Abstract
Background
In the 1980s, randomized-controlled trials showed that high-dose corticosteroid treatment did not improve the mortality of acute respiratory distress syndrome (ARDS). However, while the diagnostic criteria for ARDS have since changed, and supportive therapies have been improved, no randomized-controlled trials have revisited this issue since 1987; thus, the effect of high-dose corticosteroid treatment may be different in this era. We evaluated the effect of high-dose corticosteroid treatment in patients with ARDS using a nationwide administrative database in Japan in a retrospective and observational study.
Methods
This study was performed with a large population using the 2012 Japanese nationwide administrative database (diagnostic procedure combination). We evaluated the mortality of ARDS patients receiving or not receiving high-dose corticosteroid treatment within 7 days of hospital admission. We employed propensity score weighting with a Cox proportional hazards model in order to minimize the bias associated with the retrospective collection of data on baseline characteristics and compared the mortality between the high-dose and non-high-dose corticosteroid groups.
Results
Data from 2707 patients were used; 927 patients were treated with high-dose corticosteroid and 1780 patients were treated without high-dose corticosteroid, within 7 days of admission. After adjusting for confounds, mortality rates within 3 months were significantly higher in the high-dose corticosteroid group compared to the non-high-dose corticosteroid group (weighted hazard ratio: 1.59; 95% CI: 1.37-1.84; P < 0.001).
Conclusions
Our results suggest that high-dose corticosteroid treatment does not improve the prognosis of patients with ARDS, even in this era. However, this study has limitations owing to its retrospective and observational design.
Keywords: Acute respiratory distress syndrome, Corticosteroid, Inverse probability of treatment weighting method, Nationwide administrative database, Propensity score
Background
Acute respiratory distress syndrome (ARDS) is a critical respiratory syndrome. Recent advances in treatment strategies, such as protective mechanical ventilation techniques, have improved the mortality of patients with ARDS, according to many clinical trials [1–4]. However, no pharmacotherapies have yet been shown to be effective in improving the mortality rate; according to systematic reviews, mortalities due to ARDS were as high as 43 and 44% in 2008 and 2009, respectively, [2, 5].
A systemic inflammatory response is closely associated with the development of ARDS. Thus, anti-inflammatory corticosteroid treatment may be a logical choice for ARDS [6–8]. However, according to previous studies and meta-analyses, the efficacy of corticosteroids in ARDS is still controversial; while some reports have shown improvement in the mortality [9–13], others have not [14–16]. Furthermore, although meta-analyses have indicated the poor effectiveness of corticosteroids, they also highlight the difficulties in confirming the role of corticosteroids due to the heterogeneity of ARDS studies [8, 17]. The dosage of corticosteroids for the treatment of ARDS is also controversial. In the 1980s, a few randomized-controlled trials (RCTs) showed that high-dose corticosteroids did not improve mortality, and subsequently, for almost three decades, no RCTs have revisited this topic due to the results of these reports [14, 18]. However, the ARDS diagnostic criteria, supportive methods, and treatments for underlying diseases have changed over the years. In 1994, the American-European Consensus Conference (AECC) resulted in the development of new diagnostic criteria for ARDS [19], which were subsequently modified (Berlin definition [20]). We therefore speculated that the effectiveness of high-dose corticosteroid treatment might be different in the present era. In this retrospective and observational study, we investigated the effectiveness of high-dose corticosteroid treatment using the Japanese nationwide administrative database: the diagnostic procedure combination (DPC). To minimize the bias associated with the retrospective collection of data (i.e. the baseline characteristics in the high-dose and non-high-dose corticosteroid groups), we employed propensity score weighting with a Cox proportional hazards model [21–25].
Methods
Data source
The DPC is a case-mix patient classification system that was introduced by the Japanese government in 2002 and is linked with a lump-sum payment system [26]. It covers approximately 40% of all acute-care hospitalizations in Japan and has been actively utilized for the evaluation of treatments [27–29]. The database contains the following information: disease name, treatment costs, comorbid illnesses at admission and during hospitalization (coded by the International Classification of Diseases, 10th revision; ICD-10), patients’ age, sex, length of stay, medical procedures, intensive-care unit (ICU) admission, interventional procedures (including mechanical ventilation and hemodialysis), medications, state of consciousness according to the Japan Coma Scale (JCS) on admission, and discharge status (including in-hospital deaths) [26, 29].
Any patient identifiable information was removed from the data. This study was conducted according to guidelines laid down in the Declaration of Helsinki and was approved by Ethics Committee of Medical Research, University of Occupational and Environmental Health, Japan. Informed consent was waived because of the retrospective study design.
Patient selection
Patients who were diagnosed with ARDS, ICD-10 code J80 or pneumonia at admission (and subsequently diagnosed with ARDS as the predominant reason for hospitalization as indicated by the cost during hospitalization) and discharged within 2012 were included. Patients who were discharged and died within 7 days of hospitalization or who did not receive mechanical ventilation were excluded, as we believed that if the duration of administration was too short, the effect of high-dose corticosteroid on ARDS could not be analyzed properly, and the use of mechanical ventilation is necessary for the diagnosis according to the criteria of ARDS [19, 20].
Variables
Patients’ sex and age (years), hospital volume (number of patients with ARDS treated in 2012), emergency transport, diagnoses of sepsis, cancer, pneumonia, pancreatitis, lung or abdominal trauma, liver dysfunction (diagnosed as liver failure, hepatitis, or liver cirrhosis) at admission, hemodialysis performed within 7 days of admission, neurological dysfunction (JCS at admission of ≥100 indicating coma) [29], shock (use of a vasopressor within 7 days of admission), medication use (insulin, antithrombin III, recombinant human soluble thrombomodulin, heparin, synthetic protease inhibitors, or sivelestat within 7 days from admission), transfusion of platelets and red cells within 7 days of admission, administration of albumin and immunoglobulin within 7 days of admission, mechanical ventilation, and ICU transfer within 7 days of admission were used as variables.
Statistical analyses
The high-dose corticosteroid group was defined as the patients who received treatment with methylprednisolone at doses of > 500 mg/day for > 1 day within 7 days of admission. The definition of high-dose corticosteroid was taken from previous meta-analyses [8], and infusion of 500 mg of methylprednisolone every 12 h for < 72 h is the most common regimen of high-dose corticosteroid therapy in Japan.
The primary outcome was mortality; the secondary endpoints were the duration (in days) for which mechanical ventilation was used and the duration of ICU stay within the 28 days after admission. We employed propensity score weighting with a Cox proportional hazards model, as described previously [8, 25, 29, 30]. The propensity score was calculated using a logistic model with baseline variables that potentially influenced the use of high-dose corticosteroid, including patients’ sex and age, hospital volume, sepsis, cancer, pneumonia, pancreatitis, lung and abdominal trauma, liver dysfunction, hemodialysis, neurological dysfunction, shock, use of antithrombin III, recombinant human soluble thrombomodulin, heparin, synthetic protease inhibitors and sivelestat, platelet and red cell transfusions, albumin and immunoglobulin administration, and mechanical ventilation status.
The C-statistic was used to evaluate goodness of fit. To check the balance of the measured covariates, χ2 or Fisher’s exact tests were used for categorical data, and unpaired t-tests or Mann-Whitney U tests were used for continuous variables to evaluate the between-group (high-dose vs. non-high-dose corticosteroid group) differences before and after adjusting for confounders using propensity score weighting. The adjusted Kaplan-Meier curves were depicted, and the adjusted hazard ratio (HR) and robust 95% confidence interval (CI) were estimated in a Cox regression model [8, 25, 30, 31]. This method was performed using the IBM SPSS 22.0 (Armonk, NY, USA) and STATA/IC 14.0 (StataCorp, College Station, TX, USA) software programs. Differences of P < 0.05 were considered statistically significant in all tests.
Results
Among the data of 4982 patients diagnosed with ARDS in the DPC database (as described in the Methods), the data of 706 patients were excluded because the patients were discharged within 7 days of admission. The data of 1569 patients were also excluded because the patients did not receive mechanical ventilation within 7 days of admission. Of the remaining 2707 patients, 927 received high-dose corticosteroid treatment, and 1780 received non-high-dose corticosteroid treatment within 7 days of admission (Fig. 1).
Fig. 1.
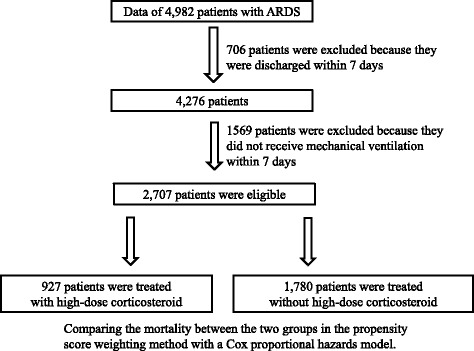
Flowchart of this study. Among the 4982 patients diagnosed with ARDS in the 2012 Japanese nationwide administrative database (diagnostic procedure combination), the data of the 2707 patients who met the inclusion criteria were used. Of these 2707 patients, 927 received high-dose corticosteroid treatment, and 1780 received non-high-dose corticosteroid treatment within 7 days of admission. We employed propensity score weighting with a Cox proportional hazards model in order to minimize the bias associated with the retrospective collection of data on baseline characteristics and compared the mortality between the two groups
To minimize the bias associated with the retrospective collection of data in the high-dose and non-high-dose corticosteroid groups, we employed propensity score weighting with a Cox proportional hazards model. The C-statistic (area under the receiver operating characteristic curve) of the propensity score was 0.72. Patient baseline characteristics, before and after adjusting for confounders, are shown in Table 1. Before adjustment, the baseline variables of patient age, hospital volume, emergency transport, sepsis, cancer, pneumonia, pancreatitis, lung and abdominal trauma, hemodialysis, neurological dysfunction, shock, insulin, antithrombin III, recombinant human soluble thrombomodulin, platelet and red cell transfusions, albumin administration, immunoglobulin administration, and ICU transfer were significantly different between the high-dose and non-high-dose corticosteroid groups. After adjusting for confounders using the IPTW method, patient baseline characteristics between the two groups were similar across these variables, although pneumonia, pancreatitis, lung and abdominal trauma, neurological dysfunction, and insulin were still significantly different between the groups. In the propensity score-weighted Cox proportional hazards model, the mortality was significantly lower in the high-dose corticosteroid group than in the non-high-dose corticosteroid group (weighted HR: 1.59; 95% CI: 1.37-1.84; P < 0.001; Fig. 2). There were no significant differences between the two groups with regard to the duration for which mechanical ventilation was used (3.5 ± 0.2 versus 3.3 ± 0.1 days, P = 0.323), and the duration of the ICU stay (13.2 ± 0.3 versus 12.8 ± 0.2 days, P = 0.330) within the 28 days after admission (Table 2).
Table 1.
Baseline characteristics of the patients treated with or without high-dose corticosteroid before and after group adjustment
| Before adjustment | After adjustment | |||||
|---|---|---|---|---|---|---|
| High-dose corticosteroid (n = 927) |
Non-high-dose corticosteroid (n = 1780) |
p-value | High-dose corticosteroid (n = 927) |
Non-high-dose corticosteroid (n = 1780) |
p-value | |
| Sex | 68.1 | 66.9 | 0.562 | 68.1 | 67.8 | 0.771 |
| Age (years) | 71.6 ± 0.5 | 67.9 ± 0.5 | < 0.001 | 67.7 ± 1.0 | 69.3 ± 0.4 | 0.05 |
| Hospital volume per year | 10.3 ± 0.3 | 13.0 ± 0.3 | < 0.001 | 12.2 ± 0.6 | 12.1 ± 0.2 | 0.806 |
| Sepsis | 12.1 | 22.1 | < 0.001 | 19.9 | 19 | 0.373 |
| Cancer | 12.3 | 8.3 | 0.001 | 10.2 | 9.7 | 0.487 |
| Pneumonia | 52.6 | 62.4 | < 0.001 | 63.6 | 60.3 | < 0.001 |
| Pancreatitis | 0.3 | 1.1 | 0.041 | 0.4 | 0.8 | 0.001 |
| Lung and abdominal trauma | 0.1 | 0.7 | 0.043 | 0.2 | 0.4 | 0.009 |
| Liver dysfunction | 2.1 | 2.2 | 0.88 | 3.0 | 2.2 | 0.392 |
| Hemodialysis | 10.1 | 13.1 | 0.023 | 14.0 | 12.4 | 0.085 |
| Neurological dysfunction | 10.6 | 22.5 | < 0.001 | 21.8 | 18.8 | 0.034 |
| Shock | 44.2 | 48.1 | 0.053 | 47.4 | 47.2 | 0.848 |
| Insulin | 61.7 | 45.9 | < 0.001 | 48.7 | 51.2 | 0.016 |
| Antithrombin III | 9.0 | 15.4 | < 0.001 | 13.9 | 13.2 | 0.537 |
| rhTM | 10.0 | 14.1 | 0.002 | 14.6 | 12.9 | 0.159 |
| Heparin | 58.2 | 61.5 | 0.094 | 62.8 | 61.7 | 0.088 |
| Protease inhibitors | 17.8 | 19.4 | 0.318 | 20.1 | 19.2 | 0.331 |
| Sivelestat | 72.9 | 56.2 | < 0.001 | 59.7 | 61.8 | 0.074 |
| Platelet transfusion | 8.0 | 10.2 | 0.045 | 9.7 | 9.7 | 0.938 |
| Red blood cell transfusion | 16.4 | 26.5 | < 0.001 | 24.8 | 23.3 | 0.241 |
| Albumin administration | 31.7 | 41.6 | < 0.001 | 38.9 | 38.5 | 0.706 |
| Immunoglobulin administration | 18.1 | 23.5 | 0.001 | 22.2 | 21.8 | 0.637 |
| Intensive-care unit | 33.3 | 32.9 | 0.852 | 33.7 | 33.4 | 0.754 |
Data are presented as the % or mean ± standard error, unless otherwise stated. Groups were adjusted using the inverse probability of treatment weighting method
rhTM recombinant human soluble thrombomodulin
Fig. 2.
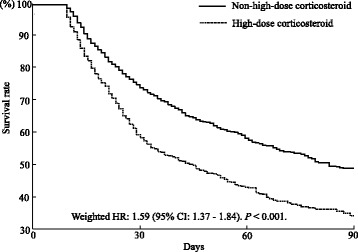
Adjusted Kaplan-Meier survival curves. In the propensity score-weighted Cox proportional hazards model, the mortality rate of the high-dose corticosteroid group was significantly lower than that of the non-high-dose corticosteroid group (weighted HR: 1.59; 95% CI: 1.37-1.84; P < 0.001)
Table 2.
Secondary endpoints of the patients treated with or without high-dose corticosteroids before and after group adjustment.
| Before adjustment | After adjustment | |||||
|---|---|---|---|---|---|---|
| High-dose corticosteroid (n = 927) |
Non-high-dose corticosteroid (n = 1780) |
p-value | High-dose corticosteroid (n = 927) |
Non-high-dose corticosteroid (n = 1780) |
p-value | |
| Duration of mechanical ventilation use within 28 days (days) | 13.3 ± 0.2 | 12.9 ± 0.2 | 0.108 | 13.2 ± 0.3 | 12.8 ± 0.2 | 0.330 |
| Duration of intensive-care unit stay within 28 days (days) | 3.4 ± 0.2 | 3.3 ± 0.1 | 0.863 | 3.5 ± 0.2 | 3.3 ± 0.1 | 0.323 |
Data are presented as the mean ± standard error. Groups were adjusted using the inverse probability of treatment weighting method
Discussion
To date, no pharmacotherapies have demonstrated robust, beneficial effects on the outcomes of patients with ARDS. In the present study, we observed the effects of high-dose corticosteroid treatment in a large number of Japanese patients with ARDS using a Japanese nationwide administrative database (DPC) by propensity score weighting with a Cox proportional hazards model. After adjusting for baseline characteristics, the mortality was found to be significantly worse in patients who received high-dose corticosteroid treatment than in those who received non-high-dose corticosteroid treatment.
The efficacy of corticosteroids is still controversial; some reports have shown improvements in mortality [9–13], while others have not [14–16]. Our results showed a higher mortality following high-dose corticosteroid use than without the use of such agents. Previous reports of the high-dose corticosteroid treatment showed that high-dose corticosteroid did not improve the mortality [7, 8, 13, 14, 16, 17, 32]. Among these, Bernard et al. showed that 30 mg/kg of body weight of methylprednisolone every 6 h for 24 h did not improve the mortality of patients with ARDS [14]. Very recently, a meta-analysis showed that high-dose corticosteroid treatment did not improve the mortality [13], and a recent retrospective propensity-matched study also showed that high-dose corticosteroid treatment increased the mortality compared to low-dose corticosteroid treatment [32]. However, the reasons for this are unclear. Weigelt et al. showed that high-dose corticosteroid treatment (30 mg/kg of body weight every 6 h for 48 h) did not prevent patients with respiratory failure from developing ARDS but did increase the risk of infectious complications [33]. In our patients, approximately 60 and 20% of patients were diagnosed with pneumonia and sepsis, respectively, at the time of admission. Thus, high-dose corticosteroids may have exacerbated infectious diseases or increased infectious complications in the present study, similar to a recent study by Takaki et al. [32]. Other than mortality, we have also shown the effects of corticosteroids on the duration for which mechanical ventilation is used and the duration of the ICU stay within the 28 days after admission, which did not differ between the groups to a statistically significant extent. On the other hand, several studies that have investigated lower dosages of corticosteroids and different protocols have shown the effects on shortening the duration of mechanical ventilation usage and ICU stay [12, 15]. Further studies might be needed to investigate the effects of high-dose corticosteroids on these secondary outcomes.
Propensity score weighting has recently been used in observational studies to assess the effect of treatment after adjusting for baseline characteristics in order to minimize the drawbacks associated with the propensity score matching method, such as sampling biases and loss of sample numbers. ARDS is relatively rare and clinically severe; therefore, it may be not easy to perform new and large RCTs, especially for the reevaluation of clinically used medications that have the potential to be clinically effective in different scenarios from previous studies. Even in retrospective studies, we speculate that propensity score weighting with a Cox proportional hazards model and a large subject population is suitable for evaluating the clinical effect of certain medications, such as high-dose corticosteroid, in patients with ARDS.
There are several limitations associated with this study, similar to previous studies using the DPC database [27–29, 34]. First, this study was observational and retrospective; however, our application of propensity score weighting with a Cox proportional hazards model reduced the effects of this limitation. Second, even after adjustment, there were still significant differences in the characteristics of the high-dose and non-high-dose corticosteroid groups, such as in the rates of pneumonia, pancreatitis, lung and abdominal trauma, neurological dysfunction and insulin. However, after adjusting for confounders, the differences between the two groups were small. For example, the frequency of neurological dysfunction in the high-dose corticosteroid and non-high-dose corticosteroid groups were 10.6% versus 22.5%, respectively, before adjustment, and 21.8% versus 18.8% after adjustment. While this remains one of the limitations of the present study, we consider these differences to be relatively small. Third, we were unable to include several clinical data, such as the peripheral blood laboratory findings, radiological findings, physiological data, including vital signs, and mechanical ventilation settings, which could also influence the mortality in patients with ARDS. Fourth, the ARDS diagnostic criteria have changed over the years [19, 20], so it was unclear which criteria had been used to diagnose ARDS in each patient in this retrospective study.
Despite these limitations, the major advantages of this study were the inclusion of a large number of patients and evaluation of the effect of high-dose corticosteroid treatment in this era. To our knowledge, this is the largest study of high-dose corticosteroid treatment for ARDS, even compared to previous meta-analyses [7, 8, 13, 17].
Conclusions
We observed a higher mortality with the administration of high-dose corticosteroids within 7 days of hospital admission in patients with ARDS than without the administration of such agents. We used a nationwide administrative database, making this the largest study to observe high-dose corticosteroid treatment for ARDS, to our knowledge. However, this study has some limitations owing to its retrospective and observational design.
Acknowledgements
Not applicable.
Funding
This research was partially supported by the Practical Research Project for Rare Intractable Diseases from the Japan Agency for Medical Research and Development (AMED), and a grant from the Ministry of Health, Labour, and Welfare of Japan, which was awarded to the study group for Diffuse Pulmonary Disorders, Scientific Research/Research on Intractable Diseases.
Availability of data and materials
The study data is available on reasonable request to the corresponding author (TK).
Abbreviations
- AECC
American-European Consensus Conference
- ARDS
Acute respiratory distress syndrome
- CI
Confidence interval
- DPC
Diagnostic procedure combination
- HR
Hazard ratio
- ICD-10
International Classification of Diseases, 10th revision
- ICU
Intensive-care unit
- IPTW
Inverse probability of treatment weighting
- JCS
Japan Coma Scale
- RCT
Randomized controlled trial
Authors’ contributions
TK had full access to all study data and takes responsibility for the integrity of the data and accuracy of the data analysis. KM, TA, TK, YF, and SM contributed to the study design, and data collection, analysis, and interpretation. HO, TO, KO, TM, HM, and KY contributed to the study design and drafting the important intellectual content of the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
This study was approved by the Ethics Committee for Medical Care and Research at the University of Occupational and Environmental Health, Japan. Informed consent was waived because of the retrospective study design.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Takashi Kido, Phone: +81-93-691-7453, Email: t-kido@med.uoeh-u.ac.jp.
Keiji Muramatsu, Email: km@med.uoeh-u.ac.jp.
Takeshi Asakawa, Email: t-asakawa@med.uoeh-u.ac.jp.
Hiroki Otsubo, Email: tsubo425@med.uoeh-u.ac.jp.
Takaaki Ogoshi, Email: togoshi@med.uoeh-u.ac.jp.
Keishi Oda, Email: oda-keishi@med.uoeh-u.ac.jp.
Tatsuhiko Kubo, Email: kubo@med.uoeh-u.ac.jp.
Yoshihisa Fujino, Email: zenq@med.uoeh-u.ac.jp.
Shinya Matsuda, Email: smatsuda@med.uoeh-u.ac.jp.
Toshihiko Mayumi, Email: mtoshi@med.uoeh-u.ac.jp.
Hiroshi Mukae, Email: hmukae@med.uoeh-u.ac.jp.
Kazuhiro Yatera, Email: yatera@med.uoeh-u.ac.jp.
References
- 1.The Acute Respiratory Distress Syndrome Network Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 2.Zambon M, Vincent JL. Mortality rates for patients with acute lung injury/ARDS have decreased over time. Chest. 2008;133(5):1120–1127. doi: 10.1378/chest.07-2134. [DOI] [PubMed] [Google Scholar]
- 3.Briel M, Meade M, Mercat A, Brower RG, Talmor D, Walter SD, Slutsky AS, Pullenayegum E, Zhou Q, Cook D, et al. Higher vs lower positive end-expiratory pressure in patients with acute lung injury and acute respiratory distress syndrome: systematic review and meta-analysis. JAMA. 2010;303(9):865–873. doi: 10.1001/jama.2010.218. [DOI] [PubMed] [Google Scholar]
- 4.Guerin C, Reignier J, Richard JC, Beuret P, Gacouin A, Boulain T, Mercier E, Badet M, Mercat A, Baudin O, et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368(23):2159–2168. doi: 10.1056/NEJMoa1214103. [DOI] [PubMed] [Google Scholar]
- 5.Phua J, Badia JR, Adhikari NK, Friedrich JO, Fowler RA, Singh JM, Scales DC, Stather DR, Li A, Jones A, et al. Has mortality from acute respiratory distress syndrome decreased over time?: a systematic review. Am J Respir Crit Care Med. 2009;179(3):220–227. doi: 10.1164/rccm.200805-722OC. [DOI] [PubMed] [Google Scholar]
- 6.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 7.Peter JV, John P, Graham PL, Moran JL, George IA, Bersten A. Corticosteroids in the prevention and treatment of acute respiratory distress syndrome (ARDS) in adults: meta-analysis. BMJ. 2008;336(7651):1006–1009. doi: 10.1136/bmj.39537.939039.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horita N, Hashimoto S, Miyazawa N, Fujita H, Kojima R, Inoue M, Ueda A, Ishigatsubo Y, Kaneko T. Impact of corticosteroids on mortality in patients with acute respiratory distress syndrome: a systematic review and meta-analysis. Intern Med. 2015;54(14):1473–1479. doi: 10.2169/internalmedicine.54.4015. [DOI] [PubMed] [Google Scholar]
- 9.Meduri GU, Headley AS, Golden E, Carson SJ, Umberger RA, Kelso T, Tolley EA. Effect of prolonged methylprednisolone therapy in unresolving acute respiratory distress syndrome: a randomized controlled trial. JAMA. 1998;280(2):159–165. doi: 10.1001/jama.280.2.159. [DOI] [PubMed] [Google Scholar]
- 10.Keel JB, Hauser M, Stocker R, Baumann PC, Speich R. Established acute respiratory distress syndrome: benefit of corticosteroid rescue therapy. Respiration. 1998;65(4):258–264. doi: 10.1159/000029273. [DOI] [PubMed] [Google Scholar]
- 11.Lee HS, Lee JM, Kim MS, Kim HY, Hwangbo B, Zo JI. Low-dose steroid therapy at an early phase of postoperative acute respiratory distress syndrome. Ann Thorac Surg. 2005;79(2):405–410. doi: 10.1016/j.athoracsur.2004.07.079. [DOI] [PubMed] [Google Scholar]
- 12.Meduri GU, Golden E, Freire AX, Taylor E, Zaman M, Carson SJ, Gibson M, Umberger R. Methylprednisolone infusion in early severe ARDS: results of a randomized controlled trial. Chest. 2007;131(4):954–963. doi: 10.1378/chest.06-2100. [DOI] [PubMed] [Google Scholar]
- 13.Yang ZG, Lei XL, Li XL. Early application of low-dose glucocorticoid improves acute respiratory distress syndrome: a meta-analysis of randomized controlled trials. Exp Ther Med. 2017;13(4):1215–1224. doi: 10.3892/etm.2017.4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bernard GR, Luce JM, Sprung CL, Rinaldo JE, Tate RM, Sibbald WJ, Kariman K, Higgins S, Bradley R, Metz CA, et al. High-dose corticosteroids in patients with the adult respiratory distress syndrome. N Engl J Med. 1987;317(25):1565–1570. doi: 10.1056/NEJM198712173172504. [DOI] [PubMed] [Google Scholar]
- 15.Steinberg KP, Hudson LD, Goodman RB, Hough CL, Lanken PN, Hyzy R, Thompson BT, Ancukiewicz M. Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N Engl J Med. 2006;354(16):1671–1684. doi: 10.1056/NEJMoa051693. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Z, Chen L, Ni H. The effectiveness of corticosteroids on mortality in patients with acute respiratory distress syndrome or acute lung injury: a secondary analysis. Sci Rep. 2015;5:17654. doi: 10.1038/srep17654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agarwal R, Nath A, Aggarwal AN, Gupta D. Do glucocorticoids decrease mortality in acute respiratory distress syndrome? A meta-analysis. Respirology. 2007;12(4):585–590. doi: 10.1111/j.1440-1843.2007.01060.x. [DOI] [PubMed] [Google Scholar]
- 18.Laggner AN, Lenz K, Base W, Druml W, Schneeweiss B, Grimm G, Sommer G, Kleinberger G. Effect of high-dose prednisolone on lung fluid in patients with non-cardiogenic lung edema. Wien Klin Wochenschr. 1987;99(7):245–249. [PubMed] [Google Scholar]
- 19.Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, Legall JR, Morris A, Spragg R. The American-European consensus conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149(3 Pt 1):818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 20.Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307(23):2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 21.Robins JM, Hernan MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11(5):550–560. doi: 10.1097/00001648-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 22.Kurita T, Yasuda S, Oba K, Odani T, Kono M, Otomo K, Fujieda Y, Oku K, Bohgaki T, Amengual O, et al. The efficacy of tacrolimus in patients with interstitial lung diseases complicated with polymyositis or dermatomyositis. Rheumatology (Oxford) 2015;54(8):1536. doi: 10.1093/rheumatology/kev192. [DOI] [PubMed] [Google Scholar]
- 23.Lunceford JK, Davidian M. Stratification and weighting via the propensity score in estimation of causal treatment effects: a comparative study. Stat Med. 2004;23(19):2937–2960. doi: 10.1002/sim.1903. [DOI] [PubMed] [Google Scholar]
- 24.Morshed S, Knops S, Jurkovich GJ, Wang J, MacKenzie E, Rivara FP. The impact of trauma-center care on mortality and function following pelvic ring and acetabular injuries. J Bone Joint Surg Am. 2015;97(4):265–272. doi: 10.2106/JBJS.N.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kido T, Muramatsu K, Yatera K, Asakawa T, Otsubo H, Kubo T, Fujino Y, Matsuda S, Mayumi T, Mukae H. Efficacy of early sivelestat administration on acute lung injury and acute respiratory distress syndrome. Respirology (Carlton, Vic) 2017;22(4):708–713. doi: 10.1111/resp.12969. [DOI] [PubMed] [Google Scholar]
- 26.Matsuda S, Ishikawa KB, Kuwabara K, Fujimori K, Fushimi K, Hashimoto H. Development and use of the Japanese case-mix system. Eur Secur. 2008;14(3):25–30. [Google Scholar]
- 27.Yasunaga H, Hashimoto H, Horiguchi H, Miyata H, Matsuda S. Variation in cancer surgical outcomes associated with physician and nurse staffing: a retrospective observational study using the Japanese diagnosis procedure combination database. BMC Health Serv Res. 2012;12:129. doi: 10.1186/1472-6963-12-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chikuda H, Yasunaga H, Takeshita K, Horiguchi H, Kawaguchi H, Ohe K, Fushimi K, Tanaka S. Mortality and morbidity after high-dose methylprednisolone treatment in patients with acute cervical spinal cord injury: a propensity-matched analysis using a nationwide administrative database. Emerg Med J. 2014;31(3):201–206. doi: 10.1136/emermed-2012-202058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iwagami M, Yasunaga H, Doi K, Horiguchi H, Fushimi K, Matsubara T, Yahagi N, Noiri E. Postoperative polymyxin B hemoperfusion and mortality in patients with abdominal septic shock: a propensity-matched analysis. Crit Care Med. 2014;42(5):1187–1193. doi: 10.1097/CCM.0000000000000150. [DOI] [PubMed] [Google Scholar]
- 30.Guo S, Fraser MW. Propensity score analysis: statistical methods and applications. 2. Thousand Oaks: Sage; 2015. [Google Scholar]
- 31.Cole SR, Hernan MA. Adjusted survival curves with inverse probability weights. Comput Methods Prog Biomed. 2004;75(1):45–49. doi: 10.1016/j.cmpb.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 32.Takaki M, Ichikado K, Kawamura K, Gushima Y, Suga M. The negative effect of initial high-dose methylprednisolone and tapering regimen for acute respiratory distress syndrome: a retrospective propensity matched cohort study. Critical Care (London, England) 2017;21(1):135. doi: 10.1186/s13054-017-1723-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weigelt JA, Norcross JF, Borman KR, Snyder WH., 3rd Early steroid therapy for respiratory failure. Arch Surg. 1985;120(5):536–540. doi: 10.1001/archsurg.1985.01390290018003. [DOI] [PubMed] [Google Scholar]
- 34.Hamada T, Yasunaga H, Nakai Y, Isayama H, Horiguchi H, Matsuda S, Fushimi K, Koike K. Continuous regional arterial infusion for acute pancreatitis: a propensity score analysis using a nationwide administrative database. Critical Care (London, England) 2013;17(5):R214. doi: 10.1186/cc13029. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The study data is available on reasonable request to the corresponding author (TK).


