Abstract
We investigated the interaction between prenatal nicotine exposure and intrauterine infection using established rat models. Beginning at gestation day (GD) 6, dams were continuously infused with either saline or 6 mg/kg/day nicotine (Nic). At GD 14, dams received either sterile broth or 105 colony-forming units Mycoplasma pulmonis (MP), resulting in four treatment groups: control (4 dams, 33 fetal units); MP only (5 dams, 55 fetal units); Nic only (5 dams, 61 fetal units), and Nic + MP (7 dams, 82 fetal units). At GD 18, nicotine exposure significantly increased (P ≤ 0.02) the percentage of amniotic fluids and fetuses infected by MP but did not impact colonization rates of maternal sites. Nicotine exposure significantly reduced the numbers of MP in the placenta required for high microbial loads (≥104 color-changing units) in the amniotic fluid (P < 0.01). Fetal inflammatory response lesions were most extensive in the Nic only and Nic + MP groups (P < 0.0001). Control and MP only placentas were interleukin (IL)10-dominant, consistent with an M2/Th2 environment. Placentas exposed to nicotine shifted to a neutral environment, with equivalent levels of interferon gamma (IFNG) and IL10. Both IL6 and tumor necrosis factor (TNF) levels in amniotic fluid were highly elevated when both nicotine and infection were present. Our study suggests that prenatal exposure to nicotine increases the risk for intrauterine infection, lowers the infectious dose required to breach the placental barrier and infect the amniotic fluid and fetus, and alters the pathology and inflammatory profile associated with maternal and fetal sites.
Keywords: nicotine, infection; rodent model, inflammation, placental pathology, pregnancy
Summary Sentence
A modifiable risk factor (nicotine) can directly impact what has been considered an immutable risk factor (infection) resulting in increased risk of pathogen transmission across the placental barrier with subsequent impacts on placental pathology and maternal/fetal immune environment.
Introduction
Maternal smoking during pregnancy is a well-established cause of perinatal complications [1–5] including preterm birth. Cigarettes contain a complex assortment of chemicals, but nicotine is one of the main pathogenic components and has the potential to impact placental function [6–9]. Accordingly, smoking cessation strategies are routinely recommended for women during pregnancy [10, 11]. Nicotine replacement therapies (NRTs), such as transdermal patches or gum [12, 13], have been recommended, but safe and effective doses of nicotine exposure via these devices during pregnancy have not been definitively established [14–16]. Electronic nicotine delivery systems (ENDS), including e-cigarettes, are also being promoted for smoking cessation [17, 18]. Unfortunately, ENDS can be perceived as a safe alternative to smoking by the general public [19–24] and are gaining popularity among young reproductive age and pregnant women [20, 21, 24, 25]. Furthermore, the level of nicotine delivered by ENDS increases significantly with user experience and duration of puffing period [26–30], and ENDS which contain higher nicotine concentrations actually result in significantly higher plasma levels of nicotine than conventional cigarettes [31]. Thus, nicotine exposure during pregnancy remains a significant health issue.
Another significant but separate risk factor for perinatal complications and preterm birth is microbial infection of the reproductive tract, both at the time of conception and during pregnancy [32–35]. Infection generally is considered to be an immutable risk factor for perinatal complications while smoking is considered a mutable risk factor (http://www.nap.edu/catalog/11622.html) [36]. Most epidemiological studies considered smoking and infection as potential confounders, and therefore excluded either smoking or infection in data analyses. Recent studies [2, 4, 37] considering both risk factors showed that women who smoke are at significantly higher risk for pathologic intrauterine infection, indicating an interaction between these risk factors.
Based on these new emerging data, this study was undertaken to evaluate the prenatal interaction between nicotine exposure and infection at the maternal:fetal interface. We hypothesized that nicotine exposure during pregnancy would lower the microbial threshold required to establish intrauterine and fetal infection. To test our hypothesis, we combined a maternal nicotine infusion model that achieves high levels of plasma nicotine and cotinine without causing fetal loss or changes in birth weight [38–41] with a defined model for intrauterine and fetal infection [42–46]. Here we report that prenatal nicotine exposure does reduce the threshold of placental microbial load required for infection of the amniotic fluid and alters both the pathology and inflammatory profile associated with maternal and fetal sites.
Materials and methods
Ethics statement
All experimental procedures involving animals were performed in accordance with the University of Florida Institutional Animal Care and Use Committee-approved protocols. The University of Florida is an AAALAC-accredited institution.
Mycoplasma preparation and culture
To ensure identical inocula for all experiments, Mycoplasma pulmonis strain X-1048 was grown to late logarithmic phase in SP4 medium, aliquoted, and frozen at –80°C. For each experiment, the stock culture was thawed and an inoculum dose of 105 colony-forming units (CFU) per 0.25 μL was used for inoculation of a rat. Dose concentration was confirmed by both optical density and culture.
Animals, husbandry, and breeding
Specific pathogen-free (SPF) adult male and female Sprague Dawley rats (Crl:SD; Charles River Laboratories International, Inc., Wilmington, MA) were used.
All animal handling took place within a laminar airflow hood. Animal rooms were maintained on a 12:12 h light:dark cycle. Rats were initially housed in an SPF barrier facility in individually ventilated cages (Microisolator, Lab Products, Inc., Maywood, NJ) and provided ad libitum access to food (LabDiet 5053, Purina Mills International, St Louis, MO) and water. Prior to receiving inoculations, rats were relocated to an ABSL-2 containment facility where they were housed singly in static filter-top cages (Microisolator, Lab Products, Inc., Maywood, NJ). Control animals were housed on separate shelves above the infected groups and were always handled first and always within a biosafety cabinet.
For breeding, females were paired with males overnight and were checked daily for evidence of copulation as determined by either visual observation of a vaginal plug or sperm on vaginal cytology. The morning of plug or sperm detection was considered the beginning of gestation day (GD) 0. Bred females were housed in pairs until surgical implantation of the osmotic pump at GD 6, after which they were housed singly.
Experimental design and treatment groups
All pregnant dams were randomly assigned to one of four treatment groups: normal saline infusion and sterile broth inoculation (control); nicotine infusion and sterile broth inoculation (Nic only); normal saline infusion and M. pulmonis inoculation (MP only); and nicotine infusion and M. pulmonis inoculation (Nic + MP).
Infusion pump implantation
At GD 6, all pregnant dams received a subcutaneous injection of carprofen (5 mg/kg; Rimadyl, Zoetis, Florham Park, NJ) prior to surgery and were anesthetized with 3%–4% isoflurane (Iso-thesia, Abbott Laboratories, Abbott Park, IL) and maintained with 1%–3% isoflurane in 100% oxygen delivered by facemask. A 28-day osmotic minipump (ALZET Osmotic Pump Model 2ML4, DURECT Corp., Cupertino, CA) was inserted subcutaneously via a small incision between the scapulae and the incision was closed with wound clips. The minipump was filled with either sterile saline (control and MP only rats; Baxter Veterinary Supplies, Round Lake, IL) or nicotine tartrate (Sigma-Aldrich, St. Louis, MO) dissolved in sterile saline at a concentration sufficient to deliver 6 mg/kg/day (Nic only and Nic + MP rats). Supplemental heat was provided by insulated heating pads (SnuggleSafe, Lenric C21 Ltd. Littlehampton, West Sussex, UK) during anesthesia and recovery.
Intravenous inoculation
Based on our previous studies [42–44, 46], GD 14 is the most efficient time point for inducing fetal infection, and intravenous (IV) administration allows for delivery of a known dose at a precise time during gestation. Consequently, at GD 14, dams were re-anesthetized (isoflurane) as described above, and placed in dorsal recumbency to allow access to the right subclavian vein. After the injection site was cleaned with a sterile 70% ethanol swab, pregnant dams received an IV inoculation via the subclavian vein with either 105 CFU M. pulmonis (MP only and Nic + MP groups) or 0.25 ml of sterile SP4 broth (control and Nic only rats), delivered slowly over 10–15 s. Pressure was applied to the injection site for 30–60 s following withdrawal of the needle.
Necropsy
Pregnant dams were euthanized at GD 18 by intraperitoneal injection of euthanasia solution (Beuthanasia-D Special, Merck Animal Health, Summit, NJ). A full gross necropsy was performed, including examination of surgical and injection sites. Tissues were collected using aseptic technique to prevent cross-contamination of tissue sites. Broths were incubated in ambient air at 37°C, and checked daily for growth to determine color-changing units (CCU). Maternal spleen, vagina, and endometrial mucosa were swabbed with calcium alginate swabs (Fisherbrand, Thermo Fisher Scientific, Inc., Waltham, MA) for culture. The uterus was opened and each fetus and associated membranes, amniotic fluid, cord, placenta, and attached uterus/mesometrium were treated as individual units for the purposes of sample collection and data analysis. Fetal units were processed identically regardless of treatment group. Each fetal unit was arbitrarily assigned a number. For all units, amniotic fluid was collected aseptically for culture and a portion of the placenta was minced in SP4 medium and serially diluted for culture. Samples from MP only and Nic + MP groups were serially diluted 10-fold in SP4 broth to 10–10; samples from control and Nic only groups were diluted to 10–3. For odd-numbered fetal units, the fetus was minced for culture, and the placenta with attached uterine wall and fetal membranes were fixed in 10% buffered formalin for histological analysis. Even-numbered fetal units were fixed in 10% buffered formalin with fetus and attached tissues intact. All fixed tissues were transferred to 70% alcohol after 48 h of fixation.
Histopathology and lesion scoring of placental tissues
Tissues were trimmed and transected so that a cross-sectional view of the placental disk with associated decidua and attached visceral yolk sac, amnion, and umbilical cord was present. Tissues were processed routinely and stained with hematoxylin and eosin. Some fetal units had missing structures that were lost during collection, handling, or processing. Tissue examined included the following areas: choriodecidua (decidual layer between metrial triangle and junctional zone, and decidua/junctional zone at margins of placental disk); subchorion (maternal blood sinuses within the labyrinth zone); chorionic plate (epithelium and stroma); visceral yolk sac (endodermal villi, vitelline vessels, and mesothelium); amnion; chorionic vessels within the plate (lumen and endothelium); lumen and endothelium of umbilical vein and umbilical arteries; and Wharton's jelly, including perivascular stroma. Lesion scores were assigned based on presence of neutrophils, with infiltration of intact neutrophils indicating acute inflammation. Fragmented neutrophils as well as necrotic or degraded cells and connective tissue indicated chronic inflammation. A score of 0–3 was assigned for both acute and chronic inflammation, with 0 indicating little to no inflammation, 1 = mild inflammation, 2 = moderate inflammation, and 3 = severe inflammation. The overall scores for each site were added together for a maximum score of 6. In a few cases where tissue damage was so advanced that neutrophils could not be assessed, a score of 7 was assigned to indicate maximum lesion severity. The maternal inflammatory response (MIR) was obtained by adding the lesion scores for the subchorion and choriodecidua; the fetal inflammatory response (FIR) was obtained by adding the lesion scores for chorionic vessels, umbilical arteries, umbilical vein, and Wharton's jelly. The inflammatory response in the chorionic plate, visceral yolk sac, and amnion tissues was considered as a mixture of both maternal and fetal responses; therefore, these lesions scores were added together to give a score for an inflammatory response potentially involving both maternal and fetal components (MIXED).
Cytokine analysis
Placental tissues were weighed and placed in a Biomasher II homogenizer (Kimble Chase). One milliliter of lysis buffer (T-PER protein extraction reagent containing HALT protease inhibitor cocktail with EDTA according to the manufacturer's instructions; Pierce Biotechnology, Inc.) per 100 mg of tissue was added and the placental tissue was mechanically homogenized. Tissue homogenates were centrifuged at 10 000 × g at 4°C and supernatants were transferred to clean tubes and frozen at –20°C prior to analysis. Placental protein extracts, amniotic fluids, and serum from dams were diluted 1:5 in the Reagent Diluent (R&D Systems) and analyzed for the presence of interferon gamma (IFNG), tumor necrosis factor (TNF), interleukin (IL6), and IL10 using rat DuoSet ELISA kits (R&D Systems). Cytokine values were expressed as pg/mg placental tissue or pg/ml amniotic fluid or serum. Standard curves were constructed using recombinant cytokines provided in the kit, and these curves were included for each assay. For placental homogenates, the recombinant proteins were diluted in Reagent Diluent:T-PER (4:1 vol/vol).
Data analysis
Culture data were compared between the two infection groups (MP only and Nic + MP) using the Mann-Whitney U test. Categorical data for culture status (positive vs. negative) were compared by Chi Square with Yates correction factor. The relationships of microbial load in placenta, amniotic fluid, and fetus were compared by Spearman r and regression models were fit to the data. Lesion scores were analyzed by Kruskal–Wallis one-way nonparametric ANOVA followed by the Dunn multiple comparison test. Distribution of severe lesions among treatment groups was assessed by the Fischer exact test. Cytokine data were transformed using log (Y+1), where Y was an individual data point and analyzed by Kruskal-Wallis one-way nonparametric ANOVA followed by the Dunn multiple comparison test. MP only and Nic + MP groups were subdivided based on infection status (culture-positive or culture-negative) of placenta and amniotic fluid. Transformed cytokine data for these subgroups were analyzed by the unpaired t test or Kruskal-Wallis one-way nonparametric ANOVA. When indicated, the Dunn test was used for relevant multiple comparisons. As an indicator of the overall M2/Th2 profile in individual placenta and amniotic fluid samples, the IL10 level was subtracted from the IFNG level. A negative value was indicative of a more M2/Th2, IL10-dominant environment; positive values were indicative of a more M1/Th1, IFNG-dominant environment, and values near zero indicated a neutral environment. The IFNG minus IL10 values were analyzed using the Kruskal-Wallis test and Dunn multiple comparison tests between groups. For all analyses, a probability of P ≤ 0.05 was considered significant.
Results
Nicotine exposure increases risk of infection in the fetal compartment (Figure 1, Table 1)
Figure 1.
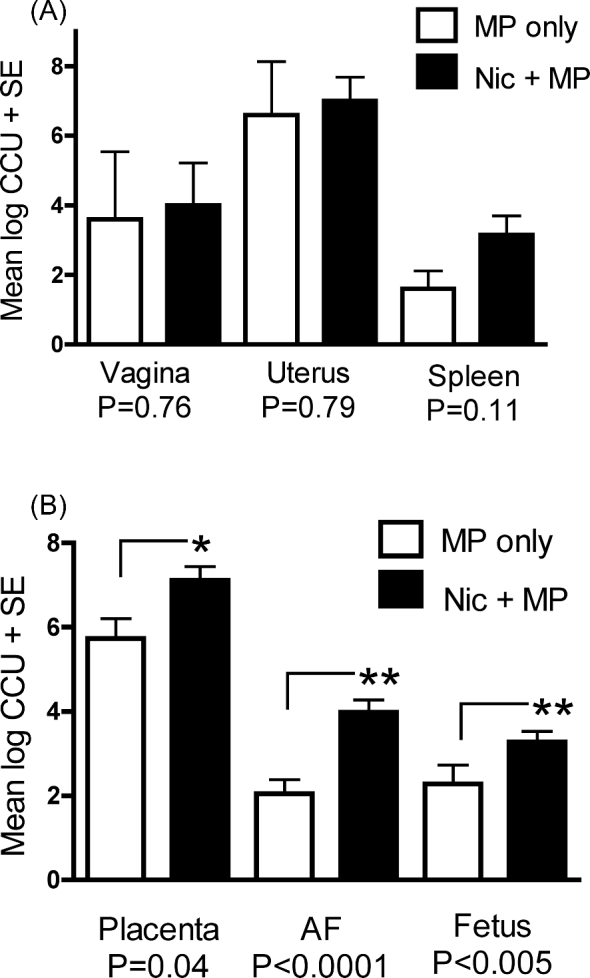
Prenatal exposure to nicotine increased microbial load in fetal but not maternal tissues. (A) Microbial load in maternal tissues was not significantly impacted by nicotine exposure. (B) Microbial load in the fetal unit [placenta, amniotic fluid (AF), and fetus] was significantly increased with maternal exposure to nicotine. Data are expressed as the mean log CCU + SE. P value < 0.05 was considered significant. Mycoplasma pulmonis was never isolated from any control or Nic only rats.
Table 1.
Nicotine did not impact the percentage of maternal sites colonized by M. pulmonis.
| Site | MP only | Nic + MP | P value |
|---|---|---|---|
| Uterus | 5/5 (100%) | 7/7 (100%) | NA |
| Placenta | 53/61 (87%) | 74/79 (94%) | 0.28 |
| Amniotic fluid | 43/57 (75%) | 73/80 (91%) | 0.02 |
| Fetus | 22/28 (79%) | 36/36 (100%) | 0.01 |
| Spleen | 4/5 (80%) | 7/7 (100%) | 0.86 |
| Vagina | 3/5 (60%) | 7/7 (100%) | 0.29 |
Statistically significant differences (Chi Square with Yates correction factor) are shown in bold. All fetal units had placental and amniotic fluid cultures performed; however, fetal cultures were available only for a subset of the total fetal units. Mycoplasma pulmonis was never isolated from any control or Nic only rats.
The mean, standard deviation, and minimum-maximum for litter sizes for the treatment groups were control, 11.5 ± 3.3, 5–16; MP only, 12.6 ± 0.9, 12–14; Nic only, 11.0 ± 3.1, 9–16; and Nic + MP, 11.9 ± 2.8, 9–16. Three control dams (fetal units = 33), four Nic only dams (fetal units = 36), five MP only dams (fetal units = 63), and seven Nic + MP dams (fetal units = 83) were used for data analysis. Exposure to nicotine (Nic + MP) did not alter the percent colonization of placenta or any of the maternal tissues (vagina, endometrial surface, spleen) when compared to MP only treatment; however, nicotine exposure did significantly increase the percentage of infected fetuses and amniotic fluids (Table 1). Although the percentage of placentas colonized by M. pulmonis was not influenced by nicotine exposure, the numbers of M. pulmonis (microbial load) recovered (Figure 1) from culture-positive placentas in the Nic + MP group was higher compared to the MP only group (P = 0.04). The microbial load in the amniotic fluids (P = 0.0001) and fetuses (P = 0.005) also was higher in Nic + MP rats compared to the MP only group. Mycoplasma pulmonis was never isolated from any Control or Nic only rats.
Nicotine exposure decreases the placental threshold associated with increased microbial load in amniotic fluid (Figure 2)
Figure 2.
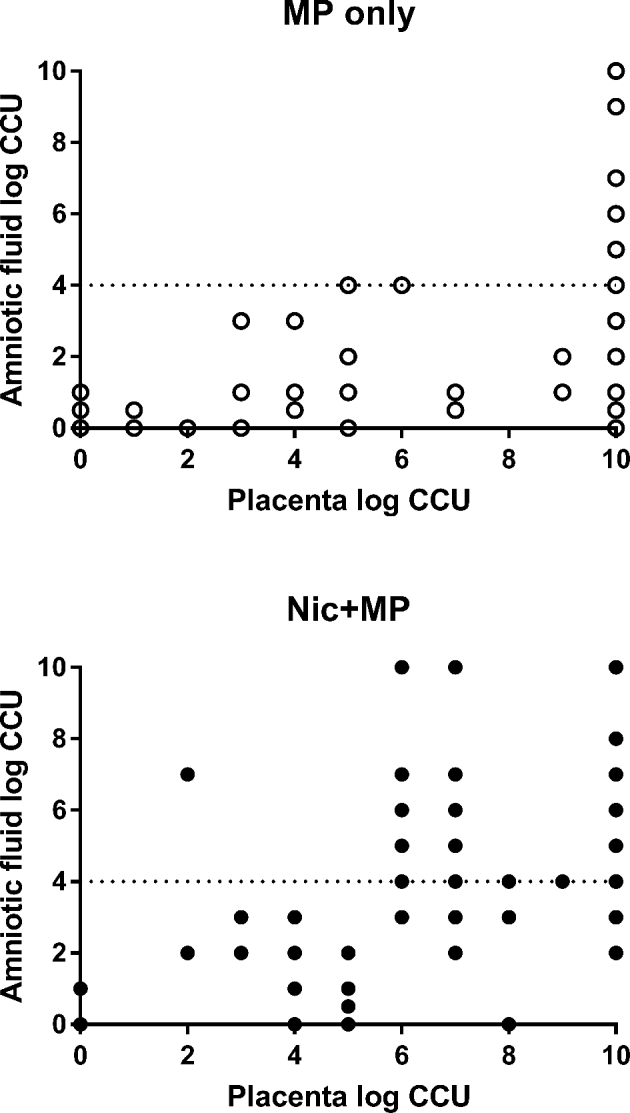
Correlation of microbial load in the placenta with microbial load in the amniotic fluid. Rats without nicotine exposure (MP only) attained levels of infection > log 4 CCU (dotted line) only when the placental load was ≥ log 10 CCU. Rats with nicotine exposure (Nic + MP) attained levels of infection > log 4 CCU (dotted line) when the placental load was ≥ log 6 CCU. Results are expressed as the log CCU recovered. Each data point represents an individual fetal unit. Mycoplasma pulmonis was never isolated from any control or Nic only rats.
Previously, we demonstrated that the microbial load in the placenta was correlated with the microbial load in the amniotic fluid [46]. To determine if nicotine altered this relationship, the microbial load of amniotic fluid as a function of microbial load in the placenta in both treatment groups was analyzed. In MP only fetal units, a high microbial load (defined as >104 CCU) was seen in the amniotic fluid only when the microbial load in the placenta was ≥109 CCU (Figure 2). In contrast, in the Nic + MP group, the threshold of placental microbial load associated with high amniotic fluid loads was much lower. Consistently high numbers of M. pulmonis were recovered from amniotic fluids with placental microbial load ≥106 CCU, and in a few instances, at even lower placental loads. When nicotine exposure was present, the relationship between placental and amniotic fluid microbial load was altered (P = 0.001, best fit regression curves; MP only, y = 0.4x – 0.1; Nic + MP, y = 0.5x + 0.8).
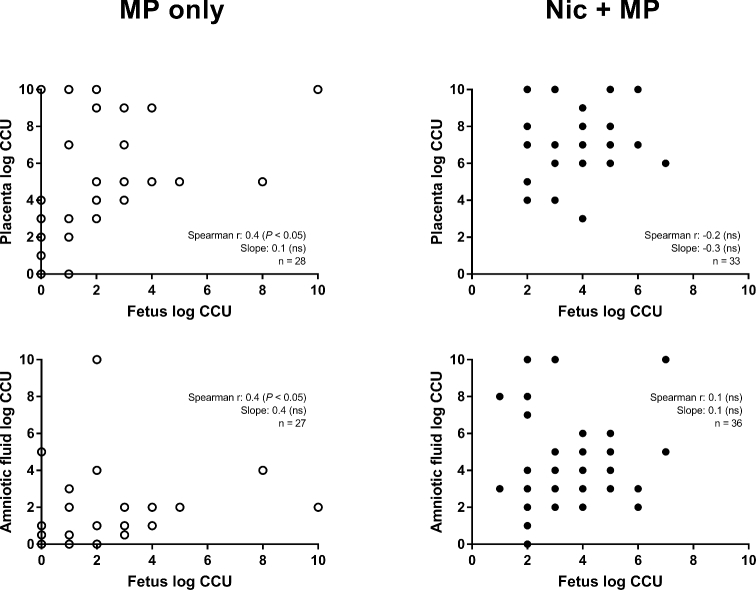
Complete culture data (Table 2, Figure 3) were available for 27 (MP only) and 33 (Nic + MP) fetal units (placenta, amniotic fluid, and fetus). Fetal colonization was more likely to occur with nicotine exposure (P < 0.05; MP only, 81.5% positive; Nic + MP, 100% positive). Furthermore, in both MP only and Nic + MP treatment groups, fetal colonization was most likely (P < 0.01) to occur when both the placenta and amniotic fluid were culture positive (Table 2). A linear correlation between fetal microbial load and both the placental (Spearman r = 0.4, P < 0.05, n = 28) and amniotic fluid (Spearman r = 0.4, P < 0.05, n = 27) microbial loads was present in the MP only group but not in the Nic + MP group (for fetus vs. placenta microbial loads: Spearman r = −0.2, P = 0.2, n = 33; for fetus vs. amniotic fluid: Spearman r = 0.1, P = 0.7, n = 36). A two-way ANOVA for treatment group (MP only and Nic + MP) and intrauterine site (placenta, amniotic fluid, and fetus) showed that both treatment group and intrauterine site, as well as an interaction between the two, contributed to variation in microbial load. Intrauterine site contributed to 78% of the variation (P < 0.0001) while treatment group contributed to 17% of the variation (P < 0.0001), and the interaction contributed to 1% of the variation (P < 0.0001).
Table 2.
Relationship of fetal culture status to presence of M. pulmonis in the placenta and amniotic fluid.
| Fetus positivea | MP only (n = 22) | Nic + MP (n = 33) |
|---|---|---|
| PL (+) AF (+)b | 19/22 (86.5%) | 32/33 (97%) |
| PL (+) AF (–) | 2/22 (9%) | 1/33 (3%) |
| PL (–) AF (+) | 0/22 (0%) | 0/33 (0%) |
| PL (–) AF (–) | 1/22 (4.5%) | 0/33 (0%) |
| Fetus negative | MP only (n = 5) | Nic + MP (n = 0) |
| PL (+) AF (+) | 3/5 (60%) | 0 |
| PL (+) AF (–) | 1/5 (20%) | 0 |
| PL (–) AF (+) | 0/5 (0%) | 0 |
| PL (–) AF (–) | 1/5 (20%) | 0 |
aFetal colonization was more likely (P < 0.05) to occur with nicotine exposure (MP only, 81.5% (22/27) culture-positive fetuses; Nic + MP, 100% (33/33) culture-positive fetuses).
bFetal colonization was most likely (P < 0.01) to occur when both the placenta and amniotic fluid were culture positive, regardless of treatment group.
Figure 3.
Correlation of microbial load in the fetus with microbial load in both the placenta and amniotic fluid. In the MP only group, there was a positive correlation between fetal microbial load and placental and amniotic fluid microbial loads (left panels). A correlation was not observed in the Nic + MP group (right panels). The correlation coefficient (Spearman r) and slope of linear regression line with corresponding P values (if < 0.05) are indicated.
Prenatal nicotine and/or infection exposure induced site-specific pathology (Figures 4 and 5; Table 3)
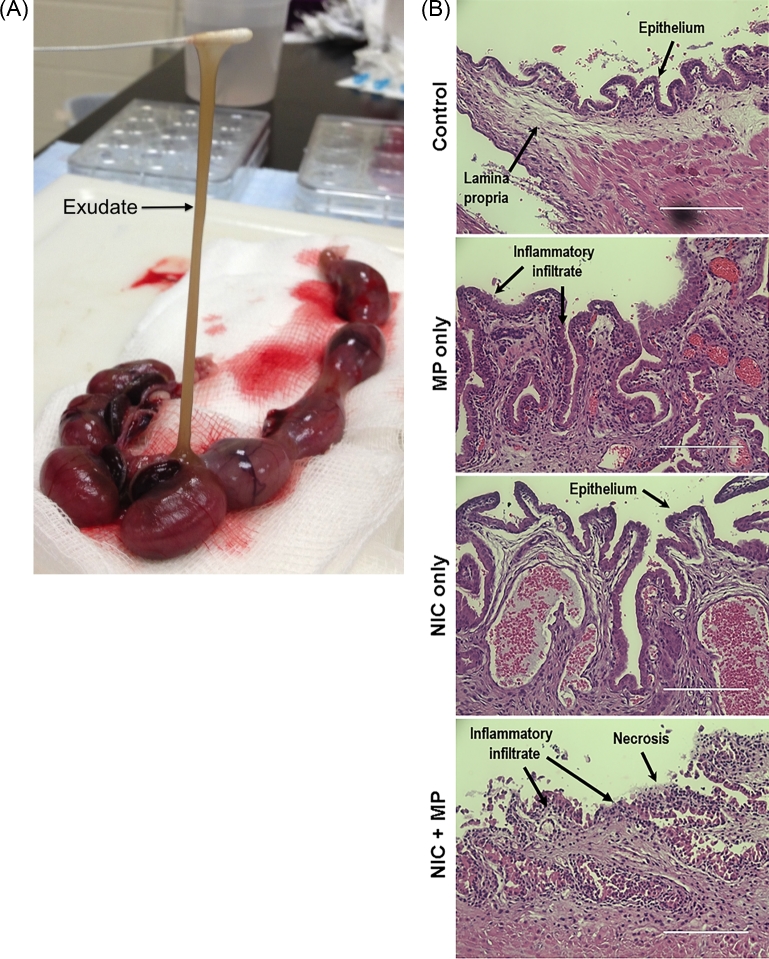
Figure 4.
Acute endometritis was observed in both MP only and Nic + MP groups. (A) Copious brown mucoid exudate (arrow) was observed in the uterine lumen of Nic + MP dams (3 of 7, 43%), but was never observed in MP only dams or controls. (B) Representative endometrial histology from control, MP only, Nic only, and Nic + MP dams. Scale bar is equivalent to 200 μm; hematoxylin and eosin stain. All microscopic images were obtained with an EVOS Auto FL imaging system (Life Technologies).
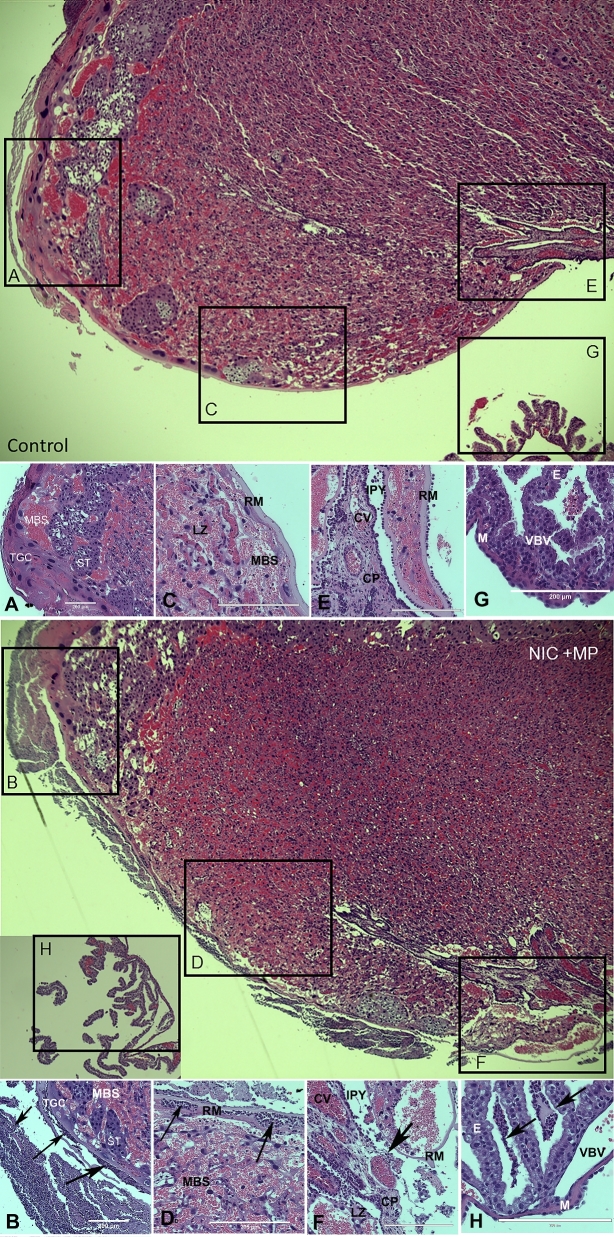
Figure 5.
Normal and representative severe histological lesions in the placenta. Low magnification images of control (top) and Nic + MP (bottom) placental disks with boxed areas indicating select sites subject to scoring: choriodecidua (boxes A and B), subchorion (boxes C and D), chorionic plate (boxes E and F), and visceral yolk sac (boxes G and H). Boxes correspond to the location of the structures shown in the high magnification image panels (control: A, C, E, G; Nic + MP: B, D, F, H). Arrows indicate neutrophils. Panel A shows normal choriodecidua. In Panel B, the decidua is obliterated by neutrophil infiltrates. Panel C shows normal subchorion. In Panel D, neutrophils infiltrate the maternal blood spaces at the periphery of the labyrinth zone. Panel E shows normal chorionic plate. Panel F shows epithelial effacement, neutrophil infiltration, and structural breakdown of the chorionic plate. Panel G shows normal visceral yolk sac. In Panel H, purulent exudate is seen among the endodermal villi of the visceral yolk sac. All images were obtained with an EVOS Auto FL imaging system (Life Technologies). Panels represent ×10 (A and B) or ×20 magnification. All scale bars are equivalent to 200 μm. Structural abbreviations: decidua (D), maternal blood sinus (MBS), trophoblastic giant cell (TGC), spongiotrophoblast (ST), labyrinth zone (LZ), Reichert's membrane (RM), intraplacental yolk sac (IPY), chorionic plate (CP), chorionic vessel (CV), endoderm (E), vitelline blood vessel (VBV), and mesothelium (M).
Table 3.
Lesion severity in sites indicative of maternal inflammatory response (MIR), fetal inflammatory response (FIR), or a potential mixture (MIXED) of both maternal and fetal components was impacted by treatment group.
| Group | MIRa,b,c | Severe MIRc | FIRa,b,c | Severe FIRc | MIXEDa,b,c | Severe MIXEDc |
|---|---|---|---|---|---|---|
| Control | 2.3 ± 1.2 | 0/12 (0%) | 3.2 ± 1.6 | 0/12 (0%) | 1.6 ± 1.4 | 0/12 (0%) |
| MP only | 4.5 ± 2.0 | 5/18 (28%) | 7.2 ± 2.6 | 0/12 (0%) | 5.5 ± 2.7 | 3/17 (18%) |
| Nic Only | 4.8 ± 1.5 | 8/26 (31%) | 13.0 ± 7.5 | 7/20 (35%) | 7.3 ± 4.7 | 8/27 (30%) |
| Nic + MP | 6.0 ± 2.1 | 17/28 (61%) | 11.0 ± 6.3 | 8/25 (32%) | 5.8 ± 4.0 | 7/28 (25%) |
Results are presented as mean lesion score ± SD, or as proportion of total having severe scores
aA score of 0–3 was assigned for both acute and chronic inflammation at a specific site; 0 = little to no inflammation, 1 = mild inflammation, 2 = moderate inflammation, and 3 = severe inflammation, with a maximum score of 6. If tissue damage was so advanced that neutrophils could not be assessed, a score of 7 was assigned to indicate maximum lesion severity.
bMIR lesion score = sum of subchorion and choriodecidua lesion scores; maximum possible score = 14; severe MIR = lesion score ≥ 6. FIR lesion score = sum of chorionic vessels, umbilical arteries, umbilical vein, and Wharton's jelly lesion scores; maximum possible score = 28; severe FIR = lesion score ≥ 12. MIXED lesion score = sum of chorionic plate, visceral yolk sac, and amnion tissues lesion scores; maximum possible score = 21; severe MIXED = lesion score ≥ 9.
cStatistically significant differences (Kruskal-Wallis) among the groups for overall lesion score are shown in bold. MIR lesion scores: All treatment groups > control, P < 0.05. FIR lesion score: both Nic only and Nic + MP > control (P < 0.0001). MIXED: all treatment groups > control (P < 0.003). Statistically significant differences (Fischer exact test) among the groups for severe lesions are shown in bold. Severe MIR: Nic + MP group > control (P < 0.0003) and both MP only and Nic only (P < 0.05) treatment groups; Nic only group > control group (P < 0.05). Severe FIR: Both Nic only and Nic + MP > both control and MP only groups (P < 0.05). Severe MIXED: Nic only (P < 0.05) > control group.
Consistent with previous studies [42, 44–46], no exudate in the uterine lumen was observed in dams from control or MP only groups; we also observed no exudate in the Nic only group. In contrast, the combination of nicotine and infection resulted in a dramatic and unexpected inflammatory response in the uterus. At gross necropsy, three of seven Nic + MP dams had copious brown mucoid exudate present in the uterine lumen (Figure 4A). The exudates were positive for growth of M. pulmonis and negative for other aerobic bacteria. Representative histological sections of the endometrium between placental implantation sites of both uninfected and infected dams are shown in Figure 4B. No inflammatory lesions within the endometrium were observed in uninfected control or Nic only dams. In contrast, both MP only and Nic + MP dams had evidence of acute endometritis, but severe necrosis was seen only in Nic + MP dams.
Lesion severity scores for sites indicative of the MIR, FIR, or a mixture (MIXED) of both maternal and fetal components are presented in Table 3. The detailed inflammatory scores for MIR (subchorion and choriodecidua), FIR (chorionic vessels, umbilical arteries, umbilical vein, and Wharton jelly), and MIXED responses (chorionic plate, visceral yolk sac, and amnion tissues) are shown in Table S1, supplementary material. For reference and orientation, low magnification placental images (Figure 5) showing the location of the choriodecidua (box A, B), subchorion (box C, D), chorionic plate (box E, F), and visceral yolk sac (box G, H) are provided. The corresponding high magnification image panels for the control placenta (A, C, E, G) are a reference for the normal architecture. Selected examples of severe lesions are shown in the high magnification image panels (B, D, F, H). The primary lesion observed was neutrophil infiltration. In severe lesions, the infiltrate was associated with severe tissue damage. For example, epithelial effacement, neutrophil infiltration, and structural breakdown of the chorionic plate are shown in Figure 5F. All treatment groups had increased MIR and MIXED lesion severity relative to controls (Table 3, P < 0.025). However, the most severe MIR lesions were seen in the Nic + MP group relative to the control (P < 0.0003), MP only (P < 0.05), and Nic only (P < 0.05) treatment groups, suggesting some synergy between the risk factors. Nicotine, alone or in concert with infection, promoted FIR (P < 0.0001), with increased lesion severity (P < 0.05) in all FIR sites (refer to Table S1, supplementary material).
Nicotine exposure alone or in combination with infection impacts cytokine levels in the placenta (Figure 6)
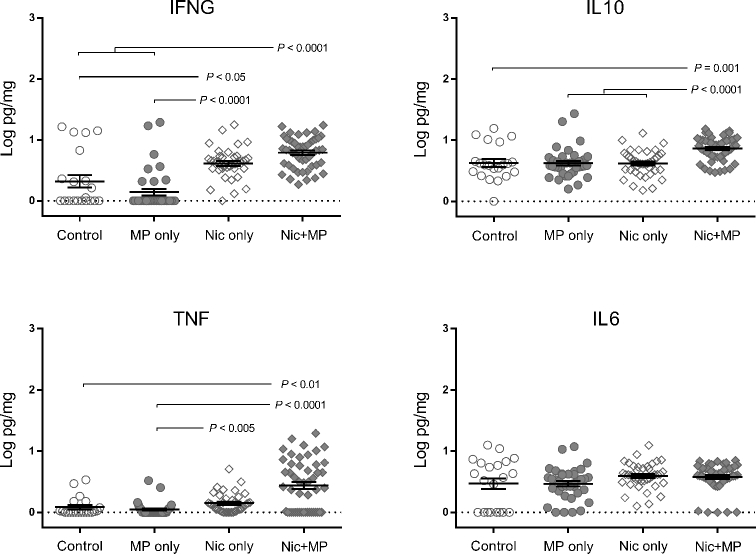
Figure 6.
Combined nicotine and infection impacted cytokine levels in the placenta. Log-transformed cytokine levels for IFNG, IL10, TNF, and IL6 are shown. Graphs show individual data points as well as bars for mean and SEM. Cytokine data for control (n = 21), Nic only (n = 39), MP only (n = 38), and Nic + MP (n = 49) placentas were analyzed by Kruskal-Wallis tests. P values < 0.05 indicate significant differences between groups.
Placental IL6 levels did not vary among groups. Relative to the control group, Nic only exposure resulted in increased placental levels of IFNG (P < 0.05) but did not alter levels of IL10 or TNF. Placental levels of IFNG and TNF were statistically higher (P < 0.01) in the Nic + MP group as compared to both control and MP only groups. Furthermore, IL10 placental levels were higher in the Nic + MP group than in all other groups (P < 0.001). In general, levels in the MP only group were comparable to control values.
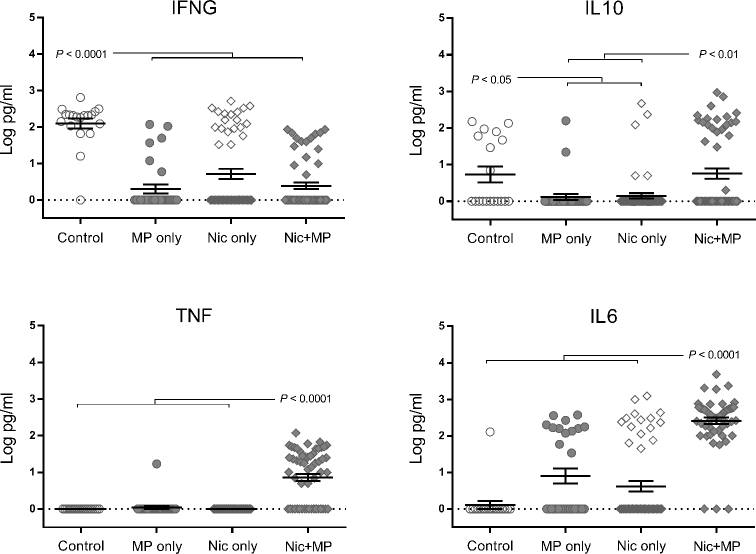
Nicotine and infection, independently and in combination, impact cytokine levels in the amniotic fluid (Figure 7)
Figure 7.
Combined nicotine and infection impacted cytokine levels in the amniotic fluid. Log-transformed cytokine levels for IFNG, IL10, TNF, and IL6 are shown. Graphs show individual data points as well as bars for mean and SEM. Data for control (n = 12), Nic only (n = 57), MP only (n = 30), and Nic + MP (n = 58) amniotic fluids analyzed by Kruskal-Wallis tests. P values < 0.05 indicate significant differences between groups.
In contrast to what was observed in the placenta, IFNG levels in amniotic fluid were decreased (P < 0.0001) in all treatment groups relative to the control group. IL10 was significantly decreased (P < 0.05) in the MP only and Nic only groups relative to the control group. However, in the Nic + MP group, the IL10 levels did not decrease in amniotic fluid and were similar to control levels. Nicotine or infection did not independently impact TNF levels, but when combined (Nic + MP), TNF levels were significantly increased (P < 0.0001) as compared to all other groups. Similarly, IL6 amniotic fluid levels in Nic + MP were increased (P < 0.0001) as compared to all other groups.
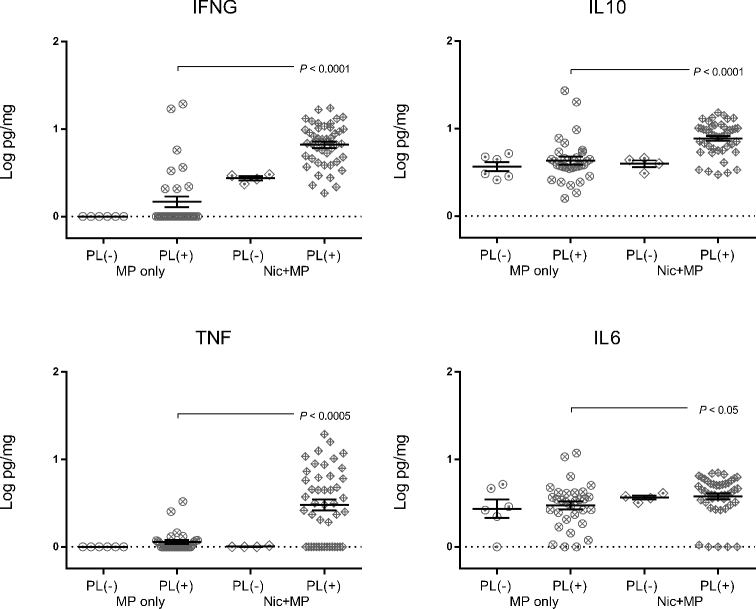
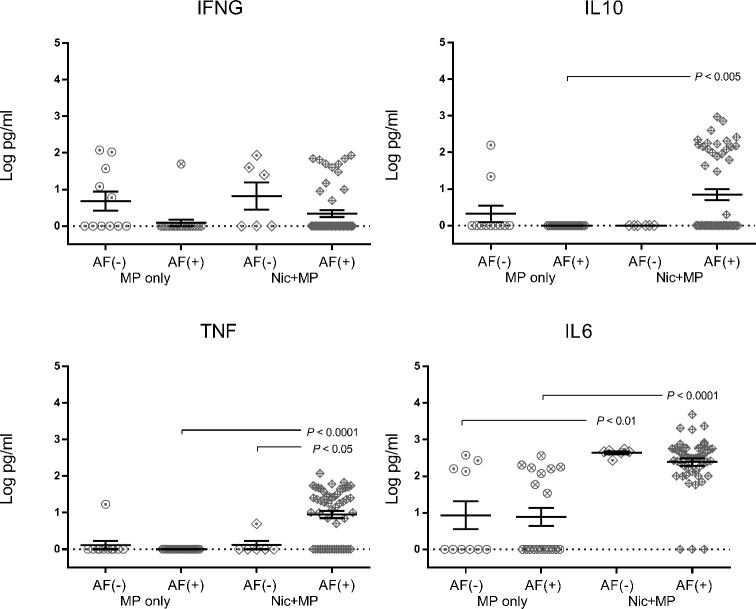
Presence of live microbes impacts cytokine levels at the site of infection (Figures 8 and 9)
Figure 8.
Cytokine levels in the placenta were impacted by nicotine and by presence of the microbe. Log-transformed cytokine levels for IFNG, IL10, TNF, and IL6 for MP only (n = 38) and Nic + MP (n = 49) placentas (PL) were further subdivided based on positive (+) and negative (–) culture status of the placenta and analyzed by the Kruskal-Wallis test. P values < 0.05 indicate significant differences between groups. Graphs show individual data points as well as bars for mean and SEM.
Figure 9.
Cytokine levels in the amniotic fluid were altered by nicotine and by presence of the microbe. Log-transformed cytokine levels for IFNG, IL10, TNF, and IL6 for MP only (n = 30) and Nic + MP (n = 58) amniotic fluids (AF) were further subdivided based on positive (+) and negative (–) culture status of the amniotic fluid and analyzed by Kruskal-Wallis test. P values < 0.05 indicate significant differences between groups. Graphs show individual data points as well as bars for mean and SEM.
We wished to determine if the cultural status of the placenta and amniotic fluid impacted cytokine levels. Therefore, the MP only and Nic + MP groups were subdivided by culture-positive and culture-negative status at the level of the placenta (Figure 8) or amniotic fluid (Figure 9). None of the cytokine levels in the MP only group were impacted by culture status of the placenta (P > 0.05). In contrast, in the Nic + MP group, IFNG and IL10 (P < 0.005) and TNF (P < 0.05) were all elevated when infection was present. Furthermore, the presence of nicotine in concert with MP infection resulted in higher placental levels of IFNG, IL10, TNF (P < 0.01), and IL6 (P < 0.05) when compared with MP only culture-positive placentas.
In the MP only group, levels of IFNG in amniotic fluid were higher when infection was absent (P < 0.05); IL10, TNF, and IL6 levels were not altered by the presence of infection. Within the Nic + MP group, when M. pulmonis was isolated from amniotic fluid, TNF (P < 0.01) levels were elevated but levels of IFNG, IL10, or IL6 were not impacted. When culture-positive amniotic fluids in the Nic + MP and MP only groups were compared, log increases in cytokine levels were observed for TNF (P < 0.0001), IL10 (P < 0.005), and IL6 (P < 0.0001) in the Nic + MP group. For IL6 (P < 0.01), the increases were also observed in the absence of microbial colonization of the amniotic fluid. These data suggest that nicotine and infection can act synergistically at the local site of infection to modulate cytokine levels.
Nicotine alters the cytokine environment in both the placenta and the amniotic fluid (Figure 10)
Figure 10.
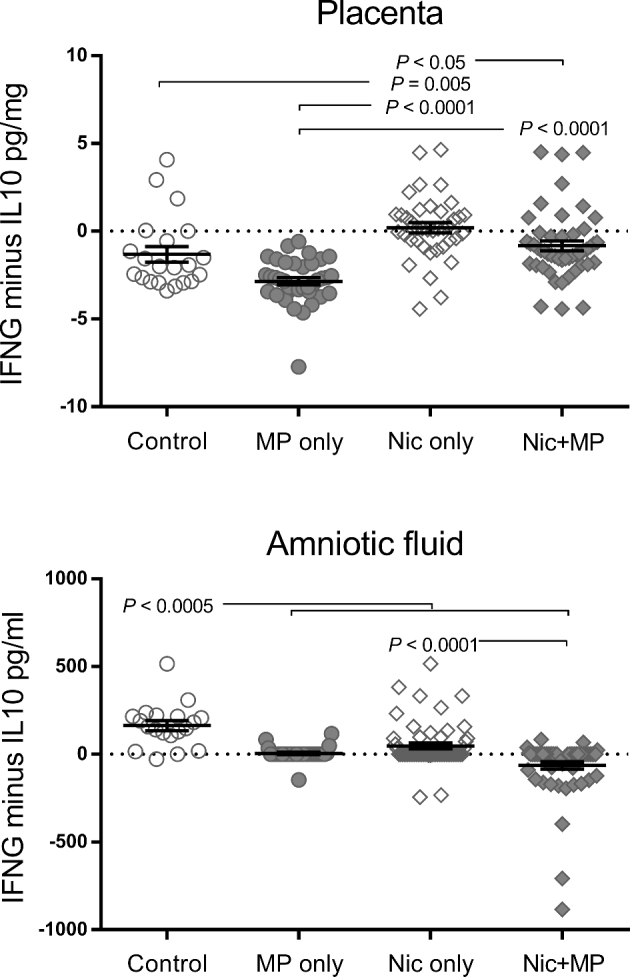
The normal environments of the placenta (M2/Th2) and amniotic fluid (M1/Th1) are impacted by nicotine, alone or in concert with infection. As an indicator of the overall M2/Th2 profile in individual placenta and amniotic fluid samples, the IL10 level was subtracted from the IFNG level. A negative value was indicative of a more M2/Th2, IL10-dominant environment; positive values were indicative of a more M1/Th1, IFNG-dominant environment, and values near zero indicated a neutral environment. Cytokine data for control (n = 21), Nic only (n = 39), MP only (n = 38), and Nic + MP (n = 49) placentas, and for control (n = 12), Nic only (n = 57), MP only (n = 30) and Nic + MP (n = 58) amniotic fluids, were analyzed by the Kruskal-Wallis test. P values < 0.05 indicate significant differences between groups. Graphs show individual data points as well as bars for mean and SEM.
Because the M2/Th2 phenotype is present in the normal pregnancy environment, we wished to determine if the combination of infection and nicotine altered key cytokines in the placenta and amniotic fluid. The IFNG-to-IL10 ratio can be used to assess the general immune environment in the placenta. In some samples, the cytokine levels were below the detection limit of our assays and were reported as zero. Therefore, we were unable to use a ratio but instead determined the difference (IFNG minus IL10) for each individual sample. A negative value was indicative of a more M2/Th2, IL10-dominant environment; a positive value was indicative of a more M1/Th1, IFNG-dominant environment, and a value near zero indicated a neutral environment. In control placentas, IL10 levels dominated over IFNG, while in Nic only placentas, the levels were equivalent and significantly different from control (P = 0.005). MP only resulted in an IL10-dominant environment similar to control. However, when both nicotine and infection were present, a shift to a neutral balance was observed (P < 0.0001). Unlike in the placenta, the general immune environment in control amniotic fluids was IFNG dominant while all other treatment groups shifted to a neutral environment (P < 0.0005). Interestingly, when both nicotine and infection were present (Nic + MP), the shift from the IFNG-dominant control environment was more pronounced, resulting in an IL10-dominant environment in the amniotic fluid (P < 0.0001).
Circulating serum cytokines were not impacted
No significant differences (Kruskal-Wallis) among treatment groups were found in maternal serum cytokine levels.
Discussion
Prenatal nicotine exposure alone has been associated with a host of adverse fetal/neonatal outcomes [8, 38–40, 47–53]. The results of this study provide new evidence demonstrating that nicotine exposure also increases the risk of fetal infection. Specifically, prenatal exposure to 6 mg/kg/day nicotine significantly increased the incidence of both amniotic fluid and fetal infection with M. pulmonis. The placental microbial load needed to trigger amniotic fluid infection was significantly decreased in Nic + MP dams, suggesting that prenatal nicotine exposure compromises the placental barrier protection against infection.
While causal relationships of adverse pregnancy outcomes can be inferred from retrospective and prospective epidemiological studies, animal models are essential to elucidate mechanisms of pathogenesis [54–57]. The rat is a good model for studies focused on the maternal:fetal interface because, similar to that in humans, this species has both hemochorial placentation and deep trophoblast invasion of spiral arteries [58]. The primary limitation of the rat as a model is that rats resorb fetuses rather than deliver prematurely. One potential caveat is that we used a high dose of nicotine in our study. The 6 mg/kg/day we chose does not impact maternal body weight, number of pups per litter, pup weight, or placental weight, but does significantly alter neurotransmitter balance within the central nervous system and postnatal behavior [38, 39, 41]. We coupled this nicotine dose with an infectious dose of M. pulmonis that results in consistent colonization of the placenta but variable colonization of the fetal compartment [45, 46]. Because there are no studies that have examined the interaction between nicotine and intrauterine microbial infection, we reasoned that using the highest dose of nicotine not associated with adverse pregnancy outcomes coupled with a moderate infectious dose would provide the best opportunity to document an interaction between nicotine and infection. In fact, in a pilot study in which both nicotine and infection were combined, at term pregnancy we saw an unexpected reduction in litter size and few surviving pups (see supplemental data). Ethical concerns for animal welfare precluded continued studies at term, but it is important to note that these severe outcomes were not observed in term dams with nicotine or infection exposure alone. Therefore, use of this model for term or postnatal studies will need to incorporate lower doses of nicotine.
One of the strengths of our model system is that we use viable M. pulmonis that can replicate, establish in the placenta, and disseminate throughout the fetal unit [42, 46, 59], similar to what is likely to occur during intrauterine infection in humans. Mycoplasma pulmonis genital disease is a naturally occurring reproductive disease in laboratory rats [60, 61]. The model is well characterized, and there is an established dose response and reproducible pattern of colonization of maternal and fetal tissues [42, 45, 46] as well as defined pathology [44, 46]. Experimentally infected dams develop varying complications as well as histological lesions similar to ureaplasmal-associated reproductive disease of humans [62–65], including chorioamnionitis, fetal infection of the lung and central nervous system, low birth weight, and fetal and neonatal death [42, 44–46, 59, 66]. Routes of infection, including both intravaginal and IV inoculation, have been defined for this model previously [43–46]. This study was designed to ensure that all inoculated dams became infected. Intravenous administration provided the precise timing and dose delivery to all pregnant dams. The uterus was infected in all dams, and there was no difference between MP only and Nic + MP with respect to numbers of microbes recovered from the uterus. Therefore, all fetal units had an equal chance of becoming infected. By design, the infectious dose chosen did not result in 100% of placentas becoming infected in the MP only group, permitting us to identify differences between the infected treatment groups. There was no difference in the percentage of placentas that were colonized between groups. What was dramatically different was the increased microbial load in the placenta in the Nic + MP group coupled with the lowered threshold required for M. pulmonis to breach the placental barrier in this group.
In this study, we used an established paradigm of prenatal nicotine exposure to model the impact of maternal smoking (either cigarettes or ENDS) on fetal infection or susceptibility to infection using viable microbes as opposed to purified endotoxin. Administration of a purified toxin like LPS is often used as a surrogate for maternal infection and can induce premature birth and/or evidence of resorption in rats and mice [55–57]. LPS intoxication stimulates innate immune responses, induces prematurity, and has provided valuable insights into the specific mechanism by which LPS alone or in concert with nicotine impacts pregnancy [67–70]. However, intoxication is not adequate to examine the mechanisms by which viable pathogens invade and breach the placental barrier. To our knowledge, very few animal studies have investigated the interaction between nicotine exposure and true maternal infection on fetal outcome.
For much of gestation, the placenta induces an M2/Th2 phenotype in decidual leukocytes [71–74]. The relative balance of IFNG to IL10 in placental tissue was used as an indicator of M2/Th2 profile. In our study, nicotine exposure altered the IFNG to IL10 balance. As expected, placentas from controls were characterized by IL10 > IFNG levels. Placental IFNG levels were increased relative to IL10 with Nic only, shifting to a neutral balance. In the MP only group, the normal IL10-dominant environment persisted, independent of the presence of live microbes in the placenta. Thus, even in the face of overt infection, the placenta was able to maintain the appropriate M2/Th2 environment. However, in the presence of nicotine and placental colonization with a live microbe, the balance was again shifted to neutral. When considered in context with lesion severity, this suggests that at 6 mg/kg/day, nicotine might prime the placental tissues to a stronger cell-mediated, M1/Th1 inflammatory response when a pathogen is encountered.
A compelling contrast was observed in the amniotic fluid. In controls, the amniotic fluid, unlike the placenta, was IFNG dominant, suggesting an M1/Th1 environment. In Nic only and MP only animals, this environment shifted to a neutral balance. When both nicotine and infection were present, the amniotic fluid environment was shifted from the M1/Th1 profile seen in the controls to become IL10 dominant. Interestingly, the Nic + MP group had dramatically increased levels of both TNF and IL6 relative to all other treatment groups. In the case of IL6, this increase was independent of the presence of M. pulmonis in the amniotic fluid. This interaction between nicotine and maternal infection to elevate IL6 levels without regard to amniotic fluid colonization is interesting. The magnitude of the increases in amniotic TNF and IL6 is striking. Levels of TNF and IL6 in amniotic fluid from the Nic + MP group were one to three logs higher than levels seen in control amniotic fluids, with TNF levels up to 117 pg/ml and IL6 levels up to 4791 pg/ml. These concentrations are comparable to those reported from human amniotic fluids with fetal inflammatory syndrome and intrauterine infection [75–77]. TNF does not cross the placental barrier; additionally, activated macrophages expressing TNF increase dramatically from GD 15 to GD 17 in the amniotic fluid of the mouse [78]. IL6 from maternal circulation does cross the placental barrier [79] but given the magnitude of the increase in IL6 in the amniotic fluid, it is unlikely that the maternal circulation or the placenta is the primary source of IL6 in the amniotic fluid. This draws into question whether it is maternal leukocytes present in the amniotic fluid or fetal cells that are the source of these cytokines. Given that IFNG levels are high in the placenta and minimal to absent in the amniotic fluid, it is possible that priming and activation occurs within the placenta prior to leukocyte entry into the amniotic fluid. Another intriguing possibility is that nonimmune fetal cells might be the source of the fetal inflammatory response [80, 81]. The cells involved in the fetal inflammatory response to infection in the rat have not been fully characterized. Thus, there is a need to determine the specific cell types producing the cytokines in response to both nicotine and infection.
In summary, our study demonstrated that nicotine exposure at 6 mg/kg/day enhances the ability of the pathogen to cross the placental barrier, colonize the amniotic fluid, and gain access to the fetus. Additional studies are needed to further elucidate the specific mechanism(s) by which nicotine facilitates microbial invasion of the fetal compartment, as well as to determine if the responses observed are dose dependent. Most importantly, these data demonstrate that a modifiable risk factor (nicotine exposure) can directly impact what has been considered an immutable risk factor (infection) for perinatal complications. This study highlights the need for continued education of reproductive age women on the potential dangers of ENDS use as well as the need for in-depth epidemiological studies on pregnancy outcomes and risks associated with ENDS use.
Supplementary data
Supplementary data are available at BIOLRE online.
Supplemental Table S1. Median lesion severity scores and interquartile range for placental sites evaluated.
Supplemental Figure S1. Adverse pregnancy outcome in term dams. A. Dam euthanized at GD 25 after failure to deliver. Arrows indicate fetuses that were removed from uterus at necropsy. Fetuses were large and misshapen, with bruising and swelling of muzzle, abdomen, and/or hind limbs. Granulomatous inflammation was widespread in placental disks. B. Dam euthanized at GD 25 after normal delivery of 2 pups at GD 22. Two macerated fetuses were retained in the uterus and were coated with viscous material. Placentas appeared necrotic. C. Upon excision of the uterus below the cervix, purulent exudate oozed from the vaginal canal.
Supplementary data are available at BIOLRE online.
Supplemental Table S1. Median lesion severity scores and interquartile range for placental sites evaluated.
Supplemental Figure S1. Adverse pregnancy outcome in term dams. A. Dam euthanized at GD 25 after failure to deliver. Arrows indicate fetuses that were removed from uterus at necropsy. Fetuses were large and misshapen, with bruising and swelling of muzzle, abdomen, and/or hind limbs. Granulomatous inflammation was widespread in placental disks. B. Dam euthanized at GD 25 after normal delivery of 2 pups at GD 22. Two macerated fetuses were retained in the uterus and were coated with viscous material. Placentas appeared necrotic. C. Upon excision of the uterus below the cervix, purulent exudate oozed from the vaginal canal.
Conflict of interest: The authors have declared that no conflict of interest exists.
References
- 1. Harding R, Maritz G. Maternal and fetal origins of lung disease in adulthood. Semin Fetal Neonatal Med 2012; 17:67–72. [DOI] [PubMed] [Google Scholar]
- 2. Mund M, Louwen F, Klingelhoefer D, Gerber A. Smoking and pregnancy - a review on the first major environmental risk factor of the unborn. Int J Environ Res Public Health 2013; 10:6485–6499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Andres RL, Day MC. Perinatal complications associated with maternal tobacco use. Semin Neonatol 2000; 5:231–241. [DOI] [PubMed] [Google Scholar]
- 4. Hayashi K, Matsuda Y, Kawamichi Y, Shiozaki A, Saito S. Smoking during pregnancy increases risks of various obstetric complications: a case-cohort study of the Japan Perinatal Registry Network database. J Epidemiol 2011; 21:61–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McEvoy CT, Spindel ER. Pulmonary effects of maternal smoking on the fetus and child: Effects on lung development, respiratory morbidities, and life long lung health. Paediatr Respir Rev 2016; 21:27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jauniaux E, Burton GJ. Morphological and biological effects of maternal exposure to tobacco smoke on the feto-placental unit. Early Hum Dev 2007; 83:699–706. [DOI] [PubMed] [Google Scholar]
- 7. Chen M, Wang T, Liao ZX, Pan XL, Feng YH, Wang H. Nicotine-induced prenatal overexposure to maternal glucocorticoid and intrauterine growth retardation in rat. Exp Toxicol Pathol 2007; 59:245–251. [DOI] [PubMed] [Google Scholar]
- 8. Holloway AC, Salomon A, Soares MJ, Garnier V, Raha S, Sergent F, Nicholson CJ, Feige JJ, Benharouga M, Alfaidy N. Characterization of the adverse effects of nicotine on placental development: in vivo and in vitro studies. Am J Physiol Endocrinol Metab 2014; 306:E443–E456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Romani F, Lanzone A, Tropea A, Tiberi F, Catino S, Apa R. Nicotine and cotinine affect the release of vasoactive factors by trophoblast cells and human umbilical vein endothelial cells. Placenta 2011; 32:153–160. [DOI] [PubMed] [Google Scholar]
- 10. Coleman T, Chamberlain C, Davey MA, Cooper SE, Leonardi-Bee J. Pharmacological interventions for promoting smoking cessation during pregnancy. Cochrane Database Syst Rev 2015; 12:CD010078. [DOI] [PubMed] [Google Scholar]
- 11. Coleman-Cowger VH, Anderson BL, Mahoney J, Schulkin J. Smoking cessation during pregnancy and postpartum: practice patterns among obstetrician-gynecologists. J Addict Med 2014; 8:14–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Aubin HJ, Luquiens A, Berlin I. Pharmacotherapy for smoking cessation: pharmacological principles and clinical practice. Br J Clin Pharmacol 2014; 77:324–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brose LS, McEwen A, West R. Association between nicotine replacement therapy use in pregnancy and smoking cessation. Drug Alcohol Depend 2013; 132:660–664. [DOI] [PubMed] [Google Scholar]
- 14. Myung SK, Ju W, Jung HS, Park CH, Oh SW, Seo H, Kim H. Efficacy and safety of pharmacotherapy for smoking cessation among pregnant smokers: a meta-analysis. Bjog 2012; 119:1029–1039. [DOI] [PubMed] [Google Scholar]
- 15. Berlin I, Grange G, Jacob N, Tanguy ML. Nicotine patches in pregnant smokers: randomised, placebo controlled, multicentre trial of efficacy. BMJ 2014; 348:g1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Slotkin TA. If nicotine is a developmental neurotoxicant in animal studies, dare we recommend nicotine replacement therapy in pregnant women and adolescents? Neurotoxicol Teratol 2008; 30:1–19. [DOI] [PubMed] [Google Scholar]
- 17. Bullen C, McRobbie H, Thornley S, Glover M, Lin R, Laugesen M. Effect of an electronic nicotine delivery device (e cigarette) on desire to smoke and withdrawal, user preferences and nicotine delivery: randomised cross-over trial. Tob Control 2010; 19:98–103. [DOI] [PubMed] [Google Scholar]
- 18. Pokhrel P, Little MA, Fagan P, Kawamoto CT, Herzog TA. Correlates of use of electronic cigarettes versus nicotine replacement therapy for help with smoking cessation. Addict Behav 2014; 39:1869–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ayers JW, Ribisl KM, Brownstein JS. Tracking the rise in popularity of electronic nicotine delivery systems (electronic cigarettes) using search query surveillance. Am J Prev Med 2011; 40:448–453. [DOI] [PubMed] [Google Scholar]
- 20. Baeza-Loya S, Viswanath H, Carter A, Molfese DL, Velasquez KM, Baldwin PR, Thompson-Lake DG, Sharp C, Fowler JC, De La Garza R 2nd, Salas R. Perceptions about e-cigarette safety may lead to e-smoking during pregnancy. Bull Menninger Clin 2014; 78:243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Benowitz NL. Emerging nicotine delivery products: Implications for public health. Ann Am Thorac Soc 2014; 11:231–235. [DOI] [PubMed] [Google Scholar]
- 22. Choi K, Forster J. Characteristics associated with awareness, perceptions, and use of electronic nicotine delivery systems among young US Midwestern adults. Am J Public Health 2013; 103:556–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Choi K, Forster JL. Beliefs and experimentation with electronic cigarettes: A prospective analysis among young adults. Am J Prev Med 2014; 46:175–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ashford K, Wiggins A, Butler K, Ickes M, Rayens MK, Hahn E. E-cigarette use and perceived harm among women of childbearing age who reported tobacco use during the past year. Nurs Res 2016; 65:408–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sieminska A, Jassem E. The many faces of tobacco use among women. Med Sci Monit 2014; 20:153–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Goniewicz ML, Hajek P, McRobbie H. Nicotine content of electronic cigarettes, its release in vapour and its consistency across batches: regulatory implications. Addiction 2014; 109:500–507. [DOI] [PubMed] [Google Scholar]
- 27. Dawkins L, Corcoran O. Acute electronic cigarette use: nicotine delivery and subjective effects in regular users. Psychopharmacology (Berl). 2014; 231:401–407. [DOI] [PubMed] [Google Scholar]
- 28. Talih S, Balhas Z, Eissenberg T, Salman R, Karaoghlanian N, El Hellani A, Baalbaki R, Saliba N, Shihadeh A. Effects of user puff topography, device voltage, and liquid nicotine concentration on electronic cigarette nicotine yield: measurements and model predictions. Nicotine Tob Res 2015; 17:150–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shihadeh A, Eissenberg T. Electronic cigarette effectiveness and abuse liability: predicting and regulating nicotine flux. Nicotine Tob Res 2015; 17:158–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hajek P, Goniewicz ML, Phillips A, Myers Smith K, West O, McRobbie H. Nicotine intake from electronic cigarettes on initial use and after 4 weeks of regular use. Nicotine Tob Res 2015; 17:175–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ramoa CP, Hiler MM, Spindle TR, Lopez AA, Karaoghlanian N, Lipato T, Breland AB, Shihadeh A, Eissenberg T. Electronic cigarette nicotine delivery can exceed that of combustible cigarettes: a preliminary report. Tob Control 2016; 25:e6–e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Berger A, Witt A, Haiden N, Kretzer V, Heinze G, Kohlhauser C. Microbial invasion of the amniotic cavity at birth is associated with adverse short-term outcome of preterm infants. J Perinat Med 2003; 31:115–121. [DOI] [PubMed] [Google Scholar]
- 33. Goldenberg RL, Andrews WW, Faye-Petersen OM, Goepfert AR, Cliver SP, Hauth JC. The Alabama Preterm Birth Study: intrauterine infection and placental histologic findings in preterm births of males and females less than 32 weeks. Am J Obstet Gynecol 2006; 195:1533–1537. [DOI] [PubMed] [Google Scholar]
- 34. Goncalves LF, Chaiworapongsa T, Romero R. Intrauterine infection and prematurity. Ment Retard Dev Disabil Res Rev 2002; 8:3–13. [DOI] [PubMed] [Google Scholar]
- 35. Romero R, Espinoza J, Kusanovic JP, Gotsch F, Hassan S, Erez O, Chaiworapongsa T, Mazor M. The preterm parturition syndrome. Bjog 2006; 113(Suppl 3):17–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Institute of medicine (U.S.) committee on understanding premature birth and assuring healthy outcomes In: Behrman RE, Butler AS (eds.) Preterm Birth: Causes, Consequences, and Prevention. Washington, DC: National Academies Press (US); 2007:1–792. [PubMed] [Google Scholar]
- 37. Aliyu MH, Weldeselasse H, August EM, Keith LG, Salihu HM. Cigarette smoking and fetal morbidity outcomes in a large cohort of HIV-infected mothers. Nicotine Tob Res 2013; 15:177–184. [DOI] [PubMed] [Google Scholar]
- 38. Boychuk CR, Fuller DD, Hayward LF. Sex differences in heart rate variability during sleep following prenatal nicotine exposure in rat pups. Behav Brain Res 2011; 219:82–91. [DOI] [PubMed] [Google Scholar]
- 39. Boychuk CR, Hayward LF. Prenatal nicotine exposure alters postnatal cardiorespiratory integration in young male but not female rats. Exp Neurol 2011; 232:212–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fuller DD, Dougherty BJ, Sandhu MS, Doperalski NJ, Reynolds CR, Hayward LF. Prenatal nicotine exposure alters respiratory long-term facilitation in neonatal rats. Respir Physiol Neurobiol 2009; 169:333–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hussein J, Farkas S, MacKinnon Y, Ariano RE, Sitar DS, Hasan SU. Nicotine dose-concentration relationship and pregnancy outcomes in rat: biologic plausibility and implications for future research. Toxicol Appl Pharmacol 2007; 218:1–10. [DOI] [PubMed] [Google Scholar]
- 42. Brown MB, Peltier M, Hillier M, Crenshaw B, Reyes L. Genital mycoplasmosis in rats: a model for intrauterine infection. Am J Reprod Immunol 2001; 46:232–241. [DOI] [PubMed] [Google Scholar]
- 43. Brown MB, Steiner DA. Experimental genital mycoplasmosis: time of infection influences pregnancy outcome. Infect Immun 1996; 64:2315–2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Peltier MR, Richey LJ, Brown MB. Placental lesions caused by experimental infection of Sprague-Dawley rats with Mycoplasma pulmonis. Am J Reprod Immunol 2003; 50:254–262. [DOI] [PubMed] [Google Scholar]
- 45. Reyes L, Shelton M, Riggs M, Brown MB. Rat strains differ in susceptibility to maternal and fetal infection with Mycoplasma pulmonis. Am J Reprod Immunol 2004; 51:211–219. [DOI] [PubMed] [Google Scholar]
- 46. Riggs MA, Maunsell FP, Reyes L, Brown MB. Hematogenous infection of Sprague-Dawley rats with Mycoplasma pulmonis: development of a model for maternal and fetal infection. Am J Obstet Gynecol 2008; 198:318e311–e317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wickstrom R. Effects of nicotine during pregnancy: human and experimental evidence. Curr Neuropharmacol 2007; 5:213–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bublitz MH, Stroud LR. Maternal smoking during pregnancy and offspring brain structure and function: review and agenda for future research. Nicotine Tob Res 2012; 14:388–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cornelius MD, Day NL. Developmental consequences of prenatal tobacco exposure. Curr Opin Neurol 2009; 22:121–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Leslie FM. Multigenerational epigenetic effects of nicotine on lung function. BMC Med 2013; 11:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Maritz GS. Nicotine and lung development. Birth Defects Res C Embryo Today 2008; 84:45–53. [DOI] [PubMed] [Google Scholar]
- 52. Spindel ER, McEvoy CT. The role of nicotine in the effects of maternal smoking during pregnancy on lung development and childhood respiratory disease: Implications for dangers of e-cigarettes. Am J Respir Crit Care Med 2016; 193:486–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wong MK, Barra NG, Alfaidy N, Hardy DB, Holloway AC. Adverse effects of perinatal nicotine exposure on reproductive outcomes. Reproduction 2015; 150:R185–R193. [DOI] [PubMed] [Google Scholar]
- 54. Furukawa S, Kuroda Y, Sugiyama A. A comparison of the histological structure of the placenta in experimental animals. J Toxicol Pathol 2014; 27:11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Burd I, Balakrishnan B, Kannan S. Models of fetal brain injury, intrauterine inflammation, and preterm birth. Am J Reprod Immunol 2012; 67:287–294. [DOI] [PubMed] [Google Scholar]
- 56. Elovitz MA, Mrinalini C. Animal models of preterm birth. Trends Endocrinol Metab 2004; 15:479–487. [DOI] [PubMed] [Google Scholar]
- 57. Kemp MW, Saito M, Newnham JP, Nitsos I, Okamura K, Kallapur SG. Preterm birth, infection, and inflammation advances from the study of animal models. Reprod Sci 2010; 17:619–628. [DOI] [PubMed] [Google Scholar]
- 58. Soares MJ, Chakraborty D, Karim Rumi MA, Konno T, Renaud SJ. Rat placentation: an experimental model for investigating the hemochorial maternal-fetal interface. Placenta 2012; 33:233–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Burton A, Kizhner O, Brown MB, Peltier MR. Effect of experimental genital mycoplasmosis on gene expression in the fetal brain. J Reprod Immunol 2012; 93:9–16. [DOI] [PubMed] [Google Scholar]
- 60. Busch K, Naglic T. Natural uterine Mycoplasma pulmonis infection in female rats. Vet Med (Praha) 1995; 40:253–255. [PubMed] [Google Scholar]
- 61. Cox NR, Davidson MK, Davis JK, Lindsey JR, Cassell GH. Natural mycoplasmal infections in isolator-maintained LEW/Tru rats. Lab Anim Sci 1988; 38:381–388. [PubMed] [Google Scholar]
- 62. Viscardi RM. Ureaplasma species: role in neonatal morbidities and outcomes. Arch Dis Child Fetal Neonatal Ed 2014; 99:F87–F92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Capoccia R, Greub G, Baud D. Ureaplasma urealyticum, Mycoplasma hominis and adverse pregnancy outcomes. Curr Opin Infect Dis 2013; 26:231–240. [DOI] [PubMed] [Google Scholar]
- 64. DiGiulio DB. Diversity of microbes in amniotic fluid. Semin Fetal Neonatal Med 2012; 17:2–11. [DOI] [PubMed] [Google Scholar]
- 65. Waites KB, Schelonka RL, Xiao L, Grigsby PL, Novy MJ. Congenital and opportunistic infections: Ureaplasma species and Mycoplasma hominis. Semin Fetal Neonatal Med 2009; 14:190–199. [DOI] [PubMed] [Google Scholar]
- 66. Peltier MR, Brown MB. Experimental genital mycoplasmosis causes increased levels of mRNA for IL-6 and TNF-alpha in the placenta. Am J Reprod Immunol 2005; 53:189–198. [DOI] [PubMed] [Google Scholar]
- 67. Elovitz MA, Mrinalini C, Sammel MD. Elucidating the early signal transduction pathways leading to fetal brain injury in preterm birth. Pediatr Res 2006; 59:50–55. [DOI] [PubMed] [Google Scholar]
- 68. Li L, Shi L, Yang X, Ren L, Yang J, Lin Y. Role of invariant natural killer T cells in lipopolysaccharide-induced pregnancy loss. Cell Immunol 2013; 286:1–10. [DOI] [PubMed] [Google Scholar]
- 69. Murphy SP, Fast LD, Hanna NN, Sharma S. Uterine NK cells mediate inflammation-induced fetal demise in IL-10-null mice. J Immunol 2005; 175:4084–4090. [DOI] [PubMed] [Google Scholar]
- 70. Robertson SA, Skinner RJ, Care AS. Essential role for IL-10 in resistance to lipopolysaccharide-induced preterm labor in mice. J Immunol 2006; 177:4888–4896. [DOI] [PubMed] [Google Scholar]
- 71. Brown MB, von Chamier M, Allam AB, Reyes L. M1/M2 macrophage polarity in normal and complicated pregnancy. Front Immunol 2014; 5:606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Svensson-Arvelund J, Ernerudh J. The role of macrophages in promoting and maintaining homeostasis at the fetal-maternal interface. Am J Reprod Immunol 2015; 74:100–109. [DOI] [PubMed] [Google Scholar]
- 73. Svensson-Arvelund J, Ernerudh J, Buse E, Cline JM, Haeger JD, Dixon D, Markert UR, Pfarrer C, De Vos P, Faas MM. The placenta in toxicology. Part II: Systemic and local immune adaptations in pregnancy. Toxicol Pathol 2014; 42:327–338. [DOI] [PubMed] [Google Scholar]
- 74. Svensson-Arvelund J, Mehta RB, Lindau R, Mirrasekhian E, Rodriguez-Martinez H, Berg G, Lash GE, Jenmalm MC, Ernerudh J. The human fetal placenta promotes tolerance against the semiallogeneic fetus by inducing regulatory T cells and homeostatic M2 macrophages. J Immunol 2015; 194:1534–1544. [DOI] [PubMed] [Google Scholar]
- 75. Kunze M, Klar M, Morfeld CA, Thorns B, Schild RL, Markfeld-Erol F, Rasenack R, Proempeler H, Hentschel R, Schaefer WR. Cytokines in noninvasively obtained amniotic fluid as predictors of fetal inflammatory response syndrome. Am J Obstet Gynecol 2016; 215:96e91–e98. [DOI] [PubMed] [Google Scholar]
- 76. Romero R, Chaemsaithong P, Korzeniewski SJ, Tarca AL, Bhatti G, Xu Z, Kusanovic JP, Dong Z, Docheva N, Martinez-Varea A, Yoon BH, Hassan SS et al. Clinical chorioamnionitis at term II: the intra-amniotic inflammatory response. J Perinat Med 2016; 44:5–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Shobokshi A, Shaarawy M. Maternal serum and amniotic fluid cytokines in patients with preterm premature rupture of membranes with and without intrauterine infection. Int J Gynaecol Obstet 2002; 79:209–215. [DOI] [PubMed] [Google Scholar]
- 78. Kobayashi K, Umezawa K, Yasui M. Apoptosis in mouse amniotic epithelium is induced by activated macrophages through the TNF receptor type 1/TNF pathway. Biol Reprod 2011; 84:248–254. [DOI] [PubMed] [Google Scholar]
- 79. Dahlgren J, Samuelsson AM, Jansson T, Holmang A. Interleukin-6 in the maternal circulation reaches the rat fetus in mid-gestation. Pediatr Res 2006; 60:147–151. [DOI] [PubMed] [Google Scholar]
- 80. D’Alquen D, Kramer BW, Seidenspinner S, Marx A, Berg D, Groneck P, Speer CP. Activation of umbilical cord endothelial cells and fetal inflammatory response in preterm infants with chorioamnionitis and funisitis. Pediatr Res 2005; 57:263–269. [DOI] [PubMed] [Google Scholar]
- 81. Kemp MW, Molloy TJ, Usuda H, Woodward E, Miura Y, Payne MS, Ireland DJ, Jobe AH, Kallapur SG, Stock SJ, Spiller OB, Newnham JP et al. Outside-in? Acute fetal systemic inflammation in very preterm chronically catheterized sheep fetuses is not driven by cells in the fetal blood. Am J Obstet Gynecol 2016; 214:281 e281-281 e210. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data are available at BIOLRE online.
Supplemental Table S1. Median lesion severity scores and interquartile range for placental sites evaluated.
Supplemental Figure S1. Adverse pregnancy outcome in term dams. A. Dam euthanized at GD 25 after failure to deliver. Arrows indicate fetuses that were removed from uterus at necropsy. Fetuses were large and misshapen, with bruising and swelling of muzzle, abdomen, and/or hind limbs. Granulomatous inflammation was widespread in placental disks. B. Dam euthanized at GD 25 after normal delivery of 2 pups at GD 22. Two macerated fetuses were retained in the uterus and were coated with viscous material. Placentas appeared necrotic. C. Upon excision of the uterus below the cervix, purulent exudate oozed from the vaginal canal.