Abstract
Background
Many interventions for the treatment of low back pain exist, but the mechanisms through which such treatments work are not always clear. This situation is especially true for biopsychosocial interventions that incorporate several different components and methods of delivery.
Objective
The study objective was to examine the indirect effects of the Cognitive Patient Education (COPE) intervention via illness perceptions, back pain myths, and pain catastrophizing on disability outcome.
Design
This study was a secondary analysis of the COPE randomized controlled trial.
Methods
Mediation analysis techniques were employed to examine the indirect effects of the COPE intervention via residualized change (baseline – posttreatment) in the 3 variables hypothesized to be targeted by the COPE intervention on posttreatment disability outcome. Pain intensity at baseline, pain duration, clinician type, and a treatment-mediator interaction term were controlled for in the analysis.
Results
Preliminary analyses confirmed that changes in pain catastrophizing and illness perceptions (not back pain myths) were related to both allocation to the intervention arm and posttreatment disability score. The treatment exerted statistically significant indirect effects via changes in illness perceptions and pain catastrophizing on posttreatment disability score (illness perceptions standardized indirect effect = 0.09 [95% CI = 0.03 to 0.16]; pain catastrophizing standardized indirect effect = 0.05 [95% CI = 0.01 to 0.12]). However, the inclusion of an interaction term led to the indirect effects being significantly reduced, with the effects no longer being statistically significant.
Limitations
This study presents a secondary analysis of variables not identified a priori as being potentially important treatment targets; other, unmeasured factors could also be important in explaining treatment effects.
Conclusions
The finding that small indirect effects of the COPE intervention via changes in illness perceptions and pain catastrophizing on posttreatment disability could be estimated indicates that these variables may be viable treatment targets for biopsychosocial interventions; however, this finding must be viewed in light of the adjusted analyses, which showed that the indirect effects were significantly reduced through the inclusion of a treatment-mediator interaction term.
Interventions to improve pain and disability in patients with low back pain (LBP) are numerous, including physical therapy, medications and psychological interventions. A recent approach developed in Australia, Explain Pain,1,2 involves teaching patients about the biological processes underpinning why they are (still) experiencing pain.2 It is defined as an educational intervention underpinned by a biopsychosocial approach to pain, where physical therapists or clinicians aim to build a strong rapport with patients and encourage them to ask questions to improve their understanding.2 Current evidence suggests that Explain Pain is effective, but that heterogeneity in intervention delivery and the small size of the randomized controlled trials currently available means that more evidence is needed to build a case for the efficacy of this intervention.2
The Cognitive Patient Education (COPE) trial3,4 aimed to investigate the effectiveness of the Explain Pain model in a Norwegian primary care population, utilizing both general practitioners and physical therapists in delivering the intervention. The trial results indicated that while a substantial decrease in functional disability occurred in both groups between baseline and posttreatment, there was no statistically significant difference between treatment and control and only a small effect size (−0.06) for the intervention.4 Further analysis of the secondary outcomes of the trial (quality of life, pain catastrophizing and illness perceptions) was carried out, and illness perceptions were found to change faster in the intervention group, but no differences between the groups were found for pain catastrophizing or quality of life measures.5
Mediation analysis, a technique used to help explain how an intervention achieved its effects,6 can also be used to help explain why an intervention was not successful.7 As there is no effect to be mediated in such situations, the term “indirect effects” is used rather than mediated effects8 to examine the effect exerted by the intervention via the potential mediating factor. Such information is important in identifying where interventions can perhaps be improved in the future,9 leading to more successful interventions that have a greater impact on people's outcomes. Formal analysis of indirect effects was not carried out as part of the analysis of secondary outcomes by Løchting et al,5 so is warranted here.
The aim of this secondary analysis was therefore to explore why the intervention did not significantly improve disability outcomes over the control intervention by using mediation analysis to examine the indirect effect of the COPE intervention via change in catastrophizing, back pain myths and illness perceptions on posttreatment disability score. This differs from Løchting et al's5 analysis in that rather than looking for between group differences in illness perceptions, pain catastrophizing and myths, we investigated whether change in these variables was important in how treatment (did not) affect the outcome of disability.
Methods
The COPE Trial
The intervention was a cluster randomized controlled trial carried out between 2009 and 2012 and involved 16 general practitioners and 20 physical therapists who were randomly assigned to carry out either the COPE intervention or usual care (control). People were recruited consecutively (110 to the intervention arm; 106 to the control arm) and those allocated to the intervention arm went on to receive a protocol of education about pain physiology, beliefs about back pain and the impact of external factors such as the environment on the pain experience. Four one-to-one, 30-minute sessions were carried out over 4 consecutive weeks, which discussed the participant's thoughts and fears about their back pain, dealing with exposure to movement and daily activities, and identifying barriers to normal functioning and the reasons behind any activity avoidance. Those in the control group also met weekly with a health care professional but received usual care, which was also available to those in the intervention group.
Mediation Analysis
Mediation analysis techniques were employed to carry out this analysis. In mediation analysis, effects can be broken down into separate paths: the c path between the treatment and outcome (without accounting for potential mediators), the a path between the intervention and the potential mediator; and the b path between the potential mediator and the outcome. The mediating (indirect) pathway is calculated as the product of paths a and b (ab).
Action Theory and Conceptual Theory
Models of indirect effects can be interpreted by breaking down the a and b paths.10,11 The action theory (a path) can be described as the intervention's ability to impact on the mediator, while conceptual theory (b path) is the mediator's ability to impact on the outcome. A strong a path would suggest that the intervention is targeting the mediator well, while a strong b path suggests that the mediator is the correct factor to be targeting in order to improve the outcome. Conversely, a weak a path would suggest that the intervention is not targeting the potential mediator strongly enough, or that the intervention itself is not effective, while a weak b path would suggest that the wrong factor is being targeted for change.11 The importance of this conceptualization lies in how it can generate discussion around how an intervention can target factors key to participant improvement (action theory) and how these proposed mediators may affect the outcome (conceptual theory).12
Theoretical Rationale for the COPE Trial
The intervention implemented in the COPE trial was described as a cognitive-based education program which aimed to improve the accuracy of knowledge of the mechanisms of pain biology.3 Health care professionals involved in the intervention arm of the study were trained in the Explain Pain treatment approach and instructed to follow a treatment manual.4 During the 4-week intervention, clinicians taught participants about how pain perceptions can be influenced by previous experience and back pain beliefs; about back pain physiology; how persistent pain may be influenced by the participants’ environment; and discussed with the participants how what they have learned from the intervention can be implemented in their daily life.4 Overall, the intervention aimed to address participant's fears about their LBP and to expose participants to normal functioning.3
Illness perceptions, or people's beliefs about their condition, can have a direct impact on emotional responses and behavior13 and poor knowledge about their condition can impact on those beliefs.13 Similarly, understanding of myths about back pain would have been addressed in that attitudes and beliefs about back pain would have been explored in this intervention and are likely to have changed had the intervention been successful, but this variable was not explored by Løchting et al.14
As the focus of the intervention overall was meant to increase the accuracy of people's knowledge about LBP, it was assumed that illness perceptions and back pain myths would be useful variables to examine as potential treatment targets. Another key aspect of the intervention was to address people's thoughts and fears about their LBP; pain catastrophizing was therefore also included as a potentially important factor. Pain catastrophizing rather than fear-avoidance was included as the intervention was based on a cognitive model, addressing participant-perceived threatening inputs to the brain,3 rather than the behavior of fear-avoidance. Pain catastrophizing has been found previously to be a mediator of treatment effect on disability outcome in randomized controlled trials for musculoskeletal pain populations,15 although methodological and design flaws precluded strong conclusions being drawn for any potential mediators. This variable was not found to change in the Løchting analysis, but as this analysis did not specifically analyze indirect effects, pain catastrophizing was included in the present analysis.
Measures: Potential Mediators
All measures were recorded using self-reported questionnaires. Data was collected at baseline, posttreatment, and 3-month and 12-month follow-up, although in the present analysis only the posttreatment follow-up point was used.
The Back Pain Myths Questionnaire16 consists of 12 items where respondents were asked whether they agreed or disagreed with each item. The number of “correct” responses are summed to give a total score. 11 items were used in the current analysis (a final item, “My view on how to manage back pain has changed in the last year” was not deemed relevant to the current analysis). A higher score indicated that more correct responses had been made, suggesting that the participant exhibited better, more factual beliefs about back pain. No information is available on the psychometric properties of this questionnaire, and the internal consistency was found to be poor within the present population (Cronbach alpha = 0.52).
The Norwegian version17 of the Pain Catastrophising Questionnaire18 was used to measure pain catastrophizing. This measure has 13 items and a score range of 0–52, with a higher score indicating a higher level of pain catastrophizing. This measure has been found to have sound psychometric properties in pain populations.19
The Brief Illness Perceptions Questionnaire20 (Brief IPQ) consists of 9 items formed from each of the illness perception subscales (identity, consequences, cause, timeline, cure/control, emotional representation, and illness comprehension). Scores range from 0 to 100, with a higher score indicating strong perceptions on a particular dimension. The psychometric properties of the Norwegian version of this measure have been explored and it has been found to have good test-retest reliability, moderate concurrent validity and good discriminant validity.14
Measures: Outcome
The Roland-Morris Disability Questionnaire (RMDQ)21 was used to measure functional low back pain-related disability. Scores range from 0 to 24, with a higher score indicating higher functional disability. This measure has been found to have good psychometric properties within this population.22,23
Potential Confounding Factors
In studies of mediating factors, there are other variables that could potentially have an impact on any mediating effects seen (confounding factors). Pain duration, pain intensity and whether participants were treated by a general practitioner or physical therapist were considered to potentially have an impact on the indirect effects of the variables being tested, and therefore were also included in the analysis of indirect effects. As the study was randomized, this adjustment was not necessary to make to the a path and therefore made to the b (mediator-outcome) path only. Finally, a treatment-mediator interaction term was also included in the analysis, as indirect effects may vary for different levels of treatment. This addition to mediation models is recommended as part of more recent developments of mediation analysis techniques.24 However, as the inclusion of interaction terms is a new development in mediation analysis, we present models both adjusted and unadjusted for this particular covariate.
Data Analysis
Descriptive analyses were carried out to examine differences between each of the variables of interest both over time and between the intervention arms. Potential mediators were represented by residualized change scores and the posttreatment score for the outcome measure was used. Residualized change scores represent the difference between the actual score at follow-up compared to what was predicted at baseline.25 This differs from raw change as it allows the confounding effect of baseline score to be controlled for. Residualized change scores have been used previously in mediation studies,26,27 and are obtained from the residual values of linear regression analysis where the follow-up score is entered as the outcome and baseline score as the predictor. Posttreatment scores for the outcome measure were used. This was to try and account for temporality in the model, which is an important aspect of causality and helps give strength to any indirect effects found.28,29 Next, linear regression analyses were performed to examine the relationships between treatment allocation and change in each of the potential mediators, and between change in each of the potential mediators and the outcome posttreatment score. This provided tests of the a and b paths separately. Finally, structural equation modelling was used to test for indirect effects (ab) of each of the potential mediators on the direct effect between treatment allocation and outcome. Linear regression and structural equation modelling require a number of assumptions to be met in order for the results of the analyses to be generalizable. Therefore, when the linear regression analyses were performed, information related to linearity and normal distribution were also obtained, with the variables included in the analyses being found to meet these assumptions.
There are also a number of assumptions relating to mediation analysis specifically. For example, the mediating pathways tested assume that there are no other unmeasured variables also on that pathway, which could be confounding any mediating effects.
Indirect effects were reported with bias-corrected bootstrapped 95% CIs, which account for the non-normal distribution of the indirect effect.8,30 One thousand bootstrap replications were used in these analyses. Indices to evaluate how well the model fits the data are also given in structural equation models, with a range of indices recommended to judge goodness of fit.31,32 In the present analysis, chi-square (x2), chi-square divided by degrees of freedom (x2/df), Comparative Fit Index (CFI), root-mean-square error of approximation (RMSEA), and standardized root-mean-square residual (SRMR) were examined. Good or adequate fit is indicated by a nonsignificant x2 value, a x2/df of between 2 and 5, a CFI of 0.96 or higher, and RMSEA and SRMR of 0.08 or lower.33,34
All analyses were conducted using SPSS PASW statistics package version 18 (IBM SPSS, Chicago, Illinois) and AMOS (add-on package for SPSS) version 19.
Role of the Funding Source
The Norwegian Research Council (ref. no. 214066) and the Norwegian Medical Association's foundation (09/1612) provided funds in support of this work. Neither of them took any part in building the design, in the conduct, or in the reporting of the study. G. Mansell is supported by National Institute for Health Research (NIHR) School for Primary Care Research Seedcorn funding. This paper presents independent research funded by the NIHR School for Primary Care Research. The views expressed in this publication are those of the authors and not necessarily those of the NIHR, the National Health Service (NHS) (UK), or the Department of Health.
Results
Examination of the mean scores of the outcome and potential mediator variables at baseline and each follow-up point, split by intervention group, are shown in Table 1. Some differences are present between pain catastrophizing and illness perception scores at baseline, with higher scores on both variables in the intervention arm, but similar decreases in scores over time are shown in disability, pain catastrophizing and illness perceptions across both treatment and control arms. Back pain myths appeared to stay relative stable over time, with little change in score. A higher proportion of those in the control arm were treated by a physical therapist compared to the treatment arm. A higher percentage of people who reported shorter pain duration were allocated to the treatment arm while a higher percentage of those reporting longer-term pain were allocated to the control arm. Pain intensity scores were similar across treatment and control groups.
Table 1.
Scores at Baseline, Posttreatment, and 12-Month Follow-up for Outcome and Potential Mediator Variables.a
| Variable | Baseline Score (n = 216) | Posttreatment Score (n = 216) | 12-mo Follow-up Score (n = 216) | |||
|---|---|---|---|---|---|---|
| Treatment (n = 110) | Control (n = 106) | Treatment (n = 110) | Control (n = 106) | Treatment (n = 110) | Control (n = 106) | |
| Outcomes | ||||||
| Disability (RMDQ) | 9.02 (3.91) | 9.66 (4.06) | 4.83 (3.98) | 5.46 (4.59) | 4.21 (4.09) | 3.21 (3.27) |
| Potential mediators | ||||||
| Pain catastrophizing | 17.97 (9.01) | 14.35 (8.72) | 11.63 (8.43) | 11.38 (8.66) | 11.49 (9.02) | 10.51 (8.73) |
| Illness perceptions | 44.50 (10.32) | 40.43 (9.29) | 33.42 (14.12) | 34.23 (12.49) | 32.40 (14.51) | 30.75 (14.09) |
| Back pain myths (no. correct) | 4.11 (1.93) | 3.81 (2.07) | 4.67 (2.32) | 3.97 (2.17) | 4.49 (2.03) | 3.89 (2.12) |
| Potential confounders | ||||||
| Treating HCP (general practitioner or physical therapist) | 60% physical therapist | 76% physical therapist | ||||
| Pain durationb | ||||||
| Acute | 60 (54.5) | 46 (43.4) | ||||
| Subacute | 21 (19.1) | 23 (21.7) | ||||
| Chronic | 29 (26.4) | 37 (34.9) | ||||
| Pain intensity | 5.23 (2.00) | 5.47 (1.73) | 3.21 (2.20) | 3.15 (2.15) | 3.13 (2.40) | 2.84 (2.12) |
aValues are reported as mean (SD) unless otherwise indicated. HCP = health care professional.
bReported as number (percentage) of participants.
Table 2 shows the differences between the treating health care professional (general practitioner or physical therapist) in terms of the scores participants received on the outcome and potential mediator variables. Those receiving physical therapy had lower baseline disability and pain catastrophizing scores than those receiving care from a general practitioner, and a larger improvement over time in disability and pain catastrophizing scores was observed in those under physical therapist care. Scores on illness perceptions, back pain myths and pain intensity were similar for both health care professional groups. Physical therapists had a much higher proportion of patients reporting long-term pain duration in the control group compared to general practitioners. Relationships between treatment allocation and change in each of the potential mediators were then examined. The results in Table 3 indicate that a very small proportion of variance was explained by each of the potential mediators (between 2% and 4%). The beta values indicate that change in illness perceptions and pain catastrophizing have the strongest association with treatment allocation (ie, allocation to the intervention arm was associated with a larger change in these 2 variables), and the 95% CIs suggest these associations are statistically significant. Change in back pain myths was also statistically significantly associated with treatment allocation, but this relationship was weaker than those found for change in illness perceptions and pain catastrophizing.
Table 2.
Scores at Baseline, Posttreatment, and 12-Month Follow-up for Outcome and Potential Mediator Variables, Split by Treating Health Care Professional.a
| Variable | Baseline Score (n = 216) | Posttreatment Score (n = 216) | 12-mo Follow-up Score (n = 216) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Physical therapist | General Practitioner | Physical therapist | General Practitioner | Physical therapist | General Practitioner | |||||||
| Treatment (n = 66) | Control (n = 81) | Treatment (n = 44) | Control (n = 25) | Treatment (n = 66) | Control (n = 81) | Treatment (n = 44) | Control (n = 25) | Treatment (n = 66) | Control (n = 81) | Treatment (n = 44) | Control (n = 25) | |
| Outcome | ||||||||||||
| Disability (RMDQ) | 8.38 (3.68) | 9.49 (3.71) | 9.98 (4.09) | 10.20 (5.11) | 4.09 (3.32) | 5.04 (4.58) | 5.93 (4.64) | 6.84 (4.44) | 3.30 (3.44) | 2.91 (2.90) | 5.57 (4.61) | 4.16 (4.19) |
| Potential mediators | ||||||||||||
| Pain catastrophizing | 17.08 (8.67) | 13.56 (8.48) | 19.32 (9.45) | 16.92 (9.16) | 9.92 (7.27) | 10.86 (8.28) | 14.20 (9.44) | 13.04 (9.77) | 9.70 (8.43) | 9.58 (8.30) | 14.18 (9.29) | 13.52 (9.55) |
| Illness perceptions | 42.71 (9.67) | 39.49 (9.87) | 47.18 (10.78) | 43.48 (6.32) | 30.86 (12.76) | 33.09 (12.76) | 37.25 (15.31) | 37.92 (11.03) | 29.61 (12.13) | 29.89 (14.57) | 36.59 (16.76) | 33.56 (12.22) |
| Back pain myths (no. correct) | 4.42 (2.02) | 4.11 (2.15) | 3.64 (1.70) | 2.84 (1.40) | 5.14 (2.47) | 4.37 (2.22) | 3.98 (1.91) | 2.68 (1.35) | 4.58 (2.03) | 4.17 (2.16) | 4.36 (2.05) | 2.96 (1.74) |
| Potential confounders | ||||||||||||
| Pain durationb | ||||||||||||
| Acute | 34 (51.5) | 32 (39.5) | 26 (59.1) | 14 (56) | ||||||||
| Subacute | 16 (24.2) | 16 (19.5) | 5 (11.4) | 7 (28.0) | ||||||||
| Chronic | 16 (24.2) | 33 (40.7) | 13 (29.5) | 4 (16.0) | ||||||||
| Pain intensity | 5.03 (1.77) | 5.38 (1.62) | 5.52 (2.30) | 5.76 (2.07) | 3.00 (1.99) | 3.21 (2.26) | 3.52 (2.46) | 2.96 (1.81) | 2.58 (1.99) | 2.56 (1.90) | 3.95 (2.73) | 3.80 (2.55) |
aValues are reported as mean (SD) unless otherwise indicated.
bReported as number (percentage) of participants.
Table 3.
Linear Regression Analysis of Univariable Associations of Change in Treatment Allocation With Residualized Change in Each Potential Mediator (a Path).
| Outcome (Potential Mediator) | Predictor | Unstandardized B (SE) | 95% CI | Standardized b | r 2 Change |
|---|---|---|---|---|---|
| Change in illness perceptions | Treatment allocation | 0.41 (0.13) | 0.14 to 0.67 | 0.20 | .04 |
| Change in pain catastrophizing | Treatment allocation | 0.33 (0.13) | 0.06 to 0.59 | 0.17 | .03 |
| Change in back pain myths | Treatment allocation | −0.27 (0.14) | −0.54 to −0.01 | −0.14 | .02 |
When relationships between change in each of the potential mediators and posttreatment RMDQ score were examined (b path of the mediation model), most variance was explained by change in illness perceptions in both treatment and control groups (Tab. 4). More variance was accounted for in the control group than in the intervention group for change in pain catastrophizing, and very little variance was explained by back pain myths. The associations between change in back pain myths and posttreatment RMDQ score in the treatment groups was also not statistically significant.
Table 4.
Linear Regression Analysis of Univariable Associations of Change Between Each Potential Mediator and Functional Outcome Score Posttreatment (b Path).a
| Outcome | Predictor (Potential Mediator) | Treatment Allocation | Unstandardized B (SE) | 95% CI | Standardized b | r 2 Change |
|---|---|---|---|---|---|---|
| RMDQ | Change in illness perceptions | Treatment | 2.07 (0.32) | 1.44 to 2.70 | 0.53 | .28 |
| RMDQ | Change in pain catastrophizing | Treatment | 1.11 (0.39) | 0.35 to 1.87 | 0.27 | .07 |
| RMDQ | Change in back pain myths | Treatment | –0.63 (0.36) | −1.31 to 0.10 | −0.16 | .03 |
| RMDQ | Change in illness perceptions | Control | 2.19 (0.43) | 1.33 to 3.05 | 0.44 | .20 |
| RMDQ | Change in pain catastrophizing | Control | 1.86 (0.41) | 1.06 to 2.67 | 0.41 | .17 |
| RMDQ | Change in back pain myths | Control | –1.02 (0.49) | −1.98 to −0.05 | −0.20 | .04 |
aRMDQ = Roland-Morris Disability Questionnaire.
At this stage, change in back pain myths was removed from the analysis as it showed very little change between baseline and posttreatment and only weak associations with either treatment allocation or posttreatment RMDQ score. Total, direct and indirect effects for the COPE intervention via change in illness perceptions and change in pain catastrophizing only were therefore calculated, adjusting for baseline scores in pain duration and pain intensity.
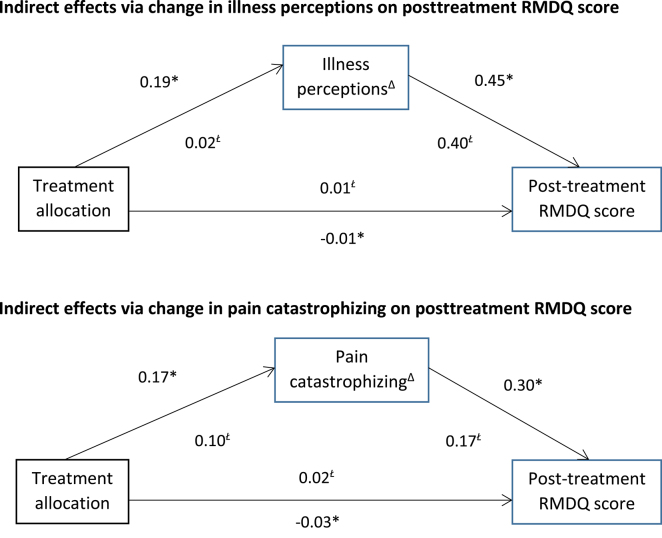
The Figure shows the direct effect of each of the variables on the other. Coefficients on the b path are stronger for both models, suggesting a strong association between change in each potential mediator and posttreatment RMDQ score. Both models were found to have a small indirect effect on posttreatment RMDQ score, which was statistically significant for both models (Tab. 5). Both models were found to have adequate fit to the data (change in illness perceptions model: x2 = 15.03, df = 6, P = .02, x2/df = 2.55, CFI = 0.90, RMSEA = 0.09, SRMR = 0.06; change in pain catastrophizing model: x2 = 15.30, df = 6, P = .02, x2/df = 2.55, CFI = 0.98, RMSEA = 0.09, SRMR = 0.06). However, when the interaction term was included in the model, the indirect effects were reduced (Tab. 5) and were no longer statistically significant. The models also fit the data less well when the term was included (change in illness perceptions model: x2 = 37.41, df = 10, P < .01, x2/df = 3.74, CFI = 0.95, RMSEA = 0.11, SRMR = 0.10; change in pain catastrophizing model: x2 = 37.40, df = 10, P < .01, x2/df = 3.74, CFI = 0.80, RMSEA = 0.11, SRMR = 0.09).
Table 5.
Total, Direct, and Indirect Effects of the COPE Intervention Via Change in Illness Perceptions and Change in Pain Catastrophizing on Posttreatment Disability Score.a
| Mediator | Effect | Modelb Without Interaction Term | Modelb With Interaction Term Added | ||
|---|---|---|---|---|---|
| Standardized Estimate (95% CI) | Unstandardized Estimate (95% CI) | Standardized Estimate (95% CI) | Unstandardized Estimate (95% CI) | ||
| Change in illness perceptions | Total (c΄) | 0.08 (−0.05 to 0.19) | 0.68 (−0.43 to 1.68) | −0.00 (−0.13 to 0.11) | −0.03 (−1.06 to 0.90) |
| Direct (c) | −0.01 (−0.13 to 0.10) | −0.08 (−1.08 to 0.89) | −0.01 (−0.13 to 0.11) | −0.08 (−1.18 to 0.89) | |
| Indirect (ab) | 0.09 (0.03 to 0.16) | 0.76 (0.29 to 1.35) | 0.01 (−0.01 to 0.03) | 0.05 (−0.07 to 0.26) | |
| Change in pain catastrophizing | Total (c΄) | 0.08 (−0.05 to 0.19) | 0.68 (−0.43 to 1.68) | −0.00 (−0.13 to 0.11) | −0.03 (−1.06 to 0.90) |
| Direct (c) | −0.03 (−0.11 to 1.14) | −0.23 (−0.92 to 1.21) | −0.02 (−0.14 to 0.09) | −0.16 (−1.21 to 0.78) | |
| Indirect (ab) | 0.05 (0.01 to 0.12) | 0.45 (0.12 to 1.10) | 0.02 (−0.00 to 0.06) | 0.14 (−0.01 to 0.50) | |
aCOPE = Cognitive Patient Education, RMDQ = Roland-Morris Disability Questionnaire.
bAdjusted for pain intensity, pain duration, and clinician type.
Figure.
Indirect effects of the Cognitive Patient Education (COPE) intervention. All estimates are standardized. Δ = change, RMDQ = Roland-Morris Disability Questionnaire. * = adjusted for baseline pain duration, pain intensity, and clinician type; Ł = additionally adjusted for treatment–mediator interaction term.
Discussion
The aim of this study was to investigate the indirect effects of change in illness perceptions, back pain myths and pain catastrophizing on the effect of an Explain Pain treatment intervention on posttreatment functional outcome. The treatment exerted its strongest indirect effect via change in illness perceptions, suggesting that this variable in particular might be an important future treatment target. Action and conceptual theory would interpret these findings as showing support for illness perceptions being an important treatment target theoretically, but that the intervention did not address these strongly enough to bring about change in the primary outcome of disability reduction. However, the addition of an interaction term to the analyses resulted in the indirect effects being reduced and no longer being statistically significant, suggesting that the effects of change in both illness perceptions and change in pain catastrophizing did indeed vary for different levels of treatment.
Comparison With Existing Literature
Little research has been conducted into mediators of treatment effect, especially in the context of analyzing why an intervention did not work. A recent systematic review15 which looked at psychological treatment mediators in psychological intervention studies of patients with musculoskeletal pain did find mediating effects of pain catastrophizing, but the studies included were reported to have a number of flaws in terms of the method of analysis used and that often potential confounders were not adjusted for. The present analysis includes a robust analysis using recommended analysis techniques. A recent example of the use of mediation analysis to investigate an unsuccessful intervention34 also did not find pain catastrophizing to have strong associations with treatment, suggesting that this factor is perhaps difficult to target effectively during treatment.
Evaluations of Explain Pain interventions specifically have been previously carried out. Lee et al35 conducted mediation analysis on audit data of the Explain Pain intervention, investigating the mediating effect of the intervention (specifically biology knowledge accuracy) via change in pain catastrophizing on subsequent improvement in pain and function, adjusting for pain duration, diagnosis, patient outcome expectations, and clinician perceptions. Change in pain catastrophizing was not found to be a mediator in their study in the adjusted analysis, and while a statistically significant indirect effect of the COPE intervention via change in pain catastrophizing was found in the present analysis, this was not as strong an effect as via change in illness perceptions.
The finding that changing illness perceptions may perhaps be more important in the effectiveness of the COPE analysis is a novel finding within Explain Pain interventions and worthy of further exploration. Few interventions targeting illness perceptions in chronic low back pain populations have been conducted; 1 study which did base their intervention on illness perceptions36 found change in 4 of the dimensions (time line cyclical, consequences, personal control and coherence), suggesting that directly targeting such factors may be amenable to change; however, few between-group differences were found in this study, which could indicate that changing illness perceptions may not necessarily lead to an improvement in outcome compared to control and that in fact other variables are responsible for the improvement seen. In the present analysis of the COPE study, the opposite conclusion could be drawn; the b path (conceptual theory) suggests that illness perceptions are the correct factor to target in leading to change in outcome, while the weak associations on the a path (action theory) suggests that illness perceptions are not being well-targeted by the intervention, which is a possible explanation for why the intervention overall was unsuccessful at reducing disability. The present findings are in line with Løchting et al's5 analysis of illness perceptions and pain catastrophizing in the COPE study, with only illness perceptions being found to be potentially important. Clearly further research is needed into illness perceptions as a treatment target.
Limitations
The analysis of indirect effects was conducted post hoc and not planned prior to the intervention being conducted, which is against current recommendations for analyzing how a treatment may work. Similarly, potential confounders of the indirect effect pathways were chosen post hoc rather than identified prior to the study commencing. The analysis of indirect effects using the product of coefficients method, a regression-based approach, does require the assumption of no missing confounders. In the present study, we identified key variables we thought could potentially be confounders, but we acknowledge that there may be a number of unmeasured and unknown confounders that were not included in this analysis. This also limits comparison with other studies such as Lee et al.34 A further issue is that the variables chosen to be investigated as potential mediators, while investigated here separately, may be interrelated with 1 variable having a causal impact on the other along the mediating pathway. However, in the COPE trial (as with many intervention studies) measures were only taken at pre- and posttreatment, not during treatment, meaning that relationships between potential mediators could not be investigated. Future mediation analyses should always be planned as part of trial development, so that factors thought to be important in explaining treatment effect, and any potential confounders of the mediating pathway, can be measured appropriately for inclusion in analysis of indirect effects. Such factors will differ depending on what the intervention is trying to target, the population involved, and who delivers the intervention, so it is very important that the trial team discuss in detail how they think the intervention will impact on the outcome of interest.
Clinical and Research Implications
This analysis has provided further insight into what might have led to the lack of treatment effect found in the COPE trial. A robust analysis of indirect effects, controlling for potential confounding factors, was conducted using recommended methods. The finding that illness perceptions are potentially important in Explain Pain interventions adds strength to the analysis of secondary outcomes of the COPE trial, and adds to a currently small evidence base for the potential importance of illness perceptions in such interventions. Future applications of the Explain Pain interventions could perhaps focus more on trying to target illness perceptions rather than pain catastrophizing, although it should be acknowledged that this analysis represents only 1 application and that further research is needed to provide support for these findings.
Author Contributions and Acknowledgments
Concept/idea/research design: M. Grotle, G. Mansell, K. Storheim, E.L. Werner
Writing: M. Grotle, I. Løchting, G. Mansell, K. Storheim, E.L. Werner
Data collection: M. Grotle, I. Løchting, K. Storheim, E.L. Werner
Data analysis: M. Grotle, G. Mansell
Project management: M. Grotle, I. Løchting, K. Storheim, E.L. Werner
Providing participants: M. Grotle, I. Løchting, K. Storheim, E.L. Werner
Providing facilities/equipment: M. Grotle, I. Løchting, K. Storheim
Providing institutional liaisons: M. Grotle, I. Løchting, K. Storheim
Clerical/secretarial support: I. Løchting
Consultation (including review of manuscript before submitting): I. Løchting, K. Storheim, E.L. Werner
The authors thank the participating general practitioners and physical therapists who contributed data for this study. The authors also thank the Norwegian Research Council and the Norwegian Medical Association for funding the COPE trial and 2 anonymous reviewers who provided very helpful comments on a previous draft of the manuscript.
Ethics Approval
No specific ethics approval was required for this secondary analysis. Ethics approval for the original study was received (S-08362a Saksnummer: 2008/10435) from the Norwegian Regional Committee for Medical Research Ethics and the Data Inspectorate.
Funding
The Norwegian Research Council (ref. no. 214066) and the Norwegian Medical Association's foundation (09/1612) provided funds in support of this work. G. Mansell is supported by National Institute for Health Research (NIHR) School for Primary Care Research Seedcorn funding. This paper presents independent research funded by the NIHR School for Primary Care Research. The views expressed in this publication are those of the authors and not necessarily those of the NIHR, the National Health Service (NHS) (UK), or the Department of Health.
Disclosure
The authors completed the ICJME Form for Disclosure of Potential Conflicts of Interest and reported no conflicts of interest.
References
- 1. Moseley GL, Nicholas MK, Hodges PW. A randomized controlled trial of intensive neurophysiology education in chronic low back pain. Clin J Pain. 2004;20:324–330. [DOI] [PubMed] [Google Scholar]
- 2. Moseley GL, Butler DS. Fifteen years of explaining pain: the past, present, and future. J Pain. 2015;16:807–813. [DOI] [PubMed] [Google Scholar]
- 3. Werner EL, Storheim K, Løchting I, Grotle M. The COPE LBP trial: cognitive patient education for low back pain—a cluster randomized controlled trial in primary care. BMC Musculoskelet Disord. 2010;11:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Werner EL, Storheim K, Løchting I, Wisløff T, Grotle M. Cognitive patient education for low back pain in primary care: a cluster randomized controlled trial and cost-effectiveness analysis. Spine. 2016;41:455–462. [DOI] [PubMed] [Google Scholar]
- 5. Løchting I, Storheim K, Werner EL, Småstuen Cvancarova M, Grotle M. Evaluation of individualised quality of life and illness perceptions in low back pain: a patient education cluster randomized controlled trial. Patient Educ Couns. 2016;99:1992–1998. [DOI] [PubMed] [Google Scholar]
- 6. Kraemer HC, Wilson GT, Fairburn CG, Agras WS. Mediators and moderators of treatment effects in randomized clinical trials. Arch Gen Psychiatry. 2002;59:877–883. [DOI] [PubMed] [Google Scholar]
- 7. Emsley R, Dunn G, White IR. Mediation and moderation of treatment effects in randomised controlled trials of complex interventions. Stat Methods Med Res. 2010;19:237–270. [DOI] [PubMed] [Google Scholar]
- 8. Hayes AF. Beyond Baron & Kenny: statistical mediation analysis in the new millennium. Communication Monographs. 2009;76:408–420. [Google Scholar]
- 9. Kazdin AE. Mediators and mechanisms of change in psychotherapy research. Annu Rev Clin Psychol. 2007;3:1–27. [DOI] [PubMed] [Google Scholar]
- 10. Chen H-T. Theory-Driven Evaluations. London, United Kingdom: Sage; 1990. [Google Scholar]
- 11. Chen H-T. Practical Program Evaluation: Theory-Driven Evaluation and the Integrated Evaluation Perspective. 2nd ed London, United Kingdom: Sage; 2015. [Google Scholar]
- 12. MacKinnon DP. Introduction to Statistical Mediation Analysis. New York, New York: Psychology Press; 2008. [Google Scholar]
- 13. Petrie KJ, Weinman J. Why illness perceptions matter. Clin Med. 2006;6:536–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Løchting I, Garratt AM, Storheim K, Werner EL, Grotle M. Evaluation of the Brief Illness Perception Questionnaire in sub-acute and chronic low back pain patients: data quality, reliability and validity. J Pain Relief. 2013;2:122. [Google Scholar]
- 15. Mansell G, Kamper SJ, Kent P. Why and how back pain interventions work: what can we do to find out? Best Pract Res Clin Rheumatol. 2013;27:685–697. [DOI] [PubMed] [Google Scholar]
- 16. Werner EL, Gross DP, Lie SA, Ihlebaek C. Healthcare provider back pain beliefs unaffected by a media campaign. Scand J Prim Health Care. 2008;26:50–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fernandes L, Storheim K, Løchting I, Grotle M. Cross-cultural adaptation and validation of the Norwegian Pain Catastrophizing Scale in patients with low back pain. BMC Musculoskelet Disord. 2012;13:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sullivan MJL, Bishop SR, Pivik J. The Pain Catastrophising Scale: development and validation. Psychol Assess. 1995;7:524–532. [Google Scholar]
- 19. Osman A, Barrios FX, Gutierrez PM, Kopper BA, Merrifield T, Grittmann L. The Pain Catastrophizing Scale: further psychometric evaluation with adult samples. J Behav Med. 2000;23:351–365. [DOI] [PubMed] [Google Scholar]
- 20. Broadbent E, Petrie KJ, Main J, Weinman J. The Brief Illness Perception Questionnaire. J Psychosom Res. 2006;60:631–637. [DOI] [PubMed] [Google Scholar]
- 21. Roland M, Morris R. A study of the natural history of back pain, part I: development of a reliable and sensitive measure of disability in low-back pain. Spine. 1983;8:141–144. [DOI] [PubMed] [Google Scholar]
- 22. Roland M, Fairbank J. The Roland-Morris Disability Questionnaire and the Oswestry Disability Questionnaire. Spine. 2000;25:3115–3124. [DOI] [PubMed] [Google Scholar]
- 23. Smeets R, Köke A, Lin C-W, Ferreira M, Demoulin C. Measures of function in low back pain/disorders: Low Back Pain Rating Scale (LBPRS), Oswestry Disability Index (ODI), Progressive Isointerial Lifting Evaluation (PILE), Quebec Back Pain Disability Scale (QBPDS), and Roland-Morris Disability Questionnaire (RDQ). Arthritis Care Res. 2011;63(suppl 11):S158–S173. [DOI] [PubMed] [Google Scholar]
- 24. VanderWeele TJ. Explanation in Causal Inference: Methods for Mediation and Interaction. Oxford, United Kingdom: Oxford University Press; 2015. [Google Scholar]
- 25. Streiner DL, Norman GR. Health Measurement Scales: A Practical Guide to Their Development and Use. 4th ed Oxford, United Kingdom: Oxford University Press; 2008. [Google Scholar]
- 26. George SZ, Zeppieri G, Cere AL et al. A randomized trial of behavioral physical therapy interventions for acute and sub-acute low back pain (NCT00373867). Pain. 2008;140:145–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Skidmore JR, Koenig AL, Dyson SJ, Kupper AE, Garner MJ, Keller CJ. Pain self-efficacy mediates the relationship between depressive symptoms and pain severity. Clin J Pain. 2015;31:137–144. [DOI] [PubMed] [Google Scholar]
- 28. Maric M, Wiers RW, Prins PJM. Ten ways to improve the use of statistical mediation analysis in the practice of child and adolescent treatment research. Clin Child Fam Psychol Rev. 2012;15:177–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kline RB. The mediation myth. Basic Appl Soc Psych. 2015;37:202–213. [Google Scholar]
- 30. MacKinnon DP, Lockwood CM, Williams J. Confidence limits for the indirect effect: distribution of the product and resampling methods. Multivariate Behav Res. 2004;39:99–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bentler PM. On tests and indices for evaluating structural models. Pers Individ Dif. 2010;42:825–829. [Google Scholar]
- 32. Byrne BM, Lam WWT, Fielding R. Measuring patterns of change in personality assessments: an annotated application of latent growth curve modelling. J Pers Assess. 2008;90:536–546. [DOI] [PubMed] [Google Scholar]
- 33. Byrne BM. Structural Equation Modelling With AMOS: Basic Concepts, Applications, and Programming. 2nd ed London, United Kingdom: Routledge; 2010. [Google Scholar]
- 34. Whittle R, Mansell G, Jellema P, van der Windt D. Applying causal mediation methods to clinical trial data: what can we learn about why our interventions (don’t) work? Eur J Pain. 2017;21:614–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lee H, McAuley JH, Hübscher M, Kamper SJ, Traeger AC, Moseley GL. Does changing pain-related knowledge reduce pain and improve function through changes in catastrophising? Pain. 2016;157:922–930. [DOI] [PubMed] [Google Scholar]
- 36. Siemonsma PC, Stuive I, Roorda LD et al. Cognitive treatment of illness perceptions in patients with chronic low back pain: a randomized controlled trial. Phys Ther. 2013;93:435–448. [DOI] [PubMed] [Google Scholar]