Abstract
Di(2-ethylhexyl) phthalate (DEHP) is a plasticizer found in polyvinyl chloride products such as vinyl flooring, plastic food containers, medical devices, and children's toys. DEHP is a ubiquitous environmental contaminant and is a known endocrine disrupting chemical. Little is known about the effects of prenatal DEHP exposure on the ovary and whether effects occur in subsequent generations. Thus, we tested the hypothesis that prenatal exposure to DEHP disrupts ovarian functions in the F1, F2, and F3 generations of female mice. To test this hypothesis, pregnant CD-1 mice were orally dosed with corn oil (vehicle control) or DEHP (20 and 200 μg/kg/day and 200, 500, and 750 mg/kg/day) daily from gestation day 10.5 until birth (7–28 dams/treatment group). F1 females were mated with untreated males to obtain the F2 generation, and F2 females were mated with untreated males to produce the F3 generation. On postnatal days 1, 8, 21, and 60, ovaries were collected and used for histological evaluation of follicle numbers and sera were used to measure progesterone, testosterone, 17β-estradiol, luteinizing hormone, and follicle stimulating hormone levels. In the F1 generation, prenatal exposure to DEHP disrupted body and organ weights, decreased folliculogenesis, and increased serum 17β-estradiol levels. In the F2 generation, exposure to DEHP decreased body and organ weights, dysregulated folliculogenesis, and disrupted serum progesterone levels. In the F3 generation, DEHP exposure accelerated folliculogenesis. These data suggest that prenatal exposure to DEHP leads to adverse multigenerational and transgenerational effects on ovarian function.
Keywords: DEHP, di(2-ethylhexyl) phthalate, phthalate, ovary, transgenerational, F3 generation, folliculogenesis, prenatal, female reproduction, follicle
Summary Sentence
Developmental exposure to di(2-ethylhexyl) phthalate adversely affects ovarian functions in multiple generations of mice.
Introduction
Phthalates are a family of synthetic chemicals that are additives and act as plasticizers to confer flexibility and prevent plastics from becoming brittle [1]. Di(2-ethylhexyl) phthalate (DEHP) is a common plasticizer found in polyvinyl chloride products such as consumer products, medical equipment, and building products [1–3]. DEHP is incorporated into a multitude of products including personal care products, medical equipment (i.e. blood and I.V. bags), car upholstery, food and beverage containers, and building materials [3]. On average, 300 million pounds of DEHP are produced annually in the United States [1]. DEHP is noncovalently bound; therefore, it readily leaches from products and into the environment after repeated use, heating, and cleaning [4]. Humans are exposed to DEHP by oral ingestion, inhalation, and dermal contact at an average range between 3 and 30 μg/kg/day [3, 5]. Studies show that 100% of human urine samples test positively for DEHP and its metabolites, indicating that humans are repeatedly and continuously exposed to DEHP [6]. This is supported by the frequent detection of DEHP in various human tissues such as blood, amniotic fluid, umbilical cord blood, breast milk, and ovarian follicular fluids in humans [3, 7–10]. This is of concern because a pregnant mother and her developing offspring can be exposed to DEHP, and DEHP is a known endocrine disrupting chemical (EDC) [1–3].
Numerous studies demonstrate that exposure to DEHP exerts adverse effects on the male reproductive system. Specifically, prenatal exposure to DEHP causes testicular dysgenesis syndrome [11–14], which is characterized by testicular cancer, low or declining semen quality, high frequencies of undescended testis, and hypospadias [15, 16]. In addition, prenatal exposure to DEHP shortens anogenital distance (an indicator of in utero sex steroid hormone level disruption) [17], decreases circulating serum testosterone levels [13], and causes pubertal abnormalities [18].
In females, the effects of prenatal exposure to DEHP are less understood than in males. However, experimental studies show that prenatal exposure to DEHP decreases litter size and pup body weight [19], disrupts sex determination, causes precocious puberty in females [20], and decreases circulating estradiol levels in females [21]. Furthermore, studies indicate that prenatal exposure to DEHP increases the time for females to become pregnant and increases pup death in mice [2], and that it is associated with reduced uterine size [22], reduced testosterone: estradiol ratio and progesterone levels [23], and advanced age of pubic hair development in young girls [24].
Studies have examined the effects of DEHP exposure on the ovary because it is an important organ for reproductive processes. Furthermore, a previous study has shown that DEHP metabolites reach the ovary [25]. Specifically, the bioactive metabolite of DEHP, mono(2-ethylhexyl) phthalate (MEHP), has been detected at approximately 9.34 ng/mL in human antral fluid [25].
The ovary is a heterogeneous organ composed of different follicle types, oocytes, corpora lutea, and interstitial tissue. Any chemical exposure that interferes with the development and function of the ovary can cause severe reproductive abnormalities. Specifically, chemicals that target the formation of the primordial follicle pool cause infertility because it depletes the finite follicle reserve used for the production of ovulatory follicles [26]. Chemicals that specifically target primary, preantral, and antral follicles may lead to temporary infertility or permanent infertility. Permanent infertility may occur when the toxicant is not removed and it continuously targets the growth and function of ovarian follicles [27]. Chemicals that target the antral follicles may also interfere with the production of sex steroid hormones, leading to infertility and other reproductive disorders [28–31]. Our laboratory has previously shown that in vitro exposure to MEHP reduces follicle growth in antral follicles and decreases sex steroid hormone biosynthesis [32]. Furthermore, we have shown that DEHP exposure (20, 200, and 750 mg/kg/day) during adulthood for 10 days accelerates primordial follicle recruitment [33] and reduces the primordial follicle pool at 9 months [34]. In addition, studies indicate that postnatal exposure to DEHP (20 and 40 μg/kg) reduces the ovarian primordial follicle pool, accelerates ovarian follicular recruitment [19], and decreases ovarian concentrations of progesterone, 17β-estradiol, and androstenedione [35].
Although the direct effects of DEHP are fairly well documented on the ovary, the effects of DEHP exposure across generations are not as well understood. Previous studies have shown that maternal DEHP exposure (0.05 mg/kg/day) reduced embryo viability over several generations in female mice [36] and prenatal exposure to a mixture of plasticizers, including DEHP, increased the presence of small and large cysts in the ovary [37].
Although previous studies have shown that DEHP exposure adversely affects the ovary, they have not assessed the impact of prenatal DEHP exposure on follicle numbers in detail, sex steroid hormone production over time, and the impact of DEHP on the ovary in subsequent generations over time. Therefore, the current study was designed to evaluate the potential effects of prenatal exposure to DEHP during the second half of pregnancy on ovarian functions in the F1, F2, and F3 generations of mice. Specifically, this study tested the hypothesis that prenatal DEHP exposure adversely affects folliculogenesis, gonadotropin hormone levels, and sex steroid hormone levels in female mice over several generations.
Materials and methods
Chemicals
DEHP (99% purity) was purchased from Sigma-Aldrich (St. Louis, MO). Stock solutions of DEHP (0.022, 0.224, 560, and 840 mg/mL) were prepared by diluting the chemical in tocopherol-stripped corn oil (MP Biomedicals, Solon, OH). These stock solutions were diluted to achieve the desired doses of 20 μg/kg/day, 200 μg/kg/day, 200 mg/kg/day, 500 mg/kg/day, and 750 mg/kg/day. DEHP concentrations were chosen based on previous studies and their environmental relevance [2, 12, 32, 33]. Specifically, DEHP at 20 μg/kg/day was selected based on the United States Environmental Protection Agency published reference safe dose (https://www.epa.gov/sites/production/files/2016-09/documents/bis-2-ethylhexyl-phthalate.pdf) and falls within the estimated human exposure range based on urinary metabolite levels [5]. DEHP at 200 μg/kg/day was used because adult exposure causes abnormal estrous cyclicity and accelerates primordial follicle recruitment in female CD-1 mice [33]. DEHP 200 mg/kg/day was chosen because adult exposure has been shown to accelerate primordial follicle recruitment in females [33]. DEHP 500 mg/kg/day was selected because this dose causes abnormalities in spermatagonial stem cells across multiple generations in male CD-1 mice [12]. DEHP 750 mg/kg/day was selected because it causes in abnormal estrous cyclicity and accelerates primordial follicle recruitment in adult female CD-1 mice [33].
Animals and study design
Adult female and male CD-1 mice (Charles River) were housed at 25°C in conventional polysulfone, ventilated cages on 12L:12D cycles. The mice were provided Teklad Rodent Diet 8604 (Harlan) and highly purified water (reverse osmosis filtered) in polysulfone water bottles ad libitum. All animal procedures were approved by the University of Illinois Institutional Animal Care and Use Committee and abide by the guidelines set forth by the National Institute of Health for the Care and Use of Laboratory Animals. Investigations using experimental animals or subjects were conducted in accordance with the Society for the Study of Reproduction's specific guidelines and standards.
At 8 weeks of age, 83 female mice (F0) were mated with control mice of the same age. The female mice were monitored twice a day for the presence of a copulatory vaginal sperm plug to confirm mating. Once a copulatory vaginal sperm plug was confirmed, the presence of which was considered gestation day (GD) 0.5, the females were weighed and individually housed. Subsequently, mice were weighed twice a week to confirm successful pregnancy. From GD 10.5 until birth of the pups, pregnant dams (F0) were orally dosed once a day with the vehicle control (tocopherol-stripped corn oil), or with DEHP (20 μg/kg/day, 200 μg/kg/day, 200 mg/kg/day, 500 mg/kg/day, 750 mg/kg/day) by placing a pipette tip with the dosing solution into the cheek pouch of the mouse. This dosing regimen was selected to mimic oral exposure to DEHP in humans [1, 33]. The doses were calculated and adjusted based on daily body weights, and delivered in 25–33 μL of tocopherol-stripped corn oil. The treatment window was chosen because it is a critical time period of ovarian development. Specifically, this is when primordial germ cells arrive at the gonad [38, 39], sex determination occurs [40], and when global demethylation and imprint erasure of primordial germ cells occur [41].
Pregnant mice were allowed to deliver naturally, and the day of birth was considered postnatal day (PND) 0. Mice born from the F0 generation were labeled the F1 generation. Female mice from the F1 generation were mated with nontreated male CD-1 mice at 3 months of age to produce the F2 generation. Females from the F2 generation were mated with nontreated male CD-1 mice at 3 months of age to produce the F3 generation. The 200 mg/kg/day treatment group was not continued past the F2 generation. Body weights in all generations were measured from PND 1–60, and at 3, 6, and 9 months of age during the collection of organs and during set intervals during adulthood. Mice were euthanized on PNDs 1 and 8 by decapitation, and on PNDs 21 and 60 by carbon dioxide asphyxiation followed by cervical dislocation.
Histological evaluation of follicle numbers
Ovaries from F1, F2, and F3 females at PND 1, 8, 21, and 60 were collected, oviducts and surrounding tissues were removed, weighed in pairs, and placed in Dietrichs fixative. Fixed ovaries were placed in paraffin and serial sectioned at 5 μm for PND 1 ovaries or at 8 μm for PND 8, 21, and 60 ovaries. The sections then were mounted on glass slides, stained with Weigert hematoxylin and picric-acid methyl blue, and covered with a glass coverslip. Every fifth section of the ovary at PND 1 or every tenth section of the ovary at PND 8, 21, and 60 was used to count the numbers of germ cells, primordial follicles, primary follicles, preantal follicles, antral follicles, and atretic follicles as described [2, 42]. Germ cells were defined as round in appearance with a nucleus. Primordial follicles were defined as follicles with an oocyte, surrounded by a single layer of squamous granulosa cells. Primary follicles consisted of an oocyte, surrounded by a single layer of cuboidal granulosa cells. Preantral follicles contained an oocyte, surrounded by multiple layers of cuboidal granulosa cells and theca cells. Antral follicles consisted of an oocyte, surrounded by numerous layers of cuboidal granulosa cells, theca cells, and a fluid filled antrum. Preantral and antral follicles were only counted if they contained a nucleus in the oocyte to avoid “double counts” due to the large follicle size that can span multiple sections. Follicles that were transitioning between stages were counted as the more immature stage. Atretic follicles were counted as preantral or antral follicles that contained 10% or greater number of apoptotic bodies. The total numbers of all follicles, total numbers of each healthy follicle type, percentages of follicle types, and percentages of atretic preantral and antral follicles were recorded. To calculate the percentages of follicle types, the number of the follicle type was divided by the total number of all follicles multiplied by 100. This calculation allows us to identify shifts in follicle pools, which may indicate either accelerated or inhibited folliculogenesis. To calculate the percentage of atretic follicles, the number of atretic preantral and atretic antral follicles were combined, divided by the number of healthy preantral and antral follicle types, and multiplied by 100. Corpora lutea were quantified at PND 60 by inspecting the individual progression of the corpora lutea throughout the ovary in all serial sections [43]. This was done to avoid double counting of corpora lutea because they do not have a landmark such as nuclear material in the oocyte observed in antral follicles. All sections were examined without knowledge of treatment group.
Analysis of sex hormone levels
Tissues and sera were collected and analyzed as described below. Mice do not cycle at PNDs 1–21; therefore, serum samples were collected at exactly 1, 8, or 21 days. Mice at PND 60 are cycling; therefore, serum samples were collected when the mice were in diestrus/metestrus to minimize fluctuations due to cycle day. All serum samples were submitted to the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core lab without knowledge of treatment groups to measure progesterone, testosterone, and 17β-estradiol using enzyme-linked immunosorbent assays and to measure follicle stimulating hormone (FSH) and luteinizing hormone (LH) using radioimmunoassay. The lowest limit of detections were 0.15 ng/mL for progesterone, 10 ng/dL for testosterone, 3 pg/mL for 17β-estradiol, 1.17 ng/mL for FSH, and 0.04 ng/mL for LH. If the assays measured less than the lowest limit of detection, the value was substituted with the lowest limit of detection/√2. The intra- and interassay coefficients of variability were less than 10% (https://med.virginia.edu/research-in-reproduction/wp-content/uploads/sites/311/2016/08/2016-INTRA-INTER-ASSAY-CVs__032316.pdf). At PND 21, low quantities of serum prevented measurements of serum progesterone levels for the F1 generation.
Statistical analyses
Data were expressed as the mean ± standard error of the mean. In all generations, data from multiple female pups originating from the same litter were averaged and combined as n = 1, and data from at least three separate litters were used in the analyses. If samples were less than 3, then statistical tests were not performed, but data were presented. Data were analyzed by comparing treatment groups with control using SPSS software (SPSS Inc., Chicago, IL). Outliers were removed using the GraphPad outlier calculator for the Grubb test (GraphPad Software Inc., La Jolla, CA). To test for cohort differences, data were tested using a general linear model univariate test. If there was an interaction effect between treatment and cohort in the tests of between-subjects effects, then the data from the first two cohorts were analyzed. If no interaction effect between treatment and cohort occurred, then all three cohorts were analyzed as one. Data that were continuous were assessed for normal distribution by the Shapiro-Wilk analysis. If data met assumptions of normal distribution and homogeneity of variance, data were analyzed by one-way analysis of variance followed by Dunnett (two-sided) post hoc comparisons. If data were not normally distributed, were presented as a percentage, and/or did not meet homogeneity of variance assumptions, the independent sample Kruskal-Wallis H followed by Mann-Whitney U nonparametric tests were performed. For all comparisons, statistical significance was determined by a P-value ≤ 0.05. In instances in which P-values were greater than 0.05, but less than 0.1, data were considered to exhibit a trend toward significance.
Results
Effects of DEHP exposure on body weights
In the F1 generation, DEHP exposure did not significantly affect body weight at any time point (Table 1). In the F2 generation, exposure to the 20 μg/kg/day dose of DEHP significantly decreased body weight at PNDs 8 and 21 compared to the control (Table 1, n = 7–20 dams/treatment group; P ≤ 0.05), but DEHP did not affect body weights at other time points. In the F3 generation, DEHP exposure did not significantly affect body weight at any time points (Table 1).
Table 1.
Effects of prenatal exposure to DEHP on body weights, measured in grams, in the F1, F2, and F3 generations of female mice.
| Generation | |||||
|---|---|---|---|---|---|
| Time point | Treatment group | Sample size | F1 | F2 | F3 |
| Control | 4–17 | 1.792 ± 0.039 | 1.942 ± 0.142 | 1.851 ± 0.046 | |
| DEHP 20 μg/kg/day | 3–10 | 1.853 ± 0.065 | 1.786 ± 0.113 | 1.751 ± 0.048 | |
| DEHP 200 μg/kg/day | 3–10 | 1.886 ± 0.072 | 1.697 ± 0.124 | 1.751 ± 0.028 | |
| PND 1 | DEHP 200 mg/kg/day | 9 | 1.800 ± 0.062 | No data | No data |
| DEHP 500 mg/kg/day | 3–10 | 1.861 ± 0.029 | 1.728 ± 0.054 | 1.790 ± 0.117 | |
| DEHP 750 mg/kg/day | 5–10 | 1.778 ± 0.033 | No data | 1.879 ± 0.099 | |
| Control | 15–20 | 6.300 ± 0.217 | 4.818 ± 0.115 | 5.116 ± 0.312 | |
| DEHP 20 μg/kg/day | 5–9 | 6.200 ± 0.321 | 4.238 ± 0.195* | 5.038 ± 0.210 | |
| DEHP 200 μg/kg/day | 3–10 | 6.142 ± 0.345 | 5.027 ± 0.209 | 4.839 ± 0.145 | |
| PND 8 | DEHP 200 mg/kg/day | 8–9 | 5.580 ± 0.268 | 4.382 ± 0.191 | No data |
| DEHP 500 mg/kg/day | 4–10 | 5.936 ± 0.192 | 5.125 ± 0.149 | 4.789 ± 0.363 | |
| DEHP 750 mg/kg/day | 5–7 | 5.740 ± 0.230 | 4.954 ± 0.218 | 5.531 ± 0.250 | |
| Control | 8–16 | 15.33 ± 0.414 | 12.38 ± 0.444 | 12.43 ± 0.758 | |
| DEHP 20 μg/kg/day | 4–7 | 14.00 ± 1.408 | 8.84 ± 0.703* | 11.78 ± 0.521 | |
| DEHP 200 μg/kg/day | 3–8 | 14.68 ± 0.757 | 12.43 ± 0.618 | 11.81 ± 0.876 | |
| PND 21 | DEHP 200 mg/kg/day | 6–8 | 14.81 ± 0.814 | 11.50 ± 0.440 | No data |
| DEHP 500 mg/kg/day | 3–5 | 13.94 ± 0.496 | 15.40 ± 1.567 | 13.02 ± 0.766 | |
| DEHP 750 mg/kg/day | 5–6 | 14.75 ± 0.618 | 12.58 ± 1.363 | 13.60 ± 0.726 | |
| Control | 7–24 | 29.66 ± 0.527 | 27.92 ± 1.056 | 29.01 ± 0.872 | |
| DEHP 20 μg/kg/day | 6–8 | 29.08 ± 1.177 | 26.99 ± 0.726 | 26.75 ± 0.492 | |
| DEHP 200 μg/kg/day | 4–7 | 28.92 ± 1.517 | 27.57 ± 0.961 | 28.29 ± 1.331 | |
| PND 60 | DEHP 200 mg/kg/day | 9 | 30.30 ± 0.513 | No data | No data |
| DEHP 500 mg/kg/day | 3–11 | 27.93 ± 0.938 | 29.72 ± 2.752 | 29.70 ± 1.688 | |
| DEHP 750 mg/kg/day | 4–6 | 28.16 ± 0.189 | 28.96 ± 1.671 | 29.25 ± 1.458 | |
| Control | 8–14 | 32.45 ± 1.105 | 29.47 ± 1.308 | 32.15 ± 0.742 | |
| DEHP 20 μg/kg/day | 7–8 | 31.66 ± 1.385 | 29.30 ± 0.510 | 29.59 ± 0.719 | |
| DEHP 200 μg/kg/day | 4–10 | 31.35 ± 0.684 | 29.15 ± 0.519 | 30.05 ± 0.484 | |
| DEHP 200 mg/kg/day | 0 | No data | No data | No data | |
| 3 month | DEHP 500 mg/kg/day | 3–13 | 30.96 ± 1.030 | 30.88 ± 0.392 | 31.25 ± 1.273 |
| DEHP 750 mg/kg/day | 6–7 | 33.55 ± 0.988 | 28.53 ± 0.821 | 31.23 ± 1.827 | |
| Control | 7–14 | 39.63 ± 1.505 | 39.97 ± 1.379 | 41.48 ± 1.461 | |
| DEHP 20 μg/kg/day | 6–8 | 39.94 ± 1.757 | 36.71 ± 1.309 | 36.92 ± 0.414 | |
| DEHP 200 μg/kg/day | 3–10 | 37.76 ± 1.302 | 39.70 ± 1.841 | 38.07 ± 1.784 | |
| DEHP 200 mg/kg/day | 0 | No data | No data | No data | |
| 6 month | DEHP 500 mg/kg/day | 3–13 | 37.18 ± 1.169 | 40.48 ± 0.273 | 40.33 ± 2.497 |
| DEHP 750 mg/kg/day | 6–7 | 40.31 ± 1.344 | 37.31 ± 0.757 | 39.01 ± 1.306 | |
| Control | 7–14 | 41.21 ± 1.377 | 42.28 ± 2.319 | 46.10 ± 2.120 | |
| DEHP 20 μg/kg/day | 6–8 | 41.19 ± 1.824 | 39.83 ± 2.255 | 41.70 ± 0.817 | |
| DEHP 200 μg/kg/day | 3–10 | 40.61 ± 1.524 | 42.54 ± 2.205 | 45.27 ± 2.739 | |
| DEHP 200 mg/kg/day | 0 | No data | No data | No data | |
| 9 month | DEHP 500 mg/kg/day | 3–13 | 39.26 ± 1.201 | 44.93 ± 1.282 | 47.90 ± 3.731 |
| DEHP 750 mg/kg/day | 6–7 | 43.61 ± 1.691 | 40.21 ± 1.316 | 43.73 ± 2.312 | |
Note: Table represent means ± standard error of the mean from 3–24 dams per treatment group. *P ≤ 0.05 (significant difference compared to the control)
Effects of DEHP on ovarian weights
In the F1 generation, the 20 μg/kg/day and 750 mg/kg/day doses of DEHP significantly decreased ovarian weight, but the 200 μg/kg/day dose of DEHP significantly increased ovarian weight at PND 21 compared to the control (Table 2, n = 3–15 dams/treatment group; P ≤ 0.05). Exposure to the 200 mg/kg/day dose of DEHP increased ovarian weight at PND 21, but this increase only trended toward significance compared to the control (Table 2, n = 8–15 dams/treatment group; P = 0.076). In the F2 generation, exposure to the 20 μg/kg/day dose of DEHP decreased ovarian weight at PND 60, but this decrease only trended toward significance (Table 2, n = 8–9 dams/treatment group; P = 0.072). Exposure to the 200 μg/kg/day dose of DEHP significantly decreased ovarian weight at PND 60 compared to the control (Table 2, n = 7–9 dams/treatment group; P ≤ 0.05). In the F3 generation, DEHP exposure did not significantly affect ovarian weight at any time points (Table 2).
Table 2.
Effects of prenatal exposure to DEHP on ovarian weights, measured in milligrams, in the F1, F2, and F3 generations of female mice.
| Generation | |||||
|---|---|---|---|---|---|
| Time point | Treatment group | Sample size | F1 | F2 | F3 |
| Control | 8–15 | 6.7 ± 0.5 | 5.0 ± 0.4 | 4.6 ± 0.5 | |
| DEHP 20 μg/kg/day | 3–7 | 3.9 ± 0.3* | 4.2 ± 0.4 | 4.1 ± 0.2 | |
| DEHP 200 μg/kg/day | 3–8 | 34.3 ± 5.9* | 5.1 ± 0.5 | 5.1 ± 0.3 | |
| DEHP 200 mg/kg/day | 6–8 | 32.8 ± 16.4∧ | 5.3 ± 0.9 | No data | |
| PND 21 | DEHP 500 mg/kg/day | 3–5 | 6.1 ± 1.0 | 6.2 ± 0.8 | 4.5 ± 0.3 |
| DEHP 750 mg/kg/day | 5–6 | 5.1 ± 0.2* | 6.8 ± 0.7 | 4.6 ± 0.4 | |
| Control | 7–24 | 15.4 ± 0.8 | 20.7 ± 1.8 | 16.9 ± 1.6 | |
| DEHP 20 μg/kg/day | 6–7 | 14.0 ± 1.2 | 16.0 ± 1.2∧ | 14.2 ± 1.4 | |
| DEHP 200 μg/kg/day | 4–7 | 14.1 ± 1.7 | 15.0 ± 0.8* | 16.0 ± 1.3 | |
| DEHP 200 mg/kg/day | 9 | 14.2 ± 1.4 | No data | No data | |
| PND 60 | DEHP 500 mg/kg/day | 3–11 | 14.6 ± 1.0 | 17.6 ± 2.0 | 17.3 ± 1.4 |
| DEHP 750 mg/kg/day | 5–6 | 14.5 ± 0.4 | 19.9 ± 1.6 | 15.3 ± 1.3 | |
Note: Ovarian weights were not collected in the F1, F2, or F3 generations at PND 1 or PND 8. Table represent means ± standard error of the mean from 3–24 dams per treatment group. *P ≤ 0.05 (significant difference compared to the control); 0.05 > ∧P ≤ 0.076 (borderline significant difference compared to the control).
Effects of DEHP on uterine weights
In the F1 generation, exposure to DEHP did not significantly affect uterine weight at PND 8, but the 200 μg/kg/day dose of DEHP significantly increased uterine weight at PND 21 compared to the control (Table 3, n = 4–16 dams/treatment group; P ≤ 0.05). In the F2 generation, exposure to the 500 mg/kg/day dose of DEHP significantly increased uterine weight at PND 8 (Table 3, n = 3–14 dams/treatment group; P ≤ 0.05). In contrast, the 200 mg/kg/day dose of DEHP decreased uterine weight at PND 8, but only trended toward significance (Table 3, n = 7–14 dams/treatment group; P = 0.084). At PND 21, exposure to the 20 μg/kg/day dose of DEHP significantly decreased uterine weight compared to the control (Table 3, n = 7–15 dams/treatment group; P ≤ 0.05). In the F3 generation, exposure to DEHP did not significantly affect uterine weight at any of the time points (Table 3).
Table 3.
Effects of prenatal exposure to DEHP on uterine weights, measured in milligrams, in the F1, F2, and F3 generations of female mice.
| Generation | |||||
|---|---|---|---|---|---|
| Time point | Treatment group | Sample size | F1 | F2 | F3 |
| Control | 7 | 1.0 ± 0.1 | |||
| DEHP 20 μg/kg/day | 7 | 1.1 ± 0.1 | |||
| DEHP 200 μg/kg/day | 3 | No data | No data | 1.1 ± 0.1 | |
| PND 1 | DEHP 200 mg/kg/day | 0 | No data | ||
| DEHP 500 mg/kg/day | 3 | 1.1 ± 0.2 | |||
| DEHP 750 mg/kg/day | 5 | 0.9 ± 0.2 | |||
| Control | 8–15 | 56.1 ± 2.8 | 3.6 ± 0.2 | 4.4 ± 0.2 | |
| DEHP 20 μg/kg/day | 5–7 | 49.3 ± 4.8 | 3.5 ± 0.2 | 4.1 ± 0.3 | |
| DEHP 200 μg/kg/day | 3–8 | 56.2 ± 4.9 | 4.0 ± 0.2 | 4.6 ± 0.5 | |
| PND 8 | DEHP 200 mg/kg/day | 7–9 | 66.8 ± 3.4 | 3.1 ± 0.1∧ | No data |
| DEHP 500 mg/kg/day | 3–5 | 61.3 ± 4.8 | 5.1 ± 0.8* | 4.0 ± 0.4 | |
| DEHP 750 mg/kg/day | 5–7 | 55.2 ± 4.8 | 4.1 ± 0.3 | 4.5 ± 0.2 | |
| Control | 8–16 | 20.6 ± 1.8 | 16.4 ± 1.4 | 15.3 ± 1.0 | |
| DEHP 20 μg/kg/day | 4–7 | 15.3 ± 2.5 | 9.3 ± 0.7* | 12.5 ± 1.2 | |
| DEHP 200 μg/kg/day | 3–7 | 206.0 ± 26.9* | 14.2 ± 0.9 | 15.1 ± 1.3 | |
| DEHP 200 mg/kg/day | 6–8 | 50.2 ± 17.1 | 16.4 ± 2.2 | No data | |
| PND 21 | DEHP 500 mg/kg/day | 3–5 | 18.7 ± 2.5 | 21.2 ± 5.4 | 17.7 ± 1.6 |
| DEHP 750 mg/kg/day | 4–6 | 23.3 ± 2.5 | 13.4 ± 1.3 | 17.5 ± 0.7 | |
| Control | 6–7 | 111.2 ± 5.6 | 128.5 ± 15.8 | 106.0 ± 7.3 | |
| DEHP 20 μg/kg/day | 4–7 | 123.2 ± 5.7 | 107.3 ± 7.5 | 116.2 ± 17.5 | |
| DEHP 200 μg/kg/day | 4 | 131.7 ± 16.7 | 153.0 ± 19.6 | 95.8 ± 3.8 | |
| DEHP 200 mg/kg/day | 0 | No data | No data | No data | |
| PND 60 | DEHP 500 mg/kg/day | 2–7 | 110.3 ± 9.2 | 103.3 ± 15.2 | 145.1 ± 33.7 |
| DEHP 750 mg/kg/day | 4–6 | No data | 118.0 ± 28.3 | 99.2 ± 2.8 | |
Note: Uterine weights were not collected in the F1 and F2 generations at PND 1. Table represent means ± standard error of the mean from 2–16 dams per treatment group. *P ≤ 0.05 (significant difference compared to the control); 0.05 > ∧P ≤ 0.084 (borderline significant difference compared to the control).
Effects of DEHP on liver weights
In the F1 generation, the 500 mg/kg/day dose of DEHP significantly increased liver weight at PND 1 compared to the control (Table 4, n = 5–17 dams/treatment group; P ≤ 0.05) and the 200 mg/kg/day dose of DEHP significantly increased liver weight at PND 8 compared to the control (Table 4, n = 8–15 dams/treatment group; P ≤ 0.05). In the F2 generation, the 500 mg/kg/day dose of DEHP significantly increased liver weight at PND 8 compared to the control (Table 4, n = 10–17 dams/treatment group; P ≤ 0.05). The 20 μg/kg/day dose of DEHP significant decreased liver weight, and the 500 mg/kg/day dose of DEHP significantly increased liver weight at PND 21 compared to the control (Table 4, n = 3–15 dams/treatment group; P ≤ 0.05). In F3 generations, exposure to DEHP did not significantly affect liver weight at any doses (Table 4).
Table 4.
Effects of prenatal exposure to DEHP on liver weights, measured in grams, in the F1, F2, and F3 generations of female mice.
| Generation | |||||
|---|---|---|---|---|---|
| Time point | Treatment group | Sample size | F1 | F2 | F3 |
| Control | 5–17 | 0.0731 ± 0.0022 | 0.0745 ± 0.0053 | 0.0721 ± 0.0027 | |
| DEHP 20 μg/kg/day | 3–7 | 0.0720 ± 0.0037 | 0.0710 ± 0.0045 | 0.0728 ± 0.0029 | |
| DEHP 200 μg/kg/day | 3–6 | 0.0733 ± 0.0033 | 0.0679 ± 0.0032 | 0.0748 ± 0.0037 | |
| PND 1 | DEHP 200 mg/kg/day | 9 | 0.0700 ± 0.0041 | No data | No data |
| DEHP 500 mg/kg/day | 3–9 | 0.0840 ± 0.0024* | 0.0721 ± 0.0027 | 0.0723 ± 0.0037 | |
| DEHP 750 mg/kg/day | 5 | 0.0760 ± 0.0024 | No data | 0.0766 ± 0.0049 | |
| Control | 8–17 | 0.1950 ± 0.0086 | 0.1234 ± 0.0088 | 0.1621 ± 0.0139 | |
| DEHP 20 μg/kg/day | 5–9 | 0.1920 ± 0.0139 | 0.1207 ± 0.0076 | 0.1481 ± 0.0093 | |
| DEHP 200 μg/kg/day | 3–10 | 0.1840 ± 0.0147 | 0.1491 ± 0.0097 | 0.1461 ± 0.0071 | |
| PND 8 | DEHP 200 mg/kg/day | 8–9 | 0.1563 ± 0.0091* | 0.1137 ± 0.0053 | No data |
| DEHP 500 mg/kg/day | 4–10 | 0.1780 ± 0.0153 | 0.1577 ± 0.0085* | 0.1475 ± 0.0077 | |
| DEHP 750 mg/kg/day | 5–7 | 0.1700 ± 0.0115 | 0.1434 ± 0.0085 | 0.1776 ± 0.0099 | |
| Control | 8–16 | 0.8154 ± 0.0256 | 0.6911 ± 0.0324 | 0.6654 ± 0.0505 | |
| DEHP 20 μg/kg/day | 4–7 | 0.7607 ± 0.0599 | 0.4541 ± 0.0445* | 0.6280 ± 0.0327 | |
| DEHP 200 μg/kg/day | 3–8 | 0.8220 ± 0.0496 | 0.6926 ± 0.0402 | 0.6305 ± 0.0708 | |
| DEHP 200 mg/kg/day | 6–8 | 0.7438 ± 0.0385 | 0.5865 ± 0.0246 | No data | |
| PND 21 | DEHP 500 mg/kg/day | 3–5 | 0.7565 ± 0.0206 | 0.9616 ± 0.1299* | 0.6928 ± 0.0413 |
| DEHP 750 mg/kg/day | 5–6 | 0.8527 ± 0.0506 | 0.6968 ± 0.1106 | 0.7607 ± 0.0441 | |
| Control | 7–23 | 1.6533 ± 0.0384 | 1.6236 ± 0.0940 | 1.7297 ± 0.0641 | |
| DEHP 20 μg/kg/day | 6–8 | 1.6687 ± 0.0630 | 1.5593 ± 0.0425 | 1.5765 ± 0.0489 | |
| DEHP 200 μg/kg/day | 4–7 | 1.5630 ± 0.1100 | 1.5649 ± 0.0901 | 1.7190 ± 0.1990 | |
| DEHP 200 mg/kg/day | 8 | 1.5885 ± 0.0358 | No data | No data | |
| PND 60 | DEHP 500 mg/kg/day | 3–11 | 1.6264 ± 0.0609 | 1.7531 ± 0.1860 | 1.7792 ± 0.1131 |
| DEHP 750 mg/kg/day | 5–6 | 1.5357 ± 0.0761 | 1.6317 ± 0.1257 | 1.7854 ± 0.1110 | |
Note: Tables represent means ± standard error of the mean from 3–23 dams per treatment group. *P ≤ 0.05 (significant difference compared to the control).
Effect of DEHP exposure on F1 ovarian morphology
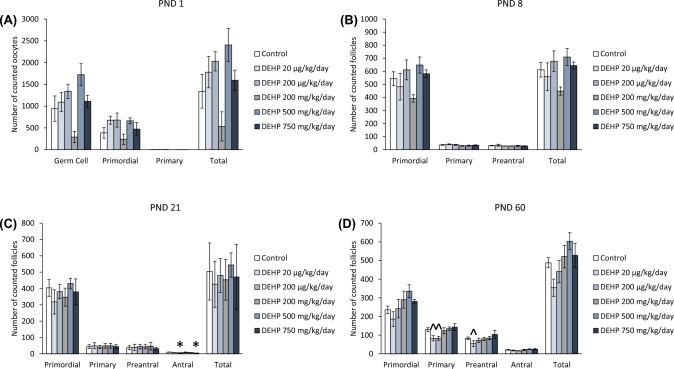
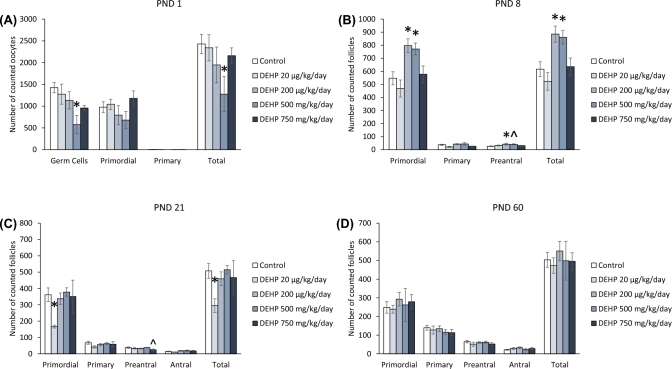
At PND 1, prenatal exposure to DEHP did not significantly affect the numbers of germ cells, or primordial, primary, and total follicles compared to the control (Figure 1A). Similarly, at PND 8, DEHP exposure did not significantly affect the numbers of primordial, primary, preantral, or total follicles compared to the control (Figure 1B). In contrast, at PND 21, the 200 μg/kg/day and 750 mg/kg/day doses of DEHP significantly decreased the numbers of antral follicles compared to the control (Figure 1C, n = 4–10 dams/treatment group; P ≤ 0.05). At PND 60, both the 20 μg/kg/day and the 200 μg/kg/day doses of DEHP decreased the number of primary follicles, but only trended toward significance (Figure 1D, n = 5–13 dams/treatment group; P = 0.071 and 0.064, respectfully). Furthermore, the 20 μg/kg/day dose of DEHP decreased the number of preantral follicles, but only trended toward significance (Figure 1D, n = 5–13 dams/treatment group: P = 0.084).
Figure 1.
The effects of prenatal exposure to DEHP on ovarian follicle numbers in the F1 generation at PND 1 (A), PND 8 (B), PND 21 (C), and PND 60 (D). Graphs represent means ± standard error of the mean from 2–14 dams per treatment group. *P ≤ 0.05 (significant difference compared to the control); 0.05 > ∧P ≤ 0.084 (borderline significant difference compared to the control). Note: In panel A, n = 2 for the DEHP 200 mg/kg/day group.
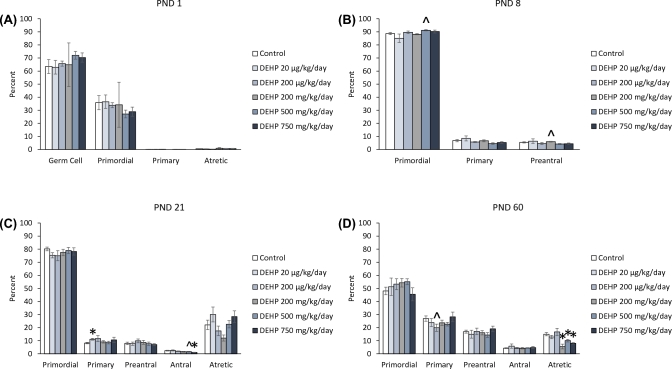
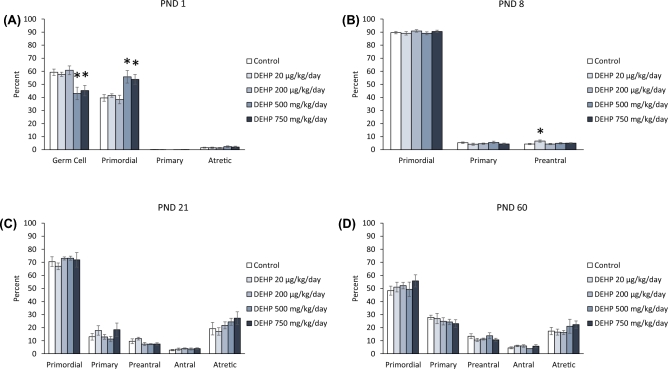
At PND 1, prenatal exposure to DEHP did not significantly affect the percentage of germ cells, or primordial, primary, and atretic follicles compared to the control (Figure 2A). In contrast, at PND 8, exposure to the 500 mg/kg/day dose of DEHP increased the percentage of primordial follicles and the 200 mg/kg/day dose of DEHP increased the percentage of preantral follicles, but both increases trended toward significance compared to the control (Figure 2B, n = 5–14 dams/treatment group; P = 0.085 and P = 0.076, respectfully). At PND 21, the 20 μg/kg/day dose of DEHP significantly increased the percentage of primary follicles and the 750 mg/kg/day dose of DEHP significantly decreased the percentage of antral follicles compared to the control (Figure 2C, n = 5–12 dams/treatment group; P ≤ 0.05). Furthermore, the 500 mg/kg/day dose of DEHP decreased the percentage of antral follicles, but the decrease only trended toward significance (Figure 2C, n = 5–12 dams/treatment group; P = 0.079). At PND 60, the 200 μg/kg/day dose of DEHP decreased the percentage of primary follicles, but the decrease only trended toward significance (Figure 2C, n = 5–13 dams/treatment group; P = 0.068). Furthermore, the 200, 500, and the 750 mg/kg/day doses of DEHP significantly decreased the percentage of atretic follicles compared to the control (Figure 2D, n = 4–12 dams/treatment group; P ≤ 0.05).
Figure 2.
The effects of prenatal exposure to DEHP on ovarian follicle percentages in the F1 generation at PND 1 (A), PND 8 (B), PND 21 (C), and PND 60 (D). Graphs represent means ± standard error of the means from 2–14 dams per treatment group. *P ≤ 0.05 (significant difference compared to the control); 0.05 > ∧P ≤ 0.085 (borderline significant difference compared to the control). Note: In panel A, n = 2 for the DEHP 200 mg/kg/day group.
Effect of DEHP exposure on F2 ovarian morphology
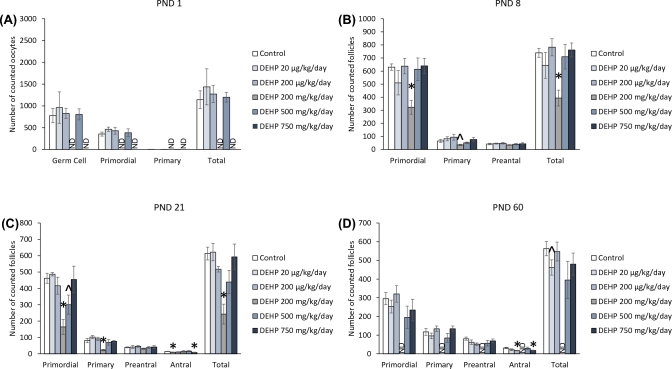
At PND 1, exposure to DEHP did not significantly affect the number of germ cells, or primordial, primary, and total follicles compared to the control (Figure 3A). In contrast, at PND 8, the 200 mg/kg/day dose of DEHP decreased the numbers of primordial, primary, and total follicles compared to the control (Figure 3B, n = 5–7 dams/treatment group; P ≤ 0.05), but the decrease in primary follicle type only trended toward significance (Figure 3B, n = 5–7 dams/treatment group; P = 0.062). At PND 21, the 200 mg/kg/day dose of DEHP significantly decreased the number of primordial, primary, and total follicles compared to the control (Figure 3C, n = 5–7 dams/treatment group; P ≤ 0.05). Furthermore, the 500 mg/kg/day dose of DEHP decreased the number of primordial follicles, but the decrease only trended toward significance (Figure 3C, n = 4–7 dams/treatment group; P = 0.09). Both the 20 μg/kg/day and the 750 mg/kg/day doses of DEHP significantly decreased the number of antral follicles at PND 21 compared to the control (Figure 3C, n = 5–7 dams/treatment group; P ≤ 0.05). At PND 60, both the 200 μg/kg/day and the 750 mg/kg/day doses of DEHP decreased the number of antral follicle numbers compared to the control (Figure 3D, n = 6–11 dams/treatment group; P ≤ 0.05). Furthermore, the 20 μg/kg/day dose of DEHP decreased the total number of follicles, but the decrease only trended toward significance (Figure 3D, n = 8–11 dams/treatment group; P = 0.075).
Figure 3.
The effects of prenatal exposure to DEHP on ovarian follicle numbers in the F2 generation at PND 1 (A), PND 8 (B), PND 21 (C), and PND 60 (D). Graphs represent means ± standard error of the mean from 3–11 dams per treatment group. *P ≤ 0.05 (significant difference compared to the control); 0.05 > ∧P ≤ 0.09 (borderline significant difference compared to the control). ND = no data
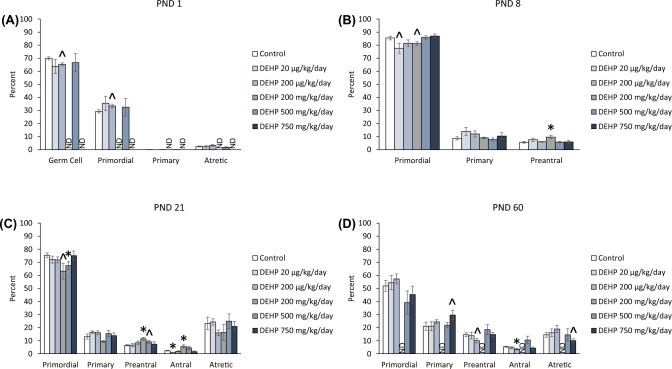
At PND 1, the 200 μg/kg/day dose of DEHP decreased the percentage of germ cells and increased the percentage of primordial follicles types, but these changes only trended toward significance (Figure 4A, n = 3–4 dams/treatment group; P = 0.077 and P = 0.077, respectfully). At PND 8, exposure to both the 20 μg/kg/day and the 200 mg/kg/day doses of DEHP decreased the percentage of primordial follicles, but the decrease only trended toward significance (Figure 4B, n = 4–7 dams/treatment group; P = 0.088 and P = 0.062). Furthermore, the 200 mg/kg/day dose of DEHP significantly increased the percentage of preantral follicles compared to controls (Figure 4B, n = 5–7 dams/treatment group; P ≤ 0.05). At PND 21, the 200 mg/kg/day of DEHP decreased the percentage of primordial follicles compared to the control, but the decrease only trended toward significance (Figure 4C, n = 5–7 dams/treatment group; P = 0.058), whereas the 500 mg/kg/day dose of DEHP significantly decreased the percentage of primordial follicles compared to the control (Figure 4C, n = 6–7 dams/treatment group; P ≤ 0.05). The 200 and 500 mg/kg/day doses of DEHP increased the percentage of preantral follicles compared to the control, with the increase in the 500 mg/kg/day trending toward significance (Figure 4C, n = 4–7 dams/treatment group; P ≤ 0.05 and P = 0.086, respectively). The 20 μg/kg/day significantly decreased the percentage of antral follicles and the 200 mg/kg/day doses of DEHP significantly increased the percentage of antral follicles compared to the control (Figure 4C, n = 4–7 dams/treatment group; P ≤ 0.05). At PND 60, the 750 mg/kg/day dose of DEHP increased the percentage of primary follicles and decreased the percentage of atretic follicles, but it only trended toward significance (Figure 4D, n = 5–11 dams/treatment group; P = 0.070 and P = 0.066, respectively). Furthermore, the 200 μg/kg/day dose of DEHP decreased the percentage of preantral and antral follicles compared to controls, but the decrease in preantral follicles only trended toward significance (Figure 4D, n = 7–11 dams/treatment group; P = 0.094 and P ≤ 0.05, respectively).
Figure 4.
The effects of prenatal exposure to DEHP on ovarian follicle percentages in the F2 generation at PND 1 (A), PND 8 (B), PND 21 (C), and PND 60 (D). Graphs represent means ± standard error of the means from 3–11 dams per treatment group. *P ≤ 0.05 (significant difference compared to the control); 0.05 > ∧P ≤ 0.094 (borderline significant difference compared to the control). ND = no data
Effect of DEHP exposure on F3 ovarian morphology
At PND 1, the 500 mg/kg/day dose of DEHP significantly decreased the number of germ cells and total number of oocytes compared to the control (Figure 5A, n = 5–7 dams/treatment group; P ≤ 0.05). At PND 8, the 200 μg/kg/day and the 500 mg/kg/day doses of DEHP increased the number of primordial, preantral, and total follicles compared to the control (Figure 5B, n = 4–5 dams/treatment group; P ≤ 0.05, except 500 mg/kg/day preantral P = 0.088). At PND 21, the 20 μg/kg/day dose of DEHP significantly decreased the number of primordial and total number of follicles compared to the control (Figure 5C, n = 4–6 dams/treatment group; P ≤ 0.05). The 750 mg/kg/day dose of DEHP decreased the number of preantral follicles, but the decrease only trended toward significance (Figure 5C, n = 5 dams/treatment group; P = 0.068). At PND 60, exposure to DEHP did not significantly affect the numbers of primordial, primary, preantral, antral, and total follicles compared to the control (Figure 5D).
Figure 5.
The effects of prenatal exposure to DEHP on ovarian follicle numbers in the F3 generation at PND 1 (A), PND 8 (B), PND 21 (C), and PND 60 (D). Graphs represent means ± standard error of the mean from 4–7 dams per treatment group. *P ≤ 0.05 (significant difference compared to the control); 0.05 > ∧P ≤ 0.088 (borderline significant difference compared to the control).
At PND 1, both the 500 mg/kg/day and the 750 mg/kg/day doses of DEHP significantly decreased the percentage of germ cells and increased the percentage of primordial follicle types compared to the control (Figure 6A, n = 5–7 dams/treatment group; P ≤ 0.05). At PND 8, the 20 μg/kg/day dose of DEHP significantly increased the percentage of preantral follicles compared to the control (Figure 6B, n = 5–7 dams/treatment group; P ≤ 0.05). At PNDs 21 and 60, DEHP exposure did not significantly affect the percentage of primordial, primary, preantral, antral, or atretic follicles compared to the control (Figures 6C and 6D).
Figure 6.
The effects of prenatal exposure to DEHP on ovarian follicle percentages in the F3 generation at PND 1 (A), PND 8 (B), PND 21 (C), and PND 60 (D). Graphs represent means ± standard error of the means from 4–7 dams per treatment group. *P ≤ 0.05 (significant difference compared to the control).
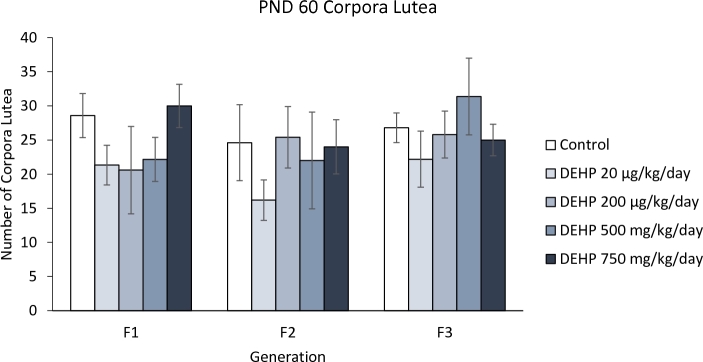
Effect of DEHP on corpora lutea in all generations
At PND 60, exposure to DEHP did not significantly affect the number of corpora lutea in the F1 generation (Figure 7). Similarly, DEHP exposure did not significantly affect the number of corpora lutea in the F2 and F3 generations (Figure 7).
Figure 7.
The effects of prenatal exposure to DEHP on corpora lutea numbers at PND 60. Graph represents mean ± standard error of the means from 4–12 dams per treatment group.
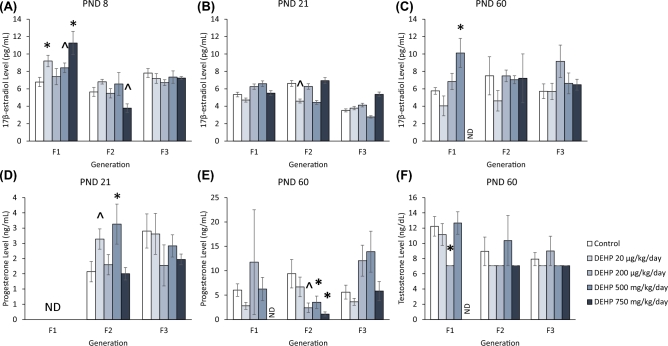
Effect of DEHP exposure on serum 17β-estradiol levels
At PND 8, the 20 μg/kg/day, 500 mg/kg/day, and 750 mg/kg/day doses of DEHP significantly increased serum 17β-estradiol levels compared to the control in the F1 generation (Figure 8A, n = 5–8 dams/treatment group; P ≤ 0.05, except for 500 mg/kg/day P = 0.057). The 750 mg/kg/day dose of DEHP decreased serum 17β-estradiol levels in the F2 generation and only trended toward significance (Figure 8A, n = 5–10 dams/treatment group; P = 0.09). DEHP exposure did not significantly affect serum 17β-estradiol levels in the F3 generation (Figure 8A).
Figure 8.
The effects of prenatal exposure to DEHP on serum sex steroid hormone levels in the F1, F2, and F3 generations. Serum 17β-estradiol levels are shown at PND 8 (A), PND 21 (B) and PND 60 (C) for the F1, F2, and F3 generations. Serum progesterone levels are shown at PND 21 (D) and PND 60 (E) for the F1, F2, and F3 generations. Serum testosterone levels are shown at PND 60 (F) for the F1, F2, and F3 generations. The serum testosterone levels were at the limit of detection and therefore do not have error bars. Graphs represent means ± standard error of the mean from 2–8 dams per treatment group. *P ≤ 0.05 (significant difference compared to the control); 0.05 > ∧P ≤ 0.091 (borderline significant difference compared to the control); ND = no data. Note: In panels B and C, n = 2 for the DEHP 500 mg/kg/day group and in panel D, n = 2 for the DEHP 750 mg/kg/day group in the F2 generation.
At PND 21, exposure to DEHP did not significantly affect serum 17β-estradiol levels in the F1 generation. Exposure to DEHP decreased serum 17β-estradiol levels in the 20 μg/kg/day treatment group in the F2 generation, but the decrease only trended toward significance (Figure 8B, n = 7–9 dams/treatment group; P = 0.074). Exposure to DEHP did not significantly affect serum 17β-estradiol levels in the F3 generation (Figure 8B)
At PND 60, exposure to the 500 mg/kg/day dose of DEHP significantly increased serum 17β-estradiol levels compared to the control in the F1 generation (Figure 8C, n = 7 dams/treatment group; P ≤ 0.05). DEHP exposure did not significantly affect serum 17β-estradiol levels in the F2 and F3 generations (Figure 8C).
Effect of DEHP exposure on serum progesterone levels
At PND 21, exposure to the 20 μg/kg/day and 500 mg/kg/day doses of DEHP significantly increased serum progesterone levels compared to the control in the F2 generation (Figure 8D, n = 5–8 dams/treatment group; P ≤ 0.05 except 20 μg/kg/day P = 0.057). In contrast, DEHP exposure did not significantly affect serum progesterone levels in the F3 generation (Figure 8D).
At PND 60, serum progesterone levels were not significantly affected by DEHP exposure in the F1 generation (Figure 8E). In contrast, exposure to 200 μg/kg/day, 500 mg/kg/day, and 750 mg/kg/day doses of DEHP significantly decreased serum progesterone levels compared to the control in the F2 generation (Figure 8E, n = 3–10 dams/treatment group; P ≤ 0.05 except 200 μg/kg/day P = 0.091). DEHP exposure did not significantly affect progesterone levels in the F3 generation (Figure 8E).
Effect of DEHP exposure on serum testosterone levels
At PND 60, exposure to 200 μg/kg/day of DEHP significantly decreased serum testosterone levels compared to the control in the F1 generation (Figure 8F, n = 4–7 dams/treatment group; P ≤ 0.05). DEHP exposure did not significantly affect serum testosterone levels in the F2 and F3 generations.
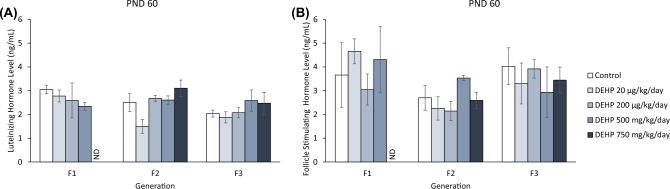
Effect of DEHP exposure on serum gonadotropin hormone levels
Exposure to DEHP did not significantly affect serum LH and serum FSH levels in the F1 generation. (Figure 9A and B). Similarly, DEHP exposure did not significantly affect serum LH and FSH levels in the F2 and F3 generations (Figure 9A and B).
Figure 9.
The effects of prenatal exposure to DEHP on serum gonadotropin hormone levels in the F1, F2, and F3 generations at PND 60. Serum luteinizing hormone levels are shown in panel (A) and serum follicle stimulating hormone levels are shown in panel (B). Graphs represent means ± standard error of the mean from 2–10 dams per treatment group. ND = no data. Note: In n = 2 for the DEHP 500 mg/kg/day group in the F2 generation.
Discussion
We have shown that prenatal exposure to DEHP during the second half of pregnancy disrupts ovarian function across generations through the maternal lineage in mice. Specifically, we provide evidence that DEHP exposure adversely affects body weights in the F2 generation, tissue weights in the F1 and F2 generations, ovarian morphology in the F1, F2, and F3 generations, and serum sex steroid hormone levels in the F1 and F2 generations. Furthermore, to our knowledge, we are the first to show adverse transgenerational effects of prenatal DEHP exposure on ovarian morphology in detail.
Interestingly, the effects caused by DEHP exposure were not the same in each generation and many effects were non-monotonic in nature. This is likely because during prenatal exposure to DEHP, the F1, F2, and F3 generations received DEHP during different developmental windows. Specifically, the F1 generation was exposed to DEHP as the developing pup. The F2 generation was exposed to DEHP as the developing ovaries within the pup. The F3 generation was not directly exposed to DEHP. Furthermore, DEHP is a known EDC, and characteristic of an EDC is that the adverse effects of a toxicant are not proportional to the dose and therefore, often do not follow a linear relationship [44]. Furthermore, because DEHP is rapidly metabolized and excreted with a half-life between 2 and 8 hours [45], it is important to note that the exposure window to DEHP is limited. Thus, it is unlikely that DEHP levels during gestation continued into the F1, F2, and F3 generations.
Our data showed that DEHP exposure decreased body weight in the F2 generation at PNDs 8 and 21, but it did not affect body weight in the F1 and F3 generations. This is in contrast to a previous study in which prenatal exposure to a phthalate mixture (200 μg/kg/day and 200 mg/kg/day) increased body weight in the F2 generation of female mice [46]. The reasons why our results differ may be because the phthalate mixture contained multiple phthalates in addition to DEHP and the different phthalates could have affected the body weight differently than DEHP alone. In addition, the dose of DEHP in the phthalate mixture was different than the doses we used in this study (approximately 41 μg and 41 mg/kg/day in the mixture vs 20 μg and 750 mg/kg/day in this study) [46].
The mechanism by which DEHP exposure decreased body weight in our study is not known. However, phthalates such as DEHP have been shown to act on peroxisome proliferator-activated receptors (PPARs) and these receptors are important for the regulation of glucose homeostasis and in turn, body weight [47]. Therefore, it is possible that DEHP may have acted on PPARs to dysregulate body weight in our study. It is interesting to note that the decrease in body weight was observed only with the lowest dose of DEHP (20 μg/kg/day), suggesting that different doses of DEHP may act on the body differently. This possibility is supported by another study that showed that prenatal DEHP exposure caused different effects on body weight at different doses. Specifically, prenatal DEHP increased body weight at 200 μg/kg/day and decreased body weight at 500 mg/kg/day in male mice [13].
In addition, our data showed that prenatal DEHP exposure affected ovarian weight in the F1 and F2 generations. Specifically, in the F1 generation, the 20 μg/kg/day and 750 mg/kg/day doses of DEHP decreased ovarian weight and the 200 μg/kg/day dose increased ovarian weight at PND 21. In the F2 generation, DEHP exposure at 200 μg/kg/day decreased ovarian weight. The reasons why DEHP causes ovarian weight changes are unknown. We speculate that DEHP exposure may alter the granulosa cell size and the granulosa cell number, or affect the interstitial cells, leading to alterations in ovarian weight. The DEHP-induced decrease in ovarian weight is similar to the DEHP-induced decrease in testes weight observed after prenatal exposure to 325 μl/L of DEHP in drinking water [48]. However, this level of DEHP in drinking water is equivalent to 30–35 mg/kg/day of DEHP and is much higher than the dose at which we observed a decrease in ovarian weight [48]. Our data differ from another study showing that prenatal DEHP exposure (0.015–405 mg/kg/day) from gestation day 6 to lactation day 21 did not affect ovarian weight [49]. The difference in results between our study and that study may be due to the different windows of exposure (during second half of gestation vs through lactation), different doses of DEHP, different animal models (mice vs rats), and different methods of exposure (gavage vs oral).
Prenatal DEHP exposure also increased uterine weight at PND 21 in the F1 generation, but it did not significantly affect uterine weight at any time points in the F2 or F3 generations. An increase in uterine weight may correlate with an increase in 17β-estradiol production. However, our data showed that at PND 21, DEHP exposure did not increase serum 17β-estradiol levels compared to controls. Thus, the DEHP-induced increase in uterine weight in the F1 generation at PND 21 may be due to developmental effects independent of 17β-estradiol levels. In contrast to our data, a study reported that in utero and lactational exposure to DEHP (0.015–405 mg/kg/day) did not alter uterine weight in the F1 generation of rats [49], suggesting the existence of species differences in the impact of prenatal DEHP exposure on uterine weight.
In our study, we counted follicle numbers and calculated the percentage of follicles at each stage of development. By examining the follicle numbers, we were able to quantify DEHP-induced changes in the number of available primordial follicles or the growth of follicles to more mature stages. By calculating the percentage of follicle types, we were able to quantify shifts in follicle pools, which may indicate either accelerated or inhibited folliculogenesis. Our data showed that the DEHP treatment disrupted ovarian follicle numbers in all three generations. In the F1 and F2 generations, exposure to DEHP decreased follicle numbers. However, in the F3 generation, DEHP exposure both decreased and increased follicle numbers, depending on the time point. Previous studies have reported that DEHP exposure during adulthood decreases the primordial follicle pool [33, 50]. These findings indicate that both prenatal and adult exposure to DEHP impact follicle numbers. The impact of prenatal exposure to DEHP is especially concerning because the DEHP-induced decrease in follicle numbers occurs across generations. It is possible that DEHP-induced losses in the finite number of primordial follicles may cause primary ovarian insufficiency, leading to declining fertility with age, and an early age of reproductive senescence. Loss of follicles was also observed in another study. Specifically, prenatal exposure to a plasticizer mixture including DEHP (750 mg/kg/day) reduced the number of primordial follicles in the F1 and F3 generations of female rats [37]. Taken together, these results suggest that prenatal DEHP exposure may lead to primary ovarian insufficiency, which is the premature loss of ovarian follicles caused by either atresia of follicular destruction [51]. Thus, future studies should examine the impact of DEHP-induced follicle losses on fertility.
Our data showed that DEHP exposure decreased folliculogenesis in adult ovaries in the F1 generation, disrupted folliculogenesis in adult ovaries in the F2 generation, and accelerated the transition of germ cells to primordial follicles in the neonatal ovary in the F3 generation. The mechanism by which DEHP exposure accelerates folliculogenesis, however, is not completely known. Our laboratory has previously demonstrated that adult exposure to DEHP (20 and 200 μg/kg/day and 20 and 750 mg/kg/day) for 10 days accelerated primordial follicle recruitment in mouse ovaries, and that this acceleration was mediated through the phosphatidylinositol 3-kinase (PI3K) signaling pathway [33]. The PI3K pathway is a critical regulator of folliculogenesis, specifically for primordial follicle activation, survival, and quiescence [33]. Thus, it is possible that prenatal exposure to DEHP activated the PI3K signaling pathway in our study. In addition, another study showed that impaired folliculogenesis correlated with reduced progesterone levels during diestrus and thus, DEHP may impair folliculogenesis by interfering with progesterone levels [52]. This possibility is supported by our data indicating that DEHP exposure reduced serum progesterone levels in the F2 generation and accelerated folliculogenesis. Furthermore, studies show that DEHP exposure (10 and 100 μM) in vitro impairs primordial follicle assembly by increasing the mRNA expression levels of pro-apoptotic gene Bax in oocytes [53]. Given that Bax plays an important role in follicle growth, it is possible that the mechanism by which DEHP impacts folliculogenesis involves Bax [54].
In addition, our data showed that prenatal exposure to DEHP decreased the percentage of atretic follicles in the F1 generation. Although the reason for the DEHP-induced reduction in atresia is unknown, a previous study showed that a mixture of phthalates containing DEHP caused the antral follicles to become arrested in the G1 state of the cell cycle [55]. At this stage, the cells are relatively resistant to apoptosis [56]. Therefore, the DEHP-induced reduction in the percentage of atretic follicles may be due to an ability of DEHP to arrest granulosa cells in the antral follicles in the G1 stage.
Our data showed that prenatal DEHP exposure did not affect the number of corpora lutea in the ovaries in any generation. This is in contrast to previous studies that have shown that DEHP exposure at much higher doses (3000 mg/kg) decreased the number of new corpora lutea in adult rats [57] and that DEHP exposure (125, 250, and 500 mg/kg/day) reduced the number of corpora lutea in pregnant mice [58]. Interestingly, one study showed that DEHP exposure (25 and 50 mg/kg) reduced the size of corpora lutea, but increased plasma progesterone levels in sheep [59]. The reasons for differences in study results likely stem from differences in exposure windows (pregnancy vs adulthood), species (mice vs ewes), and timing of evaluation (pregnant dams vs offspring).
Our data showed that DEHP exposure disrupted sex steroid hormone levels in the F1 and F2 generations. Specifically, in the F1 generation, prenatal DEHP exposure increased serum 17β-estradiol levels at PNDs 8 and 60. At PND 8, the increase in serum 17β-estradiol levels occurred at a time in which the ovary contained no antral follicles. Because antral follicles are the primary producers of 17β-estradiol, these data suggest that the DEHP-induced increase in serum 17β-estradiol at PND 8 is not due to a significant increase in antral follicle numbers. Thus DEHP may affect follicle numbers and sex steroid hormone levels via different mechanisms. One study has shown that disruption of FSH production can signal to the immature ovary and promote the synthesis of 17β-estradiol without stimulating follicular growth [60]. Therefore, it is possible that DEHP may promote FSH production and elevate serum 17β-estradiol levels. At PND 60, the ovary contains many antral follicles, but it is unlikely that the DEHP-induced increase in 17β-estradiol levels at this time point is due to a change in antral follicle numbers because we did not observe any effects of DEHP on antral follicle numbers or atresia at PND 60. Instead, it is possible that DEHP exposure may dysregulate the biosynthesis of 17β-estradiol in the ovary. Previous studies have shown that in vitro DEHP exposure (1–100 μg/mL) decreases 17β-estradiol production [32] and decreases mRNA expression of aromatase, a key steroidogenic enzyme necessary for the conversion of cholesterol to 17β-estradiol in antral follicles [61]. Therefore, DEHP exposure in vivo may affect the levels of steroidogenic enzymes, increasing the levels of serum 17β-estradiol.
Our data showed that in the F2 generation, exposure to DEHP increased serum progesterone levels at PND 21 and decreased serum progesterone levels at PND 60. It is interesting that in the F2 generation, exposure to DEHP increased serum progesterone levels even though corpora lutea were not present at PND 21. One study has shown that FSH stimulation can promote progesterone synthesis and output from granulosa cells without luteinization by upregulating enzymatic activity of 3β-hydroxysteroid dehydrogenase (3βHSD) [62]. Although we did not measure FSH or 3βHSD levels in the F2 generation at PND 21, it is possible that DEHP exposure stimulated FSH production leading to elevated progesterone levels. At PND 60, serum progesterone levels were decreased in DEHP-treated mice. Similarly, previous studies showed that in vitro DEHP exposure (1–100 μg/mL) decreased progesterone levels in antral follicles [32]. Furthermore, DEHP exposure throughout lactation (1–100 mg/kg/day) decreased serum progesterone levels in adult female rats [63]. In addition, DEHP exposure increased plasma progesterone levels by metabolic clearance and not by progesterone secretion from the corpora lutea in sheep [58]. Furthermore, DEHP exposure is associated with reduced hepatic estrogen metabolism [64]. Therefore, hormone synthesis and clearance may contribute to the altered hormone levels observed in this study and should be further explored in future studies.
It is likely that DEHP exposure affects folliculogenesis and steroidogenesis through separate mechanisms. Our data show that DEHP exposure interferes with follicle numbers, but not sex steroid hormone levels or vice versa at corresponding time points. One possible mechanism that may explain the change in sex steroid hormone levels may be a disruption of FSH and LH action either directly on the follicles or through the hypothalamus-pituitary-ovary axis. Furthermore, an alteration in the steroidogenic enzyme expression or activity in the follicles may also contribute to the altered hormone levels as demonstrated by a previous study [65]. A different mechanism to explain the change in follicle numbers may involve an oxidative stress pathway. A previous study focusing on DEHP exposure in antral follicles has shown that DEHP (10 μg/mL) significantly increases reactive oxygen species levels and decreases antioxidant enzymes to inhibit antral follicle growth [66].
Collectively, these data provide evidence that prenatal exposure to DEHP during the second half of gestation causes body weight and organ weight changes, dysregulates serum sex steroid hormone levels, and causes adverse transgenerational changes in ovarian morphology. However, further work is needed to elucidate the mechanisms underlying the effects of DEHP on folliculogenesis and steroidogenesis across generations. The mechanisms underlying the direct effects of DEHP on the F1 and F2 generations may be very different compared to the effects of DEHP on the F3 generation. In the F1 and F2 generations, it is possible that the mechanisms involve direct effects of DEHP on peroxisome proliferator-activated receptor alpha, proliferator-activated receptor gamma, or estrogen receptors [67–69]. In the F3 generation, however, it is possible that the mechanism involves epigenetic modifications to the DNA, such as DNA methylation and histone modifications [18, 37, 70–72]. Normal folliculogenesis and steroidogenesis are required for normal fertility. Therefore, future studies should also examine the fertility of mice prenatally exposed to DEHP in the F1, F2, and F3 generations.
Acknowledgments
We would like to thank the members of the Flaws laboratory for their assistance. We would also like to thank The University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Corse that is supported by the Eunice Kennedy Shriver NICHD/NIH (NCTRI) Grant P50-HD28934 for their assistance with the hormone serum sample assays.
Grant support: The work was supported by NIH P01 ES 022848, EPA RD83 543401, NIH T32 ES007326.
Conference presentation: Presented in part at the 50th Annual Meeting of the Society for the Study of Reproduction, 12–15 July, 2017, Washington D.C.
References
- 1. ATSDR Toxicological Profile for Di(2-ethylhexyl) Phthalate. U.S. Department of Health and Human Services; 2002:176–213. [Google Scholar]
- 2. Niermann S, Rattan S, Brehm E, Flaws JA. Prenatal exposure to di-(2-ethylhexyl) phthalate (DEHP) affects reproductive outcomes in female mice. Reprod Toxicol 2015; 53:23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hannon PR, Flaws JA. The effects of phthalates on the ovary. Front Endocrinol (Lausanne) 2015; 6:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Heudorf U, Mersch-Sundermann V, Angerer J. Phthalates: toxicology and exposure. Int J Hyg Environ Health 2007; 210(5):623–634. [DOI] [PubMed] [Google Scholar]
- 5. Koch HM, Calafat AM. Human body burdens of chemicals used in plastic manufacture. Philos Trans R Soc Lond B Biol Sci 2009; 364(1526):2063–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Silva MJ, Wong LY, Samandar E, Preau JL, Calafat AM, Ye X. Exposure to di-2-ethylhexyl terephthalate in a convenience sample of U.S. adults from 2000 to 2016. Arch Toxicol 2017; 91(10):3287–3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hogberg J, Hanberg A, Berglund M, Skerfving S, Remberger M, Calafat AM, Filipsson AF, Jansson B, Johansson N, Appelgren M, Hakansson H. Phthalate diesters and their metabolites in human breast milk, blood or serum, and urine as biomarkers of exposure in vulnerable populations. Environ Health Perspect 2008; 116:334–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kato K, Silva MJ, Reidy JA, Hurtz D, 3rd Malek NA, Needham LL, Nakazawa H, Barr DB, Calafat AM. Mono(2-ethyl-5-hydroxyhexyl) phthalate and mono-(2-ethyl-5-oxohexyl) phthalate as biomarkers for human exposure assessment to di-(2-ethylhexyl) phthalate. Environ Health Perspect 2004; 112:327–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Silva MJ, Reidy JA, Preau JL, Samandar E, Needham LL, Calafat AM. Measurement of eight urinary metabolites of di(2-ethylhexyl) phthalate as biomarkers for human exposure assessment. Biomarkers 2006; 11:1–13. [DOI] [PubMed] [Google Scholar]
- 10. Latini G, De Felice C Presta G, Del Vecchio A, Paris I, Ruggieri F, Mazzeo P. Exposure to Di(2-ethylhexyl)phthalate in humans during pregnancy. A preliminary report. Biol Neonate 2003; 83:22–24. [DOI] [PubMed] [Google Scholar]
- 11. Stenz L, Escoffier J, Rahban R, Nef S, Paoloni-Giacobino A. Testicular dysgenesis syndrome and long-lasting epigenetic silencing of mouse sperm genes involved in the reproductive system after prenatal exposure to DEHP. PLoS One 2017; 12:e0170441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Doyle TJ, Bowman JL, Windell VL, McLean DJ, Kim KH. Transgenerational effects of di-(2-ethylhexyl) phthalate on testicular germ cell associations and spermatogonial stem cells in mice. Biol Reprod 2013; 88:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Barakat R, Lin PP, Rattan S, Brehm E, Canisso IF, Abosalum ME, Flaws JA, Hess R, Ko C. Prenatal exposure to DEHP induces premature reproductive senescence in male mice. Toxicol Sci 2017; 156(1):96–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen J, Wu S, Wen S, Shen L, Peng J, Yan C, Cao X, Zhou Y, Long C, Lin T, He D, Hua Y et al. The mechanism of environmental endocrine disruptors (DEHP) induces epigenetic transgenerational inheritance of cryptorchidism. PLoS One 2015; 10(6):e0126403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Skakkebaek NE, Rajpert-De Meyts E, Main KM. Testicular dysgenesis syndrome: an increasingly common developmental disorder with environmental aspects: opinion. Hum Reprod 2001; 165:972–978. [DOI] [PubMed] [Google Scholar]
- 16. Hu GX, Lian QQ, Ge RS, Hardy DO, Li XK. Phthalate-induced testicular dysgenesis syndrome: Leydig cell influence. Trends Endocrinol Metab 2009; 20(3):139–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kita DH, Meyer KB, Venturelli AC, Adams R, Machado DL, Morais RN, Swan SH, Gennings C, Martino-Andrade AJ. Manipulation of pre and postnatal androgen environments and anogenital distance in rats. Toxicology 2016; 368–369:152–161. [DOI] [PubMed] [Google Scholar]
- 18. Nilsson EE, Skinner MK. Environmentally induced epigenetic transgenerational inheritance of reproductive disease. Biol Reprod 2015; 93:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang XF, Zhang LJ, Li L, Feng YN, Chen B, Ma JM, Huynh E, Shi QH, De Felici M, Shen W. Diethylhexyl phthalate exposure impairs follicular development and affects oocyte maturation in the mouse. Environ Mol Mutagen 2013; 54:354–361. [DOI] [PubMed] [Google Scholar]
- 20. Wang Y, Yang Q, Liu W, Yu M, Zhang Z, Cui X. DEHP exposure in utero disturbs sex determination and is potentially linked with precocious puberty in female mice. Toxicol Appl Pharmacol 2016; 307:123–129. [DOI] [PubMed] [Google Scholar]
- 21. Martinez-Arguelles DB, Papadopoulos V. Prenatal phthalate exposure: epigenetic changes leading to lifelong impact on steroid formation. Andrology 2016; 4(4):573–584. [DOI] [PubMed] [Google Scholar]
- 22. Su PH, Chang CK, Lin CY, Chen HY, Liao PC, Hsiung CA, Chiang HC, Wang SL. Prenatal exposure to phthalate ester and pubertal development in a birth cohort in central Taiwan: a 12-year follow-up study. Environ Res 2015; 136:324–330. [DOI] [PubMed] [Google Scholar]
- 23. Araki A, Mitsui T, Miyashita C, Nakajima T, Naito H, Ito S, Sasaki S, Cho K, Ikeno T, Nonomura K, Kishi R. Association between maternal exposure to di(2-ethylhexyl) phthalate and reproductive hormone levels in fetal blood: the Hokkaido study on environment and children's health. PLoS One 2014; 9(10):e109039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Watkins DJ, Tellez-Rojo MM, Ferguson KK, Lee JM, Solano-Gonzalez M, Blank-Goldenberg C, Peterson KE, Meeker JD. In utero and peripubertal exposure to phthalates and BPA in relation to female sexual maturation. Environ Res 2014; 134:233–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Krotz SP, Carson SA, Tomey C, Buster JE. Phthalates and bisphenol do not accumulate in human follicular fluid. J Assist Reprod Genet 2012; 29(8):773–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gore AC, Chappell VA, Fenton SE, Flaws JA, Nadal A, Prins GS, Toppari J, Zoeller RT. EDC-2: The Endocrine Society's Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocr Rev 2015; 36:E1-E150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Patel S, Zhou C, Rattan S, Flaws JA. Effects of Endocrine-Disrupting Chemicals on the Ovary. Biol Reprod 2015; 93:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Johansson HKL, Svingen T, Fowler PA, Vinggaard AM, Boberg J. Environmental influences on ovarian dysgenesis - developmental windows sensitive to chemical exposures. Nat Rev Endocrinol 2017; 13:400–414. [DOI] [PubMed] [Google Scholar]
- 29. Rattan S, Zhou C, Chiang C, Mahalingam S, Brehm E, Flaws JA. Exposure to endocrine disruptors during adulthood: consequences for female fertility. J Endocrinol 2017; 233:R109–R129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mlynarcikova A, Fickova M, Scsukova S. Impact of endocrine disruptors on ovarian steroidogenesis. Endocr Regul 2014; 48:201–224. [DOI] [PubMed] [Google Scholar]
- 31. Craig ZR, Wang W, Flaws JA. Endocrine-disrupting chemicals in ovarian function: effects on steroidogenesis, metabolism and nuclear receptor signaling. Reproduction 2011; 142:633–646. [DOI] [PubMed] [Google Scholar]
- 32. Hannon PR, Brannick KE, Wang W, Gupta RK, Flaws JA. Di(2-ethylhexyl) phthalate inhibits antral follicle growth, induces atresia, and inhibits steroid hormone production in cultured mouse antral follicles. Toxicol Appl Pharmacol 2015; 284:42–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hannon PR, Peretz J, Flaws JA. Daily exposure to Di(2-ethylhexyl) phthalate alters estrous cyclicity and accelerates primordial follicle recruitment potentially via dysregulation of the phosphatidylinositol 3-kinase signaling pathway in adult mice. Biol Reprod 2014; 90:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hannon PR, Niermann S, Flaws JA. Acute exposure to di(2-ethylhexyl) phthalate in adulthood causes adverse reproductive outcomes later in life and accelerates reproductive aging in female mice. Toxicol Sci 2016; 150:97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lai FN, Liu JC, Li L, Ma JY, Liu XL, Liu YP, Zhang XF, Chen H, De Felici M, Dyce PW, Shen W. Di (2-ethylhexyl) phthalate impairs steroidogenesis in ovarian follicular cells of prepuberal mice. Arch Toxicol 2017; 91(3):1279–1292. [DOI] [PubMed] [Google Scholar]
- 36. Pocar P, Fiandanese N, Secchi C, Berrini A, Fischer B, Schmidt J-S, Schaedlich K, Rhind SM, Zhang Z, Borromeo V. Effects of polychlorinated biphenyls in CD-1 mice: reproductive toxicity and intergenerational transmission. Toxicol Sci 2012; 126(1):213–226. [DOI] [PubMed] [Google Scholar]
- 37. Nilsson E, Larsen G, Manikkam M, Guerrero-Bosagna C, Savenkova MI, Skinner MK. Environmentally induced epigenetic transgenerational inheritance of ovarian disease. PLoS One 2012; 7(5):e36129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hirshfield AN. Development of follicles in the mammalian ovary. Int Rev Cytol 1991; 124:43–101. [DOI] [PubMed] [Google Scholar]
- 39. Pepling ME. From primordial germ cell to primordial follicle: mammalian female germ cell development. Genesis 2006; 44:622–632. [DOI] [PubMed] [Google Scholar]
- 40. Menke DB, Koubova J, Page DC. Sexual differentiation of germ cells in XX mouse gonads occurs in an anterior-to-posterior wave. Dev Biol 2003; 262:303–312. [DOI] [PubMed] [Google Scholar]
- 41. Durcova-Hills G, Capel B. Development of germ cells in the mouse. Curr Top Dev Biol 2008; 83:185–212. [DOI] [PubMed] [Google Scholar]
- 42. Berger A, Ziv-Gal A, Cudiamat J, Wang W, Zhou C, Flaws JA. The effects of in utero bisphenol A exposure on the ovaries in multiple generations of mice. Reprod Toxicol 2016; 60:39–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Barnett KR, Tomic D, Gupta RK, Babus JK, Roby KF, Terranova PF, Flaws JA. The aryl hydrocarbon receptor is required for normal gonadotropin responsiveness in the mouse ovary. Toxicol Appl Pharmacol 2007; 223:66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zoeller RT, Vandenberg LN. Assessing dose–response relationships for endocrine disrupting chemicals (EDCs): a focus on non-monotonicity. Environ Health 2015; 14:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Koch HM, Bolt HM, Angerer J. Di(2-ethylhexyl)phthalate (DEHP) metabolites in human urine and serum after a single oral dose of deuterium-labelled DEHP. Arch Toxicol 2004; 78:123–130. [DOI] [PubMed] [Google Scholar]
- 46. Zhou C, Gao L, Flaws JA. Exposure to an environmentally relevant phthalate mixture causes transgenerational effects on female reproduction in mice. Endocrinology 2017; 158:1739–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Klöting N, Hesselbarth N, Gericke M, Kunath A, Biemann R, Chakaroun R, Kosacka J, Kovacs P, Kern M, Stumvoll M, Fischer B, Rolle-Kampczyk U et al. Di-(2-ethylhexyl)-phthalate (DEHP) causes impaired adipocyte function and alters serum metabolites. PLoS One 2015; 10:e0143190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Arcadi FA, Costa C, Imperatore C, Marchese A, Rapisarda A, Salemi M, Trimarchi GR, Costa G. Oral toxicity of bis(2-ethylhexyl) phthalate during pregnancy and suckling in the Long-Evans rat. Food Chem Toxicol 1998; 36:963–970. [DOI] [PubMed] [Google Scholar]
- 49. Grande SW, Andrade AJ, Talsness CE, Grote K, Golombiewski A, Sterner-Kock A, Chahoud I. A dose–response study following in utero and lactational exposure to di-(2-ethylhexyl) phthalate (DEHP): reproductive effects on adult female offspring rats. Toxicology 2007; 229:114–122. [DOI] [PubMed] [Google Scholar]
- 50. Mu X, Liao X, Chen X, Li Y, Wang M, Shen C, Zhang X, Wang Y, Liu X, He J. DEHP exposure impairs mouse oocyte cyst breakdown and primordial follicle assembly through estrogen receptor-dependent and independent mechanisms. J Hazard Mater 2015; 298:232–240. [DOI] [PubMed] [Google Scholar]
- 51. Man L, Lekovich J, Rosenwaks Z, Gerhardt J. Fragile X-associated diminished ovarian reserve and primary ovarian insufficiency from molecular mechanisms to clinical manifestations. Front Mol Neurosci 2017; 10:290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cheng L, Shi H, Jin Y, Li X, Pan J, Lai Y, Lin Y, Jin Y, Roy G, Zhao A, Li F. Adiponectin deficiency leads to female subfertility and ovarian dysfunctions in mice. Endocrinology 2016; 157(12):4875–4887. [DOI] [PubMed] [Google Scholar]
- 53. Zhang T, Li L, Qin XS, Zhou Y, Zhang XF, Wang LQ, De Felici M, Chen H, Qin GQ, Shen W. Di-(2-ethylhexyl) phthalate and bisphenol A exposure impairs mouse primordial follicle assembly in vitro. Environ Mol Mutagen 2014; 55(4):343–353. [DOI] [PubMed] [Google Scholar]
- 54. Wang W, Craig ZR, Basavarajappa MS, Hafner KS, Flaws JA. Mono-(2-ethylhexyl) phthalate induces oxidative stress and inhibits growth of mouse ovarian antral follicles. Biol Reprod 2012; 87:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zhou C, Flaws JA. Effects of an environmentally relevant phthalate mixture on cultured mouse antral follicles. Toxicol Sci 2016; 156(1):217–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Quirk SM, Cowan RG, Harman RM, Hu CL, Porter DA. Ovarian follicular growth and atresia: the relationship between cell proliferation and survival. J Anim Sci 2004; 82(13_suppl):E40–E52. [DOI] [PubMed] [Google Scholar]
- 57. Takai R, Hayashi S, Kiyokawa J, Iwata Y, Matsuo S, Suzuki M, Mizoguchi K, Chiba S, Deki T. Collaborative work on evaluation of ovarian toxicity. 10) Two- or four-week repeated dose studies and fertility study of di-(2-ethylhexyl) phthalate (DEHP) in female rats. J Toxicol Sci 2009; 34(Suppl 1):SP111–SP119. [DOI] [PubMed] [Google Scholar]
- 58. Guo M, Lai L, Zong T, Lin Y, Yang B, Zhang L, Li M, Kuang H. Exposure to di(2-ethylhexyl) phthalate inhibits luteal function via dysregulation of CD31 and prostaglandin F2alpha in pregnant mice. Reprod Biol Endocrinol 2015; 13:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Herreros MA, Gonzalez-Bulnes A, Inigo-Nunez S, Contreras-Solis I, Ros JM, Encinas T. Toxicokinetics of di(2-ethylhexyl) phthalate (DEHP) and its effects on luteal function in sheep. Reprod Biol 2013; 13:66–74. [DOI] [PubMed] [Google Scholar]
- 60. Francois CM, Petit F, Giton F, Gougeon A, Ravel C, Magre S, Cohen-Tannoudji J, Guigon CJ. A novel action of follicle-stimulating hormone in the ovary promotes estradiol production without inducing excessive follicular growth before puberty. Sci Rep 2017; 7:46222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Gupta RK, Singh JM, Leslie TC, Meachum S, Flaws JA, Yao HH. Di-(2-ethylhexyl) phthalate and mono-(2-ethylhexyl) phthalate inhibit growth and reduce estradiol levels of antral follicles in vitro. Toxicol Appl Pharmacol 2010; 242:224–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Oktem O, Akin N, Bildik G, Yakin K, Alper E, Balaban B, Urman B. FSH Stimulation promotes progesterone synthesis and output from human granulosa cells without luteinization. Hum Reprod 2017; 32:643–652. [DOI] [PubMed] [Google Scholar]
- 63. Somasundaram DB, Selvanesan BC, Ramachandran I, Bhaskaran RS. Lactational exposure to di (2-ethylhexyl) phthalate impairs the ovarian and uterine function of adult offspring rat. Reprod Sci 2016; 23:549–559. [DOI] [PubMed] [Google Scholar]
- 64. Eagon PK, Chandar N, Epley MJ, Elm MS, Brady EP, Rao KN. Di(2-ethylhexyl)phthalate-induced changes in liver estrogen metabolism and hyperplasia. Int J Cancer 1994; 58:736–743. [DOI] [PubMed] [Google Scholar]
- 65. Lenie S, Smitz J. Steroidogenesis-disrupting compounds can be effectively studied for major fertility-related endpoints using in vitro cultured mouse follicles. Toxicol Lett 2009; 185:143–152. [DOI] [PubMed] [Google Scholar]
- 66. Wang W, Craig ZR, Basavarajappa MS, Gupta RK, Flaws JA. Di (2-ethylhexyl) phthalate inhibits growth of mouse ovarian antral follicles through an oxidative stress pathway. Toxicol Appl Pharmacol 2012; 258:288–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Lovekamp-Swan T, Davis BJ. Mechanisms of phthalate ester toxicity in the female reproductive system. Environ Health Perspect 2003; 111:139–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Lovekamp-Swan T, Jetten AM, Davis BJ. Dual activation of PPAR? and PPAR? by mono-(2-ethylhexyl) phthalate in rat ovarian granulosa cells. Mol Cell Endocrinol 2003; 201:133–141. [DOI] [PubMed] [Google Scholar]
- 69. Kay VR, Chambers C, Foster WG. Reproductive and developmental effects of phthalate diesters in females. Crit Rev Toxicol 2013; 43:200–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Skinner MK, Manikkam M, Guerrero-Bosagna C. Epigenetic transgenerational actions of environmental factors in disease etiology. Trends Endocrinol Metab 2010; 21:214–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Skinner MK. Epigenetic transgenerational inheritance. Nat Rev Endocrinol 2016; 12:68–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Hanson MA, Skinner MK. Developmental origins of epigenetic transgenerational inheritance. Environ Epigenet 2016; 2(1). [DOI] [PMC free article] [PubMed] [Google Scholar]