Abstract
Sex allocation theory applied to hermaphrodites assumes that there is a trade off between the allocation of resources to male and female functions, within a fixed reproductive resource budget. Charnov's classic resource allocation model predicts a more female-biased sex allocation when competition among different sperm donors is low due to diminishing fitness returns for male investment. By manipulating the social group size, one automatically changes the population density at which individuals live. Increasing population density may affect reproductive allocation, leading to resource competition and/or to increased concentration of harmful metabolites. This could lead to an over- or underestimation of the individual adjustment of sex allocation responses to mating opportunities. In this article, we tested the effects of density and social group size separately on female investment and body growth (considered as proxy of the overall energy budget) in the simultaneously hermaphroditic polychaete worm Ophryotrocha diadema. We manipulated social group size (i.e., monogamous and promiscuous regimes) and density (i.e., 4 levels) using a full-factorial design, to identify the underlying factor affecting female allocation (in terms of egg production) and body growth. In contrast to findings of previous experiments, we found that an increase in population density reduced body growth and egg production of hermaphrodites irrespective of social group size. We advance the hypothesis that the increase of catabolites and oxygen consumption in high-density conditions reduces the overall resource budget and this could obscure group size effects on female fecundity.
Keywords: body growth, female fecundity, mating group size, Ophryotrocha diadema, resource budget
Sex allocation theory for hermaphroditic organisms predicts that the optimal investment of the fraction of resources allocated to each sex function depends on mating group size (i.e., the average number of mating partners in a group). Indeed, simultaneous hermaphrodites are expected to adjust their reproductive resource investment to male and female functions strategically, according to variation in the number of available partners and competitors (Charnov 1982; Fischer 1984; reviewed by Schärer 2009). When the number of mates is very small (e.g., 1 pair of mates) hermaphrodites are expected to allocate as few resources as possible to sperm production (just enough to fertilize the eggs spawned by the partner) and to allocate relatively more resources to the female sex function—that is, egg production (Charnov 1982; Fischer 1984). This female-biased sex allocation increases when competition occurs between sperm from the same donor for fertilizing the eggs laid by the recipient-mating partner, a situation defined by Schärer (2009) as Local Sperm Competition (but see also Greeff et al. 2001). Indeed, producing more sperm than required for fertilizing the available eggs do not pay from the sperm donor’s perspective. In contrast, as the mating group size increases, competition will arise among the sperm of different donors. Thus, hermaphrodites are expected to allocate proportionally more resources to the male function and fewer to the female function.
Generally, all the sex allocation models for simultaneous hermaphrodites are based on the assumptions that the reproductive resource budget is fixed, invariant within individuals of a population, and that there is a direct trade off between the allocation to male and female function (Charnov 1982). However, it is very difficult to find a study model that displays those specific peculiarities. Furthermore, quantifying the effect of social group size on male and female investment is very challenging in most simultaneous hermaphroditic organisms. How to choose the right trait to be measured and how to measure it is still an issue that requires care (Schärer 2009).
Empirical tests of sex allocation predictions often investigated the effect on male and female investment of the social group size (i.e., the total number of interacting individuals), rather than that of the mating group size (i.e., the number of conspecifics which are potential mating partners), because the latter is hard to manipulate (but see Janicke et al. 2013 who succeeded in measuring the mating group size in the flatworm Macrostomum lignano). By manipulating the social group size, one automatically changes the population density (i.e., the number of individuals per area or per volume) at which individuals live, and density may itself affect reproductive allocation leading to resource competition or concentration of harmful metabolites (Schärer and Ladurner 2003). Factors strictly related to the density level in an environment, for example, food and space availability (Locher and Baur 2002; Vizoso et al. 2007), body size (Schärer et al. 2001), and stress (Hughes et al. 2003; Schärer 2009) may affect the overall resource budget of an individual, indirectly affecting the resource budget allocated into the sex functions. Thus, not controlling adequately for the density may lead to an over- or underestimate of the individual adjustment of sex allocation responses to mating opportunities.
Population density strongly influences the life history traits of several invertebrates. Laboratory studies on the fresh-water snail Biomphalaria glabrata (Thomas and Benjamin 1974) and the land snails Cepaea nemoralis (Williamson et al. 1976; Oosterhoff 1977) and Cepaea hortensis (Cameron and Carter 1979) have shown that, as population density increases, growth rate and fecundity of gastropod molluscs diminish, even when food is in excess. Likewise, the crowding effect—defined by Goser and Ratte (1994) as a density- dependent mechanism due to chemical substances released by the individuals of a population when they come into physical contact, but independent of food competition—affects negatively body size, body weight, and number of eggs produced in tapeworms (Roberts 2000) and water fleas (Preuss et al. 2009, 2010).
The protandrous simultaneous hermaphroditic polychaete worm Ophryotrocha diadema has been used extensively in the last 40 years for ecological (Simonini et al. 2009, 2010), behavioural (Sella 1988; Sella and Lorenzi 2000), and evolutionary biology studies (Lorenzi and Sella 2008). The majority of papers have focused on the role of group size on sex allocation during the hermaphroditic phase of this polychaete worm, showing that worms in the hermaphroditic phase plastically adjust their female allocation according to mating opportunities irrespective of population density (Lorenzi et al. 2005, 2014b). Likewise, Schärer and Ladurner (2003) and Brauer et al. (2007) showed that the hermaphroditic flatworms of M. lignano plastically adjust their investment in male function according to variation of social group size.
In contrast to what has been observed in the hermaphroditic phase, an increase in social group size experienced by protandrous males in the protandrous phase of O. diadema had a negative effect on their body growth and on the first egg production as soon as they reached the hermaphroditic phase. However, in the successive egg layings, the newly mature hermaphrodites adjusted their egg output to the current social conditions (Cannarsa et al. 2015). According to these authors, the reduced investment in body size and egg production at the first egg laying of worms reared in large group sizes could be related to crowding costs experienced during the protandrous phase rather than to a strategic response to social conditions (i.e., protandrous males could adjust their male investment according to the group size, which they could perceive as a cue to the level of prospective sperm competition). However, in Cannarsa et al. (2015), density effects on the number of eggs and cocoons laid, as well as on body growth, were not controlled.
In the light of these results, we investigated density effect considering group size and density as factors in a 2–way design. In previous experiments, density was considered simply as volume per bowl (Lorenzi et al. 2006, 2008) Thus, variation in density were examined only as variation in volume of water. However, a change in the social group size, maintaining the same volume of water, results also in a changed density per individual, a state that could influence the life history traits under study. Instead, in the present article, we defined density as volume of water per worm. Thus, we manipulated the density level in which individuals were reared, maintaining it identical across 2 different social group sizes. In this way, we separated the density effect from the group size effect completely. Therefore, we tested for the influence of social group size and density on female fecundity and body growth of O. diadema separately, by comparing female investment in worms reared in monogamous and promiscuous mating regimes at 4 different volumes of water per worm, thus testing 4 levels of density per worm for each mating regime. Because body growth is related to resource availability, it was considered as a proxy of the overall resource budget.
We were faced with three hypotheses. First, if female investment is mainly affected by mating opportunities, as sex allocation theory predicts, hermaphrodites will lay a smaller number of eggs when they are in large groups than when they are in pairs and their body growth is invariant, irrespective of the density level they experience. Second, density may be perceived as a cue of the mating opportunities as density dependent parameters, such as encounter rate or the concentration of waterborne signals, are reliable cues for counting the number of conspecifics in the neighborhood. Thus, we expect that both the mating regime and the density level affect female investment, being extremely correlated to each other. Worms in monogamous regime should lay more eggs than worms in promiscuous regime and within the same mating regime, worms living at the lowest density levels should produce more eggs than those living at the highest densities. At the same time, if worms adjust their female investment strategically, body size, used as a proxy for the overall resource budget should remain constant. Third, if female investment is mainly affected by population density, hermaphrodites living at high-density levels will produce a smaller number of eggs than worms living at low density, irrespective of the mating regime they experienced. According to this hypothesis, crowding effects result in an overall reduced energy budget and hence diminished body growth.
Accordingly, we could assess the contribution of a strategic sex allocation or a suffered environmental condition on the plastic female investment of the O. diadema worms. Clarifying this aspect could be of central importance in future experiments on sex allocation not only in this species.
Material and Methods
Study animal
Ophryotrocha diadema is an outcrossing protandrous hermaphroditic polychaete worm found in clusters of mussels in nutrient-rich waters in California (Åkesson 1976) and Sicily (Simonini et al. 2009, 2010) harbors. Generally, densities of Ophryotrocha populations fluctuate greatly (Prevedelli et al. 2005) and mating opportunities may vary accordingly. Population densities are reported to be generally very low (Simonini R, personal communication and Reish D, personal communication to Sella G).
Ophryotrocha diadema worms release eggs inside a jelly cocoon. Larvae develop in about a week and leave their cocoons when they are 3-segments long. The protandrous phase starts as soon as the larvae leave their cocoons, when they reach a body size of 4 chaetigerous segments (Sella 1990). Protandrous males have functional sperm and their efficiency in fertilizing the eggs laid by a hermaphrodite is positively correlated with body size (Sella 1990) and their efficiency is maximum (∼100%) starting from the body size of 10 segments onward (Sella 1990). When protandrous males compete with hermaphrodites, they can fertilize up to 30% of the hermaphrodites’ eggs sneaking in among mating pairs of mature hermaphrodites (Sella and Lorenzi 2003). The simultaneously hermaphroditic phase is reached at the body size of ∼15 body segments when individuals start producing oocytes. Oocytes can be seen in the coelom through the transparent body walls (Sella 1990). Mature worms reproduce repeatedly for nearly 13 weeks (Åkesson 1976; Premoli and Sella 1995). The number of laid eggs peaks in the third week after sexual maturity (Åkesson 1976) and then declines.
Parameters of female investment, that is, the production of oocytes, the number of eggs per cocoon, and the number of cocoons, can be easily measured, in contrast to male investment, measured as the production of sperm. Sperm production is very low (∼50 sperm per egg—Sella 1990).
Mature worms mate by pseudocopulation, a process of external fertilization in which partners maintain close physical contact before releasing their gametes. When paired, hermaphrodites regularly exchange eggs by alternating sexual roles in successive reproductive bouts (Sella 1985). In this condition, a hermaphrodite usually lays a cocoon of about 30 eggs every second or third day (Premoli and Sella 1995). Reciprocity in egg-exchange persists only as long as worms are paired and there are no other potential mates nearby (Sella and Lorenzi 2000). According to Lorenzi et al. (2006), when the number of rivals increases, hermaphrodites tend to increase the frequency of intolerant acts (bites, fast withdrawals, and pursuits) toward other hermaphrodites significantly, a behavior interpreted as part of the male investment. In this situation, multiply fertilized cocoons become common (Lorenzi et al. 2014a).
The presence of potential reproductive competitors or partners is perceived by means of waterborne chemical cues (Schleicherová et al. 2006, 2010).
Rearing methods
The experiment was carried out using a laboratory population established from worms collected at Long Beach, CA (Åkesson 1976) and renewed with worms collected in 1995 (Sella, personal communication) from the same locality.
Experimental worms were chosen from the progeny of 60 O. diadema hermaphrodites randomly chosen from the breeding masses and paired. Three hundred and fifty two larvae where selected immediately after hatching, at a body size of 4 segments, and reared in isolation during the whole protandrous phase. In this way, we avoided potential confounding effects on female investment of the costs of sperm expenditure and of complex social interactions, such as aggressive behaviors among conspecifics prior the attainment of the hermaphroditic phase. Indeed, Sella and Lorenzi (2003) found that reproductive bouts played as males during the protandrous phase negatively affected the body growth and the fitness of protandrous males.
Worms were reared in bowls containing artificial filtered seawater (34 g/l salinity), at constant temperature of 21°C and fed with chopped spinach ad libitum, thus limiting food competition. Until focal worms were isolated, we changed water every other week. Once worms had reached the hermaphroditic phase, we changed water every 10 days.
Experimental setup
We reared the newly mature hermaphrodites in 2 mating regimes (monogamy and promiscuity) and 4 density conditions. All worms entered the experiment the same day, at the same body size (i.e., 13 body segments) and the same age. Worms in the same bowl were not siblings, to avoid pseudoreplications.
We manipulated group size and density in a 2-way design. Two group sizes, monogamy and promiscuity were subject to 4 different densities (i.e., 4 water volumes per worm), which were maintained equal across the 2 mating regimes. Indeed worms reared in monogamy at a given density level had the same volume of water per worm as worms reared at the same density level in promiscuity. Density levels were the following:
very high-density level—0.5 ml of water per worm
high-density level—2.5 ml of water per worm
intermediate-density level—7.5 ml of water per worm
low-density level—25 ml of water per worm.
Once the hermaphroditic phase was attained, the newly adult worms were used for the experiment. In the promiscuous mating regime, we set up 4 replicates per density level, assigning 20 hermaphrodites to every replicate (total number of experimental worms = 320). For the very high-density level, hermaphrodites were reared in 10 ml of water; for the high-density level, in 50 ml of water; for the intermediate-density level, in 150 ml of water; and for the low-density level, 500 ml of water. In the monogamous mating regime, we set up 4 replicates per density level, assigning one pair of worms to each replicate (total number of experimental worms = 32). For the very high density level, pairs were reared in 1 ml; for the high density level, in 5 ml; for the intermediate density level, in 15 ml; and for the low density level, in 50 ml of water.
The experiment lasted for a month. We measured female fecundity by counting the number of eggs per capita. In addition, we measured the number of cocoons per capita to control whether the density level may affect the number of reproductive bouts that worms played as females.
Data on female fecundity were collected daily. Every day newly laid cocoons were removed to maintain the same level of population density in the experimental bowls. Variance in the number of offspring could be influenced by competition for resources to be invested in egg production or egg cannibalism. For this reason, we minimized both factors providing food ad libitum and by removing cocoons from parents every day. Lorenzi et al. (2008, 2014b) showed that egg mortality is not significantly different between small and large populations.
We estimated the body growth of worms by counting the number of body segments every worm had reached at the end of the experiment. Body growth was considered as the difference between the number of body segments worms had reached at the end of the experiment and the number of segments they had at the beginning of the hermaphrodite phase.
Statistical analyses
We determined whether the variables “egg number per capita” and “cocoon number per capita” showed a normal distribution by performing the Kolmogorov–Smirnov test (at P = 0.05) and Normal Q-Q plots. To test the null hypothesis—that the egg and cocoon number per capita across the 4 densities and 2 group sizes were not significantly different—we performed 2 ANOVA tests. We used the Levene test to check for heteroscedasticy. Variances resulted to be homogeneous.
In both the ANOVA tests, we applied the Tukey Post Hoc test to detect at which density levels the mean number of eggs and cocoons were significantly different from each other.
Body growth variability was analyzed using a generalized linear mixed model (GLMM) with Poisson error distribution and a log-link function. Predictor variables included density level and mating regime, while the identity of every replicate was handled as a random factor to control for similarities among worms reared in the same bowl.
We performed all the analyses using the software SPSS 22 (SPSS, Inc., Chicago, IL).
Results
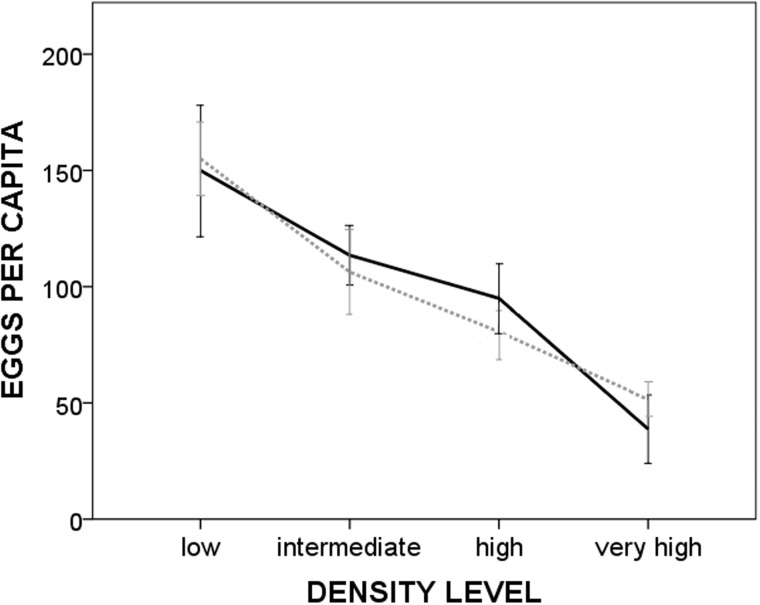
The number of eggs per capita was significantly affected by density both in monogamy and in promiscuity. Under both mating regimes, it significantly increased as the density level decreased (Table 1, Figure 1). The ANOVA test did not reveal a significant effect of the mating regime or of the interaction density*mating regime on the mean egg number per capita. The Post Hoc tests confirmed a significant difference of the mean egg number in all the pairwise comparisons of the density levels (Table 1).
Table 1.
Results of the ANOVA testing for the effect of density (expressed in ml per worm) and mating regime on the egg number per capita
| ANOVA | P | Post hoc pairwise comparisons, P | |
|---|---|---|---|
| Density | F3,24 = 53.022 | < 0.001 | |
| 0.5 ml vs. 2.5 ml | < 0.001 | ||
| 0.5 ml vs. 7.5 ml | < 0.001 | ||
| 0.5 ml vs. 25 ml | < 0.001 | ||
| 2.5 ml vs. 7.5 ml | = 0.028 | ||
| 2.5 ml vs. 25 ml | < 0.001 | ||
| 7.5 ml vs. 25 ml | < 0.001 | ||
| Mating regime | F1,24 = 0.022 | 0.884 | |
| Density*Mating regime | F3,24 = 0.988 | 0.415 |
Figure 1.
Mean egg number per capita (±SE) laid by hermaphrodites reared in monogamy and promiscuity, according to 4 density levels (very high: 0.5 ml of water per worm; high: 2.5 ml per worm; intermediate: 7.5 ml per worm; and low 25 ml per worm). Continuous line = monogamy. Dashed line = promiscuity.
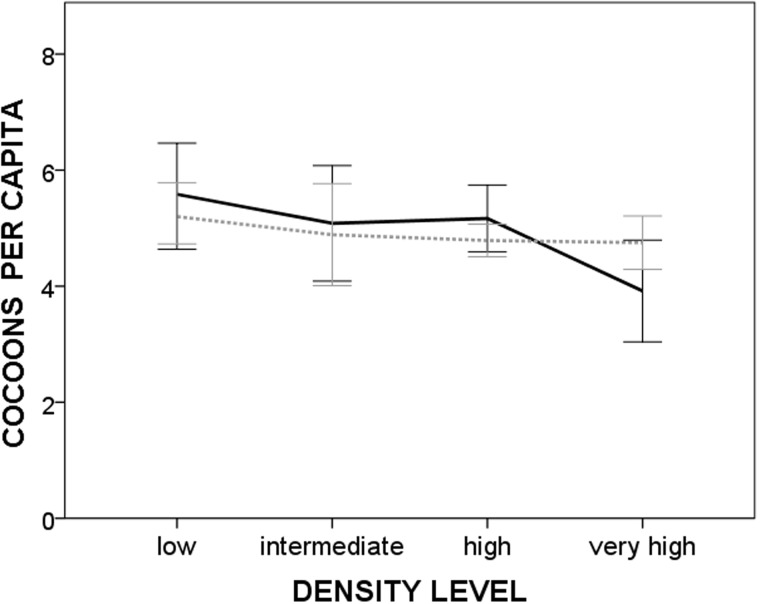
The number of cocoons per capita (Figure 2) was not affected neither by the density level nor the mating regime (ANOVA, group size: F1,24 = 0.008; P = 0,924; density: F3,24 = 1.806; P = 0.173; group size*density: F3,24 = 0.680; P = 0.505).
Figure 2.
Cocoon number (± SE) per capita laid by hermaphrodites reared in monogamy and promiscuity, according to 4 density levels (very high: 0.5 ml of water per worm; high: 2.5 ml per worm; intermediate: 7.5 ml per worm; and low 25 ml per worm). Continuous line = monogamy. Dashed line = promiscuity.
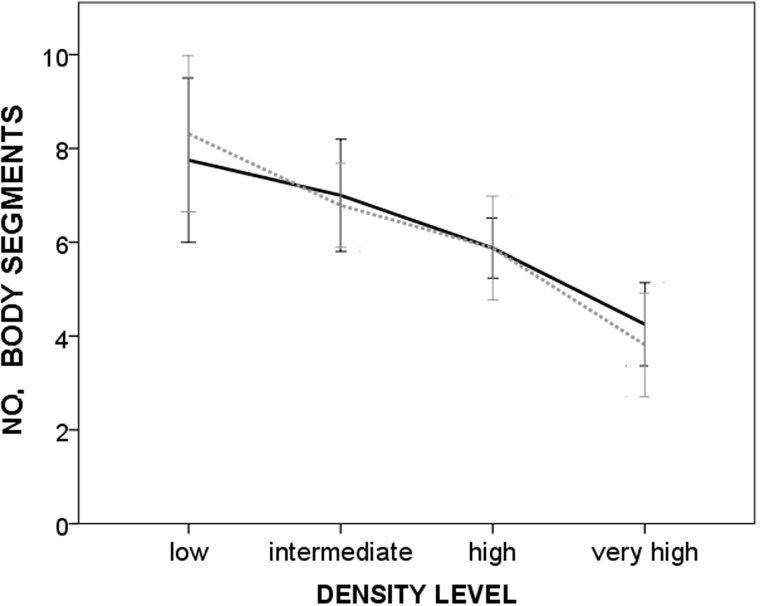
Body growth was significantly affected by density, but not by mating regime (Figure 3). There was no statistical interaction between the 2 predictors as reported in Table 2.
Figure 3.
Mean values (± SE) of body growth reached by hermaphrodites reared in monogamy and promiscuity, according to 4 density levels (very high: 0.5 ml of water per worm; high: 2.5 ml per worm; intermediate: 7.5 ml per worm; and low 25 ml per worm). Continuous line = monogamy. Dashed line = promiscuity.
Table 2.
GLMM examining the effect of density (expressed in ml per worm) on the body growth
| GLMM | P | Pairwise comparisons, P | |
|---|---|---|---|
| Density | F3,341 = 21.894 | <0.001 | |
| 0.5 ml vs. 2.5 ml | < 0.001 | ||
| 0.5 ml vs. 7.5 ml | < 0.001 | ||
| 0.5 ml vs. 25 ml | < 0.001 | ||
| 2.5 ml vs. 7.5 ml | < 0.001 | ||
| 2.5 ml vs. 25 ml | < 0.001 | ||
| 7.5 ml vs. 25 ml | < 0.001 | ||
| Mating regime | F1,341 = 0.34 | 0.560 | |
| Density*Mating regime | F1,341 = 0.164 | 0.686 | |
| Bowl identity | 0.060 |
Discussion
In our study, we documented that egg production was strongly affected by population density in O. diadema simultaneous hermaphrodites. Density affected the overall individual resource budget. The number of eggs laid by hermaphrodites increased as the population density level diminished, irrespective of the social group size. The reduction of fecundity with the increased density level may have been caused by waste products (e.g., gut discards) and catabolites. Indeed, it has been suggested that catabolites act as egg-laying inhibitory substances in opisthobranchs (Sprenger et al. 2011) and pulmonate gastropods (Jordaens et al. 2007). In addition, the rapid oxygen consumption which occurs in small amounts of water may constitute a stress agent lowering the amount of energy disposal in small organisms when they experience high levels of population density, a phenomenon reported by Hughes et al. (2003) for the bryozoan Celleporella hyalina. As the water volume increases, metabolites and catabolites are more scattered, and there is a higher amount of oxygen available in the environment. Also, newly sexually mature O. diadema hermaphrodites which had experienced a large group size—thus a high-density population—during their protandrous phase produced a significantly smaller number of eggs per cocoon at their first egg laying than worms reared in isolation in the same water volume (Cannarsa et al. 2015).
In contrast to the number of eggs per capita, the number of cocoons (i.e., the number of bouts played as a female) was not sensitive to the different levels of density nor to mating regime hermaphrodites had experienced. This is in accordance with the results of previous works (Cannarsa et al. 2015; Meconcelli et al. 2015), in which the number of cocoons was not affected by the mating regimes. Hermaphrodites in a group could tend to play more bouts as males reducing the allocation to the female function to such an extent that some individuals suppress egg production (Di Bona et al. 2010) to maximize their male investment. Our results showed that individuals behaved as females with the same frequency in pairs and in groups, and there was no preference for the male role in the promiscuous regime.
Moreover, the individuals’ body growth was affected by the density level too. The lower the density level, the larger the body length (i.e., number of segments) achieved by the experimental worms. The same relationship between body growth and population density was observed by Cannarsa et al. (2015) in protandrous males of O. diadema. Protandrous worms reared in isolation grew significantly more than those reared in group sizes larger than 1 and in the same water volume as isolated worms, thus at an increased density level. Similar results are reported for polychaete nereids species. Nesto et al. (2012) found that density has negative effects on growth rates in Hediste diversicolor. Body growth rate decreases when individuals are reared in high density. The authors suggest that the negative influence is due not only to catabolites, but also to costs related to increasing intraspecific competition. Increased density affects survival in the polychaete worms Nereis arenaceodentata (Pesh et al. 1987) and Diopatra aciculata (Safarik et al 2006) and in the water flea Daphnia magna negatively. At high densities, water fleas develop and grow more slowly and produce fewer offspring than conspecifics, which have experienced a lower density level (Preuss et al. 2009). In M. lignano (Schärer et al. 2005), reared in pairs and in groups at 2 different enclosures and resource availability, body growth was negatively affected in the small enclosures (but see Schärer and Ladurner 2003, where density did not had any effect on body size of the flatworms). In Schärer et al. (2005), the reproductive resource budget (measured by testis and ovary size) and the time of female maturation were affected negatively as well. According to Schärer et al. (2005), a difference in reproductive resources budget across the 2 group size treatments was caused by the feeding procedure that probably failed to produce similar overall resource budgets for worms reared at low and high density. As a result, such difference was probably due to uncontrolled density effects. In our experiment, we cannot infer that the difference in body growth is related to direct competition as food was given ad libitum. Moreover, the possible increased amount of interactions seemed not to have lowered the energy possibly allocated into body growth in worms reared in the promiscuous regime, as it did not affect the number of segments of the experimental individuals, which achieved a similar body size to those reared in pairs.
Several authors suggest that changes in male or female investment in hermaphrodites are phenotypically plastic changes in sex allocation in response to changes in mating regimes. In some of these studies, effects of mating regimes on fecundity and body size could not be disentangled from density effects (Trouvé et al. 1999; Tan et al. 2004; Raimondi and Martin 1991). In contrast, Schärer and Ladurner (2003) disentangled the effects of mating regime and those of density. They show how M. lignano hermaphrodites plastically increase their investment in male function in large group sizes, irrespective of density, and diminish their investment in small group sizes. Lorenzi et al. (2005) tested for effects of variation of group size (i.e., high- and low-mating opportunities) and density on the number of cocoons in O. diadema. They found that hermaphrodites plastically varied their investment in female function according to variation in group size, irrespective of density. Schleicherová et al. (2006) observed that female investment, measured as number of cocoons laid by focal worms, in groups of 12 O. diadema worms kept at the density of 0.8 ml per worm was less than that in isolated pairs at the density per 5 ml per worm. When worms were reared in water containing catabolites of the related species Ophryotrocha hartmanni, cocoon production was the same as that of worms reared in clean water. Therefore, the authors argued that the reduced female investment at the highest density was not a consequence of the stress caused by accumulation of possibly toxic catabolites, but rather of the ability of worms to evaluate group size by means of a waterborne species-specific cue that induced worms to shift their female allocation according to the group size.
Our results are different from those of Lorenzi et al. (2005) and Schleicherovà et al. (2006). They can be related to our different experimental setup. Indeed, we tested for density and group size effects on female fecundity and body growth, by taking into account both a larger variation in group sizes, a more accurate estimate of density and a longer experimental time. Where Lorenzi et al. (2005) and Schleicherová et al. (2006) used 2–12 worms; we used 2–20 worms per bowl. Where Lorenzi et al. (2005) considered density as volume of water of the rearing containers, we considered density as volume of water per worm. Indeed, Lorenzi et al. (2005) tested for variation of group size and density and found no effect of density on female allocation, but the relation between the increased group size and the density was not linear: pairs were tested in water volumes of 5 ml and 30 ml per worm respectively, while groups of 12 individuals were tested in water volumes of 0.83 ml and 5 ml per worm. Density at the high and low mating opportunities were not comparable. Moreover, in our experimental design, the time of water replacement in the experimental bowls lasted ten days, so that we could increase possible accumulation effects of harmful catabolites on the two female fecundity parameters and body growth. In contrast, in the setup of Schleicherová et al. (2006), water was changed every day. Therefore, it is possible that the use of frequently replaced water did not allow worms to perceive any crowding effect. In addition, we analysed female fecundity in a time interval of 30 days, while the former papers only took an interval of 9 days into account during which many of the experimental individuals did not lay at all.
Lorenzi et al. (2006) suggested that O. diadema worms could have a male-biased sex allocation in groups, as the energy at one’s disposal could be allocated more to male function (i.e., to male aggressive behaviors) than to female function (i.e., egg production). Even if we did not control for the interactions and aggressive behaviors among conspecifics in the experimental bowls, we could expect that hermaphrodites in group would have behaved aggressively more often than hermaphrodites in pairs, thus allocating less resources to egg production. The fact that experimental worms laid a similar number of eggs and cocoons and grew similarly irrespective of the mating regime lead us to consider that such aggressive interactions do not affect the production of gametes (female at least). We can advance the hypothesis that, probably, in large volumes of water per worm, differences in social group size may not reflect differences in mating opportunities, which are primarily affected by the actual distance among conspecifics. In contrast, in small volumes of water, it is easier for hermaphrodites to sense the number of conspecifics and consider all of them as potential mates/rivals. Moreover, in O. diadema hermaphrodites, there is a tendency to form apparently isolated pairs even in promiscuous mating regimes (Cannarsa E., personal observation). Studying the effects of density and group size on female investment in Chelidonura sandrana,Sprenger et al. (2011) find that mate encounter rate increases substantially as density and group size increase. These authors expected a mating rate close to the male optimum at large mating opportunities, but they found that the mating rate remained close to the female fitness optimum. Therefore, they advanced the hypothesis that a non-random distribution of hermaphrodites over the available volume could confound the contributions of group size and density on mating opportunities even though a rigorous experimental design was applied. This doubt holds also for O. diadema worms, which spend time in mucous trails they build along the bowl walls. Obviously, pairs of O. diadema in large volumes of water per worm were more isolated than pairs reared in smaller volumes. Therefore, the number of potential mates decreased. More observations are required to determine the real number of mating opportunities within a given density level in this species.
We found that population density considered as volume per individual strongly influences female investment in egg production and body growth. Such a result is probably related to the laboratory/experimental conditions. It is clear that such densities applied to the experimental individuals do not exist in nature. Ophryotrochadiadema worms live in rich nutrient marine water, where it is impossible to reach the concentration of catabolites and levels of oxygen consumption similar to our experimental conditions. Moreover, O. diadema worms live in extremely scattered populations (Simonini R, personal communication)—1 animal per 300 m2 of surface of fouling mussels (Sella et al. 2000)—and it is relatively hard to find in the wild the same group size we consider for our experiments. For these reasons, it could be possible that O. diadema worms were not selected under similar selective pressures and it is consequently plausible that individuals are not able to strategically allocate their resources in relation to the density level, considering it as a cue for the group size and the mating opportunities. Instead, it seemed clear that individuals suffer prohibitive conditions during the experiments, which affect their overall energy budget leading to different production of female gametes, as to a reduced body growth.
However, in the light of our results, other experiments with high rigorous experimental procedure (e.g., constant water density, observations duration, clean water replacement, etc.) are needed to compare results from different studies and avoid misleading conclusions. This study throws light on an important aspect affecting female fecundity in Ophryotrocha diadema. Our study calls for further experiments aimed at clarifying the biotic and abiotic environmental factors that affect the investment of reproductive resources in simultaneous hermaphrodites.
Acknowledgments
We thank Gabriella Sella, Dasa Schleicherovà and two anonymous referees for their helpful comments that improved a lot our manuscript. Moreover, we thank Vincent Marsicano for the English revision.
References
- Åkesson B, 1976. Morphology and life cycle of Ophryotrocha diadema, a new polychaete species from California. Ophelia 15: 23–35. [Google Scholar]
- Brauer VS, Schärer L, Michiels NK, 2007. Phenotypically flexible sex allocation in a simultaneous hermaphrodite. Evolution 61: 216–222. [DOI] [PubMed] [Google Scholar]
- Cameron RAD, Carter MA, 1979. Intra- and interspecific effects of population density on growth and activity in some Helicid land snails (Gastropoda: Pulmonata). J Anim Ecol 48: 237–246. [Google Scholar]
- Cannarsa E, Lorenzi MC, Sella G, 2015. Early social conditions affect female fecundity in hermaphrodites. Curr Zool 61: 983–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charnov EL, 1982. The Theory of Sex Allocation. Princeton: Princeton University Press. [PubMed] [Google Scholar]
- Fischer EA, 1984. Local mate competition and sex allocation in simultaneous hermaphrodites. Am Nat 124: 590–596. [Google Scholar]
- Greeff JM, Nason JD, Compton SG, 2001. Skewed paternity and sex allocation in hermaphroditic plants and animals. Proc R Soc Lond B 268: 2143–2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes RN, Manriquez PH, Bishop JDD, Burrows MT, 2003. Stress promotes maleness in hermaphroditic modular animals. Proc Natl Acad Sci USA 100: 10326–10330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janicke T, Marie-Orleach L, De Mulder K, Berezikov E, Ladurner P et al. , 2013. Sex allocation adjustment to mating group size in a simultaneous hermaphrodite. Evolution 67: 3233–3242. [DOI] [PubMed] [Google Scholar]
- Jordaens K, Dillen L, Backeljau T, 2007. Effects of mating, breeding system and parasites on reproduction in hermaphrodites: pulmonate gastropods (Mollusca). Anim Biol 57: 137–195. [Google Scholar]
- Goser B, Ratte HT, 1994. Experimental-evidence of negative interference in Daphnia magna. Oecologia 98: 354–361. [DOI] [PubMed] [Google Scholar]
- Locher R, Baur B, 2002. Nutritional stress changes sex–specific reproductive allocation in the simultaneously hermaphroditic land snail Arianta arbustorum. Funct Ecol 16: 623–632. [Google Scholar]
- Lorenzi MC, Schleicherova D, Sella G, 2006. Life history and sex allocation in the simultaneously hermaphroditic polychaete worm Ophryotrocha diadema: the role of sperm competition. Integr Comp Biol 46: 381–389. [DOI] [PubMed] [Google Scholar]
- Lorenzi MC, Schleicherová D, Sella G, 2008. Sex adjustments are not functionally costly in simultaneous hermaphrodites. Mar Biol 153: 599–604. [Google Scholar]
- Lorenzi MC, Schleicherová D, Sella G, 2014a. Multiple paternity and its benefits in non-selfing, egg-trading hermaphrodites. Acta Ethol 17: 173–179 [Google Scholar]
- Lorenzi MC, Sella G, 2008. A measure of sexual selection in hermaphroditic animals: parentage skew and the opportunity for selection. J Evol Biol 21: 827–833. [DOI] [PubMed] [Google Scholar]
- Lorenzi MC, Sella G, Schleicherová D, Ramella L, 2005. Outcrossing hermaphroditic polychaete worms adjust their sex allocation to social conditions. J Evol Biol 18: 1341–1347. [DOI] [PubMed] [Google Scholar]
- Lorenzi MC, Sella G, Schleicherová D, 2014b. Demographic costs of sex allocation: hermaphrodites perform better in sparse populations. Ital J Zool 81: 72–77. [Google Scholar]
- Meconcelli S, Cannarsa E, Sella G, 2015. Plasticity of morphological traits in response to social conditions in a simultaneous hermaphrodite. Ethol Ecol Evol 1–15. [Google Scholar]
- Nesto N, Simonini R, Prevedelli D, Da Ros L, 2012. Effects of diet and density on growth, survival and gametogenesis of Hediste diversicolor (OF Müller, 1776), (Nereididae, Polychaeta). Aquaculture 362: 1–9. [Google Scholar]
- Oosterhoff LM, 1977. Variation in growth rate as an ecological factor in the landsnail Cepaea nemoralis (L.). Neth J Zool 27: 1–132. [Google Scholar]
- Pesh CE, Zajac RN, Whitlatch RB, Balboni MA, 1987. Effect of intraspecific density on life history traits and population growth rate in Neanthes arenaceodentata (Polychaeta: Nereididae) in the laboratory. Mar Biol 96: 545–554. [Google Scholar]
- Premoli MC, Sella G, 1995. Sex economy in benthic polychaetes. Ethol Ecol Evol 7: 27–48. [Google Scholar]
- Preuss TG, Hammers-Wirtz M, Hommen U, Rubach MN, Ratte HT, 2009. Development and validation of an individual based Daphnia magna population model: the influence of crowding on population dynamics. Ecol Model 220: 310–329. [Google Scholar]
- Preuss TG, Hammers-irtz M, Ratte HT, 2010. The potential of individual based population models to extrapolate effects measured at standardized test conditions to relevant environmental conditions: an example for 3, 4-dichloroaniline on Daphnia magna. J Environ Monitor 12: 2070–2079. [DOI] [PubMed] [Google Scholar]
- Prevedelli D, Massamba-N'Siala G, Simonini R, 2005. The seasonal dynamics of six species of Dorvilleidae (Polychaeta) in the harbour of La Spezia (Italy). Mar Ecol 26: 286–293. [Google Scholar]
- Raimondi PT, Martin JE, 1991. Evidence that mating group size affects allocation of reproductive resources in a simultaneous hermaphrodite. Am Nat 138: 1206–1217. [Google Scholar]
- Roberts LS, 2000. The crowding effect revisited. J Parasitol 86: 209–211. [DOI] [PubMed] [Google Scholar]
- Safarik M, Redden AM, Schreider MJ, 2006. Density-dependent growth of the polychaete Diopatra aciculata. Sci Mar 70: 337–341. [Google Scholar]
- Schärer L, 2009. Tests of sex allocation theory in simultaneously hermaphroditic animals. Evolution 63: 1377–1405. [DOI] [PubMed] [Google Scholar]
- Schärer L, Ladurner P, 2003. Phenotypically plastic adjustment of sex allocation in a simultaneous hermaphrodite. Proc R Soc Lond B: Biol Sci 270.1518:935–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schärer L, Sandner P, Michiels NK, 2005. Trade-off between male and female allocation in the simultaneously hermaphroditic flatworm Macrostomum sp . J Evol Biol 18: 396–404. [DOI] [PubMed] [Google Scholar]
- Schärer L, Karlsson LM, Christen M, Wedekind C, 2001. Size dependent sex allocation in a simultaneous hermaphrodite parasite. J Evol Biol 14: 55–67. [DOI] [PubMed] [Google Scholar]
- Schärer L, Wedekind C, 2001. Social situation, sperm competition and sex allocation in a simultaneous hermaphrodite parasite, the cestode Schistocephalus solidus. J Evol Biol 14: 942–953 [DOI] [PubMed] [Google Scholar]
- Schleicherová D, Lorenzi MC, Sella G, 2006. How outcrossing hermaphrodites sense the presence of conspecifics and suppress female allocation. Behav Ecol 17: 1–5. [Google Scholar]
- Schleicherová D, Lorenzi MC, Sella G, Michiels NK, 2010. Gender expression and group-size: a test in a hermaphroditic and a gonochoric congeneric species of Ophryotrocha (Polychaeta). J Exp Biol 213: 1586–1590. [DOI] [PubMed] [Google Scholar]
- Sella G, 1985. Reciprocal egg trading and brood care in a hermaphroditic polychaete worm. Anim Behav 33: 938–944. [Google Scholar]
- Sella G, 1988. Reciprocation, reproductive success, and safeguards against cheating in a hermaphroditic polychaete worm Ophryotrocha diadema Åkesson, 1976. Biol Bull 175: 212–217. [Google Scholar]
- Sella G, 1990. Sex allocation in the simultaneously hermaphroditic polychaete worm Ophryotrocha diadema. Ecology 71: 27–32. [DOI] [PubMed] [Google Scholar]
- Sella G, Lorenzi MC, 2000. Partner fidelity and egg reciprocation in the simultaneously hermaphroditic polychaete worm Ophryotrocha diadema. Behav Ecol 11: 260–264. [Google Scholar]
- Sella G, Lorenzi MC, 2003. Increased sperm allocation delays body growth in a protandrous simultaneous hermaphrodite. Biological J Linn Soc 78: 149–154. [Google Scholar]
- Simonini R, Grandi V, Massamba-N'Siala G, Martino MP, Castelli A et al. , 2010. Diversity, habitat affinities and diet of Ophryotrocha species (Polychaeta, Dorvilleidae) living in Mediterranean harbour habitats. Vie Milieu 60: 27–38. [Google Scholar]
- Simonini R, Massamba-N'Siala G, Grandi V, Prevedelli D, 2009. Distribution of the genus Ophryotrocha (Polychaeta) in Italy: new records and comments on the biogeography of Mediterranean species. Vie Milieu 59: 79–88. [Google Scholar]
- Sprenger D, Lange R, Anthes N, 2011. Population density and group size effects on reproductive behaviour in a simultaneous hermaphrodite. BMC Evol Biol 11: 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan GN, Govedich FR, Burd M, 2004. Social group-size, potential sperm competition and reproductive investment in a hermaphroditic leech Helobdella papillomata (Euhirudinea: Glossiphoniidae). Evol Biol 17: 574–580. [DOI] [PubMed] [Google Scholar]
- Thomas JD, Benjamin M, 1974. The effects of population density on growth and reproduction of Biomphalaria glabrata (Gasteropoda: Pulmonata). J Anim Ecol 43: 31–50. [Google Scholar]
- Trouvé S, Jourdane J, Renaud F, Durand P, Morand S, 1999. Adaptive sex allocation in a simultaneous hermaphrodite. Evolution 53: 1599–1604. [DOI] [PubMed] [Google Scholar]
- Vizoso DB, Schärer L, 2007. Resource dependent sex allocation in a simultaneous hermaphrodite. Evol Biol 20: 1046–1055. [DOI] [PubMed] [Google Scholar]
- Williamson P, Cameron RAD, Carter MA, 1976. Population density affecting adult shell size of snail Cepaea nemoralis. Nature 263: 496–497. [DOI] [PubMed] [Google Scholar]