Abstract
The Oncology Section of the American Physical Therapy Association (APTA) developed a clinical practice guideline to aid the clinician in diagnosing secondary upper quadrant cancer-related lymphedema. Following a systematic review of published studies and a structured appraisal process, recommendations were written to guide the physical therapist and other health care clinicians in the diagnostic process. Overall clinical practice recommendations were formulated based on the evidence for each diagnostic method and were assigned a grade based on the strength of the evidence for different patient presentations and clinical utility. In an effort to maximize clinical applicability, recommendations were based on the characteristics as to the location and stage of a patient's upper quadrant lymphedema.
Secondary lymphedema is a relatively underestimated and poorly understood sequela of cancer treatment that can adversely impact quality of life.1 Cancer treatments, such as radiation therapy and resection of lymph nodes, can result in decreased lymph resorption and transport, leading to lymphatic insufficiency. When extracellular fluid is not resorbed by the impaired lymphatic system and reaches a measurable level of lymphatic insufficiency (which has been defined in a variety of ways), a patient is diagnosed with lymphedema.
The presence of secondary upper quadrant lymphedema (SUQL) can lead to significant morbidity, activity and participation restrictions, reduced quality of life, and economic hardship.2–6 In 2016 alone, it was estimated that 1.6 million people in the United States were diagnosed with cancer,7 many involving the upper quadrant, and thus a large number of cancer survivors are at risk of developing SUQL. Incidence rates of SUQL vary widely and are estimated at 6% to 70% in patients with breast cancer,8,9 5% in patients with upper extremity melanoma,10 and 73.5% in patients with head and neck cancer.11 Reasons for the wide variations in incidence rates of SUQL following cancer treatment include heterogeneity in patient and clinical characteristics and the type and extent of medical interventions. Additionally, variability across the populations studied, length of follow-up, diagnostic criteria and methods, and definitions of lymphedema contribute to inconsistencies in incidence and prevalence statistics.
Prior to establishing a plan of care, clinicians obtain a history and perform body function and structure tests and measures to establish a diagnosis, assess the stage and/or severity of the condition, and then determine the impact on activity and participation. At present, lymphedema is most often diagnosed by clinical history, physical examination of tissue quality, symptomology, and/or the presence of increased limb volume. Based on the medical history, clinicians inquire about risk factors and medical treatments known to impact lymphatic transport. As a part of differential diagnosis, clinicians also determine if other pathologies known to cause edema are likely, such as blood clot or cancer recurrence. If other causes of swelling are ruled out, clinicians then determine if disruptions of the lymphatic system have impaired lymphatic transport capacity sufficiently to diagnose lymphedema. If lymphedema—either clinically apparent or subclinical—is suspected, further assessment is warranted.
Current objective measures of SUQL include bioimpedence analysis (BIA); circumferential measurement; water displacement; and perometry and imaging, which may incorporate differences between limbs or from baseline. Clinicians may also classify the severity of the lymphatic system impairment. The International Society of Lymphology (ISL) consensus document12 classifies the severity of lymphedema by stage:
Stage 0—Subclinical state where the peripheral swelling is not visible, but lymphatic transport is impaired. Symptoms and subtle tissue changes may be noted.
Stage I—Early onset of swelling that is visible and subsides with elevation. Pitting may be present.
Stage II—Consistent volume change with pitting present. Elevation rarely reduces the swelling and progressive tissue fibrosis occurs.
Stage III—Skin changes such as thickening, hyperpigmentation, increased skin folds, fat deposits, and warty overgrowths occur. Tissue is very fibrotic and pitting is absent.
Early detection of lymphatic insufficiency, coupled with appropriate intervention, may be important to prevent progression of the condition and may provide a cost-effective approach.13,14 Diagnostic definitions of lymphedema that require a consistent volume increase, and thus being at ISL stage II or above, have the potential to hamper efforts to intervene at the early stages. Providing timely and appropriate care to patients with SUQL requires that physical therapists and other health care professionals have access to guidelines that assist in directing their assessment and management. By implementing this evidence-based practice guideline, health care professionals will be better able to detect lymphedema of the upper quadrant, both at the subclinical and clinically apparent stages, allowing this population to maintain maximum function and quality of life.
Need for Clinical Practice Guideline for Diagnosis of Secondary Upper Quadrant Lymphedema
A review of the Agency for Healthcare Quality and Research's (AHQR) National Guideline Clearinghouse and PubMed yielded few clinical practice guidelines (CPGs) or systematic reviews focusing on the assessment of patients with secondary lymphedema. In 2008, Poage et al15 provided guidelines for the nursing profession regarding cancer-related lymphedema interventions. Harris et al16,17 presented recommendations about modalities and interventions used by clinicians in the treatment of breast cancer and lymphedema—but did not include diagnosis and assessment.
In 2010, the AHRQ conducted a technology assessment of diagnostic tests and nonpharmacologic treatments for secondary lymphedema.18 The AHRQ summary stated that further research is needed in these areas. In 2013, Armer et al19 published an expert opinion guideline on assessment, risk reduction, management, and surveillance but not for the diagnosis of post-breast cancer lymphedema. Currently, few guidelines exist to help the clinician determine the most appropriate methods for diagnosing SUQL.
The Oncology Section of the American Physical Therapy Association (APTA) commissioned the writing of an evidence-based guideline for secondary lymphedema. The goals of the Guideline Development Group were to:
Describe evidence-based diagnostic methods and criteria for cancer-related SUQL.
Identify gaps in the research for the diagnosis of cancer-related SUQL.
Create reference publications for use by physical therapy professionals regardless of subspecialty and setting, as well as for other health care practitioners and students, identifying best practice related to diagnosis of SUQL.
This guideline document reflects the work of this group in developing recommendations for the detection and diagnosis of SUQL.
Methods
With the assistance of academic reference librarians from Saint Louis University and University of Southern California, search strategies were developed and executed in the following databases: Pubmed, CINAHL Plus with full text, Cochrane, AHRQ National Guideline Clearinghouse, SCOPUS, Sports Discus with full text, Physiotherapy Evidence Database (PEDRo), and Occupational Systematic Evaluation of Evidence (OTseekr). The final search terms included: Lymphedema, Elephantiasis, and truncated text words lymphedema*, lymphoedema*, elephantiasis. Articles including the terms filariasis, parasites, congenital, hereditary, as well as editorial, letter, and comment, were excluded.
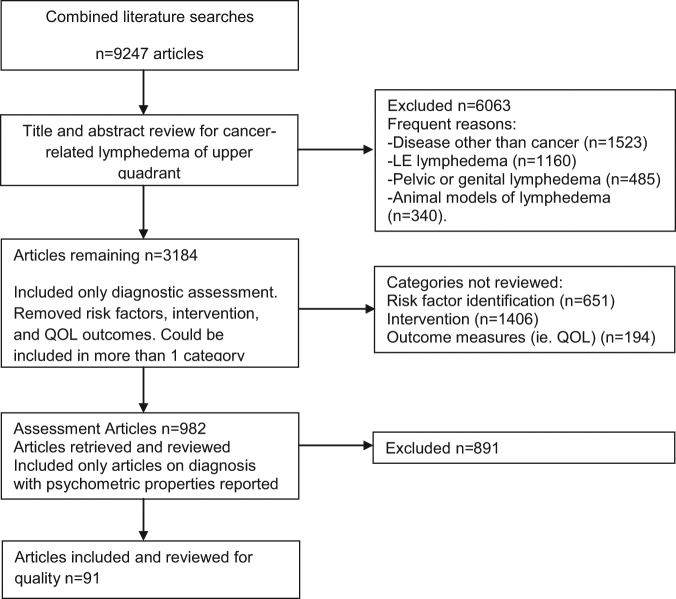
Literature published January 1, 2000, through July 5, 2015, was searched and reviewed for inclusion (Fig. 1). The search was run in 2 phases during CPG development: the initial search included studies from January 1, 2000, through June 30, 2013, and a second search included studies from July 1, 2013, through July 5, 2015. Each title and abstract was reviewed by one Guideline Development Group member for meeting the following eligibility criteria: investigated some aspect of cancer-related lymphedema of the upper quadrant, included relevant psychometrics, and the articles were written in the English language. Case studies and animal studies were excluded. Based on title and abstract reviews, articles were placed into the following categories: diagnosis and assessment, incidence and prevalence, risk factor identification, and intervention. Articles could be classified into more than one category. For this phase of the CPG development, only articles pertaining to assessment and diagnosis were retrieved and reviewed for inclusion by a Guideline Development Group member.
Figure 1.
Evidence Flow Chart
Quality Appraisal Process
As the articles were diagnostic in nature, the Quality Appraisal of Diagnostic Reliability (QAREL) checklist was used to rate the quality of reliability studies, and the Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) tool was used to rate validity and diagnostic accuracy studies.20–23 The 11-item QAREL checklist evaluates participant and examiner properties, blinding, order effects, time interval and test implementation and interpretation, and appropriateness of the statistical tests used.20,21 The QUADAS-2 evaluates appropriateness of patients (selection), choice and application of reference standard with which to compare the results of the index test, blinding of testers to results of other tests, sources of potential bias, and clinical applicability.22,23
The Guideline Development Group completed critical appraisals on 5 test articles to establish inter-rater reliability prior to initiating the article review process. Each Guideline Development Group member completed a critical appraisal for all test articles, and, when a 100% agreement was achieved, appraisers were paired to review the remaining diagnostic articles. Two appraisers from the Guideline Development Group were assigned to review each article independently and then compare results. If these 2 appraisers could not come to consensus, a third member of the Guideline Development Group was recruited to appraise the article, and consensus was achieved. A quality rating for each individual paper was assigned according to criteria established by the Centre for Evidence-Based Medicine (http://www.cebm.net/index.aspx?o=1025) for diagnostic studies and utilized in other CPGs (Tab. 1).24,25 If 2 Guideline Development Group members did not agree on the quality rating for a particular article, a third Guideline Development Group member was utilized to determine the rating.
Table 1.
| Level | >Criteria |
|---|---|
| I | Evidence obtained from high-quality diagnostic studies, prognostic or prospective studies, cohort studies or randomized controlled trials, meta-analyses or systematic reviews; critical appraisal score >50% |
| II | Evidence obtained from lesser-quality diagnostic studies, prognostic or prospective studies, cohort studies or randomized controlled trials, meta-analyses or systematic reviews (eg, weaker diagnostic criteria and reference standards, improper randomization, no blinding, <80% follow-up); critical appraisal score ≤50% |
| III | Case-controlled studies, retrospective studies, or studies of only healthy control subjects |
The evidence for each diagnostic method was then synthesized and appraised as a whole. The number of articles reviewed for each diagnostic method and the quality level of papers (Level I-III) is displayed in Table 2. Some articles reported on more than one psychometric property or more than one diagnostic method. For a diagnostic method to receive a higher evidence grade, the body of the evidence must have contained reliability, validity, and diagnostic accuracy studies. If an examination method did not have a diagnostic accuracy study, an evidence grade for that tool as an assessment of lymphedema—but not as a diagnostic measure—was assigned. Thus, the group distinguished between (1) tests and measures that could be used to support a diagnosis of lymphedema, and (2) assessment tools that provide important information about the condition at the body structure and function level but do not have evidence for their use as a diagnostic measure.
Table 2.
Number of Studies at Each Evidence Level Across Diagnostic Methods
| Diagnostic Method | Reliability | Validity | Diagnostic Accuracy | Total Number Studies | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| I | II | III | I | II | III | I | II | III | ||
| Clinical Examination | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| Questionnaires | 0 | 2 | 0 | 0 | 3 | 3 | 0 | 4 | 2 | 11 |
| Bioelectric Impedance Analysis (BIA) | 0 | 4 | 3 | 1 | 5 | 8 | 0 | 6 | 1 | 21 |
| Circumferential Measures | 8 | 12 | 3 | 1 | 6 | 7 | 0 | 5 | 1 | 30 |
| Water Displacement | 6 | 8 | 4 | 1 | 1 | 1 | 0 | 1 | 0 | 18 |
| Perometry | 2 | 4 | 0 | 0 | 1 | 5 | 0 | 0 | 0 | 10 |
| 3D Scanning | 0 | 0 | 3 | 0 | 1 | 3 | 0 | 0 | 0 | 5 |
| Tissue Dielectric Constant (TDC) | 1 | 2 | 0 | 0 | 2 | 4 | 0 | 0 | 0 | 7 |
| Ultrasound | 0 | 0 | 2 | 0 | 3 | 2 | 1 | 0 | 0 | 7 |
| Dual-Energy X-Ray Absorbiometry (DXA) | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 4 |
| Magnetic Resonance Imaging (MRI) | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 2 |
| Computed Tomography (CT) Scan | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 2 |
| Lymphoscintigraphy | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 3 |
| Lymphography | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 2 |
| Tonometry | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 |
Evidence grades were assigned based on an overall appraisal by the Guideline Development Group for all aspects of psychometric properties for each diagnostic and assessment method. Clinical utility and cost were factored into the process by expert opinion and general availability of equipment. The criteria for these evidence grades were based on previous work of APTA Academy of Pediatrics and Orthopedic Section Guideline Development Groups (Tab. 3).25,26 See eAppendix (available at https://academic.oup.com/ptj) for the quality rating for each article appraised.
Table 3.
| Grade | Recommendation | Criteria |
|---|---|---|
| A | Strong | A preponderance of Level I studies, but at least 1 Level I study directly on the topic supports the recommendation |
| B | Moderate | A preponderance of Level II studies, but at least 1 Level II study directly on the topic supports the recommendation |
| C | Weak | A single Level II study at <25% critical appraisal score or a preponderance of Level III and IV studies, including consensus statements by content experts, support the recommendation |
| D | Theoretical/Foundational | A preponderance of evidence from animal or cadaver studies, from conceptual/theoretical models/principles, or from basic science/bench research or published expert opinion in peer-reviewed journals supports the recommendation |
| EO | Expert Opinion | Recommended practice based on current clinical practice norms, exceptional situations where validating studies have not or cannot be performed, and there is clear benefit, harm, or cost and/or the clinical experience of the Guideline Development Group |
Overall clinical practice recommendations (Tab. 4) were formulated based on the evidence for each diagnostic method and were assigned a grade based on the strength of the evidence for different patient presentations and clinical utility as determined by the Guideline Development Group. In an effort to make these clinically applicable, recommendations were based on the characteristics of the location and stage of a patient's lymphedema within the upper quadrant. The categories and subcategories for practice recommendations were written for:
All patients at risk for SUQL
- Upper extremity secondary lymphedema
- - At risk, early
- - Moderate/established
- - Late
Lymphedema primarily in the hand
Trunk/breast lymphedema
Head and neck lymphedema
Table 4.
Practice Recommendations Based on Patient Presentationa
| Patient Presentation | Practice Recommendations |
|---|---|
| All Patients At Risk for Secondary Upper Quadrant Lymphedema | Self-reported symptoms of swelling, heaviness, and numbness should be investigated for early diagnosis (Grade B) |
| Palpation for fibrosis, pitting, and overall tissue quality may be clinically helpful for staging; however, it has not been investigated for diagnostic purposes (Expert Opinion) | |
| If a questionnaire is used to assist with diagnosis, the Norman Questionnaire or Morbidity Screening Tool should be considered (Grade B) | |
| At Risk/Early Upper Extremity Lymphedema (ILS Stage 0-I) | Bioimpedance Analysis: |
| Bioimpedance analysis (BIA) should be used to detect subclinical/early stage lymphedema (Grade B) | |
| – Cutpoint of >7.1 L-Dex score should be used for diagnosis of breast cancer–related lymphedema when preoperative baseline measures are not available (Grade B) | |
| – Cutpoint of >10 L-Dex score above preoperative baseline should be used for diagnosis of breast cancer–related lymphedema (Grade B) | |
| – Preoperative assessment using BIA may enhance the ability to detect changes in tissue fluid earlier indicating lymphedema (Grade B) | |
| Volume Measures: | |
| Volume determined from circumferential measurements should be used to diagnose lymphedema (Grade B) but may not capture subclinical and early-stage lymphatic transport impairment (Expert Opinion) | |
| – When using circumferential measurements, volume should be calculated (Grade B) | |
| – Calculated volume differential between sides ≥200 ml, or a volume ratio of >1.04 (affected:unaffected), will help rule in lymphedema, but values <200 ml cannot be used to rule out (Grade B) | |
| – Water displacement may be used in diagnosing lymphedema but is limited by clinical utility (Grade B) | |
| – Volume can also be assessed by perometry, but diagnostic criteria need to be evaluated for this method (Expert Opinion) | |
| – Methods of volume measurement are not interchangeable; use the same method at each time point (Grade A) | |
| Moderate or Established Upper Extremity Lymphedema (ILS Stage II) | Bioimpedance Analysis: |
| Bioimpedance analysis (BIA) is less useful in diagnosing lymphedema at this stage, and self-reported symptoms or volume measures should be used (Grade B) | |
| Accuracy with BIA in diagnosing moderate to late stage lymphedema may decline due to tissue changes/fibrosis (Expert Opinion) | |
| Volume Measures: | |
| Volume measurements should be taken and used in the diagnosis of lymphedema (Grade B) | |
| – When using circumferential measurements, volume should be | |
| calculated (Grade B) | |
| – Calculated volume differential between sides of ≥200 ml, or a volume ratio of >1.04 (affected:unaffected), will help rule in lymphedema, but values <200 ml cannot be used to rule out (Grade B) | |
| – Water displacement may be used in diagnosing lymphedema but is limited by clinical utility (Grade B) | |
| – Volume can also be assessed by perometry but diagnostic criteria need to be evaluated for this method -(Expert Opinion) | |
| – Methods of volume measurement are not interchangeable; use the same method each time point (Grade A) | |
| Late Upper Extremity Lymphedema (ILS Stage III) | As tissue changes progress, excess fluid may decrease, but excess volume may remain because of fibrosis, increased fat deposition, and other skin changes (Grade B) |
| Volume Measures: | |
| Volume measurements should be taken and used in the diagnosis of lymphedema (Grade B) | |
| – When using circumferential measurements, volume should be calculated (Grade B) | |
| – Calculated volume differential between sides of ≥200 ml, or a volume ratio of >1.04 (affected:unaffected), will help rule in lymphedema, but values <200 ml cannot be used to rule out (Grade B) | |
| – Water displacement may be used in diagnosing lymphedema but has limited clinical utility (Grade B) | |
| – Volume can also be assessed by perometry, but diagnostic criteria need to be evaluated for this method -(Expert Opinion) | |
| – Methods of volume measurement are not interchangeable; use the same method at each time point (Grade A) | |
| Ultrasound: | |
| Ultrasound should be utilized to detect underlying tissue changes (Grade B) | |
| Hand Lymphedema | Little research is available to guide diagnosis of hand lymphedema |
| Water displacement and figure of 8 method of circumferential measurement may be used for assessment but have not been studied as diagnostic tests (Expert Opinion) | |
| Trunk or Breast Lymphedema | Little research is available to guide diagnosis of truncal or breast lymphedema |
| Ultrasound has the potential to determine tissue changes consistent with different stages of lymphedema -(Expert Opinion) | |
| Tissue dielectric constant is an emerging diagnostic tool that may be useful in assisting with assessment of lymphedema (Grade C) | |
| Head and Neck Lymphedema | Modified Head and Neck External Lymphedema and Fibrosis Assessment Criteria when combined with circumferential measurements may be useful for diagnostic purposes (Expert Opinion) |
| Circumferential measurements at the upper neck point may be used in assessment (Expert Opinion) | |
| Tissue dielectric constant is an emerging diagnostic tool that may be useful in assessing lymphedema (Expert Opinion) | |
| Recommend a combined approach involving both the Modified Head and Neck External Lymphedema and Fibrosis Assessment and either circumferential measures or tissue dielectric constant (Expert Opinion) |
aILS = International Society of Lymphology.
An expert in guideline writing—as well as expert oncology clinicians (physical therapist, occupational therapist, physical medicine and rehabilitation physician, and a breast surgeon) and researchers published in the field of lymphedema detection and diagnosis—completed a review of this manuscript prior to its submission for review and publication. The CPG was also posted on the APTA Oncology Section website for public comment from practicing clinicians. Feedback on content from the expert panel of reviewers and the comments on clarity and clinical utility of the practice recommendations from the additional public commentary period were then used to revise the CPG.
Recommendations and Summary of Evidence for Individual Diagnostic Methods
Based on the evidence, a recommendation was made for each diagnostic method. These recommendations were then used to formulate the overall clinical practice recommendations presented in Table 4. Results are reported for clinical examination and patient-reported symptom assessment, BIA, volume measures, and other diagnostic measures.
Clinical Examination and Patient-Reported Symptom Assessments
Clinical Examination Recommendation
The clinician may palpate the upper quadrant for fibrosis, pitting, and overall tissue quality. Results from the palpation of the upper limb and trunk have not been investigated for diagnostic utility or accuracy. Evidence Quality: Level II validity, no reliability or diagnostic accuracy; Recommendation Strength: Expert Opinion
Clinical examination, using the Modified Head and Neck External Lymphedema and Fibrosis (HN-ELAF) assessment criteria for patients with head and neck lymphedema, may be incorporated into clinical practice and used in conjunction with circumferential measurement for diagnostic purposes. Evidence Quality: Level II reliability and validity, no diagnostic accuracy; Recommendation Strength: Expert Opinion
Summary of evidence
Although stages of secondary lymphedema and tissue fibrosis are often assessed and documented by a clinician, there is no evidence to support a tool to use for clinical examination to diagnose SUQL. One Level II study compared clinical staging with lymphoscintigraphy in patients with chronic lymphedema,27 but the authors did not provide psychometric properties.
In the head and neck region, the HN-ELAF is used to assess and grade the severity of lymphedema with outcomes ranging from grade 1 (no visible edema) to grade 5 (severe fibrosis). Reliability of the assessment criteria was 83% percent agreement for lymphedema and severity of fibrosis and 100% agreement within one grade. Deng et al28 concluded that the HN-ELAF demonstrated significant agreement between testers (k = 0.75; concordance correlation coefficient = 0.91). There were no studies to support diagnostic accuracy.
Patient-Report Symptom Assessment Recommendation
Clinically, self-reported signs of swelling, heaviness, and numbness should be investigated to facilitate early diagnosis and should signal clinicians to use other SUQL measures. Evidence Quality: Level II reliability and validity, Level II diagnostic accuracy; Recommendation Strength: Grade B
- The following questionnaires should be considered to assist in the diagnosis of SUQL:
- - Norman Questionnaire Evidence Quality: Level II reliability and diagnostic accuracy, no validity; Recommendation Strength: Grade B
- - Morbidity Screening Tool (MST) Evidence Quality: Level II validity and diagnostic accuracy, no reliability; Recommendation Strength: Grade B
Summary of evidence
Assessment tools were included in this review only if they were used as a diagnostic method. There are several such assessment tools to guide the clinician in making a diagnosis and accurately staging lymphedema. The assessment tools reviewed require a yes/no response, self-report of symptoms, and/or marking a visual analogue scale (VAS).
Hayes et al29,30 describe a self-report questionnaire measuring a response of “yes or no” to the question: “Since the diagnosis of your breast cancer have you experienced arm swelling?” A “yes” response may indicate the presence of lymphedema.29,30 No reliability or validity studies were found. Self-report demonstrated sensitivity (65.1%) and specificity (76.9%).29 In 2008, Hayes et al30 reported a sensitivity of 61.3% and a specificity 58.6% when self-report was compared with BIA for early detection.
Asim et al31 developed a 10-item questionnaire for patients to rate the severity of their symptoms (arm swelling, heaviness, or tightness). The participants responded by circling on a 4-point scale (1 = no problem, 2 = a little, 3 = quite a bit, and 4 = very much).31 There was high sensitivity (97%) and moderate specificity (55%) for this assessment tool to rate the severity of symptoms, but no reliability or validity studies are available.31
Norman et al32 developed a telephone questionnaire to determine the presence or absence of lymphedema with yes/no responses. For example, “During the past 3 months, did your right and left (hands/lower arm/upper arms) seem to you to be different size from each other?” Although this study reported good interobserver reliability (weighted kappa values of 0.83-0.84), no studies of the validity of this tool were identified.32 It demonstrated high sensitivity (93%–96%) and moderate specificity (69%–75%).32 In 2011, Smoot et al33 also reported a high sensitivity (87%) and specificity (89%) with an area under the curve (AUC) of 0.88.
Another self-report measure of swelling, the VAS (0-10 cm line) demonstrates good intrarater reliability (Interclass correlation coefficient [ICC] = .70).34 The VAS moderately correlates with circumferential measurement using truncated cone (r = .66), perometry of the affected arm (r = .65), and BIA interlimb ratio (r = .71).34 There were no diagnostic accuracy studies.
The Lymphedema Symptom Intensity and Distress Survey – Arm (LSIDS-A) is a 36-item instrument that assesses arm lymphedema and related symptoms.35 There is excellent reliability for overall intensity and distress scores of the LSIDS-A (Cronbach alpha values = .93 and .94, respectively) as well as sexuality and mood (Cronbach alpha = .90). There was moderate test-retest reliability for function intensity (ICC = .69) and distress scores (ICC = .75). Of the 36 items, 9 symptoms occurred in more than 50% of the participants with stage 2 lymphedema. Those symptoms were: swelling (90.2%), fatigue (75.7%), heavy arm (74.0%), tight arm (66.8%), appearance concerns (59.6%), decrease in physical activity (56.0%), and pain in the arm (51.9%). Convergent validity of the LSIDS-A was acceptable with the following questionnaires: Functional Assessment of Cancer Therapy (FACT)+4 (intensity rs = .41, rs = .50), Upper Limb-27 physical scores (intensity rs = .52; distress rs = .45), and Functional Assessment Screening Questionnaire (distress rs = .40). No diagnostic accuracy studies were found.
The Lymphedema and Breast Cancer Questionnaire (LBCQ) is a 19-item tool used to screen and assess lymphedema indicators, symptom frequency, and symptom management.36 Test-retest reliability (r = .98) of the LBCQ was established in healthy women. Of the 19 items, 3 symptoms were valid with a diagnosis of breast cancer–related lymphedema. They were: “heaviness in the past year” (odds ratio = 8.0), “swelling now” (odds ratio = 97.0), and “numbness in the past year” (odds ratio = 1.0). No evidence was found for diagnostic accuracy.
The Morbidity Screening Tool (MST) consists of 4 short forms that assess fatigue, upper limb function, lymphedema, and pain. There was no evidence found for reliability. Validity studies concluded that the MST poorly agreed with results from perometry (k = 0.14) and moderately agreed with results from the LBCQ (k = 0.53).37 In 2014, Bulley et al38 reported that the MST poorly correlated with results from the FACT-B (rho = 0.27) and moderately correlated with LBCQ (rho = 0.48). The MST demonstrated low sensitivity (37%) and moderate specificity (78.1%).37
Bioimpedance Analysis
Bioimpedance Analysis (BIA) Recommendation
- Bioimpedance analysis should be used to detect lymphatic transport impairments and diagnose subclinical and early stage lymphedema in patients at risk for breast cancer–related lymphedema (Stage 0 and 1). Evidence Quality: Level II reliability, validity and diagnostic accuracy; Recommendation Strength: Grade B
- - L-Dex score of >7.1 should be used as a diagnostic criteria for breast cancer–related lymphedema when no preoperative assessment is available. Evidence Quality: Level II diagnostic accuracy; Recommendation Strength: Grade B
- - L-Dex score >10 above preoperative baseline measures should be used as diagnostic criteria. Evidence Quality: Level II diagnostic accuracy; Recommendation Strength: Grade B
In moderate to late stage breast cancer–related lymphedema, as fibrosis and tissue changes occur, BIA may be utilized as a diagnostic tool; however, clinicians must be aware of the potential for decreasing extracellular fluid even with increased tissue volume. Evidence Quality: Level II diagnostic accuracy; Recommendation Strength B
Summary of evidence
Bioimpedance analysis has been used in research in the form of single and multifrequency devices to detect and measure fluid in a limb. When set at a very low frequency, BIA has the ability to measure extracellular fluid. The Imp XCA (ImpediMed Ltd, Brisbane, Queensland, Australia) single-frequency model was approved by the US Food and Drug Administration (FDA) in 2007 for clinical use.39 In 2008, a multifrequency BIA device, ImpediMed L-Dex U400 BIS (bioimpedance spectroscopy) (ImpediMed Ltd), commonly used in research, was approved by the FDA for clinical use.40 Additional BIA devices are available, such as the ImpediMed SFB7 (similar to U400), but are used primarily in research settings.
The measurement obtained from the clinical model (Impedimed L-Dex U400) is expressed as an L-Dex score. The L-Dex score is derived from the ratio of extracellular fluid differences between the affected and unaffected limbs. These measures are compared to previously established baseline measures or normative standards for quantifying lymphedema. Normal L-Dex scores lie between –10 and +10, which is equivalent to an impedance ratio range of 0.935 to 1.139 for at-risk dominant arms and 0.862 to 1.006 for at-risk nondominant arms, calculated as 3 standard deviations from the mean normative data.41 Scores outside this range are indicative of lymphedema. According to Fu et al,41 if no preoperative assessment scores are available, an L-Dex score of >7.1 can be considered diagnostic of lymphedema.
The literature includes reports on both research and clinical models of BIA. Bioimpedance analysis demonstrates excellent interrater and intrarater reliability (.95 and .99, respectively) and highly reproducible measures (covariance = .2-.268%) in research models.42–44 Newman et al45 found that BIA has the precision capable of detecting (1.65%-1.86%) the onset of extracellular fluid accumulation indicative of early development of lymphedema. Test-retest reliability using the Imp XCA, a clinical BIA device, demonstrates strong agreement in healthy and at-risk groups (ICC = .99) but only fair agreement in the group with known breast cancer–related lymphedema (ICC = .69).41 Concurrent validity has been studied using a variety of assessment methods involving both clinical and research models of BIA devices. Moderate to strong correlations have been reported between BIA data and both self-report (r = .71, research model46) and perometry results (r = .71-.93, research model34,42,43,47; r = .40-.60, clinical model48). Correlation with circumferential measurement ranged from low (clinical model)49 to moderate (.31-.52, research model,50 clinical model41).
Bioimpedence analysis demonstrates moderate to high sensitivity and specificity ranging from 0.66-1.00 and 0.84-0.98, respectively (research models,33,51–53 clinical models41,48). According to a study by Smoot et al,33 of the physical measures assessed (BIA, circumferential measurement, volume from circumferential measurement), BIA (research model) yielded the highest accuracy with an AUC of 0.88, for women whose dominant arm was the affected arm. Using a clinical model, Fu et al41 provided evidence that an L-Dex score with a diagnostic cutoff of >7.1 provides the best properties for discriminating between at-risk breast cancer survivors and survivors with breast cancer–related lymphedema with a sensitivity of 0.80, a specificity of 0.90, and an AUC of 0.86. Variances in study results were attributed to differences in the BIA device used and stages of lymphedema examined. Bioimpedence analysis may not capture tissue changes, such as fibrosis or adipose infiltration, that are seen in later-stage lymphedema.41 Adequate evidence exists to support the use of both research and clinical BIA models, thus it is recommended multifrequency or spectroscopy BIA be used in early stage and at-risk breast cancer survivors. When considering use of BIA for diagnostic or assessment purposes, the cost of electrodes and equipment should be considered.
Volume Measures
There are a number of different options for clinicians conducting a volume assessment—including circumferential measures, water displacement, and perometry—some of which have established diagnostic criteria. One of the considerations when using volume assessment as a diagnostic criterion is that body size can influence the impact of an absolute volume change (see Perometry Recommendation).54 A few authors have also considered limb asymmetry due to arm dominance when classifying volume changes. These types of considerations have been used sparingly to influence diagnostic decisions and have not been tested sufficiently in regard to diagnosis of upper extremity lymphedema. It is also important to note that different volume measurement techniques are not equivalent, and thus are not interchangeable; therefore, clinicians should use the same method for all assessments of an individual.55–58
Circumferential Measurement Recommendation
- Circumferential measurement should be used to diagnose upper extremity lymphedema (with or without hand involvement) at Stage 1 or greater. Evidence Quality: Level I reliability, validity and Level II diagnostic accuracy; Recommendation Strength: Grade B
- - A volume ratio of 1.04 may be indicative of upper extremity lymphedema. Evidence Quality: Level II diagnostic accuracy; Recommendation Strength: Grade B
- - Calculated volume differential between sides (≥200 ml) will help rule in lymphedema, but values below 200 ml cannot be used to rule out. Evidence Quality: Level II diagnostic accuracy; Recommendation Strength: Grade B
- - If preoperative measures are available, a 5% or greater volume change from baseline above and below the elbow is diagnostic of upper extremity lymphedema. Evidence Quality: Level II diagnostic accuracy; Recommendation Strength: Grade B
- - Circumferential measurement taken at any single site along the upper extremity, and specifically a ≥2 cm difference, should not be utilized as a diagnostic criterion for upper extremity lymphedema due to poor accuracy. Evidence Quality: Level II diagnostic accuracy; Recommendation Strength: Grade B
For hand lymphedema, figure of 8 method of circumferential measurement may be used as an assessment tool for determining hand volume; however, this method has not been studied as a diagnostic test. Evidence Quality: Level I reliability and validity no diagnostic accuracy; Recommendation Strength: Expert Opinion
For head and neck lymphedema, circumferential measurement taken at a single point of the upper neck (under the jawline) may be useful for assessment but has not been studied as a diagnostic test. Evidence Quality: Level I reliability, Level II validity; no diagnostic accuracy; Recommendation Strength: Expert Opinion
Summary of evidence
Circumferential measurement using a standard tape measure is a simple and easy method for obtaining the girth of a limb, which is then used to calculate limb volume.
Reliability for limb volumes calculated from circumferential measurement demonstrates excellent interrater and intrarater reliability (ICC = .93-.99) for the arm with and without the hand measurements.55,59–64 Furthermore, utilization of the single or summed frustum cone for calculating arm volumes demonstrates excellent intrarater (ICC = .96-.99) and interrater (ICC = .94-.99) reliability.55 The more widely accepted truncated Frustum cone formula assumes that the arm resembles a cone shape, rather than a cylinder, which may be a truer representation of a lymphedematous limb.63,65 Using either method can result in an overestimation of the actual limb volume. The literature suggests that the cylindrical formula may overestimate the limb volume up to 5% and the single or summed truncated cone formula by at least 100 ml.65–67 There is Level I evidence of good intertester (ICC = .84) and excellent intratester reliability (ICC = .89-.92) using the figure of 8 circumferential measurement for hand swelling due to breast cancer–related lymphedema.68 There is Level I evidence regarding the reliability of circumferential measurement in patients with head and neck cancer–related lymphedema specifically where excellent interrater reliability was demonstrated at 3 points: ear to ear (ICC = .94); upper neck (ICC = .97); and lower neck (ICC = .98).69
Volumes calculated from circumferential measurement have been found to be highly correlated with water displacement of the arm (r = .93-.98), suggesting validity for this method of determining limb volume.56–58,66 Although narrow (8 mm) and wide (15 mm) tape measures were highly correlated with water displacement (Pearson's correlation coefficient = .92 and .88 respectively), Tewari et al70 suggested the use of a narrow tape measure yields more accurate estimations of volume. Furthermore, circumferential measurement at either 4 specific points or every 10 cm along the arm is highly correlated with water displacement (r = .88-.95).71 Use of 4 specific points, as is commonly used for garment measuring, may save the clinician time during the examination. Circumferential measurements taken by physical therapists are highly correlated with patient measurements (r = .70-.81).64
A strong correlation (r = .70-.75) exists between figure of 8 for hand lymphedema with water displacement.68 There is Level III evidence to support utilizing circumferential measurement at the upper neck point (under the jaw line) to detect lymphedema in patients with head and neck cancer when compared to healthy controls (t = 2.22, P = .39).69 Additionally, 3 of the tape measurements for patients with head and neck (ear to ear; circumferential measurement of upper neck and lower neck) correlated with Moisture Meter D (MMD) readings of extracellular fluid (rs = .37-.38).69
Historically, a diagnosis of lymphedema has been established with circumferential measurement using a variety of cutpoints, including an absolute volume difference of between 75 and 200 ml or interlimb volume differences between 3% and 10%. Smoot et al33 reported that a diagnostic cutoff of ≥200ml difference between affected and unaffected limb demonstrated high specificity (1.00) but low sensitivity (0.39), with an AUC of 0.69. Additionally, Smoot et al33 found that the volume ratio of 1.04 had the highest accuracy (sensitivity = 0.67, specificity = 0.89, AUC = 0.78) for identifying existing cases of upper extremity lymphedema in a sample of 144 women with and without lymphedema after breast cancer treatment. The ratio was calculated from the circumference volume of affected/unaffected upper extremity. For example, a volume of 2000 ml in the affected upper extremity, compared with 1915 ml in the unaffected upper extremity, would be a ratio of 1.044.33 A recent systematic review of upper and lower extremity lymphedema assessment pooled data from multiple studies and found a standard error of measurement (SEM) for circumferential measurement of 2.8% and a smallest detectable change (SDC) of 6.6%.72 Thus, the 1.04 ratio, indicating a 4% difference between limbs, is above the SEM but is below the SDC and yet is within the calculated variance. A high AUC (0.76) using the 75 mL interlimb volume difference suggested good accuracy for detecting lymphedema (sensitivity = 0.67, specificity = 0.85); however, because this value is still below the SEM, use of this threshold may misdiagnose a number of individuals.33
In 2003, Bland et al73 concluded that preoperative baseline measures above and below the elbow may provide better diagnostic accuracy if using a 5% volume change from the preoperative measurement (sensitivity = 0.80, specificity = 0.71). In 2005 and 2008, Hayes et al29,30 investigated using the sum of area of circumference (SOAC) as a diagnostic criterion for upper extremity lymphedema and determined that this method is inferior to other diagnostic criteria. Thus, the debate over the most appropriate cutpoint for diagnosis continues. Currently, the best sensitivity and specificity, while being above the SEM, comes from using either the ≥200 ml volume difference or the 1.04 ratio of affected:unaffected limb.
If used to calculate limb volumes or at the upper neck point in patients’ following treatment for head and neck cancer, circumferential measurements can be useful diagnostic aids for patients with known lymphatic insults related to cancer and its treatment. Clinicians must take the time to calculate volumes from limb measurements and should understand that using criteria of >200 ml volume differential between limbs may incorrectly classify milder cases of lymphedema (eg, 150-ml interlimb difference) as not having lymphedema. Likewise, some of the criteria that are more sensitive, such as the 1.04 ratio or the 5% volume increase, may still misclassify some individuals when used as a sole diagnostic criterion. A 5% volume change in a limb may better detect lymphedema and is closer to the SDC of the measure, but it requires preoperative measurement for comparison purposes.
Water Displacement Recommendation
Water displacement may be used to diagnose lymphedema with volumetry >200 mL when compared to the contralateral arm and with volumes >10% interlimb difference. This technique is limited by clinical utility. Evidence Quality: Level 1 reliability and validity; Level II diagnostic accuracy; Recommendation Strength: Grade B
Summary of the evidence
There are numerous methods for performing water displacement as an indirect measure for lymph volume. Even though there are variations in methodology among multiple articles with high level of evidence, water displacement demonstrates excellent interrater and intrarater reliability (ICC = .97-.99).55–59,61,63,64,74–81
Due to its excellent reliability, water displacement is considered the reference standard in much of the validity and diagnostic accuracy research.56–58,63,71,79,82 Water displacement is highly correlated (r = .81-.91) with circumferential measurement for the hand and upper limb.78,80 When compared to magnetic resonance imaging (MRI) and computed tomography (CT) scans, simplified water displacement demonstrated good construct (k coefficient = .78) and concurrent validity (R = .87-.90).83
There is little evidence on the diagnostic accuracy of water displacement. Godoy et al84 combined water displacement with circumferential measurement for diagnosing lymphedema. The authors reported the highest accuracy (77.8%) and sensitivity (90.0%) and the greatest negative predictive value (93.5%) with measurements of volumetry at >200 mL when compared to the contralateral arm. The highest specificity (78.3%) was reflected with volumes >10% interlimb difference. Chen et al61 reported a minimally detectable change (MDC) as >150 mL when using water displacement clinically.
Many authors reported concerns with the use of water displacement due to time constraints and the costs involved with setup and administration of the methods.58,59,82 Due to concerns for cross-contamination in individuals with skin breakdown and open wounds, water displacement is contraindicated in this patient population.70 Although water displacement includes measurement of the hand, it does not allow for complete immersion of the upper arm. It is important for clinicians to standardize the distance of the limb when placing it in the volumeter. Despite water displacement's excellent reliability and validity, it should not be used interchangeably with other clinical measure methods.55–58
Perometry Recommendation
Perometry may be used for assessment of volume but not as a diagnostic tool for upper extremity lymphedema. This technique is limited in clinical utility. Evidence Quality: Level I reliability, Level II validity, no diagnostic accuracy; Recommendation Strength: Expert Opinion
Summary of the evidence
Perometer is an optoelectrical imaging device for measuring indirect limb volume. The arm volume is then calculated summing the volumes of elliptical segments every centimeter (cm) for 40 cm using computer software. Ancukiewicz et al85 reported that obtaining 2 measurements of each arm at each time point would reduce error in the volume ratios. There is excellent interrater and intrarater reliability with the static (ICC = .99)55 or mobile perometer (ICC = .98-.99).86
Perometry demonstrated a strong correlation with hand water volumetery (rc = .88),82 circumferential measurement, lymphometry, and BIA measures (r >.70).87 Additionally, when used on healthy women, perometry strongly correlated with circumferential measurement using truncated cone (r = .86-.98) and cylinder (r = .85-.98) formulas.88 Lee et al82 reported that use of perometry resulted in overestimated volume if participants did not hold their fingers together during testing.
There are no specific diagnostic accuracy studies, but several discrepancies in the literature exist when attempting to use perometry as a diagnostic method. Breast cancer–related lymphedema is defined as an absolute volume change that may vary with body size and shape.89 In 2010, using perometry, Czerniec et al46 defined breast cancer–related lymphedema as a 200-ml or 10% limb volume difference. Ancukiewicz et al85 reported that a relative volume change between the affected and unaffected arms expressed as a percent is more reliable in quantifying lymphedema. In 2012, Ancukiewicz et al54 reported an absolute change in arm volume (200 ml) correlated to a relative arm volume change that varied between 2.9% and 15.7% based on body size. Dylke et al88 determined that, in women over 40 years of age, an interlimb difference of >380 ml is required for diagnosis of lymphedema if the dominant arm is affected. If the clinical cut-off of 200 ml was used as previously described in the literature, 8% of their study subjects would be incorrectly diagnosed as having lymphedema. This highlights the need to consider underlying dominant versus nondominant arm asymmetry and thus reinforces the importance of baseline measurements. Stout et al13 defined breast cancer–related lymphedema as an increase of >3% limb volume as compared to a preoperative volume measurement (coefficient of determination r2 = .95).90
Clinical utility issues include equipment expense, bulkiness, and current lack of availability of the perometer through US distributors. Perometers can be used to measure volume in an efficient manner, but their diagnostic properties do not outperform other forms of volume measurement.
3D. Camera Imaging Recommendation
Although 3D camera imaging may not be utilized as a diagnostic tool, it can be used to calculate volume measurements as it has not been studied for diagnostic purposes. Evidence Quality: Level III reliability, Level II validity and no diagnostic accuracy; Recommendation Strength: Expert Opinion
Summary of evidence
A new imaging technique, using 3D imaging cameras, calculates volume measurements using different methods (6 digital single lens reflex cameras,81 positional laser and 3 cameras,91 and Microsoft Kinect™ infra-red sensor) to develop a 3D model.92
There is excellent interrater and intrarater reliability (ICC = .99).81 Correlations have been established for 3D imaging with water displacement (r = .98).81 Validity was established using water displacement as well as laser (r2 = .04); however, 3D imaging tends to overestimate volume.91 The Kinetic system is highly correlated with water displacement (r = .98), but there was only one individual with lymphedema in the sample population.93 The kinetic system could potentially be an inexpensive method for self-monitoring the upper limb, but further testing is needed in people without an established diagnosis of lymphedema, as there were no diagnostic accuracy studies.
Other Diagnostic Measures
Tissue Dielectric Constant (TDC) Recommendation
Tissue dielectric constant may not be used as a diagnostic tool for SUQL but can be utilized for assessment. This technique is limited in clinical utility. Evidence Quality: no diagnostic accuracy studies; Recommendation Strength: Expert Opinion
Breast cancer Evidence Quality: Level III reliability, Level II validity
Head and neck cancer Evidence Quality: Level I reliability, Level III validity
Summary of evidence
Tissue dielectric constant assesses changes in tissue water and skin thickness in patients at risk to moderate stages of lymphedema.94,95 In research, a MoistureMeterD (MMD) (Delfin Ltd. Kuopio, Finland) uses an ultra-high-frequency electromagnetic wave to measure the water content in the tissue.69 Higher values indicate increased levels of swelling.69 Mayrovitz et al95 found TDC normative values at the forearm for men to be 33.2 (+/- 4.0) and women to be 29.4 (+/- 2.7).
A study reported bilateral TDC forearm measurement at 2.5-mm depth had excellent intrarater reliability (ICC>.99).94 In a population of patients with head and neck cancer, Purcell et al69 reported excellent interrater and intrarater reliability (ICC = .97). Validity was established in patients with head and neck cancer using the 2.5-cm probe of the MMD on the skin 8 cm below the edge of the lower lip, and there was moderate correlation with the MD Anderson head and neck lymphedema rating scale96 (rs = .59).69 Strong correlations were found between arm TDC ratios and arm volume (r = .69) as well as segmental volume measures (r = .77). Mayrovitz et al94 demonstrated a significant relationship between inter-arm TDC ratios and number of nodes removed (r = .55) as well as patients who reported more than one symptom of swelling (r = .57). Using the TDC, a greater number of patients with breast cancer were detected to have inter-arm increases exceeding 10% that were not detected using BIA ratios, which may indicate a greater sensitivity to localized tissue water changes.93 There is no evidence on diagnostic accuracy at this time.
Measurement takes approximately 10 seconds at multiple sites and measures locally, so clinically this is an efficient technique; however, currently it has limited availability.69 The multiprobe MMD has FDA approval but the integrated-probe MMD Compact is being sold under the status of experimental use only.
Ultrasound Recommendation
Ultrasound should be utilized to detect SUQL and to identify tissue changes. Evidence Quality: Level III reliability (healthy subjects), Level II validity, Level I diagnostic accuracy; Recommendation Strength: Grade B
Summary of evidence
High-frequency ultrasound (HFUS) uses a noninvasive probe to scan the dermis and subcutis composition to evaluate edema and tissue quality.97–102 Cutaneous epifascial and subfascial tissue thickness as well as fibrosis and fluid collection can be examined in real time. Normal breast skin thickness measurements using HFUS are between 1- and 2-mm thick (mean = 1.7 mm).103 Impaired lymphatic drainage affects the echogenicity of tissue as demonstrated by the change in ultrasound images. A benefit of ultrasound is the ability to measure changes in both the extremity as well as the chest wall in patients following breast cancer treatment. Use of a 7.5-MHz probe demonstrated excellent intrarater and interrater reliability for the forearm (ICC = .82, .81) and the upper arm (ICC = .90, .72).97,99 Hwang et al98 reported excellent intrarater reliability (r = .98) and interrater reliability (r = .96). Ultrasonography reliability has been completed only in healthy participants and not yet in the population of interest.
Validity studies of ultrasound elastography on breast tissue after radiation demonstrated an increase in subcutis thickness as expected.101 An early study by Mellor et al100 found high correlations between ultrasound-measured skin thickness and arm circumference (r = .95). Another study found high correlations between ultrasound and perometry (r = .76-.79) as well as arm circumference (r = .68-.80).98 Choi et al97 found mixed results when comparing ultrasound skin thickness with arm circumferential measurement (forearm, r = .756, P = .001; upper arm, r = .54, P = .003) and BIA impedance ratios (forearm, r = .56, P = .002; upper arm, r = .50, P = .006). Measures taken at the forearm yielded the highest associations.97 Although the classification criterion was vague, Balzarini et al104 compared ultrasonography with clinical signs of edema and found that in patients with palpable soft edema, 68.4% had fluid accumulation, 64.2% had both fibrosis and medium fluid accumulation, and 76.9% had firm edema with diffuse fibrosclerosis without fluid accumulation. Another study found no significant correlations between ultrasound elastography, HFUS, and patient report.101 Thus, validity of ultrasound is mixed, potentially due to study design and the use of participants with and without SUQL.
Diagnostic accuracy of ultrasound, when compared with circumferential measurement, had moderate sensitivity at the triceps (67%) but low sensitivity at the wrist and lower forearm (40% and 33%, respectively). The specificity at these locations ranged from moderate to high (67%, 93%, and 93%, respectively).105
Ultrasonography allows the clinician to assess the soft tissue in real time with the potential ability to measure different body regions, but the evidence base for the chest wall and other areas is extremely limited. Cost of the unit is less than that of many other diagnostic imaging modalities, but the gel and gel pad need to be factored into the unit's use. In addition, physical therapists require training in use of ultrasound imaging for diagnostic purposes, as this is not a standard component for most physical therapist professional education programs.
Dual-Energy X-Ray Absorptiometry (DXA) Recommendation
Dual-energy X-ray absorptiometry may not be utilized as a diagnostic tool for SUQL but can be used for assessment to calculate arm volumes. Evidence Quality: Level I reliability, Level II validity; no diagnostic accuracy; Recommendation Strength: Expert Opinion
Summary of evidence
Volumes are calculated using DXA from 2 different sources. Newman et al45 measured the affected and nonaffected arms of patients with breast cancer–related lymphedema and noted a significant difference in fat and lean mass (increase in both by 15%, <0.0001), but only a 0.6% change in bone mass. The interrater reliability of DXA to measure arm volumes was excellent (ICC = .99).63 There are strong correlations to perometry (rp = .99) in a healthy population106 and to water displacement (r = .996) in participants with breast cancer–related lymphedema.107 There were no diagnostic accuracy studies.
DXA is easy and quick to perform, even in patients who are medically compromised, are elderly, or have skin disorders, but it does require referral for conduction of the test and interpretation.63 Several disadvantages of DXA include exposure to radiation, cost of the DXA lunar prodigy (research grade) scanner, and lack of portability.
Magnetic Resonance Imaging (MRI) Recommendation
Magnetic resonance imaging may be utilized as a diagnostic tool of SUQL. Evidence Quality: no reliability, Level III validity and diagnostic accuracy; Recommendation Strength: Grade C
Summary of evidence
With imaging, presence of edema is determined if partial or complete fluid retention is observed in subcutaneous tissue. No reliability studies were found for MRI. Validity was demonstrated for a chemical exchange saturation transfer (CEST) MRI using amide proton transfer (APT). Patients with breast cancer–related lymphedema demonstrated an increase in APT contrast in the affected arms when compared with healthy controls (P = .025; Cohen's d = 0.24).108 Mihara et al109 reported high sensitivity and specificity (100%) using T-2 weighted or short-time inversion recovery (STIR) sequence MRI in participants with ISL stage I lymphedema. Disadvantages of using an MRI include the high associated cost, need for referral for conduct of the test, and the extended time before diagnosis can be achieved, as images need to be read by a radiologist.110 Current literature shows the use of different forms of MRI scanning, and all forms require more study before a recommendation can be made for use. MRI uses a strong magnet; therefore, there are some strong precautions for MRI use in patients with pacemakers or cochlear implants, and patients with metal implants are unable to be imaged.
Computed Tomography (CT) Recommendation
Computed tomography may be utilized as a diagnostic tool for SUQL. Evidence Quality: no reliability, Level II validity, Level III diagnostic accuracy; Recommendation Strength: Grade C
Summary of evidence
Computed tomography uses special x-ray equipment to create a series of detailed body images. Each image demonstrates a thin “slice” (0.5 mm) of tissue. A fibrous component in the subcutaneous fat layer is diagnostic for lymphedema.109 Secondary changes are observed on CT in the subcutaneous tissue, skin and overgrowth of fibrous tissue as lymphedema progresses.109 Evidence for reliability and validity of CT for lymphedema diagnosis is lacking. Brorson et al111 reported that CT-computed arm volume was highly correlated with water displacement (CC [r] = .996), but this was in a small sample of subjects about to undergo liposuction surgery after failed conservative management. Diagnostic accuracy of CT images demonstrates low sensitivity (33%) and high specificity (100%).109 The high cost of equipment and need for referral for conduct of the test and radiologist interpretation makes this a more expensive diagnostic option. In addition, CT scanning exposes the patient to radiation.
Lymphoscintigraphy Recommendation
Lymphoscintigraphy may be used to detect lymphatic system impairment. Evidence Quality: Level II reliability, no validity, Level III diagnostic accuracy; Recommendation Strength: Grade C
Summary of evidence
Lymphoscintigraphy uses a low energy, high resolution, dual- head collimator-equipped GE Millennium VG (scinticamera) (GE Medical Systems-Americas, Milwaukee, Wisconsin) to produce images of lymphatic flow to observe anatomic and transport capacity abnormalities. As such, it has the potential to determine lymphatic system impairment. A radioisotope is injected between the second and third digits before taking images of the upper extremity. Dermal backflow of the radioisotope is observed and graded to diagnose lymphedema.109,112 Reliability of lymphoscintigraphy image readings, using a previous method to determine dermal back flow demonstrated adequate interobserver agreement (Fleiss k = 0.42) and poor (Cohen k = 0.06) to excellent intraobserver (Cohen k = 1.0) reliability.112 Reproducibility was excellent (ICC = .75-.85) for change of axillary uptake and change of extraction from hands.105 No validity studies were found in this patient population. The diagnostic accuracy of lymphiscintigraphy has moderate sensitivity (0.62) and high specificity (1.0) when compared with the unaffected limb.109 Clinical utility challenges include; associated costs, need for referral to another medical specialist, current low resolution, invasive nature of testing, and radiation exposure.
Lymphography Recommendation
Lymphography may be used to detect lymphatic system impairment. Evidence Quality: no reliability or validity, Level II diagnostic accuracy; Recommendation Strength: Grade C
Summary of evidence
Lymphography is a real time imaging technique where indocyanin green (ICG) dye is injected in the interdigit web space illuminating the superficial lymphatic flow and is considered to be safer than the isotope used in lymphoscintography because it is water soluble and attaches to albumin. Currently ICG is used to detect sentinel lymph nodes in breast cancer. The light emitted by the injected dye traveling in the lymphatic vessels is imaged using a specialized camera system. Normal lymphatic flow is linear and abnormalities in lymphatic flow result in a splash, stardust, or diffuse pattern.113
There are no reliability or validity studies in an at risk population. Diagnostic accuracy in patients with known lymphedema demonstrated a sensitivity of 1.0 and specificity of 1.0 when compared to CT and MRI.109 In 2013, Akita et al113 reported a high sensitivity (0.97), moderate specificity (0.55), a positive likelihood ratio of 2.15 and a negative likelihood ratio 0.05, and accuracy of 0.82 when comparing lymphography to lymphoscintigraphy. This measure needs further testing utilizing subjects without an established diagnosis of lymphedema. Compared to lymphoscintigraphy, ICG lymphography has the advantages of reduced costs and a less invasive nature,113 but it requires additional time and referral for specialized testing.
Tonometry Recommendation
Tonometry is not recommended to diagnose SUQL. Evidence Quality: Level I reliability, no validity or diagnostic accuracy; Recommendation Strength: Expert opinion
Summary of the evidence
Tonometry measures the presence of fibrosis with an increased volume of interstitial fluid. Clinicians consider the amount of tissue fibrosis as influencing choice of interventions and a prognostic indicator for potential number of visits. Presently, clinicians rely on palpation for tissue resistance to determine the degree of fibrosis. The tonometer measures fibrotic changes in tissue when placed perpendicular to the skin.
The inter/intra-rater reliability was fair to good (ICC = .66-.87) with a SEM% of 4.3-17.8%.61 When measuring breast tissue resistance a low covariance (1.29%-3.25%) was determined suggesting consistency and good reproducibility between subjects.114 In another study, significant differences between testers were found.115 Therefore, to reduce measurement error, it is recommended that the same tester conduct baseline and follow-up assessments, and that 3 measurements be taken at each time point. There were no validity and diagnostic accuracy studies.
Summary of Practice Recommendations
At present, SUQL is most often diagnosed by clinical history, physical examination of tissue quality, and a measurement of increased limb volume. Subclinical or early stage lymphedema may not display a sufficient or persistent volume change to meet this diagnostic criteria. Research indicates the importance of early detection of SUQL to minimize body function and structure impairments that may progress to functional limitations and activity and participation restrictions.13,14 For patients at risk for SUQL, symptoms of swelling, heaviness and numbness should be identified during the history, as it may assist in identifying those with subclinical or early stage lymphedema. The Norman Questionnaire and the MST should be considered to determine the presence or absence of lymphedema in conjunction with volume measures. For all patients, the physical examination should consist of observation, palpation, and other measurements. For subclinical/early stage lymphedema, BIA should be used to assist in the diagnosis of SUQL. A volume measure should also be taken, but may not be consistently increased at this point. In the moderate and late stages, circumferential measurement should be used, and water displacement may be used in some cases, to diagnosis upper extremity lymphedema (See practice recommendations in Table 4 for cutpoints). Perometry may be used for upper extremity volume assessment in the early, moderate, and late stages; however, diagnostic criteria have not been fully evaluated. In late stage, ultrasound should be utilized to detect underlying tissue changes, which may be helpful for clinicians to determine appropriate management. Clinicians need to be aware that none of the diagnostic criteria are perfect in their diagnostic accuracy, and especially patients whose measurement values fall just under or over a cutpoint have the potential to be misclassified. Thus, we encourage clinicians to cluster findings from their examination to draw a conclusion on diagnosis.
There are emerging diagnostic methods which detect tissue quality, visualize edema or evaluate structural lymphatic transport capacity. These methods include 3D camera, TDC, DXA, MRI, CT, lymphoscintigraphy, lymphography, and tonometry. Due to lack of evidence, high costs, or the invasive nature for some of these tests, these methods are not recommended to be incorporated into general clinical practice for diagnosing SUQL at this time. Lymphatic system imaging, including lymphoscintigraphy and lymphography, can be useful in determining the full extent of lymphatic system impairment and the results may assist the clinician when traditional interventions are not successful. Another emerging area is the diagnosis and assessment of lymphedema in patients treated for head and neck cancer. A combined approach of HN-ELAF, circumferential measurement at the upper neck point, and TDC may be useful for diagnostic purposes. Little research is currently available to guide the diagnosis of hand, trunk, and breast lymphedema.
Overall, based on the evidence in this CPG, there is no one diagnostic tool that can be used definitively to diagnose SUQL, but using these recommendations can facilitate early identification and should lead to an examination of activity and participation restrictions and appropriate interventions. A number of measures of activity and participation restriction and quality of life have been developed but are beyond the scope of this review. Davies et al116 provide a review of such measurement tools.
Measures of the impact of lymphedema add important information and should be included in overall patient assessment. In order to further support clinical practice in this area, The Oncology Section of the American Physical Therapy Association plans to create guidelines on risk factor identification and appropriate intervention for SUQL in the near future.
Limitations
There were several limitations in the development of this CPG. The literature was searched from January 1, 2000 through July 5, 2015; therefore, evidence from inception and newer articles may have been missed. For example, a recent systematic review of lymphedema measures72 was not included due to this limitation. Thus, it is important that clinicians also keep abreast of more recent additions to the literature. The Oncology Section plans to update this CPG every 5 years, adding the most recent literature to the development of our recommendations. In addition, papers not in the English language were excluded from this study. Of the articles retrieved from this growing body of literature, there was a lack of high-quality evidence.
It is also important to note that more research may have been published on certain diagnostic methods than on others, not because the well-published methods are superior to other measures, but because they have been used over a long period of time.
There is no single quality rating tool for all psychometric properties of diagnostic measures; therefore, multiple tools were used to review the quality of the evidence. The lack of standardization and the variations in diagnostic criteria, as well as the limited study of certain measures in a variety of cancer populations representing all stages of SUQL, may confound the findings.
Finally, this CPG was created by physical therapists, was not piloted in clinical practice, and did not include perspectives from patients or other medical and rehabilitation professionals in its inception and conduct. The authors addressed this limitation by inviting feedback from a wider audience of other physical therapists, physicians, nurses, and occupational therapists.
Implementation
As part of the dissemination and implementation of the CPG, the Guideline Development Group shared the preliminary findings at the Combined Section Meeting of the American Physical Therapy Association in 2016. We also solicited feedback on the CPG from multiple stakeholders, which in itself acts as a form of dissemination. In addition, the group is committed to the following:
Ensure open access to the CPG and reference material upon publication.
Develop a guide to implementation of the CPG in clinical practice, made available through the Oncology Section of APTA.
Present the CPG at national conferences for health care professionals, allowing dissemination to other disciplines.
Create a podcast or similar electronic media that can be used in physical therapy and other health care professional education.
Future Research Needs
There are several important directions for future research:
Further psychometric testing needs to be completed on the tools currently being used to assess and diagnose SUQL. This includes the emerging methods that lack diagnostic accuracy studies.
Much of the evidence focuses on one diagnostic method, although current practice dictates using a combination of history, symptoms, and other measurements for diagnosis. Further research is needed to determine which combination of signs, symptoms, and measures is most accurate for diagnosing SUQL.
There is a need for high-quality studies on at-risk populations as well as on the population in various stages of lymphedema.
The bulk of the evidence includes patients with upper extremity lymphedema due to breast cancer treatments. Further research is needed for diagnosing lymphedema in the trunk and in the head and neck region.
Early diagnosis is crucial to maintain quality of life and minimize upper quadrant morbidity for patients at risk for SUQL; therefore, there is a need for research to determine appropriate preoperative measurements and prospective monitoring protocols.
Supplementary Material
Supplementary data are available at PHYSTH online.
Author Contributions and Acknowledgments
Concept/idea/research design: K. Levenhagen, C. Davies, M. Perdomo, K. Ryans, L. Gilchrist
Writing: K. Levenhagen, C. Davies, M. Perdomo, K. Ryans, L. Gilchrist
Data collection: K. Levenhagen, C. Davies, M. Perdomo, K. Ryans, L. Gilchrist
Data analysis: K. Levenhagen, C. Davies, M. Perdomo, K. Ryans, L. Gilchrist
Project management: L. Gilchrist
Fund procurement: L. Gilchrist, K. Levenhagen, C. Davies, M. Perdomo, K. Ryans
Providing facilities / equipment: M. Perdomo
Providing institutional liaisons: K. Levenhagen, M. Perdomo, L. Gilchrist
Dr Davies is a Certified Lymphedema Therapist-Lymphology Association of North America (CLT-LANA), and Dr Perdomo is a Certified Lymphedema Therapist-Foldi (CLT-Foldi).
The following people reviewed a draft of the guideline and provided important feedback: Joseph Godges DPT, MA, OCS; Nicole Stout PT, DPT, CLT-LANA; Betty Smoot PT, PhD; Nancy Hutchison MD; Maria Nelson MD; Byron Schier OT; G. Steven Morris PT, PhD; Jennifer Brooks, PT, MPT, CLT-LANA; Amy Litterini PT, DPT; Margaret Rinehart Ayres, PT, PhD. Dr Stout and Dr Brooks are Certified Lymphedema Therapists-Lymphology Association of North America (CLT-LANA).
The authors also thank the librarians at each of their institutions (Saint Louis University, Baptist Health-Lexington, University of Southern California, Mercy College, St Catherine University) for their support during the literature review phase.
Disclosures
The Guideline Development Group members have no conflicts of interest to report.
Funding
This CPG was supported by a grant from the American Physical Therapy Association.
References
- 1. PDQ® Supportive and Palliative Care Editorial Board Lymphedema (PDQ®) – Health Professional Version. Bethesda, MD: National Cancer Institute; Available at: http://www.cancer.gov/about-cancer/treatment/side-effects/lymphedema/lymphedema-hp-pdq. Updated July 17, 2015. Accessed October 2, 2016. PMID: 26389244. [Google Scholar]
- 2. Boyages J, Xu Y, Kalfa S et al. . Financial cost of lymphedema borne by women with breast cancer. Psychooncology. 2016August1[Epub ahead of print]. Doi: 10.1002/pon.4239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Boyages J, Kalfa S, Xu Y et al. . Worse and worse off: the impact of lymphedema on work and career after breast cancer. Springerplus. 2016;5:657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fu MR, Ridner SH, Hu SH et al. . Psychosocial impact of lymphedema: a systematic review of literature from 2004 to 2011. Psychooncology. 2013;22:1466–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Morgan P, Doherty D, Moffatt C, Franks P. The national lymphoedema framework project. Nurs Times. 2005;101:48. [PubMed] [Google Scholar]
- 6. Paskett ED, Dean JA, Oliveri JM, Harrop JP. Cancer-related lymphedema risk factors, diagnosis, treatment, and impact: a review. J Clin Oncol. 2012;30:3726–3733. [DOI] [PubMed] [Google Scholar]
- 7. Miller KD, Siegel RL, Lin CC et al. . Cancer treatment and survivorship statistics, 2016. GA Cancer J Clin. 2016;66:271–289. [DOI] [PubMed] [Google Scholar]
- 8. Armer JM. The problem of post-breast cancer lymphedema: impact and measurement issues. Cancer Invest. 2005;23:76–83. [PubMed] [Google Scholar]
- 9. Norman SA, Localio AR, Potashnik SL et al. . Lymphedema in breast cancer survivors: incidence, degree, time course, treatment, and symptoms. J Clin Oncol. 2009;27:390–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cormier JN, Askew RL, Mungovan KS et al. . Lymphedema beyond breast cancer: a systematic review and meta-analysis of cancer-related secondary lymphedema. Cancer. 2010;116:5138–5149. [DOI] [PubMed] [Google Scholar]
- 11. Deng J, Ridner SH, Dietrich MS et al. . Prevalence of secondary lymphedema in patients with head and neck cancer. J Pain Symptom Manage. 2012;43:244–252. [DOI] [PubMed] [Google Scholar]
- 12. International Society of Lymphology The diagnosis and treatment of peripheral lymphedema: 2013 consensus document of the International Society of Lymphology. Lymphology. 2013;46:1–11. [PubMed] [Google Scholar]
- 13. Stout Gergich NL, Pfalzer LA, McGarvey C et al. . Preoperative assessment enables the early diagnosis and successful treatment of lymphedema. Cancer. 2008;112:2809–2819. [DOI] [PubMed] [Google Scholar]
- 14. Stout NL, Pfalzer LA, Springer B et al. . Breast cancer–related lymphedema: comparing direct costs of a prospective surveillance model and a traditional model of care. Phys Ther. 2012;92:152–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Poage E, Singer M, Armer J et al. . Demystifying lymphedema: development of the lymphedema putting evidence into practice card. Clin J Oncol Nurs. 2008;12:951–964. [DOI] [PubMed] [Google Scholar]
- 16. Harris SR, Hugi MR, Olivotto IA et al. . Clinical practice guidelines for the care and treatment of breast cancer: 11. Lymphedema. CMAJ. 2001;164:191–199. [PMC free article] [PubMed] [Google Scholar]
- 17. Harris SR, Schmitz KH, Campbell KL, McNeely ML. Clinical practice guidelines for breast cancer rehabilitation: syntheses of guideline recommendations and qualitative appraisals. Cancer. 2012;118(8Suppl):2312–2324. [DOI] [PubMed] [Google Scholar]
- 18. Oremus M, Walker K, Dayes I, Raina P. Diagnosis and Treatment of Secondary Lymphedema [Internet]. Rockville, MD: Agency for Healthcare Research and Quality; 2010. [PubMed] [Google Scholar]
- 19. Armer JM, Hulett JM, Bernas M et al. . Best practice guidelines in assessment, risk reduction, management, and surveillance for post-breast cancer lymphedema. Curr Breast Cancer Rep. 2013;5:134–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lucas NP, Macaskill P, Irwig I, Bogduk N. The development of a quality appraisal tool for studies of diagnostic reliability (QAREL). J Clin Epidemiol. 2010;63:854–861. [DOI] [PubMed] [Google Scholar]
- 21. Lucas N, Macaskill P, Irwig L et al. . The reliability of a quality appraisal tool for studies of diagnostic reliability (QAREL). BMC Med Res Methodol. 2013;13:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Whiting P, Rutjes AW, Reitsma JB et al. . The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol. 2003;3:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Whiting PF, Rutjes AW, Westwood ME et al. . QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529–536. [DOI] [PubMed] [Google Scholar]
- 24. Phillips B, Ball C, Sackett D et al. . Oxford Centre for Evidence-Based Medicine – levels of evidence. Available at: http://www.cebm.net/index.aspx?o=1025. Published March 2009. Accessed August 25, 2012.
- 25. Delitto A, George SZ, Van Dillen LR et al. . Low back pain. J Orthop Sports Phys Ther. 2012;42:A1–A57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kaplan SL, Coulter C, Fetters L. Physical therapy management of congenital muscular torticollis: an evidence-based clinical practice guideline—from the Section on Pediatrics of the American Physical Therapy Association. Pediatr Phys Ther. 2013;25:348–394. [DOI] [PubMed] [Google Scholar]
- 27. Lee BB, Bergan JJ. New clinical and laboratory staging systems to improve management of chronic lymphedema. Lymphology. 2005;38:122–129. [PubMed] [Google Scholar]
- 28. Deng J, Ridner SH, Wells N et al. . Development and preliminary testing of head and neck cancer related external lymphedema and fibrosis assessment criteria. Eur J Oncol Nurs. 2015;19:75–80. [DOI] [PubMed] [Google Scholar]
- 29. Hayes S, Cornish B, Newman B. Comparison of methods to diagnose lymphoedema among breast cancer survivors: 6-month follow-up. Breast Cancer Res Treat. 2005;89:221–226. [DOI] [PubMed] [Google Scholar]
- 30. Hayes S, Janda M, Cornish B et al. . Lymphedema secondary to breast cancer: how choice of measure influences diagnosis, prevalence, and identifiable risk factors. Lymphology. 2008;41:18–28. [PubMed] [Google Scholar]
- 31. Asim M, Cham A, Banerjee S et al. . Difficulties with defining lymphoedema after axillary dissection for breast cancer. N Z Med J. 2012;125:29–39. [PubMed] [Google Scholar]
- 32. Norman SA, Miller LT, Erikson HB et al. . Development and validation of a telephone questionnaire to characterize lymphedema in women treated for breast cancer. Phys Ther. 2001;81:1192–1205. [PubMed] [Google Scholar]
- 33. Smoot BJ, Wong JF, Dodd MJ. Comparison of diagnostic accuracy of clinical measures of breast cancer-related lymphedema: area under the curve. Arch Phys Med Rehabil. 2011;92:603–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Czerniec SA, Ward LC, Lee MJ et al. . Segmental measurement of breast cancer-related arm lymphoedema using perometry and bioimpedance spectroscopy. Support Care Cancer. 2011;19:703–710. [DOI] [PubMed] [Google Scholar]
- 35. Ridner SH, Dietrich MS. Development and validation of the Lymphedema Symptom and Intensity Survey-Arm. Support Care Cancer. 2015;23:3103–3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Armer JM, Radina ME, Porock D, Culbertson SD. Predicting breast cancer-related lymphedema using self-reported symptoms. Nurs Res. 2003;52:370–379. [DOI] [PubMed] [Google Scholar]
- 37. Bulley C, Gaal S, Coutts F et al. . Comparison of breast cancer-related lymphedema (upper limb swelling) prevalence estimated using objective and subjective criteria and relationship with quality of life. Biomed Res Int. 2013;2013:807569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bulley C, Coutts F, Blyth C et al. . A morbidity screening tool for identifying fatigue, pain, upper limb dysfunction and lymphedema after breast cancer treatment: a validity study. Eur J Oncol Nurs. 2014;18:218–227. [DOI] [PubMed] [Google Scholar]
- 39. US Food and Drug Administration 510(k) Premarket Notification. K050415. 2007March30 Last updated April 24, 2017. ImpediMed-Extracellular Fluid Analysis. Available at: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm?id=K050415. Accessed April 27, 2017.
- 40. US Food and Drug Administration 510(k) Premarket Notification. K080825. October 3, 2008. Available at: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn_template.cfm?id=k080825. Accessed April 27, 2017.
- 41. Fu MR, Cleland CM, Guth AA et al. . L-dex ratio in detecting breast cancer-related lymphedema: reliability, sensitivity, and specificity. Lymphology. 2013;46:85–96. [PMC free article] [PubMed] [Google Scholar]
- 42. Jain MS, Danoff JV, Paul SM. Correlation between bioelectrical spectroscopy and perometry in assessment of upper extremity swelling. Lymphology. 2010;43:85–94. [PMC free article] [PubMed] [Google Scholar]
- 43. Dylke ES, Alsobayel H, Ward LC et al. . Use of impedance ratios to assess hand swelling in lymphoedema. Phlebology. 2014;29:83–89. [DOI] [PubMed] [Google Scholar]
- 44. Svensson BJ, Dylke ES, Ward LC, Kilbreath SL. Segmental impedance thresholds for early detection of unilateral upper limb swelling. Lymphat Res Biol. 2015;13:253–259. [DOI] [PubMed] [Google Scholar]
- 45. Newman AL, Rosenthall L, Towers A et al. . Determining the precision of dual energy x-ray absorptiometry and bioelectric impedance spectroscopy in the assessment of breast cancer-related lymphedema. Lymphat Res Biol. 2013;11:104–109. [DOI] [PubMed] [Google Scholar]
- 46. Czerniec SA, Ward LC, Refshauge KM et al. . Assessment of breast cancer-related arm lymphedema: comparison of physical measurement methods and self-report. Cancer Invest. 2010;28:54–62. [DOI] [PubMed] [Google Scholar]
- 47. Ward LC, Czerniec S, Kilbreath SL. Operational equivalence of bioimpedance indices and perometry for the assessment of unilateral arm lymphedema. Lymphat Res Biol. 2009;7:81–85. [DOI] [PubMed] [Google Scholar]
- 48. Bundred NJ, Stockton C, Keeley V et al. . Comparison of multi-frequency bioimpedance with perometry for the early detection and intervention of lymphoedema after axillary node clearance for breast cancer. Breast Cancer Res Treat. 2015;151:121–129. [DOI] [PubMed] [Google Scholar]
- 49. Blaney JM, McCollum G, Lorimer J et al. . Prospective surveillance of breast cancer-related lymphoedema in the first-year post-surgery: feasibility and comparison of screening measures. Supp Care Cancer. 2015;23:1549–1559. [DOI] [PubMed] [Google Scholar]
- 50. Kim L, Jeon JY, Sung IY et al. . Prediction of treatment outcome with bioimpedance measurements in breast cancer related lymphedema patients. Ann Rehabil Med. 2011;35:687–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Berlit S, Brade J, Tuschy B et al. . Comparing bioelectrical impedance values in assessing early upper limb lymphedema after breast cancer surgery. In Vivo. 2012;26:863–867. [PubMed] [Google Scholar]
- 52. Cornish BH, Chapman M, Hirst C et al. . Early diagnosis of lymphedema using multiple frequency bioimpedance. Lymphology. 2001;34:2–11. [PubMed] [Google Scholar]
- 53. Berlit S, Brade J, Tuschy B et al. . Whole-body bioelectrical impedance analysis in assessing upper-limb lymphedema after breast cancer therapy. Anticancer Res. 2013;33:4553–4556. [PubMed] [Google Scholar]
- 54. Ancukiewicz M, Miller CL, Skolny MN et al. . Comparison of relative versus absolute arm size change as criteria for quantifying breast cancer-related lymphedema: the flaws in current studies and need for universal methodology [erratum in: Breast Cancer Res Treat. 2012;136:623]. Breast Cancer Res Treat. 2012;135:145–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Deltombe T, Jamart J, Recloux S et al. . Reliability and limits of agreement of circumferential, water displacement, and optoelectronic volumetry in the measurement of upper limb lymphedema. Lymphology. 2007;40:26–34. [PubMed] [Google Scholar]
- 56. Sander AP, Hajer NM, Hemenway K, Miller AC. Upper-extremity volume measurements in women with lymphedema: a comparison of measurements obtained via water displacement with geometrically determined volume. Phys Ther. 2002;82:1201–1212. [PubMed] [Google Scholar]
- 57. Karges JR, Mark BE, Stikeleather SJ, Worrell TW. Concurrent validity of upper-extremity volume estimates: comparison of calculated volume derived from girth measurements and water displacement volume. Phys Ther. 2003;83:134–145. [PubMed] [Google Scholar]
- 58. Taylor R, Jayasinghe UW, Koelmeyer L et al. . Reliability and validity of arm volume measurements for assessment of lymphedema. Phys Ther. 2006;86: 205–214. [PubMed] [Google Scholar]
- 59. Galland C, Auvert JF, Flahault A, Vayssairat M. Why and how post-mastectomy edema should be quantified in patients with breast cancer. Breast Cancer Res Treat. 2002;75:87–89. [DOI] [PubMed] [Google Scholar]
- 60. Galiano-Castillo N, Ariza-Garcia A, Cantarero-Villanueva I et al. . Agreement between telerehabilitation involving caregivers and face-to-face clinical assessment of lymphedema in breast cancer survivors. Support Care Cancer. 2014;22:253–258. [DOI] [PubMed] [Google Scholar]
- 61. Chen YW, Tsai HJ, Hung HC, Tsauo JY. Reliability study of measurements for lymphedema in breast cancer patients. Am J Phys Med Rehabil. 2008;87:33–38. [DOI] [PubMed] [Google Scholar]
- 62. Katz-Leurer M, Bracha J. Test-retest reliability of arm volume measurement in women with breast cancer-related lymphedema. J Lymphoedema. 2012;7:8–13. [Google Scholar]
- 63. Gjorup C, Zerahn B, Hendel HW. Assessment of volume measurement of breast cancer-related lymphedema by three methods: circumference measurement, water displacement, and dual energy X-ray absorptiometry. Lymphat Res Biol. 2010;8:111–119. [DOI] [PubMed] [Google Scholar]
- 64. Mori T, Lustman A, Katz-Leurer M. Self-measurement of upper extremity volume in women post-breast cancer: reliability and validity study. Physiother Theory Pract. 2015;31:283–287. [DOI] [PubMed] [Google Scholar]
- 65. Yamamoto T, Yamamoto N, Hara H et al. . Upper extremity lymphedema index: a simple method for severity evaluation of upper extremity lymphedema. Ann Plast Surg. 2013;70:47–49. [DOI] [PubMed] [Google Scholar]
- 66. Devoogdt N, Lemkens H, Geraerts I et al. . A new device to measure upper limb circumferences: validity and reliability. Int Angiol. 2010;29:401–407. [PubMed] [Google Scholar]
- 67. Foroughi N, Dylke ES, Paterson RD et al. . Inter-rater reliability of arm circumference measurement. Lymphat Res Biol. 2011;9:101–107. [DOI] [PubMed] [Google Scholar]
- 68. Borthwick Y, Paul L, Sneddon M et al. . Reliability and validity of the figure-of-eight method of measuring hand size in patients with breast cancer-related lymphoedema. Eur J Cancer Care (Engl). 2013;22:196–201. [DOI] [PubMed] [Google Scholar]
- 69. Purcell A, Nixon J, Fleming J et al. . Measuring head and neck lymphoedema: the “ALOHA” trial. Head Neck. 2016;38:79–84. [DOI] [PubMed] [Google Scholar]
- 70. Tewari N, Gill PG, Bochner MA, Kollias J. Comparison of volume displacement versus circumferential arm measurements for lymphoedema: implications for the SNAC trial. ANZ J Surg. 2008;78:889–893. [DOI] [PubMed] [Google Scholar]
- 71. Brorson H, Hoijer P. Standardised measurements used to order compression garments can be used to calculate arm volumes to evaluate lymphoedema treatment. J Plast Surg Hand Surg. 2012;46:410–415. [DOI] [PubMed] [Google Scholar]
- 72. Hidding JT, Viehoff PB, Beurskens CH et al. . Measurement properties of instruments for measuring of lymphedema: systematic review. Phys Ther. 2016;96:1965–1981. [DOI] [PubMed] [Google Scholar]
- 73. Bland KL, Perczyk R, Du W et al. . Can a practicing surgeon detect early lymphedema reliably? Am J Surg. 2003;186:509–513. [DOI] [PubMed] [Google Scholar]
- 74. Sagen A, Kåresen R, Risberg MA. The reliability of a simplified water displacement instrument: a method for measuring arm volume. Arch Phys Med Rehabil. 2005;86:86–89. [DOI] [PubMed] [Google Scholar]
- 75. Meijer RS, Rietman JS, Geertzen JH et al. . Validity and intra- and interobserver reliability of an indirect volume measurements in patients with upper extremity lymphedema. Lymphology. 2004;37:127–133. [PubMed] [Google Scholar]
- 76. Tsang KKW, Norte GE, Hand JW. The Assessment of Hand Volume Using a Modified Volumetric Technique. J Test Eval. 2012;40:329–333. [Google Scholar]
- 77. Megens AM, Harris SR, Kim-Sing C, McKenzie DC. Measurement of upper extremity volume in women after axillary dissection for breast cancer. Arch Phys Med Rehabil. 2001;82:1639–1644. [DOI] [PubMed] [Google Scholar]
- 78. Lette J. A simple and innovative device to measure arm volume at home for patients with lymphedema after breast cancer. J Clin Oncol. 2006;24:5434–5440. [DOI] [PubMed] [Google Scholar]
- 79. McKinnon JG, Wong V, Temple WJ et al. . Measurement of limb volume: laser scanning versus volume displacement. J Surg Oncol. 2007;96:381–388. [DOI] [PubMed] [Google Scholar]
- 80. Damstra RJ, Glazenburg EJ, Hop WC. Validation of the inverse water volumetry method: a new gold standard for arm volume measurements. Breast Cancer Res Treat. 2006;99:267–273. [DOI] [PubMed] [Google Scholar]
- 81. Erends M, van der Aa T, de Grzymala AP, van der Hulst R. Validity and reliability of three-dimensional imaging for measuring the volume of the arm. Lymphat Res Biol. 2014;12:275–281. [DOI] [PubMed] [Google Scholar]
- 82. Lee MJ, Boland RA, Czerniec S, Kilbreath SL. Reliability and concurrent validity of the perometer for measuring hand volume in women with and without lymphedema. Lymphat Res Biol. 2011;9:13–18. [DOI] [PubMed] [Google Scholar]
- 83. Sagen A, Karesen R, Skaane P, Risberg MA. Validity for the simplified water displacement instrument to measure arm lymphedema as a result of breast cancer surgery. Arch Phys Med Rehabil. 2009;90:803–809. [DOI] [PubMed] [Google Scholar]
- 84. Godoy JM, Silva SH, Godoy MF. Sensitivity and specificity of combined perimetric and volumetric evaluations in the diagnosis of arm lymphedema. Prague Med Rep. 2007;108:243–247. [PubMed] [Google Scholar]
- 85. Ancukiewicz M, Russell TA, Otoole J et al. . Standardized method for quantification of developing lymphedema in patients treated for breast cancer. Int J Radiat Oncol Biol Phys. 2011;79:1436–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Adriaenssens N, Buyl R, Lievens P et al. . Comparative study between mobile infrared optoelectronic volumetry with a Perometer and two commonly used methods for the evaluation of arm volume in patients with breast cancer related lymphedema of the arm. Lymphology. 2013;46:132–143. [PubMed] [Google Scholar]
- 87. Ridner SH, Montgomery LD, Hepworth JT et al. . Comparison of upper limb volume measurement techniques and arm symptoms between healthy volunteers and individuals with known lymphedema. Lymphology. 2007;40:35–46. [PubMed] [Google Scholar]
- 88. Dylke ES, Yee J, Ward LC et al. . Normative volume difference between the dominant and nondominant upper limbs in healthy older women. Lymphat Res Biol. 2012;10:182–188. [DOI] [PubMed] [Google Scholar]
- 89. O’Toole J, Jammallo LS, Skolny MN et al. . Lymphedema following treatment for breast cancer: a new approach to an old problem. Crit Rev Oncol Hematol. 2013;88:437–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Stout NL, Pfalzer LA, Levy E et al. . Segmental limb volume change as a predictor of the onset of lymphedema in women with early breast cancer. PM R. 2011;3:1098–1105. [DOI] [PubMed] [Google Scholar]
- 91. Ohberg F, Zachrisson A, Holmner-Rocklov A. Three-dimensional camera system for measuring arm volume in women with lymphedema following breast cancer treatment. Lymphat Res Biol. 2014;12:267–274. [DOI] [PubMed] [Google Scholar]
- 92. Lu G, DeSouza GN, Armer J et al. . A system for limb-volume measurement using 3D models from an infrared depth sensor. Proc 2013 IEEE Symp Comput Intelligence Healthcare E-Health (Cicare). 2013:64–69. [Google Scholar]
- 93. Lu G, Han K, Desouza GN et al. . A new algorithm for 3D registration and its application in self-monitoring and early detection of lymphedema. IRBM. 2014;35:370–384. [Google Scholar]
- 94. Mayrovitz HN, Weingrad DN, Lopez L. Patterns of temporal changes in tissue dielectric constant as indices of localized skin water changes in women treated for breast cancer: a pilot study. Lymphat Res Biol. 2015;13:20–32. [DOI] [PubMed] [Google Scholar]
- 95. Mayrovitz HN, Carson S, Luis M. Male-female differences in forearm skin tissue dielectric constant. Clin Physiol Funct Imaging. 2010;30:328–332. [DOI] [PubMed] [Google Scholar]
- 96. Smith BG, Lewin JS. Lymphedema management in head and neck cancer. Curr Opin Otolaryngol Head Neck Surg 2010;18:153–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Choi YH, Seo KS. Correlation among bioimpedance analysis, sonographic and circumferential measurement in assessment of breast cancer-related arm lymphedema. Lymphology. 2014;47:123–133. [PubMed] [Google Scholar]
- 98. Hwang JH, Lee CH, Lee HH, Kim SY. A new soft tissue volume measurement strategy using ultrasonography. Lymphatic Res Biol. 2014;12:89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Kim W, Chung SG, Kim TW, Seo KS. Measurement of soft tissue compliance with pressure using ultrasonography. Lymphology. 2008;41:167–177. [PubMed] [Google Scholar]
- 100. Mellor RH, Bush NL, Stanton AW et al. . Dual-frequency ultrasound examination of skin and subcutis thickness in breast cancer-related lymphedema. Breast J. 2004;10:496–503. [DOI] [PubMed] [Google Scholar]
- 101. Adriaenssens N, Belsack D, Buyl R et al. . Ultrasound elastography as an objective diagnostic measurement tool for lymphoedema of the treated breast in breast cancer patients following breast conserving surgery and radiotherapy. Radiol Oncol 2012;46:284–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Tassenoy A, De Mey J, De Ridder F et al. . Postmastectomy lymphoedema: different patterns of fluid distribution visualised by ultrasound imaging compared with magnetic resonance imaging. Physiotherapy. 2011;97:234–243. [DOI] [PubMed] [Google Scholar]
- 103. Rönkä RH, Pamilo MS, von Smitten KA, Leidenius MH. Breast lymphedema after breast conserving treatment. Acta Oncol. 2004;43:551–557. [DOI] [PubMed] [Google Scholar]
- 104. Balzarini A, Milella M, Civelli E et al. . Ultrasonography of arm edema after axillary dissection for breast cancer: a preliminary study. Lymphology. 2001;34:152–155. [PubMed] [Google Scholar]
- 105. Devoogdt N, Pans S, De Groef A et al. . Postoperative evolution of thickness and echogenicity of cutis and subcutis of patients with and without breast cancer-related lymphedema. Lymphatic Res Biol. 2014;12:23–31. [DOI] [PubMed] [Google Scholar]
- 106. Santin L, Ward LC. Agreement between dual energy X-ray absorptiometry and opto-electronic volumetry for measurement of forearm volume. Lymphat Res Biol. 2014;12:164–168. [DOI] [PubMed] [Google Scholar]
- 107. Brorson H, Ohlin K, Olsson G, Karlsson MK. Breast cancer-related chronic arm lymphedema is associated with excess adipose and muscle tissue. Lymphat Res Biol. 2009;7:3–10. [DOI] [PubMed] [Google Scholar]
- 108. Donahue MJ, Donahue PC, Rane S et al. . Assessment of lymphatic impairment and interstitial protein accumulation in patients with breast cancer treatment-related lymphedema using CEST MRI. Magn Reson Med. 2016;75:345–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Mihara M, Murai N, Hayashi Y et al. . Using indocyanine green fluorescent lymphography and lymphatic-venous anastomosis for cancer-related lymphedema. Ann Vasc Surg. 2012;26:278.e1–e6. [DOI] [PubMed] [Google Scholar]
- 110. Partsch H. Practical aspects of indirect lymphography and lymphoscintigraphy. Lymph Res Biol. 2003;1:71–74. [DOI] [PubMed] [Google Scholar]
- 111. Brorson H, Ohlin K, Olsson G, Nilsson M. Adipose tissue dominates chronic arm lymphedema following breast cancer: an analysis using volume rendered CT images. Lymphat Res Biol. 2006;4:199–210. [DOI] [PubMed] [Google Scholar]
- 112. Dylke ES, McEntee MF, Schembri GP et al. . Reliability of a radiological grading system for dermal backflow in lymphoscintigraphy imaging. Acad Radiol. 2013;20:758–763. [DOI] [PubMed] [Google Scholar]
- 113. Akita S, Mitsukawa N, Kazama T et al. . Comparison of lymphoscintigraphy and indocyanine green lymphography for the diagnosis of extremity lymphoedema. J Plast Reconstr Aesthet Surg. 2013;66:792–798. [DOI] [PubMed] [Google Scholar]
- 114. Moseley A, Piller N. Reliability of bioimpedance spectroscopy and tonometry after breast conserving cancer treatment. Lymphat Res Biol. 2008;6:85–87. [DOI] [PubMed] [Google Scholar]
- 115. Bagheri S, Ohlin K, Olsson G, Brorson H. Tissue tonometry before and after liposuction of arm lymphedema following breast cancer. Lymphat Res Biol. 2005;3:66–80. [DOI] [PubMed] [Google Scholar]
- 116. Davies C, Ryans K, Levenhagen K, Perdomo M. Breast cancer EDGE task force outcomes: quality of life and functional outcomes measures for secondary lymphedema in breast cancer survivors. Rehabil Oncol. 2014;32:7–12. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data are available at PHYSTH online.