Abstract
Background:
Platelet-rich plasma (PRP) treatment has gained popularity among different surgical specialities for improving various conditions. Androgenetic alopecia (AGA) is a common disorder, with possible psychosocial implications. Plastic surgeons have increased the practice of PRP injections for hair restoration. A meta-analysis on this topic was performed comparing local injection of PRP versus control to investigate the efficacy of local PRP injections in AGA.
Methods:
We performed a systematic literature search. The increase in number of hairs was the primary outcome. Secondary outcomes were the increase of hair thickness and the percentage increase in hair number and thickness.
Results:
Seven studies involving 194 patients were retrieved and included in the present analysis. A significantly locally increased hair number per cm2 was observed after PRP injections versus control (mean difference [MD] 14.38, 95% confidence interval [CI] 6.38–22.38, P < 0.001). Similarly, a significantly increased hair thickness cross-section per 10−4 mm2 (MD 0.22, 95% CI 0.07–0.38, P = 0.005) favoring PRP group. The pooled results did not show a significant percentage increase in hair number (MD 18.79%, 95% CI − 8.50–46.08, P = 0.18), neither hair thickness (MD 32.63%, 95% CI − 16.23–81.48, P = 0.19) among patients treated with PRP.
Conclusion:
Local injection of PRP for androgenic alopecia might be associated with an increased number of hairs in the treated areas with minimal morbidity, but there is clearly a lack of scientific evidence on this treatment modality. Further studies are needed to evaluate the efficacy of PRP for AGA.
Key words: Androgenetic alopecia, hair growth, hair restoration, platelet-rich plasma, platelet-rich plasma
INTRODUCTION
Androgenetic alopecia (AGA) also known as male pattern baldness is the most common hair loss disorder affecting up to 80% of men and up to 40% of women with Caucasian heritage. For patients, alopecia causes major discomfort due to an altered appearance with significant implications in daily living and possible leading to depression and anxiety symptoms.[1]
Platelet-rich plasma (PRP) injections for hair restoration has emerged to a popular practice among plastic surgeons because both highly demanding patients and surgeons are seeking for minimally-invasive and cost efficient treatment modalities for androgenic alopecia.[2] The scientific interest for PRP was raised in 2006 when Mishra and Pavelko managed to demonstrate the PRP efficacy in improving elbow epicondylitis, reducing the time for healing.[3] Since then, PRP local injections started to become very popular which transversally interested many medical and surgical branches.[2]
PRP is an autologous product that is manufactured by centrifugation from patients own venous blood limiting the potential risk of disease transmission. Components of PRP includes several growth factors (GF), chemokines, and cytokines, suggesting that its benefits include promotion of tissue healing in hard- and soft-tissues.[4,5] In a natural environment, platelets migrate into the inflammation site and release Alpha granuli, which are activated by platelets aggregation with a concentration of GF.[6] In addition, local injections of PRP are a highly appealing treatment modality because they can easily be administered at outpatient clinic settings, with low costs. Meanwhile, rapid and uncontrolled interest from medical and nonmedical professionals mislead to believe PRP to be a carrier of mesenchymal, stem cells, adipocytes, and bone marrow, which is obviously not.
Although there are several recent reports and small randomized controlled trials (RCT) examining the use of PRP for hair loss treatment, there are no sustained results on their overall efficacy,[7,8,9] and none of these studies have been sufficiently powered to assess the risk benefit of this modality.[10] Despite the heterogeneity of these studies and due to the lack of the previous meta-analysis specifically for comparative studies to evaluate this issue, we performed a whole comprehensive analysis hypothesizing that PRP might prove significant benefits in improving AGA.
METHODS
The objective of this review was to assess the literature on PRP outcomes for AGA, with a focus on specific clinical outcomes in a comparative view, in accordance with PRISMA statement for reporting this meta-analysis.[11] The present meta-analysis is registered in PROSPERO, an international prospective register of systematic reviews, with the reference code CRD42016041811.
Search strategy
All authors individually carried out a full systematic literature search of all records through Medline, Cochrane Library, Embase, Scopus, Google Scholar and Research Gate for any study on PRP use for hair growth therapy in androgenic alopecia from inception to August 2017.
The terms employed in the search were: “androgenic alopecia,” “hair growth,” “hair restoration,” “baldness,” “hair loss” combined with “plated-rich plasma,” “PRP;” and they were combined using Boolean operators. Each author's search results were merged, and duplicate citations were discarded. The search was performed aiming at those studies comparing outcomes of PRP treatment versus control for hair restoration. No language restrictions were applied.
Study selection
We searched for, and assessed studies comparing local injections of PRP compared to any control for AGA. Studies to be included in this review had to match predetermined criteria according to the PICOS (patients, intervention, comparator, outcomes, and study design) approach. Criteria for inclusion and exclusion are specified in Table 1. No limitations were applied on ethnicity, the age of patients or method of PRP processing. Two authors (SG and PL) independently reviewed the abstracts and articles. In addition, the reference lists of all relevant articles were scrutinized as well.
Table 1.
Patients, intervention, comparator, outcomes and study design criteria for inclusion and exclusion of studies
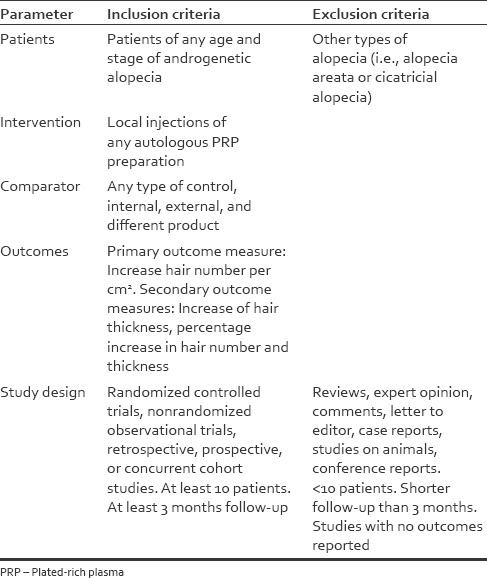
For the purpose of this analysis, the eligible studies were those reporting on quantitative outcomes on PRP compared with control treatment for AGA. Each study was independently evaluated by all three co-authors (SG, MR, PL) for inclusion or exclusion from this analysis [Table 1]. To be included, studies had to provide details on baseline characteristics, type of procedure, method of PRP processing, and outcomes on hair regrowth compared with control patients or areas in the same patient (internal control).
Data extraction
Data were independently collected by two investigators (SG and PL) and checked by a third investigator (MR) only from the retrieved articles. Disagreement on collected data was settled by consensus between these investigators. No any attempt was made to obtain specific or missing data from the authors. The following data were extracted: first author, year of publication, study design, number of patients, type of procedure, and primary and secondary measures.
The quality of the included studies was independently assessed using three investigators (SG, MR, PL) using the Cochrane Collaboration's Risk of Bias Assessment tool for RCT[12] while using the Newcastle–Ottawa Scale to evaluate the individual non-randomised studies.[13] The research team convened to resolve any disagreement on the assessment and to reach consensus.
Outcome measures
The primary outcome was the difference in number of hairs per square centimeter. Secondary outcomes were hair cross-section increase, hair regrowth, and thickness percentage increase.
All outcomes obtained from the studies were reported with the same measurements retrieved from the articles. From one article, percentages were calculated from the patients' individual data showed in the paper.[14] The patient's contralateral side was used as control in some of the included studies; whereas patients were allocated into groups where PRP was either used or not in the other studies. In both cases were accounted as one. Missing data were dealt according to previously validated estimations.[15,16]
Statistical analysis
Statistical analysis was performed using Review Manager 5.3 software (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014). Differences in continuous variables were expressed as mean difference (MD) with 95% confidence interval (CI). Heterogeneity was assessed using I2 statistic, which describes the percentage of total variation across studies that is due to heterogeneity rather than chance.[17] I2 values were evaluated as low, moderate, or high at 25%, 50%, or 75%, respectively. To perform the meta-analysis, the inverse variance statistical method was used for continuous outcome variables. In all cases, we performed random-effect analysis, which consider the variation both within- and between studies[18,19] because of the observational nature of some studies included in this analysis. A value of P < 0.05 was considered to be statistically significant.
Finally, we conducted sensitivity analyses omitting each study, in turn, using the “leave one out” methodology to determine whether the results were influenced excessively by a single study. Publication bias was assessed using the funnel plot for the primary outcome.
RESULTS
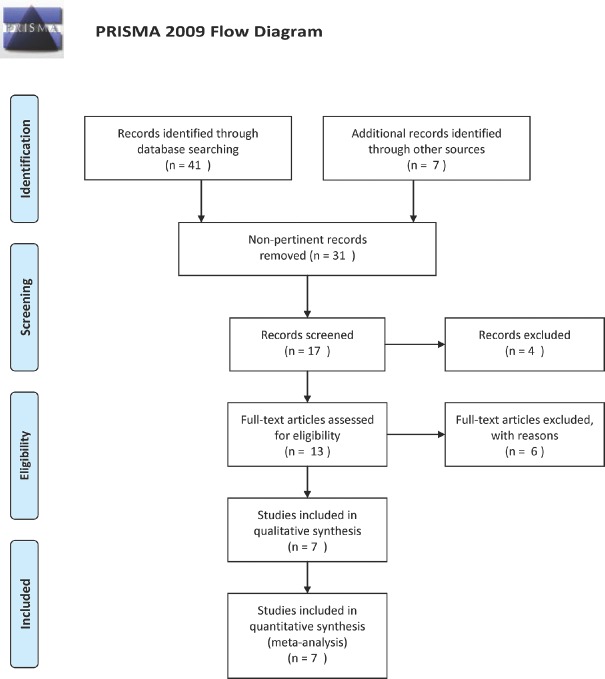
The literature search yielded seven articles[14,20,21,22,23,24,25] pertinent to this issue and sources of information on outcomes using PRP injections on scalp for AGA [Table 2]. The literature search flowchart is shown in Figure 1.
Table 2.
Characteristics of the included studies
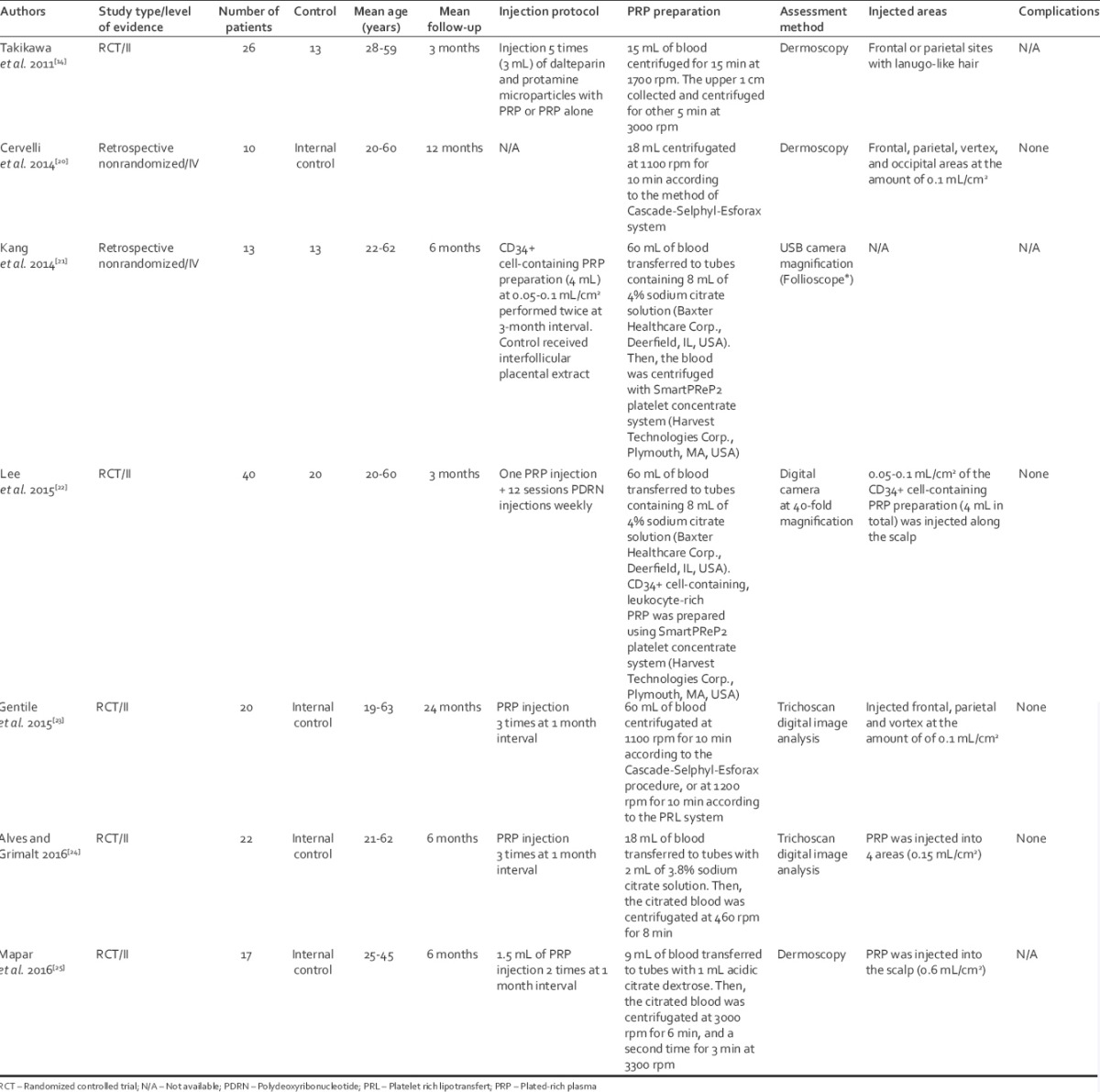
Figure 1.
Flow-chart summarizing literature search results
Five studies were RCT,[14,22,23,24,25] whereas the other two were retrospective studies.[20,21]
In the RCT, the risk of bias was either low or unclear using Cochrane Collaboration's tool for assessing the risk of bias [Supplemental Table 1 (104.1KB, pdf) ].[12] The nonRCTs were assessed with the Newcastle–Ottawa Scale for risk of bias resulting in 0 to 4 stars per category, indicating a high to low bias [Supplemental Table 2 (104KB, pdf) ].[13] The difficulty blinding participants and researchers, as well as, the presence of internal control increased the overall risk of bias.
Supplemental digital content: Risk of bias assessments - Risk of bias of randomized controlled trials using Cochrane Collaboration's Risk of Bias Assessment tool
Supplemental digital content: Risk of bias assessments - Risk of bias of nonrandomized controlled trial s using Newcastle–Ottawa Scale
The age of the patients ranged from 19 to 63 years, with a follow-up from 3 to 24 months. All the studies showed different centrifugation methods [Table 2].
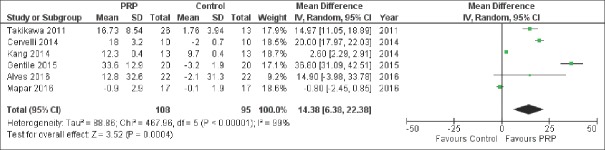
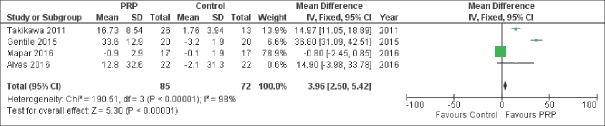
Six studies,[14,20,21,23,24,25] involving a total of 132 patients, reported results on MD of number of hairs per cm2 versus control and pooled analysis showed a significant difference between the two treatment groups [MD 14.38, 95% CI 6.38–22.38, P < 0.001; Figure 2]. Similarly, this outcome persisted when only the 4 RCT were pooled together [MD 3.96, 95% CI 2.50–5.42, P < 0.001; Figure 3].
Figure 2.
Forest plot showing the significantly increased number of hairs per cm2 compared with co
Figure 3.
Forest plot showing the significantly increased number of hairs per cm2 compared with control among randomized controlled trial studies
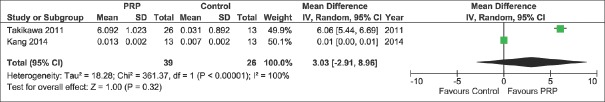
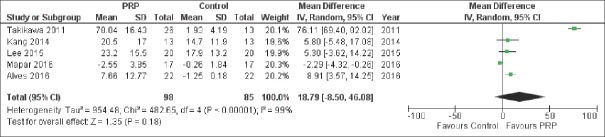
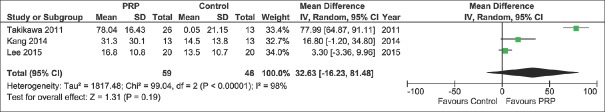
Among secondary outcomes, we also observed a significant difference between the two treatment groups concerning the hair cross section measured per 10−4 mm2 [MD 0.22, 95% CI 0.07–0.38, P = 0.005; Figure 4] favoring PRP group, but these data were reported by only 2 studies.[14,21] We did not found a significant difference between the two treatment groups concerning the percentage increase of hair number [MD 18.79%, 95% CI − 8.50–46.08, P = 0.18, Figure 5] nor hair thickness [MD 32.63%, 95% −16.23–81.48, P = 0.19; Figure 6]. Although not statistically significant, these pooled results showed a trend toward an increase of hair number [Figure 5] and hair thickness percentage [Figure 6].
Figure 4.
Forest plot showing the significantly increased thickness of hairs section expressed as 10-4/mm2 compared with control
Figure 5.
Forest plot showing the percentage increase of hair number after platelet-rich plasma treatment compared with contr
Figure 6.
Forest plot showing the percentage increase of hair thickness after platelet-rich plasma treatment compared with control
Four of the included studies[20,22,23,24] did not report any adverse effects or complications associated with PRP injections. In the other two publications, information about possible adverse effects were not reported.
Finally, the exclusion of most studies from the analysis did not materially change the summary estimates, with sensitivity analysis using the “leave one out” methodology; however, significant asymmetry in the funnel plot was observed for the primary outcome [Figure 7].
Figure 7.
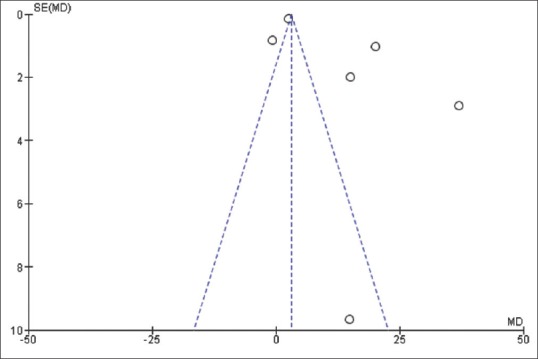
Funnel plot for bias assessment in hair number increase
DISCUSSION
The present meta-analysis, including six studies and encompassing a total of 194 patients, provides compelling evidence about PRP treatment for hair restoration in AGA. A significantly increased number of hairs per cm2 was observed after treatment with PRP [Figures 2 and 3]. Furthermore, although the included studies had a relatively small total number of patients, we found interesting results also in hair cross-section thickness and other secondary outcomes which showed a trend toward benefit Figure 4–Figure 6. This is the first pooled analysis on this emerging topic, showing overall quantitative outcomes.
PRP therapy is an appealing emerging minimally invasive therapeutic modality to enhance tissue healing. Although, used since the mid-1990s it has recently gained substantial increasing interest to provide a cost effective modality to promote the healing process. PRP is an autologous product that is manufactured from patients own venous blood limiting the potential risk of disease transmission. By definition, PRP contains concentrated the amount of platelet concentration, 1.000.000/UL platelet count, 3–8 folds superior amount as compared to the normal peripheral blood (range 150.000–350.000 UL).[24,25,26] On activation platelets undergo degranulation, and rapidly, an array of GFs are released from platelet Alpha-granuli,[26] and GF release is continued in lesser extend up to several days.[27,28] PRP also contains plasma and over 20 GFs, which include platelet-derived endothelial GF, transforming GF–β, fibroblast GF–2, vascular endothelial GF, epidermal GF, insulin-like GF–1, and, in addition, thrombin, which has biological and adhesive properties.[29] It has also been reported that PRP induces overexpression endogenous expression of GFs.[30,31] Through the complex interaction of growth and differentiation factors and along with adhesive protein factors, PRP is believed to stimulate healing by promoting regenerative chemotaxis, cell proliferation, angiogenesis, extracellular matrix formation, and collagen synthesis.[32,33]
There is no a standardized method for PRP preparation, therefore, there might be differences in product composition. This fact may lead to an altered PRP function, which might explain the controversy found into the literature. Furthermore, differences in PRP composition result from differences in the samples retrieved from person to person. Differences in the manufacturing of the inoculate result, especially from the routine of centrifugation and whether either bovine thrombin or calcium chloride is used in activation.[10,27] The used end product may vary by the used volume and the number of injections administered, as well as the color, platelet count, the number or absence of leukocytes, and its protein content.[27]
Recently, a number of reports have been published showing promising results for the treatment of AGA. Unfortunately, these studies were generally small, poorly controlled, without outcomes' objective and measured quantification and therefore, they were not included in this meta-analysis. Betsi et al.[8] treated 42 alopecia patients with PRP, five times during over 2 months showing an improvement in hair pulling test and a high overall patient satisfaction. Indeed, they found in 31% of cases some drowsiness and sensible scalp. Schiavone et al.[9] performed the largest study on this topic, including 64 male patients with AGA and they were treated with a regimen of PRP enriched with leukocytes in addition to concentrated plasma proteins. Two sequential injections were performed at initiation of the study and subsequently at three months. The evaluation was performed on the basis of global assessment of before and after photographs by unblinded assessors showing an improvement in appearance for 62 of the 64 patients.[9] Another noncontrolled, nonblinded study of 22 patients found an increase in total hair density from a mean of 143.1 at baseline to a maximum of 170.7 hairs/cm2 at 3 months follow-up.[34] Another noncontrolled, nonblinded study of 11 patients[35] detected a significant reduction in hair loss between first and fourth injection. Particularly, hair count increased from an average number of 71 hair follicular units to 93 hair follicular units, with a negative pull test was in 9 patients.[ 35]
Singhal et al.[36] performed a similar study on 10 patients also showing clinical improvement in the hair counts, thickness and root strength. They indeed had three patients complaining a mild headache after the initial procedure. More recently, Navarro et al.[9] reported an overall increase of hair density and an increase of 6.2% anagen hair follicles while a decrease of 5.1% among telogen ones on 100 patients treated with PRP, similarly to Alves and Grimalt[24] We did not attempt to pool the data existing on hair follicle cycles, as they were not consistent among the included studies. Nonetheless, PRP showed promising results also combined with hair follicular transplant to enhance the postoperative outcomes.[37,38] Particularly, Uebel studied a short series of patients comparing two areas of hair transplant with or without PRP in the root of the grafts. Two areas (2.5 cm2) were marked on the scalp and each planted with 20 grafts/cm2. After 1 year, the area implanted with the PRP-enriched grafts demonstrated a higher follicle units survival rate and density. In a murine model, Miao et al.[39] demonstrated some influence of PRP on hair regrowth when simultaneously injected with transplanted hair follicles, with consistent data, further encouraging clinical applications. Again, the data about PRP and hair growth, together with a surgical hair transplant, are sparse and heterogeneous although promising. Nevertheless, these outcomes can be explained by the physiological role of platelets degranulation during inflammation, which is stimulated by surgical hair transplantation, a traumatic event causing inflammatory response and chemotaxis.[3] For this reason, we believe that surgical transplantation of hair follicles with PRP might be more effective that PRP alone.
The results of this meta-analysis should be viewed in light of a number of limitations and potential bias influencing these findings. Only six studies were used for this pooled analysis, only four of them were randomized controlled trial[14,22,23,24,25] and two were observational[20,21] with clear heterogeneity in methods and settings [Table 2]. We wanted to include only comparative studies to better assess the efficacy of PRP, missing the outcomes of one-arm studies.
The number of patients considered was extremely small and there were differences in patients' age, devices used, centrifugation methods, control, and areas of treatment, which might be a confounding factor for the results.
Important statistical heterogeneity (I2 >75%) was found in all analysis Figure 2–Figure 6, showing important differences in methods and study settings. However, the exclusion of any study from the analysis did not materially change the summary estimates, but funnel plot for the primary outcomes showed significant asymmetry, which indicates that publication bias might have somehow influenced the results.
Other major limitations of this pooled analyses include the fact that most of the included studies used internal controls, where the patient's contralateral side or other areas served as its own control, whereas in others, patients were randomized into groups where PRP was either used or not used [Table 2]. There were differences in the treated scalp areas and in some cases, the control group was treated with placental extract[21] or dalteparin and protamine containing micro particles[14] with no placebo control.
CONCLUSION
PRP injection for local hair restoration in patients with AGA seems to increase hairs number and thickness with minimal or no collateral effects. However, the current evidence does not support this treatments modality over other treatments due to the lack of clinical evidence, established protocols (i.e., number of sessions, centrifugation, zones to be injected, etc.), and long-term follow-up outcomes.
The results of this meta-analysis should be interpreted with caution because it includes pooling many small studies and larger randomized studies should be performed to verify this perception. The medical literature does not confirm that the treatment is scientifically relevant. The addition of PRP might be useful in improving the outcomes of hair transplantation procedures, but there is no evidence whether PRP is more effective than minoxidil or finasteride treatments. Larger studies with long-term follow-up are warranted to validate this promising treatment modality.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Tabolli S, Sampogna F, di Pietro C, Mannooranparampil TJ, Ribuffo M, Abeni D, et al. Health status, coping strategies, and alexithymia in subjects with androgenetic alopecia: A questionnaire study. Am J Clin Dermatol. 2013;14:139–45. doi: 10.1007/s40257-013-0010-3. [DOI] [PubMed] [Google Scholar]
- 2.Eppley BL, Pietrzak WS, Blanton M. Platelet-rich plasma: A review of biology and applications in plastic surgery. Plast Reconstr Surg. 2006;118:147e–59e. doi: 10.1097/01.prs.0000239606.92676.cf. [DOI] [PubMed] [Google Scholar]
- 3.Mishra A, Pavelko T. Treatment of chronic elbow tendinosis with buffered platelet-rich plasma. Am J Sports Med. 2006;34:1774–8. doi: 10.1177/0363546506288850. [DOI] [PubMed] [Google Scholar]
- 4.Hammond JW, Hinton RY, Curl LA, Muriel JM, Lovering RM. Use of autologous platelet-rich plasma to treat muscle strain injuries. Am J Sports Med. 2009;37:1135–42. doi: 10.1177/0363546508330974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Virchenko O, Aspenberg P. How can one platelet injection after tendon injury lead to a stronger tendon after 4 weeks? Interplay between early regeneration and mechanical stimulation. Acta Orthop. 2006;77:806–12. doi: 10.1080/17453670610013033. [DOI] [PubMed] [Google Scholar]
- 6.Petrungaro PS. Using platelet-rich plasma to accelerate soft tissue maturation in esthetic periodontal surgery. Compend Contin Educ Dent. 2001;22:729–32. 734, 736. [PubMed] [Google Scholar]
- 7.Schiavone G, Raskovic D, Greco J, Abeni D. Platelet-rich plasma for androgenetic alopecia: A pilot study. Dermatol Surg. 2014;40:1010–9. doi: 10.1097/01.DSS.0000452629.76339.2b. [DOI] [PubMed] [Google Scholar]
- 8.Betsi EE, Germain E, Kalbermatten DF, Tremp M, Emmenegger V. Platelet-rich plasma injection is effective and safe for the treatment of alopecia. Eur J Plast Surg. 2013;36:407–12. [Google Scholar]
- 9.Navarro MR, Asín M, Martínez MA, Martinez AM, Molina C, Moscoso L, et al. Management of androgenetic alopecia: A comparative clinical study between plasma rich in growth factors and topical minoxidil. Eur J Plast Surg. 2016;1:1–8. [Google Scholar]
- 10.Leo MS, Kumar AS, Kirit R, Konathan R, Sivamani RK. Systematic review of the use of platelet-rich plasma in aesthetic dermatology. J Cosmet Dermatol. 2015;14:315–23. doi: 10.1111/jocd.12167. [DOI] [PubMed] [Google Scholar]
- 11.Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int J Surg. 2010;8:336–41. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 12.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The cochrane collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. 2014. [Last accessed on 2016 Jun 28]. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp .
- 14.Takikawa M, Nakamura S, Nakamura S, Ishirara M, Kishimoto S, Sasaki K, et al. Enhanced effect of platelet-rich plasma containing a new carrier on hair growth. Dermatol Surg. 2011;37:1721–9. doi: 10.1111/j.1524-4725.2011.02123.x. [DOI] [PubMed] [Google Scholar]
- 15.Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta-analyses can provide accurate results. J Clin Epidemiol. 2006;59:7–10. doi: 10.1016/j.jclinepi.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 16.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–48. [PubMed] [Google Scholar]
- 18.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 20.Cervelli V, Garcovich S, Bielli A, Cervelli G, Curcio BC, Scioli MG, et al. The effect of autologous activated platelet rich plasma (AA-PRP) injection on pattern hair loss: Clinical and histomorphometric evaluation. Biomed Res Int. 2014;2014:760709. doi: 10.1155/2014/760709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kang JS, Zheng Z, Choi MJ, Lee SH, Kim DY, Cho SB, et al. The effect of CD34+ cell-containing autologous platelet-rich plasma injection on pattern hair loss: A preliminary study. J Eur Acad Dermatol Venereol. 2014;28:72–9. doi: 10.1111/jdv.12062. [DOI] [PubMed] [Google Scholar]
- 22.Lee SH, Zheng Z, Kang JS, Kim DY, Oh SH, Cho SB, et al. Therapeutic efficacy of autologous platelet-rich plasma and polydeoxyribonucleotide on female pattern hair loss. Wound Repair Regen. 2015;23:30–6. doi: 10.1111/wrr.12250. [DOI] [PubMed] [Google Scholar]
- 23.Gentile P, Garcovich S, Bielli A, Scioli MG, Orlandi A, Cervelli V, et al. The effect of platelet-rich plasma in hair regrowth: A Randomized placebo-controlled trial. Stem Cells Transl Med. 2015;4:1317–23. doi: 10.5966/sctm.2015-0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alves R, Grimalt R. Randomized placebo-controlled, double-blind, half-head study to assess the efficacy of platelet-rich plasma on the treatment of androgenetic alopecia. Dermatol Surg. 2016;42:491–7. doi: 10.1097/DSS.0000000000000665. [DOI] [PubMed] [Google Scholar]
- 25.Mapar MA, Shahriari S, Haghighizadeh MH. Efficacy of platelet-rich plasma in the treatment of androgenetic (male-patterned) alopecia: A pilot randomized controlled trial. J Cosmet Laser Ther. 2016;18:452–5. doi: 10.1080/14764172.2016.1225963. [DOI] [PubMed] [Google Scholar]
- 26.Foster TE, Puskas BL, Mandelbaum BR, Gerhardt MB, Rodeo SA. Platelet-rich plasma: From basic science to clinical applications. Am J Sports Med. 2009;37:2259–72. doi: 10.1177/0363546509349921. [DOI] [PubMed] [Google Scholar]
- 27.Wroblewski AP, Melia HJ, Wright VJ. Application of platelet-rich plasma to enhance tissue repair. Oper Tech Orthop. 2010;20:98–105. [Google Scholar]
- 28.Nguyen RT, Borg-Stein J, McInnis K. Applications of platelet-rich plasma in musculoskeletal and sports medicine: An evidence-based approach. PM R. 2011;3:226–50. doi: 10.1016/j.pmrj.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 29.Marx RE. Platelet-rich plasma: Evidence to support its use. J Oral Maxillofac Surg. 2004;62:489–96. doi: 10.1016/j.joms.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 30.Anitua E, Sánchez M, Nurden AT, Nurden P, Orive G, Andía I, et al. New insights into and novel applications for platelet-rich fibrin therapies. Trends Biotechnol. 2006;24:227–34. doi: 10.1016/j.tibtech.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 31.de Mos M, van der Windt AE, Jahr H, van Schie HT, Weinans H, Verhaar JA, et al. Can platelet-rich plasma enhance tendon repair? A cell culture study. Am J Sports Med. 2008;36:1171–8. doi: 10.1177/0363546508314430. [DOI] [PubMed] [Google Scholar]
- 32.Lyras DN, Kazakos K, Agrogiannis G, Verettas D, Kokka A, Kiziridis G, et al. Experimental study of tendon healing early phase: Is IGF-1 expression influenced by platelet rich plasma gel? Orthop Traumatol Surg Res. 2010;96:381–7. doi: 10.1016/j.otsr.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 33.Lee KS, Wilson JJ, Rabago DP, Baer GS, Jacobson JA, Borrero CG, et al. Musculoskeletal applications of platelet-rich plasma: Fad or future? AJR Am J Roentgenol. 2011;196:628–36. doi: 10.2214/AJR.10.5975. [DOI] [PubMed] [Google Scholar]
- 34.Gkini MA, Kouskoukis AE, Tripsianis G, Rigopoulos D, Kouskoukis K. Study of platelet-rich plasma injections in the treatment of androgenetic alopecia through an one-year period. J Cutan Aesthet Surg. 2014;7:213–9. doi: 10.4103/0974-2077.150743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khatu SS, More YE, Gokhale NR, Chavhan DC, Bendsure N. Platelet-rich plasma in androgenic alopecia: Myth or an effective tool. J Cutan Aesthet Surg. 2014;7:107–10. doi: 10.4103/0974-2077.138352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Singhal P, Agarwal S, Dhot PS, Sayal SK. Efficacy of platelet-rich plasma in treatment of androgenic alopecia. Asian J Transfus Sci. 2015;9:159–62. doi: 10.4103/0973-6247.162713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Uebel CO, da Silva JB, Cantarelli D, Martins P. The role of platelet plasma growth factors in male pattern baldness surgery. Plast Reconstr Surg. 2006;118:1458–66. doi: 10.1097/01.prs.0000239560.29172.33. [DOI] [PubMed] [Google Scholar]
- 38.Park KY, Kim HK, Kim BJ, Kim MN. Letter: Platelet-rich plasma for treating male pattern baldness. Dermatol Surg. 2012;38:2042–4. doi: 10.1111/dsu.12037. [DOI] [PubMed] [Google Scholar]
- 39.Miao Y, Sun YB, Sun XJ, Du BJ, Jiang JD, Hu ZQ, et al. Promotional effect of platelet-rich plasma on hair follicle reconstitution in vivo. Dermatol Surg. 2013;39:1868–76. doi: 10.1111/dsu.12292. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental digital content: Risk of bias assessments - Risk of bias of randomized controlled trials using Cochrane Collaboration's Risk of Bias Assessment tool
Supplemental digital content: Risk of bias assessments - Risk of bias of nonrandomized controlled trial s using Newcastle–Ottawa Scale