Abstract
Freshwater biodiversity and ecosystem integrity are under threat from biological invasions. The “killer shrimp” Dikerogammarus villosus is a highly predatory amphipod that has spread readily across Central Europe and recently the UK and its arrival has been associated with the significant loss of resident species. Despite this, studies of its behavioral ecology are sparse, even though its behavior may contribute to its invasion success. For the first time, we investigated antipredator “fleeing” behavior in D. villosus and how this changed with water temperature. Three key patterns emerged from our analysis. First, within a particular temperature condition there are moderate but consistent among-individual differences in behavior. These are driven by a combination of mean level among-individual differences and within-individual relative consistency in behavior, and provide the key marker for animal personalities. Second, the fleeing responses were not influenced by temperature and third, regardless of temperature, all individuals appeared to habituate to a repeated nondangerous stimulus, indicating a capacity for individual learning. We suggest that the antipredator behavior of D. villosus contributes to its rapid spread and that consistent among-individual differences in behavior may promote biological invasions across heterogeneous conditions. Robustness to changing water temperatures may also be potentially advantageous, particularly in an era of global climate change, where average temperatures could be elevated and less predictable.
Keywords: invasions, Dikerogammarus villosus, animal personalities, habituation, temperature
Predator avoidance behavior has always been an important topic in behavioral ecology and, in particular, invasion biology (Sala et al. 2000; Pennuto and Keppler 2008). Predators influence the environmental impact and distribution of prey, with prey behavior, morphology, and life history all capable of change to minimize vulnerability to predators (Fraser and Huntingford 1986; MacNeil et al. 2008, 2014). Such changes often come at a cost, however, with reduced activity levels and increased vigilance potentially leading to lower feeding rates in animals such as crustaceans (Bailey et al. 2006; MacNeil et al. 2011). Invasive species may initially be naïve to many resident predators within newly invaded assemblages. Thus, they might respond inappropriately and ineffectually to predators and predation risk (Kristensen and Closs 2004). However, invasive species may also learn to quickly avoid predators and, conversely, to stop responding to novel stimuli that turn out to be nondangerous (Brown 2003). In other words, we should expect invasive species to show a high capacity for sensitization (an increase in response over repeated exposures to dangerous stimuli) and habituation (a loss response over repeated exposure to nondangerous stimuli).
Dikerogammarus villosus or “the killer shrimp” is a freshwater amphipod that has recently been referred to as a near “perfect” invader, with physiological and behavioral capabilities that have allowed it to spread rapidly throughout Central Europe from its Ponto–Caspian origins (Koback et al. 2014; Rewicz et al. 2014). Being a voracious predator of a wide range of macroinverebrate species (Dick et al. 2002), the arrival of D. villosus is often accompanied by deleterious impacts on resident biodiversity and assemblage structure (van Riel et al. 2006; MacNeil et al. 2013). In addition to its predatory behavior, D. villosus appears to be highly aggressive (apparently killing other macroinvertebrates in excess of feeding requirements), a potentially important trait in invasive animal species (Hudina et al. 2014). Despite this aggressive predatory behavior, D. villosus also has predators, such as fish, which it must avoid and efficient antipredator behavior will certainly contribute to the ability of an invader such as D. villosus to spread through new habitats. Antipredator behavior is extremely well-suited to longitudinal studies of behavior, whereby each individual in the sample is observed on multiple occasions. Such longitudinal studies seem especially well-suited to uncovering traits that might influence the potential for invasiveness (Wolf and Weissing 2012; Carere and Gherardi 2013). Behavior is typically thought of as being a highly flexible trait, such that individuals are able to modify their behavior to match the prevailing environment, an attribute referred to as behavioral plasticity (Sih et al. 2004). Consistent among-individual differences in behavior, often described as “animal personality” (Sih et al. 2004), might enhance a species invasion potential if different types of behavior correlate with the progress of different stages of biological invasion (Wolf and Weissing 2012; Juette et al. 2014). For example, risk-prone individuals might lead to rapid range expansion but risk-averse individuals might have a survival advantage in new environments. Animal personality differences, quantified by calculating repeatability (Lessells and Boag 1987), in fact derive from the balance between two sources of variation; among-individual variation in mean-level behavior and within-individual variance in behavior (Stamps et al. 2012). Animal personalities and behavioral plasticity are not mutually exclusive (Briffa et al. 2008). Nevertheless, individuals might vary in the level of plasticity that they express across environmental gradients. To date, behavioral studies of invasive species that employ the longitudinal experimental designs required to uncover these sources of behavioral variation are comparatively rare (Carere and Gherardi 2013).
For poikilothermic animals, such as D. villosus, performance is expected to be modulated by the temperature of the surrounding medium, since this will drive variation in metabolic rate (see Maazouzi et al. 2011). In amphipods, reduced activity levels, fleeing, and drifting in the water column have all been described as tactics for avoiding fish predators by reducing the frequency of encounters with predators (MacNeil et al. 2003). Furthermore, it has recently been shown that taxon- or species-level differences in 1) foraging behavior and 2) tolerance to temperature fluctuations, might drive differences among macroinvertebrates in their sensitivity to climate change (Sandin et al. 2014), such that more tolerant invasive species should be favored relative to natives under new climate change environmental regimes (Liu et al. 2011). Anticipated future temperature regimes can be characterized not only by elevated mean temperatures in the long to medium term, but also by less stable temperatures in the short term, due to heatwaves (Schär et al. 2004) and sudden instances of elevated precipitation (Trenberth 2011). Any short-term fluctuations in thermal regime, whether natural or anthropogenic in origin, could therefore lead to temporal variation in metabolic rate and hence behavior, potentially altering the susceptibility of invaders to control by predators.
We aim to address two gaps in knowledge regarding aquatic invertebrates, including invaders such as D. villosus. First, we will provide the first longitudinal study into the behavior of D. villosus and by adopting this longitudinal approach, we will be able to investigate the presence or absence of animal personalities and behavioral (thermal) plasticity. Changes in the fleeing response to a repeated stimulus will be used as an indication of sample-level behavioral plasticity. Second, we will examine the amount of thermal plasticity and repeatability under variation in temperature condition, a question relevant to the effects of daily fluctuations in a key physico-chemical parameter of freshwater systems as well as to the wider question of predicted climate change.
Materials and Methods
Behavioral observations
Sixty D. villosus were collected by hand from Grafham Water Reservoir, UK, in October 2012, and transported, in sealed containers of constantly aerated lake-water, back to the laboratory in Plymouth University. Here, they were held temporarily (2 days) in a tank of aerated lake water at 15 °C in a constant temperature room. During this period and throughout the experiment, a 12:12 h light:dark cycle was maintained in the laboratory, all behavioral observations being conducted during the light phase at a constant light intensity. Individuals were then examined for sex and obvious parasites and only healthy individuals were weighed and used in the experiment. This yielded 45 individuals for the experiment. Selected individuals were transferred to individual tanks (28 × 17 × 6 cm) of containing 1 L aerated lake water, and ∼2 cm2 of lake water-soaked leaf as a food source. Then 16 individuals (6 males, 10 females) were transferred to a 10 °C constant temperature room and 29 (10 males, 19 females) remained in the 15 °C room. After a further 2-day acclimation period, observations of fleeing behavior commenced.
Each individual was stimulated to flee by touching it lightly on the urosome with a mounted blunt-ended needle (14 cm length) from a dissection kit. This procedure did not cause any damage to the animals and provoked a known antipredator response of fleeing away from a direct stimulus (MacNeil et al. 2003). To avoid overshadowing, care was taken not to lean over tanks. The duration of swimming was timed using a stopwatch from the point at which contact with the needle was made until swimming first ceased. On some occasions, an individual would make contact with the edge of the tank before swimming stopped, but preliminary observations indicated that this did not interrupt the swimming behavior, as swimming continued along the wall of the tank. Each individual was startled five times in this way on five consecutive days. After the fifth set of fleeing responses were obtained, the temperature conditions were reversed such that tanks that started the experiment at 10 °C were moved to the 15 °C room and those that had been at 15 °C were moved to the 10 °C room. A further 2 days were allowed for acclimation to the new temperature conditions and then a further 5 fleeing responses were collected for each individual over the following 5 days as described above. Thus, 10 observations were collected for each individual, with 5 observations in each temperature condition.
The crossover design was chosen to avoid confounding effects of time in the laboratory and repeated stimulation so that any genuine effect of temperature could be disentangled from any potential habituation (or sensitization) effect. We did not directly monitor water temperature for each individual tank, relying on the ambient temperature setting of the controlled temperature rooms. A previous analysis shows that temperatures in these rooms are unlikely to show significant variation across the working bench areas (Briffa et al. 2013). At the end of the experiment, all D. villosus specimens that we collected were destroyed through immersion in boiling water, and then disposed of as biological waste.
Statistical methods
We used a general linear mixed-effects model to determine the effects of the variables in our experiment on our behavioral measure, swimming duration. The fixed effects were temperature condition (10 °C or 15 °C), the temperature-specific observation number (1–5 in each condition), sex, and individual weight. We also included interactions between treatment order, observation number, and temperature. In addition to these fixed effects, we specified random effects to account for among-individual variation in swimming behavior.
Our analysis of this model then proceeded in two stages. First, we examined the random effects structure of the model. Our initial model allowed random intercepts for each individual, individual-specific responses to temperature change and to observation number (i.e., random slopes) and for a correlation between these individual-specific intercepts and slopes. Second, having determined the random effects structure that provided the best fit with the data, we went on to assess the significance of the fixed effects in the model. Our methods for assessing the random and fixed portions of the model are described in detail below, in the following section.
To assess the random effects, we used ΔAICc (Akaike Information Criterion, corrected for small sample sizes) to compare alternative candidate models calculated using REML parameter estimation, which is the appropriate method when assessing random effects (Bolker et al. 2009). A more complex model (i.e., one that contains more random effects) was favored over a simpler one only if its AICc value was lower by three or more AICc units. Significant random intercepts denote significant repeatability and significant random slopes denote significant variation among individuals in their responses to a change in temperature or to repeated observations. We evaluated the random effects structure of the model by comparing the full model against alternatives that contained (a) no random slope for observation number, (b) no random slope for temperature, and (c) random intercepts only. The best performing model was Model (a) which contained a random slope for temperature but not for observation number. We next evaluated the correlation between intercept and slope in this model by fitting a further Model (d), which lacked this correlation. Although Model (d) performed better than the intercepts only model, it was still outperformed by Model (a). These models and their AICc values are summarized in Table 1. To quantify any significant repeatability, and its errors, we calculated ANOVA-based repeatability and its 95% confidence intervals (CIs; Nakagawa and Shielzeth 2010). This allows repeatability to be compared across temperature situations, and indeed among different studies. To assess the fixed effects, we then recalculated the model that had been determined to have the most appropriate random effects structure, this time using ML parameter estimation, which is the appropriate method when testing fixed effects (Bolker et al. 2009). In mixed-effect models, denominator degrees of freedom cannot be calculated directly from the residual sum of squares as in a linear model that lacks any random effects. One approach for assessing significance in this context is therefore to use likelihood ratio tests to compare candidate models that contain and exclude the fixed effect of interest. However, the Kenward–Roger method provides an alternative and widely used approach for estimating denominator degrees of freedom (Littell et al. 2006). This has the advantage of allowing for convenient significance testing of fixed effects via familiar F-tests, and this is the approach we used here. We assessed the normality of random effects by inspecting a plot of theoretical against sample quantiles and we assessed the homogeneity of residual variance by plotting predicted against residual values. The distribution of random effects was non-normal but Log10-transformation provided adequate normality. Thus, the analyses were performed on Log10-transformed data. Analysis was conducted within the R 3.1.0 environment (R Core Team 2014), using the packages lme4 (Bates et al. 2014), lmerTest (Kuznetsova et al. 2014), rptR (Nakagawa and Schielzeth 2010), and AICcmodavg (Mazerolle 2014).
Table 1.
Comparisons of candidate models with different random effect structures by ΔAICc value
| Model | Random effects | AICc |
|---|---|---|
| Full model | Intercept, temperature, observation | 135.3 |
| (a) | Intercept, temperature | 106.4 |
| (b) | Intercept, observation | 141.8 |
| (c) | Intercept only | 137.7 |
| (d) | Intercept, temperature, no correlation | 135.1 |
Model (a) outcompeted the simpler intercepts only model (c) and a random slope (for temperature) model that did not assume a correlation between intercept and slope (d). A model allowing random slopes for observation number (b) was worse than the intercept only model. There was no justification for the most complex model including random slopes for both temperature and observation (full model).
Results
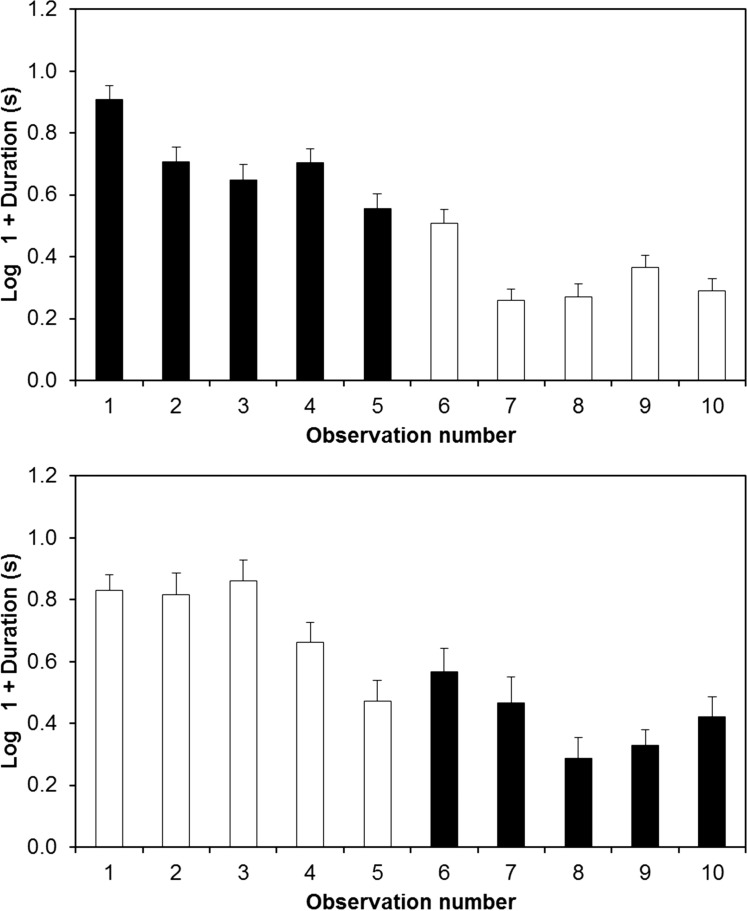
Evaluation of the random effect structure (Table 1) indicates that there might be significant among-individual variation in responses to a 5 °C change in temperature, with some individuals showing an increase in startle response duration and some showing a decrease as temperature increased. However, the main effects of the model (see below) indicate that this apparent variation in reactions to a change in temperature is in fact more likely to derive from a systematic decline in fleeing response durations across observations. Indeed, visual inspection of the data shows that individuals in the 15–10 °C group invariably gave their longest responses at 15 °C during the first part of the experiment, while for those in the 10–15 °C, this pattern was reversed (Figure 1). To further investigate this possibility, we fitted two new models separately for each treatment order, so that we could assess the random slope across temperatures independently for each block of data. For both treatment orders, a model that contained both the random slope and intercept provided a marginally better fit to the data than did a simpler version of the model containing random intercepts only. However, in each case, the ΔAICc value was too low to justify the more complex models containing random slopes over the simpler versions containing random intercepts only (15–10 °C; ΔAICc = 1.3, 10–15 °C ΔAICc = 1.1). To confirm whether these random intercepts represented consistent among-individual variation in behavior at the mean-response level, we obtained repeatability estimates by calculating the intra-class correlation coefficient and its 95% CIs. Since there was a random slope effect in our best overall model (albeit explicable by the number of observations that had taken place rather than by temperature), we should not calculate repeatability across all 10 observations (Briffa et al. 2013). Therefore, we calculated it separately for the observations conducted within in each temperature condition. There were similar patterns of moderate but significant repeatability at 10 °C (RA = 0.32, SE = 0.07, P < 0.0001; 95% CI = 0.195, 0.494) and 15 °C (RA = 0.35, SE = 0.07, P < 0.0001; 95% CI = 0.172, 0.469).
Figure 1.
Mean startle response durations. There was a significant interaction between treatment order (high to low temperature, top panel; low to high, bottom panel) and temperature (black bars = 15 °C, white bars = 10 °C). Rather than a genuine effect of temperature or treatment order, this interaction appears to reflect a general decline in startle responses over time. Error bars show standard errors. Observation numbers in this figure indicate the absolute number of times each individual had been observed, rather than the number of observations that had taken place within a given temperature condition.
To assess the fixed effects we recalculated Model (a) using ML estimation. There was no three-way interaction effect, no interaction between temperature and observation number, and no main effects of sex, weight, or observation number (Table 2). There was, however, a significant interaction between treatment order and temperature (F1,52.7 = 1.13, P < 0.0001), where individuals in the 15–10 °C showed the longest swimming duration at 15 °C and those in the 10–15 °C group showed their longest responses at 10 °C (Figure 1). The interaction effect thus confirms that, irrespective of temperature, the longest fleeing responses are given during the first 5 observations. To further confirm that this change represented a general decline in startle response duration across observations (i.e., habituation), rather than a response to temperature, we conducted a further analysis looking at the effect of the absolute observation number (1–10) and temperature, with random intercepts only. This analysis indicated that there was a decline in startle responses across the 10 observations (F1,403 = 239, P < 0.0001) and that there was no effect of temperature (F1,403 = 1.38, P = 0.24).
Table 2.
ANOVA table assessing the significance of fixed effects in Model (a)
| Characteristics | Sum of squares | Mean square | Degrees of freedom | F | P |
|---|---|---|---|---|---|
| Sex | 0.728 | 0.728 | 1,41 | 1.166 | 0.2865 |
| Weight | 0.0544 | 0.0544 | 1,41 | 0.038 | 0.8458 |
| TO | 1.8364 | 1.8364 | 1,43.56 | 151.057 | <0.0001 |
| Temperature | 0.8316 | 0.8316 | 1,83.22 | 102.964 | <0.0001 |
| Observation | 2.89 | 2.89 | 1,357 | 0.918 | 0.3385 |
| Temperature: Observation | 0.0102 | 0.0102 | 1,357 | 0.052 | 0.8202 |
| TO: Temperature | 7.7215 | 7.7215 | 1,52.7 | 113.291 | <0.0001 |
| TO: Temperature: Observation | 0.0044 | 0.0044 | 1,357 | 0.074 | 0.7856 |
TO: Treatment order.
Discussion
Three key patterns emerge from our analysis of fleeing behavior in D. villosus across multiple observations conducted at two temperatures. First, within each temperature condition there are moderate but consistent among-individual differences in behavior, as evidenced by significant random intercept effects and significant repeatability. Second, fleeing responses are not influenced by temperature. Third, regardless of temperature, or treatment order, all individuals appear to habituate to a repeated nondangerous stimulus, indicating a capacity for individual learning. Below, we first discuss the possible causation of these patterns and then we discuss our findings in the context of D. villosus as an invasive species and voracious predator.
The fleeing responses of D. villosus show the type of consistent among-individual differences typically associated with the presence of animal personalities. This is perhaps not surprising now that personality has been demonstrated in a broad range of animal taxa, including other decapod crustaceans (Gherardi et al. 2012; Juette et al. 2014). Rather more surprising were our findings in relation to temperature, specifically that a 5 °C change in temperature had no effect on behavior and individuals did not vary in how they responded to this temperature change. While D. villosus is tolerant to a wide range of temperatures (up to 30 °C), the temperature range chosen in this study (reflecting conditions in UK) is below the optimal conditions of 20–23 °C in their native range, where reproduction is inhibited below 13 °C (Bruijs et al. 2001). Where mean-level and among-individual variation in responses to temperature have been found in previous studies of ectothermic animals, it has been suggested that individuals vary in their metabolic responses to temperature (Lighton et al. 2001; Nespolo et al. 2003; Briffa et al. 2013). The lack of among-individual variation in thermal reaction norms in D. villosus may indicate a generalist strategy (Powers and Schulte 1998; Angilletta et al. 2009) for coping with temperature fluctuation in the population that we sampled. On a more general point, as in the case of a previous study (Briffa et al. 2013), this result illustrates the importance of using crossover designs in experiments on temperature. Had we, for example, subjected all animals to the 15–10 °C treatment, we might have erroneously concluded that increased escape swimming at the higher temperature is a general pattern in D. villosus (or at least would not have been able to distinguish the effect of temperature from that of observation number).
While sample-level plasticity in response to temperature variation was absent, we did see significant sample-level plasticity in response to repeated stimulation. Fleeing responses significantly declined across the 10 observations that we subjected animals to in a pattern indicative of habituation, the temporal eroding of responses to a nonthreatening stimulus. Although habituation is often assumed to be a common pattern in animals repeatedly exposed to the same stimulus, this is not always the case. In a similar recent study on another crustacean, the hermit crab Pagurus bernhardus, for example, where individuals were also subjected to repeated stimulation there was no overall pattern of decline in response (Briffa et al. 2013). Indeed, the potential for habituation in decapods appears to vary even among closely related species. In a series of studies on two grapsid crabs (reviewed in Tomsic et al. 2009), Chasmagnathus granulatus and Pachygrapsus sp., habituation to a simulated aerial predator could be induced more easily in C. granulatus. In C. granulatus, the habituation response varied with the pattern of repeated stimulation. Immediate re-exposure to the simulated threat led to a short-term reduction in response that recovered quickly following a pause in stimulation. In contrast, when there was a gap of 3 min before the next stimulus was applied there was a more gradual pattern of habituation that was more long-lived and this was ascribed to memory formation (Tomsic et al. 2009). Here, we have shown that D. villosus can habituate to repeated stimuli that are separated by far longer time periods of 24 h. Presumably, this is underpinned by the capacity to form memories of events that are separated by these comparatively large periods of time.
Consistent among-individual variation in behavior has been proposed as a factor that might contribute to a species’ invasion potential (Wolf and Weissing 2012; Juette et al. 2014). In order to reach conclusions about how personality variation may contribute to the invasiveness of D. villosus, it would be necessary to compare the repeatability estimates gained here to those for other invasive species (and perhaps the native amphipod species that it is replacing). As noted earlier, other longitudinal studies required to obtain these estimates are, in general, lacking for invasive species. While some studies have revealed behavioral differences between native and invasive species and populations (see e.g., Carere and Gherardi 2013), estimates within populations of invasive species are lacking. The presence of significant repeatability indicates that personality variation is a potential contributor to the spread of D. villosus and supports recent suggestions that animal personalities might facilitate the spread of invasive species (Juette et al. 2014). In the present study, we used animals from a single invasive population. A potential limitation is, therefore, that the lack of response to temperature per se, and the pattern of habituation observed, may be specific to this particular population. In particular, it would be interesting to analyze fleeing behavior in populations within the native Ponto–Caspian range of D. villosus. Invasive populations might represent a behavioral subset of their ancestral population due to “personality-biased” transport, establishment, and subsequent spread (Juette et al. 2014). Nevertheless, it is still pertinent that in this invasive population, we see among-individual variation, robustness against thermal regime change and a strong pattern of habituation across successive stimulation events.
Antipredator behavior is not without costs, which accrue from the energy expended on rapid locomotion and from the time lost to other behaviors such as foraging. Therefore, to maximize fitness antipredator behavior must be traded-off against other activities. Dikerogammarus villosus is a highly voracious predator (Dick et al. 2002; MacNeil et al. 2011; Rewicz et al. 2014) and understanding the causation of its antipredator behavior may help to explain this voracity. Habituation appears to be present even when stimuli are separated by relatively long intervals (∼24 h). This may enable feeding to be maintained under novel conditions and has the potential to increase the behavioral diversity at the population level (Wright et al. 2010). In addition, lack of responses to changing temperature suggest that feeding will be uninterrupted by temperature fluctuations in the environment. How this compares to other amphipod species responses to temperature needs to be investigated. Interestingly, Maazouzi et al. (2011) in a study comparing D. villosus survival, locomotory activity, oxygen consumption, and energy storage under different temperature regimes, to a native amphipod Gammarus pulex, found D. villosus better adapted to lower temperatures (5–10 °C) with a limited adjustment to temperatures above 20 °C, in contrast to G. pulex which was better adapted to intermediate temperatures (10–20 °C) with better adjustment potential to extreme temperatures (5–27 °C). This is contrary to what might have been expected when comparing a native to an invader as the invader appears to be less adapted to climate variation than the native. However, G. pulex as an invader in Northern Ireland is more tolerant of low water quality and low dissolved oxygen levels than the Irish native Gammarus duebeni celticus (MacNeil et al. 2004) and so it may well be this is a particularly tolerant species. What is clear is that more comparable studies need to be carried out for a range of native and resident amphipod taxa to allow us to assess the relative climate change sensitivity of each species. Moreover, Maazouzi et al. (2011) do not rule out the possibility of rapid adaptation to warmer freshwaters in D. villosus. In the same study, D. villosus had a higher level (up to 2-fold higher) of glycogen reserves than G. pulex, which could represent an adaptive metabolic strategy to deal with dramatic changes in environmental conditions, allowing D. villosus to invade harsh, unpredictable environments.
Here, we have demonstrated animal personality coupled with a clear pattern of habituation and lack of response to temperature variation in D. villosus. Further, longitudinal studies of behavior, incorporating the effects different stimulus repetition patterns, predator cues, and different physico-chemical parameters are clearly warranted, as are studies that investigate the effect of D. villosus on the behavior of native amphipods. The present evidence suggests that behavioral flexibility coupled with consistency in the face of fluctuating environmental parameters may contribute to the invasiveness of D. villosus. Longitudinal behavioral studies seem like an urgent priority for research in other invasive species as well as in the case of this “killer shrimp”.
Supplementary Material
Acknowledgments
We are grateful to four anonymous reviewers for their constructive comments on this study.
References
- Angilletta MJ, 2009. Thermal adaptation: A Theoretical and Empirical Synthesis. New York: Oxford University Press. [Google Scholar]
- Bailey RJE, Dick JTA, Elwood RW, MacNeil C, 2006. Predatory interactions between the invasive amphipod Gammarus tigrinus and the native opossum shrimp Mysis relicta. Journal of the North American Benthological Society 25:393–405. [Google Scholar]
- Bates D, Maechler M, Bolker B, Walker S, 2014. lme4: linear mixed-effects models using Eigen and S4. R package version 1.1-6. Available from: http://CRAN.R-project.org/package=lme4 [Google Scholar]
- Bolker BM, Brooks ME, Clark CJ, Geange SW, Poulsen JR, et al. , 2009. Generalized linear mixed models: a practical guide for ecology and evolution. Trends in Ecology and Evolution 24:127–135. [DOI] [PubMed] [Google Scholar]
- Briffa M, Rundle SD, Fryer A, 2008. Comparing the strength of behavioural plasticity and consistency across situations: animal personalities in the hermit crab Pagurus bernhardus. Proceedings or the Royal Society B: Biological Sciences 275:1305–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briffa M, Bridger D, Biro PA, 2013. How does temperature affect behaviour? Multilevel analysis of plasticity, personality and predictability in hermit crabs. Animal Behaviour 86:47–54. [Google Scholar]
- Brown GE, 2003. Learning about danger: chemical alarm cues and local risk assessment in prey fishes. Fish and Fisheries 4:227–234. [Google Scholar]
- Bruijs MCM, Kelleher B, van der Velde G, de Vaate AB, 2001. Oxygen consumption, temperature and salinity tolerance of the invasive amphipod Dikerogammarus villosus: indications of further dispersal via ballast water transport. Archiv fur Hydrobiologie 152:633–646. [Google Scholar]
- Carere C, Gherardi F, 2013. Animal personalities matter for biological invasions. Trends in Ecology and Evolution 28:5–6 . [DOI] [PubMed] [Google Scholar]
- Dick JTA, Platvoet D, Kelly DW, 2002. Predatory impact of the freshwater invader Dikerogammarus villosus (Crustacea:Amphipoda). Canadian Journal of Fisheries and Aquatic Science 59:1078–1084. [Google Scholar]
- Fraser DF, Huntingford FA, 1986. Feeding and avoiding predation hazard: the behavioral response of prey. Ethology 73:56–68. [Google Scholar]
- Gherardi F, Aquiloni L, Tricarico E, 2012. Behavioural plasticity, behavioural syndromes and animal personality in crustacean decapods: an imperfect map is better than no map. Current Zoology 58:567–579. [Google Scholar]
- Hudina S, Hock K, Žganec K, 2014. The role of aggression in range expansion and biological invasions. Current Zoology 60:401–409. [Google Scholar]
- Juette T, Cucherousset J, Cote J, 2014. Animal personality and the ecological impacts of freshwater non-native species. Current Zoology 60:417–427. [Google Scholar]
- Koback J, Jermacz L, Plachocki D, 2014. Effectiveness of zebra mussels to act as shelters from fish predators differs between native and invasive amphipod prey. Aquatic Ecology 48:397–408. [Google Scholar]
- Kristensen EA, Closs GP, 2004. Anti-predator response of naive and experienced common bully to chemical alarm cues. Journal of Fish Biology 64:643–652. [Google Scholar]
- Kuznetsova A, Brockhoff PB, Christensen RHB, 2014. lmerTest: tests for random and fixed effects for linear mixed effect models (lmer objects of lme4 package). R package version 2.0-6. Available from: http://CRAN.R–project.org/package=lmerTest [Google Scholar]
- Lessells CM, Boag PT, 1987. Unrepeatable repeatabilities: a common mistake. Auk 104:116–121. [Google Scholar]
- Lighton JRB, Brownell P, Joos B, Turner RJ, 2001. Low metabolic rate in scorpions: implications for population biomass and cannibalism. Journal of Experimental Biology 204:607–613. [DOI] [PubMed] [Google Scholar]
- Littell RC, Milliken G, Stroup W, Wolfinger R, Schabenberger O, 2006. SAS for mixed models. 2ndedn Cary, NC: SAS Institute Inc. [Google Scholar]
- Liu X, Guo Z, Ke S, Wang S, Li Y, 2011. Increasing potential risk of a global aquatic invader in Europe in contrast to other continents under future climate change. PLoS One 6(3):e18429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maazouzi C, Piscart C, Legier F, Hervant F, 2011. Ecophysiological responses to temperature of the ‘killer shrimp’ Dikerogammarus villosus: is the invader really stronger than the native Gammarus pulex. Comparative Biochemistry and Physiology 159:268–274. [DOI] [PubMed] [Google Scholar]
- MacNeil C, Dick JTA, Hatcher MJ, Dunn AM, 2003. Differential drift and parasitism in invading and native Gammarus spp. Ecography 26:467–473. [Google Scholar]
- MacNeil C, Prenter J, Briffa M, Fielding NJ, Dick JTA, et al. , 2004. The replacement of a native freshwater amphipod by an invader: roles for environmental degradation and intraguild predation. Canadian Journal of Fisheries and Aquatic Science 61:1627–1635. [Google Scholar]
- MacNeil C, Platvoet D, Dick JTA, 2008. Potential roles for differential body size and microhabitat complexity in mediating biotic interactions within invasive freshwater amphipod assemblages. Archive für Hydrobiologie 172(3):175–182. [Google Scholar]
- MacNeil C, Dick JTA, Platvoet D, Briffa M, 2011. Direct and indirect effects of species displacements; the invading amphipod crustacean Dikerogammarus villosus can disrupt aquatic ecosystem energy flow and function. Journal of the North American Benthological Society 30:38–48. [Google Scholar]
- MacNeil C, Boets P, Lock K, Goethals PLM, 2013. Potential effects of the invasive ‘killer shrimp’ Dikerogammarus villosus on macroinvertebrate assemblages and biomonitoring indices. Freshwater Biology 58:171–182. [Google Scholar]
- MacNeil C, Dick JTA, 2014. Different physicochemical tolerances, habitat utilization and predation drive patterns of coexistence and exclusion among invasive and resident amphipods. Freshwater Biology 59:1956–1969. [Google Scholar]
- Mazerolle MJ, 2014. AICcmodavg: model selection and multimodel inference based on (Q)AIC(c). R package version 2.0-1. Available from: http://CRAN.R-project.org/package=AICcmodavg. [Google Scholar]
- Nakagawa S, Schielzeth H, 2010. Repeatability for Gaussian and non-Gaussian data: a practical guide for biologists. Biological Reviews 85:935–956. [DOI] [PubMed] [Google Scholar]
- Nespolo R, Lardies MA, Bozinovic F, 2003. Intrapopulational variation in the standard metabolic rate of insects: repeatability, thermal dependence and sensitivity (Q10) of oxygen consumption in a cricket. Journal of Experimental Biology 206:4309–4315. [DOI] [PubMed] [Google Scholar]
- Pennuto C, Keppler D, 2008. Short-term predator avoidance behavior by invasive and native amphipods in the Great Lakes. Aquatic Ecology 42:629–641. [Google Scholar]
- Powers DA, Schulte PM, 1998. Evolutionary adaptations of gene structure and expression in natural populations in relation to a changing environment: a multidisciplinary approach to address the million-year saga of a small fish. Journal of Experimental Zoology 282:71–94. [PubMed] [Google Scholar]
- R Core Team, 2014. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing. ISBN 3-900051-07-0, available from: http://www.R-project.org/ [Google Scholar]
- Rewicz T, Grabowski M, MacNeil C, Bącela-Spychalska K, 2014. The profile of a ‘perfect’ invader: the case of the killer shrimp Dikerogammarus villosus. Aquatic Invasions 9:267–288. [Google Scholar]
- Sala OE, Chapin FS, Armesto JJ, Berlow E, Bloomfield J, et al. , 2000. Global biodiversity scenarios for the year 2100. Science 287:1770–1774. [DOI] [PubMed] [Google Scholar]
- Sandin L, Schmidt-Kloiber A, Svenning JC, Jeppesen E, Friberg S, 2014. A trait-based approach to assess climate change sensitivity of freshwater invertebrates across Swedish ecoregions. Current Zoology 60:221–232. [Google Scholar]
- Schär C, Vidale PL, Luthi D, Frei C, Häberli C, et al. , 2004. The role of increasing temperature variability in European summer heatwaves. Nature 427:332–336. [DOI] [PubMed] [Google Scholar]
- Sih A, Bell A, Johnson JC, 2004. Behavioral syndromes: an ecological and evolutionary overview. Trends in Ecology and Evolution 19:372–378. [DOI] [PubMed] [Google Scholar]
- Stamps JA, Briffa M, Biro PA, 2012. Unpredictable animals: individual differences in intra-individual variability (IIV). Animal Behaviour 83:1325–1334. [Google Scholar]
- Tomsic D, de Astrada MB, Sztarker J, Maldonado H, 2009. Behavioral and neuronal attributes of short- and long-term habituation in the crab Chasmagnathus. Neurobiology of Learning and Memory 92:176–182. [DOI] [PubMed] [Google Scholar]
- Trenberth KE. 2011, Changes in precipitation with climate change. Climate Research 47:123–138. [Google Scholar]
- Van Riel MC, van der Velde G, Rajagopal S, Marguillier S, Dehairs F, et al. , 2006. Trophic relationships in the Rhine food web during invasion and after establishment of the Ponto-Caspian invader Dikerogammarus villosus. Hydrobiologia 565:39–58. [Google Scholar]
- Wolf M, Weissing FJ, 2012. Animal personalities: consequences for ecology and evolution. Trends in Ecology and Evolution 27:452–461. [DOI] [PubMed] [Google Scholar]
- Wright TF, Eberhard JR, Hobson EA, Avery AL, Russello MA, 2010. Behavioral flexibility and species invasions: the adaptive flexibility hypothesis. Ethology Ecology and Evolution 22:393–404. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.