Abstract
Since reconciliation was first described more than 20 years ago, a large number of postconflict behaviors have been observed among females in many polygynous primate species. However, few studies have been conducted among males, perhaps due to the rarity with which they maintain friendly relationships with one another and their aggressive competition for resources. Although this is true for many primate males, Sichuan snub-nosed monkeys Rhinopithecus roxellana represent a potential exception as male cooperation has been known to occur. In this study, using postconflict/matched-control(PC–MC) and time-rule methods, we analyzed postconflict behavior among males and the possible occurrence of bystander affiliation or reconciliation. A total of 246 PC–MC pairs among leader males were obtained. On average, each leader male exhibited only 0.04 aggressive behaviors per observation hour, and conciliation among leader males occurred at a low rate (2.03%) relative to other primate species. The occurrence of consolation–affiliation interactions between focal males and group members other than former opponents differed significantly between PCs and MCs, which is the first time this has been confirmed among R. roxellana males. We discuss the results in light of recent theories concerning consolation in primates. The patterns of postconflict contact demonstrated that R. roxellana may be a unique species among colobines.
Keywords: Rhinopithecus roxellana, reconciliation, consolation, leader male
Reconciliation repairs damage to relationships, reduces behavioral indicators of anxiety, and the risk of aggression (Aureli et al. 2002), and is defined as friendly contact between former opponents in the minutes immediately following conflict (Aureli and de Waal 2000). Since de Waal and van Roosmalen’s (1979) research concerning postconflict behavior in chimpanzees, a large number of studies have demonstrated the occurrence of reconciliation in primate species (Majolo et al. 2005). Very few studies, however, have examined other forms of postconflict contact (i.e., bystander affiliation) or those that involve third parties (i.e., individuals not involved in the former aggression).
Bystander affiliation may be effective at mediating the costs of conflict (Fraser et al. 2009), preventing further hostility from aggressors and restoring relationships between former opponents (de Waal 1977). It is defined as the exchange of friendly contact between an opponent and other group members soon after conflict (Judge 1991). Depending on the identity of the initiator, bystander affiliation can be a form of consolation (bystander-initiated), and can be either redirected affection or postconflict contact initiated or solicited by a former opponent (Arnold and Barton 2001). Redirected affection and solicited postconflict contact, wherein the aggressor actively affiliates with a third party, were first observed in rhesus macaques (de Waal and Yoshihara 1983). Consolation is another form of bystander affiliation (de Waal and Aureli 1996), and is initiated by an uninvolved group member and directed to a victim of conflict. Consolation, like reconciliation, may alleviate the recipient’s distress (de Waal and Aureli 1996), especially if initiators are the opponents’ kin (Aureli and van Schaik 1991), and may establish the potential for short-term coalitions and so reduce the risk of further aggression for both the consoler and the victim of aggression. This behavior was first observed in chimpanzees (de Waal and Aureli 1996) and more recently in stump-tailed macaques (Call et al. 2002).
de Waal and Aureli (1996) proposed two hypotheses to account for consolatory behavior. First, the social-constraints hypothesis, which states that consolatory behavior may be more advantageous or less risky in primate species whose organization is not strictly hierarchical and whose levels of social tolerance are higher, indicating the occurrence of consolation may be related to variations in dominance style and social plasticity. Second, the social-cognition hypothesis, which states that, if a primate possesses cognition beyond a certain threshold, consolatory behavior may be observed. Although consolation has been examined in cercopithecidae and chimpanzees, few studies have been conducted on colobines (Arnold and Barton 2001).
Possessing a multi-level social structure, Rhinopithecus roxellana groups are composed of one-male units (OMUs) and associated all-male units (AMUs) (Qi et al. 2010). The OMU is the basic reproductive unit consisting of one breeding leader male, a number of adult females, and their offspring. Affliation behaviors or noticeable cooperative behaviors are often observed between leader males and members within the OMU (Zhang et al. 2012). Although leader males belong to different OMUs, affliation interactions or cooperative acts among leader males sometimes occur when males defend their females or food resources or repel other males from immigration into the unit (Zhao and Li 2009; Li and Zhao, 2007). While a series of studies on affiliation and conflict interaction have been conducted, only one study on R. roxellana has analyzed the possible occurrence of postconflict behavior, with the focus on reconciliation among females (Zhang et al. 2010 ). In the present study, we examined whether a free-ranging, provisioned group of the relatively egalitarian R. roxellana (He et al. 2013) exhibited reconciliation or affiliation behaviors involving third parties.
Based on social structure characteristics of R. roxellana and recent theories concerning postconflict behavior in primates, we predicted the following in regards to postconflict behavior among leader males. First, it is generally believed that each R. roxellana OMU represents a socially cohesive unit that engages in few direct interactions with other OMUs, as observed in other multi-level society primates (Qi et al. 2014). Thus, we predicted that the occurrence of conflicts and conciliatory tendencies among leader males should be relatively low. Second, leader males, as the only males breeding in each OMU, will often protect and support their females during a conflict and have a far strong bond with them (He et al. 2013). Initial research has also revealed that social dominance rank in males is conspicuous (Li et al. 2006), but that of adult individuals within an OMU is weak (He et al. 2013). Thus, we predicted that bystander affiliation (redirected affection, solicited postconflict contact, or consolation) after a conflict involving females should be often observed, while reconciliation and bystander affiliation involving other leader males should be limited or absent. Third, each OMU is an independent social reproduction unit in this polygynous species (Li and Zhao 2007). For leader males, mating competition is not influenced by season. Thus, we predicted that differences in conciliatory tendency among males in the mating season and nonmating season should be negligible.
Materials and Methods
Subjects and study site
Our R. roxellana study was conducted near Yuhuangmiao village (108°14'–108°14'E, 33°45'–33°50'N) in Zhouzhi National Nature Reserve, a 52 931 km2 area of temperate forest on the northern slopes of the Qinling Mountains in Shaanxi Province, China (Li et al. 2000). Rhinopithecus roxellana are found on the eastern and western slopes of the mountain along the Nancha River and consist of the West Ridge Group and East Ridge Group. Our study was carried out on the free-ranging, provisioned West Ridge Group. Eleven leader males from eleven OMUs were considered as the focal subjects in this study (Table 1). All individuals were identifiable via their prominent physical features.
Table 1.
Basic information of the focal units during observation periods
| Resident male | LP | JB | BZT | RX | DB | PK | FP | HT | JZT | SY | BB |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Female |
|
|
|
|
|
|
|
|
|
|
Full-AF (undecided) |
| Sub-adult Female |
|
||||||||||
| Juvenile |
|
|
|
|
|
|
GX |
|
J 3.5 years |
|
|
|
| |||||||||||
| Total | 10 | 10 | 11 | 6 | 11 | 8 | 5 | 3 | 6 | 4 | >5 |
Food provisioning
To aid behavioral observation, a provision site was established in October 2001 at Sanchakou (1646 masl) in Gongnigou valley, located within the home range of the West Ridge Group such that the group could also forage normally. Every morning at 9:00 am, field assistants searched for and herded the monkeys to the provision site (Zhao et al. 2008). Food was provided three times per day at 9:00 am, 12:00 am, and 2:00 pm, and consisted of apples, radishes, and corn. On average, approximately 200 g of food were provided per monkey per day, which is a small proportion of their total daily food consumption (Qi 1989; Li and Zhao 2007). After feeding and resting briefly, the focal individuals left the provision site on their own initiative and moved to the adjacent trees and surrounding area, after which we began our observations. We observed monkeys from a typical distance of between 0.5 m and 50 m.
Data collection
From September 2011 to June 2012, the study group was observed for 560 h. We considered agonistic interaction as the display of an aggressive behavior by an individual (biting, fighting, chasing, threatening, and supplanting) followed by a response from the target individual (avoiding, crouching, and retreating). For each conflict, we identified the aggressor and recipient. We collected spontaneously occurring agonistic conflicts by behavioral sampling (Martin and Bateson 2007), avoiding the influence of provisioned food, and recorded reconciliation and bystander affiliation among leader males via the postconflict/matched-control (PC–MC) method (de Waal and Yoshihara 1983). Reconciliation and bystander affiliation included embrace, hold-lumbar, hold-hand, crouch, contact sit, open-mouth, and grooming (Zhang et al. 2010).
The PC observation of the target individual and aggressor lasted for 10 min and was started ≤30 s after the conflict between the two leader males. If more than two leader males were involved in the conflict, we only recorded the first two opponents; the number of aggressors and target individuals were balanced. A 10-min MC was made on the next possible day, following the same focal individuals. If the target individuals were involved in a conflict within 3 min before a planned MC or in the first 3 min of an ongoing MC, or if the two target individuals were not observed in our visual field at the same time, we postponed the session for ≥10 min (Aureli et al. 1993). This method has been commonly used in postconflict behavioral studies (Majolo and Koyama 2006).
Data analysis
Data on reconciliation and bystander affiliation among leader males were analyzed via the PC–MC method (de Waal and Yoshihara 1983) and the time-rule method (Aureli et al. 1989). For PC–MC, the timings of the first affiliative interactions involving targets or aggressors were taken into account. The PC–MC pair were considered “attracted” if the affiliative interaction occurred only or earlier in the PC than in the MC periods; the pair were considered “dispersed” if the affiliative interaction took place only or earlier in the MC than in the PC; and the pair were considered “neutral” if the affiliative interaction occurred at the same time in the PC and MC periods or when no contact took place in either observation. Using the Wilcoxon matched-pairs signed-rank test, a skew in favor of attracted pairs indicated that reconciliation was performed between former opponents. In addition, based on the Wilcoxon matched-pairs signed-rank test, affiliative contact between the target individual and a group member other than the former opponent was compared between PCs and MCs to determine the occurrence of consolation. Conciliatory tendencies were calculated for each target animal via Veenema et al. (1994) as follows: 100 × number of (attracted pairs – dispersed pairs)/all pairs.
The time-rule method was used to determine the timing of reconciliation and bystander affiliation (Aureli et al. 1989), while the Kolmogorov–Smirnov test was used to compare contact distribution over time between the PC and MC periods.
Results
A total of 246 PC–MC pairs were analyzed (mean number of PC–MC pairs per male ± SE: 22.36 ± 4.69), including 178 in the mating season and 68 in the nonmating season. The overall frequency of conflict was low, only 0.04 aggressive behaviors per male per observation hour. Among those PC–MC pairs, 1.22% of the conflicts were polyadic, while the remaining 98.78% were dyadic; 64.23% of the observed conflicts had a clear-cut result, whereas 35.77% of conflicts were undecided.
Reconciliation was not demonstrated using the PC–MC method because the percentage of attracted pairs was not significantly higher than that of dispersed pairs (attracted pairs: Mean ± SE: 0.03 ± 0.01; dispersed pairs: Mean ± SE: 0.00 ± 0.00; Wilcoxon signed ranks test: n = 11, T = 6, P > 0.05). For consolation, however, the percentage of attracted pairs was significantly higher than that of dispersed pairs (attracted pairs: Mean ± SE: 0.87 ± 0.04; dispersed pairs: Mean ± SE: 0.09 ± 0.03; Wilcoxon signed ranks test: n = 11, t = 3, P < 0.001). Moreover, we observed redirected aggression and solicited postconflict contact following conflicts only 5 times during the PC observations. Former opponents solicited affiliation (e.g., hold-lumbar) in 20.00% of the 5 attracted PCs–MCs, in which the victim solicited help from one female within the OMU to renew aggression towards the aggressor; while former opponents initiated aggression with group members in the remaining 80.00%, including the aggressor redirecting aggressive behavior towards the victim’s OMU females twice, the victim redirecting aggressive behavior with another leader male once and with the aggressor’s OMU females once. The average conciliatory tendency of all focal individuals was 2.03%, and the conciliatory tendency in the mating season (1.69%) was not significantly lower than that in the nonmating season (2.94%).
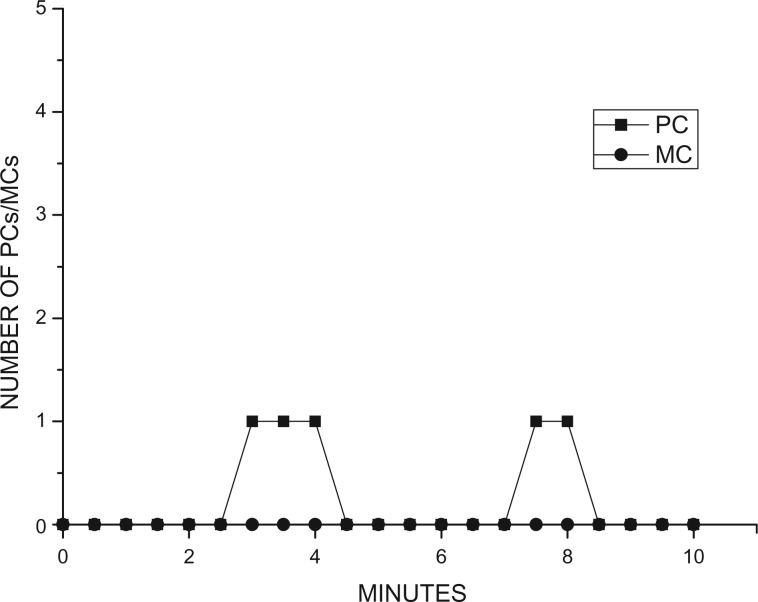
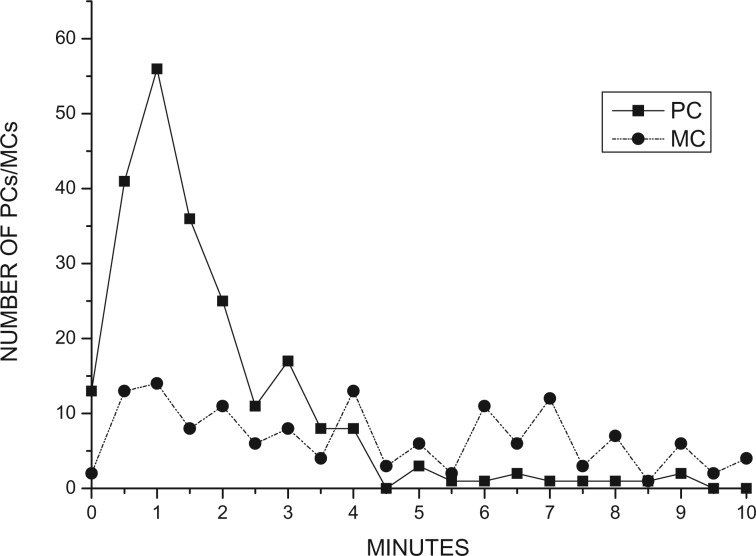
According to the time-rule method, there were insufficient cases of reconciliation, redirected affection, or solicited postconflict contact for the performance of a two-sample Kolmogorov–Smirnov test. Therefore, we could not compare the distribution over time of the first affiliative contact between former opponents in the PC periods with distribution in the MC periods (Figure 1). It was predicted that focal subjects were not expected to preferentially affiliate with former opponents in either the PC or MC periods. However, we confirmed significant differences in the distribution over time of consolation in the PC and MC periods (Kolmogorov–Smirnov test: D = 0.44, P < 0.001) (Figure 2), indicating that consolations involving leader males were performed and victims often received consolation from other members within their OMU following conflicts. In the field, we found this consolation was only initiated from the victim’s OMU females, not by other leader males.
Figure 1.
Number of PC periods and MC periods with affiliative contact of former opponents initiated or solicited in each minute (1–10 min).
Figure 2.
Number of PC periods and MC periods with consolation in each minute (1–10 min).
Discussion
This study provides the first data on reconciliation and bystander affiliation following conflict in free-ranging R. roxellana leader males. Results showed that the conciliation tendency among leader males was unexpectedly lower than those found among males in other primates species [e.g., 31% in Japanese macaques (Majolo 2005)], indicating that R. roxellana leader males exhibited more unattached relationships than those observed in some other species. On the whole, we confirmed the existence of bystander affiliation, but not the occurrence of reconciliation among leader males, which differs from other conciliatory colobine studies (Ren et al. 1991; Arnold and Barton 2001).
Postconflict behavior in free-ranging R. roxellana leader males follows a different pattern to that observed in females, even though the frequencies of aggression among males and among females are both low (Zhang et al. 2010); affiliative contact between former opponents involving another leader male occurred infrequently during the minute immediately after conflict, neither victims nor aggressors took the initiative to reconcile, and no another leader male initiated affiliation contact with the victim. These phenomena might be restricted to the social roles and functions of R. roxellana leader males. The leader males belonged to different social units as each OMU had an independent social system. Additionally, dominance rank order existed among the different units in our focal group, with each leader male able to recognize his own status in a short period of time (Li et al. 2006). These factors reduce the importance of reciprocal altruism in male behavior. Unlike females, males rarely try to establish long-lasting amicable relationships with each other (Ren et al. 1991), and consequently R. roxellana might be considered a nontolerant species with regard to relationships among leader males.
In contrast, consolation soon after conflict between victims of aggression and other group members indicated that females within their OMU often take the initiative to console victims, which favors the social-constraints hypothesis rather than the social-cognition hypothesis for this species. This may be related to social relationships and dominance style among adult individuals, or as a way in which to avoid further hostility from the aggressor toward the victim or to distract the attention of former opponents. In reconciling R. roxellana, leader males usually maintain an intimate relationship and friendly bonds with the adult females within their OMUs, and females frequently support their leader male during fights because he is the only male involved in OMU reproduction (Ren et al. 1991). To increase unit cohesion and repel challenges from other males, leader males, and females actively express their friendly relationship in cooperation or following conflict to each other (Ren et al. 1991). Thus, it is understandable that redirected affection and solicited postconflict contact occurred occasionally in this study. Former opponents initiating or soliciting friendly contact from group members might also mediate the costs of conflict and restore the tolerance of former opponents, which may be similar to that found in rhesus macaques (de Waal and Yoshihara 1983).
To date, this study is the first-reported instance of consolation in a colobine species, and is similar to results on stump-tailed macaques (Call et al. 2002) and chimpanzees (de Waal and Aureli 1996). Compared to chimpanzees (de Waal and Aureli 1996), R. roxellana cognition is lower, and falls below the threshold wherein empathetic and sympathetic responses occur (Fu et al. 2013). However, its social organization, like that of stump-tailed macaques (Call et al. 2002), is not as strictly hierarchical as other primate species and levels of social tolerance are higher (He et al. 2013). This may explain why consolation evolved in chimpanzees, stump-tailed macaques, and snub-nosed monkeys, but not in other primates. Furthermore, although R. roxellana is a seasonal breeder, no significant differences in conciliation between mating and nonmating seasons were observed. Because each OMU is relatively independent, social relationships might not be endangered by mating opportunity and might not disrupt the friendly bonds between males and females within OMUs. This suggests that the effect of season on affiliative interactions involving leader males may be very small.
In the present free-ranging R. roxellana study, former opponents engaged in affiliative interactions with group members at relatively higher rates during postconflict periods than during matched periods. As a bystander affiliative behavior, hold-lumbar was most often initiated by group members within the OMU to the victim of male–male aggression without the risk of reprisal, which has not been reported previously in colobine species. The function of this behavior may accord with previous research suggesting that consolatory begging gestures in chimpanzees are used to solicit support from other group members (de Waal and van Hooff 1981). However, the function and significance of bystander affiliation have not been demonstrated in R. roxellana. In particular, it remains unclear whether consolatory behavior after conflict is a case of emotional contagion or whether it is a response to a signal of intent or desire. Future studies should focus on the emotional change involved in affiliation interactions after conflict in free-ranging R. roxellana.
Supplementary Material
Acknowledgments
This study was supported by the Key Program of the National Natural Science Fund (31130061); National Nature Science Foundation of China (31572278; 31470214; 30970379); Foundation of Shaanxi Academy of Sciences, China (2014K-12; 2013K-34; 2013K-35; 2012K-01); Open Foundation of Key Laboratory of Resource Biology and Biotechnology in Western China of Ministry of Education (ZS14002); Western Light Talent Culture Project (2011DF05); and Cosmo Oil Eco Card Fund of Japan (2005–2015). We thank the staff of Zhouzhi National Nature Reserve for their cooperation and permission to conduct this research. We also thank the local farmers for support and assistance; Dapeng Zhao, Wen Wen Zhu, Wei Wei, Kang Huang, Weiwei Fu, Dong Zhang, Linlin Wu, and Hongyang Zhang for assistance with field observations.
Author Contributions
Haitao ZHAO and Xiaowei WANG have equal contribute to this article.
References
- Arnold K, Barton RA, 2001. Postconflict behavior of spectacled langurs Trachypithecus obscurus. Reconciliation. International Journal of Primatology 22:243–266. [Google Scholar]
- Aureli F, Cords M, van Schaik CP, 2002. Conflict resolution following aggression in gregarious animal: a predictive framework. Animal Behaviour 64:325–343. [Google Scholar]
- Aureli F, van Schaik CP, van Hooff JARAM, 1989. Functional aspects of reconciliation among captive long-tailed macaques Macaca Fascicularis. American Journal of Primatology 19:39–51. [DOI] [PubMed] [Google Scholar]
- Aureli F, van Schaik CP, 1991. Post-conflict behavior in long-tailed macaques Macaca fascicularis). I. The social events. Ethology 89:89–100. [Google Scholar]
- Aureli F, Veenema HC, van Eck CJ, van Hooff JARAM, 1993. Reconciliation, consolation and redirection in Japanese macaques Macaca fuscata. Behaviour 124:1–21. [Google Scholar]
- Call J, Aureli F, de Waal FBM, 2002. Postconflict third party affiliation in stump-tailed macaques. Animal Behavior 63:209–216. [Google Scholar]
- de Waal FBM, 1977. The organisation of agonistic relations within two captive groups of Java monkeys Macaca fascicularis. Zeitschrift fur Tierpsychologie 44:225–282. [DOI] [PubMed] [Google Scholar]
- de Waal FBM, Aureli F, 1996. Consolation, reconciliation and a possible difference between macaques and chimpanzees. In: Russon AE, Bard KA, Parker ST, editors. Reaching into thought: the minds of great apes. Cambridge: Cambridge University Press; p, 80–110. [Google Scholar]
- de Waal FBM, van Hooff JARAM, 1981. Side-directed communication and agonistic interactions in chimpanzees. Behaviour 77:164–198. [Google Scholar]
- de Waal FBM, van Roosmalen A, 1979. Reconciliation and consolation among chimpanzees. Behavioral Ecology and Sociobiology 5:55–66. [Google Scholar]
- de Waal FBM, Yoshihara D, 1983. Reconciliation and redirected affection in rhesus monkeys. Behaviour 85:224–241. [Google Scholar]
- Fu WW, Zhao DP, Qi XG, Guo ST, Wei W, et al. , 2013. Free-ranging Sichuan snub-nosed monkeys Rhinopithecus roxellana: Neophobia, neophilia, or both? Current Zoology 59:311–316. [Google Scholar]
- He HX, Zhao HT, Qi XG, Wang XW, Guo ST, et al. , 2013. Dominance rank of adult females and mating competition in Sichuan snub-nosed monkeys Rhinopithecus roxellana in the Qinling Mountains, China. Chinese Science Bulletin 58(18):2205–2211. [Google Scholar]
- Fraster ON, Koshi SE, Wittig RM, Aureli F, 2009. Why are bystanders friendly to recipients of aggression? Integrative Comparative Biology 2:285–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judge PG, 1991. Dyadic and triadic reconciliation in pigtail macaques Macaca nemestrina. American Journal of Primatology 23:225–237. [DOI] [PubMed] [Google Scholar]
- Li BG, Zhao DP, 2007. Copulation behavior within one-male groups of wild Rhinopithecus roxellana in the Qinling Mountains of China. Primates 48:190–196. [DOI] [PubMed] [Google Scholar]
- Li BG, Chen C, Ji WH, Ren BP, 2000. Seasonal home range changes of the golden snub-nosed monkey Rhinopithecus roxellana in Qinling Mountains of China. Folia Primatology 71:375–386. [DOI] [PubMed] [Google Scholar]
- Li BG, Li HQ, Zhao DP, Zhang YH, Qi XG, 2006. Study on dominance hierarchy of the Sichuan snub-nosed monkey Rhinopithecus roxellana in Qinling Mountains. Acta Theriologica Sinica 26:18–25. [Google Scholar]
- Majolo B, Koyama N, 2006. Seasonal effects on reconciliation in Macaca fuscata yakui. International Journal of Primatology 27:1383–1397. [Google Scholar]
- Majolo P, Ventura R, Koyama N, 2005. Postconflict behavior among male Japanese macaques. International Journal of Primatology 26:321–336. [Google Scholar]
- Martin P, Bateson PG, 2007. Measuring behaviour: an introductory guide. Cambridge: Cambridge University Press. [Google Scholar]
- Qi JF, 1989. The feed and reproduction of the Sichuan snub-nosed monkeys. In: Chen FG, editor. Progress in the study of Sichuan snub-nosed monkeys. Xi’an: Northwest University Press; p. 287–292. [Google Scholar]
- Qi XG, Garber PA, Ji WH, Huang ZP, Huang K, et al. , 2015. Satellite telemetry and social modeling offer new insights into the origin of primate multilevel societies. Nature Communications 5:5296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi XG, Zhang P, Li BG, Watanabe K, 2010. The diversity of polygynous social systems among multi-level societies in non-human primates. Acta Theriologica Sinica 30:322–338. [Google Scholar]
- Ren RM, Qi H, Liang B, Bao W, de Waal FBM, 1991. The reconciliation behavior of golden monkeys Rhinopithecus roxellanae in small breeding groups. Primates 32:321–327. [Google Scholar]
- Veenema HC, Das M, Aureli F, 1994. Methodological improvements for the study of reconciliation. Behavioral Process 31:29–37. [DOI] [PubMed] [Google Scholar]
- Zhang J, Zhao DP, Li BG, 2010. Postconflict behavior among females Rhinopithecus roxellana within one-male units in the Qinling Mountains, China. Current Zoology 56:222–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Li BG, Qi XG, Maclntosh AJJ, Watanabe K, 2012. A proximity-based social network of a group of Sichuan snub-nosed monkeys Rhinopithecus roxellana. International Journal of Primatology 33:1081–1095. [Google Scholar]
- Zhao DP, Li BG, 2009. Do deposed adult male Sichuan snub-nosed monkeys Rhinopithecus roxellana roam as solitary bachelors or continue to interact with former band members? Current Zoology 55(3):235–237. [Google Scholar]
- Zhao DP, Ji WH, Li BG, Watanabe K, 2008. Mate competition and reproductive correlates of female dispersal in a polygynous primate species Rhinopithecus roxellana). Behavioral Process 79:165–170. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.