Abstract
The use of computer animation in behavioral research is a state-of-the-art method for designing and presenting animated animals to live test animals. The major advantages of computer animations are: (1) the creation of animated animal stimuli with high variability of morphology and even behavior; (2) animated stimuli provide highly standardized, controlled and repeatable testing procedures; and (3) they allow a reduction in the number of live test animals regarding the 3Rs principle. But the use of animated animals should be attended by a thorough validation for each test species to verify that behavior measured with live animals toward virtual animals can also be expected with natural stimuli. Here we present results on the validation of a custom-made simulation for animated 3D sailfin mollies Poecilia latipinna and show that responses of live test females were as strong to an animated fish as to a video or a live male fish. Movement of an animated stimulus was important but female response was stronger toward a swimming 3D fish stimulus than to a “swimming” box. Moreover, male test fish were able to discriminate between animated male and female stimuli; hence, rendering the animated 3D fish a useful tool in mate-choice experiments with sailfin mollies.
Keywords: : computer animation, fish behavior, mate-choice experiment, validation, virtual fish model.
The use of artificial stimuli to study fish behavior has already a long history. Ter Pelkwijk and Tinbergen (1937; Tinbergen 1948) were 2 of the pioneers, using dummy fish to investigate courtship and agonistic behavior in three-spined sticklebacks Gasterosteus aculeatus. They showed that dead sticklebacks and schematic wooden models put on a stick and moved by hand with varying shape and belly redness could be used as visual releasers to evoke courtship and/or aggression in live male and female sticklebacks. Although they provide 3D cues, such dummy fish are very limited in their possible changes to morphology and behavior, but they are still used today (Kim and Velando 2014). Tinbergen and Perdeck (1951) used dummy heads of hering gulls Larus argentatus argentatus Pont. to investigate the begging response of chicks to the parents’ beaks. This was the first field experiment in behavioral biology. Magnus (1954) used a rotating cylinder with stripes to investigate the visual releasing stimulus for males to follow females in the silver-watched fritillary Argynnis paphia. Other dummies were used to investigate the reaction of turkeys to birds of prey (Schleidt 1961) and the begging response of black bird Turdus merula and European song thrush Turdus e. ericetorum chicks (Tinbergen and Kuenen 1939).
Thanks to rapid technical development over the last decades, we now have access to several elaborate methods to create highly realistic and varied artificial stimuli. Ward et al. (2008) used realistic stickleback replicas, made of colored resin plaster, to study decision-making strategies in shoaling fish. They automated and standardized movement by using a motorized guided line system that moved replicas through the test tank. This method was further developed leading to bio-inspired robot systems to study mate-choice, collective movement, and social networks directly within groups of live fish (Kopman et al. 2012; Landgraf et al. 2016).
Screen-based techniques for stimulus presentation, including video playback, video editing, and computer animation, are valuable alternatives in test situations in which live fish can usually choose between 2 live stimulus fish presented in separate tanks or behind glass walls. Early screen-based methods used video playbacks of live animals (Rosenthal 1999, 2000; Oliveira et al. 2000). Manipulation of video playbacks was very limited in its early stages and restricted to variation in hue or color output defined by the monitors, as was done by Rowland et al. (1995) to investigate female stickleback attentiveness toward different gray-toned and colored video sequences of male courtship. The development of video-editing software allowed more rigorous manipulations of shape and color, and limited variation in behavior of animated stimuli. Rosenthal and Evans (1998) and Körner et al. (1999) used this technique to manipulate video playbacks of Poeciliid fishes for presentation in mate-choice experiments. Not only did fish prove to be responsive toward video playback, but jumping spiders Maevia inclemens responded to prey insects, conspecifics, and heterospecifics (Clark and Uetz 1990). Clark et al. (1997) demonstrated the usability of this technique in the field and presented video-edited displaying male lizards Anolis grahami to conspecifics in the wild, who expressed natural behavior toward the video.
Advanced techniques are 2D and 3D computer animations (Woo and Rieucau 2011). Animations are more variable and the stimulus is detached from any basic, raw material. McKinnon and McPhail (1996) were one of the first using a computer generated 3D fish animation, based on morphological measurements of a male three-spined stickleback. They presented an animated rival male on a computer screen next to an aquarium containing a live test male. In presence of the animated rival, live male sticklebacks performed aggressive displays and bites to the rival. Following this new approach, it could be demonstrated that fish seemed to be similarly responsive to computer-animated models as to natural stimuli and recognized them as “real” conspecifics (e.g., Baldauf et al. 2009). Furthermore, it was shown that results obtained with virtual stimuli were congruent and reproducible with live stimuli (Rosenthal et al. 2002; Egger et al. 2011; Amcoff et al. 2013). Zbinden et al. (2003, 2004) showed that three-spined sticklebacks could be successfully put into a feigned situation of sperm competition by showing them animations of courting or brood-caring virtual males. In this test situation, sticklebacks reacted by increasing their ejaculate size, indicating the high degree of realism the animation must have had for the observing fish.
The 2D computer animations often derive from digital photographs of live animals that are then edited using various image processing software. To gain a 3D fish animation, these photographs are transferred into digital wire mesh models that can be modulated and animated using software that is also used for developing computer games and animated movies (but see Künzler and Bakker 1998, for an alternative method). With the help of animation software, different motion patterns can be specified and virtual models perform simple to complex, realistic, species-specific behavioral patterns and visual displays. For example, Clotfelter et al. (2006) designed a complex 3D Siamese fighting fish Betta splendens that was able to perform species-specific opercula displays to study mate choice.
Reasons for using animated stimuli instead of live stimulus fish, especially in mate-choice experiments, are obvious since the opportunities to manipulate virtual animals are nearly endless and do not require invasive techniques or surgery of live animals. A good example to illustrate the possibilities with virtual animals is the study of mate preferences in swordtail fish (Xiphophorus). Basolo (1990) found a female preference for sword length in swordtails by surgically manipulating sword length in sedated live males. Rosenthal and Evans (1998) took advantage of video editing to partially dissolve the swordtail from the body to investigate the underlying mechanisms of this preference. They used manipulated virtual stimulus males that had “normal” swords, only partial swords or no swords at all. They were even able to present sequences of single swords without the fish’s body, but moving as if connected to it, and found that female preference for swords reflects a bias for large apparent size. In a following study, Wong and Rosenthal (2006) used computer animated swordtails to investigate the evolution of mate preferences in swordtail fish. In this animation, the naturally swordless sheepshead swordtail Xiphophorus birchmanni was artificially equipped with a sword revealing a disdain for this trait by females of this species.
Variability of appearance is not the only advantage that virtual animals provide. Live stimulus fish used in experiments differ in their behavior and, hence, influence the test fish’s response. Live stimulus fish might not interact with the live test fish that can result in the rejection of test trials or the repetition of experiments with a new stimulus fish which is very time-consuming. Instead, behavior of virtual stimuli can be predefined and kept constant in every single trial. Recently, the need for such standardized and advanced methods gave rise to the development of free-to-use software for fish biologists, like the program anyFish 2.0 (Veen et al. 2013; Ingley et al. 2015). Müller et al. (2017) also developed user-friendly software to improve design and presentation of animated 3D fish for behavioral experiments. Their software is based on a robot operation system that enables users to steer 3D fish with a video game controller and makes it possible to implement a 3D tracking system (for details see Müller et al. 2016, 2017).
Additionally, there are remarkable studies using nonfish animals that shall be mentioned here. The complex visual display repertoire of the Australian Jacky dragon Amphibolurus muricatus inspired researchers to design an animated 3D lizard opponent to be presented during experiments to get further knowledge on the display’s significance during interaction with conspecifics (Peters and Evans, 2003; Van Dyk and Evans 2008; Woo and Rieucau 2015). To investigate avian social perception, Watanabe and Troje (2006) used an animated 3D pigeon Columba livia and demonstrated its applicability in an operant conditioning paradigm. Parr et al. (2008) showed that chimpanzees Pan troglodytes responded to and discriminated between computer-animated facial expressions of a virtual chimpanzees. Virtual chimpanzees were even able to stimulate contagious yawning in live chimpanzees, indicating an empathic response to their virtual counterparts (Campbell et al. 2009). Neave et al. (2011) studied female preference for different dance moves in 3D animations of human males, and in a recent comparative study, Dolins et al. (2014) showed that humans and chimpanzees were equally able to navigate in a 3D, virtual environment. During experiments, virtual animals can be presented via all kinds of visual devices like tablets or smartphones, but most commonly via computer monitors (CRT or LCD). There are, however, restrictions and limitations concerning stimulus presentation because devices are specially designed for the visual system of humans (Oliveira et al. 2000; Baldauf et al. 2008; Chouinard-Thuly et al. 2017). A thorough validation should, therefore, be obligatory when using virtual stimuli (see e.g., Baldauf et al. 2009; Fischer et al. 2014).
Here, we validated the custom-made simulation for animated 3D sailfin mollies Poecilia latipinna, designed by Müller et al. (2017), for the use in mate-choice experiments with live sailfin mollies. First, to address common concerns whether to use CRT or LCD monitors for the presentation of visual stimuli, we tested which monitor type (CRT or LCD) was more suitable for stimulus presentation. Second, we tested whether different stimulus presentation types (animation, video, or live fish) were equally effective to attract live fish. Third, we disentangled movement from stimulus shape by presenting a static and/or swimming animated 3D box and 3D fish because movement can influence the attractiveness of virtual stimuli (Baldauf et al. 2009; Abaid et al. 2012; Nakayasu and Watanabe 2014; Woo and Rieucau 2015). And fourth, we investigated whether sailfin molly males were able to distinguish between animated 3D males and 3D females.
Material and Methods
Study species
Sailfin mollies are small, neotropical fish inhabiting fresh- and brackwater (Meffe and Snelson 1989). They are livebearers of the family Poeciliidae without parental care, with both male and female choosing their mating partners (Schlupp and Ryan 1997; Witte and Ryan 2002). Sailfin mollies show a strong sexual dimorphism with most males exhibiting large, ornamented dorsal fins, the sailfin, and larger colorful caudal fins (Figure 1A). Male and female sailfin mollies used in experiments were mature descendants of 3 populations of wild mollies. Fish were caught from the Coleto Creek near Victoria (TX, USA) in 1998, from the Comal River in New Braunfels (TX, USA) in 2007 and from Mustang Island near Corpus Christi (TX, USA) in 2014. In the lab, the fish were kept in mixed-sex shoals and separated by populations in large housing tanks (80 × 35 × 40 cm3) under a light–dark cycle of 14:10 h and a constant temperature of 25 ± 1 °C. They were fed daily with flake food (JBL GmbH & Co. KG, Germany), frozen Artemia sp., and chironomid larvae, alternately. All experiments were performed under the German Animal Welfare Act (Deutsches Tierschutzgesetz) during 2014 and 2015, and no animals were harmed.
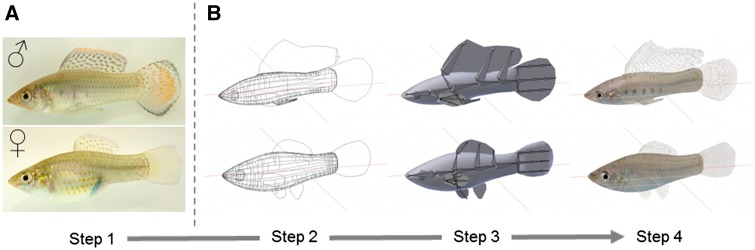
Figure 1.
Design of animated 3D male and 3D female. (A) Lateral photographs of a male and a female sailfin molly that serve as the basis (Step 1) to design the animated model. (B) Steps for 3D fish design as seen in the program Blender. The 3D wire mesh of body and fins (Step 2). Object view of the 3D fish, inner skeleton visible (Step 3). Textures for body and fins wrapped around the 3D fish (Step 4).
Video fish stimulus design
We recorded a male P. latipinna individual in a small tank (25 × 40 × 40 cm3) filled with water, and the same tank without a male using a digital camera (Canon EOS 600D, full HD movie program, 50 fps, Canon Deutschland GmbH, Germany). Tank walls were covered with blue plastic sheets, except for the front, and the ground was covered with blue-colored sand. Illumination was provided by 2 LED strips (40 cm in length, 12 V, 6,500 K) positioned above the longer sides of the tank. Short sequences were cut and combined to a video (Windows Movie Maker, Microsoft, v. 2012).
Animated 3D fish stimulus design
The 3D fish were designed with Blender (v. 2.70a, Blender Foundation, the Netherlands) and then animated and presented during experiments using custom-made software (FishCreator, FishSteering, and FishPlayer) as described in detail in Müller et al. (2017). Using FishCreator, different animated 3D fish stimuli and an artificial 3D box were created. To prevent pseudoreplication, as proposed by Rosenthal (2000), FishCreator enables generation of randomized models with textures taken from various live fish individuals. Stimulus sizes were adjusted to be within the natural range of this species (Supplementary Table 1). Measurements were taken from live males (n = 13) resulting in 4.3 ± 0.7 cm (range 3–5.7 cm) for standard length and 5.4 ± 0.9 cm (range 3.7–7 cm) for total length. Live females (n = 15) measured 3.9 ± 0.5 cm (range 3.3–5.3 cm) in standard length and 4.9 ± 0.7 cm (range 4.1–6.7 cm) in total length. In all treatments, animated 3D sailfin molly males were colorful with raised large dorsal fins. All animated 3D fish (and the 3D box) were also simulated swimming in a virtual tank when presented on screen. Color of the tank wall and the ground could be adjusted manually and then animated. Wall color was blue (105, 167, 205 RGB) in Treatment 2 and gray–white (240, 243, 218 RGB) in Treatments 3 and 4. The ground of the virtual tank was modulated to resemble the blue sand covering the experimental tank containing the live test fish. In contrast to previously used animation techniques (rotoscoping, key framing), 3D fish could be steered freely in space via gamepad using the application FishSteering. Swimming speed varied between 0 and 40 cm/s depending on the input given to the gamepad. Default swimming movements (e.g., undulatory movements, bending) were based on calculations from video analysis of live fish as described by Smielik et al. (2015). In the animation, a sailfin molly was steered to resemble a live fish swimming in a tank and interacting with another fish outside the tank (Gierszewski S, personal observation). Behaviors included (1) swimming in varying heights and depths, (2) parallel swimming at the front wall and presentation the lateral side and raised dorsal fin (in males), and (3) swimming up and down in a position vertical to the front. A movie clip showing an exemplar animation of a 3D sailfin molly male can be found in the Supplementary Material Movie1. Movements were recorded with FishSteering and then loaded into FishPlayer for presentation during experiments. Additionally, an empty virtual tank was recorded for presentation between trials and during acclimatization periods. Resolution of the animation was optimized for the LCD monitors (1920 × 1200 pixels) and presented with a frame rate of 60 fps, which is well above the estimated threshold for motion perception in fish (Fleishman and Endler 2000; Oliveira et al. 2000).
General experimental procedure
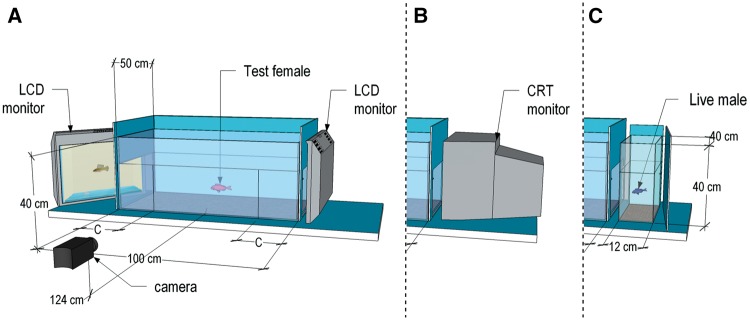
All experiments were performed using the same experimental setup in the same experimental room. The test tank (100 × 50 × 40 cm3; see Figure 2A) was divided into 3 compartments: 2 choice zones (20 cm in depth) at the outer sides of the tank and a neutral zone (60 cm) in the middle. The bottom was covered with blue-colored sand and tank walls were covered with blue plastic sheets except for the front and 2 cut-outs (Treatments 1 and 2: 40 × 25 cm2, Treatments 3 and 4: 40 × 34 cm2) on either side providing a view of the presented stimuli. Six LED strips (12 V, 6500 K) were positioned at the longer sides of the tank, 2 above the rear wall and 4 above the front wall. Water temperature was 25 ± 1 °C and water level was 25 cm deep (34 cm for Treatments 3 and 4). Depending on the treatment, stimuli were either presented on 24″ LCD monitors (EIZO Foris FX2431, EIZO Nanao AG, Austria, 1920 × 1200 pixels resolution; see Figure 2A), a 19″ CRT monitor (Samsung SyncMaster 997 MB, Samsung Electronics Display (M) (HSD), Malaysia, 85 Hz, 1280 × 960 pixels resolution; see Figure 2B), or in small tanks (40 × 40 × 12 cm3; see Figure 2C). Monitors and tanks were positioned adjacent to the choice zones of the test tank at an approximate distance of 2 cm.
Figure 2.
Overview of the experimental setup. (A) Test tank with the live test fish and 2 LCD monitors observed via camera as in Treatments 2.1, 2.2, and in Experiments 3 and 4. For illustration, the left LCD monitor is angled to show an animated scene. (B) Modified setup as used in Experiment 1. One LCD monitor was replaced by a CRT monitor. (C) Setup modified for the use in Treatment 2.3. Both LCD screens were replaced by small tanks filled with water and containing a live male or no fish. C = choice zone.
Test females were kept in small shoals separated from males several weeks prior to experiments. The day before testing, they were transferred to a 40 × 25 × 40 cm3 tank in the experimental room and kept under corresponding lighting and feeding conditions. These tanks featured blue-colored sand and blue plastic sheets on the walls. In Experiment 4, male test fish were used because they were expected to show a more distinct discrimination between the sexes because their reproductive motivation is not dependent on a reproductive cycle, as seen in females (Greven 2011). Males were not separated prior to experiments but directly taken from their home tank. This was done to prevent stress resulting from rivalry in separated male groups or isolation when kept alone. Males were assigned to a color group (“pale” or “colored”). “Pale” was defined as without or only slight black patterns and no orange patterns visible on the fins and on the body. “Colored” was defined as having distinct black and orange patterns. Assignment was done before males were taken from their home tank as colors may fade rapidly as a result of stress (Kawauchi 2006; Nilsson Sköld et al. 2013; Gierszewski S, personal observation). Color is an indicator for social status in male sailfin mollies and dependent on group constellation, with dominant males being more colorful than subordinate male. Color and size are good predictors of mating tactics, with large colorful males mostly relying on courtship and small pale males mostly using a sneaker tactic with forced copulation (Snelson 1985; Fraser et al. 2014).
During the acclimatization period of 10 min, a single test fish could swim freely and explore the test tank for 5 min. Then the fish was positioned inside a plexiglas cylinder (11 cm in diameter) in the middle of the test tank for 5 min. This procedure should guarantee an equal distance between the test fish and both stimuli, and increase the chance that test fish were aware of both stimuli before making a choice. Throughout this period, a tank (video or animation) containing no fish was shown on both sides so fish could get accustomed to the illumination emitting from the monitors. After acclimatization, both stimuli (depending on the treatment) were shown on opposite sides of the test tank. Test fish remained inside the cylinder for 1 min to watch the presented stimuli. Test fish were then released and given 5 min to choose between stimuli. We measured the time each test fish spent within choice zones with a stopwatch. After the first test trial, an intertrial interval (ITI) of 5 min was included during which test fish were gently put back into the cylinder and sides of the stimuli (and monitor types in Experiment 1) were switched to control for side bias. After the ITI, the procedure was repeated for a second test trial and time spent within the choice zones was recorded for another 5 min. Observations were done via camera (Prosilica GT1910c, Allied Vision Technologies GmbH, Germany) from a position not visible to the fish to prevent them from being stressed or influenced by the observer (Figure 2A). Stimuli were always presented simultaneously in a binary choice situation and their position (left or right) was alternated within experiments. For each test fish we measured the absolute association time (in seconds) the test fish spent with each stimulus within each choice zone. Association time is an indirect predictor for mate choice when no physical contact to the stimulus is possible and was used in different studies with this species (Witte and Noltemeier 2002; Witte and Klink 2005; Nöbel and Witte 2013). If test fish spent more than 90% of the total time (first plus second trial) in the same choice zone, even though stimuli were switched, the choice was stated as side biased and fish were excluded from analysis in accordance to other studies (Schlupp and Ryan 1997; Dosen and Montgomerie 2004; Hoysak and Godin 2007; Williams and Mendelson 2010). Standard length of each test fish and live stimulus fish was measured in centimeters after testing. We noted the standard length for all presented stimuli. For animated 3D fish and video stimuli, total length was also measured. After experiments, all test fish were returned to their home tanks. For each experiment and treatment we used new, live test fish. We performed the following experiments in the same sequence as presented below.
Experiment 1: video male on CRT versus video male on LCD monitor
Both monitor types were tested in a binary choice situation. Two identical videos of a male were presented on a LCD screen on one side and on a CRT screen on the other side of the tank (Figure 2B). With reference to former studies (e.g., Witte and Ueding 2003; Witte and Klink 2005) it is known that sailfin molly females perceive and respond to video playbacks. As screens differed in overall size, the display area of the video was adjusted to be of the same size on both screens (35 × 19.8 cm2) and resolution was set to 1280 × 960 pixels. For acclimatization and ITI, the video of an empty tank was shown so test fish could get accustomed the light emission from the monitors. White plastic sheets prevented females from viewing when position of monitor types was switched. We tested 18 females.
Experiment 2: comparison of different presentation types
In Experiment 2, we tested whether live test females differ in discrimination between a fish stimulus and a tank containing no fish when presenting these stimuli with different presentation types (animation, video, live). In each treatment, we used identical stimuli in every trial to keep stimuli as constant as possible to ensure comparability between presentation types. The side on which the fish stimulus was shown was alternated and distributed equally between left and right for all treatments.
Treatment 2.1: animated 3D male versus animated tank
In Treatment 2.1, live females were given a choice between a swimming 3D male animation on one side and a 3D animated tank (same tank but without an animated fish) on the other side, both stimuli presented on LCD screens. The tank was also shown on both sides during acclimatization and ITI. We tested 18 females.
Treatment 2.2: video male versus video tank
In Treatment 2.2, we presented live test females a video of a swimming male as 1 stimulus and a video of a tank as the alternative stimulus. Both stimuli were presented on LCD screens. During acclimatization and ITI, the tank was shown on both sides. We tested 18 females.
Treatment 2.3: live male versus real tank
In Treatment 2.3, live females could choose between a live male (presented in a real tank) and a real tank (filled with water but without a fish) as the alternative stimulus. During acclimatization and ITI, white plastic sheets prevented females from viewing the adjacent tanks. We used the same live male individual for the whole treatment for comparability between treatments with different stimulus presentation types. The live male was chosen to resemble the animated 3D male (Treatment 2.1) and the video male (Treatment 2.2). We tested 25 females.
Experiment 3: decoupling movement and shape of a stimulus
Here we tested whether live females distinguish between an animated 3D male and a 3D box (Figure 3A) that were either static or swimming. By decoupling movement and shape of the stimuli we tested how these parameters affect association time.
Figure 3.

Overview of animated 3D fish stimuli used in Experiments 3 and 4. (A) Animated 3D box and animated 3D male used in Experiment 3. (B) Different animated 3D male and 3D female sailfin mollies presented in pairs of varying combinations in Experiment 4.
Treatment 3.1: moving 3D male animation versus static 3D box animation
In this treatment, we presented live females an animation of a swimming 3D male and a static 3D box on LCD screens. The box represented dimensions (length, height, width) of the animated fish and was colored in the mean RGB value of the fish texture (207, 197, 149 RGB; see Figure 3A). The animated male was moving around the animated tank, the box was positioned in the center of the animated tank, not moving. The dorsal fin of the male was raised all the time to keep its lateral projection area constant for the duration of the experiment. We tested 23 females.
Treatment 3.2: static 3D male animation versus moving 3D box animation
In Treatment 3.2, we presented live females identical animations as used in Treatment 3.1, but now the male was static in the center of the animated tank and the box was “swimming” the identical path as the animated male did in Treatment 3.1. Lateral projection area of the fish was kept constant. We tested 22 females.
Experiment 4: animated 3D male versus animated 3D female
In Experiment 4, each live test male (pale and colored males) could choose between animations of a 3D male and a 3D female. Both animated fish were size matched and swam an identical path. FishCreator was used to generate 3 different sailfin mollies of each sex, so a total of 9 combinations of male and female animations could be presented (see Figure 3B and Supplementary Material Movie2). We tested 24 males.
Data analysis
For data analysis, we used R 3.2.2 (R Development Core Team 2015). To test for differences between association times within Experiments 1 and 4, we used paired Wilcoxon signed-rank tests. To analyze association time in Experiments 2 and 3, we used linear mixed effect (LME) models with the lme function in the nlme package (Pinheiro et al. 2015) with association time as the outcome variable. For Experiment 2, presentation type (animation, video, live), stimulus type (fish or tank), and size of the test females (SL) were fixed factors. Presentation type (PT) was equal to treatment, so treatment was not included as an additional factor. Following Crawley (2007) we used the function contrasts to define 2 orthogonal contrasts for PT: (PT1) live versus any virtual stimulus (sum of video plus animation), and (PT2) animation versus video. Identity of test fish (ID) was included as random factor. ID was nested in population. A plot of the standardized residuals against the fitted values revealed inhomogeneity of the residual variances. To account for this heteroskedasticity in the model, a weights function using the varIdent class of the lme function was included to allow different variances for each level of stimulus type and presentation type (Pinheiro and Bates 2000; Zuur et al. 2009). For Experiment 3, stimulus shape (fish or box), movement (moving or static), treatment, and test female size (SL) were set as fixed factors. Female identity (ID) was included as random factor and nested in population. We inspected model assumptions (Q/Q plots, normality of residuals, residuals against fitted values) visually. Given P values were considered significant if P ≤ 0.05.
Results
Association times measured for each experiment, total number of test fish and those showing side biases as well as all size measurements of test fish and used stimuli can be found in the Supplementary Material.
Experiment 1: video male on CRT versus video male on LCD monitor
Females (n = 16) spent significantly more time in front of the video male presented on the LCD screen than in front of the video male presented on the CRT screen (Wilcoxon signed-rank test: V = 26, P = 0.029; see Figure 4). Thus, we used only LCD monitors in the following experiments.
Figure 4.
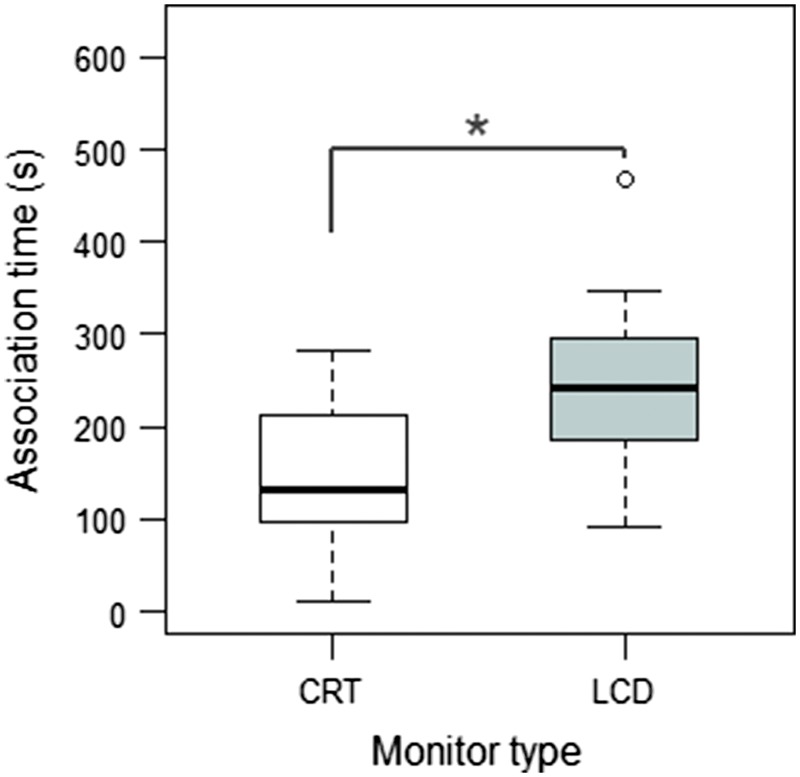
Results of Experiment 1: test of monitor type. Association times (s) for the video presentation of a swimming male on LCD and CRT screen are given. Boxplots of median, quartiles, and whiskers (1.5 × interquartile range) are shown. Circles indicate outliers. n = 16; *P ≤ 0.05.
Experiment 2: comparison of different stimulus presentation types
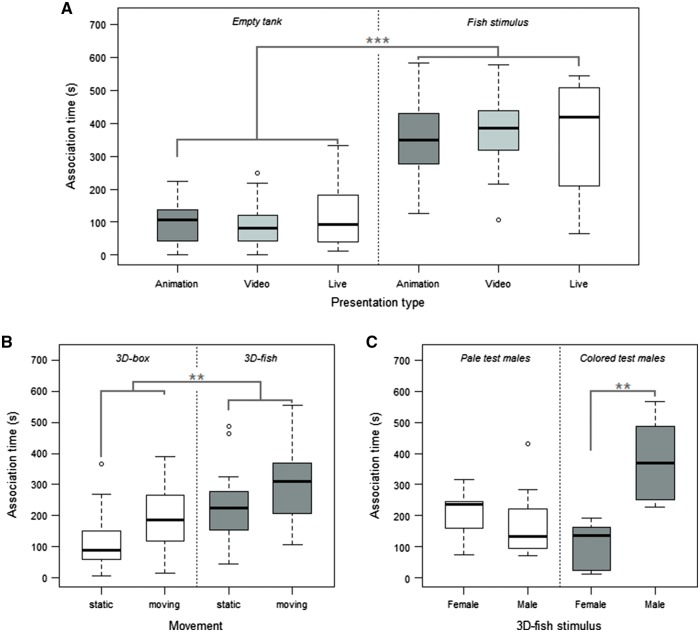
Results showed that association time was significantly affected by the stimulus type “fish” (LME: t = 11.500, P < 0.001), raising it on average by 258.4 ± 22.5 s (see Estimate in Table 1) when compared with the empty tank. Presentation type (animation, video, live) and size of the test females did not affect association time (Table 1, Figure 5A).
Table 1.
LME estimates for effects on association time in Experiments 2 and 3
| Fixed effects | Estimate | Standard error | df | t | P |
|---|---|---|---|---|---|
| Experiment 2 | |||||
| (Intercept) | 20.708 | 57.554 | 55 | 0.360 | 0.720 |
| PT1 | 13.697 | 11.883 | 55 | 1.153 | 0.254 |
| PT2 | 0.677 | 11.009 | 55 | 0.062 | 0.951 |
| Type “fish” | 258.393 | 22.477 | 55 | 11.500 | <0.001 |
| SL | 22.276 | 14.170 | 55 | 1.572 | 0.122 |
| Experiment 3 | |||||
| (Intercept) | 124.516 | 50.157 | 30 | 2.483 | 0.019 |
| Treatment “3.2” | −12.223 | 30.290 | 28 | −0.404 | 0.690 |
| Shape “fish” | 100.935 | 28.374 | 30 | 3.557 | 0.001 |
| Movement “moving” | 69.465 | 28.374 | 30 | 2.448 | 0.020 |
| SL | 0.567 | 3.947 | 28 | 0.144 | 0.887 |
Notes: Absolute association time was the outcome variable throughout. Given are estimates with standard error, degrees of freedom, t values, and P values for each fixed effect. Intercept estimates show the grand mean for each experiment. Intercept reference categories for factor estimates are “live” (PT1), “animation” (PT2), type “tank” for Experiment 2, and Treatment “3.1,” shape “box,” and movement “static” for Experiment 3. Significant values (P ≤ 0.05) are printed in bold. PT = presentation type; SL = standard length of test female.
Figure 5.
Results of Experiments 2, 3, and 4. (A) Association time obtained in Experiment 2 testing for stimulus presentation type (animation, video, live) when a tank or a fish was presented as either 3D animation, video, or live stimulus. nanimation = 15, nvideo = 16, nlive = 17. (B) Association time obtained in Experiment 3 for stimulus shape when static or moving. nbox/static = 15, nbox/moving = 17, nfish/static = 17, nfish/moving = 15. (C) Association time for the animated 3D male and 3D female stimuli in Experiment 4 for pale and colored live test males, npale = 7, ncolored = 8. Boxplots showing median, quartiles, whiskers (1.5 × interquartile range), and outliers (circles). **P < 0.01, ***P < 0.001.
Experiment 3: decoupling movement and shape of a stimulus
Association time was affected by movement (LME: t = 2.448, P = 0.02), raising it on average by about 69.5 ± 28.4 s (see Estimate in Table 1) when the stimulus (fish or box) was moving. Stimulus shape also affected association time (LME: t = 3.557, P = 0.001), raising it by about 100.9 ± 28.4 s (see Estimate in Table 1) when the animated stimulus was a fish (Figure 5B).
Experiment 4: animated 3D male versus animated 3D female
Pale test males (npale = 7) showed no significant preference for either male or female 3D animation (Wilcoxon signed-rank test: npale = 7, V = 12, P = 0.813). Colored males (ncolored = 8), however, spent significantly more time with animated male stimuli than with animated female stimuli (Wilcoxon signed-rank test: ncolored = 8, V = 36, P = 0.008; see Figure 5C). Thus, males could discriminate between animated 3D male and 3D female stimuli. Supplementary Material Movie2 illustrates an exemplar response of a colored live male toward a 3D female animation, including following, displaying and gonopodial thrusting (at minute 00:10).
Discussion
Our results showed that animated 3D sailfin mollies can be a useful tool in mate-choice studies with live sailfin mollies. The response of live females to an animated 3D stimulus was as strong as to a video male or even a live male. Movement alone was important, but females responded stronger to a swimming fish stimulus than to a box “swimming” the same path. Colored test males were able to discriminate between animated 3D male and 3D female fish, hence, validating the 3D fish as biologically relevant stimuli in choice experiments. Additionally, test females spent significantly more time in front of a male video when presented on a LCD screen than on a CRT screen.
Live test females reacted attentively toward a 3D male animation when presented together with an animated tank as an alternative stimulus and spent significantly more time in front of the male. This experimental design also served as a control for the usage of animated stimuli in previous studies with fish, showing that a fish animation was preferred over an empty scene (Künzler and Bakker 1998; Clark and Stephenson 1999; Morris et al. 2003; Kuperberg et al. 2009; Culumber and Rosenthal 2013). In comparison to different stimulus presentation types (animation, video, live), that are commonly used in behavioral experiments, test fish significantly preferred the presented fish stimulus over a tank as an alternative stimulus, irrespective of the used presentation type. Stimulus presentation in all presentation types led to a similar response in sailfin mollies, thus, our animated male seemed to be as attractive as a live male, or a video male for sailfin molly females. Our results are in accordance with the results of Qin et al. (2014) in which zebrafish Danio rerio did not differentiate between live, video, and animated fish stimuli. Clark and Stephenson (1999) also found no difference in shoaling tendency toward animated, video, or live conspecifics in the tiger barb Puntius tetrazona.
By decoupling movement and shape of an animated stimulus, we showed that movement significantly increased attractiveness of a given stimulus (both box and fish), but that a swimming fish was more attractive to females than a swimming box. The shape of a moving stimulus matters. In terms of the freely steerable nature of our animated fish, this result underlines the usability of our new approach and presents a more flexible alternative to classic rotoscoping or key framing animation techniques. FishPlayer even allows the reuse of once created swimming paths with various fish models to gain consistency between experiments. Here, one should keep in mind, that freely steered stimuli might induce an individual experimenter effect. Therefore, we are currently developing an automatic swimming mode. Movement as a critical feature to evoke a response in live fish could also be shown in cichlids. Baldauf et al. (2009) showed that both male and female cichlids Pelvicachromis taeniatus preferred a moving 2D animation of the opposite sex over a stationary one. Sometimes the manner in which a stimulus is moving seems to be even more important than its appearance. Woo and Rieucau (2015) discovered the importance of syntax for recognition of visual displays in the jacky dragon Amphibolurus muricatus. They showed that jacky dragons paid same attention toward displays of animated 3D jacky dragons independent of whether animations were highly realistic or abnormal as long as the display’s syntax was correct. A study by Abaid et al. (2012) highlighted the importance of moving speed and coordination of animated 2D zebrafish shoals for a shoaling preference in live test fish when compared to a static image.
Our results indicate that live test males were able to distinguish between animated 3D males and 3D females. Despite the assumption that test males would generally spend more time with the female animation, pale test males showed no preference for either stimulus. Colored males, however, spent significantly more time with the animated male. Animated males were large and colorful with large dorsal fins raised all the time, which might have elicited stronger agonistic responses in colorful live test males. In the given test situation, we assumed that colorful test males recognized the animated male as a rival of similar or lower quality and, hence, tried to chase him away, thus, spending more time in the choice zone in front of him. Colorful males are more dominant in their home tanks and mostly rely on courtship to attract females, but also spend lots of time chasing rival males to secure their own paternity. Pale males tend to stay close to females for copulation, but also close to dominant males as these constantly court fecund females. Here, pale males get their opportunities for sneak copulation. This might explain why pale males showed no distinct preference for either stimulus, nevertheless still discriminating between the 2 stimuli. It might be that pale and colorful males used different parameters to make their decision (e.g., quality of competitor compared to self). Discrimination between sexes of animated fish served as a control in other studies as well (Turnell et al. 2003; Baldauf et al. 2009).
Conclusion and Future Directions
The major advantages of 3D computer animations in behavior research are (1) creation of highly variable virtual animals which decreases pseudoreplication when compared to video playback. Stimuli are designed according to well-defined parameters providing high variability of morphology and appearance when compared to live test animals or videos. Parameters like shape, size, color, and behavior, can even be varied beyond natural extents. Moreover, 3D computer animations allow for specific manipulation and control of behavioral patterns, which is more difficult with 2D animation or video, and almost impossible with live animals. The 3D animations can be moved within the 3D environment as live fish do. (2) Computer-animated animals allow a high degree of standardization in test situations, and, thus, provide highly controlled, fast, and repeatable testing procedures. And (3) they allow reduction in the number of live test animals, which is in line with the three guiding principles (3Rs) of replacement, reduction, and refinement (Richmond 2010) proclaimed in the guidelines for the treatment of animals in behavioral research and teaching (ASAB 2014).
Regarding the advantages of this promising method, one has to keep in mind that a thorough validation is obligatory before using animations in tests with live animals because its usability might be species specific. Prior to experiments, it should be investigated whether “behavior” of animated animals can elicit similar responses like live stimuli to test animals. Our presented results validated the usage of animated 3D fish as a powerful tool in mate-choice tests in sailfin mollies. The next step will be to implement a 3D fish animation that can interact with a live fish in real time to further study and discover underlying mechanisms in mate-choice decisions (Müller et al. 2016). This interactive approach will open new horizons for studying fish behavior.
Supplementary Material
Acknowledgments
We thank Maria Mastoras who helped with photographing and cutting fish textures and Arndt Wellbrock for valuable comments on statistics. We thank Kathryn Dorhout and Shumail Ahmad for proofreading the manuscript and 2 anonymous reviewers for their valuable comments.
Funding
This study was funded by the Deutsche Forschungsgemeinschaft (DFG) with a grant to S.G. and K.W. ( WI 1531/12-1) and to K.M., I.S., J.M.H., and K.D.K. (KU 689/11-1).
Supplementary Material
Supplementary material can be found at http://www.cz.oxfordjournals.org/.
References
- Abaid N, Spinello C, Laut J, Porfiri M, 2012. Zebrafish Danio rerio responds to images animated by mathematical models of animal grouping. Behav Brain Res 232:406–410. [DOI] [PubMed] [Google Scholar]
- Amcoff M, Lindqvist C, Kolm N, 2013. Sensory exploitation and plasticity in female mate choice in the swordtail characin. Anim Behav 85:891–898. [Google Scholar]
- ASAB, 2014. Guidelines for the treatment of animals in behavioural research and teaching. Anim Behav 87:I–IX. [DOI] [PubMed] [Google Scholar]
- Baldauf SA, Kullmann H, Bakker TCM, 2008. Technical restrictions of computer-manipulated visual stimuli and display units for studying animal behaviour. Ethology 114:737–751. [Google Scholar]
- Baldauf SA, Kullmann H, Thünken T, Winter S, Bakker TCM, 2009. Computer animation as a tool to study preferences in the cichlid Pelvicachromis taeniatus. J Fish Biol 75:738–746. [DOI] [PubMed] [Google Scholar]
- Basolo AL, 1990. Female preference for male sword length in the green swordtail Xiphophorus helleri (Pisces: Poeciliidae). Anim Behav 40:332–338. [Google Scholar]
- Campbell MW, Carter JD, Proctor D, Eisenberg ML, de Waal FBM, 2009. Computer animations stimulate contagious yawning in chimpanzees. Proc R Soc B 276:4255–4259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouinard-Thuly L, Gierszewski S, Rosenthal GG, Reader SM, Rieucau G. et al. , 2017. Technical and conceptual considerations for using animated stimuli in studies of animal behavior. Curr Zool 63:5–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark DL, Macedonia JM, Rosenthal GG, 1997. Testing video playback to lizards in the field. Copeia 1997:421–423. [Google Scholar]
- Clark DL, Stephenson KR, 1999. Response to video and computer-animated images by the tiger barb. Environ Biol Fishes 56:317–324. [Google Scholar]
- Clark DL, Uetz GW, 1990. Video image recognition by the jumping spider Maevia inclemens (Araneae: Salticidae). Anim Behav40:884–890. [Google Scholar]
- Clotfelter ED, Curren LJ, Murphy CE, 2006. Mate choice and spawning success in the fighting fish Betta splendens: the importance of body size, display behavior and nest size. Ethology 112:1170–1178. [Google Scholar]
- Crawley MJ, 2007. The R Book. West Sussex: John Wiley & Sons Ltd. [Google Scholar]
- Culumber ZW, Rosenthal GG, 2013. Mating preferences do not maintain the tailspot polymorphism in the platyfish Xiphophorus variatus. Behav Ecol 24:1286–1291. [Google Scholar]
- Dolins FL, Klimowicz C, Kelley J, Menzel CR, 2014. Using virtual reality to investigate comparative spatial cognitive abilities in chimpanzees and humans. Am J Primatol 76:496–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosen LD, Montgomerie R, 2004. Female size influences mate preferences of male guppies. Ethology 110:245–255. [Google Scholar]
- Egger B, Klaefiger Y, Theis A, Salzburger W, 2011. A sensory bias has triggered the evolution of egg-spots in cichlid fishes. PLoS ONE 6:e25601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer S, Taborsky B, Burlaud R, Fernandez AA, Hess S. et al. , 2014. Animated images as a tool to study visual communication: a case study in a cooperatively breeding cichlid. Behaviour 151:1921–1942. [Google Scholar]
- Fleishman LJ, Endler JA, 2000. Some comments on visual perception and the use of video playback in animal behavior studies. Acta Ethol 3:15–27. [Google Scholar]
- Fraser BA, Janowitz I, Thairu M, Travis J, Hughes KA, 2014. Phenotypic and genomic plasticity of alternative male reproductive tactics in sailfin mollies. Proc R Soc B 281:20132310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greven H, 2011. Gonads, genitals, and reproductive biology In: Evans JP, Pilastro A, Schlupp I, editors. Ecology and Evolution of Poeciliid Fishes. Chicago/London: The University of Chicago Press, 3–17. [Google Scholar]
- Hoysak DJ, Godin JGJ, 2007. Repeatability of male mate choice in the mosquitofish Gambusia holbrooki. Ethology 113:1007–1018. [Google Scholar]
- Ingley SJ, Rahmani Asl M, Wu C, Cui R, Gadelhak M. et al. , 2015. anyFish 2.0: an open-source software platform to generate and share animated fish models to study behavior. SoftwareX 3–4:13–21. [Google Scholar]
- Kawauchi H, 2006. Functions of melanin-concentrating hormone in fish. J Exp Zool 305A:751–760. [DOI] [PubMed] [Google Scholar]
- Kim SY, Velando A, 2014. Stickleback males increase red coloration and courtship behaviours in the presence of a competitive rival. Ethology 120:1–9. [Google Scholar]
- Kopman V, Laut J, Polverino G, Porfiri M, 2012. Closed-loop control of zebrafish response using a bioinspired robotic-fish in a preference test. J R Soc Interface 10:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Körner KE, Lütjens O, Parzefall J, Schlupp I, 1999. The role of experience in mating preferences of the unisexual amazon molly. Behaviour136:257–268. [Google Scholar]
- Künzler R, Bakker TCM, 1998. Computer animations as a tool in the study of mating preferences. Behaviour 135:1137–1159. [Google Scholar]
- Kuperberg ES, Brown AC, Clotfelter ED, 2009. Body condition in male Betta splendens does not predict their ability to perform opercular displays under hypoxic conditions. Ethology 115:1182–1189. [Google Scholar]
- Landgraf T, Bierbach D, Nguyen H, Muggelberg N, Romanczuk P. et al. , 2016. RoboFish: increased acceptance of interactive robotic fish with realistic eyes and natural motion patterns by live Trinidadian guppies. Bioinsp Biomim 11:015001. [DOI] [PubMed] [Google Scholar]
- Magnus DBE, 1954. Experimentelle Untersuchungen am Kaisermantel zur Analyse optischer Auslösungsreize. Deut Entomol1953:58–75 [in German]. [Google Scholar]
- McKinnon JS, McPhail JD, 1996. Male aggression and colour in divergent populations of the threespine stickleback: experiments with animations. Can J Zool 74:1727–1733. [Google Scholar]
- Meffe GK, Snelson FF, 1989. Ecology and Evolution of Livebearing Fishes (Poeciliidae). Englewood Cliffs (NJ): Prentice Hall. [DOI] [PubMed] [Google Scholar]
- Morris MR, Nicoletto PF, Hesselman E, 2003. A polymorphism in female preference for a polymorphic male trait in the swordtail fish Xiphophorus cortezi. Anim Behav 65:45–52. [Google Scholar]
- Müller K, Smielik I, Hütwohl JM, Gierszewski S, Witte K. et al. , 2017. The virtual lover: variable and easily guided 3D fish animations as an innovative tool in mate-choice experiments with sailfin mollies-I. Design and implementation. Curr Zool 63:55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller K, Gierszewski S, Witte K, Kuhnert KD. 2016. Where is my mate? Real-time 3-D fish tracking for interactive mate-choice experiments. Accepted for ICPR 2016—23rd International Conference on Pattern Recognition. VAIB 2016 - Visual observation and analysis of Vertebrate and Insect Behavior Workshop Proceedings. Cancun, Mexico, 4 December, 2016.
- Nakayasu T, Watanabe E, 2014. Biological motion stimuli are attractive to medaka fish. Anim Cogn 17:559–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neave N, McCarty K, Freynik J, Caplan N, Hönekopp J. et al. , 2011. Male dance moves that catch a woman’s eye. Biol Lett 7:221–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson Sköld H, Aspengren S, Wallin M, 2013. Rapid color change in fish and amphibians: function, regulation, and emerging applications. Pigment Cell Melanoma Res 26:29–38. [DOI] [PubMed] [Google Scholar]
- Nöbel S, Witte K, 2013. Public Information influences sperm transfer to females in sailfin molly males. PLoS ONE 8:e53865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira RF, Rosenthal GG, Schlupp I, McGregor PK, Cuthill IC. et al. , 2000. Considerations on the use of video playbacks as visual stimuli: the Lisbon workshop consensus. Acta Ethol 3:61–65. [Google Scholar]
- Parr LA, Waller BM, Heintz M, 2008. Facial Expression Categorization by Chimpanzees using Standardized Stimuli. Emotion 8:216–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters RA, Evans CS, 2003. Introductory tail-flick of the Jacky dragon visual display: signal efficacy depends upon duration. J Exp Biol 206:4293–4307. [DOI] [PubMed] [Google Scholar]
- Pinheiro J, Bates D, DebRoy S, Sarkar D, R Core Team, 2015. nlme: linear and nonlinear mixed effects models R Package Version 3.1–121 [cited 2016 November 5]. Available from: http://cran.r–project.org/package=nlme.
- Pinheiro JC, Bates DM. 2000. Mixed-Effects Models in S and S-PLUS. New York: Springer. [Google Scholar]
- Qin M, Wong A, Seguin D, Gerlai R, 2014. Induction of social behavior in zebrafish: live versus computer animated fish as stimuli. Zebrafish 11:185–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team, 2015. R: a language and environment for statistical computing Vienna (Austria): R Foundation for Statistical Computing [cited 2016 November 5]. Available from http://www.r–project.org/.
- Richmond J, 2010. The three Rs In: Hubrecht R, Kirkwood J, editors. The UFAW Handbook on the Care and Management of Laboratory and Other Research Animals. Oxford: Wiley-Blackwell, 5–22. [Google Scholar]
- Rosenthal GG, 1999. Using video playback to study sexual communication. Environ Biol Fishes 56:307–316. [Google Scholar]
- Rosenthal GG, 2000. Design considerations and techniques for constructing video stimuli. Acta Ethol 3:49–54. [Google Scholar]
- Rosenthal GG, Evans CS, 1998. Female preference for swords in Xiphophorus helleri reflects a bias for large apparent size. Proc Natl Acad Sci USA 95:4431–4436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal GG, Wagner WE, Ryan MJ, 2002. Secondary reduction of preference for the sword ornament in the pygmy swordtail Xiphophorus nigrensis (Pisces: Poeciliidae). Anim Behav 63:37–45. [Google Scholar]
- Rowland WJ, Bolyard KJ, Jenkins JJ, Fowler J, 1995. Video playback experiments on stickleback mate choice: female motivation and attentiveness to male colour cues. Anim Behav 49:1559–1567. [Google Scholar]
- Schleidt WM, 1961. Reaktionen von Truthühnern auf fliegende Raubvögel und Versuche zur Analyse ihrer AAM´s. Zeitschr F Tierpsychol 18:534–560 [in German]. [Google Scholar]
- Schlupp I, Ryan MJ, 1997. Male sailfin mollies Poecilia latipinna copy the mate choice of other males. Behav Ecol 8:104–107. [Google Scholar]
- Smielik I, Müller K, Kuhnert KD. 2015. Fish motion simulation. In: Al-Akaidi M, Ayesh A, editors. ESM 2015—European Simulation and Modelling Conference Proceedings Leicester (UK): EUROSIS, 26-28 October, 2015, 392–396.
- Snelson FFJ, 1985. Size and morphological variation in males of the sailfin molly Poecilia latipinna. Environ Biol Fishes 13:35–47. [Google Scholar]
- Ter Pelkwijk JJ, Tinbergen N, 1937. Eine reizbiologische Analyse einiger Verhaltensweisen von Gasterosteus aculeatus L. Z Tierpsychol 1:193–200 [in German]. [Google Scholar]
- Tinbergen N, 1948. Social releasers and the experimental method required for their study. Wilson Bull 60:6–51. [Google Scholar]
- Tinbergen N, Kuenen DJ, 1939. Über die auslösenden und die richtungsgebenden Reizsituationen der Sperrbewegung von jungen Drosseln (Turdus m. merula L. und T. e. ericetorum Turton). Zeitschrift F Tierpsychol3:37–60 [in German]. [Google Scholar]
- Tinbergen N, Perdeck AC, 1951. On the stimulus situation releasing the begging response in the newly hatched herring gull chick (Larus argentatus argentatus Pont.). Behaviour 3:1–39. [Google Scholar]
- Turnell ER, Mann KD, Rosenthal GG, Gerlach G, 2003. Mate choice in zebrafish Danio rerio analyzed with video-stimulus techniques. Biol Bull 205:2001–2002. [DOI] [PubMed] [Google Scholar]
- Van Dyk DA, Evans CS, 2008. Opponent assessment in lizards: examining the effect of aggressive and submissive signals. Behav Ecol 19:895–901. [Google Scholar]
- Veen T, Ingley SJ, Cui R, Simpson J, Asl MR. et al. , 2013. anyFish: an open-source software to generate animated fish models for behavioural studies. Evol Ecol Res 15:361–375. [Google Scholar]
- Ward AJW, Sumpter DJT, Couzin ID, Hart PJB, Krause J, 2008. Quorum decision-making facilitates information transfer in fish shoals. Proc Natl Acad Sci USA 105:6948–6953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S, Troje NF, 2006. Towards a “virtual pigeon”: a new technique for investigating avian social perception. Anim Cogn 9:271–279. [DOI] [PubMed] [Google Scholar]
- Williams TH, Mendelson TC, 2010. Behavioral isolation based on visual signals in a sympatric pair of darter species. Ethology 116:1038–1049. [Google Scholar]
- Witte K, Klink KB, 2005. No pre-existing bias in sailfin molly females Poecilia latipinna, for a sword in males. Behaviour 142:283–303. [Google Scholar]
- Witte K, Noltemeier B, 2002. The role of information in mate-choice copying in female sailfin mollies Poecilia latipinna. Behav Ecol Sociobiol 52:194–202. [Google Scholar]
- Witte K, Ryan MJ, 2002. Mate choice copying in the sailfin molly Poecilia latipinna, in the wild. Anim Behav 63:943–949. [Google Scholar]
- Witte K, Ueding K, 2003. Sailfin molly females Poecilia latipinna copy the rejection of a male. Behav Ecol 14:389–395. [Google Scholar]
- Wong BBM, Rosenthal GG, 2006. Female disdain for swords in a swordtail fish. Am Nat 167:136–140. [DOI] [PubMed] [Google Scholar]
- Woo KL, Rieucau G, 2011. From dummies to animations: a review of computer-animated stimuli used in animal behavior studies. Behav Ecol Sociobiol 65:1671–1685. [Google Scholar]
- Woo KL, Rieucau G, 2015. The importance of syntax in a dynamic visual signal: recognition of jacky dragon displays depends upon sequence. Acta Ethol 18:255–263. [Google Scholar]
- Zbinden M, Largiadèr CR, Bakker TCM, 2004. Body size of virtual rivals affects ejaculate size in sticklebacks. Behav Ecol 15:137–140. [Google Scholar]
- Zbinden M, Mazzi D, Künzler R, Largiadèr CR, Bakker TCM, 2003. Courting virtual rivals increase ejaculate size in sticklebacks Gasterosteus aculeatus. Behav Ecol Sociobiol 54:205–209. [Google Scholar]
- Zuur A, Ieno EN, Walker N, Saveliev AA, Smith GM, 2009. Mixed Effects Models and Extensions in Ecology with R. New York: Springer. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.