Abstract
The fields of behavioral ecology, conservation science, and environmental toxicology individually aim to protect and manage the conservation of wildlife in response to anthropogenic stressors, including widespread anthropogenic pollution. Although great emphasis in the field of toxicology has been placed on understanding how single pollutants affect survival, a comprehensive, interdisciplinary approach that includes behavioral ecology is essential to address how anthropogenic compounds are a risk for the survival of species and populations in an increasingly polluted world. We provide an integrative framework for behavioral ecotoxicology using Tinbergen’s four postulates (causation and mechanism, development and ontogeny, function and fitness, and evolutionary history and phylogenetic patterns). The aims of this review are: 1) to promote an integrative view and re-define the field of integrative behavioral ecotoxicology; 2) to demonstrate how studying ecotoxicology can promote behavior research; and 3) to identify areas of behavioral ecotoxicology that require further attention to promote the integration and growth of the field.
Keywords: animal behavior, behavioral ecology, conservation, phylogenetic, pollution, toxicology.
Introduction
The last mass extinction event, induced by a rapid glaciation event, wiped out an estimated 77–90% of marine wildlife (Wilson 1989; Crutzen and Stoermer 2000; Vince 2011). Crutzen and Stoermer (2000) have called attention to a new epoch that is experiencing a human-induced sixth mass extinction event (Zalasiewicz et al. 2010; Vince 2011; Barnosky et al. 2012). Humans have modified 80% of the Earth’s land surface (Vince 2011), via massive deforestation and habitat loss, conversion of forest to cropland or savanna, hunting and overfishing, and climate change (Zalasiewicz et al. 2010; Vince 2011). As a result, Crutzen and Stoermer (2000) termed this time period as “the Anthropocene”.
Although it is clear that biodiversity is impacted by human modification of the landscape and climate change, there is another, more elusive, threat to conservation: anthropogenic pollution of the environment. Anthropogenic chemicals are ubiquitous in the environment and are therefore likely leading to the decline of wild populations of animals and creating a public health crisis (Zala and Penn 2004; Yu et al. 2011). It has been estimated that the chemical industry releases approximately 1,000 novel chemicals each year and that at least 100 million tons of chemicals are released into the environment each year (Postel 1987; Vitousek et al. 1997). Among these include endocrine disruptors, heavy metals, oil from oil spills, personal care products, pharmaceuticals, and pesticides, to name but a few (Dell’Omo 2002; Zala and Penn 2004); only a small proportion of these chemicals are thoroughly evaluated before distribution and sale (UNEP 1992; Vitousek et al. 1997). Although large-scale catastrophic pollution events are rare (such as oil spills, for example), sublethal and chronic effects of exposure to anthropogenic chemicals are common; this can result in lessened survival and reproductive success, both are essential to the viability of the individual, population (Hansen and Johnson 1999), and ecosystem (Johnston et al. 2015).
As wildlife species are exposed to multiple chemical stressors at once and many chemicals appear to influence organisms even at low concentrations, there is great potential for anthropogenic chemicals to disrupt phenotypes and influence fitness (Dell’Omo 2002; Yu et al. 2011). In addition, anthropogenic chemicals have been found in the tissues of wild animals, even in regions apparently devoid of pollution (Norstrom et al. 1998). They are known to bioaccumulate within tissues and are not only transferred and biomagnified across trophic levels, but may also be transferred across generations (i.e., parents to offspring to grandchildren to great grandchildren) (Dell’Omo 2002). Although pollution-induced extinction rates are undocumented (at least to our knowledge), anthropogenic chemicals have been responsible (at least partially) for the listing of amphibians (Davidson et al. 2001; Davidson 2004; Wake and Vredenburg 2008) and the California condor (Gymnogyps californianus, Finkelstein et al. 2012) as Critically Endangered (Wake and Vredenburg 2008). In addition, there is support indicating that endocrine-disrupting chemicals, in particular, are a factor in the decline of the Florida panther (Puma concolor coryi, Facemire et al. 1995), amphibians (Dalton 2002; Renner 2002), and marine mammals (De Guise et al. 1995). Outside of putting some populations on the brink of extinction, we propose that global anthropogenic pollution is depressing many wildlife populations and altering their evolutionary trajectories (Köhler and Triebskorn 2013; Rundlöf et al. 2015; Tüzün et al. 2015).
Historical Perspectives
Although the deleterious consequences of pollutants have been recognized since the Egyptians (Hernberg 2000) and Romans (Gilfillan 1965; Hernberg 2000), the road to understanding the implications of pollution for conservation has been a challenge. The field of ecotoxicology was born with the first scientific documentation of pollutant-induced wildlife-related mortalities at the end of the industrial revolution (Newman 1979; Rattner 2009). Reports of contaminant-induced wildlife mortalities continued after the turn of the century as the synthetic pesticide era began (Rattner 2009).
Behavioral studies, specifically, provided the first warning signs of the toxicity of dichlorodiphenyltrichloroethane (DDT) to wildlife (Zala and Penn 2004). Broley (1958) documented changes in nesting, reproductive, and courtship behaviors in bald eagles Haliaeetus leucocephalus that coincided with both population declines and exposure to DDT. Rachel Carson’s “Silent Spring” (Carson 1962) brought the dangers of DDT to the public forefront, resulting in public outcries and set in motion a much-needed focus on wildlife toxicology (Zala and Penn 2004). Even before the scientific demonstration of toxicants’ effects on wildlife, colloquial phrases such as “mad as a hatter” [referring to mercury exposure in milliners and mentioned in Lewis Carroll’s (1865) “Alice’s Adventures in Wonderland”] had made their way into everyday usage. By 1966, Warner et al. (1966) suggested that behavioral patterns should be utilized as an index of sublethal toxicity for wildlife.
Regardless of the realization that anthropogenic pollution altered behavior or that behavior was an integral player in population declines, the majority of wildlife toxicology research historically focused on lethality, acute exposure, physiological impact of exposure, and single chemical exposures (Dell’Omo 2002; Clotfelter et al. 2004; Scott and Sloman 2004; Zala and Penn 2004). In addition, the fields of ecotoxicology, wildlife toxicology, behavioral toxicology, and conservation remained disparate from each other, focusing on different biological levels of organization regardless of common aims and major areas of overlap (Hansen and Johnson 1999; Weis et al. 2001). In the last decade, calls for an integration of behavioral ecology, toxicology, and conservation have emerged to unify these fields into an integrative field: behavioral ecotoxicology (Dell’Omo 2002; Chapman 2007; Gerhardt 2007; Peeters et al. 2009; Hellou 2011). Here, we take a step further and propose the field of integrative behavioral ecotoxicology (IBET) that not only integrates across biological levels of organization but also explicitly studies and explain the influence of toxins on organisms from proximate and ultimate perspectives.
Relevance and Importance of Behavior in Ecotoxicology
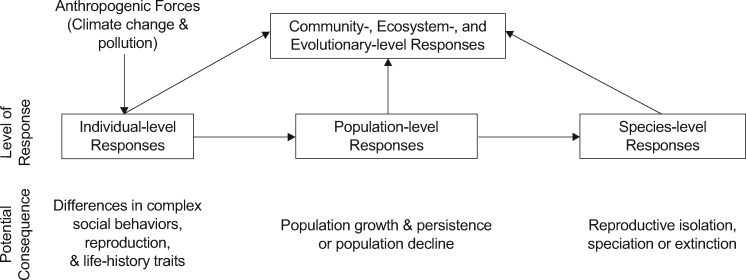
Behavior is both an assay of fitness (Scott and Sloman 2004) and an adaptive (although maladaptive at times) response to environmental stimuli (Gerhardt 2007). Therefore, it is “essential to the viability of the organism, the population, and the community,” and “its ecological importance in population maintenance is intuitively obvious” (Little 1990). There is extensive research indicating that anthropogenic chemicals extensively disrupt a wide range of behaviors in both aquatic and terrestrial animals (Little 1990). In toxicology studies, behavior is rarely incorporated as a fitness consequence or as a trait to measure toxicity (Dell’Omo 2002; Clotfelter et al. 2004). In addition, previous attempts to understand ecological or behavioral implications of contaminants did not integrate the multiple levels of organization (Figure 1), that is, physiological level, individual level, population level, species level, ecosystem level, and evolutionary levels of organization (Weis et al. 2001; Dell’Omo 2002). Even with the emergence of behavioral ecotoxicology, little integrative work in behavioral ecotoxicology has been published (but see Söffker and Tyler 2012; Brodin et al. 2014).
Figure 1.
Individual-level responses to anthropogenic forces, such as anthropogenic pollution, can disrupt complex social behaviors, reproduction, and life-history traits at the individual level. These disruptions at the individual level can therefore impact responses at the population-, species-, community-, ecosystem-, and even evolutionary-level responses.
This is surprising given the benefits of incorporating behavior in this context: (1) Behavior is an indicator of multiple levels of biological outcomes (Little 1990; Weis et al. 2001; Scott and Sloman 2004; Kane et al. 2005; Weis 2013); (2) Behavior is among the most sensitive indicators of impact of exposure, as there are noticeable changes in behavior at concentrations considered sublethal (Little 1990; Dell’Omo 2002; Zala and Penn 2004; Gerhardt 2007; Hellou 2011; Weis 2013). In fact, behavior is 10–1,000 times more sensitive than lethality measures (Gerhardt 2007; Hellou 2011). (3) Most importantly, behavior is considered an early warning tool (Hellou 2011). This is because behavior is a rapidly changing, flexible trait and so toxicologists may see responses in behavior before seeing responses in other kinds of phenotypes, or in the genome. In addition, behavior research can be non-invasive, relatively inexpensive, and does not require too much specialist equipment (Weis 1983; Zala and Penn 2004; Gerhardt 2007; Hellou 2011).
However, there are also limitations with using behavior in ecotoxicology research. For example, assessing behavior can be time consuming, less reproducible than physiological measures, and can be highly variable because of the flexibility of behaviors (Zala and Penn 2004). However, the same limitations can often be leveled at physiological measurements. In addition, in some situations it can be difficult to link individual-level variation in behavior with higher-level effects (Dell’Omo 2002; Clotfelter et al. 2004). This lack of a clear causal link between phenotype and fitness is also a common problem for interpreting molecular, cellular, and physiological metrics. Perhaps most substantially, there is resistance from policy makers as behavioral studies are sometimes, inappropriately, viewed as being less scientifically rigorous as laboratory-based physiological, cellular, and molecular methods (Weis 1983; Little 1990).
Despite these limitations, behavior can be viewed as a flexible response to an environmental stressor, such as a pollutant, and it enables an organism to survive in a constantly changing and polluted ecosystem (Kane et al. 2005; Gerhardt 2007). Using behavior, scientists can assess how anthropogenic chemicals impact the health of organisms, populations, and ecosystems in an effort to promote conservation and sustainability by incorporating multiple levels of biological organization. In addition, behavior can be used as a field of study to contribute to understanding of conservation and toxicology. We view behavior as highly integrative (Little 1990; Gerhardt 2007) and at the complementary intersection of ecology, behavior, toxicology, and conservation; therefore, we can advance these fields to solve complementary problems with behavioral ecotoxicology.
Aims of This Paper
We posit that a comprehensive, integrative approach that includes behavioral ecology is essential to address how anthropogenic chemicals are risk factors for species and population survival in an increasingly polluted world. In a complementary fashion, basic questions in animal behavior and behavioral ecology can be better addressed with a mechanistic understanding of how and when environmental toxicants alter integrated behavioral phenotypes. For example, the developmental stress hypothesis is a well-accepted framework for understanding how and why early life stress influences later-life performance (Ritchie et al. 2007), and is a hypothesis born out of studies of behavioral ecology but has been largely tested and studied through systematic exposure of organisms to toxins (Møller and Swaddle 1997).
To better integrate the fields of toxicology, conservation, and behavioral ecology, we organized and hosted a symposium at the 53rd Annual Conference of the Animal Behavior Society (Columbia, Missouri). The goals of the symposium were to: (1) address the issue that behavioral ecotoxicology is relevant and important when assessing the conservation and preservation of populations; (2) provide more integrative frameworks for the study of evolution of behaviors in light of rapid environmental change; and (3) identify areas of behavioral ecotoxicology that require further attention to facilitate the future of behavioral ecotoxicology as a discipline within the behavioral ecology, conservation, and toxicology fields.
A lack of standardization of behavioral methods and collaboration between the fields of behavioral ecology, conservation, and toxicology has contributed to a lack of unification and development of the field of behavioral ecotoxicology (Little 1990). Little (1990) first suggested that: “the greatest need of behavioral toxicologists, in regard to standardization, may be an organizational framework for formulating procedural guidelines”. Although these fields have started to come together since the 1990s there is still a need to present a unifying framework to integrate behavioral ecology, toxicology, and conservation that can provide both proximate and ultimate explanations and promote the unifying themes and goals of each field.
With a unifying framework, behavioral ecotoxicologists will be empowered to work collaboratively and across disciplines, determine important research questions, develop future directions that promote the goals of each field, and develop more focused studies (Berger-Tal et al. 2011). More importantly, a unifying framework enables behavioral ecotoxicologists to “bridge the gap between disciplines and establish a common ground in which the fields can develop and paradigms can be formed” (Berger-Tal et al. 2011). We use Tinbergen’s four questions (i.e., Tinbergen’s four postulates) as a unifying framework to not only understand better how anthropogenic pollutants affect behavior in terms of causation and mechanisms, development and ontogeny, function and fitness, evolutionary history and phylogenetic patterns, but also conservation concerns. The integration of ultimate evolutionary thinking into the sometimes proximate world of conservation and behavioral ecotoxicology is further advanced elsewhere (Swaddle 2016).
The synthesis that we summarize here is a result of presentations and discussion at a symposium held at the Animal Behavior Society 2016 annual meeting in Columbia, Missouri. The aims of the symposium were both aspirational and practical. We intended to promote an integrative view of the issues of “utility”, “adaptive value”, and “historical context” using Tinbergen’s four questions as an investigatory framework, as well as to redefine IBET. In addition, we intended to demonstrate that the study of ecotoxicology can promote behavioral research and identify areas of behavioral ecotoxicology that require further attention.
Tinbergen's Four Questions
One of the advantages of studying behavior is that it is intrinsically integrative and can render a broadly rounded view of biological functioning. Tinbergen’s four questions (or postulates) (Figure 2) are already commonly used by behavioral ecologists and conservation behaviorists to form an investigatory framework. This is because the four postulates enable both fields to answer behavioral questions in an integrative manner at different levels of biological organization while incorporating conservation in the framework (Bucholz 2007; Bateson and Laland 2013). Tinbergen suggested that we consider both proximate and ultimate explanations by asking four mutually exclusive questions about behavior to address the cause, origin, and consequence of behavioral patterns (Bucholz 2007). Tinbergen emphasized that these questions be addressed in unison with each other, rather than separately (Bateson and Laland 2013).
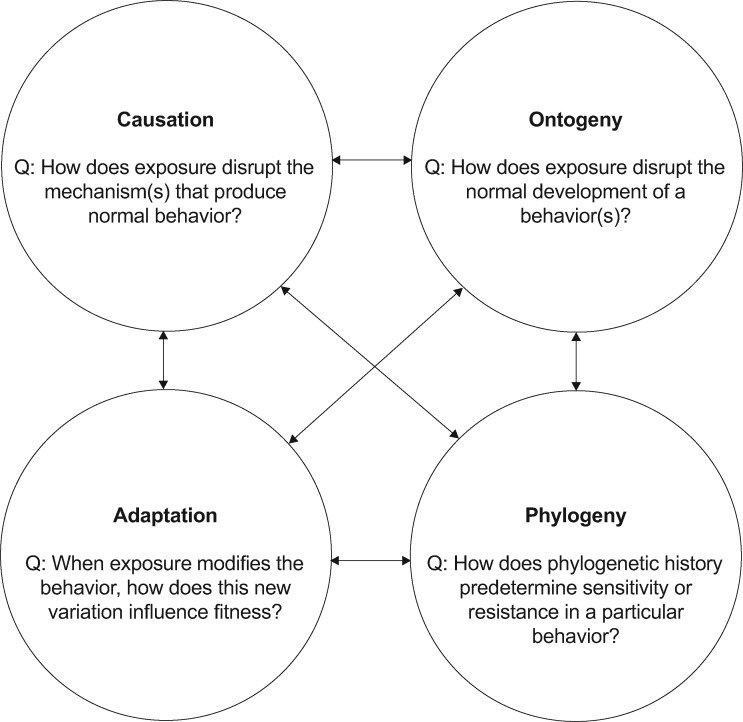
Figure 2.
An example of how a researcher could apply Tinbergen’s four questions (postulates) within our IBET framework.
These questions can be organized into either proximate or ultimate questions, in other words, “how” and “why” questions (Bucholz 2007). “How”, or proximate questions, answer “how” an individual is able to elicit a behavior via causation (e.g., learning and individual experience) and mechanism (e.g., the nervous system) or development/ontogeny (Bucholz 2007). Ultimate questions relate to the fitness of the organism. First, what is the current utility of the behavior and does it influence reproduction and survival? Second, what are the historical origins of the behavior, including deep phylogenetic patterns of origination as well as potential cultural origins in some kinds of traits (Bucholz 2007). In the following sections we review work in behavioral ecotoxicology using Tinbergen’s four questions as an investigatory framework.
Causation and mechanism
Behavior has been defined as “a sequence of quantifiable actions, operating through the central and peripheral nervous systems and cumulative manifestation of genetic, biochemical, and physiologic processes essential to life” (Kane et al. 2005). Although the genetic, biochemical, and physiological internal processes may be essential to life, other processes are equally important: cellular and molecular, hormonal processes, decision processes, and psychophysical constraints (Dell’Omo 2002).
Traditionally, environmental toxicologists have used internal processes (such as biochemical and cellular effects) as biomarkers of exposure to pollutants (Weis et al. 2001), and it is only in the last decade or so that this approach has been expanded to higher biological levels of organization by integrating aspects of behavior and ecology. Because of historical regulatory emphasis on internal processes as biomarkers of exposure, toxin exposure is most commonly assessed via changes in these internal processes. A large literature exists that thoroughly explains the role of each internal process on toxicological outcomes (e.g., neurotoxicity: Weis et al. 2001; Dell’Omo 2002; Scott and Sloman 2004; Basu and Head 2008; Levin et al. 2009; Basu 2015; and endocrine disruption: Weis et al. 2001; Clotfelter et al. 2004; Scott and Sloman 2004; Zala and Penn 2004, to name a few).
To modify Tinbergen’s causation and mechanisms question to incorporate an integrative behavioral ecotoxicological perspective, we can ask, how does exposure disrupt the mechanism(s) that produces normal behavior (Figure 2)? Although these internal processes are seemingly disparate themselves, these biological pathways are interrelated and work harmoniously to produce an integrated behavioral phenotype (Dell’Omo 2002; Scott and Sloman 2004). In addition, using this framework, we can link the various internal processes to better understand individual-level responses, as well as population-level responses and adaptive consequences. This approach is somewhat similar to the US Environmental Protection Agency’s (EPA) Adverse Outcome Pathway approach, under which molecular and cellular disruption induced by environmental toxins is linked to ecologically adverse outcomes, such as declines in reproduction and survival at the individual and population levels (Ankley et al. 2010). However, we intend to take this integration still further by not only stressing the proximate causation involved in assessing molecular and physiological effects at the cellular and organismal levels but also assessing the ultimate fitness and evolutionary consequences associated with toxin exposure. When reproduction is affected by toxin exposure we would expect to see selection pressures, which may cause evolutionary change in populations if there is heritable variation for toxin resistance. Such evolutionary change may not always lead to population declines.
Traditionally this postulate/question is asked about a fixed time scale, however, there is an overlap between development/ontogeny and mechanism/causation given that it is important to address the development of the mechanism itself (Bateson and Laland 2013). This is because both ontogeny and mechanism can impact behavioral phenotypes well beyond development (Tinbergen 1963). To make matters more complicated for integrative behavioral ecotoxicologists, these internal processes can be both conserved and taxon-specific (Dell’Omo 2002), emphasizing the need for application (and integration) of a phylogenetic approach.
Development and ontogeny
As an example of applying Tinbergen’s second postulate, we can ask the question “how did the trait (e.g., toxin resistance) develop?” Traditionally, this question has been addressed as a change in expression of the trait over the lifetime of an organism, identifying whether toxin resistance alters from early to later life stages. However, some of the more revealing questions now relate to inheritance and expression mechanisms of the trait. Can resistance of the toxin be inherited and expressed in ways beyond the simple acquisition of genetic variation from the parents? One ontogenetic way in which our knowledge of the impacts of ecotoxins has increased recently is through the study of epigenetic processes. Epigenetics refers to a suite of mechanisms that alter expression of the genome without changing nucleotide sequences. Such effects can occur through histone modification and DNA methylation, which then directly influence which genes are expressed or silenced (Basu et al. 2014). Endocrine-disrupting chemicals can have epigenetic effects, causing changes in offspring that were never directly exposed to the compounds (Crews et al. 2007). Similarly, many heavy metals (including mercury, zinc, aluminum, cadmium, lead, selenium, arsenic, and copper) induce epigenetic changes that can also be associated with harmful effects in organisms, such as increased induction of cancerous tumours in humans (Mishra et al. 2010). It appears that many of the hyper- and hypomethylation effects induced by heavy metals can be reversed (Jones and Baylin 2002), but there are also cases where ecotoxins can induce heritable epigenetic effects (Kundakovic and Champagne 2011). We are unaware of toxin-mediated epigenetic effects on behaviors, but this may be a manifestation of how many epigeneticists are largely concerned with molecular and biochemical mechanisms in model organisms and are less inherently focused on organismal-level processes.
In addition to epigenetic effects, and especially relevant to the integration of behavior with ecotoxicology, we urge researchers to consider the broad influences that parents would have on the contaminant exposure of their offspring. For example, do parental “decisions” about where to raise offspring influence contaminant exposure? High inter-year nest site fidelity and high natal-site fidelity could both lead to positive correlations of parent and offspring contaminant exposure. It may be that toxin exposure alters these return patterns.
Another way by which parents can affect the toxin exposure of offspring is through inherited cytoplasmic elements. For example, in birds (and other egg-laying organisms) toxins can be deposited in the eggs and thus expose the developing embryo to high concentrations of toxins before they even hatch out into the environment (Heinz and Hoffman 2004; Ou et al. 2015). Therefore, behavioral strategies that influence parents’ foraging and subsequent provisioning of the young can have large consequences for how and when the developing offspring are exposed to environmental contaminants. As toxin levels tend to decline in each subsequently developed egg, laying order and clutch size strategies will also influence the amount of contamination that each embryo experiences. All of which point to the importance of understanding reproductive and parental behaviors in establishing exposure risk to developing offspring.
Independent of parents, individual behavioral strategies can also fundamentally alter the expression of a trait. Many behavioral traits show substantial within-individual flexibility, degrees of developmental plasticity (which is sometimes reversible), and demonstrate heritable genetic variance (Swaddle 2016). For example, an organism’s strategy over where and when to forage could alter its exposure to an environmental toxicant (Kobiela et al. 2015), thereby intrinsically linking the development and expression of behavioral traits to ecotoxicology.
Outside of behavioral variation, the process of development could interact with the effects of a toxicant on an organism. There is growing awareness that some organisms show age- or stage-dependent sensitivity to environmental contaminants (Howdeshell 2002; Varian-Ramos et al. 2014). Hence, we encourage researchers to consider the timing and sequence of exposure relative to the development of target organisms.
Current utility and adaptive significance
Despite the focus of many toxicological studies on direct mortality, sublethal effects of contaminants on behavior are thought to be a much more sensitive endpoint (Melvin and Wilson 2013). Changes in behavior can have significant effects on individual fitness, by increasing mortality (e.g., through predator avoidance behaviors) or reducing reproductive success (e.g., through courtship behaviors). As such, many studies focus on effects of fitness-related behavioral endpoints that have some plausible connection to individual fitness. Effects of contaminants have been detected on many behaviors, including foraging behavior (Gaworecki and Klaine 2008; Mogren and Trumble 2010; Browne and Moore 2014; Tüzün et al. 2015), mating or courtship behaviors (Park et al. 2001; Mogren and Trumble 2010; Partridge et al. 2010; Seuront 2011; McKay and Maher 2012; Secondi et al. 2013), movement or activity levels (Mogren and Trumble 2010; Seuront 2011; Marentette et al. 2012; Barbee et al. 2014; Janssens et al. 2014; Tüzün et al. 2015; Brown et al. 2016; Shuman-Goodier and Propper 2016), predator avoidance (Carlson et al. 2014; Janssens et al. 2014; Justice and Bernot 2014), and social behaviors (Barbieri et al. 2013; Ward et al. 2016).
Although the connections between changes in behavior and individual fitness seem intuitive, few studies make a direct causal link between behavioral changes and reduced survival or reproductive success. There are a few studies, however, that have explicitly drawn this link (Partridge et al. 2010; Seuront 2011; Barbieri et al. 2013). For example, a study of the Argentine ant Linepithema humile revealed that colonies exposed to neonicotinoids behaved more aggressively in interspecific interactions and that this resulted in decreased colony survival (Barbieri et al. 2013). In another study, hydrocarbon contamination impacted swimming patterns in marine copepods, reducing the ability of males to follow female pheromone trails, reducing mating success (Seuront 2011).
Since behavior is linked to fitness, either through behavioral or other physiological mechanisms, there is likely strong selection occurring at contaminated sites for resistant genotypes. If genetic variation exists for sensitivity to contaminants (Buck et al. 2016), then populations with long-term exposure might have evolved adaptations to these contaminants. Such variation has been demonstrated in some cases (Pease et al. 2010; Varian-Ramos et al. 2013) and there are examples of pollutant resistant populations (Brown et al. 2016; Tüzün et al. 2015).
Behavioral adaptations may prove an important means through which populations can survive in contaminated environments. For example, one species of a freshwater pulmonate snail Physella Columbiana have evolved the ability to detect and avoid contaminated sediments, allowing them to survive in places where other species cannot (Lefcort et al. 2004). Whereas populations from uncontaminated sites are less able to avoid contaminants, suggesting that this evolved in response to selection in the contaminated areas (Lefcort et al. 2004).
Although adaptation may be important for persistence of species in contaminated sites, there may also be costs associated with adaptation. There is evidence that there are tradeoffs between such adaptations and other behaviors or processes (Varian-Ramos et al. 2014; Oziolor et al. 2016). For example, strong selection for contaminant resistance may reduce genetic variability within the population (Fasola et al. 2015) and may render these populations more susceptible to other environmental stressors, such as increasing temperatures caused by climate change (Janssens et al. 2014).
Going forward, it is important that the link between changes in behavior and individual fitness is drawn in more systems. In addition, it is important to better understand how contamination could lead to microevolution within exposed populations, as well as the implications of such evolutionary changes for population persistence and risk assessment. If behavioral adaptations exist within contaminated populations, it may result in an underestimate of the risks posed by contaminants when behaviors in exposed populations are compared with populations from uncontaminated areas (Morgan et al. 2007). To avoid this, common garden experiments could be performed to detect any adaptations to contamination that may have evolved, and/or researchers could assess the heritability of traits associated with contaminant load and contaminant resistance (Buck et al. 2016).
Historical origins
The vast number of chemicals used in commerce coupled with the Earth’s tremendous biodiversity leads us to the conclusion that ecotoxicologists will always be data limited; in other words, we will never be able to test all species against all contaminants. Ecotoxicologists have traditionally relied upon a relatively small number of surrogate (or model) species to represent the potential sensitivity of diverse faunal groups. The need to predict or extrapolate toxicity across species is very real (Barron et al. 2012; Barron et al. 2015), but we remain limited in our ability to make rational predictions.
The application of rigorous comparative and phylogenetic approaches in environmental toxicology is relatively new. First, Buchwalter et al. (2008) examined patterns of cadmium bioaccumulation kinetics and detoxification in aquatic insects: they found evolutionary history/phylogeny to be important drivers of these toxicological traits. Soon, other studies followed, examining the importance of phylogeny in determining different rates of metal efflux (Poteat et al. 2013), metal accumulation patterns (Jeffree et al. 2010; Poteat and Buchwalter 2014), and ionoregulatory traits (calcium uptake rates: Poteat and Buchwalter 2014). Other studies examined the importance of phylogeny in explaining patterns of sensitivity (toxicity) to metals (Malaj et al. 2016) and pesticides (Guenard et al. 2014). Further studies showed the potential for phylogenetic approaches in ecological monitoring and ecotoxicology (Jeffree et al. 2013; Larras et al. 2014; Keck et al. 2016).
To date, the marriage of phylogenetic perspectives with behavioral ecotoxicity remains unexplored. On the one hand, previous research has indicated that the tendency for relatives to be similar in contaminant accumulation patterns and sensitivity is pervasive (Buchwalter et al. 2008; Jeffree et al. 2010; Jeffree et al. 2013; Poteat et al. 2013; Guenard et al. 2014; Larras et al. 2014; Poteat and Buchwalter 2014; Keck et al. 2016; Malaj et al. 2016). In addition, it is expected that the neurophysiology of close relatives might be similar, as is the case for other physiological traits (Blomberg et al. 2001). However, Blomberg et al. (2003) also note that behavioral traits are relatively labile (e.g., do not follow phylogeny very closely). Therefore, it remains unclear how closely behavioral ecotoxicity endpoints would map on to phylogenies.
The Importance of Integrating Tinbergen's Four Questions
As has been observed in our advances in integrative understanding of traits that have adopted all four of Tinbergen’s questions (Bateson and Laland 2013), we propose that using such a framework will help unite disparate areas of the life and physical sciences under one banner—to thoroughly explain the interplay between behavioral science and ecotoxicology to inform conservation goals. By understanding the causal mechanisms that underlie how a contaminant affects an organism and alters a behavior, potentially through additional ontogenetic and developmental processes, to influence the behavior’s current utility and fitness function, we will be able to understand (from molecule to organismal fitness) how, when, and why a contaminant will be harmful to wildlife (and even humans). Layered on top, if we can further explore the role of phylogenetic inheritance in determining species’ behavioral and fitness responses to contaminants, we will be better able to extrapolate single population and species studies to the effects of the contaminants on communities and ecosystems. Such an integrative approach to behavioral ecotoxicology has yet to be attempted, hence our presentation of this framework.
Conservation Concerns
Recently, the EPA has reduced the amount of bioassays in non-human, non-model animals, instead increasing the use of cellular assays as a determinant for toxic effects of substances. On the one hand, this has resulted in a decrease in the number of animals sacrificed or exposed, as well as a reduction in experimental confounds (e.g., inbreeding, age, sex, and expense). From a translational point of view, this reductionism has not eliminated the significant issues of extrapolation to humans or wildlife though there are steps being taken to use computational tools to link molecular information to systems responses (Browne et al. 2015). The general reduction of observations of real systems and whole organisms has led to a narrowing of the scope on which policy decisions have been made using whole organism and behavioral data.
This transitional shift away from the standard lethal dose (LD) studies and the large-scale model-based bioassay assessments was outlined in the US National Toxicology Program’s 21st Century Roadmap for the future (National Toxicology Program 2004) and implemented reasonably recently (Collins et al. 2008). This research program has established a collaborative association with the NTP, EPA, and the National Institutes of Health Chemical Genomics Center with specializations in experimental and computational toxicology. This has led to a leap in the discovery of cellular toxicology and toxicogenomics information and will continue to promote collaborations between the fields of pathology, genetics, and toxicology for the foreseeable future (Aardema and MacGregor 2002).
Although this transition has been a positive step in understanding the toxicokinetics and metabolism of emerging threats, there are several issues with this narrow and reductionist focus in ecotoxicology. First, it lacks external validity; in other words, modern toxicology experiments are not typically applicable to relevant and realistic ecological conditions. In the framework we present here, the reductionist approach lacks an understanding of the current utility or function of traits. Some traits may influence population fitness, some may not. We need to complement cellular and molecular studies with fitness-related information to identify which concentrations of which toxicants would affect realistic populations. In addition, the current approach is a very human-centric view of toxicology. The emphasis on assays that evaluate potential impacts of chemical exposure on human tissues (e.g., using human-competent cell lines) potentially has the unintended consequence of limiting our ability to generalize toxicological findings to non-human taxa including other vertebrates. In addition, when removed from the laboratory, these compounds are in a system with complicated toxicokinetics and toxicodynamics based on interactions with other compounds in organisms. Even when the results of such studies are solely considered in a laboratory they are not always generalizable. When cellular and model-species toxicology experiments are conducted, there are constraints placed on the application of the results by necessity. In order to reduce confounding effects, both LD and sublethal dose analyses have been conducted using single pollutant types and model organisms to reduce confounding effects. Using single pollutant types is often necessary in laboratory settings. This approach allows for exposure effects to be attributed to a known source as opposed to having the question of interaction be raised. In addition, the use of model organisms has proven to be beneficial as the effects can be compared with a well-documented list of physiological responses and the outcomes of the exposure can be clearly outlined. The drawbacks of using model species and singular compound types in exposure studies are numerous though. For example, model species are at risk for low genetic diversity (hence constraining evolutionary potential) and large scale studies may not take into account the impacts of individual variation (Fields and Johnston 2005). When taken on a larger scale, this single stressor, single species experimental paradigm does not allow for regulatory decisions to be made from a wildlife toxicology and conservation biology perspective. A functioning and sustainable ecosystem is driven by diverse biotic and abiotic interaction (Cardinale et al. 2002), but if a model does not take this into account, a whole facet of the ecology of a species or a community could be lost. What we propose is a complementary set of actions that include exposure to combinations of toxicants, on model and phylogenetically related non-model systems, where we can study functional difference in the target traits. This expansion to a more ultimate-focused view of toxicant exposure is better understood when coupled with the detailed, proximate views of mechanism and development that we outlined above. It is the integration of proximate and ultimate approaches that we champion.
Additional viewpoints of how toxicology can move forward into the future could be beneficial. Adding components of behavioral ecology and conservation science to the plan for the future of toxicological risk assessment has the potential to more accurately address the impacts of the Anthropocene on human and nonhuman health. Specifically, we recommend that not solely focusing on the causal and mechanistic “how” questions that are almost exclusively funded by the NIH and EPA, we promote the inclusion of the ultimate “why” questions that are embraced by Tinbergen’s latter two questions. We must understand the current utility or function of traits in focus, and also understand their deeper evolutionary origins. Combining these ultimate questions will let us understand the impacts of toxins on individual and population fitness, while also increasing the possibility of extrapolation beyond study systems, especially to endangered biota.
Generating a model to take into account a community of species and abiotic environmental interactions (i.e., understand the trait’s current utility) can be complicated. Ideally it should take into account the interactions among predators, toxicological stressors, and other abiotic interactions for a single species in an ecosystem to understand what regulates population fitness. Selecting an indicator species that is phylogenetically representative of other important species in an at-risk ecosystem can demonstrate how the substance entering the environment will alter a variety of factors, from behavior to physiological function (Carignan and Villard 2002). The combined results from the laboratory toxicology and the ecosystem study can demonstrate the necessity for adjustments in policy and education.
Behavioral ecology would be an excellent addition to the collaborative effort behind toxicology currently underway. At face value, behavioral ecology can be noninvasive, can be correlated to mechanistic alteration such as anatomical and physiological alteration, and is often accessible for citizen science. Behavioral observation of nonhuman animals has been used and is continuing to be used in a variety of research regarding toxicity of substances (Johnson et al. 2003; Kienle et al. 2009). Using a noninvasive technique such as behavioral observation allows for minimal ecosystem disruption in the field and a further reduction of animals in the laboratory. Behavior can also be increasingly tied to internal processes occurring that are not easily seen in the wild or in a laboratory setting without euthanasia (Orger et al. 2004; Prusky et al. 2004; Lynn et al. 2007). In addition to behavior determining the impacts of the substances at that time, behavior can be used to determine larger effects such as impacts to fitness through sexual behavior, parental behavior, and predatory behavior and may lead to a better understanding of how the addition of new pollutants and compounds may impact the life history and evolutionary procedure of the species and community.
Just as importantly, some behavioral observations can be conducted by citizen scientists, bringing toxicity impacts into the language that a community member can understand. Toxicogenomics may require significant experience or scientific training to fully understand, but watching an animal behave differently than expected can be understood by most with limited training and non-specialist equipment. By incorporating behavioral ecology and conservation science practices into toxicology moving forward, the emerging field of IBET can gain external validity and aid in the preservation of the life on this planet, both human and nonhuman. Furthermore, framing the study of behavior within Tinbergen’s four questions will explicitly integrate the current focus on mechanism, causation, and development with the new areas that behavioral ecology and evolution would bring: namely current utility, adaptation, and phylogenetic patterns of toxin resistance and sensitivity.
Recommendations and Concluding Remarks
By better integrating a mechanistic and causal understanding of how and when an environmental contaminant affects the production of phenotypes (including behaviors) with detailed studies of the fitness consequences of these changes, we can integrate disparate fields to understand how contaminants affect wildlife populations. Additionally, if we can further explore how reactions to contaminant exposure change over phylogenetic trees, and how the traits that respond to contaminant exposure also vary with tree structure, we can better extrapolate beyond the common “model” systems to build an evolutionarily informed view of the effects of environmental contaminants on whole ecosystems, potentially including endangered ecosystems and populations. If there are phylogenetic patterns of the effects of toxins on species, then extrapolation from one species to another might be possible—allowing for reconstruction of among-species interactions and community analyses. Extrapolation across species is an elusive goal, but one that could be achievable if research efforts were balanced across each of Tinbergen’s four questions.
We also believe that the science of behavioral ecology will benefit from a closer integration with toxicology. In a rapidly changing Anthropocene, behaviors are likely to be the early warning signs of larger ecological and evolutionary events. By collaborating with toxicologists, behaviorists could help lead the societal need to predict what will happen to wildlife populations as the environment becomes increasingly contaminated, which will be exacerbated by global climate change. In some cases, the disruption of behaviors by specific contaminants may also render insight into what mechanism cause the production of the behavior—hence also rending basic science gains that could feed back into the more general goal of conserving populations, species, and ecosystems. Depending on the toxin and biological system being studied, there may even be extrapolations that inform public health strategies.
We thank Dr Zhiyun Jia (the executive director of Current Zoology) for inviting us to guest edit this special column in Current Zoology. We also thank the Animal Behavior Society for providing us with the opportunity and funding to organize a symposium in behavioral ecotoxicology, which was held in July 2016 at the Animal Behavior Society Annual Meeting 2016 (Columbia, Missouri). We are grateful to the speakers of this symposium (Dr David Buchwalter, Dr Frances Champagne, Dr Tyrone Hayes, Dr Jacob Kerby, Elizabeth Peterson, Dr John Swaddle, Dr Claire Varian-Ramos) for their contributions to the symposium, as well as attendees for their thoughtful feedback following the symposium. We would also like to thank Dr Frances Champagne for her feedback on this review, as well as the other authors for submitting their research to this special edition.
Funding
JPS was funded by the National Science Foundation (IOS1257590). The symposium from which this paper emerged was funded by the Animal Behavior Society.
References
- Aardema MJ, MacGregor JT, 2002. Toxicology and genetic toxicology in the new era of “toxicogenomics”: impact of “-omics” technologies. Mutat Res Fund Mol Mech Mut 499:13–25. [DOI] [PubMed] [Google Scholar]
- Ankley GT, Bennett RS, Erickson RJ, Hoff DJ, Hornung MW. et al. , 2010. Adverse outcome pathways: a conceptual framework to support ecotoxicology research and risk assessment. Environ Toxicol Chem 29:730–741. [DOI] [PubMed] [Google Scholar]
- Barbee NC, Ganio K, Swearer SE, 2014. Integrating multiple bioassays to detect and assess impacts of sublethal exposure to metal mixtures in an estuarine fish. Aquat Toxicol 152:244–255. [DOI] [PubMed] [Google Scholar]
- Barbieri RF, Lester PJ, Miller AS, Ryan KG, 2013. A neurotoxic pesticide changes the outcome of aggressive interactions between native and invasive ants. Proc R Soc B 280:20132157.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnosky AD, Hadly EA, Bascompte J, Berlow EL, Brown JH. et al. , 2012. Approaching a state shift in Earth’s biosphere. Nature 486:52–58. [DOI] [PubMed] [Google Scholar]
- Barron MG, Jackson CR, Awkerman JA, 2012. Evaluation of in silico development of aquatic toxicity species sensitivity distributions. Aquat Toxicol 116–117:1–7. [DOI] [PubMed] [Google Scholar]
- Barron MG, Lilavois CR, Martin TM, 2015. MOAtox: a comprehensive mode of action and acute aquatic toxicity database for predictive model development. Aquat Toxicol 161:102–107. [DOI] [PubMed] [Google Scholar]
- Basu N, 2015. Applications and implications of neurochemical biomarkers in environmental toxicology. Environ Toxicol Chem 34:22–29. [DOI] [PubMed] [Google Scholar]
- Basu N, Goodrich JC, Head J, 2014. Ecogenetics of mercury: from genetic polymorphisms and epigenetics to risk assessment and decision-making. Environ Toxicol Chem 33:1248–1258. [DOI] [PubMed] [Google Scholar]
- Basu N, Head J, 2008. Mammalian wildlife as complementary models in environmental neurotoxicology. Neurotoxicol Teratol 32:114–119. [DOI] [PubMed] [Google Scholar]
- Bateson P, Laland KN, 2013. Tinbergen’s four questions: an appreciation and an update. Trends Ecol Evol 28:1–7. [DOI] [PubMed] [Google Scholar]
- Berger-Tal O, Polak T, Oron A, Lubin Y, Kotler BP. et al. , 2011. Integrating animal behavior and conservation biology: a conceptual framework. Behav Ecol 22:236–239. [Google Scholar]
- Blomberg SP, Ives AR, Garland T, 2001. Detecting phylogenetic signal in comparative data. Amer Zool 41:1395. [DOI] [PubMed] [Google Scholar]
- Blomberg SP, Garland T Jr, Ives AR, 2003. Testing for phylogenetic signal in comparative data: behavioral traits are more labile. Evolution 57:717–745. [DOI] [PubMed] [Google Scholar]
- Broley C, 1958. The plight of the American bald eagle. Audubon 60:171. [Google Scholar]
- Brodin T, Piovano S, Fick J, Klaminder J, Heynen M. et al. , 2014. Ecological effects of pharmaceuticals in aquatic systems: impacts through behavioural alterations. Phil Trans R Soc B 369:20130580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DR, Bailey JM, Oliveri AN, Levin ED, Di Giulio RT, 2016. Developmental exposure to a complex PAH mixture causes persistent behavioral effects in naïve Fundulus heteroclitus (killifish) but not in a population of PAH-adapted killifish. Neurotoxicol Teratol 53:55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne P, Judson RS, Casey WM, Kleinstreuer NC, Thomas RS, 2015. Screening chemicals for estrogen receptor bioactivity using a computational model. Environ Sci Technol 26:8804–8814. [DOI] [PubMed] [Google Scholar]
- Browne AM, Moore PA, 2014. The effects of sublethal levels of 2,4-dichlorophenoxyacetic acid herbicide (2,4-D) on feeding behaviors of the crayfish O. rusticus. Arch Environ Contam Toxicol 67:234–244. [DOI] [PubMed] [Google Scholar]
- Bucholz R, 2007. Behavioral biology: an effective tool and relevant conservation tool. Trends Ecol Evol 22:401–407. [DOI] [PubMed] [Google Scholar]
- Buchwalter DB, Cain DJ, Martin CA, Xie L, Luoma SN. et al. , 2008. Aquatic insect ecophysiological traits reveal phylogenetically based differences in dissolved cadmium susceptibility. Proc Natl Acad Sci USA 105:8321–8326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck KA, Varian-Ramos CW, Cristol DA, Swaddle JP, 2016. Blood mercury levels of zebra finches are heritable: implications for the evolution of mercury resistance. PLoS ONE 11:e0162440.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinale BJ, Palmer MA, Collins SL, 2002. Species diversity enhances ecosystem functioning through interspecific facilitation. Nature 415:426–429. [DOI] [PubMed] [Google Scholar]
- Carignan V, Villard MA, 2002. Selecting indicator species to monitor ecological integrity: a review. Environ Monit Assess 78:45–61. [DOI] [PubMed] [Google Scholar]
- Carlson JR, Cristol DA, Swaddle JP, 2014. Dietary mercury exposure causes decreased escape takeoff flight performance and increased molt rate in European starlings Sturnus vulgaris. Ecotoxicology 23:1464–1473. [DOI] [PubMed] [Google Scholar]
- Carroll L, 1865. Alice’s Adventures in Wonderland. London: Macmillan. [Google Scholar]
- Carson R, 1962. Silent Spring. Cambridge (MA): Houghton Mifflin. [Google Scholar]
- Chapman PM, 2007. Introduction to perspectives: aquatic behavioral ecotoxicology-coming of age. Hum Ecol Risk Assess 13:478–480. [Google Scholar]
- Clotfelter ED, Bell AM, Levering KR, 2004. The role of animal behaviour in the study of endocrine-disrupting chemicals. Anim Behav 68:665–676. [Google Scholar]
- Collins FS, Gray GM, Bucher JR, 2008. Transforming environmental health protection. Science 319:906.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews D, Gore AC, Hsu TS, Dangleben NL, Spinetta M. et al. , 2007. Transgenerational epigenetic imprints on mate preference. Proc Natl Acad Sci USA 104:5942–5946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crutzen PJ, Stoermer EF, 2000. The “Anthropocene”. Glob Change Newsl 41:17–18. [Google Scholar]
- Dalton R, 2002. Frogs put in the gender blender by America’s favourite herbicide. Nature 416:665–666. [DOI] [PubMed] [Google Scholar]
- Davidson C, Shaffer HB, Jennings MR, 2001. Declines of the California red-legged frog: climate, UV-B, habitat, and pesticides hypotheses. Ecol Appl 11:464–479. [Google Scholar]
- Davidson C, 2004. Declining downwind: amphibian population declines in California and historical pesticide use. Ecol Appl 14:1892–1902. [Google Scholar]
- De Guise S, Martineau D, Béland P, Fourner M, 1995. Possible mechanisms of action of environmental contaminants on St. Lawrence Beluga whales Delphinapterus leucas. Environ Health Perspect 103:73–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell’Omo G, 2002. Behavioral Ecotoxicology. West Sussex: John Wiley & Sons. [Google Scholar]
- Facemire CF, Gross TS, Guillette LJ, 1995. Reproductive impairment in the Florida panther: nature or nurture? Environ Health Perspect 103:79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasola E, Ribeiro R, Lopes I, 2015. Microevolution due to pollution in amphibians: a review on the genetic erosion hypothesis. Environ Pollut 204:181–190. [DOI] [PubMed] [Google Scholar]
- Fields S, Johnston M, 2005. Whither model organism research? Science 307:1885–1886. [DOI] [PubMed] [Google Scholar]
- Finkelstein ME, Doak DF, Burnett J, Brandt J, Church M. et al. , 2012. Lead poisoning and the deceptive recovery of the critically endangered California condor. Proc Natl Acad Sci USA 109:11449–11454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaworecki KM, Klaine SJ, 2008. Behavioral and biochemical responses of hybrid striped bass during and after fluoxetine exposure. Aquat Toxicol 88:207–213. [DOI] [PubMed] [Google Scholar]
- Gerhardt A, 2007. Aquatic behavioral ecotoxicology: perspectives and limitations. Hum Ecol Risk Assess 13:481–491. [Google Scholar]
- Gilfillan SC, 1965. Lead poisoning and the fall of Rome. J Occup Med 7:53–60. [PubMed] [Google Scholar]
- Guenard G, Carsten von der OP, Carlisle WS, Lek S, Legendre P, 2014. Using phylogenetic information and chemical properties to predict species tolerances to pesticides. Proc Biol Sci 281:20133239.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen LJ, Johnson ML, 1999. Conservation and toxicology: integrating the disciplines. Conserv Biol 13:1225–1227. [Google Scholar]
- Heinz GH, Hoffman DJ, 2004. Mercury accumulation and loss in mallard eggs. Environ Toxicol Chem 23:222–224. [DOI] [PubMed] [Google Scholar]
- Hellou J, 2011. Behavioral ecotoxicology, an “early warning” signal to assess environmental quality. Environ Sci Pollut Res 18:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernberg S, 2000. Lead poisoning in a historical perspective. Am J Ind Med 38:244–254. [DOI] [PubMed] [Google Scholar]
- Howdeshell KL, 2002. A model of the development of the brain as a construct of the thyroid system. Environ Health Perspect 110:337.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssens L, Van KD, Debecker S, Bervoets L, Stoks R, 2014. Local adaptation and the potential effects of a contaminant on predator avoidance and antipredator responses under global warming: a space-for-time substitution approach. Evol Appl 7:421–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffree RA, Oberhansli F, Teyssie JL, 2010. Phylogenetic consistencies among chondrichthyan and teleost fishes in their bioaccumulation of multiple trace elements from seawater. Sci Total Environ 408:3200–3210. [DOI] [PubMed] [Google Scholar]
- Jeffree RA, Oberhaensli F, Teyssie JL, 2013. Marine radionuclide transfer factors in chordates and a phylogenetic hypothesis. J Environ Radioact 126:388–398. [DOI] [PubMed] [Google Scholar]
- Johnson MD, Kenney N, Stoica A, Hilakivi-Clarke L, Singh B. et al. , 2003. Cadmium mimics the in vivo effects of estrogen in the uterus and mammary gland. Nat Med 9:1081–1084. [DOI] [PubMed] [Google Scholar]
- Johnston EL, Mayer-Pinto M, Crowe TP, 2015. REVIEW: Chemical contaminant effects on marine ecosystem functioning. J Appl Ecol 52:140–149. [Google Scholar]
- Jones PA, Baylin SB, 2002. The fundamental role of epigenetic events in cancer. Nat Rev Genet 3:415–428. [DOI] [PubMed] [Google Scholar]
- Justice JR, Bernot RJ, 2014. Nanosilver inhibits freshwater gastropod Physa acuta ability to assess predation risk. Am Midl Nat 171:340–349. [Google Scholar]
- Kane AS, Salierno JD, Brewer SK, 2005. Fish models in behavioral toxicology: automated techniques, updates and perspectives In: Ostrander GK, editor. Methods in Aquatic Toxicology, Vol. 2 Boca Raton (FL): Lewis Publishers, 559–590. [Google Scholar]
- Keck F, Rimet F, Franc A, Bouchez A, 2016. Phylogenetic signal in diatom ecology: perspectives for aquatic ecosystems biomonitoring. Ecol Appl 26:861–872. [DOI] [PubMed] [Google Scholar]
- Kienle C, Köhler HR, Gerhardt A, 2009. Behavioural and developmental toxicity of chlorpyrifos and nickel chloride to zebrafish Danio rerio embryos and larvae. Ecotoxicol Environ Saf 72:1740–1747. [DOI] [PubMed] [Google Scholar]
- Kobiela ME, Cristol DA, Swaddle JP, 2015. Risk-taking behaviours in zebra finches affected by mercury exposure. Anim Behav 31:153–160. [Google Scholar]
- Köhler HR, Triebskorn R, 2013. Wildlife ecotoxicology of pesticides: can we track effects to the population level and beyond? Science 341:759–765. [DOI] [PubMed] [Google Scholar]
- Kundakovic M, Champagne FA, 2011. Epigenetic perspective on the developmental effects of bisphenol A. Brain Behav Immun 25:1084–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larras F, Keck F, Montuelle B, Rimet F, Bouchez A, 2014. Linking diatom sensitivity to herbicides to phylogeny: a step forward for biomonitoring? Environ Sci Technol 48:1921–1930. [DOI] [PubMed] [Google Scholar]
- Lefcort H, Abbott DP, Cleary DA, Howell E, Keller NC. et al. , 2004. Aquatic snails from mining sites have evolved to detect and avoid heavy metals. Arch Environ Contam Toxicol 46:478–484. [DOI] [PubMed] [Google Scholar]
- Levin ED, Aschner M, Heberlein U, Ruden D, Welsh-Bohmer KA. et al. , 2009. Genetic aspects of behavioral neurotoxicology. Neurotoxicology 30:741–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little EE, 1990. Behavioral toxicology: stimulating challenges for a growing discipline. Environ Toxicol Chem 9:1–2. [Google Scholar]
- Lynn SE, Egar JM, Walker BG, Sperry TS, Ramenofsky M, 2007. Fish on Prozac: a simple, noninvasive physiology laboratory investigating the mechanisms of aggressive behavior in Betta splendens. Adv Physiol Educ 31:358–363. [DOI] [PubMed] [Google Scholar]
- Malaj E, Guenard G, Schafer RB, von der Ohe PC, 2016. Evolutionary patterns and physicochemical properties explain macroinvertebrate sensitivity to heavy metals. Ecol Appl 26:1249–1259. [DOI] [PubMed] [Google Scholar]
- Marentette JR, Tong S, Wang G, Sopinka NM, Taves MD. et al. , 2012. Behavior as biomarker? Laboratory versus field movement in round goby Neogobius melanostomus from highly contaminated habitats. Ecotoxicology 21:1003–1012. [DOI] [PubMed] [Google Scholar]
- McKay JL, Maher CR, 2012. Relationship between blood mercury levels and components of male song in Nelson’s sparrow Ammodramus nelson. Ecotoxicology 21:2391–2397. [DOI] [PubMed] [Google Scholar]
- Melvin SD, Wilson SP, 2013. The utility of behavioral studies for aquatic toxicology testing: a meta-analysis. Chemosphere 93:2217–2223. [DOI] [PubMed] [Google Scholar]
- Mishra S, Prakash Dwivedi S, Singh RB, 2010. A review of epigenetic effect of heavy metal carcinogens on human health. Open Netraceuticals J 3:188–193. [Google Scholar]
- Møller AP, Swaddle JP, 1997. Asymmetry, Developmental Stability, and Evolution. Oxford: Oxford University Press. [Google Scholar]
- Mogren CL, Trumble JT, 2010. The impacts of metals and metalloids on insect behavior. Entomol Exp Appl 135:1–17. [Google Scholar]
- Morgan AJ, Kille P, Stürzenbaum SR, 2007. Microevolution and ecotoxicology of metals in invertebrates. Environ Sci Technol 41:1085–1096. [DOI] [PubMed] [Google Scholar]
- National Toxicology Program, 2004. Toxicology in the 21st Century: The Role of the National Toxicology Program. Research Triangle Park, NC: Department of Health and Human Services. [Google Scholar]
- Newman JR, 1979. Effects of industrial air pollution on wildlife. Biol Conserv 15:181–190. [Google Scholar]
- Norstrom RJ, Belikov SE, Born EW, Garner GW, Malone B. et al. , 1998. Chlorinated hydrocarbon contaminants in polar bears from eastern Russia, North America, Greenland, and Svalbard: biomonitoring of Arctic pollution. Arch Environ Contam Toxicol 35:354–367. [DOI] [PubMed] [Google Scholar]
- Orger MB, Gahtan E, Muto A, Page-McCaw P, Smear MC. et al. , 2004. Behavioral screening assays in zebrafish. Methods Cell Biol 77:53–68. [DOI] [PubMed] [Google Scholar]
- Ou L, Varian-Ramos CW, Cristol DA, 2015. Effect of laying sequence on egg mercury in captive zebra finches: an interpretation considering individual variation. Environ Toxicol Chem 34:1787–1792. [DOI] [PubMed] [Google Scholar]
- Oziolor EM, Dubansky B, Burggren WW, Matson CW, 2016. Cross-resistance in gulf killifish Fundulus grandis populations resistant to dioxin-like compounds. Aquat Toxicol 175:222–231. [DOI] [PubMed] [Google Scholar]
- Park D, Hempleman SC, Propper CR, 2001. Endosulfan exposure disrupts pheromonal systems in the red-spotted newt: a mechanism for subtle effects of environmental chemicals. Environ Health Perspect 109:669–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge C, Boettcher A, Jones AG, 2010. Short-term exposure to a synthetic estrogen disrupts mating dynamics in a pipefish. Horm Behav 58:800–807. [DOI] [PubMed] [Google Scholar]
- Pease CJ, Johnston EL, Poore AGB, 2010. Genetic variability in tolerance to copper contamination in a herbivorous marine invertebrate. Aquat Toxicol 99:10–16. [DOI] [PubMed] [Google Scholar]
- Peeters ETH, Gerhardt A, Amiard-Triquet C, 2009. Behavioral ecotoxicology: mechanisms, effects, applications and biomonitoring. Humans Ecol Risk Assess 15:7–10. [Google Scholar]
- Postel S, 1987. Defusing the Toxics Threat: Controlling Pesticides and Industrial Waste. Washington, DC: Worldwatch Institute. [Google Scholar]
- Poteat MD, Garland T, Fisher NS, Wang WX, Buchwalter DB, 2013. Evolutionary patterns in trace metal (Cd and Zn) efflux capacity in aquatic organisms. Environ Sci Technol 47:7989–7995. [DOI] [PubMed] [Google Scholar]
- Poteat MD, Buchwalter DB, 2014. Phylogeny and size differentially influence dissolved Cd and Zn bioaccumulation parameters among closely related aquatic insects. Environ Sci Technol 48:5274–5281. [DOI] [PubMed] [Google Scholar]
- Poteat MD, Buchwalter DB, 2014. Calcium uptake in aquatic insects: influences of phylogeny and metals (Cd and Zn). J Exp Biol 217:1180–1186. [DOI] [PubMed] [Google Scholar]
- Prusky GT, Alam NM, Beekman S, Douglas RM, 2004. Rapid quantification of adult and developing mouse spatial vision using a virtual optomotor system. Invest Ophthalmol Vis Sci 45:4611–4616. [DOI] [PubMed] [Google Scholar]
- Rattner BA, 2009. History of wildlife toxicology. Ecotoxicology 18:773–783. [DOI] [PubMed] [Google Scholar]
- Renner R, 2002. Amphibian declines: conflict brewing over herbicide’s link to frog deformities. Science 298:938–939. [DOI] [PubMed] [Google Scholar]
- Ritchie GRS, Kirby S, Hawkey DJC, 2007. Song learning as an indicator mechanisms: modeling the developmental stress hypothesis. J Theor Biol 251:570–583. [DOI] [PubMed] [Google Scholar]
- Rundlöf M, Andersson GK, Bommarco R, Fries I, Hederström V. et al. , 2015. Seed coating with a neonicotinoid insecticide negatively affects wild bees. Nature 521:77–80. [DOI] [PubMed] [Google Scholar]
- Scott GR, Sloman KA, 2004. The effects of environmental pollutants on complex fish behaviour: integrating behavioural and physiological indicators of toxicity. Aquat Toxicol 68:369–392. [DOI] [PubMed] [Google Scholar]
- Secondi J, Lepetz V, Cossard G, Sourice S, 2013. Nitrate affects courting and breathing but not escape performance in adult newts. Behav Ecol Sociobiol 67:1757–1765. [Google Scholar]
- Seuront L, 2011. Hydrocarbon contamination decreases mating success in a marine planktonic copepod. PLoS ONE 6:e26283.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuman-Goodier ME, Propper CR, 2016. A meta-analysis synthesizing the effects of pesticides on swim speed and activity of aquatic vertebrates. Sci Total Environ 565:758–766. [DOI] [PubMed] [Google Scholar]
- Söffker M, Tyler CR, 2012. Endocrine disrupting chemicals and sexual behaviors in fish: a critical review on effects and possible consequences. Crit Rev Toxicol 42:653–668. [DOI] [PubMed] [Google Scholar]
- Swaddle JP, 2016. Evolution and conservation behaviour In: Berger-Tal O, Saltz D, editors. Conservation Behaviour: Applying Behavioural Ecology to Wildlife Conservation and Management. Cambridge: Cambridge University Press, 35–65. [Google Scholar]
- Tinbergen N, 1963. On aims and methods of ethology. Zeitschrift Fur Tierpsychol 20:410–433. [Google Scholar]
- Tüzün N, Debecker S, Op de Beeck L, Stoks R, 2015. Urbanisation shapes behavioural responses to a pesticide. Aquat Toxicol 163:81–88. [DOI] [PubMed] [Google Scholar]
- United Nations Environment Program (UNEP), 1992. Saving Our Planet: Challenges and Hopes. Nairobi: UNEP. [Google Scholar]
- Varian-Ramos CW, Swaddle JP, Cristol DA, 2013. Genetic variation in the effects of mercury on reproduction in zebra finches. Environ Pollut 183:316–323. [DOI] [PubMed] [Google Scholar]
- Varian-Ramos CW, Swaddle JP, Cristol DA, 2014. Mercury reduces avian reproductive success and imposes selection: an experimental study with adult- or lifetime-exposure in zebra finch. PLoS ONE 9:e95674.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vince G, 2011. An epoch debate. Science 334:32–37. [DOI] [PubMed] [Google Scholar]
- Vitousek PM, Mooney HA, Lubchenco J, Melillo JM, 1997. Human domination of earth’s ecosystems. Science 277:494–499. [Google Scholar]
- Wake DM, Vredenburg VT, 2008. Are we in the midst of the sixth mass extinction? A view from the world of amphibians. Proc Natl Acad Sci USA 105:11466–11473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward AJW, Duff AJ, Horsfall JS, Currie S, 2016. Scents and scents-ability: pollution disrupts chemical social recognition and shoaling in fish. Proc R Soc B 274:101–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner RE, Peterson KK, Borgman L, 1966. Behavioral pathology in fish: a quantitative study of sublethal pesticide toxication. J Appl Ecol 3:223–247. [Google Scholar]
- Weis B, 1983. Behavioral toxicology and environmental health science: opportunity and challenge for psychology. Am Psychol 38:1174–1187. [DOI] [PubMed] [Google Scholar]
- Weis JS, 2013. Physiological, Developmental and Behavioral Effects of Marine Pollution. New York: Springer. [Google Scholar]
- Weis JS, Smith G, Zhou T, Santiago-Bass C, Weis P, 2001. Effects of contaminants on behavior: biochemical mechanisms and ecological consequences. BioScience 51:209–217. [Google Scholar]
- Wilson EO, 1989. Threats to biodiversity. Sci Amer 261:108–116. [Google Scholar]
- Yu M-H, Tsunoda H, Tsunoda M, 2011. Environmental Toxicology: Biological and Health Effects of Pollutants. New York: CRC Press. [Google Scholar]
- Zala SM, Penn DJ, 2004. Abnormal behaviours induced by chemical pollution: a review of the evidence and new challenges. Anim Behav 68:649–664. [Google Scholar]
- Zalasiewicz J, Williams M, Steffen W, Crutzen P, 2010. The new world of the Anthropocene. Environ Sci Technol 44:2228–2231. [DOI] [PubMed] [Google Scholar]