Abstract
In animals, signaling behavior is often context-dependent, with variation in the probability of emitting certain signals dependent on fitness advantages. Senders may adjust signaling rate depending on receiver identity, presence of audiences, or noise masking the signal, all of which can affect the benefits and costs of signal production. In the cooperative breeding meerkat Suricata suricatta, group members emit soft contact calls, termed as “close calls”, while foraging in order to maintain group cohesion. Here, we investigated how the close calling rate during foraging was affected by the presence of pups, that produce continuous, noisy begging calls as they follow older group members. Adults decreased their overall close call rate substantially when pups were foraging with the group in comparison to periods when no pups were present. We suggest this decrease was likely due to a masking effect of the loud begging calls, which makes the close call function of maintaining group cohesion partly redundant as the centrally located begging calls can be used instead to maintain cohesion. There was some support that adults use close calls strategically to attract specific pups based on fitness advantages, that is, as the philopatric sex, females should call more than males and more to female pups than male pups. Dominant females called more than dominant males when a pup was in close proximity, while subordinates showed no sex-based differences. The sex of the nearest pup did not affect the calling rate of adults. The study shows that meerkats modify their close call production depending on benefits gained from calling and provides an example of the flexible use of one calling system in the presence of another, here contact calls versus begging calls, within the same species.
Keywords: call rate, communication, contact calls, cooperation, flexibility, group cohesion.
Animals typically produce characteristic signals in specific contexts, but within that we find variation in the likelihood that a signal is emitted or not (Cheney et al. 1995; Rendall et al. 1999). The sender may gain fitness advantages by adjusting signaling behavior depending on potential receivers, such as those with higher rank versus lower rank (Silk et al. 2016), based on the presence of additional audiences, such as predators (Deecke et al. 2002), or as a function of noise masking the signal (Derryberry et al. 2016), all of which could affect the benefits and costs of producing the signal. As a consequence we would expect senders to be able to produce the signals flexibly, and mainly produce them when it is most beneficial to do so. In group living species, individuals may differ in their signaling plasticity, as each of them experiences costs and benefits differently depending on individual traits, such as their sex, age, rank, condition, or their spatial location in the group, or the social and ecological environments (Lemasson et al. 2013). This may also vary depending on the function of the calls, and therefore differ between contexts.
One such context is seen in group foraging, where group members are spaced out but stay in contact with each other with vocalizations (Koda et al. 2008; Kondo and Watanabe 2009; Fichtel and Manser 2010). However, by emitting contact calls, a sender reveals its position not only to other group members, but also to predators (Deecke et al. 2002). Furthermore, in groups, where group members compete for resources rather than sharing them, they may try to avoid physical contact with others, or some specific individuals, and therefore not emit calls (Gros-Louis 2004). For example, in groups where dependent young follow adults around to be fed, such as in fledgling birds (Thompson et al. 2013) or pups in mongoose species (Kunc et al. 2007; Bell 2008), only highly successful foragers may be able to feed, while the others may try to avoid being followed by hungry offspring, and keep silent. Moreover, in species where relationships differ among group members, signals may be used strategically and senders will selectively emit calls to those individuals from which they will receive the most benefits.
Noise in the environment can mask vocal signals and reduce the efficacy of contact calls in maintaining spatial coordination between foraging group members. Most studies on the masking effects on vocal signals have dealt with the influence of anthropogenic or biotic noise in the environment (Derryberry et al. 2016). However, vocalizations produced by other members of the same group using a different call type may also have a disruptive influence or masking effect, if they are emitted at the same time. In the context of group coordination vocalizations, the loud begging calls from fledglings in birds (Thompson et al. 2013) or pups in mammals (Manser and Avey 2000) are likely to mask soft contact calls. The question arises: how do these 2 communication systems affect each other, whether the more obvious and louder begging calls make the low amplitude contact calls redundant, or whether the different call types are used in a coordinated way?
Meerkats Suricata suricatta are a cooperatively breeding mongoose species that forage as a cohesive unit and maintain contact by emitting low amplitude contact calls that are referred to as close calls (Manser 1998; Gall and Manser 2017). Meerkats live in groups of, on average, 15 individuals (range 3–49), including the dominant, reproductive pair, and male and female subordinate helpers of different ages that assist with pup care and guarding (Clutton-Brock and Manser 2016). Female offspring are more likely than males to remain with the group after reaching adulthood, although both sexes can disperse to form or join new groups (Clutton-Brock 2016). Litters consist, on average, of 4 pups (range 1–7), which remain for the first 3 weeks in their underground burrow with typically one to 2 babysitters looking after them while the group is away foraging during the day. Once pups start foraging, at the age of about 4 weeks, they adopt a mobile begging system with the group; pups follow older group members during foraging and emit high rates of begging calls which indicate hunger levels (Manser et al. 2008) and elicit food provisioning from group members (Manser and Avey 2000; Brotherton et al. 2001; Kunc et al. 2007). Pups constantly emit these loud begging calls until they reach independence around 3 months of age (Manser and Avey 2000), when they are able to find enough food for themselves, and cease begging (Madden et al. 2009). While previous studies have examined pup begging behavior and food allocation by carers, little is known about how older group members alter their communication tactics in the presence of loudly begging pups. This is an important question as it examines flexibility in the production and function of a commonly used signal during the short-term presence of noisy, dependent pups.
The close calls, which meerkats only produce during foraging activities, are individually distinct and allow individuals to monitor group members spatially (Townsend et al. 2011; Reber et al. 2013). The rate of producing close calls is highly variable and is influenced by weather conditions, reproductive season, the social environment, and socio-spatial contexts (Mausbach et al. 2017). Meerkats can adjust their calling rate depending on the identity of the closest neighbor (Wolf 2014). Individuals at the leading edge of a group give more close calls than individuals at the back edge of the group, and the rate of close calling increases with decreasing proximity between group members (Engesser 2011). Preliminary observations further showed that meerkats decrease close calling rates when pups are foraging with the group (Manser 1998). Experimental studies have also demonstrated that close calls influence movement and cohesion during foraging by attracting receivers toward the caller (Gall and Manser 2017).
Here, we investigated different factors at the individual and group level to identify what explained the decrease in close calling rate of adult meerkats when pups were present. If all of the individuals decreased their calling rate, this may be related to the function of the close call to maintain group cohesion. When pups are foraging with the group, their loud and pervasive begging calls may be used by group members to guide coordinated movements and spatial positioning instead of the more quiet close calls. However, if the relative change in close calling rates differed between individuals, this could reflect differences in the direct and indirect benefits associated with cooperative care of pups. More successful foragers provide more of their found food (Clutton-Brock et al. 2002), and potentially use close calls to attract pups to follow them. As the philopatric sex, females may benefit more from attracting and provisioning female pups since larger group sizes are associated with enhanced fitness (Clutton-Brock et al. 2002) because pup growth rate is positively related to the number of carers per pup, and pup survival between emergence from the burrow and foraging independence is higher in larger groups (Russell et al. 2002). Moreover, females feed pups at a higher rate than males, feed a higher proportion of found food than males (Brotherton et al. 2001; English et al. 2008), and feed female pups more than male pups (Brotherton et al. 2001). Female pups stay closer to adults than male pups (Hollén and Manser 2006). Therefore, females may emit close calls to attract or coordinate movements during foraging with female pups as this type of cooperative behavior could provide increased long-term benefits. As such, and if close calls are used strategically to make pups follow, we predict that females will produce more close calls in the presence of pups than males and they will produce more close calls when female pups are nearby compared with when male pups are nearby.
We used paired audio recordings of individuals taken before pups started foraging with the group and when pups were foraging with the group (1) to assess the influence the presence of pups has on close call rates and (2) to determine what group- or individual-level factors may predict individual differences in the relative change in call rate in these different contexts. The group-level traits we measured include group size, litter size, the ratio of pups to carers, and the perceived predation risk within the group. At the individual level, we investigated whether the relative change in overall calling rate was affected by the caller’s sex, social rank, and relative contribution to pup provisioning and vigilance behaviors within the group. Furthermore, we examined (3) flexibility of call rates within short time windows directly associated with the close presence of a pup to determine whether these rates were influenced by the sex or rank of the caller or the sex of the nearest pup.
Materials and Methods
Study area
Research was conducted on habituated wild meerkats at the Kalahari Meerkat Project, Kuruman River Reserve (26°59′ S, 21°50′ E) near Van Zylsrus in South Africa (Clutton-Brock et al. 1998). The arid landscape at the study site consists of wind-blown dunes, dry riverbeds, and flats containing a mixture of sparsely distributed and patchy vegetation including multiple grass, shrub, and tree species. The meerkats were habituated to observers walking with the foraging groups in order to collect behavioral data and sound recordings within 0.5–1 m (Manser and Avey 2000). Data were collected from April to June 2016 and January to March 2017 on 60 adult individuals in 8 different groups. This represented 9 different breeding events (i.e., unique litters). Group size when the pups began foraging with the group ranged from 9 to 20 individuals (mean ± SD = 12.44 ± 3.64) and litter size ranged from 1 to 5 pups (mean ± SD = 3.22 ± 1.30).
Data collection
To calculate close calling rate, we conducted focal animal samples on adult meerkats while they foraged. Two focals of 5–10 min (average of 8 min) were conducted on adults in each of 2 time periods: (1) from 10 days after litter birth until the pups left the burrow and began foraging with the group (no pups period) and (2) after the pups had been foraging with the group for at least a week but were not yet independent (with pups period). Each target period for recordings was approximately 2 weeks long. Recordings were made with a directional microphone (Sennheiser ME66 with K6 powering module) with windshield (Reinhardt, Germany) fixed to a tripod leg connected to a solid state recorder (Marantz PMD661, sampling frequency 48 kHz, 24 bit). A second microphone (Joseph E-280 Dynamic Microphone) was connected to the second channel on the recorder through which the observer annotated the focal individual’s behavior and the context in which each call was produced as well as the identity and distance of nearest neighboring adult and pup. Sound recordings were only conducted in a foraging context and the recording was paused when the focal individual was engaged in non-foraging behavior for more than 30 s.
All close calls produced by the focal individual and all nearest neighbor annotations in the .wav files were manually labeled in Cool Edit Pro v2.0 (Syntrillium) and Audacity v2.1.2 (http://audacity.sourceforge.net/). The overall rate of close calls was calculated for each individual in each period by first dividing the number of close calls by the duration of the recording period, and then averaging the call rates of the 2 sound focals together for each period. Forty-eight out of 60 individuals were recorded in both time periods and subsequent analysis of overall call rate was restricted to these individuals with paired recordings. Short-term calling rates occurring during the close presence of a single pup of known sex were calculated within 10 s windows surrounding the field annotation of pup presence between 1 and 5 m from the focal individual. To be selected for analysis, no other pups or adults could be present within this time window at a distance closer than 5 m. Instances of a pup present at less than 1 m distance were not included to avoid disruptions in calling rate due to aggressive interactions or pup feeds. Since meerkats occasionally move at a quick pace during foraging, the short window duration of 10 s was selected to increase the likelihood that calls were delivered while the only nearest neighbor was the specific pup described in the annotation.
We also attempted to record all pup provisioning events, bouts of anti-predator vigilance, and alarm events during foraging sessions for an average of 10 h per group per week. These observations were made on days adjacent to the day on which we recorded vocalizations. Thus, these data permitted us to make comparisons between periods of having the groups foraging without the pups and the period with pups, and calculate relative contributions by different individuals to these cooperative behaviors. We were careful to avoid any systematic bias in the data by constantly moving around among all different group members and covering the spatial range of the group equally. During these observation periods, observers noted the initiator of feeding events to pups as well as the recipient, the occurrence and duration of vigilance behaviors displayed by each individual, and the number of predator alarm events produced by the group. Each individual’s relative contribution to pup provisioning within their group was calculated by dividing the number of times an individual provisioned a pup with food by the total number of pup provisioning events recorded for the group in the first 20 days after the pups started foraging with the group. Similarly, each individual’s relative contribution to group vigilance behavior was calculated by dividing the total number of vigilance events per individual by the total number of vigilance events observed by all group members within their group during a 40-day period surrounding the date that the pups started foraging with the group (i.e., 20 days before and after this date). Group-wide alarm event rates for this same time period were calculated by dividing the total number of alarm events observed at each group by the total number of hours each group was observed during this 40 day period.
Statistical analysis
A Wilcoxon signed ranks test was used to compare the overall close call rate of each individual during sound focal samples when there were no pups to their overall close call rate when there were pups foraging with the group. Wilcoxon rank sum tests were used to test for significant differences in the overall call rate between the sexes and between dominants and subordinates within the 2 separate time periods. Non-parametric tests were used because the data were not normally distributed, as determined by both visual inspection of QQ plots and Shapiro–Wilk tests of normality. The relative change in call rate between the No Pup and With Pup periods was calculated as a percentage change for each individual as follows:
A linear mixed-effects model fit by maximum likelihood (with Satterthwaite approximations of degrees of freedom and P values) was used to determine what factors may partially explain the lower close call rate when pups were foraging with the group. Percentage change in close call rate between the 2 periods was the response variable and group nested within year was included as a random intercept term to account for repeated measurements within the same group and year. Focal ID was not included as a random effect because there was only one data point per individual. Predictor variables added to the model as main effects included sex, social rank (dominant or subordinate), relative contribution to pup feeding, relative contribution to vigilance behavior, group alarm rate, group size, litter size, and pup to carer ratio were included. The interaction term between sex and rank was also included. Age was not included in the model as a fixed effect because the age distribution was strongly associated with social rank (e.g., younger subordinates and older dominants). Prior to analysis of overall call rates or percentage change in call rate, one extreme outlying data point was removed which was in the 99.9% percentile of the data distribution. Model assumptions were examined graphically with QQ plots and plots of fitted values versus standardized residuals.
A mixed model regression with Poisson distribution was used to assess what factors influence short-term call rates within narrow time windows directly associated with the close presence of a pup during the period when pups were foraging with the group. Here, we tested if the number of close calls produced within the 10 s windows of time surrounding each nearest neighbor annotation was influenced by the sex and rank of the caller and the sex of the nearest pup. Fixed effects within the full model included the sex and rank of the caller, the sex of the nearest pup present at a distance of 1–5 m, and the interactions between these factors. Focal ID nested within group was included as a random effect (intercept). Year was initially included as a random effect as well, with group and focal ID nested within it, but was subsequently removed as it displayed very little variation and likelihood ratio tests confirmed its inclusion did not improve model fit. To examine the influence of focal sex more closely, the dataset was subsequently split into dominant and subordinate subsets for further analysis. Model outcomes were examined for over- and under-dispersion by comparing the sum of squared Pearson residuals to the residual degrees of freedom. Zero-inflation was assessed by comparing the proportion of zero counts observed in the dataset to the proportion of zero counts predicted by the Poisson distribution. As there were no issues with dispersion or zero-inflation detected, the final models were run with the generalized log linear mixed model regression with Poisson distribution (fit by maximum likelihood, Laplace approximation).
Stepwise backward model selection procedures were used to determine the best fit models for both the linear and Poisson mixed effects models. Best fit models were compared with null models using likelihood ratio tests (within anova functions), with secondary comparisons conducted using AICc scores. Boxplots were used to visualize the data: the lower and upper hinges correspond to the first and third quartiles, the middle line represents the median, and the whiskers extend to the smallest and largest values within 1.5 times the interquartile range. All tests were run in R Studio v1.0.136 with R version 3.3.1 (R Core Team 2016), using the lmer and glmer functions in the lme4 package (Bates et al. 2015).
Results
Close calling rate of adults depending on pups’ presence
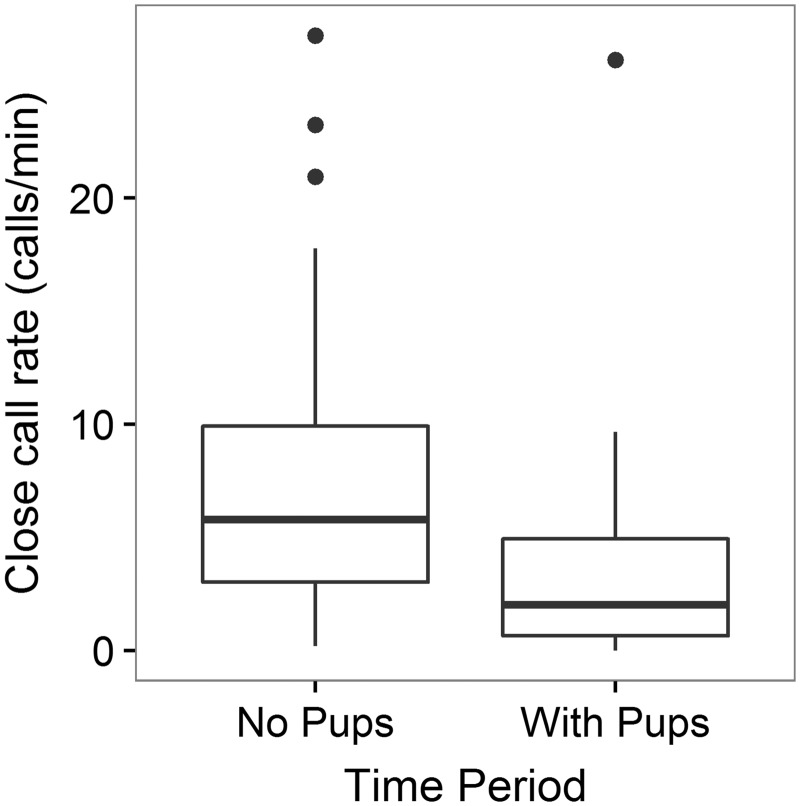
There was a significant decrease in close call rate between the period before pups foraged with the group and the period when pups were foraging with the group (MedianNoPups = 5.80 calls/min, MedianWithPups = 2.04 calls/min; Wilcoxon signed rank test, V48 = 1,129, P < 0.0001, Figure 1).
Figure 1.
Overall close call rate of adults before (no pups) versus when pups were foraging with (with pups) the group.
What factors predict change in overall call rate?
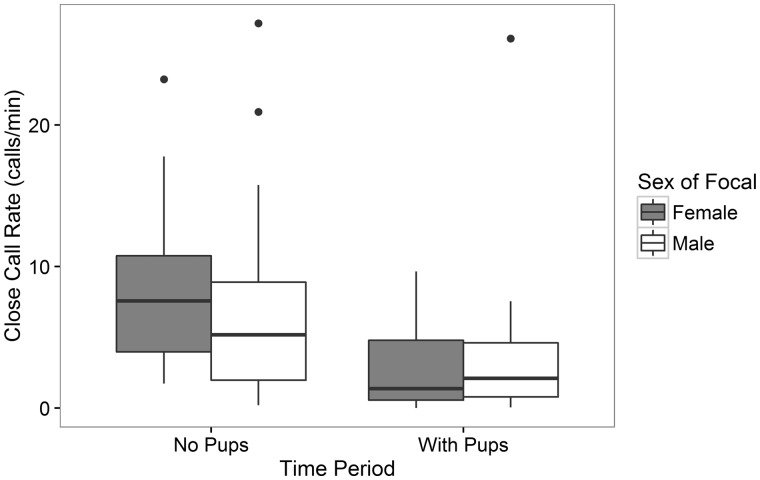
Sex of the caller significantly influenced the percentage change of call rate between the 2 conditions (t39.74 = 2.05, P = 0.047, Table 1). While females exhibited a 65.6% reduction in call rate after pups started foraging with the group, males only reduced their call rate by 45.8%. This reduced model with focal sex as the only remaining fixed effect was a significantly better fit than the null model (χ21 = 4.032, P = 0.045) and was less complex but similar in fit to the next best fit reduced model with focal sex, group alarm rate, and group count as fixed effects (χ21 = 4.066, P = 0.131, see Table 1 for full, reduced, and null models). Although females had a higher median call rate than males in the period before pups were foraging with the group (MedianFemales = 7.58 calls/min, MedianMales = 5.12 calls/min) and a slightly lower median call rate than males after pups started foraging with the group (MedianFemales = 1.38 calls/min, MedianMales = 2.11 calls/min), there was no significant difference between the sexes in overall call rate within either period (no pups: W48 = 352, P = 0.177; with pups: W48 = 257, P = 0.712, Figure 2). Thus, although females showed a greater relative change in their call rate between the 2 conditions, there was no difference between the sexes in overall call rate within each condition. Similarly, there was no significant difference in overall call rate between dominant and subordinate individuals within each condition (no pups: W48 = 198, P = 0.163; with pups: W48 = 280, P = 0.733).
Table 1.
Results of linear mixed effects model selection for factors predicting percentage change of close call rate between the no pups and with pups conditions
| Fixed effects | Value | Standard error | DF | t value | P value | AICc | logLik |
|---|---|---|---|---|---|---|---|
| Null model | 479.673 | −235.360 | |||||
| (Intercept) | −55.043 | 7.527 | – | −7.313 | <0.0001 | ||
| Best fit model | 478.152 | −233.344 | |||||
| (Intercept) | −65.552 | 8.813 | 15.66 | −7.438 | <0.0001 | ||
| Sex M | 19.706 | 9.604 | 39.74 | 2.052 | 0.0468 | ||
| Next best fit model | 479.494 | −231.311 | |||||
| (Intercept) | −96.678 | 32.194 | 6.49 | −3.003 | 0.022 | ||
| Sex M | 19.748 | 9.628 | 41.08 | 2.051 | 0.047 | ||
| Group alarm rate | 32.872 | 15.545 | 7.53 | 2.115 | 0.070 | ||
| Group count | −4.264 | 1.960 | 7.30 | −2.176 | 0.065 | ||
| Full model | 495.577 | −229.273 | |||||
| (Intercept) | −114.981 | 41.606 | 47.00 | −2.764 | 0.008 | ||
| Sex M | 23.571 | 17.521 | 47.00 | 1.345 | 0.185 | ||
| Rank SUB | −4.390 | 14.605 | 47.00 | −0.301 | 0.765 | ||
| Pup carer ratio | 110.154 | 78.285 | 47.00 | 1.407 | 0.166 | ||
| Pup feed ratio | −56.515 | 57.204 | 47.00 | −0.988 | 0.328 | ||
| Vigilance ratio | −21.577 | 50.699 | 47.00 | −0.426 | 0.672 | ||
| Group alarm rate | 30.652 | 17.837 | 47.00 | 1.718 | 0.092 | ||
| Group count | −1.244 | 3.867 | 47.00 | −0.322 | 0.749 | ||
| Pup count | −14.415 | 13.519 | 47.00 | −1.066 | 0.292 | ||
| SexM:RankSUB | 0.884 | 20.812 | 47.00 | 0.042 | 0.966 |
Figure 2.
Call rates of males and females before (no pups) versus when pups were foraging with (with pups) the group.
Factors influencing short-term call rate when pups are nearest neighbors
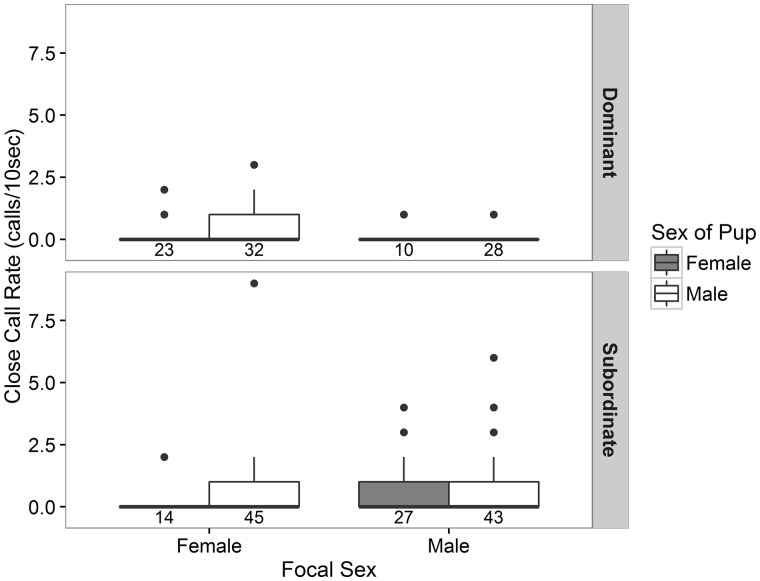
The short-term call rate of adults when pups were in close proximity was not significantly affected by focal sex, focal rank, pup sex, or their interactions within the full model. Additionally, there was no significant difference between the null model and the full model (χ21 = 5.707, P = 0.574) or between the null model and any reduced model. However, when examining call rates of only dominant individuals, the sex of the caller significantly influenced the call rate during periods when a pup was the nearest neighbor at a distance of 1–5 m. Dominant females called at a significantly higher rate near close pups than dominant males (2.205 calls/min vs. 0.727 calls/min; Zdfresid 89 = −2.327, P = 0.020, Figure 3). The sex of the pup did not affect call rate and was removed from the final model. The best fit reduced model, with focal sex as the main effect, tended toward being significantly different from the null model (χ21 = 3.325, P = 0.068) and possessed the lowest AICc score.
Figure 3.
The number of close calls produced by dominant individuals (top) and subordinate individuals (bottom) within the 10 s time window when a pup was the only neighbor between 1 and 5 m. Boxplot color represents the sex of the nearest neighbor (gray = female pup; white = male pup). The numbers under each boxplot represent the number of time windows included in the analysis for each combination (e.g., there were 14 time windows where a female pup was the nearest neighbor to a subordinate female).
Neither focal sex nor sex of the pup predicted call rate by subordinate individuals, although there was a non-significant trend for subordinates to call more in the presence of male pups than female pups (Zdfresid 124 = 1.715, P = 0.086, Figure 3). The best fit reduced model for subordinates included focal sex and pup sex as the main effects. This model tended toward being significantly different from the null model (χ21 = 4.874, P = 0.087) and possessed the lowest AICc score.
Discussion
In meerkats, group members produced fewer close calls when they had pups foraging with them in comparison to periods when no pups were foraging with the group. There was a significant difference in the relative change in close call rate between the 2 periods as a function of the caller’s sex: females reduced calling rate more than males. This may have been because females appeared to call more than males when pups were not foraging with the group, while when pups were foraging with the group, both females and males called at about the same rate (Figure 2). Within each period, however, there was no significant difference in overall call rate between the sexes. The relative change in calling rate between these 2 periods was not significantly affected by other individual-level factors such as social rank, relative pup provisioning, or relative vigilance behavior, or other group-level factors such as group size, litter size, pup to carer ratio, or group alarm rates. However, within the period when pups were foraging in close proximity, dominant females had higher short-term close calling rates than dominant males.
There are several reasons that may explain the reduction in the rate of emitting close calls when pups are foraging with the group. Pups are typically located in the center of the group and are constantly emitting loud begging calls. Thus, the soft close calls are likely to be masked by these begging calls. The function of the close calls to maintain group cohesion becomes at least partly redundant, because group members can direct themselves toward the center of the group using the loud begging calls rather than the highest rate of close calls (Gall and Manser 2017). There was also no effect of group size, litter size, or pup–carer ratio, suggesting, as soon as meerkats had a begging pup with them they reduced the rate of emitting close calls. These results support the hypothesis that the decreased calling rate was because of the redundant function of close calls to maintain group cohesion when begging pups emitting the loud calls were present.
The other explanation, that older group members may selectively use close calls to attract the attention of specific pups where they gain the largest fitness benefit, was partially supported. We predicted that as the philopatric sex, females would produce more close calls in the presence of pups than males and would produce more close calls when female pups were close compared with when male pups were close. The sex of the caller significantly predicted call rate when a close pup was present: dominant females called more in the presence of a close pup than dominant males. These results suggest that dominant females call more than dominant males when pups are near because they gain a greater fitness benefit by doing so, potentially from attracting pups for provisioning. This is supported by the observation that female parents provision pups more than male parents (Brotherton et al. 2001). However, subordinate females and males showed no difference in their calling behaviors in the presence of a close pup, despite the fact that female helpers provision pups more than male helpers (Clutton-Brock et al. 2001) and female helpers provision a similar proportion of food to pups as female parents (Brotherton et al. 2001). Although females provide more food to female pups than male pups (Brotherton et al. 2001), the sex of the nearest close pup did not affect the calling rate of adult group members. Furthermore, when pups were foraging with the group, the overall call rate was similarly low for all different individuals, independent of sex or rank. Since older group members not only have to feed pups, but also forage for themselves, they may try to avoid having pups following them as long as they are not satiated to a certain level themselves. This is supported by the fact that the least successful foragers provided the least amount of prey items to the pups (Clutton-Brock et al. 2001) but not supported by the lack of a correlation between the relative change in close call rate and generosity of carers. Experiments are needed to identify whether close calls attract pups to follow the vocalizing carer.
This adjustment of close call rate in meerkats to the pups’ presence indicates the flexibility and control of senders on the production of this signal type. Furthermore, it strongly suggests that the reduction in close call rate is due to a masking effect of the obvious loud begging calls, which make the close call function of maintaining group cohesion partly redundant. The few close calls emitted when pups were present may still have the function to attract pups, with dominant females potentially attracting more pups than dominant males, although with the current sample size we have no clear evidence that these calls are used to attract specific pups by the different social categories of adult group members. This study suggests that meerkats adjust their close call production to the masking effect due to pup begging calls, and therefore according to the benefit of emitting these calls in the specific situation. It provides an example of the flexible use of one calling system in the presence of another, here contact calls versus begging calls, within the same species.
Ethical Note
All data collection adhered to ASAB guidelines. All research was conducted under the permission of the ethical committee of Pretoria University (Permit number: EC031-13) and the Northern Cape Conservation Service (FAUNA 192/2014), South Africa.
Acknowledgments
We thank the Kalahari Research Trust for permission to work at the Kuruman River Reserve, and the neighboring farmers to use their land. Many thanks also to Tim Clutton-Brock, Dave Gaynor, Tim Vink for the organization of the field site, data base and their input on the field work, and the managers Laura Meldrum and Chris Duncan, the volunteers of the KMP for maintaining the habituation and basic data collection on the meerkats, and Talitha Gray for assisting on audio recordings. Furthermore, we thank the Northern Cape Department of Environment and Nature Conservation for permission to conduct the research. We are grateful to Alexsandra Brendle, Dominik del Castillo, and Sabrina Engesser for their assistance with processing acoustic recordings. We are also thankful to Dan Blumstein and Arik Kershenbaum for their suggestions and edits which helped improve the manuscript.
Funding
This study was funded by the University of Zurich within the employment of M.B.M. This paper has relied on records of individual identities and/or life histories maintained by the Kalahari Meerkat Project (KMP), which has been supported by the European Research Council [Grant No. 294494 to T.H. Clutton-Brock since 1 July 2012], the University of Zurich and the Mammal Research Institute at the University of Pretoria. The long-term field site KMP was financed by the Universities of Cambridge and Zurich.
References
- Bates B, Maechler M, Bolker B, Walker S, 2015. Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48. [Google Scholar]
- Bell MB, 2008. Strategic adjustment of begging effort by banded mongoose pups. Proc R Soc B 275:1313–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brotherton PNM, Clutton-Brock TH, O’riain MJ, Gaynor D, Sharpe L. et al. , 2001. Offspring food allocation by parents and helpers in a cooperative mammal. Behav Ecol 12:590–599. [Google Scholar]
- Cheney DL, Seyfarth RM, Silk JB, 1995. The role of grunts in reconciling opponents and facilitating interactions among adult female baboons. Anim Behav 50:249–257. [Google Scholar]
- Clutton-Brock TH, Gaynor D, Kansky R, MacColl ADC, McIlrath G. et al. , 1998. Costs of cooperative behaviour in suricates Suricata suricatta. Proc R Soc Lond B 265:185–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clutton-Brock TH, Brotherton PNM, O’riain MJ, Griffin AS, Gaynor D. et al. , 2001. Contributions to cooperative rearing in meerkats. Anim Behav 61:705–710. [Google Scholar]
- Clutton-Brock TH, Russell AF, Sharpe LL, Young AJ, Balmforth Z. et al. , 2002. Evolution and development of sex differences in cooperative behavior in meerkats. Science 297:253–256. [DOI] [PubMed] [Google Scholar]
- Clutton-Brock TH, Manser M, 2016. Meerkats: cooperative breeding in the Kalahari In: Koenig WD, Dickinson JL, editors. Cooperative Breeding in Vertebrates: Studies of Ecology, Evolution, and Behavior. Cambridge: Cambridge University Press, 294–317. [Google Scholar]
- Clutton-Brock TH, 2016. Mammal Societies. John Wiley & Sons, p. 404. [Google Scholar]
- Deecke VB, Slater PJ, Ford JK, 2002. Selective habituation shapes acoustic predator recognition in harbour seals. Nature 420:171–173. [DOI] [PubMed] [Google Scholar]
- Derryberry EP, Danner RM, Danner JE, Derryberry GE, Phillips JN. et al. , 2016. Patterns of song across natural and anthropogenic soundscapes suggest that white-crowned sparrows minimize acoustic masking and maximize signal content. PLoS ONE 11:e0154456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engesser S, 2011. Function of ‘close’ calls in group foraging carnivore Suricata suricatta [MSc thesis]. [Zurich (Switzerland)]: University of Zurich.
- English S, Kunc HP, Madden JR, Clutton-Brock TH, 2008. Sex differences in responsiveness to begging in a cooperative mammal. Biol Lett 4:334–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fichtel C, Manser M, 2010. Vocal communication in social groups In: Kappeler PM, editor. Animal Behaviour: Evolution and Mechanisms. Berlin: Springer, 29–54. [Google Scholar]
- Gall G, Manser MB, 2017. Group cohesion in foraging meerkats: follow the moving ‘vocal hot spot’. R Soc Open Sci 4:170004.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gros-Louis J, 2004. The function of food-associated calls in white-faced capuchin monkeys Cebus capucinus from the perspective of the signaller. Anim Behav 67:431–440. [Google Scholar]
- Hollén LI, Manser MB, 2006. Ontogeny of alarm call responses in meerkats Suricata suricatta: the roles of age, sex and nearby conspecifics. Anim Behav 72:1345–1353. [Google Scholar]
- Koda H, Shimooka Y, Sugiura H, 2008. Effects of caller activity and habitat visibility on contact call rate of wild Japanese macaques Macaca fuscata. Am J Primatol 70:1055–1063. [DOI] [PubMed] [Google Scholar]
- Kondo N, Watanabe S, 2009. Contact calls: information and social function. Jpn Psychol Res 51:197–208. [Google Scholar]
- Kunc HP, Madden JR, Manser MB, 2007. Begging signals in a mobile feeding system: the evolution of different call types. Am Nat 170:617–624. [DOI] [PubMed] [Google Scholar]
- Lemasson A, Guilloux M, Barbu S, Lacroix A, Koda H, 2013. Age- and sex-dependent contact call usage in Japanese macaques. Primates 54:283–291. [DOI] [PubMed] [Google Scholar]
- Madden JR, Kunc HJP, English S, Clutton-Brock TH, 2009. Why do meerkat pups stop begging? Anim Behav 78:85–89. [Google Scholar]
- Manser MB, 1998. The evolution of auditory communication in suricates Suricata suricatta [PhD thesis]. [ Cambridge (UK)]: University of Cambridge. [Google Scholar]
- Manser MB, Avey G, 2000. The effect of pup vocalisations on food allocation in a cooperative mammal, the meerkat Suricata suricatta. Behav Ecol Sociobiol 48:429–437. [Google Scholar]
- Manser MB, Madden JR, Kunc HP, English S, Clutton-Brock TH, 2008. Signals of need in a cooperatively breeding mammal with mobile offspring. Anim Behav 76:1805–1813. [Google Scholar]
- Mausbach J, Braga Goncalves I, Heistermann M, Ganswindt A, Manser MB, 2017. Meerkat close calling patterns are linked to sex, social category, season and wind, but not fecal glucocorticoid metabolite concentrations. PLoS ONE 12:e0175371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reber SA, Townsend SW, Manser MB, 2013. Social monitoring via close calls in meerkats. R Soc Proc B 280:20131013. https://www.R-project.org/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team, 2016. R: A Language and Environment for Statistical Computing Vienna: R Foundation for Statistical Computing. http://dx.doi.org/10.1098/rspb.2013.1013.
- Rendall D, Seyfarth RM, Cheney DL, Owren MJ, 1999. The meaning and function of grunt variants in baboons. Anim Behav 57:583–592. [DOI] [PubMed] [Google Scholar]
- Russell AF, Clutton-Brock TH, Brotherton PNM, Sharpe LL, McIlrath Gm, Dalerum FD, Cameron EZ, Barnard JA, 2002. Factors affecting pup growth and survival in co-operatively breeding meerkats Suricata suricatta. J Anim Ecol 71:700–709. [Google Scholar]
- Silk JB, Seyfarth RM, Cheney DL, 2016. Strategic use of affiliative vocalizations by wild female Baboons. PLoS ONE 11:e0163978.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend SW, Allen C, Manser MB, 2011. A simple test of vocal individual recognition in wild meerkats. Biol Lett 8:179–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AM, Raihani NJ, Hockey PAR, Britton A, Finch FM. et al. , 2013. The influence of fledgling location on adult provisioning: a test of the blackmail hypothesis. R Soc Proc B 280:20130558.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf C, 2014. Acoustic cues to hierarchical social structures in subordinate female meerkats [M.Sc. thesis]. [Greifswald (DE)]: Ernst Moritz Arndt University of Greifswald.