Abstract
A host of abiotic factors modify fish social behavior. However, few studies have characterized the effects of temperature on behavior. In this study, brown trout Salmo trutta fry were reared at 5 different temperatures (4°C, 6°C, 8°C, 10°C, and 12°C). In order to characterize group structure, 3 behavioral parameters were investigated: group social structure (based on inter-individual distances), inter-individual relationships (based on physical contacts), and individual activity. These behavioral parameters were studied at the emergence stage, which corresponds to a switch from a social gregarious life in the gravel to a solitary one in the water column. Data analysis showed that the inter-individual distances increased with increasing temperature, particularly the nearest neighbor distance. The mean number of physical contacts between fry increased at both low and high temperatures. At high temperatures, most fry moved apart from each other after a physical contact. Swimming activity decreased at both the lower and upper temperatures (18% of activity at 4°C, 38% at 8°C, and 12% at 12°C). This study showed that temperature modifies brown trout fry activity, inter-individual relationships, and social behavior, which all affect group cohesion before emergence and can influence their survival and dispersal.
Keywords: early development, emergence, freshwater species, group structure, relationships
Social relationships and interactions between animals are basic elements of social structure (Hinde 1976). Relationships are based on the succession of interactions between 2 individuals, and relationships between several individuals create a group with a particular structure (Hinde 1976). The organization within social groups has consequences on several functions, including reproduction, predator avoidance, and feeding resource exploitation (Guevara et al. 2011). Group structure is a trade-off between 2 opposing forces (benefits and disadvantages) (Miller and Gerlai 2007). In vertebrates, living in a group can have an impact on individual behavior, which can modify interactions with congeners. This can lead to a decrease in individual predation risk (Beauchamp 2008; Samuk et al. 2011) and to an increase in individual food availability (Sonerud et al. 2001), or mating opportunities (Bijleveld et al. 2010). In fish, the benefits of group living have been widely studied (Tien et al. 2004). More effective foraging is one of the most well-known benefits (Pitcher et al. 1982; Ranta and Juvonen 1993), but protection from predators is also improved (Miller and Gerlai 2007). Various mechanisms could explain this improved protection, such as dilution of the risk or a confusion effect (Landeau and Terborgh 1986; Pitcher and Parrish 1993), the early detection of predators (Godin et al. 1988) or coordinated evasive manoeuvres (Magurran and Pitcher 1987). However, there are also some disadvantages to group living. Competition for food can occur between congeners when resources are less available; this is known as competition by exploitation (Pitcher and Parrish 1993). Competition by interference, when individual foraging is less efficient among individuals in a tight group, can also take place (Ryer and Olla 1998).
Environmental temperature is a crucial factor for ectotherms whose body temperature regulation depends on it (Weetman et al. 1999). Larvae of freshwater species are a good biological model to the impact of temperature because they are less thermo tolerant (capable of living within a narrow range of temperature) than juveniles or adults (Kamler 2002; Ojanguren and Braña 2003). In fish, both individual behavior (Biro et al. 2010; Rey et al. 2015) and group structure (Flierl et al. 1999). Most studies on the effects of temperature on fish behavior focused on either individual activity or on predator–prey relationships. It has been shown that individual activity increased with temperature (Fukuhara 1990; Biro et al. 2010), whereas others concluded that swimming ability decreased when temperature increased (Johansen and Jones 2011). In addition, other studies demonstrated that activity decreased at low temperatures, increased to a peak and then decreased as temperature approached the upper thermal limit (Myrick and Cech 2000; Ojanguren and Braña 2000; Claireaux et al. 2006). Concerning the predator–prey relationships, Malavasi et al. (2013) showed that the percentage of time spent freezing (an antipredator response) decreased when temperature increased in the sea bass Dicentrarchus labrax. Fish larvae are also a model to investigate the effects of temperature on social behavior and group structure because many species exhibit these behaviors only during their early life stages, as they often become solitary as they get older. Temperature affects these behaviors. For instance, the appearance of a predator was associated with a significant increase in shoaling index (shoal cohesiveness) depending on the temperature applied. In the guppy Poecilia reticulata, Weetman et al. (1998) demonstrated that the interaction between the presence of a predator and temperature had a significant impact on their social antipredator behavior. As group structure is dependent on the interactions between individuals (Hinde 1976), and as temperature may affect individual behavior, temperature could directly influence the social group.
Some studies reported that temperature could modify inter-individual relationships, that is, aggressive behavior increased with temperature (Biro et al. 2010; Zhao and Feng 2015). In guppy and walleye pollock Theragra chalcogramma, the distance to the nearest neighbor within a group increased with higher temperatures (Weetman et al. 1998; Hurst 2007). Temperature also affected the aggressive relationships between Atlantic salmon Salmo salar juveniles, and at lower temperatures (4.6°C), there was less aggression (Gibson 2015). These previous studies were carried out on juveniles or adults and this is the first time that this type of study focused on the early life stages of a fish.
The aim of this study was to test the effects of temperature on the individual activity and social behavior at emergence of the larvae (known as fry) of a cold stenothermal fish, the brown trout Salmo trutta. After hatching, fry feed on the yolk sac and when the yolk reserves decrease, they leave the gravel and enter into the water column, a phenomenon known as “emergence,” which occurs at approximately 65 ± 19 days post hatching (dph) at 8°C (Teletchea et al. 2009). Under natural conditions, emergence is followed by the dispersal of the fry (Gaudin and Héland 1995; Héland et al. 1995) and depends on the local abiotic conditions such as warming water temperature (Côté and Green 2012). It is well known that this phenomenon depends on fry development, and is strongly affected by temperature, but nothing is known about the social changes occurring during emergence. Based on previous studies on other species (Weetman et al. 1998; Hurst 2007; Biro et al. 2010; Gibson 2015), we hypothesized that a rise in temperature during the first stages of development would accelerate emergence and be followed by faster fry dispersal. Thus, the resulting collapse in group structure would be characterized by greater inter-individual distances and more aggressive behavior between fry.
Materials and Methods
Rearing conditions
Eggs were obtained from 7 wild females (each fertilized independently by 7 wild males) caught 2 years previously and put in an earthen pond (water temperature: 8°C) located at the INBO (International Network of Basin Organizations) at Linkebeek (Belgium). Fry were raised at the UR AFPA (Unit of Animal Research and Functionality of Animal Products—University of Lorraine (http://www.urafpa.fr/), France), in 5 independent incubators (110 × 64 × 186 cm, 300 L) each including 9 racks (45 × 7 × 12 cm), in the same hatchery room. Each incubator had a flow rate of 4 m3 h−1 and water was UV sterilized. Approximately 6,000 eggs were put in each incubator (around 650 per rack); for this we mixed eggs from the 7 females. Each incubator had its own recirculated water and temperature control system. The physicochemical properties of the water were monitored daily to ensure optimal conditions: oxygen saturation was checked every morning (82.8 ± 9.8%); ammonia and nitrite concentrations were lower than 0.05 and 0.01 mg L−1, respectively; pH was controlled at 8.00 ± 0.05. Incubators were kept under natural photoperiodic conditions (ranging from 8 h light/16 h dark in January to 12 h light/12 h dark in May).
Five water temperatures were applied in the 5 different incubators with a difference of 2°C and 4°C below and above the temperature recorded in the earthen breeding ponds (8°C). In each incubator, the temperature was maintained constant throughout the experiment (4 ± 0.3°C, 6 ± 0.4°C, 8 ± 0.4°C, 10 ± 0.4°C, and 12 ± 0.4°C).
Protocol
The study ran from January to May 2014. Depending on the temperature, development times were very different, so emergence did not occur at the same time. Tests were performed at emergence for each temperature. Fry emerged at 24–25 dph at 4°C, 19–20 dph at 6°C, 15–16 dph at 8°C, 13–14 dph at 10°C, and 9–10 dph at 12°C. The fry tested were 24.81 ± 0.12 mm, 23.97 ± 0.08 mm, 24.80 ± 0.08 mm, 24.15 ± 0.10 mm, and 22.93 ± 0.27 mm in total length (N = 10 fish/group), respectively (stage 32 of the Vernier Table, 1969) (Réalis-Doyelle et al. 2016). At emergence fry were not fed; they lived on their yolk sac.
A circular arena (diameter = 30 cm and height = 5 cm with water 1.5 cm deep) was used to observe fry social behavior. Water in the arena was the same as that of each incubator. The room temperature was maintained at 10°C. The bottom of the arena was translucent and lit at 5–10 l× from underneath to avoid fry shadows during the recording. This light intensity was chosen from observations that fry are more active at both dawn and dusk (Jonsson and Jonsson 2009). The test room was a cold chamber to maintain the water temperature in the arena constant throughout the test.
Ten fry were collected with a beaker (and not with a net to avoid drying) from an incubator and placed in the center of the arena. After a 30-min acclimatization period, fry were filmed for 1 h with a camcorder (Sony, Handycam, DCR-SR72E) placed 50 cm above the arena. Two arenas were followed simultaneously with 2 camcorders. We filmed 10 groups for each tested temperature except for 12°C (we had only 2 groups due to the low survival rate of fry at this temperature—less than 2%). At the end of each experiment, fish were euthanized with MS 222 (200 mg L−1; AVMA 2007).
Quantification of fish behavior
Group structure
To analyze fry group structure, we extracted images from the videos at 5-min intervals for each group (13 images per video). The position of each fry in each image was mapped using its XY coordinates and determined by its center of mass using “ImageJ” software.
For group structure, we used 3 parameters to characterize each individual: the nearest neighbor distance, the mean of inter-individual distances and the variance of these inter-individual distances. For the nearest neighbor distance, because each member of the group has only 1 nearest neighbor, this parameter is not dependent on the size of the group and it is also less dependent on the distribution of the fish within the group (Buske and Gerlai 2011). This parameter is representative of the dispersion of individuals in the environment. The nearest neighbor distances (NND) were averaged for each image (ΣN/10) for 1 group of 10 individuals. Then, the mean values of 13 images were averaged to give the mean neighbor distance for 1 group at a given temperature. The mean and the standard error (SE) of this NND were calculated for the all groups for each temperature. Based on the NND, we also determined the structure of the group (fry can be dispersed, aggregated, or randomly distributed, see Online Appendix 1 for calculations). For this, we compiled the images showing aggregative and/or dispersive distribution of the fry within the group at each temperature (Online Appendix 1). We then calculated the percentage of aggregated and dispersed cases in the total number of images (130 images were taken for 4°C, 6°C, 8°C, and 10°C and 26 for 12°C). For a given temperature, we ensured that the number of images put in 1 particular category (aggregative, dispersive, or random) was the same between groups so that the data for a given temperature was not dependent on a particular group.
The second parameter used to characterize group structure was the mean inter-individual distance (D). It is the distance between 1 fry and all the other fry in the group. This parameter is representative of group cohesion (Buske and Gerlai 2011). In contrast to the nearest neighbor distance, it is dependent on the size of the group and on the distribution of fry within the group (Buske and Gerlai 2011). The mean inter-individual distance was then calculated by first measuring the distances between a given fry called focal fry and the other group members. These distances were thus averaged for a focal fry (ΣD/9); then the values obtained for all the fry of a group were averaged to obtain 1 value per image (Σ (ΣD/9)/10). For each group, the mean and SE were calculated from 13 images. The mean and the SE of these distances were calculated for each temperature (10 groups).
The third parameter was the variance of the distances between a focal fry and all other group members (V). This parameter is a classical metric to explain group homogeneity (Buske and Gerlai 2011). The 10 variances for each individual of a group were averaged (ΣV/10) for an image; then it was averaged for 13 images. Finally, the mean and SEs of these variances were calculated for each temperature (10 groups).
Inter-individual relationships and activity
Inter-individual relationships and individual activity were measured using the “The Observer XT10” software (Noldus, The Netherlands). The parameters characterizing inter-individual relationships and activity (see below) were obtained from three 2-min samples taken from the 1-h video of each individual group. The three 2-min samples were: the first 2 min, from the 30th min to 32th min and from the 58th min to 60th min.
Inter-individual relationships, defined as a physical contact between 2 individuals, were recorded as well as the behavior of the 2 fry afterwards. A physical contact was defined as a collision between 2 individuals, which could be a frontal or a lateral collision. After a physical contact, there were 4 scenarios: 1) the 2 individuals stayed in contact motionless, 2) the fry that initiated the physical contact stayed motionless and the other moved, 3) inversely, the first fry moved and the other one stayed motionless, or 4) both individuals moved apart.
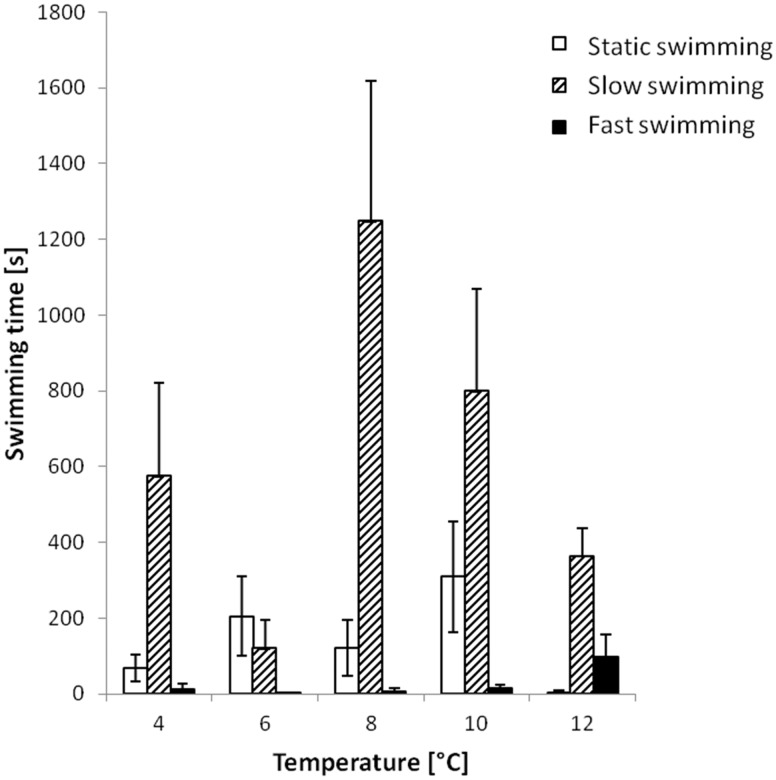
During emergence, fry must improve their swimming ability and capacity to forage further into the water column (Sanchez-Hernandez et al. 2011). Three different types of swimming activity were considered: static swimming, slow swimming, and fast swimming. Static swimming was defined by a tail flick, but which did not propel the fry more than its body length for more than 1 s. Slow swimming was the same as static except the fry moved more than its body length for more than 1 s. Fast swimming was defined as rapid displacement of the fry, with the tail flick no longer visible and fry moving more than its body length in less than 1 s. For each fry, we took into account the duration of each swimming type and total swimming was defined as the sum of the 3 modes: we then calculated the proportion of each swimming type relative to the total swimming duration for each temperature.
Data analysis
For all variables, we tested the assumption of normality (Shapiro test) and of homogeneity of variances (Levene test). As the data did not always fit with a normal distribution pattern considering the small (N = 10) and unequal sample sizes (4 sets with 10 groups and 1 set with 2 groups), we chose to use the one-way non-parametric analysis of variance test (Kruskal–Wallis H test) for independent data. In the case of significant temperature effects between the 5 temperatures tested, post hoc comparison tests (permutation tests) were performed to investigate which temperature groups differed from the others.
In order to determine the group structure and the distribution of individuals in the group, we used the nearest neighbor method (derived from Clarck and Evans 1954) based on the mean of distances between a point and its nearest neighbor. This method allowed the distribution—aggregated, dispersed, or random—of a population of individuals spread on a given surface to be determined (Online Appendix 1). We tested logarithmic and linear regressions to describe the distribution of data as a function of temperature: the curve of best fit had the highest regression coefficient. Comparisons for outcomes of individuals after contact between temperatures were made with chi-square.
All statistical tests were performed with “R” software (version 3.0.3) and results are presented as mean ± SE for histogram of activity and mean, median, and outliers in box plots for the other parameters. In the graphs, results are presented from the lowest to the highest temperature. Results were considered significant at P < 0.05.
Compliance with ethical standards
This article does not contain any studies with human participants performed by any of the authors. During all procedures, we took care to minimize handling and stress as much as possible for the study animals. All fish treatments and procedures used in this study were in accordance with the general guidelines of the Council of European Communities (1986, No. 86/609/CEE) and the French Animal Care Guidelines (Animal approval No. C54-547-18).
Results
Group social structure
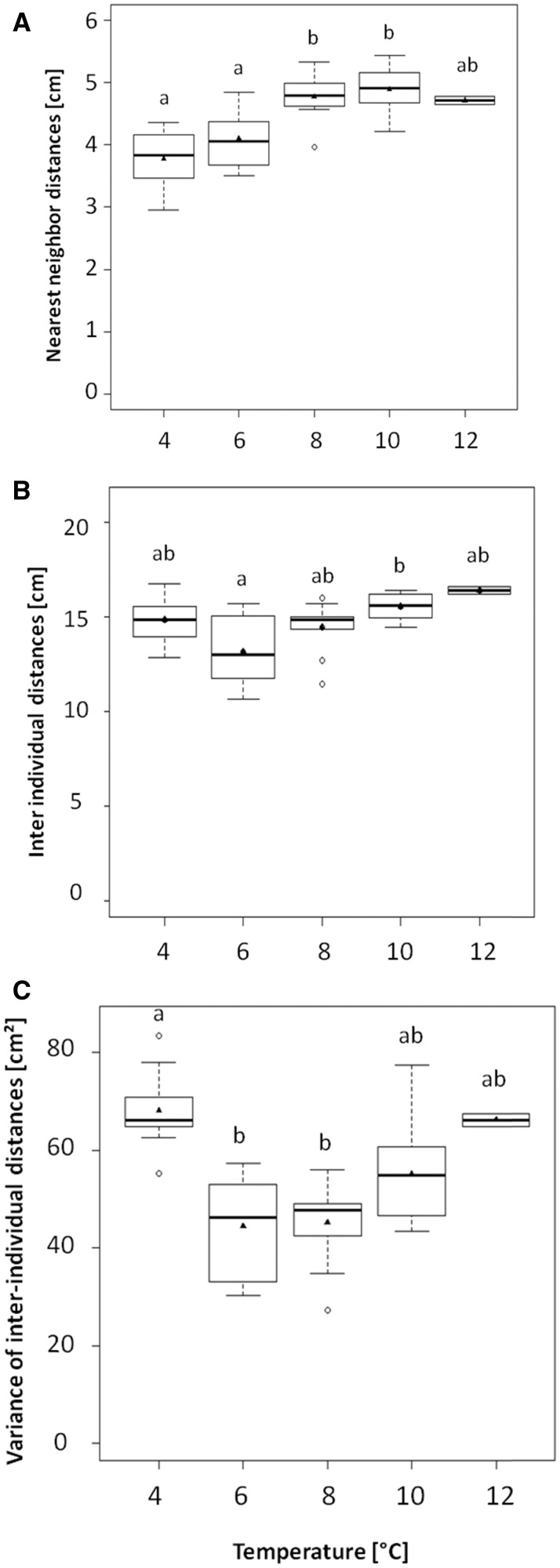
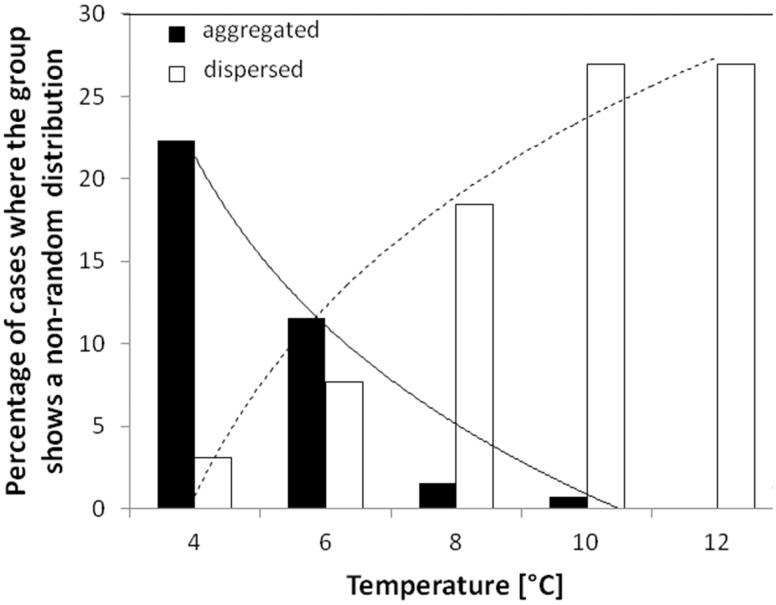
The nearest neighbor distances were different for the 5 tested temperatures (H = 25.05, df = 4, P < 0.0001). Significantly bigger distances were observed at 8°C and 10°C than at both 4°C (Z = −3.42, P < 0.001 and Z = −3.58, P < 0.001, respectively; Figure 1A) and 6°C (Z = −2.86, P = 0.004 and Z = −3.15, P = 0.001, respectively; Figure 1A). When the temperature increased, the tendency to aggregate decreased, while the tendency to disperse increased (Figure 2). Relatively to the temperature, the 2 distributions (dispersed and aggregated) of the fry in groups followed a logarithmic regression (dispersed: R2 = 0.94, P = 0.006; aggregated: R2 = 0.92, P = 0.009; Figure 2).
Figure 1.
Three measures (mean ± SE) of group structure: (A) nearest neighbor distances, (B) inter-individual distances, and (C) variance of inter-individual distances. The black line is the median, the black triangle is the mean, and the white dot is the outsider. Different letters indicate significant differences between temperatures at P < 0.05 using post hoc permutation tests.
Figure 2.
Distribution of individuals as a function of temperature with logarithmic regression. Fry are aggregated or dispersed in the arena.
For the inter-individual distances, there was an overall difference between temperatures (H = 14.58, df = 4, P = 0.005). At 6°C, inter-individual distances were significantly shorter than at 10°C (Z = 2.96, P = 0.003). No difference was observed between the other temperatures (Figure 1B).
There was also a global difference between temperatures (H = 23.83, df = 4, P < 0.0001) for the variance of the inter-individual distances. At 4°C, the variance of inter-individual distances was significantly higher than at 6°C (Z = 3.54, P < 0.001) and 8°C (Z = 3.58, P < 0.001). No difference was observed between the other temperatures (Figure 1C).
Inter-individual relationships and activity
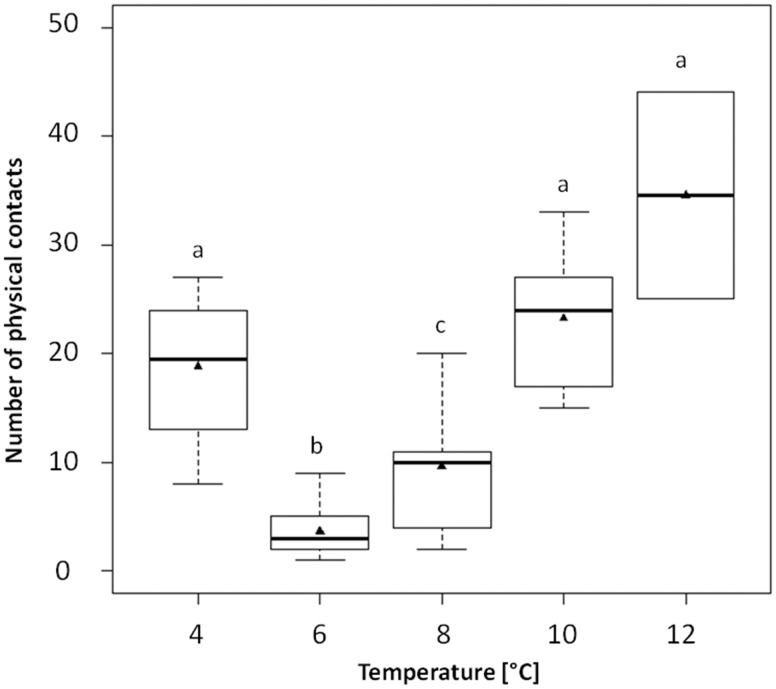
The number of physical contacts between individuals was significantly different depending on the temperature tested (H = 29.29, df = 4, P < 0.0001). At 4°C, there were significantly more physical contacts than at 6°C and 8°C. At 6°C, there were fewer physical contacts than at 8°C, 10°C, and 12°C. At 8°C, there were fewer physical contacts than at 10°C and 12°C (Table 1, Figure 3).
Table 1.
Permutation test (post hoc test) results for comparison of the number of physical contacts as a function of temperature
| Temperatures (°C) | Physical contacts |
|||||
|---|---|---|---|---|---|---|
| 4 | 6 | 8 | 10 | 12 | ||
| Physical contacts | 4 | — | Z = 3.72 | Z = 2.70 | — | — |
| P < 0.0001 | P < 0.006 | |||||
| 6 | — | Z = −2.54 | Z = −3.94 | Z = −3.09 | ||
| P = 0.01 | P < 0.0001 | P = 0.001 | ||||
| 8 | — | Z = −3.32 | Z = −2.74 | |||
| P < 0.001 | P = 0.006 | |||||
| 10 | — | |||||
| 12 | — | |||||
Figure 3.
Number of physical contacts (mean ± SE) between fry as a function of temperature. The black line is the median and the black triangle is the mean. Different letters indicate significant differences between temperatures at P < 0.05 using post hoc permutation tests.
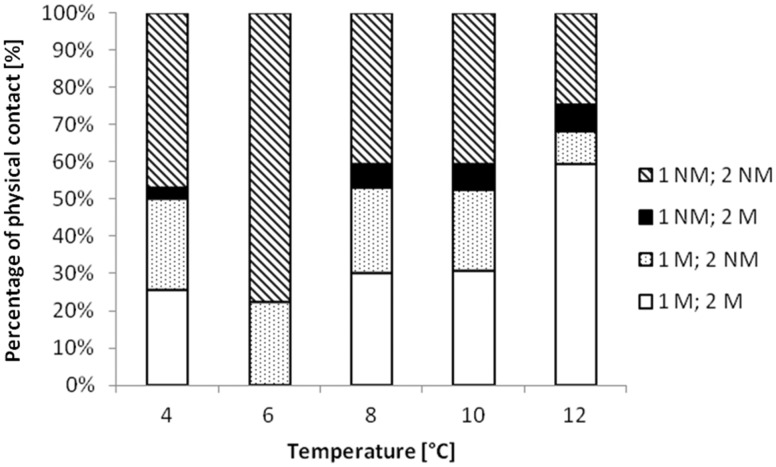
After a physical contact, fry could either stay in contact motionless (scenario 1) or move apart (scenario 2); there was no significant difference for either of the 2 other scenarios (scenarios 2 and 3, see the methods). Differences were observed between the 5 temperature groups with the 2 fry staying together (χ2 = 29.19, df = 4, P < 0.0001) or moving apart (χ2 = 45.71, df = 4, P < 0.0001) (Figure 4). At 12°C, more individuals moved apart after a contact than at other temperatures (4°C: Z = −2.67, P = 0.007; 8°C: Z = −2.68, P = 0.01; and 10°C: Z = −2.30, P = 0.02); at 6°C no individual moved apart.
Figure 4.
Percentage of each type of physical contacts among the total number of contacts at each temperature. 1 represents the fry that instigated the physical contact and 2 is the fry that was subjected to the contact. M (= Move) is when the fry moved after contact, NM (= Not Move) is when the fry stayed motionless after contact.
The total activity also varied depending on the temperature (H = 30.90, df = 4, P < 0.0001). At 4°C (18%), it was higher than at 6°C (9%), but lower than at 8°C (38%) and 10°C (31%). At 8°C and 10°C, it was higher than at 6°C and at 12°C (12%) (Table 2). There were differences in fast swimming time among the 5 temperatures (H = 18.40, df = 4, P = 0.001). At 12°C, the fry swam fast for longer than at 4°C, 6°C, 8°C, and 10°C (Table 2, Figure 5). For slow swimming time, there was also a global difference among the 5 temperatures (H = 32.85, df = 4, P < 0.0001). At 4°C, there was more slow swimming time than at 6°C and less time at 8°C. At 6°C, there were shorter periods of slow swimming than at 8°C, 10°C, and 12°C. At 8°C, there was more slow swimming time than at 10°C (Table 2, Figure 5). Static swimming was different for the 5 tested temperatures (H = 24.46, df = 4, P < 0.0001). At 4°C, there was less static swimming time than at 6°C and 10°C. At 8°C, there was less static swimming time than at 10°C (Table 2, Figure 5).
Table 2.
Summary of statistical analyses of activity using permutation tests (post hoc test)
| Activity | Temperature (°C) | 4 | 6 | 8 | 10 | 12 |
|---|---|---|---|---|---|---|
| Total activity | 4 | — | Z = 2.81 | Z = −3.33 | Z = −2.70 | — |
| P = 0.004 | P < 0.001 | P = 0.007 | ||||
| 6 | — | Z = −3.90 | Z = −3.68 | — | ||
| P < 0.001 | P < 0.001 | |||||
| 8 | — | — | Z = 2.41 | |||
| P = 0.01 | ||||||
| 10 | — | Z = 2.02 | ||||
| P = 0.03 | ||||||
| 12 | — | |||||
| Fast swimming | 4 | — | — | — | — | Z = 2.75 |
| P = 0.005 | ||||||
| 6 | — | — | — | Z = 3.01 | ||
| P = 0.002 | ||||||
| 8 | — | — | Z = 2.92 | |||
| P = 0.003 | ||||||
| 10 | — | Z = 2.84 | ||||
| P = 0.004 | ||||||
| 12 | — | |||||
| Slow swimming | 4 | — | Z = 3.47 | Z = −3.25 | — | — |
| P < 0.001 | P = 0.001 | |||||
| 6 | — | Z = −3.97 | Z = −3.82 | Z = −2.55 | ||
| P < 0.001 | P < 0.001 | P = 0.01 | ||||
| 8 | — | Z = 2.55 | — | |||
| P = 0.01 | ||||||
| 10 | — | — | ||||
| 12 | — | |||||
| Static swimming | 4 | — | Z = −2.95 | — | Z = −3.35 | — |
| P = 0.003 | P < 0.001 | |||||
| 6 | — | — | — | — | ||
| 8 | — | Z = 2.84 | — | |||
| P = 0.004 | ||||||
| 10 | — | — | ||||
| 12 | — |
Figure 5.
Swimming activity duration (mean ± SE) for each swimming type (static, slow, and fast swimming) as a function of temperature (see “Materials and methods” section).
Discussion
To our knowledge, the present study is one of the very few that has investigated the effects of temperature on the social behavior of fish larvae (e.g., Héland et al. 1995; Hurst 2007). While investigating the effects of temperature on individual activity, we showed that it had a marked effect on the social interactions between individuals and thus the group structure. The temperature had a strong impact on fry behavior at emergence. During this period, fry must disperse to find food and acquire a foraging site that could be associated with a territory. Territory possession is essential for food exploitation and survival (Elliott 1990). Territory is difficult to measure, but the nearest neighbor distances and the inter-individual relationships (i.e., aggressive behavior) provide an estimate of the size of the defended area.
Furthermore, during emergence fry must improve their swimming ability and their ability to forage more often in the water column (Sanchez-Hernandez et al. 2011). Our results suggest that brown trout fry already begin to show territorial behavior. Temperature appears to have an effect on the organization of this behavior at this stage.
Activity and physical contact
It is widely known that water temperature affects individual fish activity. This was generally studied by observing swimming performance (e.g., Beamish 1978; Ojanguren et al. 2001). The maximum swimming speed is usually reduced at low temperatures, increases to a peak at an optimum temperature and then decreases when the temperature approaches the upper thermal limit (Myrick and Cech 2000; Ojanguren and Braña 2000; Koumoundouros et al. 2002; Claireaux et al. 2006). In our study, we did not measure the maximum swimming speed, but the total activity showed a characteristic curve with higher values around an optimum temperature and lower values at both higher and lower temperatures. However, we did observe that this curve was skewed asymmetrically toward low temperatures. Indeed, at 4°C, individual activity increased relative to 6°C but was less than at 8°C. Ojanguren and Braña (2000) observed a similar pattern while studying juvenile brown trout. They concluded that brown trout might maintain some swimming activity below the lowermost (5.5°C) experimental temperature but probably not above the uppermost one (26°C). In our study there were more physical contacts at both 4°C and 12°C than at 8°C. However, the underlying causes of these differences can be interpreted from observations of the outcomes of the physical contacts. Thus, fry may increase activity at 4°C to maintain their internal temperature and not as an expression of territorial behavior. Indeed, at 4°C, the fish remained motionless after a physical contact whereas at 12°C they moved away from each other. Furthermore, at 12°C, there was more speed swimming suggesting that the relationships between individuals were more aggressive to defend a foraging site. At this stage (emergence) abiotic factors (temperature) could affect this behavior more than fry developmental stage (Valdimarsson and Metcalfe 2001).
Group structure
The nature, quality, and patterning of relationships can be described based on observations of the group structure (Hinde 1976). In our study, the nearest neighbor distance analysis showed that it increased with temperature (between 4°C and 10°C). In addition, dispersal within the group increased with temperature, whereas aggregation decreased. This is consistent with another study that demonstrated that the distance to the nearest neighbor increased by approximately 32% between 2°C and 9°C in walleye pollock juveniles (Hurst 2007). In our study, the distance to the nearest neighbor increased by about 16% between 4°C and 12°C. For all tested temperatures, fry were at the same biological stage (emergence) but showed different group structures. It is known that after emergence, when searching for food, individuals should stay away from conspecifics to avoid competition (Pitcher and Parrish 1993), but with dispersal, individuals are more at risk of being predated (Landeau and Terborgh 1986; Weetman et al. 1999). In our study, at 12°C, the dispersal of fry in the arena could have the same consequences. The present data support the conclusion that an increase in temperature promotes fry dispersal in the environment at emergence.
Possible ecological implications
Several studies on ectothermic species showed that abiotic factors can often have an impact on relationships between individuals and consequently group structure patterns: risk of predation in anura Phrynomantis microps (Spieler 2003) and fish (Pitcher et al. 1988; Ryer and Olla 1998; Miller and Gerlai 2007), or food reduction in fish (Ryer and Olla 1995, 1998). Our study demonstrated that the group social structure varied at emergence and physical contacts increased with temperature. This is consistent with studies on other animal groups. For example, at low temperature female spiders were less active and showed less aggressive behaviors, and were more tolerant toward conspecifics (Pruitt et al. 2011). In contrast, at higher temperatures, females exhibited increased activity levels, as well as aggressiveness toward conspecifics (Pruitt et al. 2011). Moreover, in coral reef fish, swimming speed increased with temperature and contributed to fish dispersal (Green and Fisher 2004).
Climate change models forecast a global increase of up to 4.8°C on the Earth surface, but with local, seasonal, or day/night cycles of positive and negative variations. In this context, Buisson (2009) showed that temperature changes will affect freshwater fish species and climate warming could strongly modify their distribution. Furthermore, temperature could have a stronger impact on freshwater fish than marine species because the network structure of drainage basins constrains their dispersal abilities (Buisson et al. 2008). Freshwater fish are limited to the river basin they currently live it. Consequently, dispersal phenomena (which start at the emergence stage with acquisition of territory) begin earlier at higher temperatures; this could result in a time gap between prey and predators and modify behavioral responses to the absence of food or a new resource.
Overall, our findings suggest that temperature directly affects fry behavior and thus raise questions on the future survival of brown trout. Indeed, the shift in time of emergence due to increasing temperature causes early dispersal. However, early-dispersing fry may not be ready to live on their own, that is, to escape from predators and defend a territory. Group living may be highly beneficial, for example, by minimizing predation risks (Pritchard et al. 2001). Several studies showed that larger groups face less predation risks due to different factors: increased predator confusion (Landeau and Terborgh 1986), collective vigilance (Godin et al. 1988), and social information transfer (Mathis et al. 1996). It can thus be inferred that an increase in temperature will increase the risk of predation in brown trout. An increase in temperature also leads to a shift in the time of arrival of fry in the water column. Prey may not yet be present or another species could take the ecological niche as it has been shown for pikeperch Sander lucioperca (Ginter 2012).
Brown trout are found in the cold rivers of mountainous and coastal areas in North-West France. It is one of the fish species that could be the most affected by climate change. In her study, Buisson (2009) took into account the distribution of different species as a function of several environmental data and 2 climatic data—temperature and rainfall—to predict the future distribution of trout. No river sections analyzed would be favorable to it and it could disappear from most rivers (Buisson 2009) leaving it confined to the most apical parts of Brittany’s highlands and rivers. The study was conducted on adults and juveniles, which have broader thermal limits than fry (Kamler 2002; Ojanguren and Braña 2003). In our study, we showed that temperature significantly affects fry behavior. The population structure could be affected at an early life stage inducing more competition between individuals, and, if the population of fry is affected by an increase of temperature, the future recruitment of juveniles and adults could also be affected.
Supplementary Material
Acknowledgments
We would like to thank François Olivier (University of Lorraine, Institute Elie Carcan) for his statistical help, Emilie Réalis-Doyelle for maintaining our fishes in good health, and Anthony Millot for his analysis of activity video. We would like to thank Leigh Gebbie (LKG Scientific Editing and Translation, Brisbane QLD Australia) for her help to improve the English of this article.
Funding
This research was supported by the University of Lorraine and the ONEMA (French National Agency for Water and Aquatic Environments) through a grant to T.C.
Supplementary Material
Supplementary material can be found at http://www.cz.oxfordjournals.org/.
Conflict of Interest
None declared.
References
- AVMA, 2007. Agents and methods of euthanasia by species. In: American Veterinary Medical Association, editor. Guidelines on Euthanasia (Formerly Report of the AVMA Panel on Euthanasia). Beltsville: National Agricultural Library, 28. [Google Scholar]
- Beamish FWH, 1978. Swimming capacity, locomotion. In: Hoar WS, Randall DJ, editors. Fish Physiology. New York: Academic Press, 101–187. [Google Scholar]
- Beauchamp G, 2008. What is the magnitude of the group-size effect on vigilance? Behav Ecol 19:1361–1368. [Google Scholar]
- Bijleveld AI, Egas M, Van Gils JA, Piersma T, 2010. Beyond the information centre hypothesis: communal roosting for information on food, predators, travel companions and mates? Oikos 119:277–285. [Google Scholar]
- Biro PA, Beckmann C, Stamps JA, 2010. Small within-day increases in temperature affects boldness and alters personality in coral reef fish. Proc Roy Soc B 277:71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buisson L, 2009. Poissons des rivières françaises et changement climatique: impacts sur la distribution des espèces et incertitudes des projections [PhD thesis]. Université de Toulouse, France. [Google Scholar]
- Buisson L, Thuiller W, Lek S, Lim P, Grenouillet G, 2008. Climate change hastens the turnover of stream fish assemblages. Glob Chang Biol 14:2232–2248. [Google Scholar]
- Buske C, Gerlai R, 2011. Early embryonic ethanol exposure impairs shoaling and the dopaminergic and serotoninergic system in adult zebrafish. Neurotoxicol Teratol 33:698–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claireaux G, Couturier C, Groison AL, 2006. Effect of temperature on maximum swimming speed and cost of transport in juvenile European sea bass Dicentrarchus labrax. J Exp Biol 209:3420–3428. [DOI] [PubMed] [Google Scholar]
- Clark PJ, Evans FC, 1954. Distance to nearest neighbour as a measure of spatial relationships in populations. Ecology 35:445–453. [Google Scholar]
- Côté IM, Green SJ, 2012. Potential effects of climate change on a marine invasion: the importance of current context. Curr Zool 58:1–8. [Google Scholar]
- Elliott JM, 1990. Mechanisms responsible for population regulation in young migratory trout, Salmo trutta. III Role of territorial behaviour. J Anim Ecol 59:803–818. [Google Scholar]
- Flierl G, Gruenbaum D, Levin S, Olson D, 1999. From individuals to aggregations: the interplay between behavior and physics. J Theor Biol 196: 397–454. [DOI] [PubMed] [Google Scholar]
- Fukuhara O, 1990. Effects of temperature on yolk utilization, initial growth, and behavior of unfed marine fish-larvae. Mar Biol 106:169–174. [Google Scholar]
- Gaudin R, Héland M, 1995. Stratégies d'utilisation de l'habitat par les alevins post émergents de truite commune Salmo trutta et de saumon atlantique Salmo salar. B Fr Peche Piscic 337–339. [Google Scholar]
- Gibson RJ, 2015. Some behavioural and ecological factors affecting distribution, biomass and production of juvenile Atlantic salmon. Ecol Fresh Fish 24:397–411. [Google Scholar]
- Ginter K, 2012. The diet of juvenile pikeperch Sander lucioperca in lakes Peipsi and Võrtsjärv: relations between long–term changes in the fish communities and food resources in large shallow lakes [PhD thesis]. Estonian University of Life Sciences. [Google Scholar]
- Godin JGJ, Classon LJ, Abrahams MV, 1988. Group vigilance and shoal size in a small characin fish. Behaviour 104:29–40. [Google Scholar]
- Green BS, Fisher R, 2004. Temperature influences swimming speed, growth and larval duration in coral reef fish larvae. J Exp Mar Biol Ecol 299:115–132. [Google Scholar]
- Guevara J, Gonzaga O, Vasconcellos-Neto J, Avilès L, 2011. Sociality and resource use: insights from a community of social spiders in Brazil. Behav Ecol 22:630–638. [Google Scholar]
- Héland M, Gaudin P, Bardonnet A, 1995. Mise en place des premiers comportements et utilisation de l’habitat après l’émergence chez les salmonidés d’eau courante. B Fr Pêche Piscic 337/338/339:191–197. [Google Scholar]
- Hinde RA, 1976. Interactions, relationships and social structure. Man 11:1–17. [Google Scholar]
- Hurst TP, 2007. Thermal effects on behavior of juvenile walleye pollock Theragra chalcogramma: implications for energetic and food web models. Can J Fish Aquat Sci 64:449–457. [Google Scholar]
- Johansen JL, Jones GP, 2011. Increasing ocean temperature reduces the metabolic performance and swimming ability of coral reef damselfishes. Glob Chang Biol 17:2971–2979. [Google Scholar]
- Jonsson B, Jonsson N, 2009. A review of the likely effects of climate change on anadromous Atlantic salmon Salmo salar and brown trout Salmo trutta, with particular reference to water temperature and flow. J Fish Biol 75:2381–2447. [DOI] [PubMed] [Google Scholar]
- Kamler E, 2002. Ontogeny of yolk-feeding fish: an ecological perspective. Rev Fish Biol Fisher 12:79–103. [Google Scholar]
- Koumoundouros G, Sfakianakis DG, Divanach P, Kentouri M, 2002. Effect of temperature on swimming performance of sea bass juveniles. J Fish Biol 60:923–932. [DOI] [PubMed] [Google Scholar]
- Landeau L, Terborgh J, 1986. Oddity and the “confusion effect” in predation. Anim Behav 34:1372–1380. [Google Scholar]
- Magurran AE, Pitcher TJ, 1987. Provenance, shoal size and sociobiology of predator: evasion behaviour in minnow shoals. Proc Roy Soc B 299:439–465. [Google Scholar]
- Malavasi S, Cipolato G, Cioni C, Torricelli P, Alleva E et al. , 2013. Effects of temperature on the antipredator behavior and on the cholinergic expression in the European sea bass Dicentrarchus labrax juveniles. Ethology 119:592–604. [Google Scholar]
- Mathis A, Chivers DP, Smith RJF, 1996. Cultural transmission of predator recognition in fish: intraspecific and interspecific learning. Anim Behav 51:185–201. [Google Scholar]
- Miller NY, Gerlai R, 2007. Quantification of shoaling behaviour in zebrafish Danio rerio. Behav Brain Res 184:157–166. [DOI] [PubMed] [Google Scholar]
- Myrick CA, Cech JJ, 2000. Swimming performance of four California stream fishes: temperature effects. Environ Biol Fish 58:289–295. [Google Scholar]
- Ojanguren AF, Braña F, 2000. Thermal dependence of swimming endurance in juvenile brown trout. J Fish Biol 56:1342–1347. [Google Scholar]
- Ojanguren AF, Braña F, 2003. Thermal dependence of embryonic growth and development in brown trout. J Fish Biol 62:580–590. [Google Scholar]
- Ojanguren AF, Reyes-Gavilan FG, Braña F, 2001. Thermal sensitivity of growth, food intake and activity of juvenile brown trout. J Therm Biol 26:165–170. [DOI] [PubMed] [Google Scholar]
- Pitcher TJ, Lang SH, Turner JA, 1988. A risk-balancing trade-off between foraging rewards and predation hazard in a shoaling fish. Behav Ecol Sociobiol 22:225–228. [Google Scholar]
- Pitcher TJ, Magurran AE, Winfield IJ, 1982. Fish in larger shoals find food faster. Behav Ecol Sociobiol 10:149–151. [Google Scholar]
- Pitcher TJ, Parrish JK, 1993. Functions of shoaling behavior in teleosts. In: Pitcher TJ, editor. Behavior of Teleost Fishes. London: Chapman & Hall, 363–439. [Google Scholar]
- Pritchard VL, Lawrence J, Butlin RK, Krause J, 2001. Shoal choice in zebrafish, Danio rerio: the influence of shoal size and activity. Anim Behav 62:1085–1088. [DOI] [PubMed] [Google Scholar]
- Pruitt JN, Demes KW, Dittrich-Reed DR, 2011. Temperature mediates shifts in individual aggressiveness, activity level, and social behaviour in a spider. Ethology 117:318–325. [Google Scholar]
- Ranta E, Juvonen SK, 1993. Interference affects food-finding rate in schooling sticklebacks. J Fish Biol 43:531–535. [Google Scholar]
- Réalis-Doyelle E, Pasquet A, De Charleroy D, Fontaine P, Teletchea F, 2016. Effect of temperatures on the survival, the development and the body condition of a cold stenothermal fish, brown trout (Salmo trutta). PLoS ONE11:e0155487 doi:10.1371/journal.pone.0155487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey S, Digka N, MacKenzie S, 2015. Animal personality relates to thermal preference in wild-type Zebrafish Danio rerio. Zebrafish12:243–249. [DOI] [PubMed] [Google Scholar]
- Ryer CH, Olla BL, 1995. Influences of food distribution on fish foraging behavior. Anim Behav 49:411–418. [Google Scholar]
- Ryer CH, Olla BL, 1998. Shifting the balance between foraging and predator avoidance: the importance of food distribution for a schooling pelagic forager. Environ Biol Fish 52:467–475. [Google Scholar]
- Samuk KM, LeDue EE, Avilès L, 2011. Sister clade comparisons reveal reduced maternal care behavior in social cobweb spiders. Behav Ecol 23:35–43. [Google Scholar]
- Sanchez-Hernandez J, Vieira-Lanero R, Servia MJ, Cobo F, 2011. Feeding habits of four sympatric fish species in the Iberian Peninsula: keys to understanding coexistence using prey traits. Hydrobiologia 667:119–132. [Google Scholar]
- Sonerud GA, Smedshaug CA, Brathen O, 2001. Ignorant hooded crows follow knowledgeable roost-mates to food: support for the information centre hypothesis. Proc Roy Soc B 268:827–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spieler M, 2003. Risk of predation affects aggregation size: a study with tadpoles of Phrynomantis microps (Anura: Microhylidae). Anim Behav 65:179–184. [Google Scholar]
- Teletchea F, Fostier A, Kamler E, Gardeur JN, Le Bail PY et al. , 2009. Comparative analysis of reproductive traits in 65 freshwater fish species: application to the domestication of new fish species. Rev Fish Biol Fisher 19:403–430. [Google Scholar]
- Tien JH, Levin SA, Rubenstein DI, 2004. Dynamics of fish shoals: identifying key decision rules. Evol Ecol Res 6:555–565. [Google Scholar]
- Vernier M, 1969. Chronological table of the embryonic development of rainbow trout Salmo gairdneri (Rich 1836). Ann Embryol Morphog 2:495–520. [Google Scholar]
- Valdimarsson SK, Metcalfe NB, 2001. Is level of aggression and dispersion in territorial fish dependent on light intensity? Anim Behav 61:1143–1149. [Google Scholar]
- Weetman D, Atkinson D, Chubb JC, 1998. Effects of temperature on anti-predator behavior in the guppy Poecillia reticulata. Anim Behav 55:1361–1372. [DOI] [PubMed] [Google Scholar]
- Weetman D, Atkinson D, Chubb JC, 1999. Water temperature influences the shoaling decisions of guppies Poecilia reticulata under predation threat. Anim Behav 58:735–741. [DOI] [PubMed] [Google Scholar]
- Zhao D, Feng P, 2015. Temperature increase impacts personality traits in aquatic non-native species: implications for biological invasion under climate change. Curr Zool 61:966–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.