Abstract
Background:
The prevalence of obesity is increasing worldwide. Obese children without hypertension are becoming an important health challenge.
Aims:
Complications of obesity in adults are well established, but in obese children, cardiac dysfunction has not been reported clinically.
Settings and Design:
The present crosssectional study investigates subclinical systolic and diastolic dysfunction using echocardiographic modalities.
Materials and Methods:
Twentyfive youngsters with body mass index (BMI) >30 and 25 healthy children with BMI <25 were assigned into case and control group, respectively. In all participants, complete cardiovascular examination, electrocardiography, and echocardiography were fulfilled. Echocardiography surveys included standard, pulsed wave Doppler (PWD), and tissue Doppler imaging (TDI).
Statistical Analysis Used:
SPSS software, version 24.
Results:
The two groups were matched for age and sex. The resting heart rate and blood pressure were markedly higher in the obese group (P = 0.0001) though they were within the normal range in either category. Ejection fraction in the two groups was similar. Left ventricular (LV) mass (P = 0.0001), LV mass index (P = 0.029), left atrialtoaortic diameter ratio (P = 0.0001), and LV enddiastolic diameter (P = 0.008) were significantly greater in the case group, indicating cardiomegaly and subclinical systolic and diastolic dysfunction. Except for the aortic velocity, all PWD variables were considerably lower in the case group, suggesting subclinical diastolic dysfunction. All TDI parameters varied significantly between the two categories. There was a direct correlation between isovolumetric relaxation time and BMI.
Conclusions:
Obesity in children without hypertension is associated with subclinical systolic and diastolic cardiac dysfunction. We propose the evaluation of blood pressure as well as myocardial performance using PWD and TDI in all obese children without hypertension, regularly.
Keywords: Doppler, echocardiography, pediatric obesity, ventricular dysfunction
INTRODUCTION
The prevalence of obesity is increasing globally. Nowadays, complications of obesity among adults are well described.[1] Occasionally, obesity in adults dates back to childhood and adolescence in origin. A probe into some clinical variables of obese children can help in early diagnosis of upcoming problems.[2] Investigation of obese patients without hypertension may disclose some unknown aspects of obesity, thereby preventing unfavorable outcomes in the future. It may lead to adopting new strategies in the prophylaxis of cardiovascular sequelae in this high-risk category of children. Some complications of obesity occur gradually during a lifetime. Obesity without hypertension can depress cardiac function.[3] The severity and duration of enhanced body mass index (BMI) have a positive impact on the development of cardiac dysfunction.[4] We assessed cardiac function in the obese juveniles without high blood pressure by means of standard, pulsed wave Doppler (PWD), and tissue Doppler imaging (TDI) echocardiography.
MATERIALS AND METHODS
This cross-sectional study was carried out on the obese children from January 2014 to February 2015 in our pediatric cardiology clinic. The study was approved by our Institutional Review Board. Twenty-five obese juveniles with BMI >30 (obese group) and 25 healthy children with normal weight and BMI <25 (control group) were selected. Children ages were between 5 and 15 years. Matching was done for age and gender between the two groups. BMI was calculated by the formula; BMI = weight (kg)/height (m2). Informed written consent was obtained from parents or guardians of all minors participated in our research.
All participants in the two groups underwent thorough cardiovascular examination. At first, heart rate (HR) was taken during 1 min. Then, blood pressure (BP) was measured from the right arm with proper sized cuff, three times, each after 20 min of resting. Blood pressure more than a percentile of 95 was considered as hypertension.[4]
Laboratory tests were taken from all cases including complete blood count, liver function test, fasting blood glucose, fasting serum insulin, thyroid function test, and homeostatic model assessment of insulin resistance (HOMA-IR).
Blood glucose and insulin were measured after 12 h of fasting. HOMA-IR rate was calculated by the formula; HOMA-IR = fasting insulin (mIU/mL) × fasting blood glucose (mmol/L)/22.5.
All individuals involved in the investigation underwent a chest X-ray, electrocardiography, and echocardiography.
Exclusion criteria
Obese children who had systolic or diastolic blood pressure greater than the 90th percentile, cases with BMI <30 and >25, sleep apnea, thyroid disorders, abnormal blood lipid profile, cardiac structural abnormalities, decreased ejection fraction (EF), systemic diseases, and treatment with antiobesity drugs were excluded from the analysis.
Echocardiography measurements
Echocardiography was performed after 15 min of resting by one physician. Vivid 3 (GE Vingmed Ultrasound) with 8 and 5 MHZ probe was employed during the procedure. Standard echocardiography was perfected for every child. Left ventricular (LV) end-diastolic diameter (LVEDD), left atrium (LA), and aortic diameter were determined in the parasternal long view by the M-mode. EF was measured precisely by the M-mode modality as well. LV mass index (LVMI) was calculated by LVM/body surface area (g/m2). To assess the subclinical systolic and diastolic dysfunctions, PWD and TDI were utilized. All calculations in standard echocardiography, TDI, and PWD were obtained from the average of three cycles.
Pulsed wave Doppler echocardiography
PWD echocardiography was used to evaluate the diastolic and systolic cardiac functions. Parameters of early diastolic mitral velocity (E), late diastolic mitral velocity (A), E/A ratio, and early deceleration time (E-DT) were calculated. To increase the accuracy of formulations and better quality, a sample value was placed 1–3 mm at the tip of the mitral valve. Measurements were obtained in the four-chamber view in the lateral decubitus position. Likewise, aortic flow velocity was achieved in five-chamber view.
Tissue Doppler imaging echocardiography
TDI echocardiography was accomplished to evaluate the diastolic and systolic functions of the heart. To appraise the subclinical systolic and diastolic dysfunction, the early (Em) and late (Am) diastolic myocardial velocities, Em/Am ratio, systolic myocardial velocity (Sm), isovolumetric relaxation time (IVRT), and E/Em ratio were estimated.
We placed a sample volume 5 mm above the mitral valve annulus in the outer rim to prevent noise. Sample volume size was 2 ml, and the high frame rate image (HFR) was >100 frames/s and mean Nyquist limit was 10 ± 20 cm/s.
The S-wave velocity time integral (S-VTI) was calculated by marking velocity–time integral systolic S-wave obtained by TDI. The end-diastolic distance from the mitral annulus to the LV apex was designated as L0. We used displacement index (DI) for gauging the LV longitudinal movement.
Longitudinal DI was calculated by dividing S-VTI by L0 (S-VTI/L0).[5,6]
Statistical analysis
Data were analyzed using the software SPSS version 24 (SPSS, Inc., Chicago, IL, USA). Descriptive statistics are presented as mean ± standard deviation (SD); Chi-square test was used to compare sex distribution. Independent samples Student t-test was used to compare continuous variables as in age. Analysis of covariance was used to remove the intervening effect of systolic BP while examining the group differences. Pearson's linear correlation coefficient analysis was used to assess the relationship between BMI, echocardiography, and other laboratory parameters. P < 0.05 was considered statistically significant.
RESULTS
Table 1 summarizes the clinical characteristics of the case and control groups. The study included 25 obese (64% male; mean age: 10.41 ± 3.52 years) and 25 normal weight children (56% male; mean age: 9.92 ± 3.51 years). Although HR and BP were within the normal range in either group, there were significant differences in terms of HR and BP between the two categories (P = 0.0001), i.e., the resting heart rate and blood pressure were significantly higher in obese children.
Table 1.
Comparison of the clinical characteristics in the case and control groups
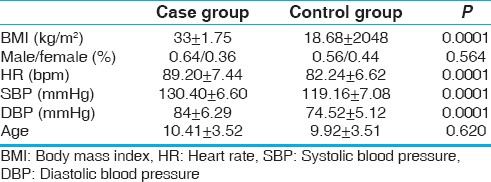
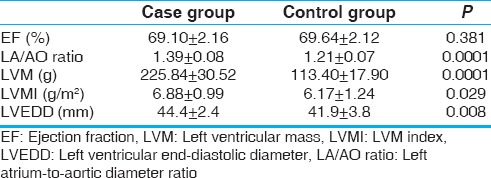
Table 2 compares the standard echocardiographic parameters between the two groups. The EF in the two categories was similar and within the normal range. However, LA-to-aortic diameter ratio (P = 0.0001), LVM (P = 0.0001), LVMI (P = 0.029), and LVEDD (P = 0.008) were significantly greater in the case group as compared to controls. In other words, in obese children without hypertension, standard echocardiography revealed cardiomegaly and increased LV diameters.
Table 2.
Comparison of standard echocardiographic parameters between the two groups
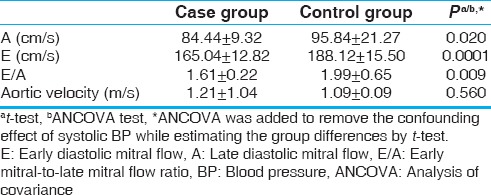
PWD echocardiography parameters are listed in Table 3. Aortic velocity was not different between the two groups (P = 0.560). E-wave (P = 0.0001), A-wave (P = 0.020), and E/A ratio (P = 0.009) were significantly lower in the case group. Otherwise stated, in nonhypertensive obese children, a significant diastolic dysfunction was observed.
Table 3.
Comparison of pulsed wave Doppler parameters in the two groups
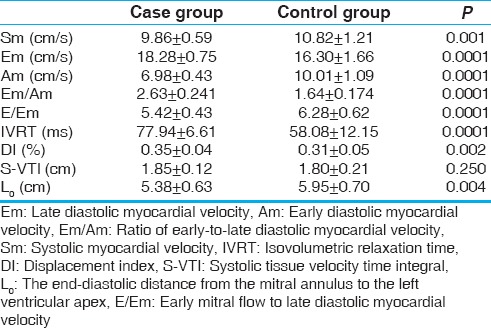
TDI echocardiography parameters are presented in Table 4. S-VTI parameter between the two groups was not different significantly (P = 0.25). Differences in Em, Am, Sm, IVRT, DI, E/Em ratio, and L0 between the two groups were statistically significant. This finding showed that, even in the absence of hypertension, significant reduction of LV systolic function occurs as impairment of longitudinal myocardial motion, and diastolic dysfunction arises as impairment in cardiac relaxation and compliance.
Table 4.
Comparison of the tissue Doppler image parameters in the two groups
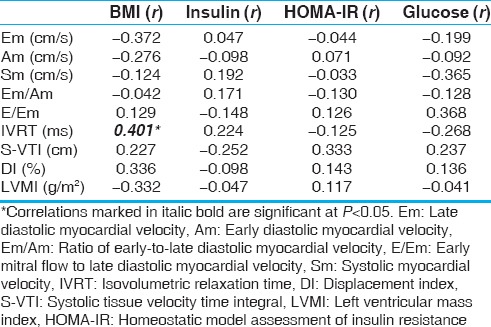
The results of correlation between the BMI and laboratory variables with some of the echocardiographic parameters in the case group are displayed in Table 5. Because of concision, all echocardiography parameters have not been presented in the table, though included in our calculations, thoroughly. There was a direct positive connection solely between IVRT and BMI.
Table 5.
Pearson's correlations between the body mass index, laboratory variables, and some echocardiographic parameters in the case group
DISCUSSION
The number of obese individuals in the young age is on the rise steadily, and this situation can be seen in our country too. Having determined the effects of obesity on cardiac function, the importance of weight reduction and lifestyle changes would become more evident. Obesity without systemically increased blood pressure is a unique clinical situation in which one can contemplate merely the effect of obesity on the cardiac function without confounding effects of hypertension. Complications such as diabetes, hypertension, coronary atherosclerosis, sleep apnea, LV hypertrophy, and cardiac dysfunction have been delineated in the adults' obesity. Most fat adults have high blood pressure with ensuing LV hypertrophy. However, there are little clinical data regarding systolic and diastolic cardiac dysfunction with or without hypertension in fat children. Factors such as insulin resistance, high blood sugar, and impaired autonomic nervous system might contribute to impaired cardiac contractility in patients with metabolic syndromes.[6]
Referring to our results, the baseline BP was below the 90th percentile in the case group yet higher than the BP in healthy children. The heart rate in the obese cases was considerably higher than the control group. There was no overt clinical depression of myocardial function in either category. Our study suggests blood pressure, and heart rate monitoring should be done in the obese children at frequent intervals to detect early stages of hypertension.
Until recently, traditional echocardiographic methods were used to evaluate the performance of systolic and diastolic phases of cardiac contractions. Currently, PWD and TDI have emerged as new methods to assay systolic and diastolic heart failure.[5,7]
Based on our findings, among obese children, standard echocardiography did not show abnormalities in EF. Left atrium size in the case group was larger than nonobese counterparts. This finding confirmed that cardiac volume in the obese children is enlarging gradually though there was not documented systolic dysfunction in the standard echocardiography. Nevertheless, increased LV diameters and mass have been demonstrated in our study in the obese children. Despite the lack of high blood pressure, obese youngsters had significantly greater LVM than healthy children. The increase in LVM predisposes the child to subclinical systolic and diastolic dysfunction. Our study could not detect the correlation between BMI, LVM, and LVMI with other laboratory tests [Table 5].
LVEDD was significantly larger in obese cases in comparison to healthy children. The increase in LVEDD may be the result of volume overload secondary to increased BMI (BMI >30). A relationship between obesity and diastolic dysfunction in the obese children has been suggested.[8,9] In the course of our study, the diastolic function of obese participants was assessed by PWD and TDI. Mitral E/A ratio, A- and E-wave velocity decreased dramatically in the case group as compared to control category. All parameters pertaining to diastolic function in TDI and PWD were impaired in the case group as well [Tables 3 and 4]. Em, Am, Em/Am ratio, and IVRT differed statistically significant between the healthy and obese groups. In other words, diastolic dysfunction occurs in the obese participants as impaired relaxation and compliance. In the obese group, there was a statistically significant dampening in E/Em variable that indicates impaired LV filling in diastolic phase.
We evaluated the systolic function by the TDI variables including S-VTI, Sm, and DI. S-VTI is a parameter of the myocardial systolic function.[10] We could not detect a statistically significant difference between the two groups in terms of S-VTI. We did not show a correlation between S-VTI and laboratory parameters with BMI, either. Sm was significantly lower in the case group, notwithstanding aortic velocity was identical among categories. Moreover, our findings did not show a significant correlation between Sm and laboratory parameters with BMI [Table 5].
DI is a parameter for evaluating LV longitudinal movement and systolic dysfunction due to ventricular deformation. DI has a relation to EF and longitudinal global systolic dysfunction.[5] In fact, DI indicates LV longitudinal linear motion. It has been found that DI is in direct connection with BMI and HR. LV deformations in obese children are generally subclinical and may improve with treatment.[11] As compared to S-VTI, DI is less dependent on the age and body size.[7] Of TDI parameters, DI is known to be correlated with mortality and cardiac complications. To determine the subclinical systolic dysfunction, DI may be a more sensitive criterion than S-VTI as false-negative results for S-VTI have been reported in some instances.
Our study revealed a statistically significant difference with respect to DI between the two groups. DI increased in the obese group. However, there was no correlation between DI, laboratory parameters, and BMI [Table 5].
In addition, preclinical regional deformation may occur in BMI >30.
PWD and TDI parameters determine the form of subclinical systolic and diastolic dysfunction. Clinical cardiac dysfunction usually does not occur in the fat children. According to the values of DI, Sm, S-VTI, and IVRT parameters in the present study, the subclinical systolic and diastolic dysfunction was detected in the obese group without elevated blood pressure.
As noted earlier, we also examined the relationship between BMI, fasting blood sugar, and insulin to all variables of standard, PWD, and TDI echocardiography. Based on our results, only IVRT was found to be correlated with the BMI and this relation was positive. We did not find a connection between laboratory criteria such as HOMA-IR, fasting glucose, and insulin to other variables of standard, PWD, and TDI echocardiography [Table 5]. HOMA-IR is generally impaired before the clinical onset of diabetes.[10,12] HOMA-IR is an index of insulin resistance in adults, which is used in children, as well.[12,13] There is insulin resistance in the fleshy children. This is a risk factor for type II diabetes and the occurrence of high blood pressure.[12] In obese adults, there has been a strong correlation between insulin resistance and obesity with myocardial deformation. In other studies, this link was between insulin resistance and LVM as a sign of myocardial dysfunction and deformation,[13,14] but we did not discover this connection, likely due to inadequate sample size to achieve statistical power. On top of obesity, insulin resistance in children is affected by several factors including gender and pubertal status.[15] A more solid association between obesity indices and HOMA-IR levels was observed in males than in females.[16] In a study by Ling et al.,[17] gender (females), BMI, and waist circumference had the most significant positive correlations with fasting insulin and HOMA-IR. In an investigation by Cozzolino et al.,[18] 89 consecutive obese children were evaluated by TDI technique to delineate the relationship between BMI, insulin resistance, and cardiac function. In conclusion, insulin resistance had a corroborative effect on the negative impact of obesity on myocardial performance and cardiac autonomic regulation. In other words, subclinical dysfunction of myocardium and cardiac autonomic system dysregulation were correlated with the degree of insulin resistance.
These findings suggest that in children, obesity without having high blood pressure could result in subclinical systolic and diastolic cardiac dysfunction.
Study limitations
The number of obese children was relatively low, so we recommend more studies with greater samples in the future. Likewise, the obese individuals usually have poor echocardiographic views making interpretation of results more difficult. Lack of long-term follow-up of obese cases, to evaluate the effects of probable weight loss on clinical and echocardiographic variables, was another drawback of our research. Furthermore, the obese minors were not under 24-h blood pressure monitoring to strictly determine the variations in systolic and diastolic blood pressure.
CONCLUSIONS
Our findings suggest that obese children without hypertension could develop systolic and diastolic dysfunction of the heart. It appears that LV systolic dysfunction takes place due to ventricular deformation and increased LVM and LVMI. These findings emphasized the importance of prevention of obesity and its ensuing complications in juveniles. Thus, we recommend measurement of blood pressure, as well as, cardiac performance monitoring using echocardiography methods in the obese children on a regular basis.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Zeybek C, Celebi A, Aktuglu-Zeybek C, Onal H, Yalcin Y, Erdem A, et al. The effect of low-carbohydrate diet on left ventricular diastolic function in obese children. Pediatr Int. 2010;52:218–23. doi: 10.1111/j.1442-200X.2009.02940.x. [DOI] [PubMed] [Google Scholar]
- 2.Gnavi R, Spagnoli TD, Galotto C, Pugliese E, Carta A, Cesari L, et al. Socioeconomic status, overweight and obesity in prepuberal children: A study in an area of Northern Italy. Eur J Epidemiol. 2000;16:797–803. doi: 10.1023/a:1007645703292. [DOI] [PubMed] [Google Scholar]
- 3.Movahed MR, Saito Y. Obesity is associated with left atrial enlargement, E/A reversal and left ventricular hypertrophy. Exp Clin Cardiol. 2008;13:89–91. [PMC free article] [PubMed] [Google Scholar]
- 4.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114:555–76. [PubMed] [Google Scholar]
- 5.Roberson DA, Cui W. Tissue Doppler imaging measurement of left ventricular systolic function in children: Mitral annular displacement index is superior to peak velocity. J Am Soc Echocardiogr. 2009;22:376–82. doi: 10.1016/j.echo.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 6.Kibar AE, Pac FA, Ballı S, Oflaz MB, Ece I, Bas VN, et al. Early subclinical left-ventricular dysfunction in obese nonhypertensive children: A tissue Doppler imaging study. Pediatr Cardiol. 2013;34:1482–90. doi: 10.1007/s00246-013-0674-8. [DOI] [PubMed] [Google Scholar]
- 7.Sutherland GR, Stewart MJ, Groundstroem KW, Moran CM, Fleming A, Guell-Peris FJ, et al. Color Doppler myocardial imaging: A new technique for the assessment of myocardial function. J Am Soc Echocardiogr. 1994;7:441–58. doi: 10.1016/s0894-7317(14)80001-1. [DOI] [PubMed] [Google Scholar]
- 8.Di Bello V, Santini F, Di Cori A, Pucci A, Palagi C, Delle Donne MG, et al. Obesity cardiomyopathy: Is it a reality. An ultrasonic tissue characterization study? J Am Soc Echocardiogr. 2006;19:1063–71. doi: 10.1016/j.echo.2006.03.033. [DOI] [PubMed] [Google Scholar]
- 9.Willens HJ, Chakko SC, Lowery MH, Byers P, Labrador E, Gallagher A, et al. Tissue Doppler imaging of the right and left ventricle in severe obesity (body mass index & gt; 35 kg/m2) Am J Cardiol. 2004;94:1087–90. doi: 10.1016/j.amjcard.2004.06.076. [DOI] [PubMed] [Google Scholar]
- 10.Levent E, Gökşen D, Ozyürek AR, Darcan S, Coker M. Usefulness of the myocardial performance index (MPI) for assessing ventricular function in obese pediatric patients. Turk J Pediatr. 2005;47:34–8. [PubMed] [Google Scholar]
- 11.Uluçay A, Tatli E. Myocardial performance index. Anadolu Kardiyol Derg. 2008;8:143–8. [PubMed] [Google Scholar]
- 12.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC, et al. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 13.Koç F, Tokaç M, Kaya C, Kayrak M, Yazıcı M, Karabaǧ T, et al. Diastolic functions and myocardial performance index in obese patients with or without metabolic syndrome: A tissue Doppler study. Turk Kardiyol Dern Ars. 2010;38:400–4. [PubMed] [Google Scholar]
- 14.Di Salvo G, Pacileo G, Del Giudice EM, Natale F, Limongelli G, Verrengia M, et al. Abnormal myocardial deformation properties in obese, non-hypertensive children: An ambulatory blood pressure monitoring, standard echocardiographic, and strain rate imaging study. Eur Heart J. 2006;27:2689–95. doi: 10.1093/eurheartj/ehl163. [DOI] [PubMed] [Google Scholar]
- 15.Kurtoǧlu S, Hatipoǧlu N, Mazıcıoǧlu M, Kendirici M, Keskin M, Kondolot M, et al. Insulin resistance in obese children and adolescents: HOMA-IR cut-off levels in the prepubertal and pubertal periods. J Clin Res Pediatr Endocrinol. 2010;2:100–6. doi: 10.4274/jcrpe.v2i3.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lim SM, Choi DP, Rhee Y, Kim HC. Association between obesity indices and insulin resistance among healthy Korean adolescents: The JS high school study. PLoS One. 2015;10:e0125238. doi: 10.1371/journal.pone.0125238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ling JC, Mohamed MN, Jalaludin MY, Rampal S, Zaharan NL, Mohamed Z, et al. Determinants of high fasting insulin and insulin resistance among overweight/Obese adolescents. Sci Rep. 2016;6:36270. doi: 10.1038/srep36270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cozzolino D, Grandone A, Cittadini A, Palmiero G, Esposito G, De Bellis A, et al. Subclinical myocardial dysfunction and cardiac autonomic dysregulation are closely associated in obese children and adolescents: The potential role of insulin resistance. PLoS One. 2015;10:e0123916. doi: 10.1371/journal.pone.0123916. [DOI] [PMC free article] [PubMed] [Google Scholar]


