Synopsis
Current dental restorative materials are typically inert and replace missing tooth structures. This article reviews recent efforts in the development of a new generation of bioactive materials designed to not only replace the missing tooth volume, but also possess therapeutic functions. Composites and bonding agents with remineralizing and antibacterial characteristics have shown promise in replacing lost minerals, inhibiting recurrent caries, neutralizing acids, repelling proteins, suppressing biofilms and acid production, demonstrating a low cytotoxicity similar to current resins, and protecting dental pulp and promoting tertiary dentin formation. This new class of bioactive materials shows promise to reverse lesions and inhibit caries.
Keywords: Bioactive composites, bonding agents, antibacterial monomers, remineralization, calcium phosphate nanoparticles, silver nanoparticles, oral biofilms, caries inhibition
Introduction
Dental caries is a prevalent disease worldwide, and composites have become increasingly popular for restoring teeth damaged by caries, largely because of their esthetics [1–7]. Composite compositions and properties have been substantially improved, yielding longer clinical lifetimes [8–14]. Nonetheless, recurrent caries along the tooth-composite interfaces remains a predominant reason for failure and replacement of restorations [15,16]. A study showed that for Class I composite restorations, secondary caries was the cause in 113 out of 129 cases of failure (88%), followed by fracture [17]. Another study showed that for Class II restorations, secondary caries was a primary cause for failure (73.9%), followed by lost restorations (8.0%) and material fracture (5.3%) [18]. Contributing factors to composite restoration failures include:
Composites tend to accumulate more biofilms than other restorative materials [19]. “The percentage mutans streptococci of total CFU count in plaque was higher on composite (mean 13.7) and amalgam (mean 4.3) than on glass-ionomer (mean 1.1) restorations” [20]. “Resin composites inherently enhance bacterial growth” [21], and “there is a potential impact of composite resins on the ecology of microorganisms in the dental plaque biofilm”, due to an increased biofilm buildup on composites [22].
The composite-tooth bonded interface is the weak link of the restoration, often forming microgaps and allowing microleakage over time in vivo, providing a site for bacterial invasion that may lead to recurrent caries [23–25].
To overcome these problems, efforts have been devoted to developing a new generation of bioactive dental materials containing additives that have remineralizing and antimicrobial capabilities.
Dental composites and adhesives with remineralizing properties
Dental resins containing calcium phosphate (CaP) filler particles were developed with remineralizing capabilities [26–30]. The CaP particle sizes ranged from about 1 μm to 55 μm in traditional CaP-containing resins [26–28]. These composites released supersaturating levels of calcium (Ca) and phosphate (P) ions and were shown to remineralize tooth lesions in vitro [26,27]. One study showed that whisker-reinforced CaP composite, which was proposed for use in atraumatic restorative treatments (ART composite), remineralized natural dentin as well as dentin with artificial caries [31]. To improve the load-bearing properties, a stronger barium-glass filler was also incorporated into a composite containing amorphous calcium phosphate (ACP), yielding improvement in flexural strength and elastic modulus, with no adverse influence on ion release profiles [32].
One drawback of traditional CaP composites was that they were mechanically weak, with flexural strength about half that of unfilled resin [26,27]. A material with such a low strength was not acceptable for use as a restorative [26]. More recently, CaP nanoparticles of sizes of about 100 nm were synthesized via a spray-drying technique and loaded into dental resins [29,30]. The CaP nanocomposite achieved Ca and P ion releases similar to those of traditional CaP containing composites, while possessing much greater mechanical properties [29,30].
In an in vitro study [33], enamel was first demineralized, then a composite containing nanoparticles of amorphous calcium phosphate (NACP) was used to remineralize the enamel lesions. A cyclic demineralization (pH 4) and remineralization (pH 7) regimen showed that NACP nanocomposite achieved an enamel remineralization that was 4-fold that of a commercial fluoride-releasing composite control [33]. This was likely because NACP neutralized the acid and raised the pH during demineralization, and enhanced remineralization by providing Ca and P ions. Another study investigated the caries-inhibition effect of NACP composite via an in situ model in 25 human participants [34]. NACP composite substantially reduced caries formation in situ under oral biofilms which were supplied with sucrose eight times per day to allow the biofilms to produce acids. The enamel mineral loss at the margins around NACP composite was only 1/3 of the mineral loss around the control composite (Fig. 1) [34]. The mechanism for this caries reduction was likely the neutralizing effect of NACPs on acids released by the biofilms, thereby protecting the tooth structures from demineralization. However, one major limitation of CaP-containing resins was that the Ca and P ion release was short-term, typically lasting for only a couple of months, which limits their commercialization potential. Recently, this shortcoming was overcome with the development of novel rechargeable CaP-containing resins (see Section 3).
Figure 1.
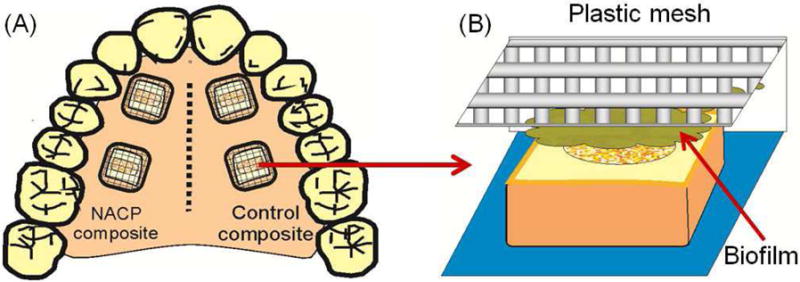
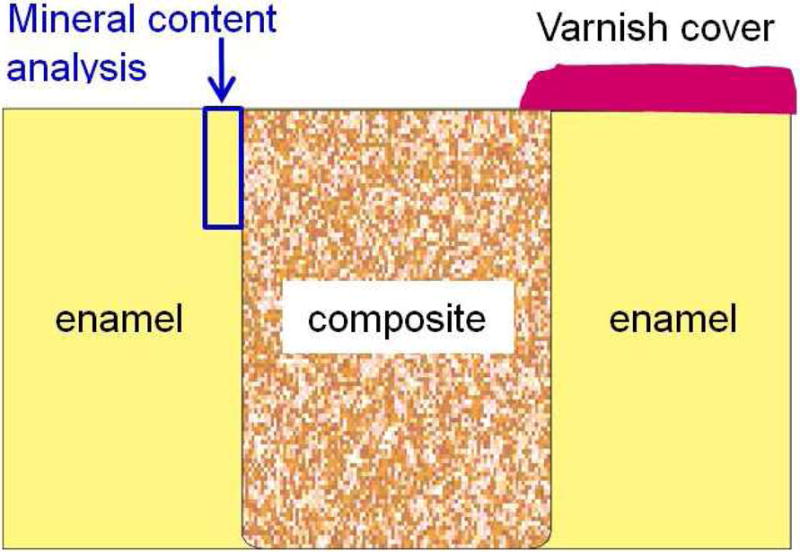
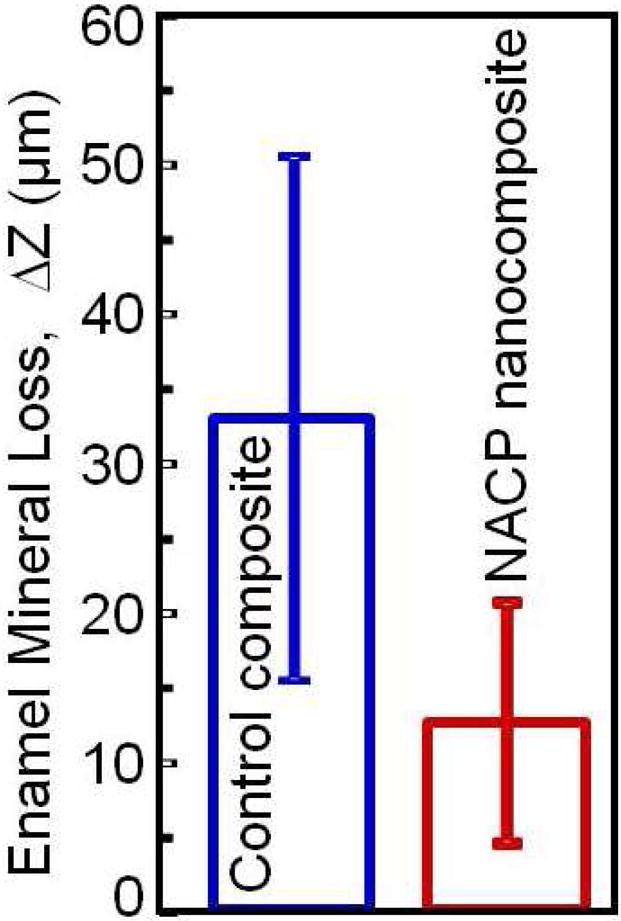
Human in situ caries inhibition via NACP composite. (A) One hundred bovine enamel slabs of 5×5×2 mm were obtained. A cavity of 2 mm in diameter and 1.5 mm in depth was prepared and restored with a composite. 25 volunteers wore palatal devices each containing four slabs: two filled with NACP nanocomposite on one side, and two filled with control composite on the other side. (B) A plastic mesh with 1 mm space protected the biofilms in situ. (C) Enamel slabs were cut to sections for microradiography. (D) Enamel mineral loss around NACP nanocomposite was reduced to nearly 1/3 of that around control composite (p < 0.05).
Figure 1A, 1B, 1C: From Melo MA, Weir MD, Rodrigues LK, et al. Novel calcium phosphate nanocomposite with caries-inhibition in a human in situ model. Dent Mater 2013;29(2):233; with permission.
Figure 1D: Adapted from Melo MA, Weir MD, Rodrigues LK, et al. Novel calcium phosphate nanocomposite with caries-inhibition in a human in situ model. Dent Mater 2013;29(2):237; with permission.
Besides composites, NACP have also been incorporated into dental adhesives [35]. NACP mass fractions of up to 40% were mixed into an adhesive without decreasing the dentin bond strength [35]. After acid-etching the dentin and during bonding, the small sizes of NACP allowed them to flow with the adhesive into dentinal tubules. SEM examination revealed many resin tags with numerous NACP infiltrated into the tubules. The NACP could release Ca and P ions to remineralize the remnants of lesions in the prepared tooth cavity, as well as neutralize acids and raise the local pH in the case of marginal gap formation and microleakage with bacterial invasion at the margins [36]. Indeed, a NACP composite was able to quickly raise the pH from a cariogenic pH of 4 to a safe pH of greater than 6, while other restorative materials including a glass ionomer cement failed to raise the pH to higher than 4 [36]. Therefore, CaP-containing dental composites and adhesives with improved mechanical properties and remineralizing capabilities are promising candidates to combat recurrent caries.
With the purpose of enhancing the bonded interface durability, a study investigated the effect of NACP and an antibacterial monomer on dentin bonding after biofilm degradation [37]. The bonding agent and composite contained NACP as well as other antibacterial agents. Twin resin-dentin bonded interface specimens were prepared using extracted human teeth. Flexural strength was evaluated after 1-day, 7-day water-storage and 7-day biofilm challenge. Cyclic loading of specimens was performed after 1-day water-storage and 7-day biofilm challenge using a universal testing system with a four-point bending configuration. The results showed that biofilm challenge significantly reduced the flexural strength and fatigue resistance of the resin-dentin interface of the control group. However, the antibacterial and remineralizing materials were able to reduce the cariogenic impact of the biofilm, thereby improving the mechanical strength and fatigue resistance of the dentin-resin bonded interface [37].
Besides NACP, sol-gel processed bioactive glasses (BAG) that release calcium and phosphate ions have also been incorporated into dental restorative materials. Such experimental materials have also been tested for their antimicrobial effect against Streptococcus sobrinus (ATCC33478), Streptococcus mutans (ATCC25175) and Enterococcus faecalis (ATCC19433) [38]. Bacterial suspensions were independently incubated with bioactive glass in particulate form (< 3 μm) for 4 and 24 hours. Viability was determined by colony-forming units. At 4 hours, all BAG groups produced an order of magnitude reduction in all three types of bacteria. After 24 hours, all BAG groups produced a significant reduction in S. sobrinus colonies, but no further reduction in S. mutans. BAG groups also significantly reduced E. faecalis compared to the control. At 4 hours, an increase in the pH was noted for the BAG groups (to pH 9) which could also have contributed to the bactericidal effect [38]. In addition, a separate study examined the effect of BAG incorporation in a composite on bacterial biofilms penetrating into marginal gaps of simulated tooth fillings in vitro during cyclic mechanical loading [39]. The results showed that the average depth of bacterial penetration into the marginal gap for the BAG group was significantly less than that without BAG. Hence, dental composite incorporating BAG may be promising to hinder the development and propagation of recurrent caries at the tooth-restoration interfaces [39].
In addition to composites and adhesives, several calcium silicate cements are commercially available for direct and indirect pulp-capping treatment. Biodentine (Septodent, Lancaster, PA) is used for pulp capping, permanent dentin restorations, and temporary enamel restorations, etc. It contains tricalcium silicate, dicalcium silicate, and calcium carbonate as fillers, polycarboxylate as a water-reducing agent and calcium chloride as an accelerator. Biodentine has a relatively rapid setting time of 9–12 min, which helps minimize potential premature dissolution of the cement. Furthermore, effective sealing is obtained via micromechanical interlocking of crystals in the dentin tubules. Like other calcium silicate cements, Biodentine exhibits significant calcium ion release and the ability to increase the local pH. Biodentine can induce the formation of dentin bridges and the repair of pulpal tissues. In addition, an alternate approach is to combine the ion-releasing, sealing, and dentin-repairing aspects of calcium-silicate cements with light-cured resin systems. Materials such as TheraCal (BISCO Dental Products, Schaumburg, IL) contain calcium silicate, fumed silica, and photopolymerizable methacrylate resins and can be used for direct and indirect pulp capping and as a liner under other restorative materials. TheraCal is effective at sealing the dental pulp area and has a rapid setting time due to light-curing properties. The rapid setting time is important because it prevents premature dissolution of unset material, which can occur with traditional calcium silicate cements. Another commercial product, ACTIVA (Pulpdent, Watertown, MA), has been used as a base/liner as well as in bulk fill, post and core build up procedures. ACTIVA consists of a resin-modified glass ionomer, a proprietary bioactive ionic resin, a rubberized resin and is photopolymerizable. This composition allows for the release of calcium, phosphate and fluoride, enhances wear-resistance and fracture-resistance, protects against microleakage and has antibacterial properties. In addition, an antibacterial methacryloyloxydodecylpyridinium bromide (MDPB)-based bonging agent Clearfil Protect Bond (Kuraray Dental, New York, NY) is also available for clinical use. Therefore, new bioactive materials are gradually being commercialized as the dental industry finds these antibacterial and remineralizing functions worthy of pursuit.
Rechargeable composite and adhesive with long-term Ca/P ion release
A major drawback of CaP composites is that the Ca and P ion release lasts for only weeks to months, and then diminishes over time [26–30]. However, to have practical implications in vivo, the CaP-containing restoration needs to be effective for much longer than a few months, and in fact must continue to release Ca and P ions to suppress enamel and dentin demineralization for many years. Therefore, it would be highly desirable for the CaP composite to be able to repeatedly recharge and re-release Ca and P ions, thereby providing these ions indefinitely with long-term caries-inhibition capability.
Recently, an experimental rechargeable CaP dental composite was developed [40]. Three NACP nanocomposites were tested using resins including:
bisphenol A glycidyl dimethacrylate (BisGMA) and triethylene glycol dimethacrylate (TEGDMA) (referred to as BT group);
pyromellitic glycerol dimethacrylate (PMGDM) and ethoxylated bisphenol A dimethacrylate (EBPADMA) (referred to as PE group);
BisGMA, TEGDMA, and Bis[2-(methacryloyloxy)ethyl] phosphate (BisMEP) (referred to as BTM group).
BisGMA and TEGDMA were frequently used in dental resins. PMGDM and BisMEP were selected because both are acidic adhesive monomers and may chelate with Ca and P ions from a recharge solution to achieve the recharge capability. For each group, the Ca and P ion release was first exhausted, then the exhausted specimens were recharged to measure the re-release for 7 days as one cycle. This was then repeated for six cycles as described in Fig. 2A. The results (Fig. 2B) showed that the ion recharge capability was the greatest for PE group. For each recharge cycle, the ion re-release reached similarly high levels, showing that the ion re-release did not decrease with increasing the number of recharge cycles [40].
Figure 2.
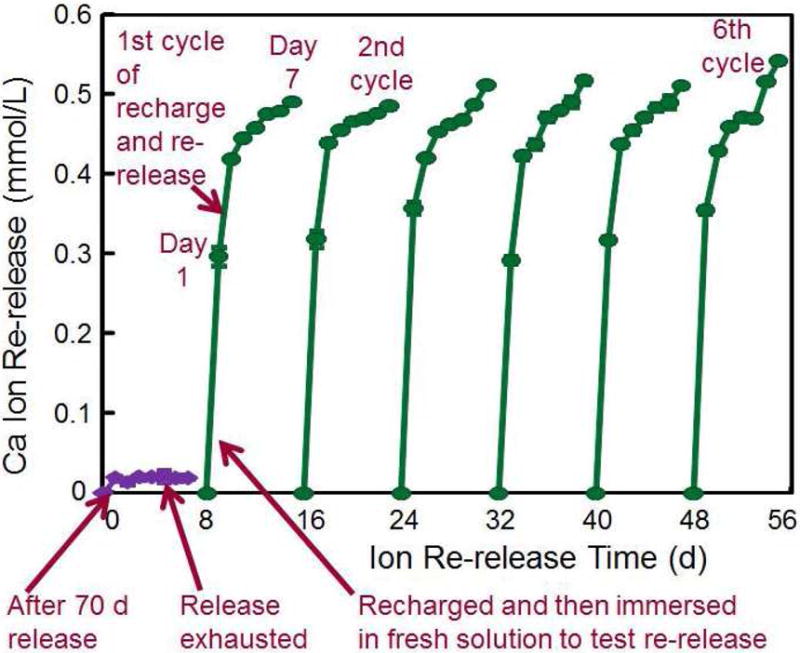
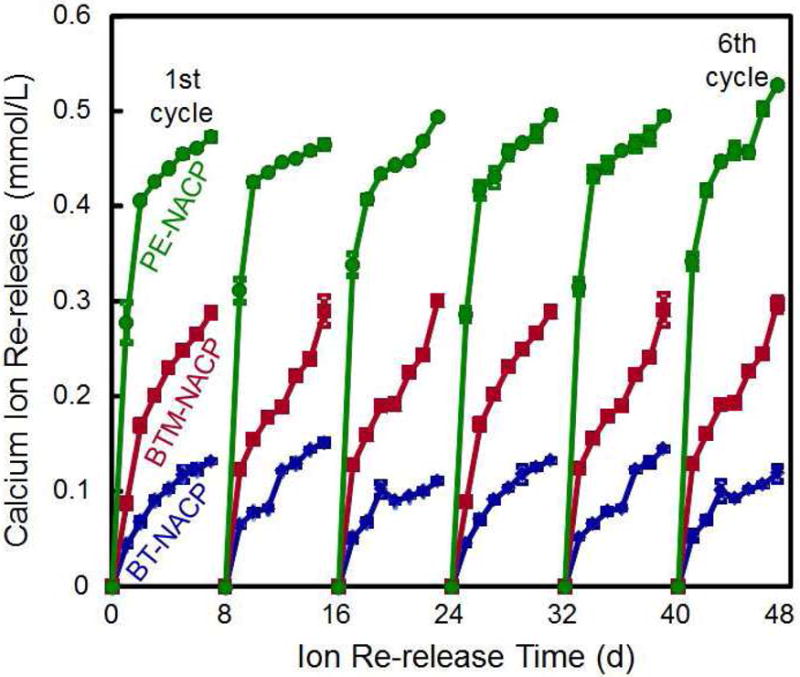
Rechargeable NACP composite for long-term Ca and P ion release. (A) NACP composite was immersed in a pH 4 solution for 70 d to exhaust the ion release (lower left arrow). Then the specimens were immersed in a new pH 4 solution to confirm that the ion release was exhausted (lower middle arrow). Specimens were recharged in a recharge solution, then tested for ion re-release for 7 d (third arrow at the bottom of A). This constituted the first recharge/re-release cycle. This process was repeated for 6 cycles. (B) Three NACP nanocomposites with six cycles of recharge/re-release, showing no decrease in ion release with increasing recharge cycles (p > 0.1).
Figure 2A: From Zhang L, Weir MD, Chow LC, et al. Novel rechargeable calcium phosphate dental nanocomposite. Dent Mater 2016;32(2):288; with permission.
Figure 2B: From Zhang L, Weir MD, Chow LC, et al. Novel rechargeable calcium phosphate dental nanocomposite. Dent Mater 2016;32(2):289; with permission.
In addition, a rechargeable CaP bonding agent was also developed with the purpose of suppressing recurrent caries at the bonded tooth-restoration interfaces [41]. The Ca and P ion release from the adhesive resin remained high and did not decrease with increasing the number of recharge and re-release cycles. After the third recharge cycle, specimens without any further recharge exhibited a continuous Ca and P ion release for 2–3 weeks [41]. Therefore, it appeared to be possible to recharge the restoration only once per week to have sustained ion re-release for at least a week. These results demonstrated the potential for sustained Ca and P ion release for an NACP nanocomposite and NACP adhesive to potentially achieve a long-term caries-inhibition capability.
Antibacterial dental composites and bonding agents
Dental caries is one of the most common bacterial infections in humans and is a dietary carbohydrate-modified bacterial infectious disease [42]. Tooth demineralization is caused by acid generated by bacterial biofilms (dental plaque) in the presence of fermentable carbohydrates [43]. One approach to this problem has been the development and synthesis of antibacterial quaternary ammonium methacrylates (QAMs) and their incorporation into resins for use in dental restorative systems [44–49]. Pioneering work by Imazato et al. yielded MDPB which could be co-polymerized and covalently bonded in the resin matrix, thus becoming immobilized to provide long-term contact-inhibition against oral bacteria [50,51]. A commercially available MDPB-containing bonding agent (Clearfil Protect Bond, Kuraray Dental) was shown to possess potent antibacterial activity against S. mutans, Lactobacillus casei and Actinomyces naeslundii, and was able to eradicate residual bacterial inside dentinal tubules of prepared tooth cavities [50]. In addition, several other antimicrobial formulations were also developed, including a methacryloxylethyl cetyl dimethyl ammonium chloride (DMAE-CB)-containing adhesive [52], quaternary ammonium polyethylenimine (PEI) nanoparticles for antimicrobial dental composites [53], antibacterial glass ionomer cements [54], and antibacterial nanocomposites and bonding agents incorporating a quaternary ammonium dimethacrylate (QADM) [55–57]. Besides composites, bonding agents are important in adhering resin-based restorations to tooth structures [24,58]. Previous studies have greatly enhanced the bonding agent compositions and procedures, leading to greater tooth-restoration bond strengths [24,59–63]. To reduce caries at the tooth-restoration margins, antibacterial bonding agents were formulated [50–52]. They can kill the residual bacteria in the prepared tooth cavity, and inhibit new bacteria at the tooth-restoration interfaces when marginal leakage occurs [44,50,51]. Several meritorious antibacterial bonding agents containing QAMs were shown to effectively suppress bacteria attachment and substantially reduce biofilm growth [44,50,51,56,57,64,65].
QAM resins possess positively-charged quaternary amine N+ which can interact with the negatively-charged membrane of bacteria, leading to membrane disruption and cytoplasmic leakage [53]. It is postulated that long-chained quaternary ammonium compounds can be especially effective by inserting into the bacterial membrane, resulting in physical disruption and bacteria death [66]. One study developed antimicrobial glass ionomer materials which showed stronger antimicrobial activity with increasing alkyl chain length [54]. A recent study on bonding agents demonstrated that the oral biofilm viability and colony-forming unit counts (CFU) were substantially decreased with increasing alkyl chain length (CL) [67]. This was also shown in a dental composite, where a dental plaque microcosm biofilm model using human saliva was employed to evaluate the antibacterial activity [68]. QAM incorporation did not compromise the flexural strength and elastic modulus of the NACP nanocomposites. Increasing the CL from 3 to 16 greatly enhanced the antibacterial activity of NACP nanocomposite, and a nanocomposite with CL16 reduced the biofilm CFU counts of total microorganisms, total streptococci, and mutans streptococci by two orders of magnitude (Fig. 3) [68]. The NACP nanocomposite containing the antibacterial monomer with CL16 appeared to be promising to obtain the double benefits of being antibacterial and having remineralization capabilities to combat caries.
Figure 3.
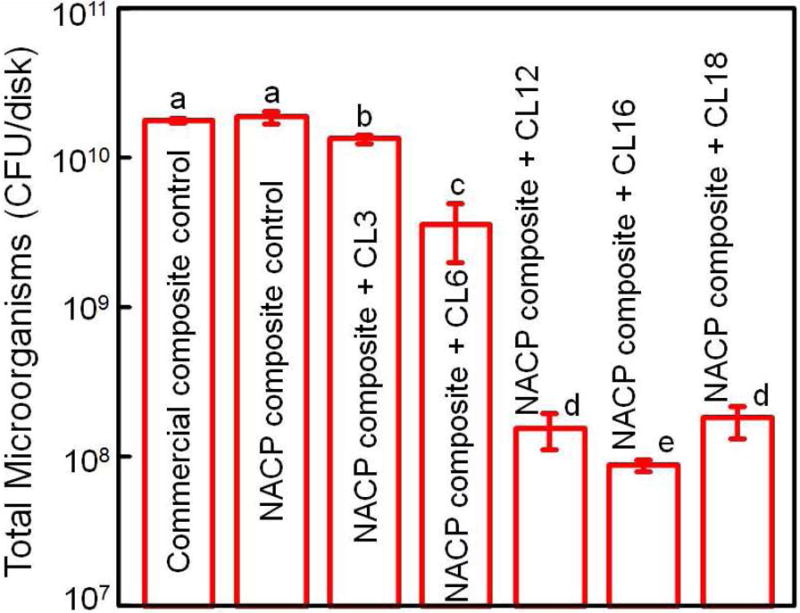
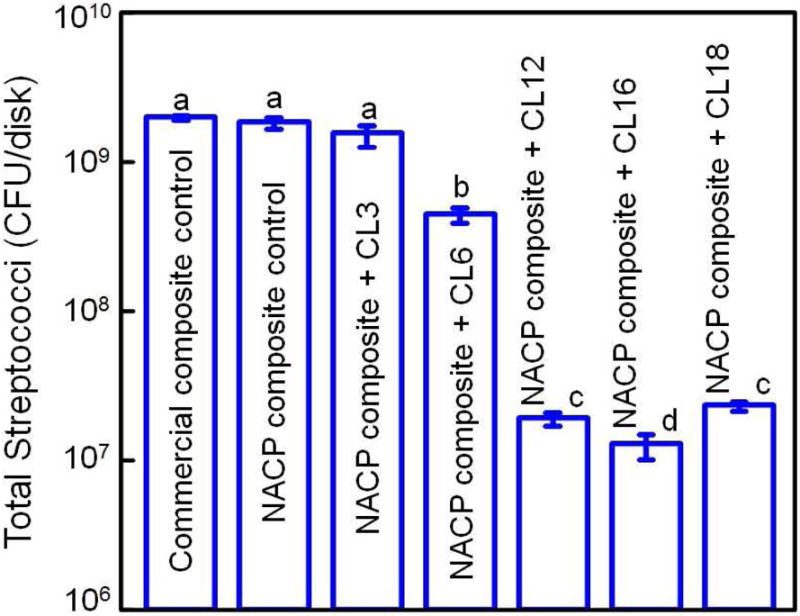
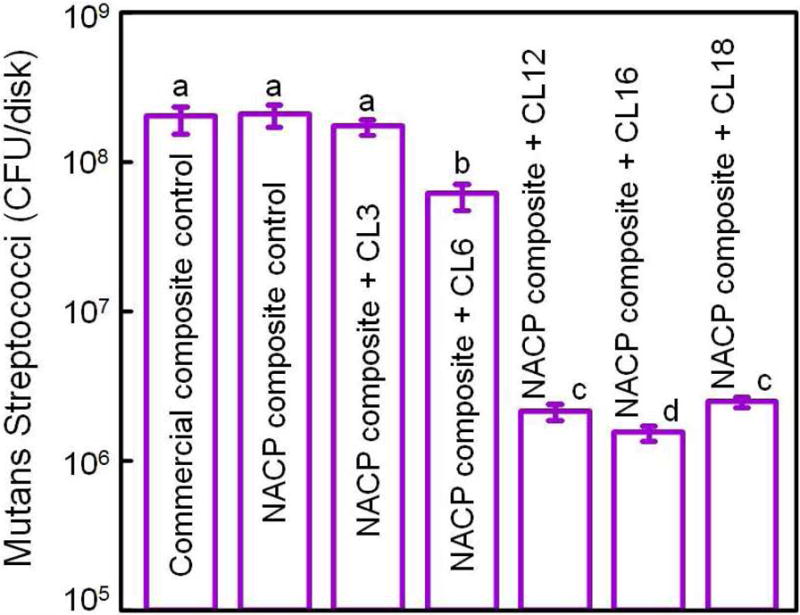
Dental plaque microcosm biofilm model with colony-forming unit (CFU) counts of 2-day biofilms on composites: (A) total microorganisms, (B) total streptococci, and (C) mutans streptococci (mean ± sd; n = 6). In each plot, values with dissimilar letters are significantly different from each other (p < 0.05). The two control composites had the highest CFU counts. Increasing CL from 3 to 16 decreased the CFU counts significantly (p < 0.05). Note the log scale for the y-axis.
Adapted from Zhang K, Cheng L, Weir MD, et al. Effects of quaternary ammonium chain length on the antibacterial and remineralizing effects of a calcium phosphate nanocomposite. Int J Oral Sci 2016; 8(1):50; with permission.
To further increase the antibacterial potency, an antibacterial monomer dimethylaminododecyl methacrylate (DMADDM) was combined with nanoparticles of silver (NAg) in an experimental bonding agent containing NACP [69]. Due to the small size of NACP and NAg, these nanoparticles readily flowed into dentinal tubules to form resin tags (Fig. 4A–C) [69], which could potentially kill residual bacteria in the tubules and remineralize the remnants of caries damaged tissues in the prepared tooth cavity [64,69,70]. The strong antibacterial activity was maintained during 6 months of water-aging, with no decrease in the killing potency at 6 months, compared to that at 1 d [69]. During the 6 months of water-aging, a commercial bonding agent control lost 35% of the dentin bond strength (Fig. 4D) [69]. However, the new antibacterial bonding agents showed no loss in dentin bond strength over 6 months, likely due to the anti-enzyme properties and the inhibition of matrix metalloproteinases (MMPs) thereby protecting the hybrid layer via the antibacterial monomer [71–74]. Therefore, the new antibacterial bonding agent shows promise to inhibit biofilms and caries at the margins, with the potential to provide a stronger and longer-lasting bonded interface, in addition to the potential remineralization effect from NACP.
Figure 4.
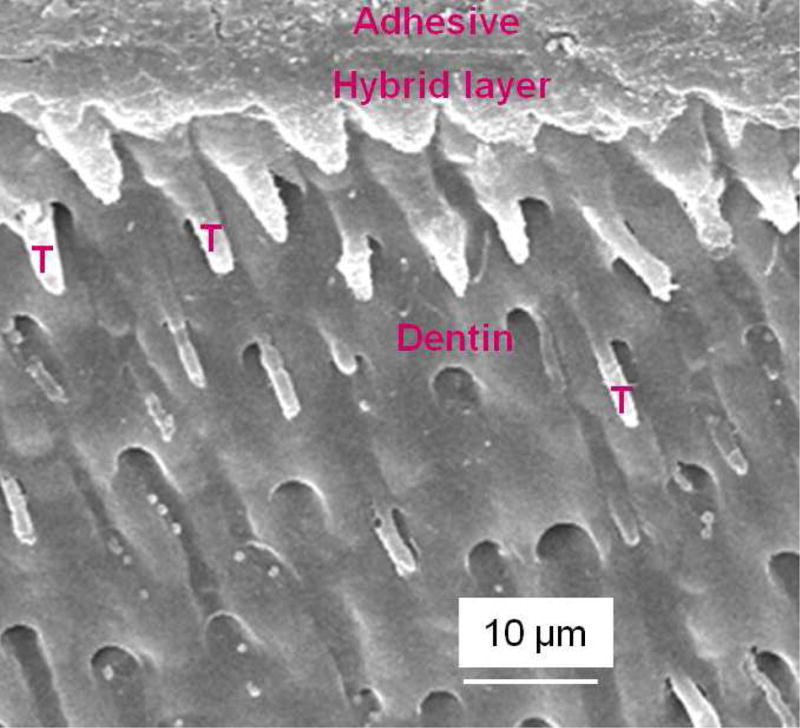
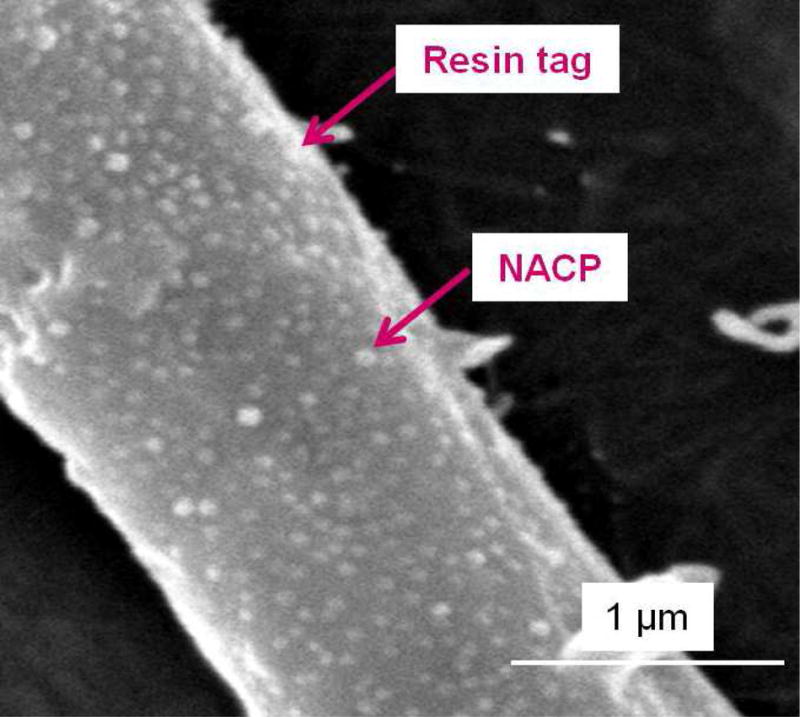

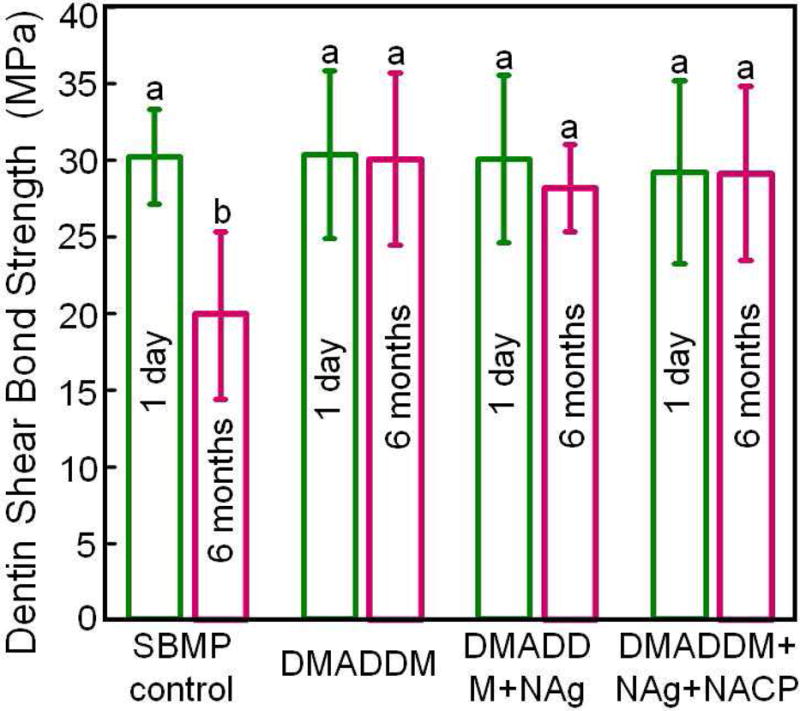
Dentin bonding. (A) SEM of dentin-adhesive interface for DMADDM+NAg+NACP group (T = resin tag). (B) Higher magnification SEM of a resin tag showing NACP in tubules. (C) Higher magnification TEM indicating NAg and NACP in resin tag. (D) Dentin shear bond strengths (mean ± sd; n = 10). Dissimilar letters indicate significantly different values (p < 0.05). There was a 35% loss in bond strength for commercial group in water-aging for 6 months. There was no bond strength loss for groups containing DMADDM, NAg and NACP.
From Zhang K, Cheng L, Wu EJ, et al. Effect of water-aging on dentin bond strength and anti-biofilm activity of bonding agent containing new monomer dimethylaminododecyl methacrylate. J Dent 2013;41(6):504–513; with permission.
Protein repellent and antibacterial composites, adhesives and cements
A dental resin, when placed in the oral environment in the presence of normal salivary flow, is quickly coated with a pellicle that is comprised of a layer of selectively adsorbed salivary proteins [75]. Early colonizing oral bacteria, such as mutans streptococci, adhere to resin and tooth surfaces via this layer, and this is the initial step in biofilm formation [76]. Therefore, it would be highly desirable to develop a new composite that can repel proteins, thereby hindering bacterial attachment. To this end, poly(ethylene glycol) (PEG) and two pyridinium group-containing methacrylate monomers were immobilized to silicon wafer surfaces to achieve protein-repellent activity [77]. Hydrophilic surfaces are usually more resistant to protein adsorption and bacterial adhesion than hydrophobic surfaces. 2-methacryloyloxyethyl phosphorylcholine (MPC) is a methacrylate with a phospholipid polar group in the side chain, and is one of the most common biocompatible and hydrophilic biomedical polymers [78]. MPC shows excellent resistance to protein adsorption and bacterial adhesion, and has been used in artificial blood vessels, artificial hip joints and microfluidic devices [78–82]. The MPC polymer coating renders the surfaces extremely hydrophilic, prevents the adhesion of proteins, and inhibits the adhesion of bacteria [78–80].
Recently, MPC was incorporated into an experimental dental composite to render it protein-repellent [83]. Incorporation of 3% MPC and 1.5% DMAHDM into the composite achieved protein-repellent and antibacterial capabilities without compromising the mechanical properties. The composite showed protein adsorption that was only 1/10 that of a commercial composite (Fig. 5A) [83]. Lactic acid production by biofilms formed on the material was also greatly reduced (Fig. 5B). The biofilm CFU on the composite with 3% MPC + 1.5% DMAHDM was more than three orders of magnitude lower than that of the commercial control (Fig. 5C). Further, incorporation of MPC and DMAHDM achieved biofilm reduction efficacy that was much greater than that using MPC or DMAHDM alone [83]. The reason for the synergistic effect obtained when using MPC and DMAHDM together is likely that the salivary protein coating on resin surfaces in vivo could reduce the contact-inhibition efficacy of DMAHDM. Hence, due to the protein-repellent function of MPC, the resin surface had adsorbed much less protein, and therefore was more exposed to direct contact against bacteria and their biofilms, thereby increasing the contact-inhibition efficacy of DMAHDM. Therefore, using MPC plus DMAHDM together may have wide applicability to other dental materials.
Figure 5.
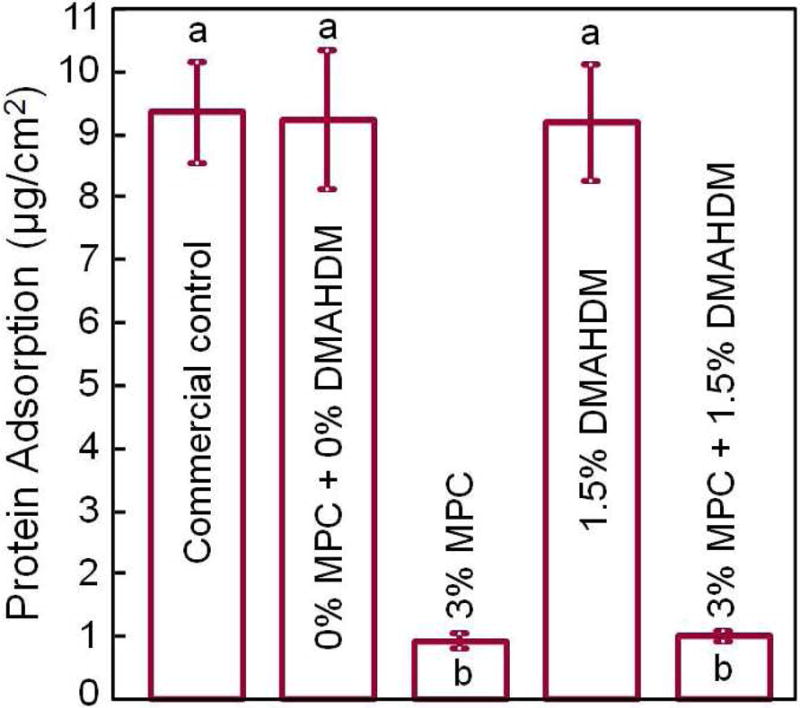
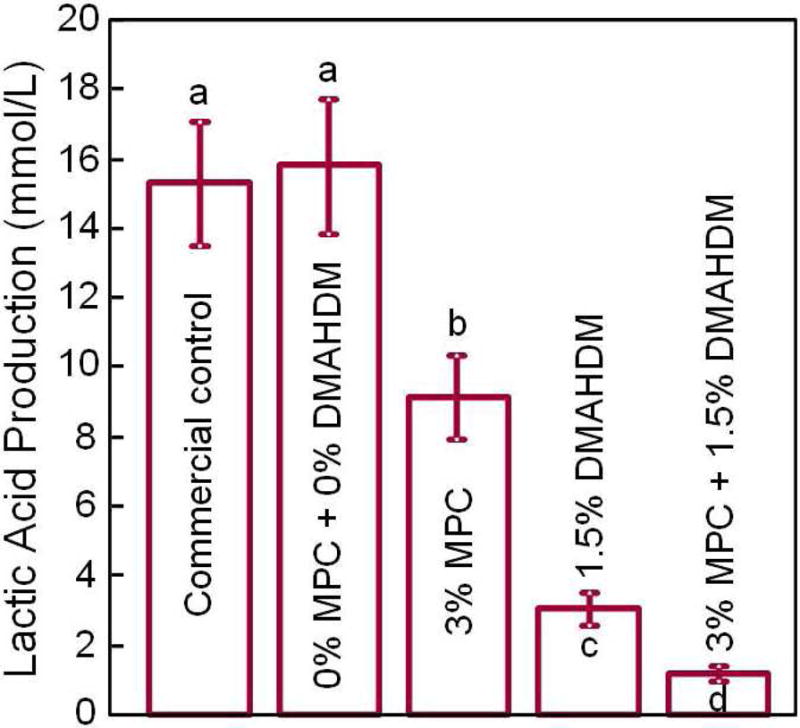
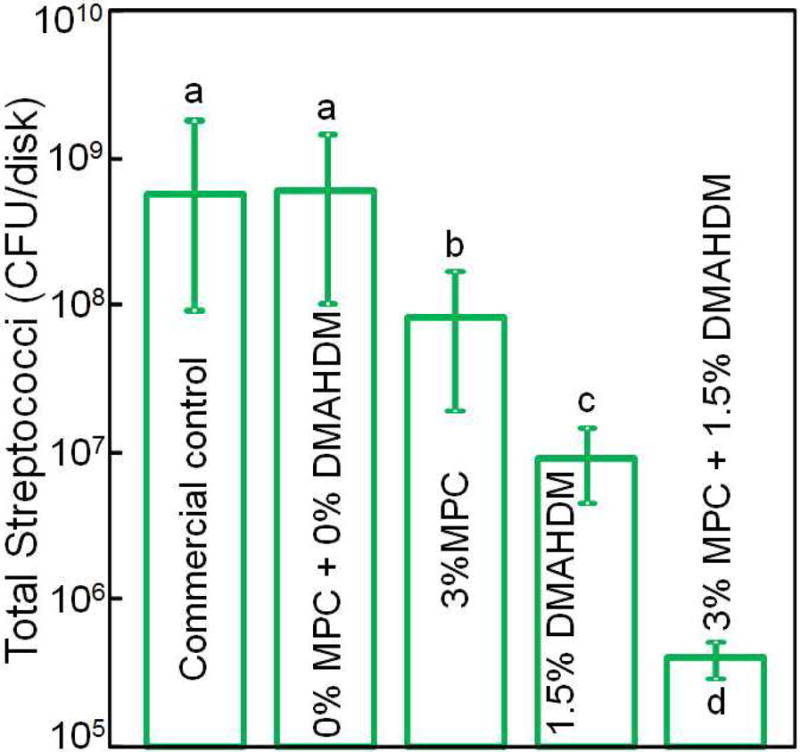
Protein-repellent. (A) Bovine serum albumin adsorption onto composite surface. The composite with 3% MPC and the composite with 3% MPC + 1.5% DMAHDM both had protein adsorption about 1/10 that of commercial composite. In each plot, dissimilar letters indicate significantly different values (p < 0.05). (B) Lactic acid production by biofilms was greatly reduced via MPC and DMAHDM. (C) Biofilm total streptococci CFU on composite with 3% MPC + 1.5% DMAHDM was more than 3 orders of magnitude lower than commercial control.
Adapted from Zhang N, Ma J, Melo MA, et al. Protein-repellent and antibacterial dental composite to inhibit biofilms and caries. J Dent 2015;43(2):225–234; with permission.
In addition to restorative composites, a bonding agent incorporating combined MPC and DMAHDM has also been tested [84]. Adding 7.5% MPC and 5% DMAHDM into the primer and adhesive did not adversely affect the dentin shear bond strength, while the protein adsorption was nearly 20-fold less than that of a control. Biofilm CFU on the resin with 7.5% MPC + 5% DMAHDM was 4 orders of magnitude less than that of the control [84]. Consistent with the results obtained for the composite [83], the use of double agents (protein-repellent MPC + antibacterial DMAHDM) synergistically in the dental adhesive achieved much stronger inhibition of biofilms than using each agent alone.
The protein-repellent method was further applied to developing a bioactive orthodontic cement [85]. Orthodontic treatments often cause white spot lesions in enamel due to biofilm accumulation and acid production [86]. Resin-modified glass ionomer cements (RMGIs) possess fluoride release and clinically-acceptable enamel bond strength, and have been used as orthodontic cements [87]. However, white spot lesions around orthodontic brackets are still common, which jeopardizes the health and esthetics of the teeth. This indicates that fluoride ions alone cannot prevent enamel demineralization [86]. In a recent study, four bioactive agents (MPC, DMAHDM, NAg, and NACP) were incorporated into a RMGI [85]. The incorporation of MPC into RMGI reduced the protein adsorption by an order of magnitude. Via DMAHDM and NAg, the biofilm CFU was reduced by 3 orders of magnitude. This method appeared to be promising to yield a protein-repellent, antibacterial and remineralizing orthodontic cement [85]. The combined use of four bioactive agents (MPC, DMAHDM, NAg, and NACP) may lead to the prevention of enamel white spot lesions as well as caries-inhibition effects in other dental materials systems, which warrants further in vitro and in vivo investigations.
Biocompatibility of antibacterial and remineralizing resins
For novel bioactive materials to be used in tooth restorations in patients, investigations are needed to ensure that the new materials are non-cytotoxic and compatible with the dentin-pulp complex. Several studies have investigated the biocompatibility of antibacterial monomers and resins [88–91]. MDPB was shown to have a relatively low level of toxicity to human pulpal cells, with a cytotoxicity similar to the dimethacrylate monomer, TEGDMA, which has long been used in dentistry [88]. Another study showed that MDPB incorporation into a primer did not cause an increase in toxicity to pulpal cells [89]. Another study compared the cytotoxicity of MDPB with BisGMA, and showed that the inhibitory effects of MDPB on the proliferation and mineralization of odontoblast-like MDPC-23 rodent-derived cells were lower than BisGMA, indicating that MDPB was less cytotoxic than BisGMA [90]. In addition, an in vivo study investigated MDPB-containing primer in tooth cavities in dogs [92]. The results showed that restorations with experimental primer containing MDPB caused little or no pulpal inflammation in vivo [92].
These results are consistent with a report on dimethylaminododecyl methacrylate (DMADDM) which had a median lethal concentration of between 20 to 40 μg/mL, about 20-fold higher than that of a BisGMA control, indicating that DMADDM had a much lower cytotoxicity than BisGMA [91]. Another study tested the in vitro cytotoxicity of a series of QAMs including DMAHDM against human gingival fibroblasts and odontoblast-like MDPC-23 cells [67]. The results showed that increasing monomer concentration increased the cytotoxicity, and increasing the CL also increased the cytotoxicity. However, all the tested antibacterial monomers had less cytotoxicity than BisGMA, and similar cytotoxicity to those of HEMA and TEGDMA [67]. Furthermore, the testing using eluents from the polymerized resin specimens containing QAMs also showed that the fibroblast and odontoblast cytotoxicity was similar to commercial dental resin controls (without QAMs) [67].
The majority of studies evaluating the biocompatibility of antibacterial dental monomers have been conducted in vitro. However, several animal studies have also been performed, using models employing monkeys [93], dogs [94], ferrets [95], and rats [96]. Ethical and cost considerations make rodent models more preferable. A study showed that, after pulp-capping, the rat molar pulp healing was histologically similar to that in humans and other animal species [96]. Several studies used rats with maxillary molar models to examine Class I [96] and Class V restorations [97]. A recent study investigated antibacterial and remineralizing restorations containing DMADDM and NACP in a rat tooth cavity model [98]. NACP and DMADDM were incorporated into a composite and an adhesive. Four types of restorations were tested:
Control composite and control adhesive;
Control composite + DMADDM, and control adhesive + DMADDM;
Control composite + NACP, and control adhesive + NACP;
Control composite + DMADDM + NACP, control adhesive + DMADDM + NACP [98].
Occlusal deep cavities were prepared in the first molars of rats and restored with one of the four groups (Fig. 6A, 6B). The NACP group and DMADDM+NACP group showed less inflammatory response in the pulp, with more tertiary dentin formation, than the control (Fig. 6C, 6D). At 30 days, the NACP group and DMADDM+NACP group had tertiary dentin thickness that was 4 to 6 times that of the control (Fig. 6E) [98]. Groups with or without DMADDM had similar pulpal responses and tertiary dentin thickness (TDT), indicating that DMADDM had no significant effect on pulpal inflammation and TDT. All the samples containing NACP, whether with or without DMADDM, yielded milder pulpal inflammation and much greater TDT, indicating that the presence of NACP was beneficial to the pulp, likely due to Ca and P ion release [98]. Therefore, the combined use of NACP and DMADDM in composites and adhesives are promising as a new therapeutic restorative system with the potential to not only combat oral pathogens and cariogenic biofilm acids, but also facilitate the healing of the dentin-pulp complex [98].
Figure 6.
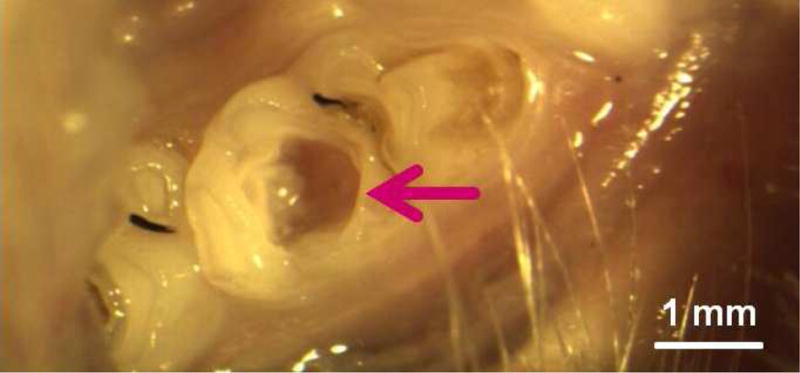
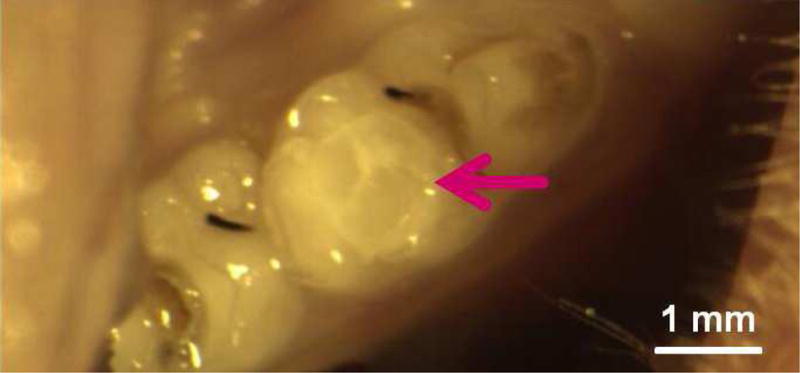
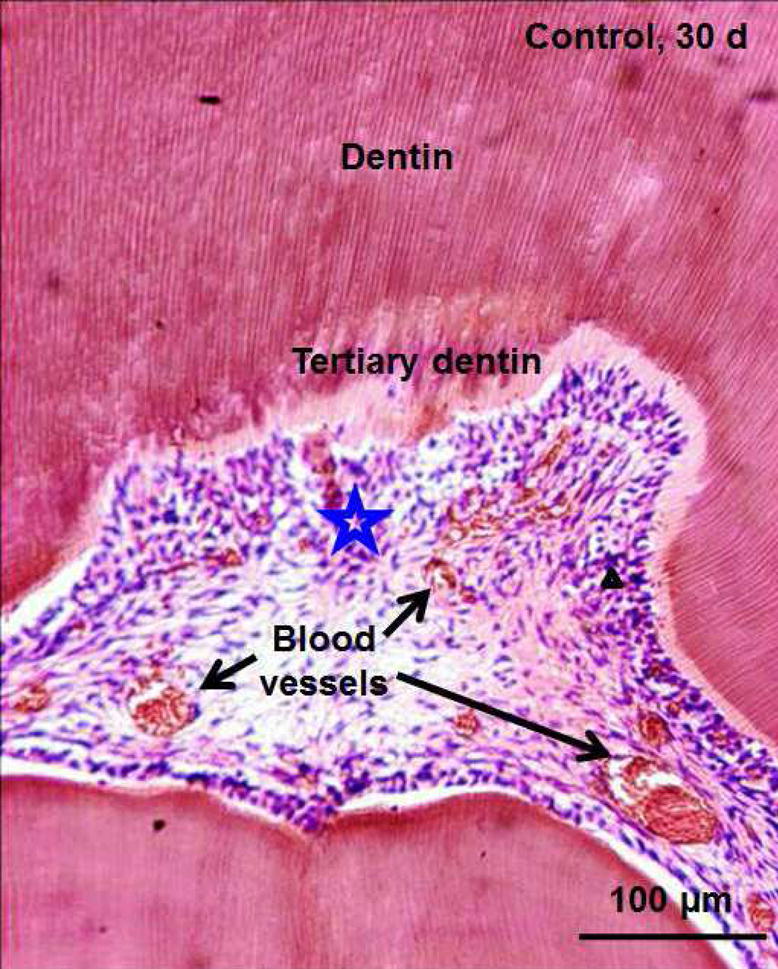
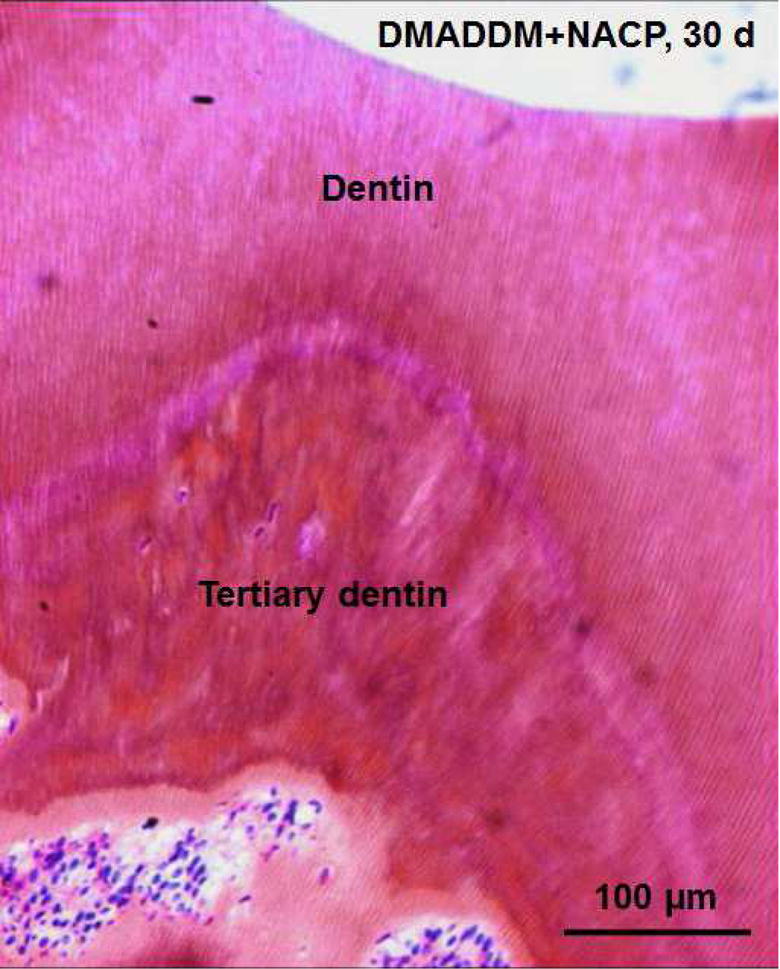
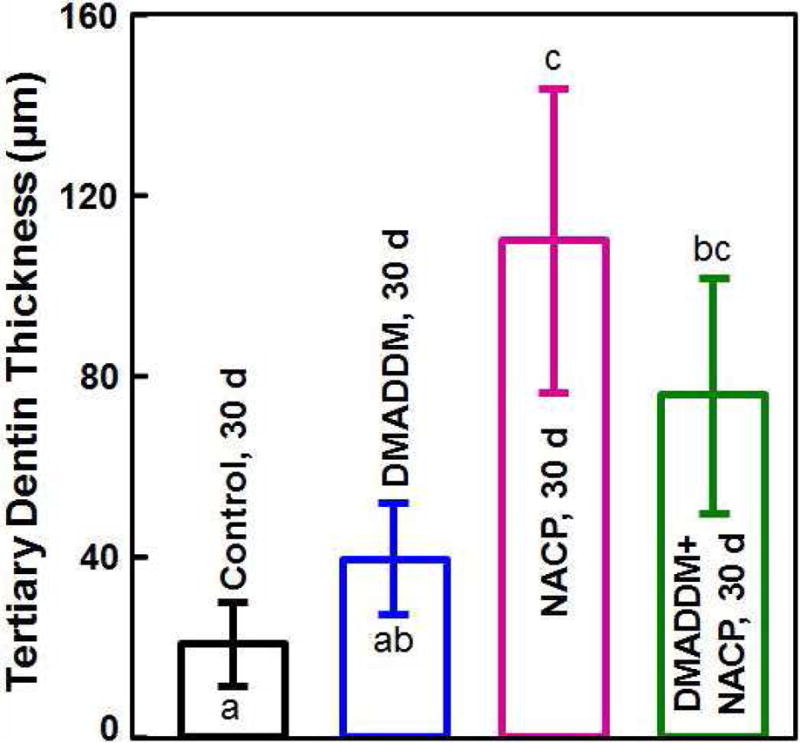
In vivo biocompatibility of antibacterial and remineralizing composite and adhesive. (A) Rat tooth model, in which the right and left molars were used. (B) Occlusal cavity was restored with bonding agent and composite. H&E images at 30 d for (C) control, and (D) DMADDM + NACP. Star indicates inflammatory cells. Blood vessels are indicated by arrows. Control group exhibited slight inflammation. NACP group and DMADDM + NACP group showed normal pulp without inflammatory response, along with greater tertiary dentin thicknesses. (E) Tertiary dentin thickness data. Different letters indicate significantly different values (p < 0.05).
Figures 6A, 6B, and 6E: Adapted from Li F, Wang P, Weir MD, et al. Evaluation of antibacterial and remineralizing nanocomposite and adhesive in rat tooth cavity model. Acta Biomater 2014;10(6):2804–2813; with permission.
Figures 6C and 6D: From Li F, Wang P, Weir MD, et al. Evaluation of antibacterial and remineralizing nanocomposite and adhesive in rat tooth cavity model. Acta Biomater 2014;10(6):2810; with permission.
Regarding future work, while current restorative materials are relatively inert and replace the missing tooth structures, it would be highly desirable for future restorative materials to not only replace the missing tooth volume, but also be bioactive and possess beneficial therapeutic properties. The development of new bioactive restorative materials including remineralizing and antibacterial characteristics, while still in a relatively early stage, has achieved significant progress. Nonetheless, further studies are needed to improve and optimize the new bioactive materials, and investigate their antibacterial and remineralization efficacy in human in situ or in vivo models under clinically-relevant conditions. More studies are also needed to thoroughly understand the remineralizing and antibacterial mechanisms and systematically establish the structure-property-clinical performance relationships for the new class of bioactive restorative materials.
Conclusions
Currently available dental composites and bonding agents are typically bio-inert and replace the missing volume of the tooth, but lack bioactivity to interact with either oral bacteria or pulp cells, despite biofilm acids and recurrent caries being a major cause of restoration failures. Several key approaches are being investigated to develop a new generation of bioactive dental composites and bonding agents with therapeutic functions. One approach incorporates calcium phosphate nanoparticles into composites and adhesives to remineralize existing lesions and inhibit future caries from occurring. A second approach develops antibacterial composites and bonding agents by copolymerizing QAMs in resins, to suppress biofilm growth and acid production. A third approach imparts a protein-repellent capability to resins, to repel proteins from the surface, thereby making it more difficult for bacteria to attach to the surface. A forth approach combines multiple bioactive agents for synergistic effects. For example, the use of both protein-repellent and antibacterial agents has been shown to result in a much greater reduction in biofilm growth than using a single agent alone. Furthermore, combining calcium phosphate nanoparticles with protein-repellent and antibacterial agents could yield a resin with not only much less biofilm plaque buildup and acid production, but also with remineralization and acid-neutralizing capabilities. In vitro cell studies and in vivo animal models have indicated that these antibacterial and remineralizing materials not only possessed cytotoxicity similar to, or less than, existing dental monomers and resins, but also induced milder pulpal inflammation and facilitated the healing of the dentin-pulp complex. This new class of bioactive materials with remineralizing and antibacterial properties is promising to reverse tooth decay, regain lost minerals and inhibit recurrent caries.
Key points.
-
◆
Secondary caries is a primary reason for restoration failures. It is beneficial to develop a new generation of bioactive composites and bonding agents with therapeutic functions.
-
◆
Calcium phosphate nanoparticles in composites and adhesives can remineralize existing lesions and inhibit future caries from occurring.
-
◆
Antibacterial resins can suppress biofilms and acid production. Protein-repellent resins can make it more difficult for bacteria to attach to the surface dental materials.
-
◆
Combining multiple bioactive agents synergistically can lead to much greater reductions in oral biofilms than using a single agent, while also achieving remineralization.
-
◆
The new antibacterial and remineralizing dental materials not only possess cytotoxicity similar to existing dental monomers and resins, but also can induce milder pulpal inflammation and facilitate the healing of dentin-pulp complex in animal models.
Acknowledgments
We thank Drs. Lei Cheng, Mary Anne S. Melo, Satoshi Imazato, Jack Ferracane, Ashraf F. Fouad, Joseph M. Antonucci, Nancy J. Lin, Sheng Lin-Gibson, Laurence C. Chow, Xianju Xie, Ling Zhang, Fang Li and Lin Wang for discussions and experimental help. Many of the studies cited in this article were supported by NIH R01DE17974 (HX), National Natural Science Foundation of China grant 81400540 (KZ), and a seed fund (HX) from the University of Maryland School of Dentistry.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement
There is no conflict of interest for all authors.
References
- 1.Bayne SC, Thompson JY, Swift EJ, Stamatiades P, Wilkerson M. A characterization of first-generation flowable composites. J Am Dent Assoc. 1998;129:567–577. doi: 10.14219/jada.archive.1998.0274. [DOI] [PubMed] [Google Scholar]
- 2.Watts DC, Marouf AS, Al-Hindi AM. Photo-polymerization shrinkage-stress kinetics in resin-composites: methods development. Dent Mater. 2003;19:1–11. doi: 10.1016/s0109-5641(02)00123-9. [DOI] [PubMed] [Google Scholar]
- 3.Drummond JL. Degradation, fatigue, and failure of resin dental composite materials. J Dent Res. 2008;87:710–719. doi: 10.1177/154405910808700802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lynch CD. Successful posterior composites. London: Quintessence Publishing Co; 2008. [Google Scholar]
- 5.Ferracane JL. Resin composite - State of the art. Dent Mater. 2011;27:29–38. doi: 10.1016/j.dental.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 6.Demarco FF, Correa MB, Cenci MS, Moraes RR, Opdam NJM. Longevity of posterior composite restorations: Not only a matter of materials. Dent Mater. 2012:87–101. doi: 10.1016/j.dental.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 7.Ferracane JL, Hilton TJ. Polymerization stress–is it clinically meaningful? Dent Mater. 2016;32:1–10. doi: 10.1016/j.dental.2015.06.020. [DOI] [PubMed] [Google Scholar]
- 8.Xu X, Burgess JO. Compressive strength, fluoride release and recharge of fluoride-releasing materials. Biomaterials. 2003;24:2451–61. doi: 10.1016/s0142-9612(02)00638-5. [DOI] [PubMed] [Google Scholar]
- 9.Samuel SP, Li S, Mukherjee I, Guo Y, Patel AC, Baran GR, Wei Y. Mechanical properties of experimental dental composites containing a combination of mesoporous and nonporous spherical silica as fillers. Dent Mater. 2009;25:296–301. doi: 10.1016/j.dental.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 10.Milward PJ, Adusei GO, Lynch CD. Improving some selected properties of dental polyacid-modified composite resins. Dent Mater. 2011;27:997–1002. doi: 10.1016/j.dental.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 11.Hosoya Y, Shiraishi T, Odatsu T, Nagafuji J, Kotaku M, Miyazaki M, Powers JM. Effects of polishing on surface roughness, gloss, and color of resin composites. J Oral Sci. 2011;53:283–291. doi: 10.2334/josnusd.53.283. [DOI] [PubMed] [Google Scholar]
- 12.Wei YJ, Silikas N, Zhang ZT, Watts DC. Hygroscopic dimensional changes of self-adhering and new resin-matrix composites during water sorption/desorption cycles. Dent Mater. 2011;27:259–66. doi: 10.1016/j.dental.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 13.Ferracane JL, Giannobile WV. Novel biomaterials and technologies for the dental, oral, and craniofacial structures. J Dent Res. 2014;93:1185–6. doi: 10.1177/0022034514556537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stansbury JW, Idacavage MJ. 3D printing with polymers: Challenges among expanding options and opportunities. Dent Mater. 2016;32:54–64. doi: 10.1016/j.dental.2015.09.018. [DOI] [PubMed] [Google Scholar]
- 15.Deligeorgi V, Mjor IA, Wilson NH. An overview of reasons for the placement and replacement of restorations. Prim Dent Care. 2001;8:5–11. doi: 10.1308/135576101771799335. [DOI] [PubMed] [Google Scholar]
- 16.National Institute of Dental and Craniofacial Research (NIDCR) announcement # 13-DE-102. Dental Resin Composites and Caries. 2009 Mar 5; [Google Scholar]
- 17.Pallesen U, van Dijken JW, Halken J, Hallonsten AL, Höigaard R. A prospective 8-year follow-up of posterior resin composite restorations in permanent teeth of children and adolescents in Public Dental Health Service: reasons for replacement. Clin Oral Investig. 2014;18:819–827. doi: 10.1007/s00784-013-1052-x. [DOI] [PubMed] [Google Scholar]
- 18.Bernardo M, Luis H, Martin MD, Leroux BG, Rue T, Leitão J, DeRouen TA. Survival and reasons for failure of amalgam versus composite posterior restorations placed in a randomized clinical trial. J Am Dent Assoc. 2007;138:775–783. doi: 10.14219/jada.archive.2007.0265. [DOI] [PubMed] [Google Scholar]
- 19.Bourbia M, Ma D, Cvitkovitch DG, Santerre JP, Finer Y. Cariogenic bacteria degrade dental resin composites and adhesives. J Dent Res. 2013;92:989–994. doi: 10.1177/0022034513504436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Svanberg M, Major IA, Ørstavik D. Mutans streptococci in plaque from margins of amalgam, composite and glass ionomer restorations. J Dent Res. 1990;69:861–864. doi: 10.1177/00220345900690030601. [DOI] [PubMed] [Google Scholar]
- 21.Beyth N, Bahir R, Matalon S, Domb AJ, Weiss EI. Streptococcus mutans biofilm changes surface-topography of resin composites. Dent Mater. 2008;24:732–736. doi: 10.1016/j.dental.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 22.Khalichi P, Singh J, Cvitkovitch DG, Santerre JP. The influence of triethylene glycol derived from dental composite resins on the regulation of Streptococcus mutans gene expression. Biomaterials. 2009;30:452–459. doi: 10.1016/j.biomaterials.2008.09.053. [DOI] [PubMed] [Google Scholar]
- 23.Breschi L, Mazzoni A, Ruggeri A, Cadenaro M, Di Lenarda R, De Stefano Dorigo E. Dental adhesion review: aging and stability of the bonded interface. Dent Mater. 2008;24:90–101. doi: 10.1016/j.dental.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 24.Spencer P, Ye Q, Park JG, Topp EM, Misra A, Marangos O, Wang Y, Bohaty BS, Singh V, Sene F, Eslick J, Camarda K, Katz JL. Adhesive/dentin interface: The weak link in the composite restoration. Annals Biomed Eng. 2010;38:1989–2003. doi: 10.1007/s10439-010-9969-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khvostenko D, Salehi S, Naleway SE, Hilton TJ, Ferracane JL, Mitchell JC, Kruzic JJ. Cyclic mechanical loading promotes bacterial penetration along composite restoration marginal gaps. Dent Mater. 2015;31:702–10. doi: 10.1016/j.dental.2015.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Skrtic D, Antonucci JM, Eanes ED, Eichmiller FC, Schumacher GE. Physiological evaluation of bioactive polymeric composites based on hybrid amorphous calcium phosphates. J Biomed Mater Res. 2000;53B:381–391. doi: 10.1002/1097-4636(2000)53:4<381::aid-jbm12>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 27.Dickens SH, Flaim GM, Takagi S. Mechanical properties and biochemical activity of remineralizing resin-based Ca-PO4 cements. Dent Mater. 2003;19:558–566. doi: 10.1016/s0109-5641(02)00105-7. [DOI] [PubMed] [Google Scholar]
- 28.Langhorst SE, O’Donnell JNR, Skrtic D. In vitro remineralization of enamel by polymeric amorphous calcium phosphate composite: Quantitative microradiographic study. Dent Mater. 2009;25:884–891. doi: 10.1016/j.dental.2009.01.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu HHK, Sun L, Weir MD, Antonucci JM, Takagi S, Chow LC. Nano dicalcium phosphate anhydrous-whisker composites with high strength and Ca and PO4 release. J Dent Res. 2006;85:722–727. doi: 10.1177/154405910608500807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu HHK, Weir MD, Sun L, Moreau JL, Takagi S, Chow LC, Antonucci JM. Strong nanocomposites with Ca, PO4 and F release for caries inhibition. J Dent Res. 2010;89:19–28. doi: 10.1177/0022034509351969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang B, Flaim G, Dickens SH. Remineralization of human natural caries and artificial caries-like lesions with an experimental whisker-reinforced ART composite. Acta Biomater. 2011;7:2303–9. doi: 10.1016/j.actbio.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marovic D, Tarle Z, Hiller KA, Müller R, Rosentritt M, Skrtic D, Schmalz G. Reinforcement of experimental composite materials based on amorphous calcium phosphate with inert fillers. Dent Mater. 2014;30:1052–60. doi: 10.1016/j.dental.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 33.Weir MD, Chow LC, Xu HHK. Remineralization of demineralized enamel via calcium phosphate nanocomposite. J Dent Res. 2012;91:979–984. doi: 10.1177/0022034512458288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Melo MAS, Weir MD, Rodrigues LKA, Xu HHK. Novel calcium phosphate nanocomposite with caries-inhibition in a human in situ model. Dent Mater. 2013;29:231–240. doi: 10.1016/j.dental.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Melo MAS, Cheng L, Zhang K, Weir MD, Rodrigues LKA, Xu HHK. Novel dental adhesives containing nanoparticles of silver and amorphous calcium phosphate. Dent Mater. 2013;29:199–210. doi: 10.1016/j.dental.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moreau JL, Sun L, Chow LC, Xu HHK. Mechanical and acid neutralizing properties and inhibition of bacterial growth of amorphous calcium phosphate dental nanocomposite. J Biomed Mater Res Part B. 2011;98:80–88. doi: 10.1002/jbm.b.31834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Melo MAS, Orrego S, Weir MD, Xu HHK, Arola DD. Designing multiagent dental materials for enhanced resistance to biofilm damage at the bonded interface. ACS Applied Mater & Interfaces. 2016 doi: 10.1021/acsami.6b01923. [DOI] [PubMed] [Google Scholar]
- 38.Salehi S, Davis HB, Ferracane JL, Mitchell JC. Sol-gel-derived bioactive glasses demonstrate antimicrobial effects on common oral bacteria. Am J Dent. 2015;28:111–5. [PubMed] [Google Scholar]
- 39.Khvostenko D, Hilton TJ, Ferracane JL, Mitchell JC, Kruzic JJ. Bioactive glass fillers reduce bacterial penetration into marginal gaps for composite restorations. Dent Mater. 2016;32:73–81. doi: 10.1016/j.dental.2015.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang L, Weir MD, Chow LC, Antonucci JM, Chen J, Xu HHK. Novel rechargeable calcium phosphate dental nanocomposite. Dent Mater. 2016;32:285–293. doi: 10.1016/j.dental.2015.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang L, Weir MD, Hack G, Fouad AF, Xu HHK. Rechargeable dental adhesive with calcium phosphate nanoparticles for long-term ion release to inhibit caries. J Dent. 2015;43:1587–1595. doi: 10.1016/j.jdent.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.ten Cate JM. Biofilms, a new approach to the microbiology of dental plaque. Odontology. 2006;94:1–9. doi: 10.1007/s10266-006-0063-3. [DOI] [PubMed] [Google Scholar]
- 43.Totiam P, Gonzalez-Cabezas C, Fontana MR, Zero DT. A new in vitro model to study the relationship of gap size and secondary caries. Caries Res. 2007;41:467–473. doi: 10.1159/000107934. [DOI] [PubMed] [Google Scholar]
- 44.Imazato S, Ehara A, Torii M, Ebisu S. Antibacterial activity of dentine primer containing MDPB after curing. J Dent. 1998;26:267–271. doi: 10.1016/s0300-5712(97)00013-4. [DOI] [PubMed] [Google Scholar]
- 45.Imazato S. Bio-active restorative materials with antibacterial effects: new dimension of innovation in restorative dentistry. Dent Mater J. 2009;28:11–19. doi: 10.4012/dmj.28.11. [DOI] [PubMed] [Google Scholar]
- 46.Antonucci JM, Zeiger DN, Tang K, Lin-Gibson S, Fowler BO, Lin NJ. Synthesis and characterization of dimethacrylates containing quaternary ammonium functionalities for dental applications. Dent Mater. 2012;28:219–228. doi: 10.1016/j.dental.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weng Y, Howard L, Guo X, Chong VJ, Gregory RL, Xie D. A novel antibacterial resin composite for improved dental restoratives. J Mater Sci. 2012;23:1553–1561. doi: 10.1007/s10856-012-4629-z. [DOI] [PubMed] [Google Scholar]
- 48.Xu X, Wang Y, Liao S, Wen ZT, Fan Y. Synthesis and characterization of antibacterial dental monomers and composites. J Biomed Mater Res Part B: Applied Biomater. 2012;100:1151–1162. doi: 10.1002/jbm.b.32683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Imazato S, Ma S, Chen JH, Xu HHK. Therapeutic polymers for dental adhesives: Loading resins with bio-active components. Dent Mater. 2014;30:97–104. doi: 10.1016/j.dental.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Imazato S, Kinomoto Y, Tarumi H, Ebisu S, Tay FR. Antibacterial activity and bonding characteristics of an adhesive resin containing antibacterial monomer MDPB. Dent Mater. 2003;19:313–319. doi: 10.1016/s0109-5641(02)00060-x. [DOI] [PubMed] [Google Scholar]
- 51.Imazato S, Kuramoto A, Takahashi Y, Ebisu S, Peters MC. In vitro antibacterial effects of the dentin primer of Clearfil Protect Bond. Dent Mater. 2006;22:527–532. doi: 10.1016/j.dental.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 52.Li F, Chen J, Chai Z, Zhang L, Xiao Y, Fang M, Ma S. Effects of a dental adhesive incorporating antibacterial monomer on the growth, adherence and membrane integrity of Streptococcus mutans. J Dent. 2009;37:289–296. doi: 10.1016/j.jdent.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 53.Beyth N, Yudovin-Farber I, Bahir R, Domb AJ, Weiss EI. Antibacterial activity of dental composites containing quaternary ammonium polyethylenimine nanoparticles against Streptococcus mutans. Biomaterials. 2006;27:3995–4002. doi: 10.1016/j.biomaterials.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 54.Xie D, Weng Y, Guo X, Zhao J, Gregory RL, Zheng C. Preparation and evaluation of a novel glass-ionomer cement with antibacterial functions. Dent Mater. 2011;27:487–496. doi: 10.1016/j.dental.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 55.Cheng L, Weir MD, Xu HH, Antonucci JM, Kraigsley AM, Lin NJ, Lin-Gibson S, Zhou X. Antibacterial amorphous calcium phosphate nanocomposites with a quaternary ammonium dimethacrylate and silver nanoparticles. Dent Mater. 2012;28:561–572. doi: 10.1016/j.dental.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cheng L, Zhang K, Melo MA, Weir MD, Zhou X, Xu HH. Anti-biofilm dentin primer with quaternary ammonium and silver nanoparticles. Dent Mater. 2012;91:598–604. doi: 10.1177/0022034512444128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang K, Melo MA, Cheng L, Weir MD, Bai Y, Xu HH. Effect of quaternary ammonium and silver nanoparticle-containing adhesives on dentin bond strength and dental plaque microcosm biofilms. Dent Mater. 2012;28:842–852. doi: 10.1016/j.dental.2012.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pashley DH, Tay FR, Breschi L, Tjaderhane L, Carvalho RM, Carrilho M, Tezvergil-Mutluay A. State of the art etch-and-rinse adhesives. Dent Mater. 2011;27:1–16. doi: 10.1016/j.dental.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Spencer P, Wang Y. Adhesive phase separation at the dentin interface under wet bonding conditions. J Biomed Mater Res. 2002;62:447–456. doi: 10.1002/jbm.10364. [DOI] [PubMed] [Google Scholar]
- 60.Ikemura K, Tay FR, Endo T, Pashley DH. A review of chemical-approach and ultramorphological studies on the development of fluoride-releasing dental adhesives comprising new pre-reacted glass ionomer (PRG) fillers. Dent Mater J. 2008;27:315–329. doi: 10.4012/dmj.27.315. [DOI] [PubMed] [Google Scholar]
- 61.Ritter AV, Swift EJ, Jr, Heymann HO, Sturdevant JR, Wilder AD., Jr An eight-year clinical evaluation of filled and unfilled one-bottle dental adhesives. J Am Dent Assoc. 2009;140:28–37. doi: 10.14219/jada.archive.2009.0015. [DOI] [PubMed] [Google Scholar]
- 62.Van Meerbeek B, Yoshihara K, Yoshida Y, Mine A, De Munck J. State of the art of self-etch adhesives. Dent Mater. 2011;27:17–28. doi: 10.1016/j.dental.2010.10.023. [DOI] [PubMed] [Google Scholar]
- 63.Blum IR, Hafiana K, Curtis A, Barbour ME, Attin T, Lynch CD, Jagger DC. The effect of surface conditioning on the bond strength of resin composite to amalgam. J Dent. 2012;40:15–21. doi: 10.1016/j.jdent.2011.10.019. [DOI] [PubMed] [Google Scholar]
- 64.Imazato S, Tay FR, Kaneshiro AV, Takahashi Y, Ebisu S. An in vivo evaluation of bonding ability of comprehensive antibacterial adhesive system incorporating MDPB. Dent Mater. 2007;23:170–176. doi: 10.1016/j.dental.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 65.Hiraishi N, Yiu CK, King NM, Tay FR. Effect of chlorhexidine incorporation into a self-etching primer on dentine bond strength of a luting cement. J Dent. 2010;38:496–502. doi: 10.1016/j.jdent.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 66.Simoncic B, Tomcis B. Structures of novel antimicrobial agents for textiles - a review. Textile Res J. 2010;80:1721–1737. [Google Scholar]
- 67.Li F, Weir MD, Xu HK. Effects of quaternary ammonium chain length on antibacterial bonding agents. J Dent Res. 2013;92:932–938. doi: 10.1177/0022034513502053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang K, Cheng L, Weir MD, Bai YX, Xu HHK. Effects of quaternary ammonium chain length on antibacterial and remineralizing calcium phosphate nanocomposite. Int J Oral Sci. 2016;30(8):45–53. doi: 10.1038/ijos.2015.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang K, Cheng L, Wu EJ, Weir MD, Bai Y, Xu HHK. Effect of water-aging on dentin bond strength and anti-biofilm activity of bonding agent containing antibacterial monomer dimethylaminododecyl methacrylate. J Dent. 2013;41:504–513. doi: 10.1016/j.jdent.2013.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cheng L, Zhang K, Weir MD, Liu H, Zhou X, Xu HHK. Effects of antibacterial primers with quaternary ammonium and nano-silver on S. mutans impregnated in human dentin blocks. Dent Mater. 2013;29:462–472. doi: 10.1016/j.dental.2013.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Donmez N, Belli S, Pashley DH, Tay FR. Ultrastructural correlates of in vivo/in vitro bond degradation in self-etch adhesives. J Dent Res. 2005;84:355–359. doi: 10.1177/154405910508400412. [DOI] [PubMed] [Google Scholar]
- 72.Breschi L, Mazzoni A, Nato F, Carrilho M, Visintini E, Tjaderhane L, Ruggeri A, Jr, Tay FR, Dorigo Ede S, Pashley DH. Chlorhexidine stabilizes the adhesive interface: a 2-year in vitro study. Dent Mater. 2010;26:320–325. doi: 10.1016/j.dental.2009.11.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tezvergil-Mutluay A, Agee KA, Uchiyama T, Imazato S, Mutluay MM, Cadenaro M, Breschi L, Nishitani Y, Tay FR, Pashley DH. The inhibitory effects of quaternary ammonium methacrylates on soluble and matrix-bound MMPs. J Dent Res. 2011;90:535–540. doi: 10.1177/0022034510389472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li F, Majd H, Weir MD, Arola DD, Xu HHK. Inhibition of matrix metalloproteinase activity in human dentin via novel antibacterial monomer. Dent Mater. 2015;31:284–292. doi: 10.1016/j.dental.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lendenmann U, Grogan J, Oppenheim FG. Saliva and dental pellicle – a review. Adv Dent Res. 2000;14:22–28. doi: 10.1177/08959374000140010301. [DOI] [PubMed] [Google Scholar]
- 76.Donlan RM, Costerton JW. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbio Reviews. 2002;15:167–93. doi: 10.1128/CMR.15.2.167-193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Müller R, Eidt A, Hiller KA, Katzur V, Subat M, Schweikl H, Imazato S, Ruhl S, Schmalz G. Influences of protein films on antibacterial or bacteria-repellent surface coatings in a model system using silicon wafers. Biomaterials. 2009;30:4921–9. doi: 10.1016/j.biomaterials.2009.05.079. [DOI] [PubMed] [Google Scholar]
- 78.Ishihara K, Nomura H, Mihara T, Kurita K, Iwasaki Y, Nakabayashi N. Why do phospholipid polymers reduce protein adsorption? J Biomed Mater Res. 1998;39:323–30. doi: 10.1002/(sici)1097-4636(199802)39:2<323::aid-jbm21>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 79.Lewis AL. Phosphorylcholine-based polymers and their use in the prevention of biofouling. Colloids and Surfaces B: Biointerfaces. 2000;18:261–75. doi: 10.1016/s0927-7765(99)00152-6. [DOI] [PubMed] [Google Scholar]
- 80.Sibarani J, Takai M, Ishihara K. Surface modification on microfluidic devices with 2-methacryloyloxyethyl phosphorylcholine polymers for reducing unfavorable protein adsorption. Colloids and Surfaces B: Biointerfaces. 2007;54:88–93. doi: 10.1016/j.colsurfb.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 81.Kuiper KK, Nordrehaug JE. Early mobilization after protamine reversal of heparin following implantation of phosphorylcholine-coated stents in totally occluded coronary arteries. Am J Cardiology. 2000;85:698–702. doi: 10.1016/s0002-9149(99)00843-7. [DOI] [PubMed] [Google Scholar]
- 82.Lewis AL, Tolhurst LA, Stratford PW. Analysis of a phosphorylcholine-based polymer coating on a coronary stent pre-and post-implantation. Biomaterials. 2002;23:1697–706. doi: 10.1016/s0142-9612(01)00297-6. [DOI] [PubMed] [Google Scholar]
- 83.Zhang N, Ma J, Melo MAS, Weir MD, Bai YX, Xu HHK. Protein-repellent and antibacterial dental composite to inhibit biofilms and caries. J Dent. 2015;43:225–234. doi: 10.1016/j.jdent.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang N, Weir MD, Bai YX, Xu HHK. Development of novel dental adhesive with double benefits of protein-repellent and antibacterial capabilities. Dent Mater. 2015;31:845–54. doi: 10.1016/j.dental.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 85.Zhang N, Weir MD, Chen C, Melo MAS, Bai YX, Xu HHK. Orthodontic cement with protein-repellent and antibacterial properties and the release of calcium and phosphate ions. J Dent. 2016 doi: 10.1016/j.jdent.2016.05.001. http://dx.doi.org/10.1016/j.jdent.2016.05.001. [DOI] [PubMed]
- 86.Chapman JA, Roberts WE, Eckert GJ, Kula KS, Gonza´ lez-Cabezas C. Risk factors for incidence and severity of white spot lesions during treatment with fixed orthodontic appliances. Am J Orthod Dentofacial Orthop. 2010;138:188–94. doi: 10.1016/j.ajodo.2008.10.019. [DOI] [PubMed] [Google Scholar]
- 87.Rogers S, Chadwick B, Treasure E. Fluoride-containing orthodontic adhesives and decalcification in patients with fixed appliances: a systematic review. Am J Orthod Dentofacial Orthop. 2010;138:390.e1–8. doi: 10.1016/j.ajodo.2010.02.025. [DOI] [PubMed] [Google Scholar]
- 88.Imazato S, Ebi N, Tarumi H, Russell RR, Kaneko T, Ebisu S. Bactericidal activity and cytotoxicity of antibacterial monomer MDPB. Biomaterials. 1999;20:899–903. doi: 10.1016/s0142-9612(98)00247-6. [DOI] [PubMed] [Google Scholar]
- 89.Imazato S, Tarumi H, Ebi N, Ebisu S. Cytotoxic effects of composite restorations employing self-etching primers or experimental antibacterial primers. J Dent. 2000;28:61–67. doi: 10.1016/s0300-5712(99)00039-1. [DOI] [PubMed] [Google Scholar]
- 90.Nishida M, Imazato S, Takahashi Y, Ebisu S, Ishimoto T, Nakano T, Yasuda Y, Saito T. The influence of the antibacterial monomer 12-methacryloyloxydodecylpyridinium bromide on the proliferation, differentiation and mineralization of odontoblast-like cells. Biomaterials. 2010;31:1518–32. doi: 10.1016/j.biomaterials.2009.11.023. [DOI] [PubMed] [Google Scholar]
- 91.Li F, Weir MD, Fouad AF, Xu HH. Time-kill behaviour against eight bacterial species and cytotoxicity of antibacterial monomers. J Dent. 2013;41:881–891. doi: 10.1016/j.jdent.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Imazato S, Kaneko T, Takahashi Y, Noiri Y, Ebisu S. In vivo antibacterial effects of dentin primer incorporating MDPB. Oper Dent. 2004;29–4:369–375. [PubMed] [Google Scholar]
- 93.Cox CF, White KC, Ramus DL, Farmer JB, Snuggs HM. Reparative dentin: factors affecting its deposition. Quintessence Int. 1992;23:257–270. [PubMed] [Google Scholar]
- 94.Tziafas D, Alvanou A, Papadimitriou S, Gasic J, Komnenou A. Effects of recombinant basic fibroblast growth factor, insulin-like growth factor-II and transforming growth factor-beta 1 on dog dental pulp cells in vivo. Arch Oral Biol. 1998;43:431–444. doi: 10.1016/s0003-9969(98)00026-0. [DOI] [PubMed] [Google Scholar]
- 95.Smith AJ, Tobias RS, Plant CG, Browne RM, Lesot H, Ruch JV. In vivo morphogenetic activity of dentine matrix proteins. J Biol Buccale. 1990;18:123–129. [PubMed] [Google Scholar]
- 96.Dammaschke T. Rat molar teeth as a study model for direct pulp capping research in dentistry. Lab Anim. 2010;44:1–6. doi: 10.1258/la.2009.008120. [DOI] [PubMed] [Google Scholar]
- 97.Kawagishi E, Nakakura-Ohshima K, Nomura S, Ohshima H. Pulpal responses to cavity preparation in aged rat molars. Cell Tissue Res. 2006;326:111–122. doi: 10.1007/s00441-006-0230-4. [DOI] [PubMed] [Google Scholar]
- 98.Li F, Wang P, Weir MD, Fouad AF, Xu HHK. Evaluation of novel antibacterial and remineralizing nanocomposite and adhesive in rat tooth cavity model. Acta Biomater. 2014;10:2804–2813. doi: 10.1016/j.actbio.2014.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]


