Abstract
The chief goal of combination therapy using guided tissue regeneration along with bone grafts is to optimally and predictably regenerate the periodontal attachment apparatus. The evolution of regenerative therapy along with careful patient selection and treatment planning can have positive results even in cases which are not ideal. The present case report describes a tooth with a questionable prognosis treated successively by endodontic then periodontal therapy using an amniotic membrane along with a bone graft to treat an intrabony defect. The follow-up, 12 months later showed a resolution of the interradicular lesion and a radiographic bone fill.
Keywords: Amniotic membrane, guided tissue regeneration, intrabony defect, periobone-G, periodontic-endodontic lesion
INTRODUCTION
For 30 years, intrabony periodontal defects have been treated successfully by selective cell repopulation based guided tissue regeneration (GTR).[1] Nonresorbable membranes made way for bioabsorbable membranes, of which collagen membranes are used commonly to avoid a second surgical procedure for removal and membrane contamination.[2] However, predictability of GTR can be enhanced by the evolution of both membranes and surgical procedures.
Initially, placental tissues were used in ophthalmic surgery, then became commercially available for dentistry in 2008.[3,4] This placental allograft is a dehydrated amnion-chorion cryopreserved laminate and is a composite membrane comprised of pluripotent cellular elements embedded in a semi-permeable membranous structure; with unique regenerative properties. Amniotic membrane (AM) closely mimics basement membrane of human oral mucosa and provides a source of stem cells, immunomodulatory, anti-inflammatory, and antiscarring effects among many more which make it an exciting option for the future of periodontal regeneration.
This case report describes a tooth with questionable prognosis treated successively by endodontic and periodontal therapy, using an AM with bone graft to treat an intrabony defect.
CASE REPORT
A 34-year-old female patient reported with a chief complaint of mobility and dull tooth pain in relation to 11 [Figure 1]. Patient gave a history of trauma to the maxillary region due to an accident, 2 months prior. Clinical examination showed inflammation extending till the attached gingiva and pain on percussion in relation to 11. It was supraerupted, with Grade II mobility and thick band of calculus. Probing pocket depth (PD) of 6 mm and clinical attachment loss (CAL) of 8 mm was seen in relation to 11. Radiographically, an intrabony defect with radiolucency in the mesiodistal region was seen [Figure 2]. A positive response to percussion test suggested that the inflammation of periodontal ligament could be of pulpal or periodontal origin. Pulp sensitivity testing using pulse oximeter showed 11 to be vital.
Figure 1.
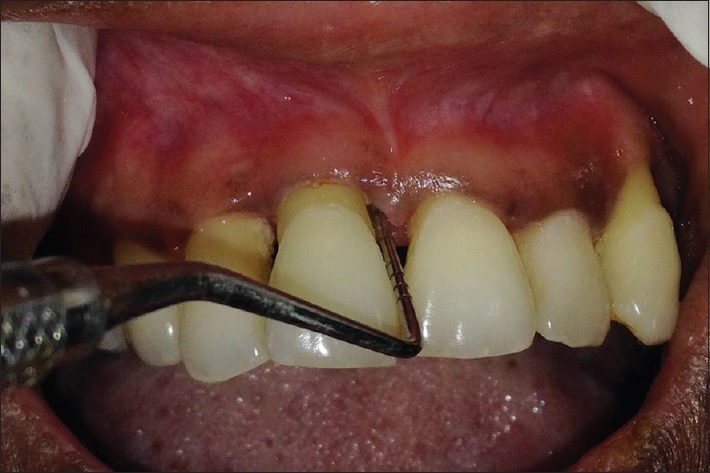
Preoperative view in relation to 11
Figure 2.
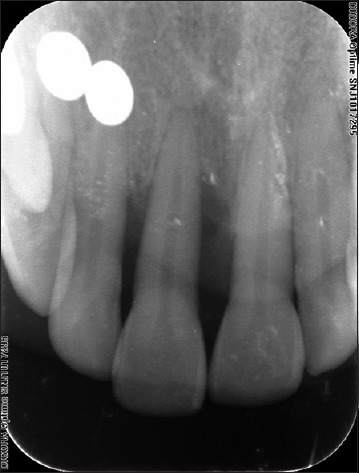
Preoperative radiograph
Considering 11 was vital with Grade II mobility, the patient was advised root canal treatment (RCT) and splinting of the tooth followed by periodontal regenerative therapy. The patient's written consent, and ethical approval was attained from the Institutional Committee (MADC/IRB/2015/94).
At the first appointment, periodontal parameters of PD, mobility, and radiographic assesment of bone loss were carried out. Full mouth scaling and root planing followed by oral hygiene instructions were given. Antibiotics (amoxicillin 500 mg thrice daily/5 days) and analgesics (Ibuprofen 400 mg thrice daily/3 days) were prescribed. The patient then underwent RCT of 11 [Figure 3] and splinting was done consecutively (13–23) within a week [Figure 4]. After reevaluation at 3rd month, periodontal regenerative therapy was initiated. After local anesthesia, a Bard-Parker No. 15 blade was used in relation to 13–23 to make the crevicular incisions. A full-thickness flap was raised, defect debridement, and thorough root planing were done. The defect was filled using the bone graft (Periobone-G) [Figure 5]. The AM was trimmed and tucked underneath the flap margins, allowing it to adapt to and cover the defect and interproximal area [Figures 6 and 7]. This AM upon contact with tissue fluid, develops advantageous self-adhering properties and was placed without shrivelling. The mucoperiosteal flap was repositioned and sutured [Figure 8].
Figure 3.
Obturation in relation to 11
Figure 4.
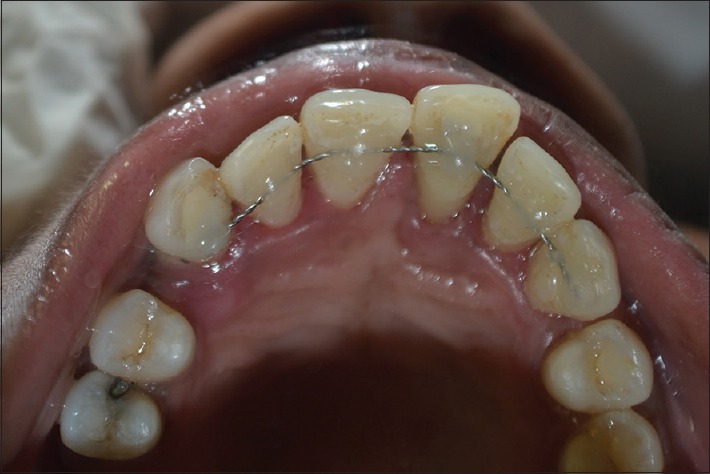
Splinting done from 13 to 23
Figure 5.
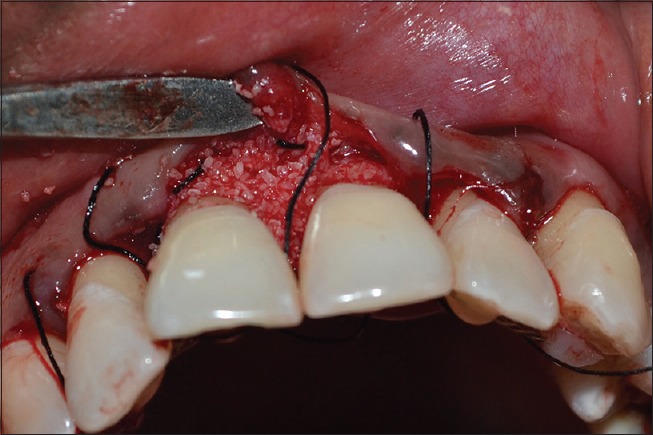
Flap reflected (presutured) and Bone graft (Periobone-G) placed in relation to 11
Figure 6.

Amniotic membrane
Figure 7.
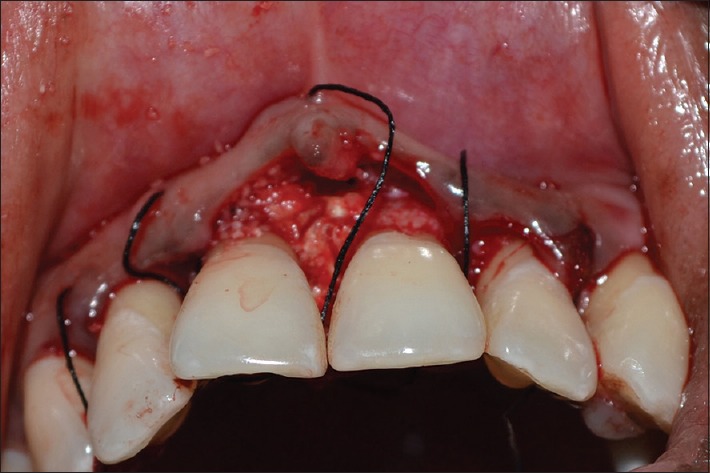
Amniotic membrane placed in relation to 11
Figure 8.
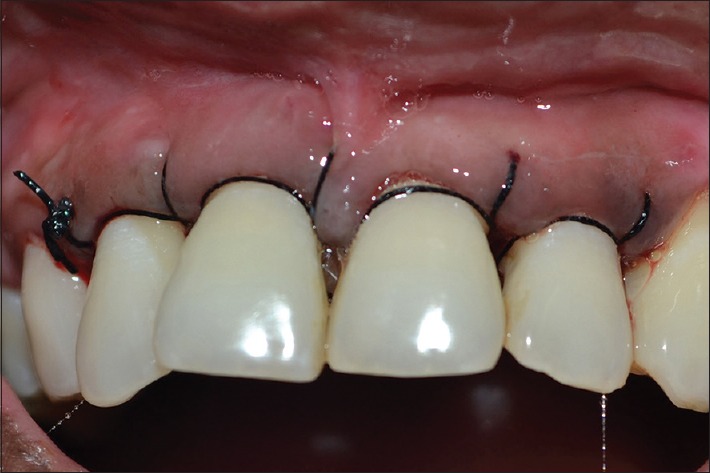
Sutures placed
Postsurgical instructions were given; systemic antibiotics and analgesics were also prescribed. The patient was given instructions to rinse everyday with a 0.2% chlorhexidine solution for 7 days. The patient was recalled for appointments which were scheduled once in 10 days for the 1st month, and later at the 9th and 12th month.
RESULTS
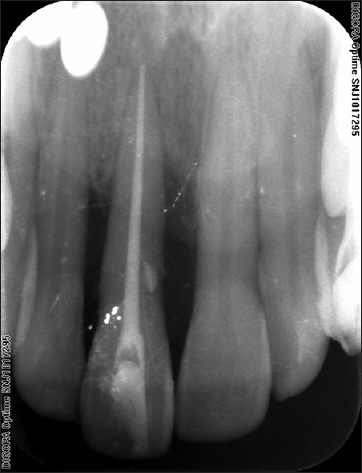
After 10 days, the sutures were removed, and healing was found to be satisfactory. When the patient was revealuated at the 9th and 12th month radiographic bone fill and soft tissue healing was detected [Figure 9]. The probing depth had reduced from 6 to 3 mm Periapical radiographs taken postoperatively at the 9th and 12th month indicated radiographic bone fill in relation to 11 [Figures 10 and 11].
Figure 9.
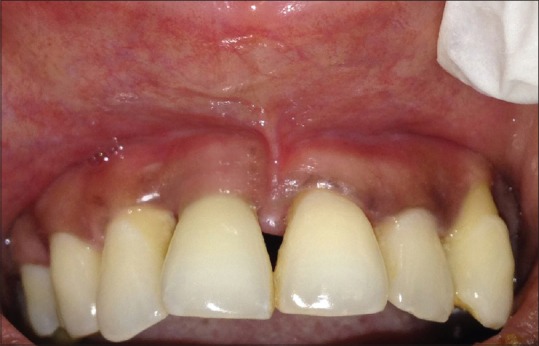
Twelfth month postoperative
Figure 10.
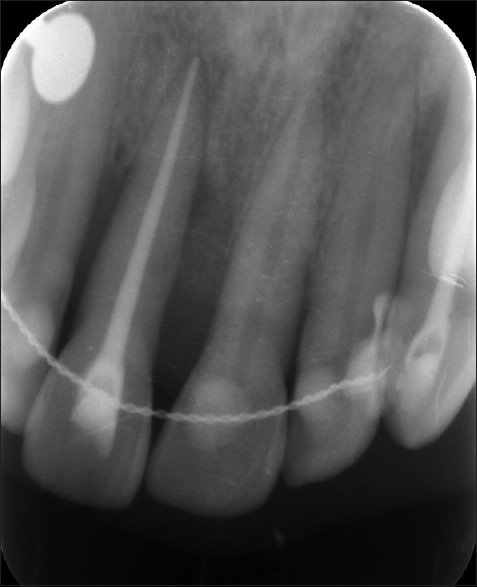
Postoperative radiograph after 9 months
Figure 11.
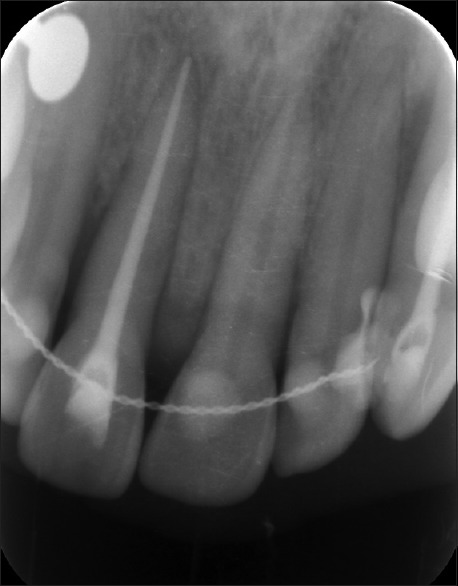
Postoperative radiograph after 12 months
DISCUSSION
Meticulous treatment planning is essential for a successful treatment and the initial endodontic therapy and splinting ruled out future complications which could compromise the sequentially planned periodontal therapy. The regenerative procedure consisted of bone grafts in combination with a freeze-dried irradiated AM for GTR. Till date, there have been no reported cases highlighting the application and success of AM alone or in conjunction with graft material for the treatment of intrabony defects, especially when it is also in relation to a splinted and endodontically treated tooth.
The primary principle of biological healing revolved around the use of mechanical barriers to facilitate selective cell repopulation of the root surface by periodontal ligament cells which was brought forth by Melcher.[5] The clinical relevance of AM for GTR is in line with the current concepts and goals of a biomechanical GTR. Commonly used collagen membranes primarily act as physiological barriers and are considered to be biologically inactive. However, the AM used in this case report not only facilitates the cellular migration but also improves overall regeneration with its rich source of pluripotent stem cells, specialized proteins such as fibronectin and collagens, cytokines such as vascular endothelial growth facto and platelet-derived growth factor, reduction of postsurgical scarring and also shows remarkable immunotolerance, antimicrobial and bacteriostatic barrier activity.[6] The AM also shows excellent bone inductive potential due to the presence of mesenchymal progenitor cells which show osteogenic and adipogenic differentiation. In addition, the AM not only contains the graft material but also resorbs without voids.[7] Previous studies have shown that the volume of bone fill and new bone formation was greatest when AM was used in conjunction with bone graft than when compared to bone graft alone.[8] The results of our case report correlate with several studies that show that AM used in conjunction with bone graft appears to have remarkable synergistic properties.
The bone graft used for this case was demineralized bone matrix hydroxyapatite (HA) material. This bone graft was chosen as it acts as an osteoconductive bone void filler which both fills the defects and acts as a scaffold for bone formation. The HA bone graft along with its superior bone conductive abilities enable the migration of osteogenic cells extending from the present bone surfaces into the neighboring bone graft.[9] Filling the defect facilitates angiogenesis and stabilization of the blood clot; as well as prevents the soft tissues from collapsing into the defect.
Kothiwale et al.[10] used bio-oss and AM to treat Grade II furcation defects in comparison with demineralized freeze-dried bone allografts and AM. The results showed an increase in CAL and a significant reduction in PD as well as an amount of bone fill seen in both groups, at the 9 month period of follow-up, without any significant differences.
CONCLUSION
Since this was the first case report of its nature, there was a lack of sample size and histological data for comparison; longitudinal studies that compare placental and other GTR membranes with bone grafts are also required to claim the superior efficacy of AM. Within these limitations, this case report demonstrated the use of AM as an effective barrier when used in conjunction with bone grafts to treat an intrabony defect.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given her consent for her images and other clinical information to be reported in the journal. The patient understand that name and initial will not be published and due efforts will be made to conceal identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Bystrom A, Happonen RP, Sjogren U, Sundqvist G. Healing of periapical lesions of pulpless teeth after endodontic treatment with controlled asepsis. Endod Dent Traumatol. 1987;3:58–63. doi: 10.1111/j.1600-9657.1987.tb00543.x. [DOI] [PubMed] [Google Scholar]
- 2.Choukroun J, Diss A, Simonpieri A, Girard MO, Schoeffler C, Dohan SL, et al. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part V: Histologic evaluations of PRF effects on bone allograft maturation in sinus lift. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:299–303. doi: 10.1016/j.tripleo.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 3.De Rotth A. Plastic repair of conjunctival defects with fetal membrane. Arch Opthalmol. 1940;23:522–5. [Google Scholar]
- 4.Niknejad H, Peirovi H, Jorjani M, Ahmadiani A, Ghanavi J, Seifalian AM, et al. Properties of the amniotic membrane for potential use in tissue engineering. Eur Cell Mater. 2008;15:88–99. doi: 10.22203/ecm.v015a07. [DOI] [PubMed] [Google Scholar]
- 5.Melcher AH. On the repair potential of periodontal tissues. J Periodontol. 1976;47:256–60. doi: 10.1902/jop.1976.47.5.256. [DOI] [PubMed] [Google Scholar]
- 6.Lafzi A, Farahani RM, Shoja MM, Tubbs RS. Amniotic membrane: A potential candidate for periodontal guided tissue regeneration? Med Hypotheses. 2007;69:454. doi: 10.1016/j.mehy.2006.12.022. [DOI] [PubMed] [Google Scholar]
- 7.Gomes JA, Romano A, Santos MS, Dua HS. Amniotic membrane use in ophthalmology. Curr Opin Ophthalmol. 2005;16:233–40. doi: 10.1097/01.icu.0000172827.31985.3a. [DOI] [PubMed] [Google Scholar]
- 8.Samandari MH, Adibi S, Khoshzaban A, Aghazadeh S, Dihimi P, Torbaghan SP, et al. Human amniotic membrane, best healing accelerator and the choice of bone induction for vestibuloplasty technique (an animal study) Transplant Res Risk Manage. 2011;3:1–8. [Google Scholar]
- 9.Kenney EB, Lekovic V, Han T, Carranza FA, Jr, Dimitrijevic B. The use of porous hydroxyapatitie implant in periodontal defects. I. Clinical results after 6 months. J Periodontol. 1985;56:82–8. doi: 10.1902/jop.1985.56.2.82. [DOI] [PubMed] [Google Scholar]
- 10.Kothiwale SV, Anuroopa P, Gajiwala AL. Clinical and radiological evaluation of DFDBA with amniotic membrane versus bovine derived xenografts with amniotic membrane in human periodontal grade II furcation defects. Cell Tissue Bank. 2009;10:317–26. doi: 10.1007/s10561-009-9126-3. [DOI] [PubMed] [Google Scholar]


