Abstract
Acquired cutis laxa (ACL) is a rare connective tissue disorder characterized by pendulous and coarsely wrinkled skin. There have been few cases of its association to monoclonal immunoglobulin deposition disease (MIDD), which constitutes the light chain (LCDD), heavy chain (HCDD), and light and heavy chain (LHCDD) deposition disease. MIDD predominantly involves the kidney. Skin is the next common organ to be affected by HCDD, which presents as ACL. We report the case of a 40-year-old male who presented with ACL associated with LHCDD. The clinical features of ACL in the present case appeared prior to the development of clinical features related to LHCDD.
Keywords: Acquired cutis laxa, light and heavy chain deposition disease, monoclonal immunoglobulin deposition disease
Introduction
Acquired cutis laxa (ACL) is a rare connective tissue disorder characterized by loose, inelastic skin. It may be related to malignancies such as multiple myeloma, paraproteinemia, lymphomas, monoclonal gammopathy of undetermined significance, and monoclonal immunoglobulin deposition disease (MIDD).[1,2,3,4] Based on the type of deposits, MIDD is described as light-chain deposition disease (LCDD), light and heavy chain deposition disease (LHCDD), and heavy-chain deposition disease (HCDD), which are essentially similar in clinical and pathologic terms.[5] There have been only few reported cases of ACL in association with MIDD.[6] We describe a case of ACL with LHCDD.
Case Report
A 40-year-old man presented with progressive flaccidity of skin, mainly on his face, neck, and trunk since two years. He gave a history of developing anasarca, breathlessness, anemia, and fatigue 1 year back. At the time of presentation to our institute, he had bilateral pedal edema with pallor and prematurely aged appearance. There was no family history of cutis laxa. He was diagnosed with hypertension 2 months back, and had a history of heavy drinking and smoking for the past 20 years, which he stopped 2 months back. Cutaneous examination revealed loose hanging ear lobes, blepharochalasis, lax nasolabial folds, and increased folds over the neck, axilla, and trunk [Figures 1–3].
Figure 1.
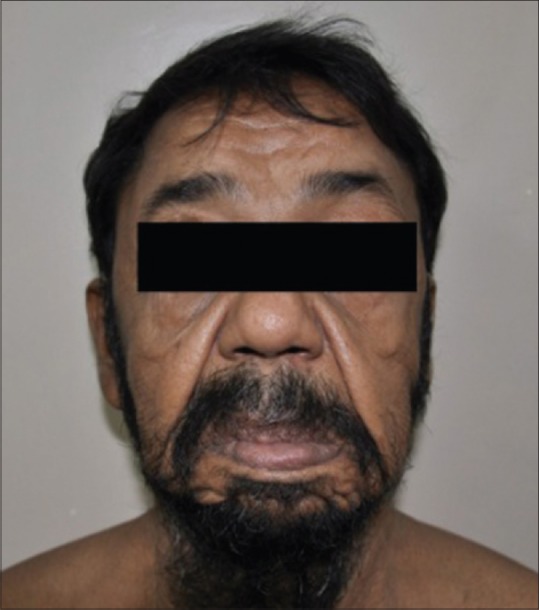
Lax nasolabial folds
Figure 3.
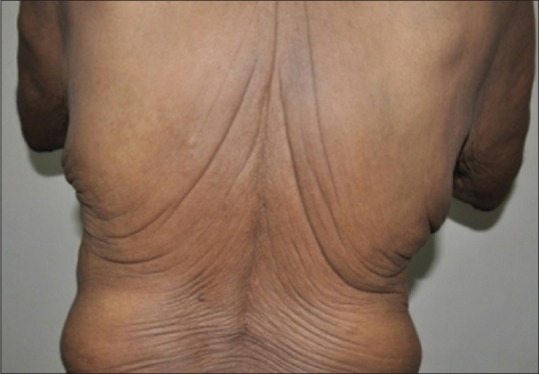
Increased cutaneous folds over the back
Figure 2.
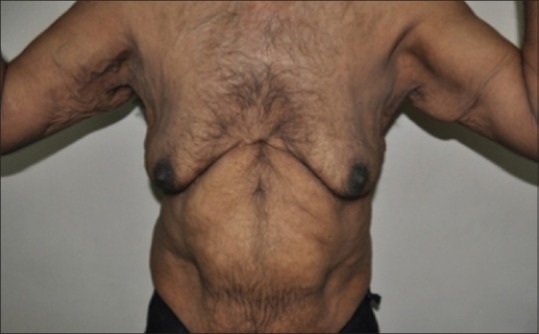
Increased cutaneous folds over the neck, chest, and axillary region
Further investigations revealed nephrotic range proteinuria (24-hour urine protein - 9.1 g), hematuria, raised urine β2 microglobulin levels, presence of urine Bence–Jones proteins, raised levels of blood urea (98 mg%) and serum creatinine (1.61 mg%), reduced total protein (4.8 gm/dl) and albumin (2.3 gm/dl), low hemoglobin (7.7 g/dl), normal calcium, high LDH, and uric acid levels. Serum electrophoresis revealed “M-band” - 0.3 g% in gamma region. Immunofixation electrophoresis showed IgG-κ, with elevation of free kappa, normal lambda chain levels (κ = 100 mg/L, λ = 24 mg/L), and raised κ/λ ratio of 4.2. Bone marrow examination revealed 9% plasma cells with occasional immature forms. Radiological evaluation was negative for lytic lesions. Two-dimensional echo showed a large pericardial effusion with no valvular prolapse. Ultrasonography of the abdomen pelvis was normal. Renal biopsy showed nodular glomerulosclerosis with positive staining on immunohistochemistry for monoclonal kappa and IgG. Skin biopsy showed markedly reduced elastic fibers [Figures 4 and 5]. Staining for amyloid in both renal and skin biopsy was negative.
Figure 4.
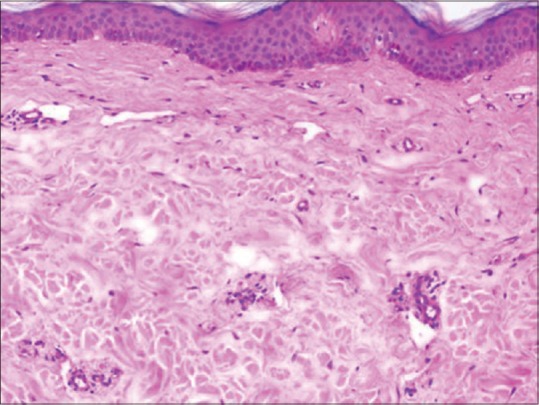
Normal thickness of epidermis with no evident dermal inflammatory infiltrate and normal collagen bundles. Elastin fiber changes are not well appreciated on H and E staining. H and E, ×10
Figure 5.
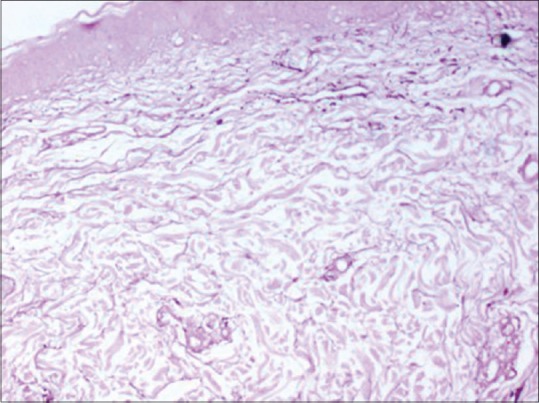
Marked reduction in elastic fibers in the superficial and deep reticular dermis with mild reduction of elastic fibers in the papillary dermis. Orcein stain, ×10
Based on the above investigations, he was diagnosed to have ACL with LHCDD.
He was started on chemotherapy with Cy-BorD protocol (weekly doses of inj. dexamethasone 20 mg, tab. cyclophosphamide 300 mg/m2, inj bortezomib 2 mg). He was suggested facelift surgery, but he did not consent for the same.
During the 6th cycle of therapy, he showed significant improvement clinically as well as in laboratory parameters with an elevation in Hb (8.9 g%), total protein (6.9 g/dL), albumin (4.4 g/dL), and reduction in serum creatinine (1.25 mg%), blood urea (92 mg%), 24-hour urine protein (2.5 gm), and urine Bence–Jones proteins were negative. Serum electrophoresis showed faint band in gamma region. Immunofixation electrophoresis showed IgG κ – end of gamma. Unfortunately, we lost the patient to follow-up after the 6th cycle.
Discussion
MIDD is characterized by immunoglobulin deposits along basement membranes in most tissues, especially in the glomerulus and renal tubule.[5] MIDD includes LCDD, LHCDD, and HCDD. Skin is the next organ commonly affected by HCDD, which presents as ACL. Heart and liver are involved in 25% of the cases of LCDD and LHCDD. It may also involve the cutaneous, lymphoreticular, respiratory, gastrointestinal, and musculoskeletal system.[5]
LHCDD can be a variant of LCDD with monoclonal light chain associated with a normal heavy chain. However, truncated heavy chain with CH1 deletion have also been reported.[7] It can manifest as renal failure, nephrotic range proteinuria, hematuria, hypertension, or M component (blood or urine). The glomerular pattern of injury, classically presents as nodular glomerulosclerosis with monotypic deposits of light chains, usually kappa, and heavy chains.[5]
The redundant skin in ACL is caused by defective elastin synthesis, function, or degradation.[8] Deposition of immune complexes cause release of inflammatory cytokines which destroy the elastic fibers.[1] Excess light chains may also alter elastin production by activation of alternative complement pathway.[9] However, some case reports show lack of immunoglobulin deposition on the elastin fibers.[1,2] Furthermore, the severity of ACL does not seem to correlate with the serum levels of monoclonal paraprotein.[3]
ACL can present years before the clinical or serological manifestations of the related systemic disease,[3] as seen in our case. Extracutaneous cardiopulmonary, gastrointestinal, vesicourinary, and skeletal involvement may occur;[10] however, none were involved in our case.
Histopathological examination shows diminished elastic fibers in the dermis on orcein staining. Giant cells phagocytosing the elastic fibers, variable inflammatory reaction, or immunoglobulin deposits may be present in the dermis.
Facial uplift, rhytidectomy, blepharoplasty, and botulinum toxin can help improve cutaneous symptoms.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Yadav T, Dongre A, Khopkar U. Acquired cutis laxa of face with multiple myeloma. Indian J Dermatol Venereol Leprol. 2014;80:454. doi: 10.4103/0378-6323.140310. [DOI] [PubMed] [Google Scholar]
- 2.Gverić T, Barić M, Bulat V, Situm M, Pusić J, Huljev D, et al. Clinical Presentation of a Patient with Localized Acquired Cutis Laxa of Abdomen: A Case Report. Dermatol Res Pract 2010. 2010:e402093. doi: 10.1155/2010/402093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim DP, Klein PA. Acquired cutis laxa in a 55-year-old female with multiple myeloma and serologic evidence of systemic lupus erythematosus. Dermatol Online J. 2011;17:8. [PubMed] [Google Scholar]
- 4.Harrington CR, Beswick TC, Susa JS, Pandya AG. Acquired cutis laxa associated with heavy chain deposition disease. J Am Acad Dermatol. 2008;59(5 Suppl):S99–101. doi: 10.1016/j.jaad.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 5.Ronco P, Plaisier E, Mougenot B, Aucouturier P. Immunoglobulin Light (Heavy)-Chain Deposition Disease: From Molecular Medicine to Pathophysiology-Driven Therapy. Clin J Am Soc Nephrol. 2006;1:1342–50. doi: 10.2215/CJN.01730506. [DOI] [PubMed] [Google Scholar]
- 6.O’Malley JT, D’Agati VD, Sherman WH, Grossman ME. Acquired cutis laxa associated with heavy chain deposition disease involving dermal elastic fibers. JAMA Dermatol. 2014;150:1192–6. doi: 10.1001/jamadermatol.2014.725. [DOI] [PubMed] [Google Scholar]
- 7.Cohen C, El-Karoui K, Alyanakian MA, Noel LH, Bridoux F, Knebelmann B. Light and heavy chain deposition disease associated with CH1 deletion. Clin Kidney J. 2015;8:237–9. doi: 10.1093/ckj/sfv002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nygaard RH, Maynard S, Schjerling P, Kjaer M, Qvortrup K, Bohr VA, et al. Acquired Localized Cutis Laxa due to Increased Elastin Turnover. Case Rep Dermatol. 2016;13:42–51. doi: 10.1159/000443696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joss N, Boulton-Jones JM, More I. Premature ageing and glomerulonephritis. Nephrol Dial Transplant. 2001;16:615–8. doi: 10.1093/ndt/16.3.615. [DOI] [PubMed] [Google Scholar]
- 10.New HD, Callen JP. Generalized acquired cutis laxa associated with multiple myeloma with biphenotypic IgG-λ and IgA-κ gammopathy following treatment of a nodal plasmacytoma. Arch Dermatol. 2011;147:323–8. doi: 10.1001/archdermatol.2011.26. [DOI] [PubMed] [Google Scholar]


