Abstract
Background. Continued racial/ethnic health disparities were recently described as “the most serious and shameful health care issue of our time.” Although the 2014 US Affordable Care Act–mandated national insurance coverage expansion has led to significant improvements in health care coverage and access, its effects on life expectancy are not yet known. The Veterans Health Administration (VHA), the largest US integrated health care system, has a sustained commitment to health equity that addresses all 3 stages of health disparities research: detection, understanding determinants, and reduction or elimination. Despite this, racial disparities still exist in the VHA across a wide range of clinical areas and service types.
Objectives. To inform the health equity research agenda, we synthesized evidence on racial/ethnic mortality disparities in the VHA.
Search Methods. Our research librarian searched MEDLINE and Cochrane Central Registry of Controlled Trials from October 2006 through February 2017 using terms for racial groups and disparities.
Selection Criteria. We included studies if they compared mortality between any racial/ethnic minority and nonminority veteran groups or between different minority groups in the VHA (PROSPERO# CRD42015015974). We made study selection decisions on the basis of prespecified eligibility criteria. They were first made by 1 reviewer and checked by a second and disagreements were resolved by consensus (sequential review).
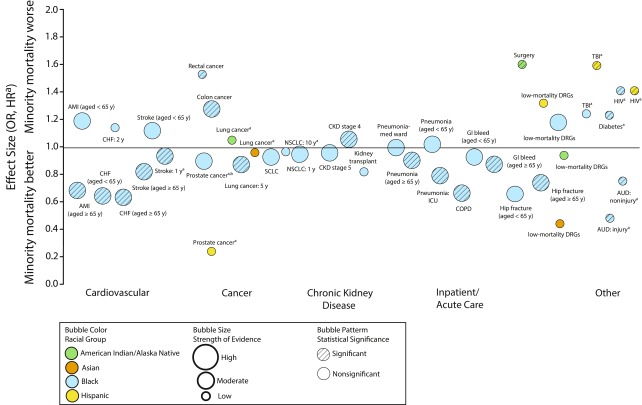
Data Collection and Analysis. Two reviewers sequentially abstracted data on prespecified population, outcome, setting, and study design characteristics. Two reviewers sequentially graded the strength of evidence using prespecified criteria on the basis of 5 key domains: study limitations (study design and internal validity), consistency, directness, precision of the evidence, and reporting biases. We synthesized the evidence qualitatively by grouping studies first by racial/ethnic minority group and then by clinical area. For areas with multiple studies in the same population and outcome, we pooled their reported hazard ratios (HRs) using random effects models (StatsDirect version 2.8.0; StatsDirect Ltd., Altrincham, England). We created an evidence map using a bubble plot format to represent the evidence base in 5 dimensions: odds ratio or HR of mortality for racial/ethnic minority group versus Whites, clinical area, strength of evidence, statistical significance, and racial group.
Main Results. From 2840 citations, we included 25 studies. Studies were large (n ≥ 10 000) and involved nationally representative cohorts, and the majority were of fair quality. Most studies compared mortality between Black and White veterans and found similar or lower mortality for Black veterans. However, we found modest mortality disparities (HR or OR = 1.07, 1.52) for Black veterans with stage 4 chronic kidney disease, colon cancer, diabetes, HIV, rectal cancer, or stroke; for American Indian and Alaska Native veterans undergoing noncardiac major surgery; and for Hispanic veterans with HIV or traumatic brain injury (most low strength).
Author’s Conclusions. Although the VHA’s equal access health care system has reduced many racial/ethnic mortality disparities present in the private sector, our review identified mortality disparities that have persisted mainly for Black veterans in several clinical areas. However, because most mortality disparities were supported by single studies with imprecise findings, we could not draw strong conclusions about this evidence. More disparities research is needed for American Indian and Alaska Native, Asian, and Hispanic veterans overall and for more of the largest life expectancy gaps.
Public Health Implications. Because of the relatively high prevalence of diabetes in Black veterans, further research to better understand and reduce this mortality disparity may be prioritized as having the greatest potential impact. However, other mortality disparities affect thousands of veterans and cannot be ignored.
PLAIN-LANGUAGE SUMMARY
Although the Veterans Health Administration (VHA), the largest US health care system, is committed to equal health and health care quality for all veterans, some veterans in racial/ethnic minority groups still have poorer health outcomes and reduced services. To help the VHA focus future health equity research, a group of researchers at the US Department of Veterans Affairs (VA) Evidence-Based Synthesis Program Coordinating Center systematically reviewed up-to-date evidence on how rates of death compare between racial/ethnic minority and nonminority veteran groups and between different minority groups in the VHA. Although this review found few differences among Black and White veterans, there are some important health conditions in which mainly Black veterans have higher death rates than do White veterans: stage 4 chronic kidney disease, colon cancer, diabetes, HIV, rectal cancer, and stroke. Because diabetes is the most common among these conditions, we suggested it as a top priority for future VHA research. Although there is a lot of research on Black veterans, more disparities research is needed for other racial/ethnic minority groups, including American Indian and Alaska Native, Asian, and Hispanic veterans.
Continued racial/ethnic health disparities were recently described as “the most serious and shameful health care issue of our time.”1(p1569) On the basis of National Center for Health Statistics’ data on deaths in 2014, the 11 largest gaps in life expectancy between Blacks and Whites may include HIV; homicide; essential hypertension and hypertensive renal disease; nephritis, nephrotic syndrome, and nephrosis; prostate cancer; diabetes; septicemia; breast cancer; cerebrovascular disease; colorectal cancer; and diseases of the heart.2,3 Lack of insurance and poorer access to health care have long been considered major contributors to racial/ethnic disparities.4 Although the 2014 US Affordable Care Act–mandated national insurance coverage expansion has led to significant improvements in health care coverage and access, its effects on life expectancy are not yet known.5
Accordingly, a top research priority for the National Institute on Minority Health and Health Disparities is premature or excessive mortality.6 Other national efforts to eliminate disparities include provisions in Healthy People 2020, the Affordable Care Act, and the VA Commission on Care report. These promote greater racial/ethnic diversity of health care professionals and funding for research to reduce racial/ethnic health disparities.7,8
The Veterans Health Administration (VHA), the largest US integrated health care system, strives to provide equal access to care to all veterans. Racial/ethnic diversity is increasing in the VHA user population, with 23.5% of veterans coming from racial/ethnic minority groups in fiscal year 2013.9 The VHA has a sustained commitment to quality improvement and health equity10,11 that addresses all 3 stages of health disparities research: detection, understanding determinants, and reduction or elimination.12 Despite this, racial disparities still exist in the VHA across a wide range of clinical areas and service types.9,13 In June 2016, the Commission on Care recommended increasing resources to, and institutional prioritization of, health equity to more effectively eliminate health care disparities among veterans.8
To support the VHA’s efforts to better understand the scale and determinants of disparities in racial/ethnic mortality and to develop interventions to reduce disparities, investigators from the Veterans Affairs (VA) Evidence-Based Synthesis Program Coordinating Center conducted a systematic review of mortality disparities specific to the VHA.
METHODS
This work is an updated and expanded version of a VHA-funded report. We provide an update on what research and implementation priorities have emerged since (1) the VA Evidence-Based Synthesis Program’s 2007 systematic review Racial and Ethnic Disparities in the VA Health Care System,13,14 and (2) the VA Evidence-Based Synthesis Program’s 2011 systematic review Interventions to Improve Minority Health Care and Racial and Ethnic Disparities.15 Complete details on the scope and methods of our original report are available in the full evidence report.16
Topic Development
The VA Evidence-Based Synthesis Program Coordinating Center investigators consulted with VHA stakeholders to identify the population, comparator, outcome, timing, setting, and study design characteristics of interest. These eligibility criteria are presented in Table A (available as a supplement to the online version of this article at http://www.ajph.org). Our original study protocol is registered with the International Prospective Register of Systematic Reviews (PROSPERO ID# CRD42015015974). We reported this work in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement.17
Search Strategy and Study Selection
Our research librarian searched MEDLINE (via PubMed), and the Cochrane Central Register of Controlled Trials from October 9, 2006, to February 2, 2017, using terms for racial groups and disparities. Study selection was made by 1 reviewer on the basis of the prespecified criteria presented in Table A and checked by another (dual sequential review). We resolved disagreements by consensus.
Data Abstraction and Quality Assessment
We abstracted data on each of the eligibility criteria items (Table A). Two reviewers (K. P. and either E. M. or J. A.) sequentially used methods from the Drug Effectiveness Review Project18 to rate the internal validity of all studies. We resolved disagreement via consensus. To summarize our internal validity ratings, we used Microsoft Excel 2010 (Microsoft Corp., Redmond, WA) to calculate descriptive statistics and generate a stacked bar graph.
Data Synthesis
Two reviewers (K. P. and either E. M. or J. A.) sequentially graded the strength of evidence on the basis of the Agency for Healthcare Research and Quality’s Methods Guide for Comparative Effectiveness Reviews and resolved disagreements via consensus.19 This approach incorporates 5 key domains: study limitations (including study design and aggregate quality), consistency, directness, precision of the evidence, and reporting biases. Ratings ranged from high to insufficient, reflecting our confidence that the evidence reflected the true effect. For example, the overall strength of evidence rating might be “moderate” for a single, adequately powered well-done study. This is because we would still be uncertain whether a single study presented the definitive picture of the magnitude or direction of a disparity (unknown consistency).
We synthesized the evidence qualitatively by grouping studies first by racial/ethnic minority group and then by clinical area. For areas with multiple studies in the same population and outcome, we pooled their reported hazard ratios (HRs) using random effects models (StatsDirect version 2.8.0; StatsDirect Ltd., Altrincham, England). We created an evidence map using a bubble plot format to represent the evidence base in 5 dimensions: odds ratio (OR) or HR of mortality for racial/ethnic minority group versus Whites (y-axis), clinical area (x-axis), strength of evidence (bubble size), statistical significance (bubble pattern), and racial group (bubble color). We converted effect estimates of ORs for survival to mortality by taking the inverse of the OR and estimates with the racial/ethnic minority group as the reference (i.e., White vs Black) were converted to White as the reference racial group (i.e., Black vs White) by taking the inverse of the estimate.
For studies with mortality outcomes at various time points, we have reported the longest follow-up outcome. To focus on the best-quality and most consistent evidence, we excluded from the map effect estimates comparing different minority groups (i.e., Hispanic vs Black), studies we rated as poor quality, and effect estimates other than ORs or HRs (i.e., number of days to death,20 risk differences).21 We created the map using Excel, Tableau version 9.0.3 (Tableau Version 9.0.3. [2015] Seattle, WA) and Adobe Illustrator 2015.3. (Adobe Systems, San Jose, CA).
RESULTS
Searches resulted in 2840 potentially relevant articles (Figure A, available as a supplement to the online version of this article at http://www.ajph.org). Of these, we included 25 studies in 29 publications. Table 1 summarizes the characteristics of the included studies. Most studies compared mortality between Black and White veterans across a variety of clinical areas. Studies were large (n ≥ 10 000) and involved nationally representative cohorts.
TABLE 1—
Characteristics of Included Studies: United States, October 9, 2006–February 2, 2017
| Characteristic | No. (%) |
| Race/Ethnicitya | |
| Black | 24 (96) |
| Hispanic | 5 (20) |
| Asian | 2 (8) |
| AI/AN | 3 (12) |
| Clinical areaa | |
| Cardiovascular | 4 (16) |
| Chronic heart failure | 2 (8) |
| Stroke | 2 (8) |
| Cancer | 7 (28) |
| Lung | 4 (16) |
| Prostate | 3 (12) |
| Colorectal | 1 (4) |
| Rectal | 1 (4) |
| Chronic kidney disease | 4 (16) |
| Inpatient/acute care | 5 (20) |
| Other | 6 (24) |
| Alcohol use disorders | 1 (4) |
| Diabetes | 2 (8) |
| HIV | 1 (4) |
| Kidney transplant | 1 (4) |
| TBI | 1 (4) |
| Study site | |
| National | 23 (92) |
| Regional | 2 (8) |
| Sample size | |
| < 1000 | 3 (12) |
| 1000–5000 | 6 (24) |
| 5001–10 000 | 1 (4) |
| 10 001–100 000 | 7 (28) |
| > 100 000 | 8 (32) |
| Study quality | |
| Good | 8 (32) |
| Fair | 16 (64) |
| Poor | 1 (4) |
Note. AI/AN = American Indian/Alaska Native.
Total > 25 because some studies evaluated more than 1 racial group or clinical area.
We rated 8 studies as good quality,22–29 1 as poor quality,30 and the remainder as fair quality20,21,31–44 (Figure B, available as a supplement to the online version of this article at http://www.ajph.org). Frequent methodological limitations among fair quality studies included unclear handling of missing data and lack of adjustment for hospital-level variables or region. The poor quality study did not adjust for any potential confounders. We rated the strength of the evidence as low for the majority of findings because most were supported by a single, imprecise, fair quality study. We rated findings with multiple studies that were good quality and adequately powered as moderate or high strength of evidence.
Black Veterans
Twenty-four studies (in 27 publications) compared mortality between Black and White veterans (Table 2). The majority of studies focused on patients with cancer (7 studies),20,26,32,34,36,37,43 chronic kidney disease (CKD; 4 studies in 6 publications),22,24,29,40,47,48 inpatient or acute care (5 studies),21,23,25,27,28 and cardiovascular disease (4 studies).21,28,38,44 These studies suggested a potentially increased risk of mortality for Black veterans following stroke and for those with colorectal cancer, stage 4 CKD, diabetes, and HIV (Figure 1; Table 2).
TABLE 2—
Summary of Mortality Findings Among Black vs White Veterans: United States, October 9, 2006–February 2, 2017
| Clinical Area | Study | VHA Data Source | Sample Size | Mortality Risk: Black vs White | Strength of Evidence |
| Cardiovascular | |||||
| Stroke | |||||
| AMI or CHF for age < 65 y | Volpp et al.28 | 1 national study (1996–2002) | 284 974 | 30-d mortality: | Moderate |
| Stroke: OR = 1.12 (95% CI = 0.95, 1.32) | |||||
| AMI: OR = 1.19 (95% CI = 0.99, 1.43) | |||||
| CHF: OR = 0.71 (95% CI = 0.62, 0.82) | |||||
| AMI or CHF for age ≥ 65 y | Volpp et al.28 | 1 national study (1996–2002) | 284 974 | 30-d mortality: | Moderate |
| Stroke: OR = 0.81 (95% CI = 0.74, 0.89) | |||||
| AMI: OR = 0.75 (95% CI = 0.67, 0.84) | |||||
| CHF: OR = 0.70 (0.65, 0.76) | |||||
| Polsky et al.21 | 1 national study (1998–2002) | NR | 2-y mortality: | Low | |
| Stroke: Risk difference = 2.5 (P < .05)a,d | |||||
| AMI: Risk difference = −1.0 (P > .05)d | |||||
| CHF: Risk difference = −2.8 (P < .05)d | |||||
| CHF | Jones et al.44 | 1 national study (1995–1999) | 898 | 2-y HR = 1.14 (95% CI = 0.86, 1.50) | Low |
| Stroke | Kamalesh et al.38 | 1 national study (1990–1997) | 55 094 | 1-y mortality: HR = 0.94 (95% CI:0.91, 0.98)c | Moderate |
| Cancer | |||||
| Colon | Samuel et al.26 | 1 national study (2001–2004) | 4 642 | 3-y OR = 1.28 (95% CI = 1.04, 1.56)a,b | Moderate |
| Lung | Samuel et al.26 | 1 national study (2001–2004) | 21 907 | 1y mortality: | Moderate |
| NSCLC: OR = 0.95 (95% CI = 0.87, 1.04)b | |||||
| 3 668 | 1y mortality: | ||||
| SCLC: OR = 0.93 (95% CI = 0.72, 1.22)b | |||||
| Lung (NSCLC) | Williams et al.43 | 1 national study (2001–2010) | 18 466 | 10-y mortality: HR = 0.97 (95% CI = 0.93, 1.02) | Low |
| Ganti et al.36 | 1 national study (1995–2009) | 81 823 | 5-y mortality: HR = 0.94 (95% CI = 0.92, 0.96) | Moderate | |
| Zullig et al.20 | 1 national study (2006–2007) | 2 190 | Days from diagnosis to death at 2 y: HR = 1.31 (95% CI = 1.14, 1.50)d | Low | |
| Prostate | Daskivich et al.32 | 1 regional study (1998–2004) | 1 122 | 6.6-y mortality: HR = 0.60 (0.28, 1.26)d | Moderate |
| Freeman et al.34 | 1 national study (1986–1990) | 102 | 5.7-y mortality: HR = 1.36 (95% CI = 0.62, 2.96)d | ||
| Graham-Steed et al.37 | 1 national study (1991–2006) | 1 270 | 11–16-y mortality: HR = 0.90 (95% CI = 0.58, 1.40) | ||
| Rectal | Samuel et al.26 | 1 national study (2001–2004) | 1 301 | 3-y OR = 1.52 (95% CI = 1.00, 2.33)a,b | Low |
| Chronic kidney disease | |||||
| CKD | |||||
| Stage 1 and 2 combined | Choi et al.22 | 1 national study (2001–2005) | 1 595 557 | 5-y mortality: HR = 1.15 (95% CI = 1.11, 1.18)a,d | Low |
| Kovesdy et al.24 | 1 national study (2004–2006) | 3 072 966 | 7.9-y mortality: HR = 0.76 (95% CI = 0.75, 0.77)d | ||
| Stage 3a | Kovesdy et al.40 | 1 national study (2004–2006) | 382 727 | 5-y mortality: HR = 0.88 (95% CI = 0.81, 0.97)d | Insufficient |
| Choi et al.22 | 1 national study (2001–2005) | 283 664 | 5-y mortality: HR = 1.32 (95% CI = 1.27, 1.36)a,d | ||
| Stage 3b | Kovesdy et al.40 | 1 national study (2004–2006) | 149 815 | 5-y mortality: HR = 0.81 (95% CI = 0.71, 0.92)d | Insufficient |
| Choi et al.22 | 1 national study (2001–2005) | 104 092 | 5-y mortality: HR = 1.21 (95% CI = 1.18, 1.27)a,d,e | ||
| Stage 4 | Kovesdy et al.,40 Choi et al.22 | 2 national studies (2001–2006) | 62 116 | 5-y mortality: HR = 1.07 (95% CI = 1.00, 1.13)a,b | Moderate |
| Stage 5 | Kovesdy et al.,40 Choi et al.22 | 2 national studies (2001–2006) | 8 678 | 5-y mortality: | Moderate |
| Stage 5 = HR = 0.96 (95% CI = 0.83, 1.11)f | |||||
| Kidney transplant | Taber et al.,29 Taber al.45 | 1 national study (2001–2007) | 4 918 | 6-y mortality: OR = 0.84 (95% CI = 0.67, 1.06) | Low |
| Inpatient and acute care | |||||
| COPD | Sarrazin et al.25 | 1 national study (2002–2006) | 50 979 | In-hospital or 30-d mortality: OR = 0.69 (95% CI = 0.62, 0.77) | Moderate |
| Low-mortality DRGs | Shimada et al.27 | 1 national study (2001–2005) | 294 381 | In-hospital mortality: OR = 1.18 (P > .05) | Moderate |
| Pneumonia | Frei et al.23 | 1 national study (2002–2007) | 5 172 | 30-d mortality: | Moderate |
| ICU: OR = 0.82 (95% CI = 0.68, 0.99) | |||||
| 35 706 | 30-d mortality: | ||||
| Medical ward: OR = 0.98 (95% CI = 0.87, 1.10) | |||||
| Aged < 65 y | Volpp et al.28 | 1 national study (1996–2002) | 284 974 | 30-d mortality: | Moderate |
| Pneumonia: OR = 1.09 (95% CI = 0.98, 1.21) | |||||
| GI bleed: OR = 0.93 (95% CI = 0.78, 1.10) | |||||
| Hip fracture: OR = 0.66 (95% CI = 0.28, 1.55) | |||||
| Aged ≥ 65 y | Volpp et al. 28 | 1 national study (1996–2002) | 284 974 | 30-d mortality: | Moderate |
| Pneumonia: OR = 0.90 (95% CI = 0.85, 0.95) | |||||
| GI bleed: OR = 0.88 (95% CI = 0.79, 0.99) | |||||
| Hip fracture: OR = 0.73 (95% CI = 0.58, 0.90) | |||||
| Polsky et al.21 | 1 national study (1998–2002) | NR | 2-y mortality: | Low | |
| Pneumonia: Risk difference = 0.3 (P > .05)d | |||||
| GI bleed: Risk difference = 0.4 (P > .05)d | |||||
| Hip fracture: Risk difference = 2.3 (P > .05)d | |||||
| Other | |||||
| Alcohol use disorders | Fudalej et al.35 | 1 national study (2000–2006) | 2 545 | 5-y injury-related mortality: HR = 0.46 (95% CI = 0.41, 0.52)c | Low |
| 19 381 | 5-y noninjury related mortality: HR = 0.76 (95% CI = 0.72, 0.78)c | ||||
| Diabetes | Kokkinos et al.39 | 1 regional study (1986–2007) | 3 148 | 7-y HR = 1.23 (95% CI = 1.02, 1.47)a | Low |
| Diabetes | Lynch et al.30 | 1 national study (2002–2006) | 533 895 | 5-y mortality: 24% vs 20%d | Insufficient |
| HIV | McGinnis et al.41 | 1 national study (1999–2001) | 5 193 | 2-y HR = 1.41 (95% CI = 1.19, 1.66)a | Low |
| Traumatic brain injury | Egede,33 Dismuke et al.46 | 1 national study (2006) | 9 633 | 2-y mortality: HR = 1.25 (95% CI = 0.90, 1.73) | Low |
Note. AMI = acute myocardial infarction; CHF = chronic heart failure; CI = confidence interval; CKD = chronic kidney disease; COPD = chronic obstructive pulmonary disease; DRG = diagnosis related group; GI = gastrointestinal; HR = hazard ratio; ICU = intensive care unit; NR = not reported; NSCLC = non–small cell lung cancer; OR = odds ratio; SCLC = small-cell lung cancer; VHA = Veterans Health Administration.
Disparity.
Converted original data from survival to mortality.
Converted original data from White versus Black to Black versus White.
Not included in map.
Estimated from figure.
Veterans Affairs Evidence-Based Synthesis Program pooled.
FIGURE 1—
Evidence Map of Risk of Mortality for Racial/Ethnic Minorities vs Whites in the Veterans Health Administration: United States, October 9, 2006–February 2, 2017
Note. AMI = acute myocardial infarction; AUD = alcohol-use disorder; CHF = congestive heart failure; CKD = chronic kidney disease; COPD = chronic obstructive pulmonary disease; DRGs = low-mortality diagnosis–related groups; GI = gastrointestinal; HR = hazard ratio; ICU = intensive care unit; NSCLC = non–small cell lung cancer; OR = odds ratio; SCLC = small-cell lung cancer; TBI = traumatic brain injury.
aDenotes a hazard ratio and not an odds ratio.
bDisease-specific mortality.
Cardiovascular disease.
Four studies evaluated mortality in Black veterans with acute myocardial infarction,21,28 heart failure,21,28,44 or stroke.21,28,38 The only disparity we identified was an increased risk of death for Black veterans aged 65 years and older at 2 years after stroke (Table 2, not shown in map because study reported risk difference and not an HR or OR).21 Otherwise, risk of mortality in Black veterans with cardiovascular conditions was similar or lower than was that of Whites and varied by age and time. For example, at 30 days after stroke, risk of mortality was similar for Black and White veterans aged younger than 65 years28 but lower for Blacks aged 65 years and older.28 There were no differences in the mortality rate of White and Black veterans at 1 year after stroke,38 but risk was higher at 2 years.21
Among Black veterans with heart failure, 30-day mortality was lower than among White veterans regardless of age28 and 2-year mortality was similar.44 In Black veterans aged 65 years and older with acute myocardial infarction, 30-day mortality was lower than was that in Whites and 2-year mortality was similar.21 In veterans younger than 65 years with acute myocardial infarction, 30-day mortality was similar in Blacks and Whites.28
Cancer.
Compared with White veterans, Black veterans with colorectal cancer had significantly higher rates of 3-year mortality (Figure 1; Table 2).26 This finding comes from a good quality but small study that adjusted for age, gender, marital status, history of cancer, Charlson comorbidity score, and diagnosis year. The study authors concluded that the disparity was attributable to within-hospital differences, because additional adjustment for between-hospital differences did not affect the findings.
We found no evidence of mortality disparities in studies of veterans with prostate or lung cancer. For prostate cancer, among 3 studies,32,34,37 the largest and longest term found similar prostate cancer mortality at 11 to 16 years for Black and White veterans after adjusting for age, comorbidity, and D’Amico score.37 Mortality was also similar between Black and White veterans with prostate cancer when measured on the basis of death attributable to all causes at 5.7 years in 2 Chicago, Illinois, VHA hospitals34 and when measured on the basis of prostate cancer causes at 6.6 years at 2 California VHA hospitals.32 For early- to late-stage non–small cell lung cancer, risk of mortality was consistently similar or lower in Black veterans compared with White veterans across 4 studies with follow-up ranging from 1 to 10 years.20,26,36,43
The largest study included 14 791 Black veterans with stages 1 to 4 non–small cell lung cancer.36 After adjustment for age, histology type, clinical stage, family history of cancer, and type of treatment, this study found a significantly lower risk of 5-year mortality for Black veterans (HR = 0.94; 95% CI = 0.92, 0.96).36
A more recent, smaller study (n = 2838 Black veterans, 15 628 White veterans) focused on early stage non–small cell lung cancer and evaluated mortality according to initial treatment received.43 Ten-year mortality in Black veterans was similar overall compared with White veterans (HR = 0.97; 95% CI = 0.93, 1.02) after adjustment for age, stage, histologic diagnosis, comorbidity index, period, region, and tobacco use. Ten-year mortality in Black veterans compared with White veterans was also similar in those who underwent an operation (HR = 0.94; 95% CI = 0.87, 1.01) and lower in those who underwent radiation (HR = 0.90; 95% CI = 0.82, 0.98) or other or no treatment (HR = 0.89; 95% CI = 0.81, 0.97).43
In a small cohort of patients with small cell lung cancer, there was no significant difference between Black and White veterans in 1-year mortality after adjustment for age, gender, marital status, cancer history, Charlson comorbidity score, year of diagnosis, chronic obstructive pulmonary disease, and hospital fixed effects.26
Chronic kidney disease.
Evidence from 4 studies (in 6 publications)22,24,29,40,47,48 suggested that mortality disparity risk varied by CKD stage, but the precise pattern of variation remained unclear because of unexplained inconsistencies among studies (Table 2). Two national studies had consistent findings for CKD stages 4 and 5. Pooled data from these studies suggested an increased risk of mortality in Black compared with White veterans with CKD stage 4 and similar risk of mortality between Black and White veterans with CKD stage 5 (Table 2; Figure 1).22,40 However, risk of mortality was inconsistent among studies for CKD stages 1 to 3.22,24,40 A 2009 study of a cohort from 2000 to 2001 found higher mortality risks for Black veterans with CKD stages 1 to 3b.22
However, subsequent studies of cohorts from 2004 to 2006 found lower mortality risks for Black veterans with CKD stages 1, 2,24 3a, and 3b.40 For CKD stages 1 and 2, the lower mortality risk for Black veterans was attenuated in older patients47 and limited to those with serum calcium levels of at least 8.8 milligrams per deciliter.42 We agree with Kovesdy et al.40 that the explanation for the differences between studies in mortality risk is unclear because of the absence of unadjusted HRs for comparison in the 2009 study. Risk of mortality was similar for Black and White veteran kidney transplant recipients.29,45
Inpatient/acute care.
Compared with White veterans, risk of mortality was equal or lower for Black veterans hospitalized for chronic obstructive pulmonary disease exacerbation,25 gastrointestinal bleeding,21,28 hip fracture,21,28 pneumonia,21,23,28 and low-risk diagnosis–related groups, a variety of conditions that had a mortality risk of less than 0.5% on the basis of Agency for Healthcare Research and Quality Indicators software (Table 2; Figure 1).27,49 We rated the strength of evidence as moderate because, generally, each condition was evaluated in a single, adequately powered, well-done study. In subgroup analyses we found that mortality risk varied by age28 and setting.23 In patients with pneumonia, mortality risk was equal between Black and White veterans when admitted to a medical ward21,23,28 but lower for Black veterans when admitted to an intensive care unit.23 In patients with pneumonia, gastrointestinal bleeds, and hip fractures, mortality risk was equal between Black and White veterans who were younger than 65 years, but in Black veterans aged 65 years and older, mortality risk was lower at 30 days after hospitalization28 and equal at 2 years after hospitalization.21
Alcohol use disorders.
Black veterans with alcohol use disorders had lower risks of 5-year injury-related and non–injury-related mortality than did White veterans (Figure 1; Table 2).35 Injury-related causes of death included accidents, suicide, and homicide. The most frequent noninjury causes of death included heart disease (21.5%), malignant neoplasms (19.8%), and other medical conditions (22.6%). The strength of the finding was low because it was from a single study with some methodological limitations.
Traumatic brain injury.
Black and White veterans with traumatic brain injury (TBI) had a similar risk of 2-year mortality.33,46 However, the strength of the evidence was low because it was from a single study that was likely underpowered to detect a difference; the event rates were low (2.69% for Black veterans [n = 1748] and 2.93% for White veterans [n = 7885]).
Diabetes.
In male veterans with type 2 diabetes (who took an exercise tolerance test conducted between July 1986 and November 2007), Black race was associated with a 23.0% higher mortality within 7 years (Table 2; Figure 1).39 The mortality rate was 29.9% in Black veterans and 21.7% in White veterans. This difference was consistent across different fitness categories, but an exercise capacity–related reduction in mortality appeared to be attenuated in Black veterans. No information was provided about the duration of diabetes in the sample, but mean age was 61 years, body mass index was 30 kilogram per meter squared, rate of cardiovascular disease was 50%, and 42% were smokers. We rated evidence as low strength because it was from a single study with some important methodological limitations.
HIV.
In a nationwide sample of HIV-positive veterans, there was low-strength evidence that Black race was associated with a 41% higher rate of mortality 2 years after diagnosis.41 This finding came from evaluation of data from the Veterans Aging Cohort 3-Site Study (VACS 3). The median age of the sample was 50 years, 98.7% were male, 10.3% had mental illness, and 54.1% were currently smoking. A limitation of this study was that survival estimates were adjusted only for age. It was unclear whether potential differences in comorbidities or HIV illness severity may have contributed to racial differences.
American Indian and Alaska Native Veterans
Four studies compared mortality between American Indian or Alaska Native (AI/AN) and White veterans with lung cancer36 and during acute inpatient care (Table 3).27,31,50 The only disparity found was greater 30-day all-cause postoperative mortality among AI/AN veterans than among White veterans after major noncardiac surgery (Table 3).31 A 2009 reanalysis that evaluated risk of postoperative mortality attributable to specific complications was inconclusive because of a lack of adjustment for potential confounding factors.50 AI/AN veterans had similar in-hospital mortality in low-mortality diagnosis–related groups27 and similar 5-year mortality among patients with lung cancer36 (low strength) compared with White veterans.
TABLE 3—
Summary of Mortality Findings Among Other Race Versus White Veterans: United States, October 9, 2006–February 2, 2017
| Clinical Area | Study | Data Source | No. | Mortality Risk | Strength of Evidence |
| AI/AN | |||||
| Lung cancer | Ganti et al.36 | 1 national study (1995–2009) | 67 323 | 5-y mortality: HR = 1.05 (95% CI = 0.93, 1.20) | Low |
| Low-mortality DRGs | Shimada et al.27 | 1 national study (2001–2005) | 236 369 | In-hospital mortality: OR = 0.94 (P > .05) | Low |
| Major noncardiac surgery | Alvord et al. 31,50 | 1 national study (1991–2002) | 4 419 | 30-d mortality: OR = 1.56 (95% CI = 1.04, 2.35)a | Low |
| Asian | |||||
| Lung cancer | Ganti et al.36 | 1 national study (1995–2009) | 67 332 | 5-y mortality: HR = 0.96 (95% CI = 0.84, 1.09) | Low |
| Low-mortality DRGs | Shimada et al.27 | 1 national study (2001–2005) | 236 845 | In-hospital mortality: OR = 0.44 (P > .05) | Low |
| Hispanic | |||||
| Prostate cancer | Daskivich et al.32 | 1 regional study (1998–2004) | 720 | 6.6-y mortality: HR = 0.24 (95% CI = 0.03, 1.82) | Low |
| Low-mortality DRGs | Shimada et al.27 | 1 national study (2001–2005) | 244 397 | In-hospital mortality: OR = 1.32 (P > .05) | Low |
| Diabetes | Lynch et al.30 | 1 national study (2002–2006) | 115 791 | 5-y mortality: 19% vs 20% | Insufficient |
| HIV | McGinnis et al.41 | 1 national study (1999–2001) | 3 003 | 2-y mortality: HR = 1.41 (95% CI = 1.06, 1.86)a | Low |
| Traumatic brain injury | Egede et al.,33 Dismuke et al.49 | 1 national study (2006–2009) | 8 199 | 2-y mortality: HR = 1.61 (95% CI = 1.00, 2.58)a | Low |
Note. AI/AN = American Indian/Alaska Native; CI = confidence interval; DRG = Diagnosis Related Group; HR = Hazard Ratio; OR = Odds Ratio; NR = ; VHA = Veterans Health Administration.
Disparity.
Asian Veterans
Two studies compared mortality between Asian and White veterans with lung cancer36 and during acute inpatient care (Table 3).27 Asian veterans had similar 4-year mortality among patients with lung cancer36 and similar in-hospital mortality in low-mortality diagnosis related groups compared with White veterans.27 The strength of this evidence was low, because each finding was supported by only a single small study.
Hispanic Veterans
Five studies (in 6 publications) compared mortality outcomes between Hispanic and White veterans in the clinical areas of HIV,41 inpatient or acute care,27 prostate cancer,32 TBI,33,46 and diabetes30 (Table 3). Mortality disparities were found for Hispanic veterans with HIV41 and TBI.33,46 The authors of the TBI study found that Hispanic veterans with TBI had significantly lower service utilization, particularly neurology, than did their White counterparts and that this was a partial mediator of the mortality disparity.49 Hispanic veterans had similar in-hospital mortality for patients in low-mortality diagnosis–related groups27 and similar mortality compared with White veterans in patients with prostate cancer32; however, these single studies may have been inadequately powered to detect a difference. There was insufficient evidence to draw conclusions about how Hispanic and White veterans with type 2 diabetes differ in risk of mortality because the available study did not adjust for any potential confounding factors.30
Black Veterans vs Other Veteran Minority Groups
One study examined ethnicity as 1 of several risk factors for injury-related and noninjury-related mortality in veterans with alcohol use disorders.35 This study compared risk in Black veterans with that in a group of veterans that combined all other racial/ethnic groups except Whites. Compared with Black veterans, veterans identifying as races other than White had similar non–injury-related mortality (HR = 0.97; 95% CI = 0.92, 1.01), but higher mortality rates for injury-related deaths (HR = 1.59; 95% CI = 1.40, 1.80).35 The utility of this finding was unclear, however, because there was no information about the composition of the “other ethnicity” category.
DISCUSSION
Findings from this review suggest that the VHA’s equal access health care system has reduced certain racial/ethnic mortality disparities compared with health care delivered outside the VHA. The majority of studies found racial/ethnic mortality equality23,28,29,32,34,37,43,44,46 or even lower mortality23,24,28,36,38 for mostly Black veterans compared with White veterans in areas where there have been reported disparities in the private sector. Researchers have suggested that such findings—contradicting previous observations that White veterans tend to have better outcomes than do Black veterans (disparity paradox)—may be because Black veterans are less severely ill, which may be unmeasured and unadjusted for.12 The proposed mechanism for this is that White veterans may only approach the VHA for care later in their illness process, after exhausting other private sector health care options, whereas Black veterans may use the VHA earlier because they have less access to the private sector. We found the opposite to be true. In studies reporting better outcomes for Black veterans than for White veterans, 78% had some measure of illness severity. Of these, 71% of the studies found Black veterans to have greater illness severity than did White veterans at baseline and to have higher levels of comorbidities as well. In all these cases, the differences were adjusted for in multivariate analyses.
As in the private sector, however, mortality disparities were detected in the VHA for Black veterans with stage 4 CKD,22,24 colon and rectal cancer,26 diabetes,39 HIV,41 and stroke21; for AI/AN veterans undergoing noncardiac major surgery31; and for Hispanic veterans with HIV41 or TBI.33,49 Among these, the mortality disparity in Black veterans with diabetes may have the greatest impact.39 Although the size of the diabetes mortality disparity was modest (HR or OR = 1.23; 95% CI = 1.02, 1.47), diabetes was the third most commonly diagnosed condition in racial/ethnic minorities in fiscal year 2013.9 Therefore, the mortality disparity in Black veterans with diabetes may be considered the highest priority for further research.39,51 HRs for other mortality disparities ranged from 1.0722,40 to 1.52.26 Although they were not among the top 20 most diagnosed conditions (prevalence 12% to < 20%), they are not ignorable deaths; they represent thousands of racial/ethnic minority veterans.9
Evidence Base Limitations
Limitations of the evidence base include few studies for all racial/ethnic minority groups other than Black veterans. Only 21% evaluated Hispanic veterans, 13% evaluated AI/AN veterans, 8% evaluated Asian veterans, and none evaluated Hawaiian or Pacific Islanders. Only 1 study compared mortality risk among different racial/ethnic minority groups.35 Studies have neglected to investigate a few of the 11 largest causes of gaps in life expectancy between Blacks and Whites, including septicemia, breast cancer, essential hypertension, and hypertensive renal disease. We could not draw strong conclusions about the mortality disparities we identified because of a few important methodological limitations of this body of evidence. For example, most mortality disparities were supported by single studies with imprecise findings.
Additionally, the studies that found racial disparities in mortality most often controlled for age, gender, and socioeconomic status, but these measures do not constitute a complete and accurate list of important potential confounding factors. Health disparities are caused by multiple, complex factors, including physiologic, genetic, behavioral, environmental, and cultural differences among racial groups—factors that can interact in various ways to affect health.52 Because minority groups are often characterized by multiple disparities or vulnerabilities (homelessness, mental health, etc.), it is difficult to identify how each of these, alone or in combination, influences health outcomes. Statistical models can be used to control for potentially confounding vulnerabilities in the population, but thorough and accurate measurement of all potential factors can be impossible. Also, studies rarely reported changes between sequential models with increasing levels of adjustment, which could better disentangle contributing factors.
More disparities research is needed for AI/AN, Asian, Hawaiian and Pacific Islander, and Hispanic veterans overall, as well as for studies comparing different racial/ethnic minority groups. Additionally, more research is needed to investigate more of the 11 largest gaps in life expectancy between Blacks and Whites.2 Finally, for the mortality disparities identified in Black veterans with stage 4 CKD, colon cancer, diabetes, HIV, rectal cancer, and stroke; for AI/AN veterans undergoing noncardiac major surgery; and for Hispanic veterans with HIV or TBI, higher quality studies are still needed that address the identified methodological limitations. For example, analyses should adjust for potential confounding from a broader range of clinical, demographic, social, and system factors. More explicit comparison of changes from unadjusted results to those from sequential models with increasing levels of adjustment may better support identification of disparity determinants.
For the mortality disparity in the potentially highest-impact area of diabetes in Black veterans, we suggest conducting a larger and broader replication study of a more recent cohort. This is because the current supporting study was conducted in men only, involved data from only 2 VA medical centers (Washington, DC, and Palo Alto, CA), and is potentially outdated—the cohort was from 1986 to 2007. Future diabetes disparity research should also explore cardiorespiratory fitness as a predictor of mortality. Although the diabetes study authors were able to rule out differences in obesity and waist circumference as determinants, they suggested further research on the potential roles of differences in vascular reactivity, medical therapy adherence, and genetics. We recognize that the detailed data needed to explore such causes may be difficult to collect.13
Review Limitations
A main limitation of our review is its scope. Although we focused on 1 of the National Institute on Minority Health and Health Disparities highest-priority outcomes of mortality, we did not evaluate other important patient health and process outcomes, nor did we comprehensively evaluate their determinants or potential interventions to reduce them.
A second limitation of our review is that our reliance on few electronic databases—MEDLINE and the Cochrane Central Registry of Controlled Trials—may have increased the risk of publication bias. However, we think it is unlikely that we missed any large studies of veterans that would have meaningfully altered our conclusions.53
Conclusions
Although the VHA’s equal access health care system has reduced many racial/ethnic mortality disparities in the private sector, our review identified mortality disparities that have persisted for Black veterans in several clinical areas, for AI/AN veterans undergoing noncardiac major surgery, and for Hispanic veterans with HIV. However, a few important limitations of the evidence precluded drawing strong conclusions about these findings. Because of the relatively high prevalence of diabetes in Black veterans, further research to better understand and reduce this mortality disparity may be prioritized as having the greatest potential impact. More disparities research is still needed for AI/AN, Asian, and Hispanic veterans overall, as well as for studies comparing different racial/ethnic minority groups.
ACKNOWLEDGMENTS
This article is based on work supported by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Quality Enhancement Research Initiative, Evidence-Based Synthesis Program (project 09-199).
We would like to thank Ana Quiñones, PhD, for providing subject matter expertise; Julia Haskin, MA, Edwin Reid, MS, MAT, MFA, and Donald Bourne, MPH, for searching and editorial support; and Rose Relevo, MLIS MS for searching support.
Our database and all coding and analysis procedures are available to others by request to K. Peterson.
HUMAN PARTICIPANT PROTECTION
Institutional review board approval was not needed for this project, because no human participants were involved in this study.
Footnotes
See also Ibrahim, p. 299.
REFERENCES
- 1.Feldman M. Leading the way in health disparities research. J Gen Intern Med. 2015;30(11):1569. doi: 10.1007/s11606-015-3498-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fuchs VR. Black gains in life expectancy. JAMA. 2016;316(18):1869–1870. doi: 10.1001/jama.2016.14398. [DOI] [PubMed] [Google Scholar]
- 3.Kochanek KD, Murphy SL, Xu J, Tejada-Vera B. Deaths: final data for 2014. Natl Vital Stat Rep. 2016;65(4):1–122. [PubMed] [Google Scholar]
- 4.Chen J, Vargas-Bustamante A, Mortensen K, Ortega AN. Racial and ethnic disparities in health care access and utilization under the affordable care act. Med Care. 2016;54(2):140–146. doi: 10.1097/MLR.0000000000000467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sommers BD, McMurtry CL, Blendon RJ, Benson JM, Sayde JM. Beyond health insurance: remaining disparities in US health care in the post-ACA era. Milbank Q. 2017;95(1):43–69. doi: 10.1111/1468-0009.12245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perez-Stable E. NIMHD contributions to and priorities for interventions to reduce health disparities and opportunities for synergy. Paper presented at: Partners in Advancing Health Equity in the VA Healthcare System. Philadelphia; September 21, 2016.
- 7.National Partnership for Action to End Health Disparities. The Affordable Care Act resource kit. 2014. Available at: http://minorityhealth.hhs.gov/npa/materials/affordablecareactresourcekit.pdf. Accessed March 13, 2017.
- 8.Commission on Care. Final Report of the Commission on Care. Washington, DC: 2016. [Google Scholar]
- 9.Office of Health Equity. National Veteran Health Equity Report: FY 2013. Washington, DC: US Department of Veterans Affairs; 2016. [Google Scholar]
- 10.Ibrahim SA, Egede LE, Uchendu US, Fine MJ. The struggle for health equity: the sustained effort by the VA healthcare system. Am J Public Health. 2014;104(suppl 4):S514–S516. doi: 10.2105/AJPH.2014.302199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uchendu US. Institutional journey in pursuit of health equity: Veterans Health Administration’s Office of Health Equity. Am J Public Health. 2014;104(suppl 4):S511–S513. doi: 10.2105/AJPH.2014.302183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kilbourne AM, Switzer G, Hyman K, Crowley-Matoka M, Fine MJ. Advancing health disparities research within the health care system: a conceptual framework. Am J Public Health. 2006;96(12):2113–2121. doi: 10.2105/AJPH.2005.077628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saha S, Freeman M, Toure J, Tippens KM, Weeks C, Ibrahim S. Racial and ethnic disparities in the VA health care system: a systematic review. J Gen Intern Med. 2008;23(5):654–671. doi: 10.1007/s11606-008-0521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saha S, Freeman M, Toure J, Tippens KM, Weeks C. Racial and ethnic disparities in the VA healthcare system: a systematic review. 2007. Available at: https://www.hsrd.research.va.gov/publications/esp/RacialDisparities-2007.pdf. Accessed March 13, 2017. [PubMed]
- 15.Quiñones AR, O’Neil M, Saha S, Freeman M, Henry SR, Kansagara D. Interventions to improve minority health care and racial and ethnic disparities. 2011. Available at: https://www.hsrd.research.va.gov/publications/esp/healthcare-disparities.cfm. Accessed March 13, 2017. [PubMed]
- 16.Peterson K, McCleery E, Waldrip K, Helfand M. Evidence brief: update on prevalence of and interventions to reduce racial and ethnic disparities within the VA. 2015. Available at: http://www.hsrd.research.va.gov/publications/esp/HealthDisparities.cfm. Accessed March 13, 2017. [PubMed]
- 17.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McDonagh MS, Jonas DE, Gartlehner G et al. Methods for the drug effectiveness review project. BMC Med Res Methodol. 2012;12:140. doi: 10.1186/1471-2288-12-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berkman ND, Lohr KN, Ansari M . Grading the Strength of a Body of Evidence When Assessing Health Care Interventions for the Effective Health Care Program of the Agency for Healthcare Research and Quality: An Update. Rockville, MD: Agency for Healthcare Research and Quality; 2013. [PubMed] [Google Scholar]
- 20.Zullig LL, Carpenter WR, Provenzale DT et al. The association of race with timeliness of care and survival among Veterans Affairs health care system patients with late-stage non–small cell lung cancer. Cancer Manag Res. 2013;5:157–163. doi: 10.2147/CMAR.S46688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Polsky D, Jha AK, Lave J et al. Short- and long-term mortality after an acute illness for elderly Whites and Blacks. Health Serv Res. 2008;43(4):1388–1402. doi: 10.1111/j.1475-6773.2008.00837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choi AI, Rodriguez RA, Bacchetti P, Bertenthal D, Hernandez GT, O’Hare AM. White/Black racial differences in risk of end-stage renal disease and death. Am J Med. 2009;122(7):672–678. doi: 10.1016/j.amjmed.2008.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frei CR, Mortensen EM, Copeland LA et al. Disparities of care for African-Americans and Caucasians with community-acquired pneumonia: a retrospective cohort study. BMC Health Serv Res. 2010;10:143. doi: 10.1186/1472-6963-10-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kovesdy CP, Norris KC, Boulware LE et al. Association of race with mortality and cardiovascular events in a large cohort of US veterans. Circulation. 2015;132(16):1538–1548. doi: 10.1161/CIRCULATIONAHA.114.015124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sarrazin MV, Cannon KT, Rosenthal GE, Kaldjian LC. Racial differences in mortality among veterans hospitalized for exacerbation of chronic obstructive pulmonary disease. J Natl Med Assoc. 2009;101(7):656–662. doi: 10.1016/s0027-9684(15)30974-3. [DOI] [PubMed] [Google Scholar]
- 26.Samuel CA, Landrum MB, McNeil BJ, Bozeman SR, Williams CD, Keating NL. Racial disparities in cancer care in the Veterans Affairs health care system and the role of site of care. Am J Public Health. 2014;104(suppl 4):S562–S571. doi: 10.2105/AJPH.2014.302079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shimada SL, Montez-Rath ME, Loveland SA, Zhao S, Kressin NR, Rosen AK. Racial Disparities in Patient Safety Indicator (PSI) Rates in the Veterans Health Administration. Rockville, MD: Agency for Healthcare Research and Quality; 2008. [PubMed] [Google Scholar]
- 28.Volpp KG, Stone R, Lave JR et al. Is thirty-day hospital mortality really lower for Black veterans compared with White veterans? Health Serv Res. 2007;42(4):1613–1631. doi: 10.1111/j.1475-6773.2006.00688.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taber DJ, Gebregziabher M, Payne EH, Srinivas T, Baliga PK, Egede LE. Overall graft loss versus death-censored graft loss: unmasking the magnitude of racial disparities in outcomes among US kidney transplant recipients. Transplantation. 2017;101(2):402–410. doi: 10.1097/TP.0000000000001119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lynch CP, Gebregziabher M, Zhao Y, Hunt KJ, Egede LE. Impact of medical and psychiatric multi-morbidity on mortality in diabetes: emerging evidence. BMC Endocr Disord. 2014;14:68. doi: 10.1186/1472-6823-14-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alvord LA, Rhoades D, Henderson WG et al. Surgical morbidity and mortality among American Indian and Alaska Native veterans: a comparative analysis. J Am Coll Surg. 2005;200(6):837–844. doi: 10.1016/j.jamcollsurg.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 32.Daskivich TJ, Kwan L, Dash A, Litwin MS. Racial parity in tumor burden, treatment choice and survival outcomes in men with prostate cancer in the VA healthcare system. Prostate Cancer Prostatic Dis. 2015;18(2):104–109. doi: 10.1038/pcan.2014.51. [DOI] [PubMed] [Google Scholar]
- 33.Egede LE, Dismuke C, Echols C. Racial/ethnic disparities in mortality risk among US veterans with traumatic brain injury. Am J Public Health. 2012;102(suppl 2):S266–S271. doi: 10.2105/AJPH.2011.300176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Freeman VL, Durazo-Arvizu R, Arozullah AM, Keys LC. Determinants of mortality following a diagnosis of prostate cancer in Veterans Affairs and private sector health care systems. Am J Public Health. 2003;93(10):1706–1712. doi: 10.2105/ajph.93.10.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fudalej S, Bohnert A, Austin K, Barry K, Blow F, Ilgen M. Predictors of injury-related and non-injury-related mortality among veterans with alcohol use disorders. Addiction. 2010;105(10):1759–1766. doi: 10.1111/j.1360-0443.2010.03024.x. [DOI] [PubMed] [Google Scholar]
- 36.Ganti AK, Subbiah SP, Kessinger A, Gonsalves WI, Silberstein PT, Loberiza FR., Jr Association between race and survival of patients with non–small-cell lung cancer in the United States Veterans Affairs population. Clin Lung Cancer. 2014;15(2):152–158. doi: 10.1016/j.cllc.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 37.Graham-Steed T, Uchio E, Wells CK, Aslan M, Ko J, Concato J. ‘Race’ and prostate cancer mortality in equal-access healthcare systems. Am J Med. 2013;126(12):1084–1088. doi: 10.1016/j.amjmed.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kamalesh M, Shen J, Tierney WM. Stroke mortality and race: does access to care influence outcomes? Am J Med Sci. 2007;333(6):327–332. doi: 10.1097/MAJ.0b013e318065c101. [DOI] [PubMed] [Google Scholar]
- 39.Kokkinos P, Myers J, Nylen E et al. Exercise capacity and all-cause mortality in African American and Caucasian men with type 2 diabetes. Diabetes Care. 2009;32(4):623–628. doi: 10.2337/dc08-1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kovesdy CP, Quarles LD, Lott EH et al. Survival advantage in Black versus White men with CKD: effect of estimated GFR and case mix. Am J Kidney Dis. 2013;62(2):228–235. doi: 10.1053/j.ajkd.2012.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McGinnis KA, Fine MJ, Sharma RK et al. Understanding racial disparities in HIV using data from the veterans aging cohort 3-site study and VA administrative data. Am J Public Health. 2003;93(10):1728–1733. doi: 10.2105/ajph.93.10.1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lu JL, Molnar MZ, Ma JZ et al. Racial differences in association of serum calcium with mortality and incident cardio-and cerebrovascular events. J Clin Endocrinol Metab. 2016;101(12):4851–4859. doi: 10.1210/jc.2016-1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Williams CD, Salama JK, Moghanaki D, Karas TZ, Kelley MJ. Impact of race on treatment and survival among U.S. veterans with early stage lung cancer. J Thorac Oncol. 2016;11(10):1672–1681. doi: 10.1016/j.jtho.2016.05.030. [DOI] [PubMed] [Google Scholar]
- 44.Jones LG, Sin MK, Hage FG et al. Characteristics and outcomes of patients with advanced chronic systolic heart failure receiving care at the Veterans Affairs versus other hospitals: insights from the beta-blocker evaluation of survival trial (BEST) Circ Heart Fail. 2015;8(1):17–24. doi: 10.1161/CIRCHEARTFAILURE.114.001300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Norris KC, Mensah GA, Boulware LE et al. Age, race and cardiovascular outcomes in African American veterans. Ethn Dis. 2016;26(3):305–314. doi: 10.18865/ed.26.3.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Taber DR, Robinson WR, Bleich SN, Wang YC. Deconstructing race and gender differences in adolescent obesity: Oaxaca-blinder decomposition. Obesity (Silver Spring) 2016;24(3):719–726. doi: 10.1002/oby.21369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Taber DJ, Hunt KJ, Fominaya CE et al. Impact of cardiovascular risk factors on graft outcome disparities in Black kidney transplant recipients. Hypertension. 2016;68(3):715–725. doi: 10.1161/HYPERTENSIONAHA.116.07775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Averill RF, Goldfield N, Hughes JS All patient refined diagnosis-related groups (APR-DRGs) 2003. Available at: https://www.hcup-us.ahrq.gov/db/nation/nis/APR-DRGsV20MethodologyOverviewandBibliography.pdf. Accessed March 13, 2017.
- 49.Dismuke CE, Gebregziabher M, Egede LE. Racial/ethnic disparities in VA services utilization as a partial pathway to mortality differentials among veterans diagnosed with TBI. Glob J Health Sci. 2015;8(2):260–272. doi: 10.5539/gjhs.v8n2p260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alvord LA, Henderson WG, Benton K, Buchwald D. Surgical outcomes in American Indian veterans: a closer look. J Am Coll Surg. 2009;208(6):1085–1092. doi: 10.1016/j.jamcollsurg.2009.02.058. [DOI] [PubMed] [Google Scholar]
- 51.Fuchs J, Kouyate A, Kroboth L, McFarland W. Growing the pipeline of diverse HIV investigators: the impact of mentored research experiences to engage underrepresented minority students. AIDS Behav. 2016;20(suppl 2):249–257. doi: 10.1007/s10461-016-1392-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marmot M, Allen JJ. Social determinants of health equity. Am J Public Health. 2014;104(suppl 4):S517–S519. doi: 10.2105/AJPH.2014.302200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Halladay CW, Trikalinos TA, Schmid IT, Schmid CH, Dahabreh IJ. Using data sources beyond PubMed has a modest impact on the results of systematic reviews of therapeutic interventions. J Clin Epidemiol. 2015;68(9):1076–1084. doi: 10.1016/j.jclinepi.2014.12.017. [DOI] [PubMed] [Google Scholar]