red and black rectangle areas in E, respectively; F1 and F2, enlarged images from the red and black rectangle areas in F, respectively; red *, embryo; LE, uterine luminal epithelium; GE, uterine glandular epithelium; Str, stroma; Dec, decidual zone; scale bar, 100 μm (C, D), 200 μm (E, F), or 50 μm (E1, E2, F1, F2). (G) Atp6v0d2 mRNA expression in pseudopregnant and pregnant uterus at D3 22:00 h. N = 4–5.
Uterine epithelial acidification upon implantation initiation
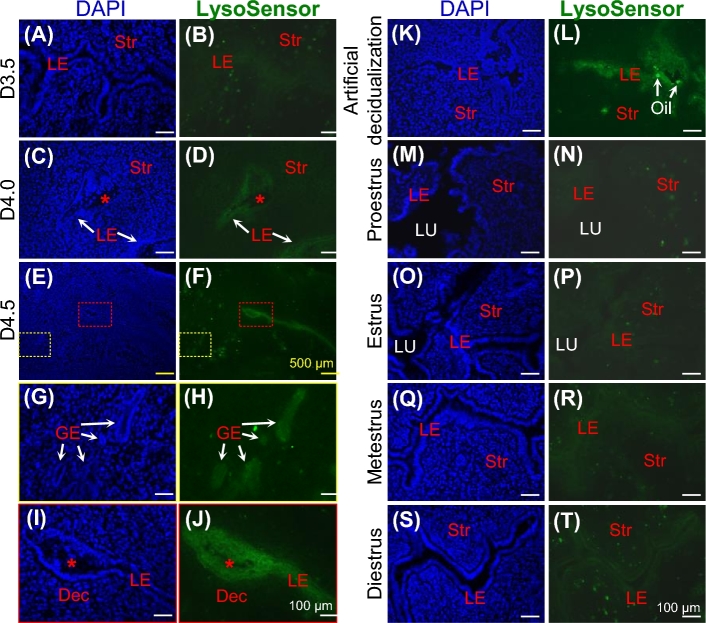
Atp6v0d2 encodes a tissue-specific d subunit of V-ATPase that regulates the acidity of extracellular lumen pH and pH of intracellular organelles [50]. LysoSensor Green DND-189 was used to detect intracellular acidity because it becomes fluorescent only when it is inside acidic compartments (pKa ∼ 5.2) [32]. This fluorescent dye detected basal levels of fluorescence in preimplantation pregnant D0.5 and D2.5 uteri (data not shown), basal level fluorescence (data not shown), or above basal level fluorescence in the D3.5 uterine epithelium (Figure 2A and B); detectable signals in D4.0 uterine epithelium (Figure 2C and D); stronger signals in D4.5 uterine epithelium, including LE at both implantation site and interimplantation site and GE (Figure 2E–J); as well as in the D5.5 remaining uterine epithelium but not in the D5.5 implantation chamber where the LE surrounding the embryo had disappeared (data not shown). Strong fluorescence was also detected in the pseudopregnant D4.5 uterine horns with artificial decidualization induced by intrauterine oil infusion (Figure 2K and L) but not in the contralateral uterine horns without oil infusion (data not shown). Fluorescence at basal levels was detected in the nonpregnant uteri at different estrus stages, although the fluorescence in the uterine epithelium at metestrus and diestrus appeared to be above the basal level (Figure 2M–T). LysoSensor Green DND-189 staining did not appear to label the uterine stroma and myometrium during early pregnancy (Figure 2 and data not shown). These data demonstrated increased uterine epithelial acidification during early stages of embryo implantation in mice.
Figure 2.
Uterine epithelial acidification during embryo implantation, artificial decidualization, and estrous cycle in WT mice. D4.0, gestation day 4.0, embryo attachment; DAPI, staining nuclei; LysoSensor Green DND-189, reflecting intracellular acidity; LE, uterine luminal epithelium; GE, uterine glandular epithelium; Str, stroma; Dec, decidual zone; LU, uterine lumen; red *, embryo; cross sections (10 μm) of frozen uteri; scale bars, 100 μm (A–D, G–T), or 500 μm (E, F); left panel, DAPI staining of nuclei; right panel, LysoSensor Green DND-189 staining of uterine sections in left panel. (A, B) D3.5 uterus. (C, D) D4.0 uterus. (E, F) D4.5 uterus. (G, H) Enlarged from the yellow rectangles in E and F, respectively. (I, J) Enlarged from the red rectangles in E and F, respectively. (K, L) D4.5 uterus from oil-infused artificial decidualization. Bright green dots in the lumen area were infused oil droplets. (M–T) Different stages of estrous cycle.
Embryo implantation and fertility in Atp6v0d2−/− females
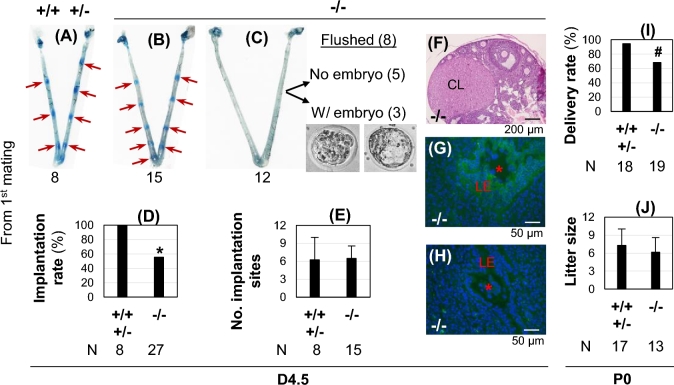
Since upregulation of Atp6v0d2 correlated with increased uterine epithelial acidification (Figures 1 and 2), did Atp6v0d2 subunit play a role in embryo implantation? Atp6v0d2−/− females (2 months old) from first mating had significantly reduced implantation rate compared to their littermate control females (Figure 3A–D). The 15 Atp6v0d2−/− mice with detectable implantation sites had a comparable number of implantation sites to the control (Figure 3E). Among the 12 Atp6v0d2−/− females without implantation sites, the reproductive tracts of 8 females were flushed, 3 with embryos and 5 without embryos (Figure 3C). Interestingly, all the flushed embryos had intact zona pellucida (Figure 3C), which is normally present in D3.5 embryos but not D4.5 embryos [28, 51, 52]. In addition, all five Atp6v0d2−/− females without implantation sites or embryos had corpora lutea (Figure 3F), suggesting ovulation had occurred. LysoSensor Green DND-189 detected uterine epithelial acidification in the Atp6v0d2−/− uteri with implantation sites indicated by blue dye reaction (Figure 3G) but not in those without detectable implantation sites, even with the presence of an embryo (Figure 3H). Fertility test indicated marginally reduced (P = 0.0897, two-tailed Fisher exact test) delivery rate from the first mating in Atp6v0d2−/− females compared to that from the littermate control (Figure 3I). However, the litter size from those delivered Atp6v0d2−/− females was not significantly different from the litter size in the control group (Figure 3J). The Atp6v0d2−/− females that did not get pregnant from the first mating eventually became pregnant from the subsequent matings and delivered pups within 6 weeks after cohabitation. They had comparable gestation periods and produced comparable litter sizes as the control (data not shown). These data demonstrate that Atp6v0d2-deficiency affected embryo implantation and fertility in some mice from the first mating only and did not affect the overall fertility, and that uterine epithelial acidification was always associated with successful embryo implantation.
Figure 3.
Embryo implantation and fertility in Atp6v0d2−/− females from the first mating. +/+ / +/–, WT and heterozygotes as control; –/–, Atp6v0d2−/−; control females and Atp6v0d2−/− females were mated with the same stud males; 8, 15, etc., numbers of mice in each condition/group; red arrow, implantation site; A–H, data from gestation day 4.5 (D4.5); I–J, data from postnatal day 0 (P0). (A) D4.5 control uterus. (B) D4.5 Atp6v0d2−/− uterus with implantation sites. (C) D4.5 Atp6v0d2−/− uterus without an implantation site. Among the 12 uteri without an implantation site, 8 were flushed, 5 of them with no embryos flushed and remaining 3 with embryos flushed. All the flushed embryos had zona pellucida. (D) D4.5 implantation rate, expressed as the number of mice with implantation site/total number of plugged mice × 100. *P < 0.05. (E) Number of implantation sites from the uteri with implantation sites on D4.5. (F) Histology of an ovary from an Atp6v0d2−/− mouse without embryo or implantation site as seen in Figure 3C. CL, corpus luteum; scale bar, 200 μm. (G, H) Merged images of DAPI staining (blue) and LysoSensor Green DND-189 staining (green) in uteri with (G) or without (H) detectable implantation sites. LE, uterine luminal epithelium; *, embryo; uterine cross sections (10 μm); scale bar, 50 μm. (I) Delivery rate, expressed as the number of mice delivered pups/total number of plugged mice × 100. #P = 0.0897 (two-tailed Fisher exact test). (J) Litter size at birth. (E, J) Error bar, standard deviation.
Gene expression in Atp6v0d2−/− uterus and luminal epithelium
Since Atp6v0d2 encodes only one of the 14 subunits for a functional V-ATPase, it was possible that there were compensatory regulations, especially of Atp6v0d1 that also encodes a d subunit, for the loss of Atp6v0d2 in the uterus and/or LE during embryo implantation. Since the difference in mRNA levels was only observed in some V0 subunits but not any of the V1 subunits between D3.5 LE and D4.5 LE from microarray [4], all the V0 subunits, together with several lysosomal counter ion channels, were examined using realtime PCR. There were no significant changes in mRNA levels between D3.5 WT and D3.5 Atp6v0d2−/− uteri except an upregulation of Atp6v0a3 in the D3.5 Atp6v0d2−/− uteri; no significant difference in mRNA levels was detected between D4.5 WT and D4.5 Atp6v0d2−/− uteri with implantation sites (Figure 4A). Since Atp6v0d2 was mainly detected in the LE and potential changes in LE could be obscured in the whole uterus, D4.5 LE was isolated from WT uteri with implantation sites (+/+ IS), Atp6v0d2−/− uteri with implantation sites (–/– IS) or without implantation sites (–/– no IS). No significant difference except <1 fold reduction of Atp6v0a3, Atp6v0a4, and Atp6v0e1 in the +/+ IS compared to –/– IS was observed (Figure 4B). Several genes, Atp6v0a1, Atp6v0a2, Atp6v0a3, Atp6v0d1, Atp6v0e1, Tpcn1, and Cftr, had <3 fold increased expression in the –/– no IS compared to –/– IS (Figure 4B), which might be related to the implantation status because some of these genes, such as Atp6v0a1, Atp6v0d1, and Cftr, showed reduced mRNA levels in D4.5 LE compared to preimplantation D3.5 LE in the microarray analysis [4]. These data indicated no obvious compensatory regulation of related genes at the transcriptional level but did not exclude potential compensatory regulation at other levels than transcription.
Figure 4.
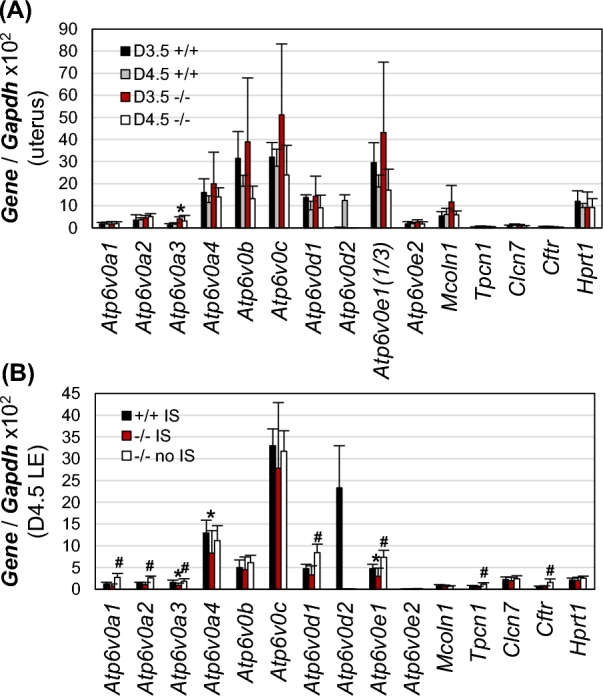
Realtime PCR detecting mRNA levels of V-ATPase V0 subunits and counter ion channels in Atp6v0d2−/− uterine LE and uterus. +/+, wild type; –/–, Atp6v0d2−/−; IS, implantation site; error bar, standard deviation; N = 6–8. (A) mRNA levels of V0 subunits and counter ion channels in D3.5 and D4.5 WT and Atp6v0d2−/− uteri with implantation sites. Atp6v0e1 was shown one-third of its value; *P < 0.05, compared to D3.5 +/+. (B) mRNA levels of V0 subunits and counter ion channels in D4.5 WT LE (+/+ IS) and Atp6v0d2−/− LE (–/– IS) with implantation sites and D4.5 Atp6v0d2−/− LE without implantation sites (–/– no IS). *P < 0.05, compared to +/+ IS; #P < 0.05, compared to –/– IS.
Effect of V-ATPase inhibitor bafilomycin A1 on embryo implantation
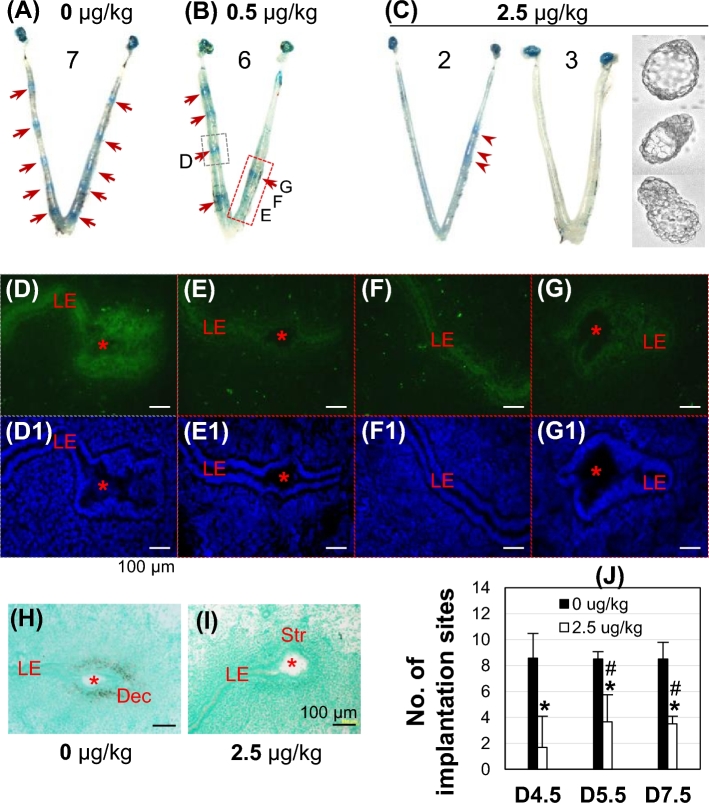
Since Atp6v0d2 is only one of many subunits for a functional V-ATPase, the nonessential role of Atp6v0d2 in embryo implantation and female fertility (Figure 3) could not rule out potential function of V-ATPase in uterine epithelial acidification and embryo implantation. Local uterine fat pad injection of 0.5 or 2.5 μg/kg bafilomycin A1 dose-dependently suppressed embryo implantation detected on D4.5 (Figure 5A–C). A uterus from 0.5 μg/kg bafilomycin A1-injected group had four implantation sites on the uninjected side and one implantation site on the injected side (Figure 5B). The sections from the injected side (Figure 5B, red rectangle) had dim green fluorescence in the LE surrounding an embryo without blue dye reaction (Figure 5E, E1), at interimplantation site (Figure 5F, F1), or surrounding an embryo with clear blue dye reaction (Figure 5G, G1), an indication of embryo implantation. The green fluorescence in the LE (Figure 5D, D1) from the uninjected side (Figure 5B, gray rectangle) was stronger than that from the bafilomycin A1-injected site (Figure 5E–G1). Bafilomycin A1 had a similar effect on the green fluorescence intensity in the GE (data not shown) as that in LE. The green fluorescence in the D4.5 uterine epithelium was further lower to barely detectable from mice treated with 2.5 μg/kg bafilomycin A1, especially those without detectable implantation sites (data not shown). These data indicated the inhibitory effect of bafilomycin A1 on uterine epithelial acidification.
Figure 5.
Effects of V-ATPase inhibitor bafilomycin A1 on embryo implantation and uterine epithelial acidification in WT mice. (A–C) Representative uterine images from 0 (A), 0.5 (B), and 2.5 μg/kg (C) bafilomycin A1-treated groups detected on D4.5. Red arrow, defined implantation site; red arrowhead, delayed implantation site; 7, 6, 2, 3, numbers of mice in each condition/group. Embryos in C flushed from the uterine horns without implantation sites. Bafilomycin A1 was injected into uterine fat pad on the right uterine horn on D3 at 18:00 h, 4–6 h before implantation initiation [49]. (D–G) LysoSensor Green DND-189 staining of the implantation site indicated by the gray rectangle (D) and the approximate uterine segments in the red rectangle (no blue dye reaction in the uterine segments for E and F and a clear implantation site for G) in B. (D1–G1) DAPI staining of the sections in D–G, respectively. (H, I) In situ hybridization of decidualization marker Abp1 in an implantation site from A (H) and a faint implantation site in C (I). (D–I) Red star, embryo; LE, uterine luminal epithelium; Str, stroma; Dec, decidual zone; scale bar, 100 μm. (J) Number of implantation sites in vehicle and 2.5 μg/kg bafilomycin A1-treated groups detected on D4.5, D5.5, and D7.5. Error bar, standard deviation; *P < 0.05, compared to vehicle-treated group; #P < 0.05 compared to bafilomycin A1-treated group on D4.5; N = 3–7.
The effect of bafilomycin A1 on embryo implantation was confirmed by uterine expression of decidualization marker Abp1 [48], which was detected in the primary decidual zone of an implantation site in a vehicle-injected uterus (Figure 5A and H), but not in the stromal area of an implantation site in a 2.5 μg/kg bafilomycin A1-treated uterus (Figure 5C and I). The numbers of implantation sites were also determined on D5.5 and D7.5 in vehicle and 2.5 μg/kg bafilomycin A1-treated groups. Bafilomycin A1 treatment significantly reduced the numbers of implantation sites at all three time points compared to vehicle control (Figure 5J). No significant difference in the numbers of implantation sites was observed between D5.5 and D7.5 in the 2.5 μg/kg bafilomycin A1-treated group, but at these two time points, the numbers of implantation sites were significantly higher than that at D4.5 (Figure 5J) and the implantation sites in the D4.5 2.5 μg/kg bafilomycin A1-treated group were faint (Figure 5C), indicating delayed implantation. These data revealed that uterine local fat pad administration of V-ATPase inhibitor bafilomycin A1 not only suppressed uterine epithelial acidification, but also delayed implantation for some embryos, and prevented implantation for other embryos.
Effect of V-ATPase inhibitor bafilomycin A1 on decidualization
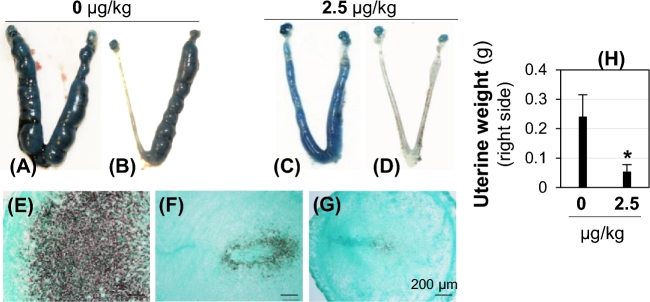
To eliminate any potential effect of bafilomycin A1 on embryos in implantation (Figure 5) and to further demonstrate the effect of bafilomycin A1 on uterine function during embryo implantation, uterine fat pad injection of 2.5 μg/kg bafilomycin A1 was performed in an oil-induced artificial decidualization mouse model. Suppression of artificial decidualization upon bafilomycin A1 treatment was indicated by significant reduction of the right (treated side) uterine horn weight on D7.5 in the treated group compared to that in the control group (Figure 6A–D and H), as well as reduced expression of a uterine decidualization marker Abp1 [48] in the treated group (Figure 6F and G) compared to that in the control group (Figure 6E).
Figure 6.
Effect of bafilomycin A1 on artificial decidualization detected on pseudopregnant D7.5 WT mice. Artificial decidualization was induced by intraluminal oil injection on right side and bafilomycin A1 treatment was also on the right side via uterine fat pad injection. (A–D) Representative uteri from vehicle (A, B) or bafilomycin A1 injection (C, D). A and C shows decidualization on both uterine horns due to oil leakage from the right side. (E–G) Detection of decidualization marker Abp1 on right uterine horns by in situ hybridization. E from B; F from C; G from D; Scale bar, 200 μm. (H) Weight of right uterine horn (injected side). Error bar, standard deviation; N = 4; *P < 0.05.
Discussion
This study demonstrates a novel phenomenon that uterine epithelium becomes more acidic during the initial stages of embryo implantation. Increased uterine epithelial acidity is readily detectable at the time of embryo attachment ∼D4.0 in natural pregnant uterus and in artificially decidualized pseudopregnant D4.5 uterus by LysoSensor Green DND-189 dye, indicating that uterine epithelial acidification is associated with early uterine preparation for embryo implantation in the presence of an embryo or other stimulus, such as oil, in the uterus.
Although no report is available in the literature about uterine epithelial acidification upon embryo implantation in any species, the following studies imply that uterine epithelial acidification might be a common phenomenon. Striking increases in enzymatic activity of acid phosphatase, which is stored in the lysosome and requires optimal acidic pH, were observed in the uterine LE during initial stages of embryo implantation in rat, mouse, and rabbit [53]. Increased lysosomal numbers and activity were found in D5.5 rat uterine epithelium (postimplantation initiation) compared to nonpregnant state (estrus or diestrus stages) [22]. Transmission electron microscopic study of bovine endometrium detected lysosomes and autolysosomes in the LE at the initial stages of embryo implantation [54]. Three V-ATPase subunits were highly expressed in the bovine LE during early stages of embryo implantation and became pericellularly localized, instead of the apical localization seen in the nonpregnant LE [21]. Although no human uterine epithelial data are available, microarray data (identifier GSE6364) of human endometrium at proliferative and secretory phases in normal and endometriosis patients revealed significant upregulation of several highly expressed V-ATPase subunits in the secretory (receptive) phase but such upregulation was abolished in the endometrium of endometriosis patients [55]. The underlying mechanisms for uterine epithelial acidification upon embryo implantation have never been investigated.
LysoSensor Green DND-189 becomes fluorescent only when it is inside acidic compartments (pKa ∼ 5.2) [32]. Since the lysosome is the most acidic intracellular organelle with pH < 5 [50, 56], uterine epithelial acidification detected by LysoSensor Green DND-189 most likely indicates lysosomal acidification, although acidification of other intracellular organelles could not be ruled out. Lysosome acidity is mainly maintained by ATP-driven proton pump V-ATPase coupled with additional ion transporters to dissipate the transmembrane voltage built up by V-ATPase [57].
V-ATPase is a complex enzyme composed of multiple subunits [14]. Some subunits have multiple isoforms, e.g., a subunit has four isoforms (a1, a2, a3, and a4) identified and d subunit has two isoforms (d1 and d2) identified [58, 59]. Despite amino acid sequence similarity, different V-ATPase subunits can have differential expression patterns. For example, a1 is ubiquitously expressed [59]; a2 is highly expressed in the lung, kidney, and spleen [60]; a3 is mainly detectable in osteoclasts [15]; and a4 is expressed in the kidney and male reproductive tract [18, 58]; d1 is ubiquitously expressed [14], whereas d2 is more dominant in kidney, lung, and osteoclast [61]. The expression patterns of the V-ATPase subunits in the peri-implantation uterus had not been previously investigated. Our previous microarray analysis [4] and this study demonstrated the most dramatic upregulation of Atp6v0d2 mRNA, moderate differential mRNA expression of some other V0 subunits, and no change of mRNA levels of V1 subunits in the uterine LE upon embryo implantation, indicating the differential expression and regulation patterns of V-ATPase subunits in the uterine LE.
Although the precise functions of each V-ATPase subunit are not fully understood, the differential expression patterns of V-ATPase subunits would suggest potentially different functions of the subunits. Indeed, limited data have demonstrated that mutations in ATP6V1B1 cause distal renal tubular acidosis in patients [62], mutations in ATP6V0A4 are associated with atypical progressive sensorineural hearing loss and distal renal tubular acidosis [59, 63], while Atp6v0d2-deficiency in mice results in impaired osteoclast fusion and increased bone formation [24]. Although Atp6v0d2 mRNA was highly upregulated in the LE upon embryo implantation, Atp6v0d2−/− females had reduced implantation rate only from the first mating and they had comparable overall fertility as age-matched control, indicating that Atp6v0d2 was not essential for embryo implantation or female fertility. V-ATPase, together with other ion channels, such as cystic fibrosis transmembrane regulator, Cl−/HCO3− exchanger (SLC26A6), sodium-hydrogen exchanger-1, CA isoenzymes II and XII [64, 65], and possibly epithelial Na+ channel [66], also regulates uterine lumen pH. Based on the reduced D4.5 implantation rate and retained zona pellucida of D4.5 blastocysts from Atp6v0d2−/− females without implantation sites in the first mating, Atp6v0d2-deficiency might initially affect the uterine lumen pH, thus timely blastocyst development and hatching from zona pellucida for embryo implantation in some Atp6v0d2−/− mice, which subsequently adapted to Atp6v0d2-deficiency and had normal fertility.
It was noticed that Atp6v0d2 mRNA in the D4.5 LE at the implantation chamber was lower than its level in the D4.5 LE at interimplantation site, whereas D4.5 LE acidification at implantation site was comparable, if not stronger, than that at interimplantation site; and that upregulation of Atp6v0d2 mRNA upon embryo implantation was mainly detected in the LE but not in the GE, yet GE and LE had comparable acidification upon embryo implantation. These observations suggest that Atp6v0d2 subunit may not play an important role in LE acidification at the implantation site or in GE acidification, and different mechanisms may be involved in regulating acidification of LE at the implantation site and the interimplantation site, and acidification of GE upon embryo implantation.
V-ATPase activity is regulated at multiple levels, such as transcriptional and/or translational regulation of subunits, domain assembly, alternative subunits (e.g., Atp6v0d1 also encodes a d subunit), and targeting of subunits to specific membranes [11–13, 67]. No significant compensatory transcriptional regulation of V0 subunits (especially Atp6v0d1) and counter ion channels in D4.5 Atp6v0d2−/− LE coupled with no obviously impaired fertility in Atp6v0d2−/− females suggests nonessential role of Atp6v0d2 subunit in embryo implantation, and/or compensatory regulations of other V-ATPase subunits and/or counter ions at levels other than transcription, such as translation, domain assembly, etc. [11–13, 67]. The data from Atp6v0d2−/− females cannot exclude potential function of V-ATPase as a whole unit in embryo implantation.
To determine the potential function of V-ATPase on embryo implantation, we took advantage of a pharmacological approach using the specific V-ATPase inhibitor bafilomycin A1 [68] coupled with uterine fat pad injection [39–45]. We noticed that the lower dose of bafilomycin A1 (0.5 μg/kg) via uterine fat pad injection caused an adverse effect mainly on the injected side, while the higher dose (2.5 μg/kg) had an adverse effect on both uterine horns, suggesting that sufficient levels of the drug might be transported to the uninjected uterine horn through the local uterine blood supply. The data from uterine fat pad injection of bafilomycin A1 demonstrated a critical role of V-ATPase for uterine function in embryo implantation. However, with this approach, the roles of V-ATPase in different uterine compartments (e.g., uterine epithelium, stroma, myometrium) on embryo implantation could not be differentiated.
This study demonstrated the novel phenomenon of uterine epithelial acidification upon embryo implantation and the involvement of V-ATPase in regulating uterine epithelial acidification and uterine preparation for embryo implantation. V-ATPase plays an essential role in regulating lysosomal acidification [23], which affects the functions of lysosomes in intracellular metabolism and nutrient homeostasis in many cell types [69]. The molecular mechanisms for lysosomal acidification in uterine epithelium as well as the functions of uterine epithelial acidification in the initial uterine-embryo communications and uterine epithelial-stromal paracrine communications remain to be investigated.
Acknowledgments
Authors thank the Office of the Vice President for Research, Interdisciplinary Toxicology Program, and Department of Physiology and Pharmacology at University of Georgia. This work was supported by the National Institutes of Health grants to XY: NIH R15HD066301 and NIH R01HD065939 (co-funded by Office of Research on Women's Health (ORWH) and Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD).
Conflict of interest statement: The authors declare that there are no conflicts of interest.
References
- 1. Jones CJ, Aplin JD. Glycosylation at the fetomaternal interface: does the glycocode play a critical role in implantation? Glycoconj J 2009; 26:359–366. [DOI] [PubMed] [Google Scholar]
- 2. Schlafke S, Enders AC. Cellular basis of interaction between trophoblast and uterus at implantation. Biol Reprod 1975; 12:41–65. [DOI] [PubMed] [Google Scholar]
- 3. Yoshinaga K. A sequence of events in the uterus prior to implantation in the mouse. J Assist Reprod Genet 2013; 30:1017–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Xiao S, Diao H, Zhao F, Li R, He N, Ye X. Differential gene expression profiling of mouse uterine luminal epithelium during periimplantation. Reprod Sci 2014; 21:351–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Denker HW. Implantation: a cell biological paradox. J Exp Zool 1993; 266:541–558. [DOI] [PubMed] [Google Scholar]
- 6. Spencer TE. Biological roles of uterine glands in pregnancy. Semin Reprod Med 2014; 32:346–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Murphy CR. Uterine receptivity and the plasma membrane transformation. Cell Res 2004; 14:259–267. [DOI] [PubMed] [Google Scholar]
- 8. Xiao S, Diao H, Zhao F, Li R, He N, Ye X. Differential gene expression profiling of mouse uterine luminal epithelium during periimplantation. Reprod Sci 2013; 21:351–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lee W. Electron microscopic observations of the rat endometrium during the implantation period, with special reference to its sex steroid hormone regulation (author's transl). Nihon Naibunpi Gakkai Zasshi 1975; 51:938–966. [DOI] [PubMed] [Google Scholar]
- 10. Toei M, Saum R, Forgac M. Regulation and isoform function of the V-ATPases. Biochemistry 2010; 49:4715–4723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Forgac M. Structure and properties of the vacuolar (H+)-ATPases. J Biol Chem 1999; 274:12951–12954. [DOI] [PubMed] [Google Scholar]
- 12. Qi J, Wang Y, Forgac M. The vacuolar (H+)-ATPase: subunit arrangement and in vivo regulation. J Bioenerg Biomembr 2007; 39:423–426. [DOI] [PubMed] [Google Scholar]
- 13. Smith AN, Lovering RC, Futai M, Takeda J, Brown D, Karet FE. Revised nomenclature for mammalian vacuolar-type H+ -ATPase subunit genes. Mol Cell 2003; 12:801–803. [DOI] [PubMed] [Google Scholar]
- 14. Sun-Wada GH, Wada Y, Futai M. Diverse and essential roles of mammalian vacuolar-type proton pump ATPase: toward the physiological understanding of inside acidic compartments. Biochim Biophys Acta 2004; 1658:106–114. [DOI] [PubMed] [Google Scholar]
- 15. Toyomura T, Murata Y, Yamamoto A, Oka T, Sun-Wada GH, Wada Y, Futai M. From lysosomes to the plasma membrane: localization of vacuolar-type H+-ATPase with the a3 isoform during osteoclast differentiation. J Biol Chem 2003; 278:22023–22030. [DOI] [PubMed] [Google Scholar]
- 16. Da Silva N, Shum WW, Breton S. Regulation of vacuolar proton pumping ATPase-dependent luminal acidification in the epididymis. Asian J Androl 2007; 9:476–482. [DOI] [PubMed] [Google Scholar]
- 17. Herak-Kramberger CM, Sabolic I, Blanusa M, Smith PJ, Brown D, Breton S. Cadmium inhibits vacuolar H(+)ATPase-mediated acidification in the rat epididymis. Biol Reprod 2000; 63:599–606. [DOI] [PubMed] [Google Scholar]
- 18. Pietrement C, Sun-Wada GH, Silva ND, McKee M, Marshansky V, Brown D, Futai M, Breton S. Distinct expression patterns of different subunit isoforms of the V-ATPase in the rat epididymis. Biol Reprod 2006; 74:185–194. [DOI] [PubMed] [Google Scholar]
- 19. Blomqvist SR, Vidarsson H, Soder O, Enerback S. Epididymal expression of the forkhead transcription factor Foxi1 is required for male fertility. EMBO J 2006; 25:4131–4141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yeung CH, Breton S, Setiawan I, Xu Y, Lang F, Cooper TG. Increased luminal pH in the epididymis of infertile c-ros knockout mice and the expression of sodium-hydrogen exchangers and vacuolar proton pump H+-ATPase. Mol Reprod Dev 2004; 68:159–168. [DOI] [PubMed] [Google Scholar]
- 21. Skinner MA, MacLaren LA, Wildeman AG. Stage-dependent redistribution of the V-ATPase during bovine implantation. J Histochem Cytochem 1999; 47:1247–1254. [DOI] [PubMed] [Google Scholar]
- 22. Kirk AT, Murphy CR. Increase in lysosomal numbers and activity in the rat uterine luminal and glandular epithelium during early pregnancy: a histochemical study. Acta Anat (Basel) 1991; 141:63–69. [DOI] [PubMed] [Google Scholar]
- 23. Ishida Y, Nayak S, Mindell JA, Grabe M. A model of lysosomal pH regulation. J Gen Physiol 2013; 141:705–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lee SH, Rho J, Jeong D, Sul JY, Kim T, Kim N, Kang JS, Miyamoto T, Suda T, Lee SK, Pignolo RJ, Koczon-Jaremko B, et al. v-ATPase V0 subunit d2-deficient mice exhibit impaired osteoclast fusion and increased bone formation. Nat Med 2006; 12:1403–1409. [DOI] [PubMed] [Google Scholar]
- 25. Diao H, Li R, El Zowalaty AE, Xiao S, Zhao F, Dudley EA, Ye X. Deletion of lysophosphatidic acid receptor 3 (Lpar3) disrupts fine local balance of progesterone and estrogen signaling in mouse uterus during implantation. Biol Reprod 2015; 93:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Diao H, Aplin JD, Xiao S, Chun J, Li Z, Chen S, Ye X. Altered spatiotemporal expression of collagen types I, III, IV, and VI in Lpar3-deficient peri-implantation mouse uterus. Biol Reprod 2011; 84:255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ye X, Herr DR, Diao H, Rivera R, Chun J. Unique uterine localization and regulation may differentiate LPA3 from other lysophospholipid receptors for its role in embryo implantation. Fertil Steril 2011; 95:2107–2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ye X, Hama K, Contos JJ, Anliker B, Inoue A, Skinner MK, Suzuki H, Amano T, Kennedy G, Arai H, Aoki J, Chun J. LPA3-mediated lysophosphatidic acid signalling in embryo implantation and spacing. Nature 2005; 435:104–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Xiao S, Li R, Diao H, Zhao F, Ye X. Progesterone receptor-mediated regulation of n-acetylneuraminate pyruvate lyase (NPL) in mouse uterine luminal epithelium and nonessential role of NPL in uterine function. PLoS One 2013; 8:e65607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Diao H, Xiao S, Li R, Zhao F, Ye X. Distinct spatiotemporal expression of serine proteases prss23 and prss35 in periimplantation mouse uterus and dispensable function of prss35 in fertility. PLoS One 2013; 8:e56757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Diao H, Xiao S, Zhao F, Ye X. Uterine luminal epithelium-specific proline-rich acidic protein 1 (PRAP1) as a marker for successful embryo implantation. Fertil Steril 2010; 94:2808–2811. [DOI] [PubMed] [Google Scholar]
- 32. Ishiguro K, Ando T, Watanabe O, Goto H. Novel application of low pH-dependent fluorescent dyes to examine colitis. BMC Gastroenterol 2010; 10:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Perzov N, Padler-Karavani V, Nelson H, Nelson N. Characterization of yeast V-ATPase mutants lacking Vph1p or Stv1p and the effect on endocytosis. J Exp Biol 2002; 205:1209–1219. [DOI] [PubMed] [Google Scholar]
- 34. Bigler L, Spirli C, Fiorotto R, Pettenazzo A, Duner E, Baritussio A, Follath F, Ha HR. Synthesis and cytotoxicity properties of amiodarone analogues. Eur J Med Chem 2007; 42:861–867. [DOI] [PubMed] [Google Scholar]
- 35. Teichgraber V, Ulrich M, Endlich N, Riethmuller J, Wilker B, De Oliveira-Munding CC, van Heeckeren AM, Barr ML, von Kurthy G, Schmid KW, Weller M, Tummler B et al. Ceramide accumulation mediates inflammation, cell death and infection susceptibility in cystic fibrosis. Nat Med 2008; 14:382–391. [DOI] [PubMed] [Google Scholar]
- 36. Byers SL, Wiles MV, Dunn SL, Taft RA. Mouse estrous cycle identification tool and images. PLoS One 2012; 7:e35538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Li R, Diao H, Zhao F, Xiao S, El Zowalaty AE, Dudley EA, Mattson MP, Ye X. Olfactomedin 1 deficiency leads to defective olfaction and impaired female fertility. Endocrinology 2015; 156:3344–3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Li R, Zhao F, Diao H, Xiao S, Ye X. Postweaning dietary genistein exposure advances puberty without significantly affecting early pregnancy in C57BL/6J female mice. Reprod Toxicol 2014; 44:85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Diao H, Xiao S, Howerth EW, Zhao F, Li R, Ard MB, Ye X. Broad gap junction blocker carbenoxolone disrupts uterine preparation for embryo implantation in mice. Biol Reprod 2013; 89:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yoshinaga K. Effect of local application of ovarian hormones on the delay in implantation in lactating rats. J Reprod Fertil 1961; 2:35–41. [DOI] [PubMed] [Google Scholar]
- 41. Yoshinaga K. On the delayed implantation in the lactating pregnant rat. III. The local action of progesterone. Jpn J Anim Reprod 1961; 6:152–154. [Google Scholar]
- 42. Yoshinaga K. On the delayed implantation in the lactating pregnant rat. IV. The local action of estrogen. Jpn J Anim Reprod 1961; 6:155–158. [Google Scholar]
- 43. Yoshinaga K, Pincus G. Local effect of estrogen on cholesterol synthesis in the uterus of ovariectomized rats. Steroids 1963; 1:656–663. [Google Scholar]
- 44. Ferrando G, Nalbandov AV. Relative importance of histamine and estrogen on implantation in rats. Endocrinology 1968; 83:933–937. [DOI] [PubMed] [Google Scholar]
- 45. Psychoyos A. New remarks on the determinism of ovum implantation. C R Hebd Seances Acad Sci 1962; 254:4360–4362. [PubMed] [Google Scholar]
- 46. Drose S, Altendorf K. Bafilomycins and concanamycins as inhibitors of V-ATPases and P-ATPases. J Exp Biol 1997; 200:1–8. [DOI] [PubMed] [Google Scholar]
- 47. Lim H, Paria BC, Das SK, Dinchuk JE, Langenbach R, Trzaskos JM, Dey SK. Multiple female reproductive failures in cyclooxygenase 2-deficient mice. Cell 1997; 91:197–208. [DOI] [PubMed] [Google Scholar]
- 48. Liang XH, Zhao ZA, Deng WB, Tian Z, Lei W, Xu X, Zhang XH, Su RW, Yang ZM. Estrogen regulates amiloride-binding protein 1 through CCAAT/enhancer-binding protein-beta in mouse uterus during embryo implantation and decidualization. Endocrinology 2010; 151:5007–5016. [DOI] [PubMed] [Google Scholar]
- 49. Diao H, Paria BC, Xiao S, Ye X. Temporal expression pattern of progesterone receptor in the uterine luminal epithelium suggests its requirement during early events of implantation. Fertil Steril 2011; 95:2087–2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mindell JA. Lysosomal acidification mechanisms. Annu Rev Physiol 2012; 74:69–86. [DOI] [PubMed] [Google Scholar]
- 51. Zhao F, Li R, Xiao S, Diao H, Viveiros MM, Song X, Ye X. Postweaning exposure to dietary zearalenone, a mycotoxin, promotes premature onset of puberty and disrupts early pregnancy events in female mice. Toxicol Sci 2013; 132:431–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Xiao S, Diao H, Smith MA, Song X, Ye X. Preimplantation exposure to bisphenol A (BPA) affects embryo transport, preimplantation embryo development, and uterine receptivity in mice. Reprod Toxicol 2011; 32:434–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sengupta J, Roy SK, Manchanda SK. Hormonal control of implantation: a possible role of lysosomal function in the embryo-uterus interaction. J Steroid Biochem 1979; 11:729–744. [DOI] [PubMed] [Google Scholar]
- 54. Leiser R, Wille KH. Cytochemical establishment of acid phosphatase in the bovine endometrium and trophoblast during implantation. Anat Embryol (Berl) 1975; 148:159–173. [DOI] [PubMed] [Google Scholar]
- 55. Burney RO, Talbi S, Hamilton AE, Vo KC, Nyegaard M, Nezhat CR, Lessey BA, Giudice LC. Gene expression analysis of endometrium reveals progesterone resistance and candidate susceptibility genes in women with endometriosis. Endocrinology 2007; 148:3814–3826. [DOI] [PubMed] [Google Scholar]
- 56. Casey JR, Grinstein S, Orlowski J. Sensors and regulators of intracellular pH. Nat Rev Mol Cell Biol 2010; 11:50–61. [DOI] [PubMed] [Google Scholar]
- 57. Xu H, Ren D. Lysosomal physiology. Annu Rev Physiol 2015; 77:57–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Oka T, Murata Y, Namba M, Yoshimizu T, Toyomura T, Yamamoto A, Sun-Wada GH, Hamasaki N, Wada Y, Futai M. a4, a unique kidney-specific isoform of mouse vacuolar H+-ATPase subunit a. J Biol Chem 2001; 276:40050–40054. [DOI] [PubMed] [Google Scholar]
- 59. Wagner CA, Finberg KE, Breton S, Marshansky V, Brown D, Geibel JP. Renal vacuolar H+-ATPase. Physiol Rev 2004; 84:1263–1314. [DOI] [PubMed] [Google Scholar]
- 60. Peng SB, Li X, Crider BP, Zhou Z, Andersen P, Tsai SJ, Xie XS, Stone DK. Identification and reconstitution of an isoform of the 116-kDa subunit of the vacuolar proton translocating ATPase. J Biol Chem 1999; 274:2549–2555. [DOI] [PubMed] [Google Scholar]
- 61. Smith AN, Borthwick KJ, Karet FE. Molecular cloning and characterization of novel tissue-specific isoforms of the human vacuolar H(+)-ATPase C, G and d subunits, and their evaluation in autosomal recessive distal renal tubular acidosis. Gene 2002; 297:169–177. [DOI] [PubMed] [Google Scholar]
- 62. Karet FE, Finberg KE, Nelson RD, Nayir A, Mocan H, Sanjad SA, Rodriguez-Soriano J, Santos F, Cremers CW, Di Pietro A, Hoffbrand BI, Winiarski J et al. Mutations in the gene encoding B1 subunit of H+-ATPase cause renal tubular acidosis with sensorineural deafness. Nat Genet 1999; 21:84–90. [DOI] [PubMed] [Google Scholar]
- 63. Li X, Chai Y, Tao Z, Li L, Huang Z, Li Y, Wu H, Yang T. Novel mutations in ATP6V0A4 are associated with atypical progressive sensorineural hearing loss in a Chinese patient with distal renal tubular acidosis. Int J Pediatr Otorhinolaryngol 2012; 76:152–154. [DOI] [PubMed] [Google Scholar]
- 64. Karim K, Giribabu N, Muniandy S, Salleh N. Vacuolar-ATPase (V-ATPase) mediates progesterone-induced uterine fluid acidification in rats. J Membr Biol 2016; 249:65–76. [DOI] [PubMed] [Google Scholar]
- 65. Gholami K, Muniandy S, Salleh N. In-vivo functional study on the involvement of CFTR, SLC26A6, NHE-1 and CA isoenzymes II and XII in uterine fluid pH, volume and electrolyte regulation in rats under different sex-steroid influence. Int J Med Sci 2013; 10:1121–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ruan YC, Guo JH, Liu X, Zhang R, Tsang LL, Dong JD, Chen H, Yu MK, Jiang X, Zhang XH, Fok KL, Chung YW et al. Activation of the epithelial Na+ channel triggers prostaglandin E(2) release and production required for embryo implantation. Nat Med 2012; 18:1112–1117. [DOI] [PubMed] [Google Scholar]
- 67. O’Callaghan KM, Ayllon V, O’Keeffe J, Wang Y, Cox OT, Loughran G, Forgac M, O’Connor R. Heme-binding protein HRG-1 is induced by insulin-like growth factor I and associates with the vacuolar H+-ATPase to control endosomal pH and receptor trafficking. J Biol Chem 2010; 285:381–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Yoshimori T, Yamamoto A, Moriyama Y, Futai M, Tashiro Y. Bafilomycin A1, a specific inhibitor of vacuolar-type H(+)-ATPase, inhibits acidification and protein degradation in lysosomes of cultured cells. J Biol Chem 1991; 266:17707–17712. [PubMed] [Google Scholar]
- 69. Colacurcio DJ, Nixon RA. Disorders of lysosomal acidification-The emerging role of v-ATPase in aging and neurodegenerative disease. Ageing Res Rev 2016; 32:75–88. [DOI] [PMC free article] [PubMed] [Google Scholar]