Abstract
Uterine fibroids (UFs, AKA leiomyoma) are the most important benign neoplastic threat to women's health, with costs up to hundreds of billions of health care dollars worldwide. Uterine fibroids caused morbidities exert a tremendous health toll, impacting the quality of life of women of all ethnicities, especially women of color. Clinical presentations include heavy vaginal bleeding, pelvic pain, bulk symptoms, subfertility, and obstetric complications. Current management strategies heavily lean toward surgical procedures; nonetheless, the choice of treatment is generally subject to patient's age and her desire to preserve future fertility. Women with UF who desire to maintain future fertility potential face a dilemma because of the limited treatment choices that are currently available to help them achieve that goal. Recently, ulipristal acetate the first of the promising family of oral selective progesterone receptor modulators has been approved for UF treatment in Europe, Canada, and several other countries and is under review for possible approval in the USA. In this review article, we discuss recent advances in the management options against UF with a bend toward oral effective long-term treatment alternatives who are particularly suited for those seeking to preserve their future fertility potential. We also explore the transformative concept of primary and secondary UF prevention using these new anti-UF agents. We envision a remarkable shift in the management of UF in future years from surgical/invasive treatment to orally administrated options; clearly, this potential shift will require additional intense clinical research.
Keywords: uterine fibroids, selective progesterone receptor modulators, ulipristal acetate, fertility preservation, treatment, prevention
Summary Sentence
We focus on oral long term anti-UF treatment options which can benefit those seek to preserve future fertility. We explore the transformative concept of primary/secondary UF prevention using these agents. We envision a futuristic shift in the UF management from invasive treatment to oral one.
Abbreviations
- ActRIIB
Activin receptor type
- Alk4
Activin receptor-like kinase 4
- ART
Assisted reproductive techniques
- AUB
Abnormal uterine bleeding
- BMD
Bone mineral density
- COCs
Combined oral contraceptives
- E2
Estrogen
- EC
Emergency Contraceptive
- ECM
Extracellular matrix
- EMA
European Medicines Agency
- ER
Estrogen receptor
- GnRH
Gonadotropin releasing hormone
- HIFU
High intensity focused ultrasound
- HMB
Heavy menstrual bleeding
- IGFRI
Insulin-like growth factor receptor-I
- IR
Insulin receptor
- IVF
In Vitro Fertilization
- LA
Leuprolide acetate
- P4
Progesterone
- PAECs
Progesterone receptor modulators associated endometrial changes
- PEARL
PGL4001 (UPA) efficacy assessment in reduction of symptoms due to uterine leiomyomata
- PK
Pharmacokinetic
- PR
Progesterone receptor
- SPRMs
Selective progesterone receptor modulator
- TGFβ
Transforming growth factor β
- TNF-α
Tumor necrosis factor alpha
- UF
Uterine fibroids
- UPA
Ulipistal acetate
- VEGF
Vascular endothelial growth factor
Introduction
Uterine fibroids (UFs) or leiomyoma are considered the most common benign solid monoclonal smooth muscle tumors in women of reproductive age with a prevalence rate of 70%–80% in women by 50 years of age, making it a substantial health care burden with significant quality-of-life impact [1]. Uterine fibroids originate from myometrium when a normal myometrial stem cell is sufficiently altered eventually leading to the emergence of a somatic mutation such as Med12 mutation, and converting that stem cell into a fibroid tumor initiating cell [2–4]. Each fibroid lesion is an independent mutagenic event as evidenced recently by the detection of an assortment of Med12 mutation in different UF lesion in the same uterus [5,6]. Patients with UF develop various symptoms over time such as bulk symptoms include pelvic pressure and pain, dysmenorrhea, dyspareunia, and constipation [7], but the most common UF-related symptoms is heavy menstrual bleeding (HMB), as 80% of women with UFs experience menorrhagia and menometrorrhagia which often leads to iron deficiency anemia [7]. Additionally, various obstetrical complications such as miscarriage, premature labor, postpartum hemorrhage, and placental abruption can also be provoked by UF [8]. In the USA alone, UFs have a total economic cost estimated to range between 6 and 34 billion dollars annually. This embraces direct costs of management such as surgery, hospital admissions, outpatient visits, and medications, in addition to the indirect costs attributed to loss of wages, disability, and other obstetric complications [9].
Uterine fibroids and fertility
Uterine fibroids have a negative impact on female infertility. Uterine fibroids are present in 5%–10% of women with infertility and remarkably are the only detectable cause of infertility in up to 2.5% of these cases [10]. Most of these cases (65%) are attributed to inadequate endometrial receptivity to embryo implantation secondary to deleterious effects of UFs on endometrium [11]. Uterine fibroids may also affect both transport of sperms and uterine contractility, this was confirmed indirectly via promising fertility performance after removal of UFs [12]. Many studies were conducted to address the possible effect of UFs on the outcome of assisted reproductive techniques (ART), including in vitro fertilization and intracytoplasmic sperm injection, as compared to those without UFs. These studies showed a reduced rate of implantation, pregnancy, and live birth with increase in miscarriage rate among women with UFs, especially submucosal and intramural lesions [13,14]. Current literature call for removal of submucous (type 0) fibroid and possibly cavity distorting intramural fibroid (types 1–2) to optimize ART supported pregnancy outcomes. While removal of intramural fibroids (noncavity distorting, types 3–5) of any size and especially if less than 4 cm is still controversial [7,15–20].
Role of progesterone in pathogenesis of uterine fibroids
Female sex steroids, estrogen (E2) and progesterone (P4), play a major role in UF pathogenesis [21]. This has been proven epidemiologically, clinically, and at the molecular level [22–24]. Size of UF is increased at early stage of pregnancy along with the increase in circulating E2 and P4, while a paradoxical stabilization and eventual decrease in size are observed during late pregnancy and postpartum period, which is attributed to the increase in myometrial differentiation and extracellular matrix remodeling [25–27]. Interestingly, high parity was found to be protective against UF relative to nulliparous women [28] while early menarche increases the risk of UF development [29].
Classically, UFs were thought to be mainly E2-dependent tumors, based on their chronological association with the reproductive age besides the overexpression of estrogen receptors (ERs) alpha as well as aromatase enzyme in UFs relative to normal myometrium [30,31]. Furthermore, encouraging anti-fibroid findings using medications that decrease E2 production such as gonadotropin releasing hormone (GnRH) analogs [32], aromatase inhibitors [33], and selective ER modulators [34] supported that notion.
Recently, another important role for E2 has been identified, which is to support both progesterone receptors (PR A & B) induction and facilitating PR ligands action on target cells [35]. Uterine fibroid cells exhibit an increase in the expression of both PR isoforms in response to estradiol [36]. Interestingly, the overexpression of dominant-negative (nonfunctional) ER results in decrease in the PR expression in human UF cells [37]. It is therefore suggested that E2 primary role in UF pathogenesis is to maintain PR levels and it is indeed P4 that promotes UF growth and progression [38].
A growing number of clinical and experimental studies support the pivotal role of P4 in UF growth and development [39]. For example, the mitotic activity in UFs is higher during the secretory phase of the menstrual cycle (when P4 is dominant) than during the proliferative phase (when E2 is dominant) [40], and also increased proliferation of UF cells in vitro when exposed to both E2 and P4 [41]. Finally, a UF xenograft animal model showed that P4 is imperative for proliferation of UF tumor cells and formation of UF lesions [38].
Progesterone actions on the female reproductive system are mainly mediated via PR which is synthesized from a single gene and expressed as two main protein isoforms (PR-A, PR-B) [42]. Both PR A/B are extensively expressed in UFs as compared to normal myometrium from the same patient [43]. PR-B is the transcriptional activator of the progesterone-responsive genes, while PR- A is the ligand-dependent repressor of PR-B transcriptional activity [44]. Progesterone action is found to be tissue selective as it stimulates growth of UF in the uterus while inhibits the growth of endometrium. This tissue selectivity is highly dependent on differential recruitment of PRs and associated transcriptional co-regulators to gene promoters in different target tissues [45].
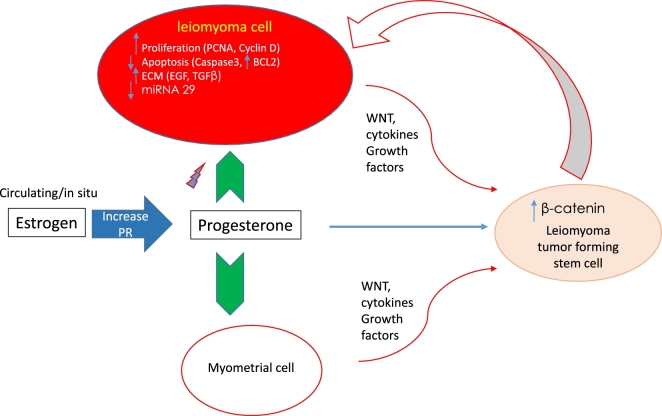
Additionally, P4 has been recognized to influence the activity of many other signaling pathways via rapid onset (seconds to minutes) extracellular cytoplasmic nongenomic mechanisms prompted by its binding to membrane-bound receptors [46]. This includes important pathways such as WNT/β-catenin pathway [35] and PI3K/AKT pathway [47]. All are crucial pathways for UF growth and progression (Figure 1).
Figure 1.
Role of progesterone in uterine fibroids pathogenesis. Progesterone, in response to estrogen, affects different cellular functions such as proliferation, apoptosis, and extracellular matrix deposition, either directly on fibroid cell via progesterone receptors or indirectly via paracrine effect on fibroid stem cells which give rise to more fibroid cells. Abbreviations: PCNA, proliferating cell nuclear antigen; BCL2, B-cell lymphoma 2; ECM, extracellular matrix; EGF, epidermal growth factor; TGFβ, transforming growth factor β; PR, Progesterone receptor.
Studies also showed that P4 plays an important role in regulation of growth factors levels and the differential expression of their receptors in UF such as insulin receptor (IR), insulin-like growth factor receptor-I (IGFRI), IGF-RII, epidermal growth factor, platelet-derived growth factor including its receptor, and transforming growth factor β ligands (TGFβ) and its receptors especially TGF-β3/R [48,49]. Importantly, progesterone plays a key role in the proliferation and apoptosis processes in UF as shown in increasing levels of proliferating cell nuclear antigen (PCNA), which is associated with cell proliferation, anti-apoptotic B-cell lymphoma 2 (BCL-2) gene, and the decrease in level of cleaved caspase 3 which is crucial for apoptosis [50,51] (Figure 1).
Interestingly, E plus P, not E alone, suppressed miRNA-29b expression level which belongs to miRNA 29 family [52]. This family was recently found to be less expressed in UF relative to myometrium and this downregulation contributes to increased collagen level in UF tissues [53].
Figure 1 summarizes the different roles of progesterone on UF pathogenesis.
Treatment options for uterine fibroids
Many women, if given the option, would prefer medical treatment for their UFs over a surgical solution to avoid the possible risks associated with surgery, and preserve their uterus for future fertility and also for psychological/feminine reasons [54,55]. Selecting a specific UF treatment primarily depends on patient's age, her symptoms, her preferences, and most importantly her reproductive plans. Currently, there are limited number of treatment options available for UF patients who desire future fertility [56,57].
Detailed description of surgical and traditional non-surgical treatment options against UF is beyond the scope of this review article and has been addressed with excellent reports recently [48,58]. In this article, we will focus on UF treatment options with a bend toward their effect on preservation of fertility potential.
Surgical interventions
Hysterectomy
Uterine fibroids are the leading gynecological cause of hospital admission, as ∼200 000 of the 600 000 hysterectomies performed each year in the USA are due to UFs [59]. Hysterectomy completely removes the fibroids, however, deprives these women of being able to naturally conceive for the rest of their lives. In addition, minor and major surgical complications can occur [60,61]. Currently, due to various social and financial reasons, many women postpone their first pregnancy to the later part of their reproductive years, so the request for uterine preservation, to maintain fertility, is becoming an increasingly urgent need in the gynecologist's daily practice in women with symptomatic UFs [62].
Myomectomy
Another surgical option to manage women with symptomatic UF is myomectomy, with a rate of ∼30 000 conducted annually in USA [9]. It aims to remove tumor only and retains the uterus. Yet, it is still considered a major surgical operation with potential morbidity and significant risks of UF recurrence [63]. Furthermore, there is a high risk of postoperative adhesion formation which makes the positive impact of myomectomy on UF-related infertility rather doubtful [64]. Laparoscopic or hysteroscopic radiofrequency myomectomy is a relatively recent modality with limited data on its impact on subsequent fertility [65]. Hysteroscopic myomectomy for intracavitary submucous (FIGO type 0–1) is one area where high-quality evidence strongly suggests positive impact on subsequent fertility [66].
Non-surgical interventions
Less invasive procedures such as uterine artery embolization uses embolus to block blood flow to the tumor, which consequently reduce fibroid size and its associated symptoms [67]. However, it has been connected to potential complications such as premature ovarian failure, chronic vaginal discharge, occasional pelvic sepsis, and may have limited efficacy when the fibroids are large [67]. Additionally, there is a debate regarding its effect on future fertility, with a general acceptance in the field that it should not be considered for those who plan future pregnancy [68].
Magnetic resonance-guided focused ultrasound also known as high intensity focused ultrasound (HIFU) uses focused ultrasound energy to thermally ablate UF tissue [69]. HIFU may be effective in select cases, but a recent randomized placebo-controlled study showed its limited durable effect and the high rate of additional subsequent fibroid related procedures [70]. Generally, nonhysterectomy procedures are typically associated with a high rate of symptoms recurrence from either regrowth of pre-existing fibroid or new UF tumor formation.
Pharmacological treatment
Therapeutic drugs may offer excellent alternative options for many UF patients, including those who desire more conservative management approach, women approaching menopause (perimenopause), and particularly for young UF patients who wants to preserve their future fertility. Drug-based approaches have been traditionally used as preoperative adjuvant to reduce fibroid volume and not for long-term courses. Current medical therapies either fail to fully resolve symptoms or are associated with unacceptable side effects that limit their long-term use [57]. Current investigations in the field foresee UF drug treatment options for a major role beyond short-term presurgical adjuvant therapy, but rather as a viable long-term treatment options with sustained effectiveness, safety, affordability, and most importantly fertility preservation capability. Herein, we will briefly describe these various hormonal treatment modalities with special emphasis on ulipistal acetate (UPA).
Combined oral contraceptives and levonorgestrel intrauterine system
Gynecologists generally considered the use of combined oral contraceptive pills (COCs) as their first choice to control UF-related abnormal uterine bleeding (AUB); this is based on their suppressive effect on endometrial proliferation besides being accessible with low cost and relatively good safety profile [71]. However, COCs have limited efficacy as well as lack of ability to reduce tumor size [71]. Following the same concept, FDA approved in 2009 the use of levonorgestrel intrauterine system to treat women with HMB. However, studies have shown conflicting results on its efficacy in controlling UF-related bleeding [72–75].
Gonadotropin releasing hormone agonists and antagonists
GnRH agonist was one of the first medical therapies to be used in UF treatment. In 1999, the FDA approved the short-term use of leuprolide acetate (LA) as a preoperative hematologic improvement adjunct in women with symptomatic UF who are accompanied with anemia. Its action is based on induction of hypoestrogenic state as a result of pituitary GnRH receptor downregulation with subsequent decrease of gonadal steroids, thus putting patient in pseudomenopause state and reducing fibroid size and symptomatology [1,9,71,76]. However, it causes a wide range of side effects such as hot flushes, vaginal dryness, and mood swings to serious ones as bone demineralization and decreased bone mineral density (BMD), thus limiting LA use to a maximum of 3- to 6-month duration [77]. Being expensive besides the rapid recurrence of UF symptoms within 3 months of treatment cessation limits the use of this approach for long-term therapy [78].
GnRH antagonists as cetrorelix and ganirelix have been used with advantage over the agonists of bypassing the initial flare effect due to receptor stimulation (up to 15 days), thus allowing them to show faster improvement of bleeding pattern [77,79]. Yet, several reasons prohibit widespread use of the antagonists generally in symptomatic treatment of UF such as high price, requirement of daily administration, and lack of clinical trial-based evidence of their superiority over the agonist [78,80].
Currently, several phase III randomized controlled clinical trials are being conducted to evaluate the utility of novel orally active GnRH antagonist such as elagolix, relugolix, and OBE2109 either with or without add-back therapy in women with symptomatic UF [81–83].
Selective estrogen receptor modulators
Although preclinical data appeared to be promising regarding the use of selective ER modulators such as tamoxifen or raloxifene in the treatment of UFs, clinical trial results were unsatisfactory [34].
Selective progesterone receptor modulators
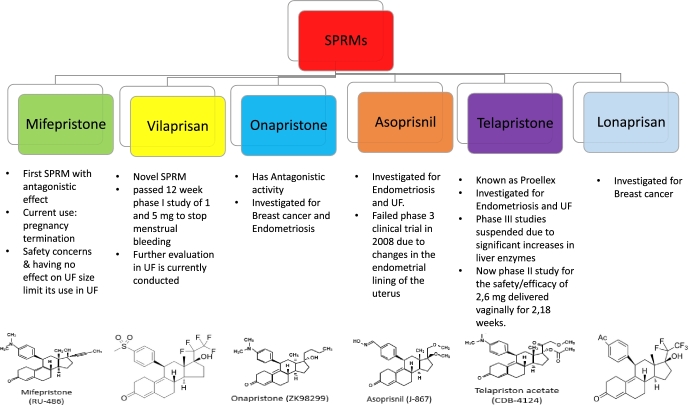
Selective progesterone receptor modulators (SPRMs) are relatively new class of synthetic steroid ligands with a PR-target and tissue-selective effects of mixed agonist and antagonist activities [84]. They have several current indications including emergency contraception (EC), termination of pregnancy, premenstrual syndrome, and assisted reproduction [85]. Furthermore, as they may have direct effects on endometrial and fibroid cells, they are also investigated for therapeutic utility against UF, AUB, dysmenorrhea, endometriosis, and more recently breast cancer prevention [86]. Mifepristone was the first member of this class that has expanded to include asoprisnil, onapristone, UPA, lonaprisan, vilaprisan, and telapristone (Figure 2). The agonistic/antagonistic nature of interaction between each ligand and PR, with subsequent effects on target genes, is based on cell type, molecular environment, and selective recruitment of co-activators or co-repressors. Furthermore, SPRMs have minimal effect on serum estrogen levels and so they are not expected to induce menopausal-like symptoms or subsequent bone loss [84,85,87,88]. Figure 2 highlights the SPRM family members, other than UPA, and their current research direction.
Figure 2.
SPRMs family members other than ulipristal acetate. List of different members of selective progesterone receptor modulators family, other than ulipristal acetate, with their chemical structures, main characteristics, and current research direction. SPRM, selective progesterone receptor modulator.
Pending additional clinical trial evaluation, SPRMs are poised to provide additional options in the management choices against UF and may provide viable alternative to surgery for women seeking fertility preservation (medical myomectomy). In the following sections, we will briefly describe the state of the art of the commonly studied SPRMs against UFs with emphasis on UPA as the forefront SPRM already approved for use against UF in Europe, Canada, and several other countries.
Mifepristone
Mifepristone was the first SPRM, with predominantly, almost pure, antagonistic effect, to be investigated in UF management [89]. A recent systematic review concludes its efficacy in reducing bleeding and improving quality of life albeit without significant reduction in UF volume. Unfortunately, safety concerns were raised due to its associated risk of endometrial hyperplasia and therefore it is no longer recommended for UF management and its current use is primarily for pregnancy termination [89,90].
Asoprisnil
Asoprisnil was developed for symptomatic treatment of endometriosis, UF, and dysfunctional uterine bleeding. It resulted in reduction in fibroid size and improvement in HMB. Unfortunately, it has not been taken further in clinical trials in recent years due to failing phase III clinical trial in 2008 which is attributed to unsafe changes in the endometrial lining of the uterus [91–93].
Telapristone
Also known as Proellex, telapristone has been evaluated for treatment of symptoms associated with endometriosis and UF. However, phase III studies were suspended because of significant increases in liver enzymes [94]. At present time, there is an ongoing phase II clinical trial started on 2014 that aims to evaluate both safety and efficacy of lower oral as well as vaginal doses of telapristone acetate [95].
Vilaprisan
A novel SPRM which recently passed a 12-week phase I clinical trial successfully, in which most of the women who took the medication at daily dose of 1–5 mg reported absence of menstrual bleeding. These results supported the initiation of advanced clinical trials to evaluate vilaprisan in women with symptomatic UFs [96].
Ulipristal acetate
It is our belief that the interest in this oral agent will soon increase in the USA as a novel and much-needed therapy for the medical management of UFs. UPA (Figure 3) is being evaluated not only as a presurgical adjuvant but also for long-term use with special utility in women with symptomatic UFs who are seeking fertility preservation. Hence, the present work aims at providing a comprehensive summary of its main features as clinical pharmacology, pharmacokinetic (PK) properties, and safety as well as its clinical utility in UF management.
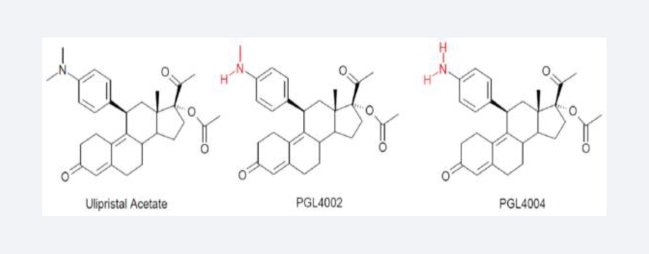
Figure 3.
Chemical structures of ulipristal acetate and its metabolites.
Pharmacology
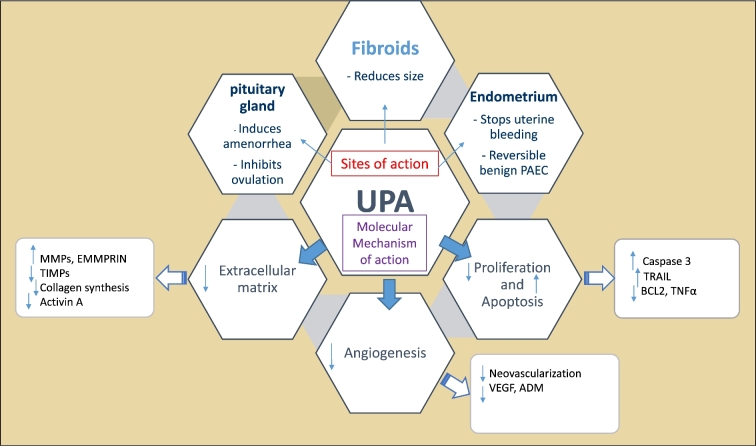
UPA is a synthetic steroid derived from 19-norprogesterone, and has tissue-specific mixed agonist/antagonist effects with noted preferential binding in the uterus, cervix, ovaries, and hypothalamus [97]. UPA is characterized by its superior selectivity for PRs, even higher than P4 itself, and it increases apoptosis and decreases proliferation via numerous mechanisms including increase in alkaline phosphatase activity, upregulation of cleaved caspase-3, and downregulation of both tumor necrosis factor alpha (TNF-α) and Bcl-2 expression [98]. UPA also induces apoptosis by activating the mitochondrial and TNF-related apoptosis-inducing ligand (TRAIL) pathways and eliciting endoplasmic reticulum stress [98]. It was also shown that UPA suppresses the expression of angiogenic factors such as vascular endothelial growth factor (VEGF) and adrenomedullin in cultured human fibroid cells [99]. Furthermore, UPA reduces collagen deposition in the extracellular matrix (ECM), resulting in shrinkage of ECM volume [100]. UPA was also shown to inhibit the expression and functions of activin A in UF cells which was proven to increase ECM expression. It also decreases activin binding proteins, follistatin, activin receptor type (ActRIIB), and activin receptor-like kinase 4 (Alk4) mRNA expressions. In addition, it was able to block the activin A-induced increase in fibronectin or VEGF-A mRNA expression [101]. Moreover, ECM deposition in UF is also reduced by UPA due to increasing both matrix metalloproteinases (MMPs) and ECM metalloproteinase inducer (EMMPRIN) while reducing tissue inhibitor of metalloproteinases (TIMPs) [100,102]. Finally, UPA also increases the ratio of progesterone receptor isoforms (PR-A/PR-B) as it decreases PR-B receptor expression while increases PR-A so UPA inhibits progesterone-mediated effects on fibroid cells [98–101, 103–108]. Figure 4 summarizes UPA mechanisms of action presented in the literature so far.
Figure 4.
Mechanism of action of ulipristal acetate (UPA). UPA binds to pituitary gland, endometrium, and uterine fibroids to elicit its actions via modulations of several markers that regulate different cell functions such as proliferation, apoptosis, extracellular matrix deposition, and angiogenesis. Abbreviations: PAEC, Progesterone Receptor Modulators associated Endometrial Changes; UPA, Ulipristal Acetate; TRAIL, TNF-related apoptosis-inducing ligand; BCL2, B-cell lymphoma 2; TNF, Tumor Necrosis Factor Alpha; VEGF, Vascular Endothelial Growth Factor; ADM, Adrenomedullin; MMPs, Matrix Metalloproteinases; EMMPRIN, Matrix Metalloproteinase Inducer; TIMP, Tissue Inhibitor of Metalloproteinases.
A retrospective study of tissues collected from women treated preoperatively with UPA versus placebo-treated controls demonstrated that UPA supported low proliferation rate in UF cells, stimulated cell death, and intensely reduced ECM in UF lesions [102].
Pharmacokinetics
UPA has a good oral bioavailability, mainly excreted in feces, and less than 10% is excreted in the urine. It is extensively metabolized by the liver via CYP3A4-mediated N-demethylation giving rise to its main metabolites N-monodemethylated (PGL4002) and N-didemethylated UPA (PGL4004) (Figure 3). Therefore, concomitant use of potent CYP3A4 inducers such as rifampicin, phenytoin, and phenobarbital or inhibitors such as ketoconazole and erythromycin is not recommended to avoid either treatment failure or toxicity, respectively [109,110].
By assessing the PK of a single oral dose of UPA (5 mg) in healthy female volunteers, UPA rapidly reached peak concentrations within 1 h after administration, with the apparent terminal half-life time (t1/2) ranging from 35 to 43 h, thus allowing one oral administration per day dosage [111]. Multiple dosing of UPA exhibited a PK profile consistent with that of a once-daily regiment [111].
Rate of absorption of UPA is pH dependent so concomitant administration with drugs that increase gastric PH as proton pump inhibitors can slightly modify PK parameters [112]. No dramatic changes are observed when administrated either with or without food [113]. Interestingly, trough UPA concentrations were generally comparable in healthy participants and UF patients [114,115].
As AUB is a frequent symptom of UF with concomitant iron deficiency anemia, iron salts are commonly prescribed in UF patients. These salts are traditionally known to inhibit the bioavailability of other concurrently taken drugs. However, PK studies concluded that its effect is minimal on UPA bioavailability and of no clinical significance. As UPA is mainly metabolized in liver, it is not recommended for patients with moderate-to-severe hepatic impairment unless closely monitored. However, it is safe, without dose modification, in mild cases [116].
Although conception is unlikely during UPA intake due to its ovulation suppression, it is still contraindicated in pregnancy as the safety data regarding its teratogenicity are unknown. So backup contraceptive favorably barrier methods such as condoms must be used. While COCs or progestin only pills are not recommended, they might reduce UPA therapeutic effect [117].
Indications
In February 2012, European medicines agency (EMA) approved the use of 5 mg UPA tablets (Esmya) as a preoperative treatment for moderate-to-severe symptoms of UF in adult women of reproductive age, with a treatment duration limited to 3 months which extended to two courses of 3-month treatments in early 2014 [83]. In May 2015, UPA was approved for long-term intermittent treatment as follows, the first cycle starts with the first days of menstruation and then the subsequent course should begin with the next menstruation. In 2013, UPA received a Health Canada approval for the same indication of EMA under the name Fibristal [110,118].
In 2010, the FDA in the USA licensed the use of UPA at the dose of 30 mg (Ella) as an EC, due to its inhibition of ovulation and rapid effects on endometrium that may play a role in the prevention of implantation. Approval for UPA by FDA for the treatment of UF is pending and anticipated in near future, likely in 2018.
Advantages over gonadotropin releasing hormone analogs****
UPA can aid the operative treatment of UFs by reducing fibroid volume especially in cases of large UFs that exceed 6 cm in diameter, multiple fibroids, or fibroids of unfavorable localization such as cervical UF. After approximately 7 days of UPA administration, a significant reduction in bleeding occurs and amenorrhea ensues with subsequent increase in hemoglobin level, which decreases or eliminates the necessity of blood transfusions, “autotransfusion effect.” [119]
In women treated with UPA, circulating estradiol levels are maintained in the midfollicular range throughout the treatment duration, unlike GnRH agonists which decreased serum E2 to postmenopausal levels. Thus, UPA use avoids the annoying climacteric side effects, such as BMD loss and vasomotor symptoms, and in turn improves patient satisfaction and compliance [115]. After treatment discontinuation, the ongoing effects of fibroid volume reduction appear to be more prolonged with UPA than with GnRH agonists [120]. Moreover, UPA has not been linked to increased risk of thromboembolic events unlike other anti-UF hormonal therapeutics [114]. Finally, GnRH analogs are expensive and in most cases require additional hormonal add-back therapy, while UPA shows improved quality of life and cost-effectiveness (discussed later in Pharmacoeconomics section) [115].
Pivotal clinical trials of ulipristal acetate
Several clinical trials have evaluated the efficacy of UPA in treatment of UFs in terms of ability to reduce menstrual blood loss and reaching amenorrhea. They also evaluated its ability to reduce uterus and fibroid size as well as its impact on quality of life [114,115,121–128].
The most widely cited studies investigating anti-UF use of UPA are the European phase III studies, PGL4001 (UPA) Efficacy Assessment in Reduction of Symptoms Due to Uterine Leiomyomata (PEARLs I/II/III/IV) [114,115,121,125,129]. In 2016, this same group of investigators published an extension study with longer duration [121]. Table 1 summarizes all five UPA anti-UF seminal randomized, double-blinded, controlled multicenter phase III trials.
Table 1.
Summary of PEARL I-IV pivotal trials (with extensions).
| POC | PEARL I 2012 [114] | PEARL II 2012 [115] | PEARL III with extension 2014 [129] | PEARL IV 2015 [125] | PEARL IV “extension” 2016 [121] |
|---|---|---|---|---|---|
| Study objective | To study efficacy and safety of ulipristal acetate (UPA) versus placebo for symptomatic uterine fibroid (UF) treatment before surgery | To study efficacy and side-effect profile of UPA as compared with those of leuprolide acetate (LA) for the treatment of symptomatic uterine fibroids before surgery | To investigate the efficacy and safety of UPA for long-term treatment of symptomatic UF | To study the efficacy and safety of two 12-week courses of UPA for intermittent treatment of symptomatic UF | To study the efficacy and safety of four 12-week courses of UPA for intermittent treatment of symptomatic UF |
| Treatment, patient number, dosage regimen, and duration | UPA 5 mg/day (96 patients), UPA 10 mg/day (98 patients), placebo/day (48 patients) for 13 weeks then perform surgery | UPA 5 mg/day (95 patients), UPA 10 mg/day (100 patients); LA 3.75 mg/month (95 patients) for 3 month | Four 3-month courses of UPA 10 mg daily, immediately followed by 10-day double-blind treatment with norethisterone acetate (10 mg daily) or placebo (209 patients start first course and 107 patients complete the four course) | Two repeated 12-week treatment courses (separated by a drug-free interval of daily 5 or 10 mg of UPA (451 patients: 228 patients take 5 mg, 223 patients take 10 mg) | Four repeated 12-week treatment courses of daily 5 or 10 mg UPA (451 patients) |
| Primary outcome | Efficacy of UPA in term of control of uterine bleeding, reduction of fibroid volume | UPA is not inferior to LA in reducing the uterine bleeding in term of proportion of patients with controlled bleeding at end of study | Amenorrhea at the end of each UPA course | Amenorrhea at the end of both UPA courses | Endometrial safety in term of frequency of nonphysiological changes of biopsies and confirm efficacy of UPA |
| Secondary outcome | Bleeding pattern, amenorrhea, hemoglobin, hematocrit, and ferritin values, pain, quality of life | Bleeding pattern, amenorrhea, hemoglobin, hematocrit, and ferritin values, pain, quality of life | Reduction of the three largest fibroids | Reduction of the three largest fibroids | General safety, laboratory parameters, amenorrhea, controlled bleeding, fibroid volume, quality of life, and pain |
| Tolerability of UPA | Tolerability of UPA | Pain | Pain | ||
| Quality of life | Quality of life | ||||
| Notes | All patients received 80 mg iron supplementation once daily during the active treatment | Iron supplementation was left to the discretion of the treating physician | Double-blinded and placebo-controlled study toward the administration of progestin after the end of each UPA treatment course | Compliance with intermittent treatment is good, and symptomatic improvement and fibroid volume shrinkage can be largely maintained during the off-treatment periods | Data focus on the new findings from treatment courses 3 and 4 as well as the four treatment courses combined |
| —Fibroid-related menorrhagia was evaluated by the Pictorial Blood Assessment Chart (PBAC) score and was considered significant for inclusion when it was higher than 100 on days 1–8 of menstruation —Fibroid-associated anemia was considered significant when the hemoglobin level was lower than 10.2 g/dl |
|||||
| Outcome | PEARL I 2012 | PEARL II 2012 | PEARL III with extension 2014 | PEARL IV 2015 | PEARL IV extension 2016 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UPA 5 mg/day | UPA10 mg/day | placebo | UPA 5 mg/day | UPA10 mg/day | LA | UPA 10 mg Course 1 | UPA 10 mg Course 2 | UPA 10 mg Course 3 | UPA 10 mg Course 4 | UPA 5 mg 2 course | UPA10mg 2 courses | UPA 5 mg 4 courses | UPA10 mg 4 courses | |
| PBAC < 75 at 13 weeks | 91% (P < 0.001) | 92% (P < 0.001) | 19% | 90% | 98% | 89% | Not reported | 81.1% | 86% | 67.1% | 71.9% | |||
| Amenorrhea at end of treatment | 73% (P < 0.001) | 82% (P < 0.001) | 6% | 75% | 89% | 80% | 79.5% | 88.5% | 88.2% | 89.7% | 62% | 73% | 48.7% | 60.5% |
| Reduction in UF volume at 13 weeks | −21% (P = 0.002) | −12% (P = 0.002) | +3% | −36% | −42% | −53% | −45.1% | −63.2% | −67% | −72.1% | −54% | −58% | −71.8% | −72.7% |
| Days to amenorrhea | 50% within 10 days | 70% within 10 days | 7 | 5 | 21 | 4 | 2 | 3 | 3 | 5 after both cycles | 4 after first 6 after 2nd cycle | Not reported | ||
| Notes | —Hgb was higher in UPA-treated patients | —E level maintained in mid-follicular range avoiding postmenopausal symptoms while with LA decreased to postmenopausal levels —Patients who did not undergo surgery, UPA showed a more sustained effect on UF volume reduction during the following 6 months without treatment than did LA |
—All endometrial biopsies showed benign histology without hyperplasia; NETA did not affect fibroid volume or endometrial histology —Women could either attend a final follow-up visit 12 weeks later (PEARL III) or, if they wished to enroll in the PEARL III extension study to obtain up to three further courses of UPA (and NETA/placebo), each separated by an off-treatment period including a full menstrual cycle |
The primary null hypothesis for this study was that there would be no difference in the percentage of subjects who were in amenorrhea at the end of both treatment courses 1 and 2 for 10 mg of UPA compared with 5 mg of UPA | The primary null hypothesis was that there would be no difference in the percentage of subjects who are in amenorrhea at the end of all four treatment courses for UPA 10 mg compared with UPA 5 mg | |||||||||
| Conclusion | UPA treatment for 13 weeks effectively controlled excessive bleeding due to UF and reduced the size of the fibroids | Both daily 5-mg and 10-mg UPA were noninferior to once-monthly LA in controlling uterine bleeding and were significantly less likely to cause hot flashes | —Repeated 3-month UPA courses effectively control bleeding and shrink fibroids in patients with symptomatic UF —10-day progestin courses reduced the magnitude of menstrual bleeding during the off-treatment periods and also brought forward menstruation return |
Repeated 12-week courses of daily oral UPA (5 and 10 mg) effectively control bleeding and pain, reduce fibroid volume, and restore QoL in patient with symptomatic fibroids | With the recently registered indication of intermittent treatment with UPA, the absence of endometrial and laboratory safety findings associated with long-term therapy is of special interest to clinicians | |||||||||
Interestingly, PEARL studies demonstrated that efficacy of UPA treatment is still maintained even during the off-treatment periods, which allows intermittent long-term UF treatment with advantages of rapid bleeding control and progressive fibroid reduction [115,130].
The first US-based phase III clinical trial was completed to assess the efficacy and safety of UPA (5 and 10 mg) vs a placebo (VENUS 1 study). The end points were amenorrhea and activity score in premenopausal women. This study showed promising results in terms of rate of and time to amenorrhea without any reported adverse events that required drug discontinuation [131]. In the same study, efficacy of UPA for UF treatment was explored in different racial (black vs non-Black) and BMI (≥30 kg/m2 vs <30 kg/m2) groups with results highlighting efficacy of UPA regardless of race and BMI.
Safety evaluation
In PEARL III trial, treatment emergent adverse events occurred in 120 women (57.4%), but only 8 women (3.8%) had severe adverse events, including headache (16.3%) that lead to treatment withdrawal in five cases and abdominal pain (5.3%) but its incidence did not increase over time. No safety concerns in relation to liver function, other laboratory safety tests, hormone levels, ovarian or breast imaging, and ECGs were reported [114,115,129,132].
Endometrial safety and progesterone receptor modulators associated endometrial changes****
UPA was associated with an increase in endometrial overgrowth known as PR modulators associated endometrial changes (PAECs). These are benign histologic changes of endometrial glands that appear dilated or cystic; nevertheless, the cells lack mitotic activity and changes are reversible within few weeks to a maximum of 6 months post-therapy. Moreover, the absence of stromal breakdown and glandular crowding makes it distinctive from hyperplasia. The National institute of health sponsored a workshop to further discuss PAEC in women taking UPA and other SPRMs with the help of expert gynecologic pathologists. The workshop concluded that no specimen fits the criteria of atypical hyperplasia or endometrial carcinoma and presenting changes differ from classic unopposed E2 effect in terms of absence of mitosis and its reversibility. Another study re-analyzed PEARL I and II endometrium tissues and recommended that pathologists must be aware of these PEAC changes to avoid initial misdiagnosis [1,114,115,121,125,129,133–136].
Interestingly, a recent case report was published regarding a prolonged exposure to UPA for 5 years in a woman with benign metastasizing UFs, endometrial biopsies were collected at established intervals for endometrial safety assessment, and the result indicated absence of any evidence of endometrial hyperplasia or neoplasia [137].
Studies on the rate of pregnancy after completing UPA therapy have been conducted. These studies demonstrated that the endometrium is of sufficient quality for blastocyst implantation [138]. Patients treated with UPA were able to conceive quickly and easily, pregnancy rate in one study was 71%, and all the babies were born healthy [138].
Teratogenicity
There is limited data available about UPA teratogenicity when used as ECs due to its high efficiency. Besides, if conceptions were to happen, most women choose to terminate it [139]. From the little data available when used as a treatment for UFs, none reported any teratogenic effect [140,141].
Pharmacoeconomics
Pharmacoeconomic and outcome research studies were, and still being, conducted to study the cost-effectiveness of UPA as either an add-on or alternative therapy option to surgery, with positive results showing a favorable cost-effectiveness ratio [142]. A Canadian economic study evaluated the cost utility of preoperative UPA administration relative to LA in women with moderate-to-severe symptoms of UFs. It showed an emphasizing domination of UPA strategy over LA, as it provided patients with more quality-adjusted life years (0.177 versus 0.165) at a lower cost ($1273 versus $1366) with fewer side effects and faster bleeding control [143]. A similar study was conducted in Mexico, concluding that UPA is a cost-effective alternative to surgery, as 21% of the patients treated with UPA avoided hysterectomy, which translates to $47,614,017 USD being saved for every 1000 patients. [144].
Clinical applications and future directions
As UPA finds its way as a viable treatment option for women with symptomatic UF, it is clear that a new treatment paradigm is evolving. It is anticipated that UPA will not only be beneficial in the treatment of moderate to severe UF, but also it can be exploited to postpone/eliminate the need for surgery especially in women desiring fertility preservation.
Primary/secondary prevention of uterine fibroids with ulipristal acetate***
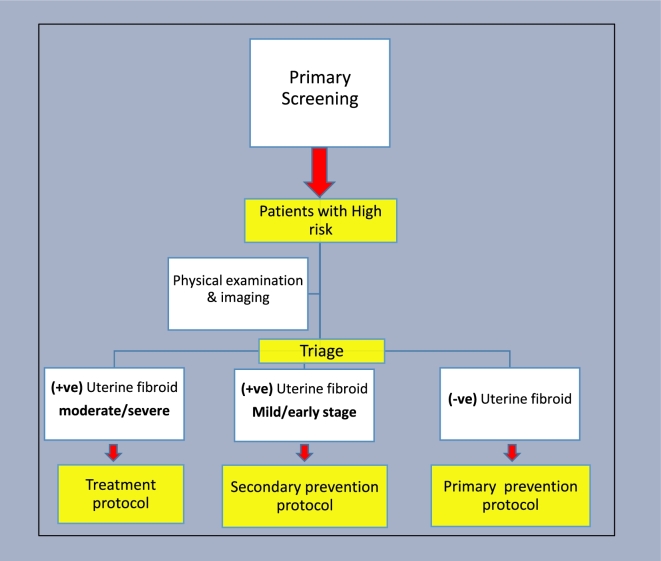
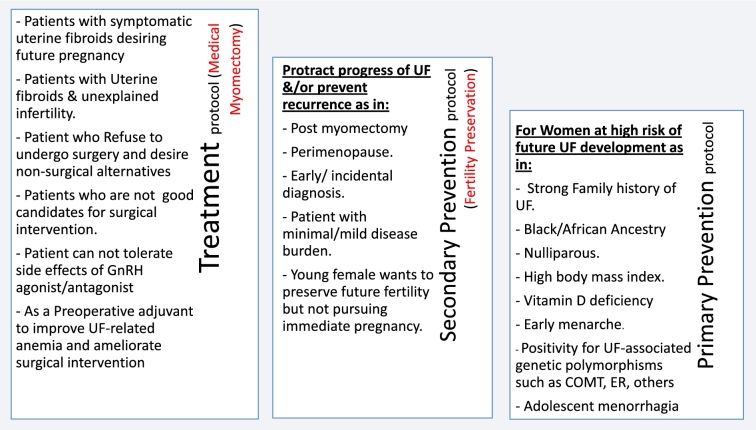
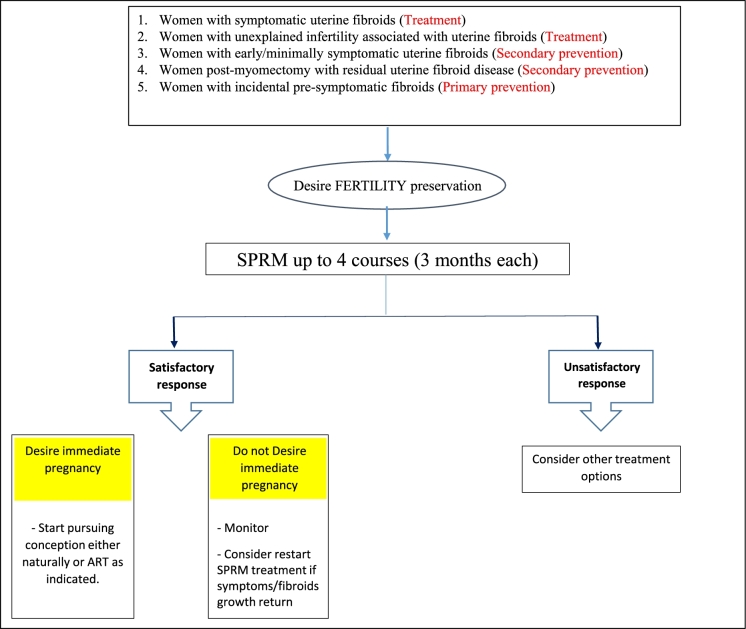
Aiming for a comprehensive use of UPA, we thought about developing a UF-specific risk assessment triage algorithm (Figure 5), with possible subsequent use of UPA for UF primary prevention in women at highest risk for future UF development. To apply such algorithm, asymptomatic reproductive age women can be screened for well-established UF risk factors (listed in Table 2). Women deemed to be at high UF risk, determined based on additional clinical research to determine the contribution of each risk factor individually as well as combination thereof, can then be further evaluated by appropriate examination and imaging modalities. Based on these tests, women can be triaged into one of three possible scenarios: (1) women with moderate-to-severe UF burden can then enter a treatment protocol and offer various UF-specific treatment options including oral treatment options; (2) women with mild/early-stage UF disease may benefit from a secondary prevention protocol to either halt fibroids progress or appearance of new ones. This same secondary prevention approach can also be applied for women with incidental finding of UF, patients after myomectomy to decrease the rate of recurrence, or young women with symptomatic UF but no immediate plans for pregnancy; (3) women at high risk for future UF development but currently with UF-free uteri (in imaging studies) may be activated in a primary prevention protocol to preclude or delay the development of UF. Figure 6 summarizes these case scenarios that may benefit from availability of UPA (and other oral-specific anti-UF therapies) in the future. To the extent of available data from clinical trials to date, these cases who desire fertility preservation can be safely treated with UPA, up to four cycles, 3 months each, followed by close monitoring and retreatment as needed (Figure 7). Clearly, these proposals will need to be carefully vetted in well-designed clinical trials and likely to evolve and undergo multiple tuning as collective experience with UPA and other novel anti-UF treatments accumulates. We envision a remarkable shift in the management of UF in future years from surgical/invasive treatment to orally administrated options; clearly, this potential shift will require additional intense clinical research.
Figure 5.
Uterine fibroid-specific risk assessment triage algorithm. Patient deemed to be at high risk of UF development will be further investigated and triaged into one of the three possible scenarios with subsequent protocols to be initiated.
Table 2.
Risk factors for uterine fibroid.
| Risk Factor | Reference |
|---|---|
| Age | [145] |
| Ethnicity (black vs. non-Black) | [146–148] |
| Obesity/overweight | [149–151] |
| Vitamin D deficiency/insufficiency | [152] |
| COMT polymorphism | [153] |
| ER polymorphism | [154] |
| Early menarche | [29] |
| Parity | [28] |
| Tobacco, caffeine, and alcohol | [148] |
| Family history of uterine fibroids | [155,156] |
| Higher TGF-β3 serum concentrations | [156] |
Figure 6.
Clinical applications of ulipristal acetate in uterine fibroid (UF) treatment/prevention. List of case scenarios that may benefit from availability of ulipristal acetate in the three different protocols of treatment, primary, and secondary prevention.
Figure 7.
Proposed treatment algorithm for ulipristal acetate use in uterine fibroid-related clinical profiles who desire fertility preservation. Different uterine fibroid-related clinical profiles who desire future fertility will administer ulipristal acetate or other oral agents for a 3-month cycle, up to four cycles, followed by close monitoring and retreatment as needed. ART, assisted reproductive techniques; SPRM, selective progesterone receptor modulator.
Conclusions
UPA will most likely usher the era of oral long-term treatment for women with symptomatic UFs and can be exploited for safe nonsurgical fertility preservation (medical myomectomy) as well. It may also support the transformational concept of UF primary and/or secondary prevention in presymptomatic/early symptomatic women, respectively. The field has advanced enough and is ripe to develop a robust UF risk assessment tool to identify women who are either racially, genetically, biochemically, or anthropometrically predisposed to future UF development. Further studies are urgently needed to delineate the appropriate place of UPA as well as other SPRMs in the anti-UF armament.
Conflict of interest: Ayman Al-Hendy is a consultant for Allergan plc, Bayer, Repros, and AbbVie.
References
- 1. Segars JH, Parrott EC, Nagel JD, Guo XC, Gao X, Birnbaum LS, Pinn VW, Dixon D. Proceedings from the Third National Institutes of Health International Congress on Advances in Uterine Leiomyoma Research: comprehensive review, conference summary and future recommendations. Hum Reprod Update 2014; 20(3):309–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yang Q, Diamond MP, Al-Hendy A. Early life adverse environmental exposures increase the risk of uterine fibroid development: role of epigenetic regulation. Front Pharmacol 2016; 7:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yang Q, Al-Hendy A. Developmental environmental exposure alters the epigenetic features of myometrial stem cells. Gynecol Obstet Res 2016; 3(2):e1–e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yang Q, Diamond MP, Al-Hendy A. Converting of myometrial stem cells to tumor-initiating cells: mechanism of uterine fibroid development. Cell Stem Cells Regen Med 2016; 2(1):e103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Makinen N, Mehine M, Tolvanen J, Kaasinen E, Li Y, Lehtonen HJ, Gentile M, Yan J, Enge M, Taipale M, Aavikko M, Katainen R et al. MED12, the mediator complex subunit 12 gene, is mutated at high frequency in uterine leiomyomas. Science 2011; 334(6053):252–255. [DOI] [PubMed] [Google Scholar]
- 6. Halder SK, Laknaur A, Miller J, Layman LC, Diamond M, Al-Hendy A. Novel MED12 gene somatic mutations in women from the Southern United States with symptomatic uterine fibroids. Mol Genet Genomics 2015; 290(2):505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pritts EA, Parker WH, Olive DL. Fibroids and infertility: an updated systematic review of the evidence. Fertil Steril 2009; 91(4):1215–1223. [DOI] [PubMed] [Google Scholar]
- 8. Rice JP, Kay HH, Mahony BS. The clinical significance of uterine leiomyomas in pregnancy. Am J Obstet Gynecol 1989; 160(5 Pt 1):1212–1216. [DOI] [PubMed] [Google Scholar]
- 9. Cardozo ER, Clark AD, Banks NK, Henne MB, Stegmann BJ, Segars JH. The estimated annual cost of uterine leiomyomata in the United States. Am J Obstet Gynecol 2012; 206(3):211.e1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Surrey ES, Lietz AK, Schoolcraft WB. Impact of intramural leiomyomata in patients with a normal endometrial cavity on in vitro fertilization-embryo transfer cycle outcome. Fertil Steril 2001; 75(2):405–410. [DOI] [PubMed] [Google Scholar]
- 11. Olive DL, Pritts EA. Fibroids and reproduction. Semin Reprod Med 2010; 28(3):218–427. [DOI] [PubMed] [Google Scholar]
- 12. Rackow BW, Taylor HS. Submucosal uterine leiomyomas have a global effect on molecular determinants of endometrial receptivity. Fertil Steril 2010; 93(6):2027–2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sunkara SK, Khairy M, El-Toukhy T, Khalaf Y, Coomarasamy A. The effect of intramural fibroids without uterine cavity involvement on the outcome of IVF treatment: a systematic review and meta-analysis. Hum Reprod 2010; 25(2):418–429. [DOI] [PubMed] [Google Scholar]
- 14. Donnez J, Jadoul P. What are the implications of myomas on fertility? A need for a debate? Hum Reprod 2002; 17(6):1424–1430. [DOI] [PubMed] [Google Scholar]
- 15. Metwally M, Cheong YC, Horne AW. Surgical treatment of fibroids for subfertility. Cochrane Database Syst Rev 2012; 11:CD003857. [DOI] [PubMed] [Google Scholar]
- 16. Galliano D, Bellver J, Diaz-Garcia C, Simon C, Pellicer A. ART and uterine pathology: how relevant is the maternal side for implantation? Hum Reprod Update 2015; 21(1):13–38. [DOI] [PubMed] [Google Scholar]
- 17. Casini ML, Rossi F, Agostini R, Unfer V. Effects of the position of fibroids on fertility. Gynecol Endocrinol 2006; 22(2):106–109. [DOI] [PubMed] [Google Scholar]
- 18. Bosteels J, Weyers S, Puttemans P, Panayotidis C, Van Herendael B, Gomel V, Mol BW, Mathieu C, D’Hooghe T. The effectiveness of hysteroscopy in improving pregnancy rates in subfertile women without other gynaecological symptoms: a systematic review. Hum Reprod Update 2010; 16(1):1–11. [DOI] [PubMed] [Google Scholar]
- 19. Vilos GA, Allaire C, Laberge PY, Leyland N, Special C. The management of uterine leiomyomas. J Obstet Gynaecol Can 2015; 37(2):157–178. [DOI] [PubMed] [Google Scholar]
- 20. American Association of Gynecologic Laparoscopists: Advancing Minimally Invasive Gynecology Worldwide AAGL practice report: practice guidelines for the diagnosis and management of submucous leiomyomas. J Minim Invasive Gynecol 2012; 19(2):152–171. [DOI] [PubMed] [Google Scholar]
- 21. Borahay MA, Al-Hendy A, Kilic GS, Boehning D. Signaling pathways in leiomyoma: understanding pathobiology and implications for therapy. Mol Med 2015; 21:242–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Englund K, Blanck A, Gustavsson I, Lundkvist U, Sjoblom P, Norgren A, Lindblom B. Sex steroid receptors in human myometrium and fibroids: changes during the menstrual cycle and gonadotropin-releasing hormone treatment. J Clin Endocrinol Metab 1998; 83(11):4092–4096. [DOI] [PubMed] [Google Scholar]
- 23. Marshall LM, Spiegelman D, Goldman MB, Manson JE, Colditz GA, Barbieri RL, Stampfer MJ, Hunter DJ. A prospective study of reproductive factors and oral contraceptive use in relation to the risk of uterine leiomyomata. Fertil Steril 1998; 70(3):432–439. [DOI] [PubMed] [Google Scholar]
- 24. Tsigkou A, Reis FM, Lee MH, Jiang B, Tosti C, Centini G, Shen FR, Chen YG, Petraglia F. Increased progesterone receptor expression in uterine leiomyoma: correlation with age, number of leiomyomas, and clinical symptoms. Fertil Steril 2015; 104(1):170–175e1. [DOI] [PubMed] [Google Scholar]
- 25. Rosati P, Exacoustos C, Mancuso S. Longitudinal evaluation of uterine myoma growth during pregnancy. A sonographic study. J Ultrasound Med 1992; 11(10):511–515. [DOI] [PubMed] [Google Scholar]
- 26. Hammoud AO, Asaad R, Berman J, Treadwell MC, Blackwell S, Diamond MP. Volume change of uterine myomas during pregnancy: do myomas really grow? J Minim Invasive Gynecol 2006; 13(5):386–390. [DOI] [PubMed] [Google Scholar]
- 27. Ciavattini A, Di Giuseppe J, Stortoni P, Montik N, Giannubilo SR, Litta P, Islam MS, Tranquilli AL, Reis FM, Ciarmela P. Uterine fibroids: pathogenesis and interactions with endometrium and endomyometrial junction. Obstet Gynecol Int 2013; 2013:173184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Baird DD, Dunson DB. Why is parity protective for uterine fibroids? Epidemiology 2003; 14(2):247–250. [DOI] [PubMed] [Google Scholar]
- 29. Khan AT, Shehmar M, Gupta JK. Uterine fibroids: current perspectives. Int J Womens Health 2014; 6:95–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ishikawa H, Reierstad S, Demura M, Rademaker AW, Kasai T, Inoue M, Usui H, Shozu M, Bulun SE. High aromatase expression in uterine leiomyoma tissues of African-American women. J Clin Endocrinol Metab 2009; 94(5):1752–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nierth-Simpson EN, Martin MM, Chiang TC, Melnik LI, Rhodes LV, Muir SE, Burow ME, McLachlan JA. Human uterine smooth muscle and leiomyoma cells differ in their rapid 17beta-estradiol signaling: implications for proliferation. Endocrinology 2009; 150(5):2436–2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lethaby A, Vollenhoven B, Sowter M. Pre-operative GnRH analogue therapy before hysterectomy or myomectomy for uterine fibroids. Cochrane Database Syst Rev 2001; 2:CD000547. [DOI] [PubMed] [Google Scholar]
- 33. Shozu M, Murakami K, Segawa T, Kasai T, Inoue M. Successful treatment of a symptomatic uterine leiomyoma in a perimenopausal woman with a nonsteroidal aromatase inhibitor. Fertil Steril 2003; 79(3):628–631. [DOI] [PubMed] [Google Scholar]
- 34. Deng L, Wu T, Chen XY, Xie L, Yang J. Selective estrogen receptor modulators (SERMs) for uterine leiomyomas. Cochrane Database Syst Rev 2012; 10:CD005287. [DOI] [PubMed] [Google Scholar]
- 35. Bulun SE, Moravek MB, Yin P, Ono M, Coon JST, Dyson MT, Navarro A, Marsh EE, Zhao H, Maruyama T, Chakravarti D, Kim JJ et al. Uterine leiomyoma stem cells: linking progesterone to growth. Semin Reprod Med 2015; 33(5):357–365. [DOI] [PubMed] [Google Scholar]
- 36. Hodges LC, Houston KD, Hunter DS, Fuchs-Young R, Zhang Z, Wineker RC, Walker CL. Transdominant suppression of estrogen receptor signaling by progesterone receptor ligands in uterine leiomyoma cells. Mol Cell Endocrinol 2002; 196(1-2):11–20. [DOI] [PubMed] [Google Scholar]
- 37. Hassan MH, Salama SA, Arafa HM, Hamada FM, Al-Hendy A. Adenovirus-mediated delivery of a dominant-negative estrogen receptor gene in uterine leiomyoma cells abrogates estrogen- and progesterone-regulated gene expression. J Clin Endocrinol Metab 2007; 92(10):3949–3957. [DOI] [PubMed] [Google Scholar]
- 38. Ishikawa H, Ishi K, Serna VA, Kakazu R, Bulun SE, Kurita T. Progesterone is essential for maintenance and growth of uterine leiomyoma. Endocrinology 2010; 151(6):2433–2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cermik D, Arici A, Taylor HS. Coordinated regulation of HOX gene expression in myometrium and uterine leiomyoma. Fertil Steril 2002; 78(5):979–984. [DOI] [PubMed] [Google Scholar]
- 40. Kawaguchi K, Fujii S, Konishi I, Iwai T, Nanbu Y, Nonogaki H, Ishikawa Y, Mori T. Immunohistochemical analysis of oestrogen receptors, progesterone receptors and Ki-67 in leiomyoma and myometrium during the menstrual cycle and pregnancy. Virchows Arch A Pathol Anat Histopathol 1991; 419(4):309–315. [DOI] [PubMed] [Google Scholar]
- 41. Matsuo H, Kurachi O, Shimomura Y, Samoto T, Maruo T. Molecular bases for the actions of ovarian sex steroids in the regulation of proliferation and apoptosis of human uterine leiomyoma. Oncology 1999; 57 (Suppl 2):49–58. [DOI] [PubMed] [Google Scholar]
- 42. Robinson-Rechavi M, Escriva Garcia H, Laudet V. The nuclear receptor superfamily. J Cell Sci 2003; 116(Pt 4):585–586. [DOI] [PubMed] [Google Scholar]
- 43. Fujimoto J, Hirose R, Ichigo S, Sakaguchi H, Li Y, Tamaya T. Expression of progesterone receptor form A and B mRNAs in uterine leiomyoma. Tumour Biol 1998; 19(2):126–131. [DOI] [PubMed] [Google Scholar]
- 44. Maruo T, Ohara N, Yoshida S, Nakabayashi K, Sasaki H, Xu Q, Matsuo H, Sitruk-Ware R, Yamada H. Lessons learned from the preclinical drug discovery of asoprisnil and ulipristal for non-surgical treatment of uterine leiomyomas. Expert Opin Drug Discov 2011; 6(9):897–911. [DOI] [PubMed] [Google Scholar]
- 45. Patel B, Elguero S, Thakore S, Dahoud W, Bedaiwy M, Mesiano S. Role of nuclear progesterone receptor isoforms in uterine pathophysiology. Hum Reprod Update 2015; 21(2):155–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chabbert-Buffet N, Meduri G, Bouchard P, Spitz IM. Selective progesterone receptor modulators and progesterone antagonists: mechanisms of action and clinical applications. Hum Reprod Update 2005; 11(3):293–307. [DOI] [PubMed] [Google Scholar]
- 47. Kovacs KA, Lengyel F, Kornyei JL, Vertes Z, Szabo I, Sumegi B, Vertes M. Differential expression of Akt/protein kinase B, Bcl-2 and Bax proteins in human leiomyoma and myometrium. J Steroid Biochem Mol Biol 2003; 87(4-5):233–240. [DOI] [PubMed] [Google Scholar]
- 48. Stewart EA, Laughlin-Tommaso SK, Catherino WH, Lalitkumar S, Gupta D, Vollenhoven B. Uterine fibroids. Nat Rev Dis Primers 2016; 2:16043. [DOI] [PubMed] [Google Scholar]
- 49. Soloff MS, Jeng YJ, Izban MG, Sinha M, Luxon BA, Stamnes SJ, England SK. Effects of progesterone treatment on expression of genes involved in uterine quiescence. Reprod Sci 2011; 18(8):781–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yin XJ, Wang G, Khan-Dawood FS. Requirements of phosphatidylinositol-3 kinase and mammalian target of rapamycin for estrogen-induced proliferation in uterine leiomyoma- and myometrium-derived cell lines. Am J Obstet Gynecol 2007; 196(2):176.e1-5. [DOI] [PubMed] [Google Scholar]
- 51. Ohara N, Morikawa A, Chen W, Wang J, DeManno DA, Chwalisz K, Maruo T. Comparative effects of SPRM asoprisnil (J867) on proliferation, apoptosis, and the expression of growth factors in cultured uterine leiomyoma cells and normal myometrial cells. Reprod Sci 2007; 14(8 Suppl):20–27. [DOI] [PubMed] [Google Scholar]
- 52. Qiang W, Liu Z, Serna VA, Druschitz SA, Liu Y, Espona-Fiedler M, Wei JJ, Kurita T. Down-regulation of miR-29b is essential for pathogenesis of uterine leiomyoma. Endocrinology 2014; 155(3):663–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Marsh EE, Steinberg ML, Parker JB, Wu J, Chakravarti D, Bulun SE. Decreased expression of microRNA-29 family in leiomyoma contributes to increased major fibrillar collagen production. Fertil Steril 2016; 106(3):766–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Clarke-Pearson DL, Geller EJ. Complications of hysterectomy. Obstet Gynecol 2013; 121(3):654–673. [DOI] [PubMed] [Google Scholar]
- 55. Lonnee-Hoffmann R, Pinas I. Effects of hysterectomy on sexual function. Curr Sex Health Rep 2014; 6(4):244–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Cook H, Ezzati M, Segars JH, McCarthy K. The impact of uterine leiomyomas on reproductive outcomes. Minerva Ginecol 2010; 62(3):225–236. [PMC free article] [PubMed] [Google Scholar]
- 57. Owen C, Armstrong AY. Clinical management of leiomyoma. Obstet Gynecol Clin North Am 2015; 42(1):67–85. [DOI] [PubMed] [Google Scholar]
- 58. Donnez J, Dolmans MM. Uterine fibroid management: from the present to the future. Hum Reprod Update 2016; 22(6):665–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wu JM, Wechter ME, Geller EJ, Nguyen TV, Visco AG. Hysterectomy rates in the United States, 2003. Obstet Gynecol 2007; 110(5):1091–1095. [DOI] [PubMed] [Google Scholar]
- 60. Altman D, Granath F, Cnattingius S, Falconer C. Hysterectomy and risk of stress-urinary-incontinence surgery: nationwide cohort study. Lancet 2007; 370(9597):1494–1499. [DOI] [PubMed] [Google Scholar]
- 61. Sozeri-Varma G, Kalkan-Oguzhanoglu N, Karadag F, Ozdel O. The effect of hysterectomy and/or oophorectomy on sexual satisfaction. Climacteric 2011; 14(2):275–281. [DOI] [PubMed] [Google Scholar]
- 62. Gambadauro Dealing with uterine fibroids in reproductive medicine. J Obstet Gynaecol, 2012; 32(3):210–216. [DOI] [PubMed] [Google Scholar]
- 63. Fauconnier A, Chapron C, Babaki-Fard K, Dubuisson JB. Recurrence of leiomyomata after myomectomy. Hum Reprod Update 2000; 6(6):595–602. [DOI] [PubMed] [Google Scholar]
- 64. Khaund A, Lumsden MA. Impact of fibroids on reproductive function. Best Pract Res Clin Obstet Gynaecol 2008; 22(4):749–760. [DOI] [PubMed] [Google Scholar]
- 65. Vilos GA, Allaire C, Laberge PY, Leyland N, Special C, Vilos AG, Murji A, Chen I. The management of uterine leiomyomas. J Obstet Gynaecol Can 2015; 37(2):157–181. [DOI] [PubMed] [Google Scholar]
- 66. Jayakrishnan K, Menon V, Nambiar D. Submucous fibroids and infertility: Effect of hysteroscopic myomectomy and factors influencing outcome. J Hum Reprod Sci 2013; 6(1):35–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Freed MM, Spies JB. Uterine artery embolization for fibroids: a review of current outcomes. Semin Reprod Med 2010; 28(3):235–241. [DOI] [PubMed] [Google Scholar]
- 68. Torre A, Paillusson B, Fain V, Labauge P, Pelage JP, Fauconnier A. Uterine artery embolization for severe symptomatic fibroids: effects on fertility and symptoms. Hum Reprod 2014; 29(3):490–501. [DOI] [PubMed] [Google Scholar]
- 69. Al Hilli MM, Stewart EA. Magnetic resonance-guided focused ultrasound surgery. Semin Reprod Med 2010; 28(3):242–9. [DOI] [PubMed] [Google Scholar]
- 70. Jacoby VL, Kohi MP, Poder L, Jacoby A, Lager J, Schembri M, Rieke V, Grady D, Vittinghoff E, Coakley FV. PROMISe trial: a pilot, randomized, placebo-controlled trial of magnetic resonance guided focused ultrasound for uterine fibroids. Fertil Steril 2016; 105(3):773–780. [DOI] [PubMed] [Google Scholar]
- 71. Singh SS, Belland L. Contemporary management of uterine fibroids: focus on emerging medical treatments. Curr Med Res Opin 2015; 31(1):1–12. [DOI] [PubMed] [Google Scholar]
- 72. Mercorio F, De Simone R, Di Spiezio Sardo A, Cerrota G, Bifulco G, Vanacore F, Nappi C. The effect of a levonorgestrel-releasing intrauterine device in the treatment of myoma-related menorrhagia. Contraception 2003; 67(4):277–280. [DOI] [PubMed] [Google Scholar]
- 73. Machado RB, de Souza IM, Beltrame A, Bernardes CR, Morimoto MS, Santana N. The levonorgestrel-releasing intrauterine system: its effect on the number of hysterectomies performed in perimenopausal women with uterine fibroids. Gynecol Endocrinol 2013; 29(5):492–495. [DOI] [PubMed] [Google Scholar]
- 74. Maruo T, Ohara N, Matsuo H, Xu Q, Chen W, Sitruk-Ware R, Johansson ED. Effects of levonorgestrel-releasing IUS and progesterone receptor modulator PRM CDB-2914 on uterine leiomyomas. Contraception 2007; 75(6 Suppl):S99–103. [DOI] [PubMed] [Google Scholar]
- 75. Song H, Lu D, Navaratnam K, Shi G. Aromatase inhibitors for uterine fibroids. Cochrane Database Syst Rev 2013; 10:CD009505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Doherty L, Mutlu L, Sinclair D, Taylor H. Uterine fibroids: clinical manifestations and contemporary management. Reprod Sci 2014; 21(9):1067–1092. [DOI] [PubMed] [Google Scholar]
- 77. Kumar P, Sharma A. Gonadotropin-releasing hormone analogs: Understanding advantages and limitations. J Hum Reprod Sci 2014; 7(3):170–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Sabry M, Al-Hendy A. Medical treatment of uterine leiomyoma. Reprod Sci 2012; 19(4):339–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Conn PM, Ulloa-Aguirre A. Pharmacological chaperones for misfolded gonadotropin-releasing hormone receptors. Adv Pharmacol 2011; 62:109–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Gonzalez-Barcena D, Alvarez RB, Ochoa EP, Cornejo IC, Comaru-Schally AM, Schally AV, Engel J, Reissmann T, Riethmuller-Winzen H. Treatment of uterine leiomyomas with luteinizing hormone-releasing hormone antagonist Cetrorelix. Hum Reprod 1997; 12(9):2028–2035. [DOI] [PubMed] [Google Scholar]
- 81. clinicaltrials.gov , A Global Phase 3 Study to Evaluate the Safety and Efficacy of Elagolix in Subjects With Moderate to Severe Endometriosis-Associated Pain. https://clinicaltrials.gov/ct2/show/NCT01931670, 2016. last viewed July, 2017 (NCT01931670). [Google Scholar]
- 82. Myovant Sciences Myovant Sciences Announces Presentation of Positive Phase 2 Data for Relugolix in Women with Heavy Menstrual Bleeding and Uterine Fibroids at the Annual Meeting of the American Congress of Obstetricians and Gynecologists. http://investors.myovant.com/news-releases/2017/05-07-2017-215955962, 2017. Last viewed July 2017. [Google Scholar]
- 83. Obseva ObsEva SA Initiates Phase 3 Clinical Program for OBE2109 in Uterine Fibroids. http://www.obseva.com/news/obseva-sa-initiates-phase-3-clinical-program-for-obe2109-in-uterine-fibroids, 2017. last viewed July 2017. [Google Scholar]
- 84. Lusher SJ, Raaijmakers HC, Vu-Pham D, Dechering K, Lam TW, Brown AR, Hamilton NM, Nimz O, Bosch R, McGuire R, Oubrie A, de Vlieg J. Structural basis for agonism and antagonism for a set of chemically related progesterone receptor modulators. J Biol Chem 2011; 286(40):35079–35086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Bouchard Selective progesterone receptor modulators: a class with multiple actions and applications in reproductive endocrinology, and gynecology. Gynecol Endocrinol 2014; 30(10):683–684. [DOI] [PubMed] [Google Scholar]
- 86. Wagenfeld A, Saunders PT, Whitaker L, Critchley HO. Selective progesterone receptor modulators (SPRMs): progesterone receptor action, mode of action on the endometrium and treatment options in gynecological therapies. Expert Opin Ther Targets 2016; 20(9):1045–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Bouchard P, Chabbert-Buffet N, Fauser BC. Selective progesterone receptor modulators in reproductive medicine: pharmacology, clinical efficacy and safety. Fertil Steril 2011; 96(5):1175–1189. [DOI] [PubMed] [Google Scholar]
- 88. Donnez J, Arriagada P, Donnez O, Dolmans MM. Current management of myomas: the place of medical therapy with the advent of selective progesterone receptor modulators. Curr Opin Obstet Gynecol 2015. 27(6):422–431. [DOI] [PubMed] [Google Scholar]
- 89. Tristan M, Orozco LJ, Steed A, Ramirez-Morera A, Stone P. Mifepristone for uterine fibroids. Cochrane Database Syst Rev 2012;8:CD007687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Shen Q, Hua Y, Jiang W, Zhang W, Chen M, Zhu X. Effects of mifepristone on uterine leiomyoma in premenopausal women: a meta-analysis. Fertil Steril 2013; 100(6):1722–1726e1-10. [DOI] [PubMed] [Google Scholar]
- 91. Wilkens J, Chwalisz K, Han C, Walker J, Cameron IT, Ingamells S, Lawrence AC, Lumsden MA, Hapangama D, Williams AR, Critchley HO. Effects of the selective progesterone receptor modulator asoprisnil on uterine artery blood flow, ovarian activity, and clinical symptoms in patients with uterine leiomyomata scheduled for hysterectomy. J Clin Endocrinol Metab 2008; 93(12):4664–4671. [DOI] [PubMed] [Google Scholar]
- 92. Chwalisz K, Larsen L, Mattia-Goldberg C, Edmonds A, Elger W, Winkel CA. A randomized, controlled trial of asoprisnil, a novel selective progesterone receptor modulator, in women with uterine leiomyomata. Fertil Steril 2007; 87(6):1399–412. [DOI] [PubMed] [Google Scholar]
- 93. ClinicalTrials.gov , Safety of Treatment of Uterine Fibroids with Asoprisnil. https://clinicaltrials.gov/ct2/show/NCT00156208, 2008. last viewed July, 2017 (NCT00156208). [Google Scholar]
- 94. Luo X, Yin P, Coon VJ, Cheng YH, Wiehle RD, Bulun SE. The selective progesterone receptor modulator CDB4124 inhibits proliferation and induces apoptosis in uterine leiomyoma cells. Fertil Steril 2010; 93(8):2668–2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. ClinicalTrials.gov A Phase 2, Study to Evaluate the Safety and Efficacy Proellex® (Telapristone Acetate) Administered Vaginally in the Treatment of Uterine Fibroids. https://clinicaltrials.gov/ct2/show/NCT02323646, 2016. last viewed June, 2017 (NCT02323646). [Google Scholar]
- 96. Schutt B, Kaiser A, Schultze-Mosgau MH, Seitz C, Bell D, Koch M, Rohde B. Pharmacodynamics and safety of the novel selective progesterone receptor modulator vilaprisan: a double-blind, randomized, placebo-controlled phase 1 trial in healthy women. Hum Reprod 2016; 31(8):1703–1712. [DOI] [PubMed] [Google Scholar]
- 97. Donnez J, Donnez O, Courtoy GE, Dolmans MM. The place of selective progesterone receptor modulators in myoma therapy. Minerva Ginecol 2016; 68(3):313–320. [PubMed] [Google Scholar]
- 98. Yoshida S, Ohara N, Xu Q, Chen W, Wang J, Nakabayashi K, Sasaki H, Morikawa A, Maruo T. Cell-type specific actions of progesterone receptor modulators in the regulation of uterine leiomyoma growth. Semin Reprod Med 2010; 28(3):260–273. [DOI] [PubMed] [Google Scholar]
- 99. Xu Q, Ohara N, Chen W, Liu J, Sasaki H, Morikawa A, Sitruk-Ware R, Johansson ED, Maruo T. Progesterone receptor modulator CDB-2914 down-regulates vascular endothelial growth factor, adrenomedullin and their receptors and modulates progesterone receptor content in cultured human uterine leiomyoma cells. Hum Reprod 2006; 21(9):2408–2416. [DOI] [PubMed] [Google Scholar]
- 100. Xu Q, Ohara N, Liu J, Amano M, Sitruk-Ware R, Yoshida S, Maruo T. Progesterone receptor modulator CDB-2914 induces extracellular matrix metalloproteinase inducer in cultured human uterine leiomyoma cells. Mol Hum Reprod 2008; 14(3):181–191. [DOI] [PubMed] [Google Scholar]
- 101. Ciarmela P, Carrarelli P, Islam MS, Janjusevic M, Zupi E, Tosti C, Castellucci M, Petraglia F. Ulipristal acetate modulates the expression and functions of activin a in leiomyoma cells. Reprod Sci 2014; 21(9):1120–1125. [DOI] [PubMed] [Google Scholar]
- 102. Courtoy GE, Donnez J, Marbaix E, Dolmans MM. In vivo mechanisms of uterine myoma volume reduction with ulipristal acetate treatment. Fertil Steril 2015; 104(2):426–434e1. [DOI] [PubMed] [Google Scholar]
- 103. Talaulikar VS, Manyonda IT. Ulipristal acetate: a novel option for the medical management of symptomatic uterine fibroids. Adv Ther 2012; 29(8):655–663. [DOI] [PubMed] [Google Scholar]
- 104. Donnez J, Donnez O, Dolmans MM. With the advent of selective progesterone receptor modulators, what is the place of myoma surgery in current practice? Fertil Steril 2014; 102(3):640–648. [DOI] [PubMed] [Google Scholar]
- 105. Islam MS, Protic O, Giannubilo SR, Toti P, Tranquilli AL, Petraglia F, Castellucci M, Ciarmela P. Uterine leiomyoma: available medical treatments and new possible therapeutic options. J Clin Endocrinol Metab 2013; 98(3):921–934. [DOI] [PubMed] [Google Scholar]
- 106. Gentry CC, Okolo SO, Fong LF, Crow JC, Maclean AB, Perrett CW. Quantification of vascular endothelial growth factor-A in leiomyomas and adjacent myometrium. Clin Sci (Lond) 2001; 101(6):691–695. [PubMed] [Google Scholar]
- 107. Hague S, Zhang L, Oehler MK, Manek S, MacKenzie IZ, Bicknell R, Rees MC. Expression of the hypoxically regulated angiogenic factor adrenomedullin correlates with uterine leiomyoma vascular density. Clin Cancer Res 2000; 6(7):2808–2814. [PubMed] [Google Scholar]
- 108. Attardi BJ, Burgenson J, Hild SA, Reel JR. In vitro antiprogestational/antiglucocorticoid activity and progestin and glucocorticoid receptor binding of the putative metabolites and synthetic derivatives of CDB-2914, CDB-4124, and mifepristone. J Steroid Biochem Mol Biol 2004; 88(3):277–288. [DOI] [PubMed] [Google Scholar]
- 109. W.L. Inc , FIBRISTAL(TM) PRODUCT MONOGRAPH. http://www.fibristal.ca/docs/Fibristal_Product_Monograph_E.pdf, 2013. last viewed July, 2017. [Google Scholar]
- 110. A.S.P. Co , FIBRISTAL® ulipristal acetate tablet, 5 mg monogrsh. http://www.fibristal.ca/docs/Fibristal_Product_Monograph_E.pdf, 2015. last viewed July, 2017. [Google Scholar]
- 111. Melis GB, Piras B, Marotto MF, Orru MM, Maricosu G, Pilloni M, Guerriero S, Angiolucci M, Lello S, Paoletti AM. Pharmacokinetic evaluation of ulipristal acetate for uterine leiomyoma treatment. Expert Opin Drug Metab Toxicol 2012; 8(7):901–908. [DOI] [PubMed] [Google Scholar]
- 112. Pohl O, Osterloh I, Lecomte V, Gotteland JP. Changes in gastric pH and in pharmacokinetics of ulipristal acetate - a drug-drug interaction study using the proton pump inhibitor esomeprazole. Int J Clin Pharmacol Ther 2013; 51(1):26–33. [DOI] [PubMed] [Google Scholar]
- 113. Watson Laboratories Inc Watson Laboratories study UL1101: An Open-Label, Crossover, Comparative Bioavailability Study of 10 mg Ulipristal Acetate After a High Fat Meal or Under Fasting Conditions. Study Report 2011. [Google Scholar]
- 114. Donnez J, Tatarchuk TF, Bouchard P, Puscasiu L, Zakharenko NF, Ivanova T, Ugocsai G, Mara M, Jilla MP, Bestel E, Terrill P, Osterloh I et al. Ulipristal acetate versus placebo for fibroid treatment before surgery. N Engl J Med 2012; 366(5):409–420. [DOI] [PubMed] [Google Scholar]
- 115. Donnez J, Tomaszewski J, Vazquez F, Bouchard P, Lemieszczuk B, Baro F, Nouri K, Selvaggi L, Sodowski K, Bestel E, Terrill P, Osterloh I et al. Ulipristal acetate versus leuprolide acetate for uterine fibroids. N Engl J Med 2012; 366(5):421–432. [DOI] [PubMed] [Google Scholar]
- 116. Watson Laboratories Inc Watson Laboratories study UL1103: An Open-Label, Crossover Study to Investigate the Effects of Oral Iron Administration on the Pharmacokinetics of a Single 10mg Dose of Ulipristal Acetate in Healthy Female Subjects. Study Report 2012. [Google Scholar]
- 117. Gemzell-Danielsson K, Meng CX. Emergency contraception: potential role of ulipristal acetate. Int J Womens Health 2010; 2:53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. E.M. Agency , Esmya -Summary of Product Characteristics. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Summary_for_the_public/human/002041/WC500124089.pdf, 2012. last updated June, 2017. [Google Scholar]
- 119. Grzechocinska B, Gadomska H, Zygula A, Wielgos M. Application of ulipristal acetate in female patients with uterine fibroids. Neuro Endocrinol Lett 2014; 35(3):175–178. [PubMed] [Google Scholar]
- 120. Maybin JA, Critchley HO. Medical management of heavy menstrual bleeding. Womens Health (Lond) 2016; 12(1):27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Donnez J, Donnez O, Matule D, Ahrendt HJ, Hudecek R, Zatik J, Kasilovskiene Z, Dumitrascu MC, Fernandez H, Barlow DH, Bouchard P, Fauser BC et al. Long-term medical management of uterine fibroids with ulipristal acetate. Fertil Steril 2016; 105(1):165–173e4. [DOI] [PubMed] [Google Scholar]
- 122. Willame A, Marci R, Petignat P, Dubuisson J. Myoma migration: an unexpected “effect” with Ulipristal acetate treatment. Eur Rev Med Pharmacol Sci 2016. 20(8):1439–1444. [PubMed] [Google Scholar]
- 123. Olejek A, Olszak-Wasik K, Czerwinska-Bednarska A. Long-term intermittent pharmacological therapy of uterine fibroids - a possibility to avoid hysterectomy and its negative consequences. Prz Menopauzalny 2016; 15(1):48–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Luyckx M, Pirard C, Fellah L, Dereume A, Mhallem M, Debieve F, Squifflet J. Long-term nonsurgical control with ulipristal acetate of multiple uterine fibroids, enabling pregnancy. Am J Obstet Gynecol 2016; 214(6):756.e1-2. [DOI] [PubMed] [Google Scholar]
- 125. Donnez J, Hudecek R, Donnez O, Matule D, Arhendt HJ, Zatik J, Kasilovskiene Z, Dumitrascu MC, Fernandez H, Barlow DH, Bouchard P, Fauser BC et al. Efficacy and safety of repeated use of ulipristal acetate in uterine fibroids. Fertil Steril 2015; 103(2):519–527e3. [DOI] [PubMed] [Google Scholar]
- 126. Ferrero S, Racca A, Tafi E, Alessandri F, Venturini PL, Leone Roberti Maggiore U. Ulipristal acetate before high complexity hysteroscopic myomectomy: a retrospective comparative study. J Minim Invasive Gynecol 2016; 23(3):390–395. [DOI] [PubMed] [Google Scholar]
- 127. Kalampokas T, Kamath M, Boutas I, Kalampokas E. Ulipristal acetate for uterine fibroids: a systematic review and meta-analysis. Gynecol Endocrinol 2016; 32(2):91–96. [DOI] [PubMed] [Google Scholar]
- 128. Trefoux Bourdet A, Luton D, Koskas M. Clinical utility of ulipristal acetate for the treatment of uterine fibroids: current evidence. Int J Womens Health 2015; 7:321–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Donnez J, Vazquez F, Tomaszewski J, Nouri K, Bouchard P, Fauser BC, Barlow DH, Palacios S, Donnez O, Bestel E, Osterloh I, Loumaye E et al. Long-term treatment of uterine fibroids with ulipristal acetate. Fertil Steril 2014; 101(6):1565–1573e1-18. [DOI] [PubMed] [Google Scholar]
- 130. Murji A, Whitaker L, Chow TL, Sobel ML. Selective progesterone receptor modulators (SPRMs) for uterine fibroids. Cochrane Database Syst Rev 2017; 4:CD010770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Simon J, Catherino WH, Segars JH, Blakesley R, Chan A, Sniukiene V, Al-Hendy A. First US-based phase 3 study of ulipristal acetate (UPA) for symptomatic uterine fibroids (UF): results of VENUS-I Fertil Steril 2016; 106(3):e376. [Google Scholar]
- 132. Communal L, Vilasco M, Hugon-Rodin J, Courtin A, Mourra N, Lahlou N, Dumont S, Chaouat M, Forgez P, Gompel A. Ulipristal acetate does not impact human normal breast tissue. Hum Reprod 2012; 27(9):2785–2798. [DOI] [PubMed] [Google Scholar]
- 133. Williams AR, Bergeron C, Barlow DH, Ferenczy A. Endometrial morphology after treatment of uterine fibroids with the selective progesterone receptor modulator, ulipristal acetate. Int J Gynecol Pathol 2012; 31(6):556–569. [DOI] [PubMed] [Google Scholar]
- 134. Williams AR, Critchley HO, Osei J, Ingamells S, Cameron IT, Han C, Chwalisz K. The effects of the selective progesterone receptor modulator asoprisnil on the morphology of uterine tissues after 3 months treatment in patients with symptomatic uterine leiomyomata. Hum Reprod 2007; 22(6):1696–16704. [DOI] [PubMed] [Google Scholar]
- 135. Wilkens J, Williams AR, Chwalisz K, Han C, Cameron IT, Critchley HO. Effect of asoprisnil on uterine proliferation markers and endometrial expression of the tumour suppressor gene, PTEN. Hum Reprod 2009; 24(5):1036–1044. [DOI] [PubMed] [Google Scholar]
- 136. Mutter GL, Bergeron C, Deligdisch L, Ferenczy A, Glant M, Merino M, Williams AR, Blithe DL. The spectrum of endometrial pathology induced by progesterone receptor modulators. Mod Pathol 2008; 21(5):591–598. [DOI] [PubMed] [Google Scholar]
- 137. Levy G, Elkas J, Armstrong AY, Nieman LK. Endometrial effects of prolonged therapy with the selective progesterone receptor modulator ulipristal acetate: a case report. J Reprod Med 2016; 61(3-4):159–162. [PMC free article] [PubMed] [Google Scholar]
- 138. Luyckx M, Squifflet JL, Jadoul P, Votino R, Dolmans MM, Donnez J et al. First series of 18 pregnancies after ulipristal acetate treatment for uterine fibroids. Fertil Steril 2014; 102(5):1404–1409. [DOI] [PubMed] [Google Scholar]
- 139. Glasier A. The rationale for use of ulipristal acetate as first line in emergency contraception: biological and clinical evidence. Gynecol Endocrinol 2014; 30(10):688–690. [DOI] [PubMed] [Google Scholar]
- 140. Hild SA, Reel JR, Hoffman LH, Blye RP. CDB-2914: anti-progestational/anti-glucocorticoid profile and post-coital anti-fertility activity in rats and rabbits. Hum Reprod 2000; 15(4):822–829. [DOI] [PubMed] [Google Scholar]
- 141. Tarantal AF, Hendrickx AG, Matlin SA, Lasley BL, Gu QQ, Thomas CA, Vince PM, Van Look PF. Effects of two antiprogestins on early pregnancy in the long-tailed macaque (Macaca fascicularis). Contraception 1996; 54(2):107–115. [DOI] [PubMed] [Google Scholar]
- 142. Nagy B, Timar G, Jozwiak-Hagymasy J, Kovacs G, Meresz G, Vamossy I, Agh T, Laszlo A, Voko Z, Kalo Z. The cost-effectiveness of ulipristal acetate tablets in treating patients with moderate to severe symptoms of uterine fibroids. Eur J Obstet Gynecol Reprod Biol 2014; 175:75–81. [DOI] [PubMed] [Google Scholar]
- 143. Tsoi B, Blackhouse G, Ferrazzi S, Reade CJ, Chen I, Goeree R. Incorporating ulipristal acetate in the care of symptomatic uterine fibroids: a Canadian cost-utility analysis of pharmacotherapy management. Clinicoecon Outcomes Res 2015; 7:213–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Paladio-Hernandez J, Rosas R, Pozo L, Del Cid O, Robles Valencia JA. Economic evaluation of ulipristal acetate for the treatment of patients with moderate and severe symptoms of uterine fibroids before surgery in Mexico. Value Health 2015; 18(7):A834. [Google Scholar]
- 145. Peddada SD, Laughlin SK, Miner K, Guyon JP, Haneke K, Vahdat HL, Semelka RC, Kowalik A, Armao D, Davis B, Baird DD. Growth of uterine leiomyomata among premenopausal black and white women. Proc Natl Acad Sci USA 2008; 105(50):19887–19892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Othman EE, Al-Hendy A. Molecular genetics and racial disparities of uterine leiomyomas. Best Pract Res Clin Obstet Gynaecol 2008; 22(4):589–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Wise LA, Palmer JR, Spiegelman D, Harlow BL, Stewart EA, Adams-Campbell LL, Rosenberg L. Influence of body size and body fat distribution on risk of uterine leiomyomata in U.S. black women. Epidemiology 2005; 16(3):346–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Wise LA, Palmer JR, Harlow BL, Spiegelman D, Stewart EA, Adams-Campbell LL, Rosenberg L. Risk of uterine leiomyomata in relation to tobacco, alcohol and caffeine consumption in the Black Women's Health Study. Hum Reprod 2004; 19(8):1746–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Takeda T, Sakata M, Isobe A, Miyake A, Nishimoto F, Ota Y, Kamiura S, Kimura T. Relationship between metabolic syndrome and uterine leiomyomas: a case-control study. Gynecol Obstet Invest 2008; 66(1):14–17. [DOI] [PubMed] [Google Scholar]
- 150. Marshall LM, Spiegelman D, Barbieri RL, Goldman MB, Manson JE, Colditz GA, Willett WC, Hunter DJ. Variation in the incidence of uterine leiomyoma among premenopausal women by age and race. Obstet Gynecol 1997; 90(6):967–973. [DOI] [PubMed] [Google Scholar]
- 151. Shikora SA, Niloff JM, Bistrian BR, Forse RA, Blackburn GL. Relationship between obesity and uterine leiomyomata. Nutrition 1991; 7(4):251–255. [PubMed] [Google Scholar]
- 152. Sabry M, Halder SK, Allah AS, Roshdy E, Rajaratnam V, Al-Hendy A. Serum vitamin D3 level inversely correlates with uterine fibroid volume in different ethnic groups: a cross-sectional observational study. Int J Womens Health 2013; 5:93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Al-Hendy A, Salama SA. Catechol-O-methyltransferase polymorphism is associated with increased uterine leiomyoma risk in different ethnic groups. J Soc Gynecol Investig 2006; 13(2):136–144. [DOI] [PubMed] [Google Scholar]
- 154. Al-Hendy A, Salama SA. Ethnic distribution of estrogen receptor-alpha polymorphism is associated with a higher prevalence of uterine leiomyomas in black Americans. Fertil Steril 2006; 86(3):686–693. [DOI] [PubMed] [Google Scholar]
- 155. Van Voorhis BJ, Romitti PA, Jones MP. Family history as a risk factor for development of uterine leiomyomas. Results of a pilot study. J Reprod Med 2002; 47(8):663–669. [PubMed] [Google Scholar]
- 156. Ciebiera M, Wlodarczyk M, Slabuszewska-Jozwiak A, Nowicka G, Jakiel G. Influence of vitamin D and transforming growth factor beta3 serum concentrations, obesity, and family history on the risk for uterine fibroids. Fertil Steril 2016; 106:1787–1792. [DOI] [PubMed] [Google Scholar]