Abstract
A closed-loop teleprompter system was used to isolate and manipulate social interactivity in the natural courtship interactions of pigeons Columbia livia. In Experiment 1, a live face-to-face real-time interaction between 2 courting pigeons (Live) was compared to a played back version of the video stimulus recorded during the pairs Live interaction. We found that pigeons were behaving interactively; their behavior depended on the relationships between their own signals and those of their partner. In Experiment 2, we tested whether social interactivity relies on spatial cues present in the facing direction of a partner’s display. By moving the teleprompter camera 90° away from its original location, the partner’s display was manipulated to appear as if it is directed 90° away from the subject. We found no effect of spatial offset on the pigeon’s behavioral response. In Experiment 3, 3 time delays, 1 s, 3 s, and 9 s, a Live condition, and a playback condition were chosen to investigate the importance of temporal contiguity in social interactivity. Furthermore, both opposite-sex (courtship) and same-sex (rivalry) pairs were studied to investigate whether social-context affects social interactivity sensitivity. Our results showed that pigeon courtship behavior is sensitive to temporal contiguity. Behavior declined in the 9 s and Playback conditions as compared to Live condition and the shorter time delays. For males only, courtship behavior also increased in the 3-s delay condition. The effect of social interactivity and time delay was not observed in rivalry interactions, suggesting that social interactivity may be specific to courtship.
Keywords: courtship behavior, pigeons, social interactivity, spatial offset, time delay, video playback.
Non-verbal social interaction is ubiquitous in nature. From defense and foraging to courtship and mating, visual communications play a key role in survival and reproduction. To date, the study of visual, non-verbal social interaction has generally focused on investigating the meaning of the discrete behavioral actions and gestures as sources of communication (e.g., Wosegien and Lamprecht 1989). Although this approach is valuable, visual interaction is a dynamic and continuously unfolding process, and may contain other types of information. The spacing, timing, and statistical relationships between visual signals are potentially rich sources of information in a complex social world.
Social interactivity can be defined by the degree to which social actions depend on the social signals immediately preceding it, as well as the entire series of preceding signals and the relationships between them (Fong et al. 2003). Four levels of social interactivity can be described: non-interactive behavior is unrelated to other behaviors, socially reactive behavior is “released” by a social stimulus but is not necessarily communicative in function (Tinbergen 1952), socially responsive behavior functions as a communicative reply to a preceding social signal (Tinbergen 1962), and interactive behavior may be influenced, not only by the stimuli in the signals immediately preceding it, but by a number of previous signals as well as the relationships between them (Fong et al. 2003).
Ethologists studying vocal interactions have used interactive playback methods, where an audio recording is played back in relation to the subjects own behavior in order to simulate natural back and forth interactions. As a result, the ethology and neuroscience of social interactivity in the vocal domain has undergone a surge of progress (e.g., Mennill et al. 2002; Burt and Beecher 2008; Kao et al. 2008). In contrast, interactive phenomena between visual, non-verbal cues during social interaction are much less understood. Although there are unique challenges to studying social interactivity in the visual domain, in order to fully understand social interaction, it is essential to gain an understanding of the structural patterns underlying visual dialogues.
In the field of video playback research, where video has been developed as a tool to study visual communication in non-human animals, the importance of social interactivity has become a reoccurring point of discussion (Kodric-Brown and Nicoletto 1997; Patton et al. 2009). By using video images in place of live-acting social partners, ethologists can vary and control, at least to some degree, the dynamic features of social signals. By observing a subject’s natural reaction toward these stimuli, one can begin to understand the complex meaning of visual signals. These methods have been vital in building knowledge about a broad range of interests, including multimodal signaling (O'Loghlen and Rothstein 2010, 2012), sexual selection (Moravec et al. 2010), group influences on behavior (Rieucau and Giraldeau 2009) social preference behavior (Bird and Emery 2008), and behavioral plasticity and development (Balsby and Dabelsteen 2002). Video playback methods have been employed for studying visual communications in a diverse variety of animal species, including invertebrates (Aizawa 1998), fish (Rosenthal et al. 1996), reptiles (Van Dyk and Evans 2008), and birds (Bird and Emery 2008).
One problem of video playback methods is that pre-recording behavior necessarily precludes the formation of natural reciprocity and responsiveness between the subject and the partner displayed on video. Any social signal under investigation is necessarily stripped of the social reactions and responses that it is likely to elicit in a truly interactive setting. In destroying the natural relationships between reciprocal signals, the meaning of the signal under investigation may change.
Several researchers using video playback to study visual communication in animals have noted that subject behavior differs in response to conspecifics presented on video as compared to live conspecifics presented through clear glass (Macedonia et al. 1994; D’eath and Dawkins 1996; Kodric-Brown and Nicoletto 1997; Fleishman and Endler 2000; Oliveira et al. 2000; Swaddle et al. 2006). For instance, when viewing conspecifics across clear glass, hens Gallus domesticus spend more time in proximity with familiar cage mates than with strangers. However, if the same familiar and unfamiliar conspecifics are shown on video, hens show no such preference (D’eath and Dawkins 1996). Female zebra finches Taeniopygia guttata prefer their mates over other males across clear glass, but behave indiscriminately toward these stimuli if they are presented as a video playback (Swaddle et al. 2006). Male Anolis lizards are equally aggressive toward males of their own species and males of other species, but when intraspecific and interspecific opponents are displayed as video playback stimuli male lizards suddenly aggress more toward the members of their own species (Macedonia et al. 1994). Female swordtail fish Xiphophorus helleri prefer males with long tails to a greater extent if these males are shown as playback stimuli than if the males interact live across clear glass (Trainor and Basolo 2000). Together, these findings suggest that animals are behaving interactively. When signal relations that typify partner responsiveness are stripped from the video playback, subject behavior changes.
Of course, interactive behavior is only one of many possible explanations for differences in behavioral response toward the video image of a partner and a real partner presented live across glass. Other differences in the stimulus conditions that could account for the behavioral effects include the properties and visual quality of the display in terms of parameters such as color, luminance, depth, flicker (reviewed in D’eath 1998; Fleishman and Endler 2000; Oliveira et al. 2000; Schlupp 2000), and motion quality (Ware et al. 2015) as well as the lack or mis-presentation of other sensory qualities such as olfactory and auditory cues. Additionally, video playback partners are usually not filmed exactly as would occur during the live face-to-face interaction in the clear glass condition. Thus, partners presented on video may not exhibit as much subject directed and motivated behavior as live partners do. Ultimately, the clear glass/playback comparison confounds the question of whether social interactivity influences behavior with the effects of the reduced realism of screen displays.
The possibility that pigeon behavior is sensitive to the relationships between their own signals and those of their social partner has received mixed support (Friedman 1977; Shimizu 1998; Toda and Watanabe 2008; Patton et al. 2009). Friedman (1977) showed that the growth of the female ring dove’s (Streptopelia risoria, a close relative of the pigeon) reproductive physiology (specifically their follicular development) was found to depend on the female’s ability to interact with a male partner. Friedman arranged female doves around a single male to engage in courtship and nest soliciting interactions. One of these females viewed the male through clear glass so that the male could react and respond interactively toward her and another female viewed the male through 1-way glass, so that she could see him but he could not see or respond to her. Thus both females received the same visual cues from the male, but the potential for social interactivity to develop in their communication with the male differed between them. The male’s orientation toward these 2 female subjects may have also been different. The results showed that females viewing a male across clear glass had greater follicular development than the females viewing a male across 1-way glass. In this case, social interactivity appeared to impact the female’s reproductive development. However, it is also possible that these physiological effects were due to the differences in male facing direction that each female experienced during the interaction, rather than by social interactivity alone.
Shimizu (1998) presented male pigeons with a female partner shown either through clear glass or as a video playback stimulus on a screen. The pigeons’ behavioral responses did not show evidence of being socially interactive: the number of bows and coos the male exhibited did not differ between conditions. Yet, in another study with the exact same experimental conditions that also used brain-imaging techniques, the “visual association areas” in the male’s brain appeared to discriminate between females presented across clear glass and female partners displayed on video (Patton et al. 2009). Note, however, that these studies also confounded effects of the social interactivity manipulation with potential effects of the 2 different display methods. Nonetheless, these findings corroborate those of Friedman (1977), described above, showing evidence that social interactivity affects the females on a physiological level in the ring dove.
The current work uses a method of testing the effects of social interactivity on behavior that achieves both experimental control and a high degree of naturalness. We use a double closed-loop teleprompter interface. This paradigm was pioneered in work studying human infant–mother interactions and is an established way to conduct a controlled manipulation of social interactivity (Murray and Trevarthen 1985; Muir and Hains 1999; Nadel et al. 1999; Bigelow and DeCoste 2003; Striano et al. 2006). The setup enables 2 subjects to see each other in real time from viewpoints that mimic the position of the respective other bird (Live condition). Recordings of the footage which was streamed to the other bird in this Live condition can be later played back in the Playback condition. Being aware that we compromise some of the naturalness as a consequence of using video technology, we take care that the degree of image degradation is the same in all conditions of our experiments.
Using the double closed-loop teleprompter interface, we present 3 experiments. In Experiment 1, we compared pigeons’ courtship responses across the Live and Playback conditions to investigate whether pigeons were behaving interactively.
Finding that this manipulation significantly affects courtship behavior, in Experiment 2 we further investigated the effect of spatial offset of the partner’s image from the veridical viewpoint of the subject bird. In Experiment 2, the partner’s facing direction in relation to the location of the subject bird was varied by changing the angle of the horizontal camera position by 90°. So, if courtship behavior is directed at all, then the partner would appear to direct its courtship 90° away from the subject. In addition, we cross the experimental factor of facing direction with the manipulation of social interactivity, using Live and Playback conditions from Experiment 1. This design can assess the influence of facing direction and signal relations, both separately and in combination, on pigeon courtship behavior.
In Experiment 3, we also tested the effect of temporal contiguity, testing how sensitive pigeons are to various time delays in the transmission of signals between their own actions and those of their partner. Having shown that social interactivity does influence courtship behavior in Experiments 1 and 2, we also used Experiment 3 to investigate if these findings generalize to another social behavior, same-sex rivalry interactions. In the third experiment we manipulate the temporal contiguity of pigeon interactions by comparing circle walking behavior across Live, 1-s delay, 3-s delay, 9-s delay and Playback conditions. The 3 time delays were chosen to approximate the duration of visual signals in the pigeon’s behavioral repertoire (the bow, the circle and the circle walking bout, respectively). In addition, by staging both same-sex and opposite-sex interactions in Experiment 3, we also test the pigeon’s sensitivity to temporal manipulations across 2 different social contexts, courtship and competitive rivalry interactions. This design can assess the pigeon’s sensitivity to the timing of signal relations in both opposite-sex and same-sex interactions.
Materials and Methods
Subjects
Eighteen male and female homing pigeons Columbia livia were selected from a pigeon aviary, 23 m2, containing a colony of 70 pigeons assembled from racing breeders in the Kingston (Ontario, Canada) area. All birds were between 3 and 14 years old. Birds were selected to participate in the study if they exhibited active courtship and maintained it under experimental conditions. Beginning 2 weeks prior to the study, the subjects were housed individually in standard steel rabbit cages (60 × 46 × 40 cm) so that they were visually isolated from the other birds in the room. Birds were kept on a feed of cracked corn and standard pigeon grains. Their light cycle was kept such that it approximated Eastern Ontario natural dawn–dusk light cycle. All experimental protocols had been approved by the Queen’s University Animal Care Committee.
Apparatus
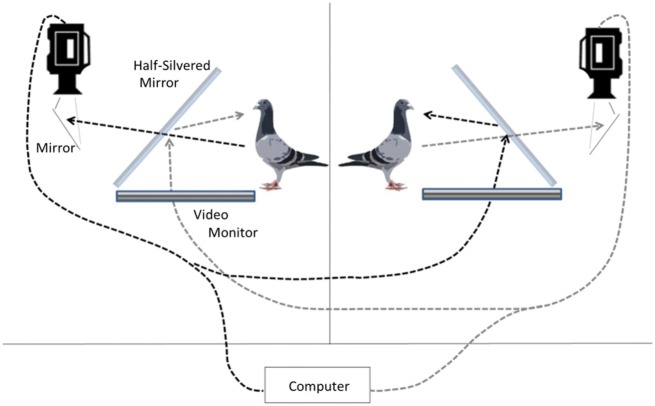
The double closed-loop teleprompter setup, shown in Figures 1 and 2, consisted of 2 teleprompter setups in 2 different rooms. Each consisted of a 19” Samsung LCD Syncmaster 1,701 monitor which laid flat and faced up toward a half-silvered mirror (64 × 55 cm) mounted at a 45° angle with respect to the horizontal plane. A Sony Handycam video camera (National Television System Committee (NTSC), frame rate: 30 fps interlaced) was placed directly behind the half-silvered mirror and fixed so that it pointed vertically downwards inside the teleprompter, filming the subject bird at eye level by way of a small mirror placed a few inches below the camera (45° to the camera and 45° to the bird). The purpose of this mirror was to compensate for the mirror flip that occurs on the half-silvered glass when the video image is projected. The teleprompter was housed in an aluminum frame (60 cm wide × 64 cm tall × 67 cm long). To make the interior of the teleprompter dark, black Choroplast plastic board was used to cover the top and the sides of the apparatus. This ensured that the bird saw only the reflection of the other bird on the half-silvered mirror and could not see the interior of the teleprompter where the video camera was housed. The same black Choroplast board was used as a background placed behind each stimulus bird as it was being filmed. While in the teleprompter setup, the subjects were placed in 46 × 46 × 46 cm cages made of a thin steel framing that was covered with mist netting on all sides. The camera and teleprompter setup were calibrated to make the partner’s image appear life-size. The final image was projected approximately 80 cm away from each pigeon, as measured at eye level to the approximate location where the image would appear after being reflected onto the half-silvered mirror.
Figure 1.
The double closed-loop teleprompter apparatus. Two teleprompters enabled live social interaction over a video interface, allowing each subject to be filmed from a hidden camera placed behind the live video image of the other subject. Black dotted and long-dash grey lines denote the course of visual information flow through the video channel from 1 bird to the other in either direction. The video camera inside the teleprompter films 1 pigeon off a mirror and through a pane of 1-way glass. The video then streams into the teleprompter apparatus of the other subject, as well as into the control room where the experimenter can observe.
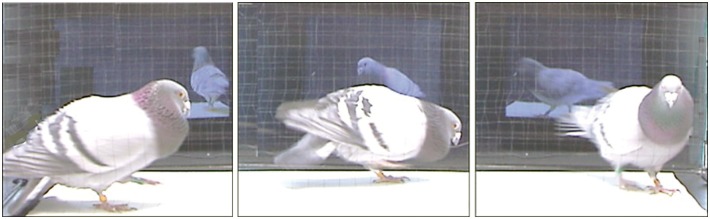
Figure 2.
Pigeon courtship in the double closed-loop teleprompter apparatus. The double teleprompter interface allowed us to film the stimulus partner while they were interacting with a live conspecific. When these stimuli were played back in the teleprompter during the experiment, they contained socially motivated that was oriented directly toward the subject bird at the same position as the original partner. Here a male pigeon is seen interacting with a female partner on screen.
Procedure
Before the experiments began, birds were habituated for 30 min daily to the experimental apparatus until they appeared comfortable and responded with courtship behavior to videos of conspecifics. During a typical experimental trial the subject was placed in the teleprompter apparatus and the monitor was switched on at trial onset. No bird was run more than once every 4 h, and the experiments always took place between 8 AM and 6 PM. Ambient noise (a radio receiver running at moderate volume) was used to mask environmental noise during the experiment. An observation camera was placed in the room to record the subject’s behavior for coding and analysis. There was no audio connection between the 2 rooms. Sound could not travel naturally between the 2 rooms either.
Automatic behavioral coding
An automatic coding technique measured the motion energy of the subject pigeon’s visual display behavior on video. This technique assesses the motion energy from the video using optical flow analysis algorithms embedded in the EyesWeb Open Software Platform Motion Analysis Library. This software is available for download at www.eyesweb.software.informer.com (Camurri et al. 2004). This technique is described in Ware et al. (2015).
The time series of motion energy values was processed to estimate each subject’s (1) total circle walking duration, (2) average bout duration, and (3) number of circle walking bouts. Although these measurements are redundant (total bout duration is the product of the other 2), together their pattern is informative: total circle walking duration gives an overall measure of display behavior, whereas the latter 2 measures provide information about the pattern of display and indicate whether circle walking occurs in a few long bouts or in many short bouts.
Experimental stimuli and design
Experiment 1
In the double closed-loop teleprompter apparatus, 6 male and 6 female subjects were either exposed to a real-time socially interactive partner (Live) or to a video recording of that partner obtained during the pair’s corresponding Live interaction (Playback). A Digital Video Recorder was used to obtain uncompressed video footage of the Live condition for Playback. Therefore, the composition, sequence, and intensity of the partner’s social behavior were identical across the Live and Playback conditions; the only difference was that the Live partner represented a responsive, socially interactive partner and the Playback partner did not.
The 6 male and 6 female subjects were paired in every possible opposite-sex combination to yield 36 pairs of birds. For half of the 36 pairs, the female acted as the subject and the male was the stimulus partner. In these trials the male was filmed during the Live condition and this footage was used for the Playback condition of that female subject. For the other 18 pairs, the male acted as the subject and the female was the stimulus partner. Thus, all 12 subjects interacted with the Live and Playback stimuli of 3 different partners, yielding 72 trials. A second round of experimental trials was conducted where the subject–partner roles for each pair were switched, yielding a total of 144 trials. Thus, each pigeon experienced Live and Playback conditions with 6 different partners.
Each trial contained 6 min of social interaction, which was split into three, 2-min segments, referred to here as trial phases, and each trial phase was interspersed by a 1-min wait period. The reason for using this stimulus-on, stimulus-off procedure was to stimulate as much circle walking as possible. Birds tend to behave more intensely at stimulus onset.
Experiment 2
Subjects for Experiment 2 were 6 male and 6 female pigeons. We manipulated 2 experimental factors with 2 levels each: the partner’s facing direction was either veridical (0°) or rotated by 90°, and social interactivity was either intact (Live) or not (Playback). With these 2 factors, 4 conditions were created: Live−0°, Live−90°, Playback−0° and Playback−90°.
The 90° spatial offset condition was achieved by placing a second tripod for filming the partner at a camera angle located 90° away from the camera inside the teleprompter apparatus. Whether the tripod was placed to the left or the right of the stimulus pigeon was counter-balanced across conditions. Because the 90° offset condition involved removing the camera from the teleprompter apparatus, a pane of glass was fitted on a 45° angle in front of the camera lens to match the filming quality obtained from the camera within the teleprompter. Black chloroplast plastic board was placed on the top and the sides of this camera setup to prevent glare on the glass from the room lights.
Social interactivity was manipulated as in Experiment 1; subjects were either exposed to a real-time socially interactive partner (Live) or to a video recording of that partner obtained during the pair’s corresponding Live interaction (Playback). A Digital Video Recorder was used to obtain uncompressed video footage of the Live condition for Playback.
Each trial contained 6 min of continuous social interaction. All 36 pairs underwent all 4 conditions twice, so that the female and the male each acted as the subject once for all 4 conditions. This yielded a total of 288 experimental trials.
Experiment 3
Experiment 3 was run twice, first with 12 birds (Block 1) and then with 6 more subjects (Block 2). There were no differences in the experimental design between the 2 blocks. For courtship interactions, all possible combinations of male and female birds were used yielding 36 pairs in Block 1 and 9 pairs in Block 2. For rivalry interactions, all possible combinations of 2 male birds or 2 female birds yielded 15 male–male and 15 female–female pairs in Block 1 (30 pairs in total), and 3 male–male and 3 female–female pairs in Block 2 (6 pairs in total).
This experiment contained 5 conditions. As in Experiments 1 and 2, the Live condition was a real-time interaction, and the Playback condition is the presentation of video playback that was previously recorded during the pair’s Live condition. Here, however, we added 3 time delay conditions to assess the sensitivity of behavior to the timing of social interactivity; the delays used were 1 s, 3 s, and 9 s. We tested all 5 conditions in 2 different social contexts: opposite-sex (courtship) interactions and same-sex (rivalry) interactions.
In Block 1, each dyad from all courtship and rivalry interactions experienced all 5 experimental conditions, yielding 180 opposite sex (courtship) and 150 same sex (rivalry trials). In Block 2, each pair underwent each condition twice, yielding 90 opposite-sex and 30 same-sex interactions in Block 2. In combining Block 1 and Block 2, there were 270 opposite-sex and 180 same-sex trials in total. Each single trial lasted 6 min.
Analogous to the 2 previous experiments, the time delay and video playback conditions were always implemented as unidirectional manipulations. This means that only 1 subject experienced a manipulated video feed, whereas the other subject viewed their partner in real time. However, in a closed-loop communication circuit, a unidirectional manipulation is assumed to always affect both subjects bi-directionally, in an equivalent fashion. For example, for a time delay travelling from the female camera to the male’s stimulus display, the male bird will experience social responses that are delayed in time and the female will experience the same social delay due to the male’s delayed response behavior. The same reasoning can be made for the Playback condition. In the Playback condition, pigeon A sees a video playback of pigeon B and pigeon B sees a real-time rendering of pigeon A (who is interacting with the video playback of pigeon B). Neither subject can influence the behavior of its partner, although the partner’s behavior is otherwise either identical or motivationally equivalent to the control condition. Therefore, in contrast to Experiments 1 and 2, in every trial of Experiment 3 both pigeons were considered to be a “subject” as well as a “partner.” In doing so, the data available for analysis were doubled, resulting in a total of 540 courtship and 360 rivalry subject videos for analysis. The directionality of temporal delays and Playback manipulations were counterbalanced across all subjects.
Data analysis
Experiment 1
We conducted three 2-way, [2 (sex) × 2 (interactivity)] mixed-effects Analysis of Variance (ANOVAs), where sex is a between-subjects factor, interactivity is a within-subject factor and the subject was treated as the random variable. The 3 ANOVAs correspond to measures of circle walking behavior: the average bout duration, the number of bouts, and their product, which amounts to the total circle walking duration. Main effects were evaluated using an alpha level of 0.05 (N = 12).
To further explore the influence of trial duration on the effect of social interactivity on total circle walking duration, a 2-way [2 (interactivity) × 3 (trial phase)] repeated measures ANOVA was added to the analysis. Pairwise Bonferroni corrected t-tests were used to assess differences between the Live and Playback condition in each of the 3 trial phases.
Experiment 2
Three 3-way mixed-effects ANOVAs [2 (sex) × 2 (facing direction) x 2 (interactivity)] were performed to evaluate the effect of spatial relations on courtship behavior. The 3 ANOVAs correspond to the subject’s average bout duration, number of circle walking bouts, and their product, total circle walking duration. Bird sex was treated as a between-subjects factor, facing direction and interactivity were treated as within-subject factors, and the random variable was the subject (N = 12). Main effects were evaluated with a significance level of 0.05. To further investigate main effects pairwise t-tests were used to compare the Live−90°, Playback−0°, and Playback−90° with the Live−0° condition. A Bonferroni corrected alpha level of 0.0167 was used to evaluate significance.
Experiment 3
As in Experiments 1 and 2, the measures of total circle walking duration, average bout duration and number of circle walking bouts, were used to analyze subjects’ circle walking behavior. After verifying that there were no differences in the data collected in Block 1 and Block 2, we combined all data together for analysis. We conducted three 3-way [2 (sex) × 2 (social context) × 5 (time delay)] mixed-effects ANOVAs corresponding to each of the 3 measures of circle walking behavior. Sex was treated as a between-subjects factor, social context and time delay were within-subject factors, and the bird’s identity was the random variable (N = 18). Significant interactions were further explored with a series of 2-way and 1-way ANOVAs. An alpha value of 0.05 was used to assess significance in the ANOVAs.
Main effects of time delay conditions on behavior were further investigated with pairwise t-tests to compare the circle walking measures in each time delay condition (1-s, 3-s, and 9-s delays) as well as the Playback condition with those measures in the Live condition. A Bonferroni corrected alpha of 0.0125 was used to assess significance in these cases.
Results
Experiment 1
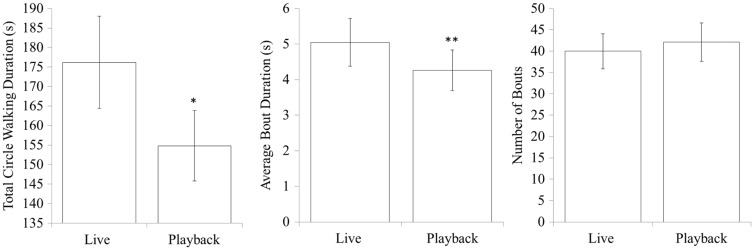
The results revealed a main effect of social interactivity on total circle walking duration, which was significantly longer in the Live condition (F1, 10 = 6.86, P = 0.026). This difference was entirely carried by a difference in average bout duration (F1, 10 = 10.47, P = 0.009). There was no effect of social interactivity on the number of circle walking bouts (Figure 3).
Figure 3.
Circle walking across Live and Playback conditions. Pigeons’ total circle walking duration and average bout duration varied significantly depending on whether their social partner was displayed Live in the teleprompter apparatus or displayed as a Playback of the previously captured footage (recorded during the Live condition). Error bars indicate standard error of the mean. Significant differences between Live and Playback conditions are indicated by asterisks over the Playback data point, *P < 0.05, **P< 0.01, ***P > 0.001.
The results also reveal strong effects of sex on circle walking behavior. Overall circle walking duration was about the same between the 2 sexes, but male birds demonstrated only about half the number of bouts as females (F1, 10 = 536.52, P < 0.001). This was compensated for by average bout durations, which were much longer in males than in females (F1, 10 = 8.53, P = 0.015; Figure 4). There was no interaction between sex and social interactivity.
Figure 4.
Sex differences in circle walking. Male and female pigeons each have distinct patterns of circle walking. Significant differences between male and female circle walking are indicated by asterisks over the female data point, *P < 0.05, **P < 0.01, ***P > 0.001.
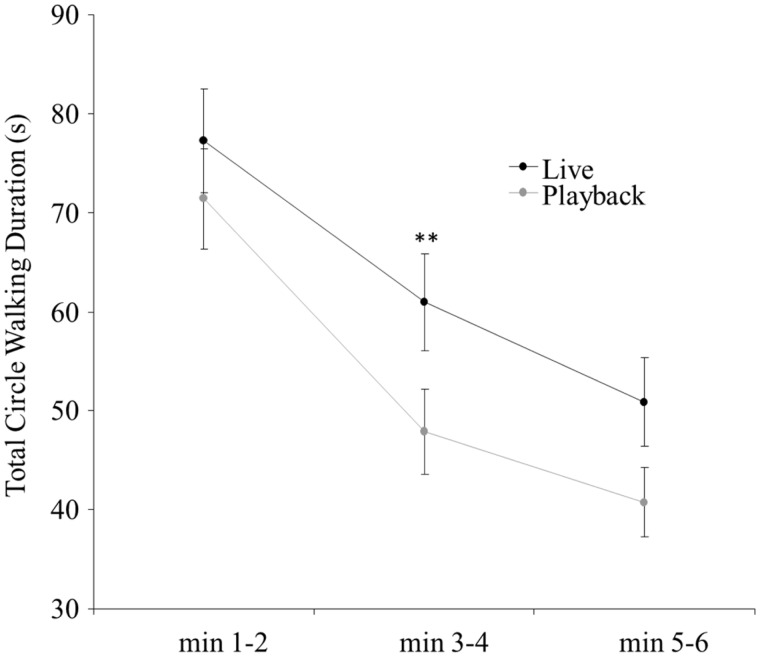
In addition to a replication of the effect of social interactivity, the second ANOVA also showed a significant effect of trial phase (F2, 11 = 68.24, P < 0.001), where circle walking was observed to decline as the trial proceeded. The interaction between interactivity and trial phase just barely missed reaching significance (F2, 22 = 3.138, P = 0.063) but points to larger effects of interactivity in later phases (Figure 5). Follow-up Bonferroni-corrected t-tests showed a significant difference between Live and Playback conditions only for Phase 2 (3–4 min, t11 = 3.881, P = 0.003).
Figure 5.
Courtship exhibits greater sensitivity to social interactivity as the trial proceeds. Total circle walking duration is shown over three, 2-min trial segments. The difference in responses toward the Live and Playback stimuli increases as the trial proceeds. Error bars indicate standard error. Significant differences in circle walking between the Live and Playback conditions at each time point are indicated by asterisks, **P < 0.01.
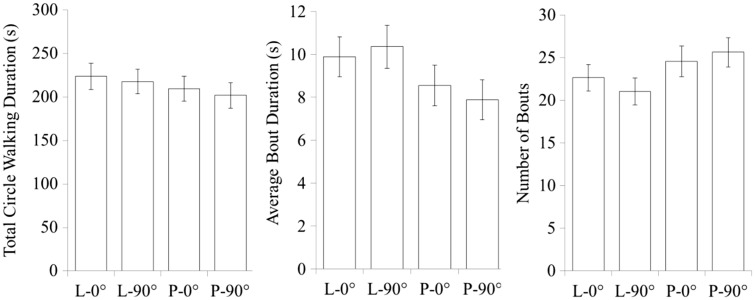
Experiment 2
There was no significant effect of facing direction on any measure of subject circle walking duration (Figure 6). The study did, however, replicate the effect of social interactivity on total circle walking duration, F1,10 = 16.79, P = 0.002, average bout duration, F1,10 = 15.46, P = 0.009, and the number of bouts, F1,10 = 11.05, P = 0.008.
Figure 6.
The effect of social interactivity and partner directionality on circle walking. The results show that pigeon circle walking is sensitive to social interactivity but not to the cues present in the partner’s facing direction. These findings suggest that a pigeon’s interactive circle walking does not depend on signal relationships that require partners to face each other directly. Error bars indicate standard error.
Sex differences in circle walking behavior were also similar to those observed in Experiment 1. Males circle-walked for longer average bout durations, F1,10 = 12.83, P = 0.005, but exhibited fewer bouts, F1,10 = 21.03, P = 0.001, than females.
Experiment 3
Social context
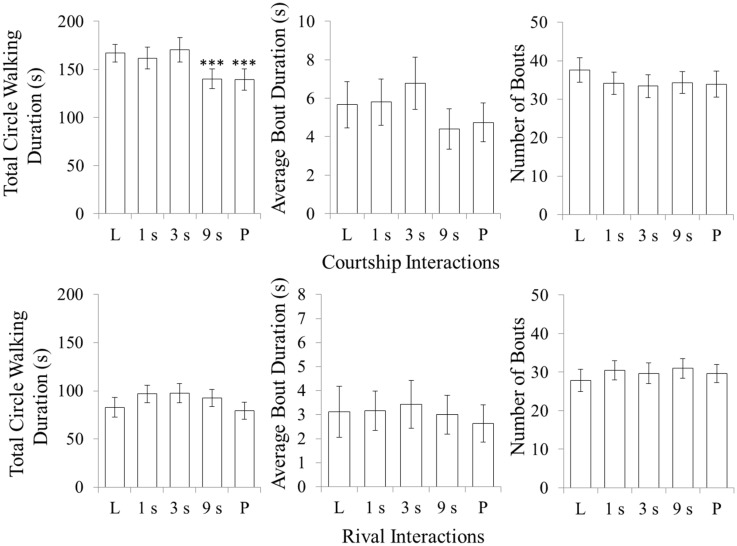
There was a main effect of social context, F1,16 = 53.83, P < 0.001, on total circle walking duration. Circle-walking duration was much longer in the courtship context than in the rivalry context. Differences are mainly due to longer bout durations in the courtship condition, F1, 16 = 10.21, P = 0.006 (Figure 7).
Figure 7.
The effect of social interactivity on circle walking depends on the social context. Pigeon circle walking is sensitive to social interactivity and temporal contiguity manipulations in courtship but not in competitive interactions between rivals. The total circle walking duration, average bout duration and total number of bouts are shown for each time delay condition across opposite-sex (courtship) and same-sex (rivalry) interactions. In opposite-sex interactions only, the 9-s delay condition and the Playback condition produced significant effects on circle walking. Error bars indicate standard error. Significant differences between a time delay condition and the Live condition are indicated by asterisks over the time delay data point, *P < 0.05, **P < 0.01, ***P > 0.001.
Time delay
There was a main effect of time delay on total circle walking duration F (4, 64) = 5.46, P = 0.003 in the current experiment. Circle walking activity was higher in the Live condition and with short (≤3 s) delays, and lower for the 9-s delay and the playback condition. Again, this effect is mainly carried by differences in average bout duration F4, 64 = 6.55, P < 0.001.
Interaction effect in total circle walking duration
There was a significant interaction between the effects of social context and time delay on total circle walking duration, F4, 64 = 3.53, P = 0.011. Two follow-up 2-way ANOVAs, conducted for each social context separately, revealed a main effect of time delay on total circle walking duration in opposite-sex courtship interactions, F4, 64 = 7.75, P < 0.001, and no significant effect in same-sex rivalry interactions. Follow-up t-tests revealed that total circle walking duration in courtship interactions significantly decreased between the Live and 9-s conditions, t17 = 4.29, P < 0.001, as well as between the Live and Playback conditions, t17 = 4.14, P = 0.001 (Figure 7).
Interaction in average bout duration
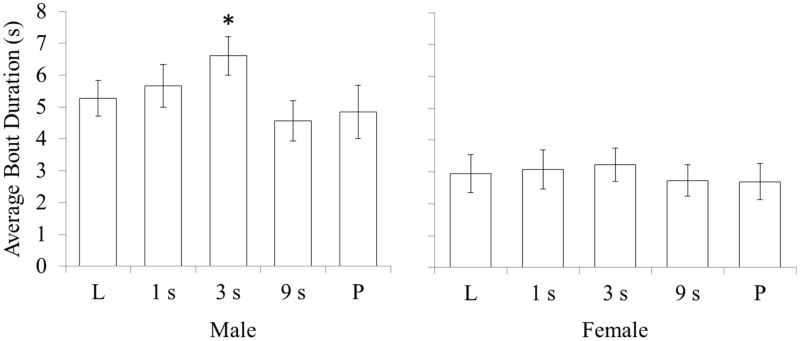
There was a significant interaction between sex, social context and time delay, F4, 64 = 3.36, P = 0.036, on average bout duration. Follow-up ANOVAs, conducted in each sex and each social context separately, revealed a significant effect of time delay on male average bout duration in courtship interactions, F4, 32 = 5.01, P = 0.003, but no effect in females or in any rivalry interactions; t-tests on the male courtship data revealed a marginally significant increase between Live and 3 s, t8 = −2.51, P = 0.036, in male average bout duration (Figure 8).
Figure 8.
Interactions between sex and temporal contiguity on average bout duration in opposite-sex courtship interactions. In opposite-sex courtship interactions, male pigeons, but not female pigeons, show a marginally significant increase in their average bout duration in the 3-s delay condition. Error bars indicate standard error. Significant differences between a time delay condition and the Live condition are indicated by asterisks over the time delay data point, *P < 0.05, **P < 0.01, ***P > 0.001.
Sex
The effects of pigeon sex on circle walking behavior were similar to those found in Experiment 2 (Figure 6). Males and females significantly differed in total circle walking duration, F1, 16 = 11.42, P = 0.004, and average bout duration, F1, 16 = 61.13, P < 0.001. There was no significant effect of sex on number of bouts.
Discussion
Unlike previous methods used for testing the influence of signal relations on behavior (e.g., Friedman 1977; Kodric-Brown and Nicoletto 1997; Trainor and Basolo 2000), the present study controlled all aspects of the social stimulus (including the color, depth, motion, luminance, and behavioral content of the partners’ image). In our contrast between Live and Playback conditions, the only difference was the ability or inability for the social partner to engage interactively with the subject bird. Experiment 1 was therefore the first conclusive demonstration of interactive behavior in the visual communication channel of a non-human species. Furthermore, the results of Experiments 2 and 3 suggest that temporal contiguity but not spatial offset is key to the social interactivity observed in pigeon courtship. Experiment 3 showed that the threshold at which deviations from veridical timing interfere with social interactivity is somewhere between 3 s and 9 s. Sensitivity to temporal contiguity or the manipulation of social interactivity more generally was found to be specific to courtship and was not observed in same-sex rivalry interactions.
The findings of Experiment 1 show that pigeon courtship behavior depends on social interactivity; their behavior is sensitive to the relationship between their own actions and those of their partner. This result suggests that social interaction is not merely a chain of reciprocal signals where each is successively “released” by stimuli present in the signal preceding it. Rather, the social dynamic itself and the nature of the relationships between signals can influence behavior.
The results also demonstrate sex differences in the pigeon’s circle walking behavior. Males circle in long bouts, whereas females tend to circle in shorter but more frequent circle walking bouts. These sex differences in courtship are important to document as they likely help shape and define the signal relationships that drive the social interactivity observed here.
The present results contrast with those of Shimizu (1998) and Patton et al. (2009), who did not find significant differences in male pigeon courtship behavior toward females presented either live (across a pane of glass) or on video playback. There are 2 main methodological differences that might explain this discrepancy: (1) the behavioral measure used and (2) the length of the experimental trial.
First, instead of using circle walking duration to operationalize courtship intensity, Shimizu (1998) and Patton et al. (2009) measured the frequency of discrete behavioral signals like coos, tail drags and bowing. The circle walk might be considered to be the “dynamic scaffolding” of the courtship display, providing a structure for other discrete signals to be transmitted in an organized and efficient manner. It is possible that, due to its structural role in courtship, circle walking exhibits greater sensitivity to the manipulation of social interactivity than other behavioral measures do.
The second major methodological difference between this study and previous work is the length of the experimental trial used. Shimizu (1998) and Patton et al. (2009) both measured behavior during 2-min interactions and the present study measured behavior over 6 min of interaction. The change in sensitivity to temporal contiguity over time can be seen in Figure 5. In the first 2 min of the trial, courtship levels appear near ceiling, and there is no significant behavioral discrimination between a responsive and a non-responsive partner. As social interaction proceeds into the second 2 min of the trial, courtship behavior appears to become progressively sporadic and less predictable. Our data suggest that it is in this phase, during 3–4 min, that courting pigeons are most likely to be influenced by disruptions of social interactivity. In the last 2 min of the interaction courtship behavior begins to decline overall, which results in a corresponding reduction in behavioral sensitivity to social interactivity.
Overall, Experiment 1 provided evidence that social interactivity is significant for maintaining courtship behavior. This prompted Experiments 2 and 3 to gain additional insight into the inter-signal spacing and timing of the pigeons circle walking interactions
In Experiment 2, we found that pigeon circle walking duration was not significantly sensitive to spatial offsets and resulting changes in facing direction. Behavioral outcomes did not significantly differ when the partner’s facing direction was oriented 90° away from the subject.
The pigeon’s insensitivity to facing direction was somewhat surprising. We know that pigeons can perceive the directionality of conspecific walking behavior using biological motion cues (Troje and Aust 2013). It is possible that the dynamics of courtship display that incite conspecific responses do not vary appreciably with the display’s orientation. In other words, there may be a lack of behaviorally relevant directionality cues in pigeon’s circle walking behavior. This might reflect selection for display features that influence recipients located at various possible viewing angles. A similar behavioral insensitivity to signal orientation has also been observed in the Jacky dragon lizard’s (Amphibolurus muricatus) visual displays of aggression (Peters and Evans 2007). Alternatively, it is possible that this finding is attributable to assay insensitivity. Pigeon’s circle walking behavior may not be the best measure to capture this effect. For example, when Galoch and Bischof (2007) played videos of female zebra finches (T.guttata) to male birds, they found that the male’s courtship song and his proximity to the monitor was not sensitive to social context but the males’ beak wiping behavior and “Tit” and “Tet” calls were (Galoch and Bischof 2007). It is possible, as in Galoch’s study, that other measures of pigeon courtship behaviors, if measured, would exhibit sensitivity to facing direction.
In Experiment 3, none of the time delay manipulations, or the Playback condition, affected display behavior during same-sex rivalry interactions. It is possible that social interactivity is not behaviorally significant in pigeon’s rivalry interactions. It is also possible that rivalry interactions are sensitive to social interactivity or timing, or both, but that the assay used in this experiment is insensitive to the behavioral effects. Indeed, our results are somewhat inconsistent with evidence for time sensitive social interactivity between rivals in other species, including the vocal interactions of multiple songbirds (McGregor et al. 1992; Dabelsteen et al. 1997; Peake et al. 2005; Burt and Beecher 2008) and the visual interactions of male Jacky dragon lizards A.muricatus (Macedonia et al. 1994; Ord and Evans 2002).
Temporal contiguity between signals appears to represent a meaningful parameter characterizing the pigeon’s social interactivity in courtship interactions, but not in same-sex rivalry encounters. In courtship, the results showed that the 9 s and Playback conditions disrupted circle walking behavior. For the male pigeon, the average length of circle walking bouts marginally increased when communications were delayed by 3 s. Here we will briefly discuss 2 potentially interdependent processes that might explain this pattern of results.
First, interactivity might help to structure the social dynamics of courtship interactions. More specifically, pigeon courtship interactions may constitute a perceptual crossing event (Di Paolo et al. 2008; Auvray et al. 2009; Froese and Di Paolo 2010). Perceptual crossing occurs when 2 behaviors of the same nature simultaneously “cross,” as in eye contact, mutual touch, or mutual action (Di Paolo et al. 2008). Perceptual crossing events can trigger mutually congruent social responses that increase the likelihood that the perceptual crossing event will re-occur, thereby potentiating the “ongoingness” of the interaction (Auvray et al. 2009). Perceptual crossing in circle walking could drive stable, self-sustaining social dynamics, and when these processes are disrupted under Playback or time delay conditions, circle walking behavior may decrease.
Our results suggest that a behavioral action that has duration longer than 3 s and up to 9 s is a possible candidate for a perceptual crossing dynamic. This time frame rules out many candidates such as eye contact, head-bobbing, bowing, or the fine spatiotemporal dynamics of circles, because these perceptual crossings would be uncoupled by shorter delays. A 3–9 s timeframe leaves only one obvious behavioral candidate for perceptual crossing, the circle walking bout. If courting pigeons are mutually stimulated by the sight of an opposite-sex conspecific circle walking, the simultaneous action of circle walking could trigger a mutually congruent social “release” that potentiates circle walking in both pair members at the same time. By potentiating circle walking behavior, the perceptual crossing event of simultaneous circle walking (SCW) increases the likelihood that SCW will re-occur, over and over again. These dynamics trigger a feed-forward cycle, which can drive coherent, self-potentiating social dynamics in spite of individual or inter-individual variations (Froese and Di Paolo 2010). Froese has called this the “constitutive autonomy” of the interaction process where, in this case, there exists a reciprocal dependency between the individual pigeon’s circle walking behavior and the overall circle walking dynamics of the system of interaction (Froese et al. 2007). When the SCW between pigeons is disrupted by a 9-s time delay manipulation or playback condition, any moment that 1 pigeon becomes motivated to engage, the other animal may be experiencing a different social event altogether. This unshared experience would create a mismatch in the dynamics of mutual influence, thus destroying the stability of circle walking dynamics and their influence on individual circle walking duration.
SCW dynamics also lends a potential explanation for the increase in circle duration in response to the 3-s delay has on male behavior. The sex specificity of this effect may lie in the fact that males perform their circle walking differently than females do. Males typically add circles to create longer bouts, whereas females perform short bouts more frequently. Instead of uncoupling SCW, the 3-s delay could have the effect of delaying the temporal position of female responses as they occur within the male’s display behavior. If male bout termination depends on placement of female circle walking within the male bout, under the 3-s conditions, the male pigeon would receive this stimulation later, potentially causing him to extend his circle walking bout a little longer.
The second mechanism that might explain the sensitivity to timing in pigeon courtship has to do with the disruption of specific signal–response pairs that function in courtship interaction. The female signaling hypothesis—that female signals may be an important force modulating male courtship intensity (Borgia and Coleman 2000; Patricelli et al. 2002; Royle and Pike 2010)—is one well-supported model of signal–response relations that could help explain our results. This hypothesis predicts that courting females exhibit 2 types of signaling behavior (Patricelli et al. 2006). One female signal is designed to trigger an increase in male display, functioning to gain greater access to the male’s fitness information when the female feels sexually motivated. In the pigeon, the female’s circle walking behavior would be an obvious candidate to fulfill this function. This would produce SCW, discussed above.
Another female signal would be designed to trigger a decrease in male circle walking when the female feels threatened, functioning to mitigate the damages of male aggression on the outcomes of the female’s mate search and the male’s courtship efforts (Borgia and Coleman 2000; Patricelli et al. 2006). Such an “appeasement” signal elicits a pause in the male’s display, functioning to reduce the aggressive components of the male’s display, and enable females to recover and re-invigorate their engagement in the courtship interaction. A good candidate for an appeasement display in pigeons is the head-nod. The head-nod is a rapid gesture frequently displayed by female pigeons that does not extend as low as bowing behavior but is deeper than head bobbing. Wosegien and Lamprecht (1989) showed that head-nodding appeases male aggression. They used the human fist to mimic the head-nodding action, while recording male subjects’ aggressive pecks toward the nodding hand. They found that the nodding action reduces the number of aggressive male pecks toward the hand both immediately and progressively over repeated trials, as compared to the control situation in which the hand was rotated horizontally instead of “nodding” vertically (Wosegien and Lamprecht 1989). Although head-nodding was not measured here, throughout the present experiment, females were frequently observed to head-nod in the pauses between circle walking bouts. It is possible that the function of head-nods in this context may lie in modulating male circle walking intensity. When social interactivity or temporal contiguity is manipulated, a head-nodding female would not experience the male’s response in a timely manner after attempting to appease the male’s display. The male would continue to circle walk and the female would remain in a defensive state. Under the 9 s and Playback conditions, this may have caused a reduction of female circle walking duration, which eventually would result in a diminished male display as well.
Pigeon courtship behavior seems to be interesting and complex enough to provide a rich animal model for the study of social interactivity. On the other hand, it is stereotypic in many respects, can be elicited in the lab, responses can be quantified in principled ways and using video technology similar to the one we used here, many stimulus aspects can be controlled at least to some degree. Particularly exciting is the potential for exploring the possibility of multiple mechanisms underlying social interactivity, as well as the neural basis and function of these mechanisms. In addition to using interactive video playback, other promising methods of social behavior, such as robotic models (Krause et al. 2011), and animations (Watanabe and Troje 2006; Woo and Rieucau 2011), are being continuously developed to create realistic experiences of social interaction.
Acknowledgments
We thank Dr. Barrie Frost for fruitful discussions, as well as Sharon David and Lisa Wilberforce for assistance in veterinary services.
Funding
This work was funded by a Natural Sciences and Engineering Research Council of Canada Discovery Grant held by N.F.T.
References
- Aizawa N, 1998. Synchronous waving in an ocypodid crab Ilyoplax pusilla: analyses of response patterns to video and real crabs. Mar Biol 131:523–532. [Google Scholar]
- Auvray M, Lenay C, Stewart J, 2009. Perceptual interactions in a minimalist virtual environment. New Ide Psychol 27:32–47. [Google Scholar]
- Balsby TJS, Dabelsteen T, 2002. Female behaviour affects male courtship in whitethroats Sylvia communis: an interactive experiment using visual and acoustic cues. Anim Behav 63:251–257. [Google Scholar]
- Bigelow AE, DeCoste C, 2003. Sensitivity to social contingency from mothers and strangers in 2-, 4-, and 6-month-old infants. Infancy 4:111–140. [Google Scholar]
- Bird CD, Emery NJ, 2008. Using video playback to investigate the social preferences of rooks- Corvus frugilegus. Anim Behav 76:679–687. [Google Scholar]
- Borgia G, Coleman SW, 2000. Co-option of male courtship signals from aggressive display in bowerbirds. Proc R Soc B Biol Sci 267:1735–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt JM, Beecher MD, 2008. The social interaction role of song in song sparrows: implications for signal design. Comp Cog Behav Rev 3:86–98. [Google Scholar]
- Camurri A, Mazzarino B, Volpe G, 2004. Analysis of expressive gesture: the eyesweb expressive gesture processing library In: Camurri A, Volpe G, editors. Gesture-Based Communication in Human-Computer Interaction. Berlin/Heidelberg: Springer, 469–470. [Google Scholar]
- D’eath RB, 1998. Can video images imitate real stimuli in animal behaviour experiments? Biol Rev Cam Philoso Soc 73:267–292. [Google Scholar]
- D’eath RB, Dawkins MS, 1996. Laying hens do not discriminate between video images of conspecifics. Anim Behav 52:903–912. [Google Scholar]
- Dabelsteen T, McGregor PK, Holland J, Tobias JA, Pedersen SB, 1997. The signal function of overlapping singing in male robins. Anim Behav 53:249–256. [Google Scholar]
- Di Paolo EA, Rohde M, Iizuka H, 2008. Sensitivity to social contingency or stability of interaction? Modelling the dynamics of perceptual crossing. New Ide Psychol 26:278–294. [Google Scholar]
- Fleishman LJ, Endler JA, 2000. Some comments on visual perception and the use of video playback in animal behaviour studies. Acta Ethol 3:15–27. [Google Scholar]
- Fong T, Nourbakhsh I, Dautenhahn K, 2003. A survey of socially interactive robots. Robo Auto Sys 42:143–166. [Google Scholar]
- Friedman MB, 1977. Interactions between visual and vocal courtship in the neuroendocrine response of female doves. J Comp Physiol Psychol 91:1408–1416. [Google Scholar]
- Froese T, Di Paolo EA, 2010. Modelling social interaction as perceptual crossing: an investigation into the dynamics of the interaction process. Conn Sci 22:43–68. [Google Scholar]
- Froese T, Virgo N, Izquierdo E, 2007. Autonomy: a review and a reappraisal Proceedings of the 9th European Conference on Advances in Artificial Life. Lisbon, Portugal: Springer. [Google Scholar]
- Galoch Z, Bischof H-J, 2007. Behavioural responses to video playbacks by zebra finch males. Behav Proc 74:21–26. [DOI] [PubMed] [Google Scholar]
- Kao MH, Wright BD, Doupe AJ, 2008. Neurons in a forebrain nucleus required for vocal plasticity rapidly switch between precise firing and variable bursting depending on social context. J Neurosci 28:13232–13247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodric-Brown A, Nicoletto P, 1997. Repeatability of female choice in the guppy: response to live and videotaped males. Anim Behav 54:369–376. [DOI] [PubMed] [Google Scholar]
- Krause J, Winfield AF, Deneubourg JL, 2011. Interactive robots in experimental biology. Tren Ecol Evol 26:369–375. [DOI] [PubMed] [Google Scholar]
- Macedonia JM, Evans CS, Losos JB, 1994. Male Anolis lizards discriminate video-recorded conspecific and heterospecific displays. Anim Behav 47:1220–1223. [Google Scholar]
- McGregor PK, Dabelsteen TD, Shepherd M, Pederson SB, 1992. The signal value of matched singing in great tits: evidence form interactive playback experiments. Anim Behav 43:987–998. [Google Scholar]
- Mennill DJ, Ratcliffe LM, Boag PT, 2002. Female eavesdropping on male song contests in songbirds. Science 296:873–873. [DOI] [PubMed] [Google Scholar]
- Moravec ML, Striedter GF, Burley NT, 2010. “Virtual Parrots” confirm mating preferences of female budgerigars. Ethology 116:961–971. [Google Scholar]
- Muir D, Hains S, 1999. Young infants’ perception of adult intentionality: adult contingency and eye direction In: Rochat P, editor. Early Social Cognition: Understanding Others in the First Months of Life. London: Lawrence Erlbaum Associates, 155–187. [Google Scholar]
- Murray L, Trevarthen C, 1985. Emotional regulation of interactions between two-month-olds and their mothers In: Feild TM, Fox NA, editors. Social Perception in Infants. Norwood, New Jersey: Ablex Publishing Corporation, 177–197. [Google Scholar]
- Nadel J, Carchon I, Kervella C, Marcelli D, Reserbat-Plantey D, 1999. Expectancies for social contingency in 2-month-olds. Devel Sci 2:164–173. [Google Scholar]
- O'Loghlen AL, Rothstein SI, 2010. It’s not just the song: male visual displays enhance female sexual responses to song in brown–headed cowbirds. Condor 112:615–621. [Google Scholar]
- O'Loghlen AL, Rothstein SI, 2012. When less is best: female brown-headed cowbirds prefer less intense male displays. PLoS ONE 7:e36130.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira RF, Rosenthal GG, Schlupp I, McGregor PK, Cuthill IC. et al. , 2000. Considerations on the use of video playbacks as visual stimuli: the Lisbon workshop consensus. Acta Ethol 3:61–65. [Google Scholar]
- Ord TJ, Evans CS, 2002. Interactive video playback and opponent assessment in lizards. Behav Proc 59:55–65. [DOI] [PubMed] [Google Scholar]
- Patricelli GL, Coleman SW, Borgia G, 2006. Male satin bowerbirds Ptilonorhynchus violaceus adjust their display intensity in response to female startling: an experiment with robotic females. Anim Behav 71:49–59. [Google Scholar]
- Patricelli GL, Uy JAC, Walsh G, Borgia G, 2002. Sexual selection: male displays adjusted to female’s response. Nature 415:279–280. [DOI] [PubMed] [Google Scholar]
- Patton TB, Husband SA, Shimizu T, 2009. Female stimuli trigger gene expression in male pigeons. Soc Neurosci 4:28–39. [DOI] [PubMed] [Google Scholar]
- Peake TM, Matessi G, McGregor PK, Dabelsteen T, 2005. Song type matching, song type switching and eavesdropping in male great tits. Anim Behav 69:1063–1068. [Google Scholar]
- Peters RA, Evans CS, 2007. Active space of a movement-based signal: response to Jacky dragon Amphibolurus muricatus display is sensitive to distance, but independent of orientation. J Exp Biol 210:395–402. [DOI] [PubMed] [Google Scholar]
- Rieucau G, Giraldeau L-A, 2009. Video playback and social foraging: simulated companions produce the group size effect in nutmeg mannikins. Anim Behav 78:961–966. [Google Scholar]
- Rosenthal GG, Evans CS, Miller WL, 1996. Female preference for a dynamic trait in the green swordtail Xiphophorus helleri. Anim Behav 51:811–820. [Google Scholar]
- Royle N, Pike T, 2010. Social feedback and attractiveness in zebra finches. Behav Ecol Sociobiol 64:2015–2020. [Google Scholar]
- Schlupp I, 2000. Are there lessons from negative results in studies using video playback? Acta Ethol 3:9–13. [Google Scholar]
- Shimizu T, 1998. Conspecific recognition in pigeons using dynamic video images. Behaviour 135:43–53. [Google Scholar]
- Striano T, Henning A, Stahl D, 2006. Sensitivity to interpersonal timing at 3 and 6 months of age. Inter Stud 7:251–271. [Google Scholar]
- Swaddle JP, McBride L, Malhotra S, 2006. Female zebra finches prefer unfamiliar males but not when watching non-interactive video. Anim Behav 72:161–167. [Google Scholar]
- Tinbergen N. 1952. Derived Activities; Their Causation, Biological Significance, Origin, and Emancipation During Evolution. Q Rev Biol 27 (1):1. [DOI] [PubMed] [Google Scholar]
- Tinbergen N, 1962. Social Behaviour in Animals with Special Reference to Vertebrates. London/New York: Methuen Co. Ltd./John Wiley Sons. [Google Scholar]
- Toda K, Watanabe S, 2008. Discrimination of moving video images of self by pigeons Columba livia. Anim Cog 11:699–705. [DOI] [PubMed] [Google Scholar]
- Trainor BC, Basolo AL, 2000. An evaluation of video playback using Xiphophorus helleri. Anim Behav 59:83–89. [DOI] [PubMed] [Google Scholar]
- Troje NF, Aust U, 2013. What do you mean with “direction”? Local and global cues to biological motion perception in pigeons. Vis Res 79:47–55. [DOI] [PubMed] [Google Scholar]
- Van Dyk DA, Evans CS, 2008. Opponent assessment in lizards: examining the effect of aggressive and submissive signals. Behav Ecol 19:895–901. [Google Scholar]
- Ware E, Saunders DR, Troje NF, 2015. The influence of motion quality on responses towards video playback stimuli. Biol Open 4:803–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S, Troje N, 2006. Towards a virtual pigeon: A new technique for investigating avian social perception. Anim Cog 9:271–279. [DOI] [PubMed] [Google Scholar]
- Woo KL, Rieucau G, 2011. From dummies to animations: a review of computer-animated stimuli used in animal studies. Behav Ecol Sociobiol 65:1671–1685. [Google Scholar]
- Wosegien A, Lamprecht J, 1989. Nodding: an appeasement behavior of pigeons Columba Livia. Behaviour 108:44–56. [Google Scholar]