Abstract
An intriguing question in behavioral biology is whether consistent individual differences (called animal personalities) relate to variation in cognitive performance because commonly measured personality traits may be associated with risk-reward trade-offs. Social insects, whose learning abilities have been extensively characterized, show consistent behavioral variability, both at colony and at individual level. We investigated the possible link between personality traits and learning performance in the carpenter ant Camponotus aethiops. Exploratory activity, sociability, and aggression were assessed twice in ant foragers. Behaviors differed among individuals, they were partly repeatable across time and exploratory activity correlated positively with aggression. Learning abilities were quantified by differential conditioning of the maxilla-labium extension response, a task that requires cue perception and information storage. We found that exploratory activity of individual ants significantly predicted learning performance: “active-explorers” were slower in learning the task than “inactive-explorers”. The results suggest for the first time a link between a personality trait and cognitive performance in eusocial insects, and that the underlying individual variability could affect colony performance and success.
Keywords: ants, associative learning, cognition, exploratory activity, personality
Both humans and non-human animals show inter-individual variation in levels of behavioral expression, that is, frequency, duration, or intensity of a given behavior. When these differences are consistent and predictable over time and/or across different situations, they are defined as “personality differences” (Réale and Dingemanse 2012). The term “behavioral syndrome” is often considered as equivalent but it refers more strictly to suites of correlated traits across contexts (Sih et al. 2004). Cognition is by definition the acquisition, processing, storage, and use of information from the social and physical environment (Shettleworth 2010). Since personality differences are primarily based on how individuals explore, gain information, interact, and respond to environmental challenges (both social and non-social), there might be a functional link between personality and cognition. Importantly, both personality differences and variation in cognitive abilities have ecological and evolutionary consequences (Dukas 2004; Carere and Maestripieri 2013).
The interaction between personality and cognition has been poorly explored and only recently a research agenda has been put forward (Griffin et al. 2015). Ivan Pavlov first proposed that personality types were markers for different strategies in associative learning (see Carere and Locurto 2011), and more recently it has been proposed that behavioral types (e.g. bold/fast explorers versus shy/slow explorers) are associated with cognitive speed-accuracy trade-offs (Sih and Del Giudice 2012). In particular, concerning learning and memory, Sih and Del Giudice (2012) predict that fast-exploring individuals would be more successful than slow-exploring individuals at learning new activity-based tasks (e.g. operant conditioning), but would be less performant in information collection and storage and slower in reversal learning because they follow a high-speed/low-accuracy cognitive strategy. These predictions are supported by a growing number of empirical studies, which have been carried out in vertebrates. For instance, in guppies, Poecilia reticulata, bolder individuals are faster learners in conditioning tasks (Trompf and Brown 2014). In chickadees, Poecile atricapillus, fast-explorers are faster learners than slow-explorers in conditioning tasks (Guillette et al. 2009), but in reversal learning, slow-explorers are quicker than fast-explorers (Guillette et al. 2011). Also in cavies, Cavia aperea, bold, active, and aggressive individuals are faster in associative learning (operant conditioning), but the less aggressive individuals are faster in reversal learning (Guenther et al. 2014).
The interplay between personality and cognition has not been investigated in invertebrates, although these show personality differences (Carere and Maestripieri 2013). We believe that social insects are promising models for cognition and personality studies based on the following reasons. Social insect colonies contain groups of individuals (castes) performing different tasks, such as nursing, foraging, and nest maintenance (Hölldobler and Wilson 1990) and there is evidence for personality at the colony, caste, and individual level (Chapman et al. 2011; Wray et al. 2011; Jandt et al. 2013; Kühbandner et al. 2014; Blight et al. 2016), raising questions about the adaptive value of a mix of personality types within colonies (Pinter-Wollman 2012; Jeanson and Weidenmüller 2014). Social insects have also evolved remarkable cognitive abilities, including non-elemental learning, and established protocols are available to study learning and memory in social insects such as bees and ants (d’Ettorre 2013; Giurfa 2013). Some studies addressed learning speed and foraging success, for instance, in bumblebees there is colony variation in learning speed and fast-learners were shown to be more successful foragers than slow-learners (Raine and Chittka 2008).
Here, we used the carpenter ant Camponotus aethiops as study organism because these ants show individual associative learning abilities (Guerrieri and d’Ettorre 2010; Bos et al. 2012) and inter-individual variation in learning performance (Perez et al. 2013). We tested whether personality traits, assessed repeatedly with three different behavioral tests, predict learning performance. Exploratory activity, sociability, and aggression were measured and tested for individual consistency. We define as “active-explorers” those ants that spent more time moving during the exploratory activity test (open field) relative to “inactive-explorers” (for details see Materials and Methods and Supplementary Material).
To evaluate learning performance we choose an associative learning task (classical olfactory conditioning of the maxilla-labium extension response, Guerrieri and d’Ettorre 2010) performed with harnessed ants, which allows controlling factors that could influence learning in experiments with free-walking ants, such as the number and the duration of conditioned and unconditioned stimuli presentations and the inter-trial interval. We focused on a single caste, the foragers, to reduce variation due to age (foragers are the oldest workers) and possible variation that could be linked to different sensitivities toward stimuli, which may vary between castes. For instance, ant foragers show higher responsiveness to sucrose than intra-nidal workers and sucrose responsiveness is positively correlated with learning performance (Perez et al. 2013; see e.g. Scheiner et al. 2001 for honeybees). Foragers work outside the nest in a fluctuating environment where learning abilities are relevant. Ants and other social insects need to find and remember rewarding food sources, such as extra-floral nectaries. Foragers that are able to associate a particular plant odor with the reward (high-quality nectar) will increase foraging efficiency, with a positive impact at the colony level. Indeed, in bumblebees, efficiency in associative learning under laboratory conditions is positively correlated with foraging performance under field conditions (Raine and Chittka 2008).
We predicted (i) to find consistent individual differences within the same behavioral test over time and association between different behaviors (in particular exploratory activity and aggression), and that (ii) active-explorers are slower learners than inactive-explorers in a differential olfactory conditioning task, which requires accurate perception of cues and information storage (as predicted by Sih and Del Giudice 2012).
Materials And Methods
Animals and housing
Three queenright colonies (C1, C2, and C3) of C. aethiops, collected in 2013 near Toulouse (Midi-Pyrénées, France, latitude 43.5°, longitude 1.516667°), were each housed in two Fluon®-coated plastic boxes connected by a hose. Colonies were kept under laboratory conditions (22 ± 2 °C, L12/D12, 40% humidity). One box, the nest, had a plaster floor and was darkened by cardboard; the other, the foraging area, was exposed to light. Colonies were fed twice a week with Bhatkar diet (Bhatkar and Whitcomb 1970) and water was provided ad libitum. Two weeks before the learning task, the colonies were deprived of sucrose and crickets, Acheta domestica, were provided instead in order to increase their motivation for sucrose reward.
Personality tests
Personality traits were evaluated by testing three of the five behavioral categories most commonly used (Réale et al. 2007): exploratory activity, sociability, and aggression, repeated 3 weeks later to assess individual consistency over time.
Ants were individually marked with dots of paint (uniPAINT©) on their thorax 3 days before the experiments started. Personality traits were evaluated with three consecutive tests carried out on each ant during 1 week (one test per day with one day break between tests to minimize possible carry-over effects). After each test, the ants were immediately returned to their respective colony. Three weeks later, the tests were repeated to assess individual consistency over time. The order in which the different ants performed the tests was randomized (for each colony, test, and session).
Exploratory activity was evaluated in a circular open-field arena (Ø 11.5 cm) away from the nest (Supplementary Figure S1a). An ant was introduced into an acclimatization tube (2 cm diameter) for 120 s. Then, the tube was removed and the time of mobility was measured for 300 s.
Sociability was quantified in a circular apparatus (Ø 5 cm) placed in the foraging area of the nest, familiar to the ants (Supplementary Figure S1b). A single nestmate forager “target” ant per colony was randomly selected to measure the sociability of the tested ants. The target ant was introduced into the apparatus 600 s before the beginning of the test to allow familiarization with the apparatus. Then, each tested ant was introduced in the acclimatization tube individually, as above. After removing the tube, the duration of the following social interactions (from tested ant to target ant) was measured for 300 s: antennal contact, trophallaxis (exchange of liquid food), and proximity (maximum distance of 1 cm between the two ants); the sociability score was calculated as the sum of the duration of these interactions.
Aggression was evaluated in a circular apparatus (Ø 5 cm, Supplementary Figure S1c). A target ant from a different colony than the tested ant (non-nestmate) was used as “intruder” (a different target ant for each tested ant). These target ants were killed by freezing at −20 °C on the same day of the test. The use of a dead target ant allows removing its active influence on the tested ant without stopping the tested ant to show agonistic behaviors (Stroeymeyt et al. 2010). The target ant was placed near the wall of the apparatus and the tested ant into the acclimatization tube. The two ants were separated of about 1 cm, allowing immediate perception of the target ant by the tested ant (Brandstaetter et al. 2008). After removing the tube, the duration of mandible opening, bite, and gaster-flexing was measured for 180 s. An aggression index was calculated as duration of: (mandible opening * 1) + (bite * 2) + (gaster-flexing * 3); the score 1, 2, 3 was given according to the increasing aggression level of these behaviors (similarly to Errard and Hefetz 1997).
During all tests, data were directly recorded by a trained single operator (E.U.) with Etholog© (Ottoni 2000).
Learning task
The learning task was differential olfactory conditioning of the maxilla-labium extension response (MaLER) Guerrieri and d'Ettorre et al. 2010), performed 2 weeks after the last personality test. Ants were harnessed individually (Supplementary Material and Supplementary Figure S2) and were subjected to differential conditioning, in which ants had to learn to respond differentially to two conditioned stimuli (CS+ and CS−). The two CS were octanal and hexanol (Sigma Aldrich, France), floral scents that are pertinent for C. aethiops ants, which feed in part on extra-floral nectaries. These odors are generally well discriminated by this ant species (Bos et al. 2012; Perez et al. 2015), and are therefore useful to study learning abilities by overcoming low learning performances that could be due to perceptual similarity. The CS+ odor was rewarded with the unconditioned stimulus (US), a sucrose solution (50% weight/weight), while the CS− odor was not rewarded. To have a balanced design, half of the ants received octanal as CS+ and hexanol as CS− and the opposite for the other half.
The acquisition phase consisted of 10 pseudo-randomized trials (5CS+ and 5CS− presentations) per each ant (see Supplementary Material for trial description). An acquisition score (AS) representing learning performance for each CS was calculated. For each trial, when MaLER was visible upon the presentation of the CS, a response score (RS) of 1 was attributed; if MaLER was not visible the RS was 0. An AS for the CS+ and the CS− was calculated (AS+ and AS−, respectively; Perez et al. 2013). The AS+ corresponds to the sum of the RSs to the CS+ which have been weighted in a decreasing order; while the AS− corresponds to the sum of the RSs to the CS− which have been weighted in an increasing order:
The AS+ is positive and varies between 0 (no MaLER) and 10 (MaLER from trial 2 to 5), while the AS− varies between −10 (MaLER from trial 2 to 5) and 0 (no MALER). The AS− is negative because it indicates a better performance when it approaches zero.
The weighting of both ASs allows emphasizing differences in learning performance. For example, a response at the second trial for the CS+ indicates a better performance than a response at the fourth trial because of the lower number of paired CS–US associations experienced at the second trial. For the CS−, ants are not expected to respond. Thus, a response to the CS− at the last trial underlines a lower learning performance than a response at the second trial. The response to each CS in the very first trial was not included in the formula as both CS were initially neutral for ants; therefore, no conditioned response could be recorded.
In our experiments, the AS− remained low and did not vary enough among individuals to be used as indicator of individual learning performance (34 out of 45 individuals showed an AS− equal to 0, which means no response to the CS−). Indeed, ants discriminated well the two CS and learned the differential conditioning task. Thus, the AS− was not included in further analysis and only the AS+ (mean ± SD = 6.489 ± 3.195) was used to assess the possible associations between learning performances and behavioral tests.
Statistical analyses
Statistics were performed with R (version 3.0.1). Initial sample size was n = 90 (30 ants/colony). Forty-one individuals died during the experimental period. Sixty-eight individuals could undergo the personality tests of which 45 underwent both personality and learning tests. This mortality rate falls within the natural range, since foragers are the oldest workers (a similar mortality rate was found in other studies, e.g. Kühbandner et al. 2014); moreover, four individuals did not respond enough to sucrose (details in Supplementary Material). Thus, the individuals undergoing both personality and learning tests were 45 (colony1 = 16; colony2 = 16; colony3 = 13). Colony identity was included as random factor when running a generalized linear mixed model (GLMM) (see below) and the variance explained by the term “colony” was different from zero in all our models.
Personality tests. Intra-class correlations were calculated to assess repeatability and their 95% confidence intervals (CIs) with LMM-based calculations by R package rptR with 1000 bootstrap steps with individual as a random factor (Nagakawa and Schielzeth 2010). P values were calculated by 1000 permutations. Pearson correlation coefficient was used to test the associations between the mean scores of the three behavioral variables (log transformed).
Learning tasks. Learning performance (acquisition curves) was analyzed with a GLMM with a binomial error structure and a logit link function (R package lme4, Bates et al. 2013). RSs (0 or 1 for each trial) were used as response variable. The stimulus (CS+ or CS−) and the nature of CS+ (octanal or hexanol) were included as fixed factors. “Trials” were included as covariate and “colony” (C1, C2, C3) and individual identity as random factors to account for within-colony similarities and repeated measures (at individual level). Interactions between fixed factors and covariate were included in the model to detect differences in the response slope along trials for each stimulus according to the nature of CS+. Non-significant interactions were removed from the model, which was then recalculated. A post-hoc analysis was performed (by applying the same GLMM without the factor stimulus to the, respectively, reduced set of data) to detect possible changes in the ant responses along successive trials: we expect that the level of response to the CS+ increases with successive trials while the level of response to CS− does not change and remain low.
Personality and learning. We used the three original variables (scaled) and applied a generalized linear mixed-effects model (Poisson distribution, log-link function) to test the predictive effect of exploratory activity, sociability, and aggression on learning performance (AS+, response variable), including colony identity as random factor (R package lme4, Bates et al. 2013). Since there were indications for overdispersion, we included case-level random effects. Variances were homogeneous as verified visually by plotting residuals versus fitted values. P values were calculated by Wald type III test. Further, we calculated variance inflation coefficients to check for collinearities among predictor variables. The coefficients were always lower than 1.5 indicating no interference of collinearities.
Results
Personality tests
The variables showed a low-to-moderate repeatability over time: exploratory activity, R = 0.11, CI = (0, 0.37), P = 0.22; sociability, R = 0.19, CI = (0, 0.45), P = 0.10; aggression, R = 0.39, CI = (0.11, 0.62), P = 0.006. Exploratory activity and aggression were moderately and positively correlated (r = 0.30, P = 0.04), whereas sociability did not correlate with exploratory activity or aggression (r = 0.19, P = 0.44; r = 0.03, P = 0.84).
Learning task
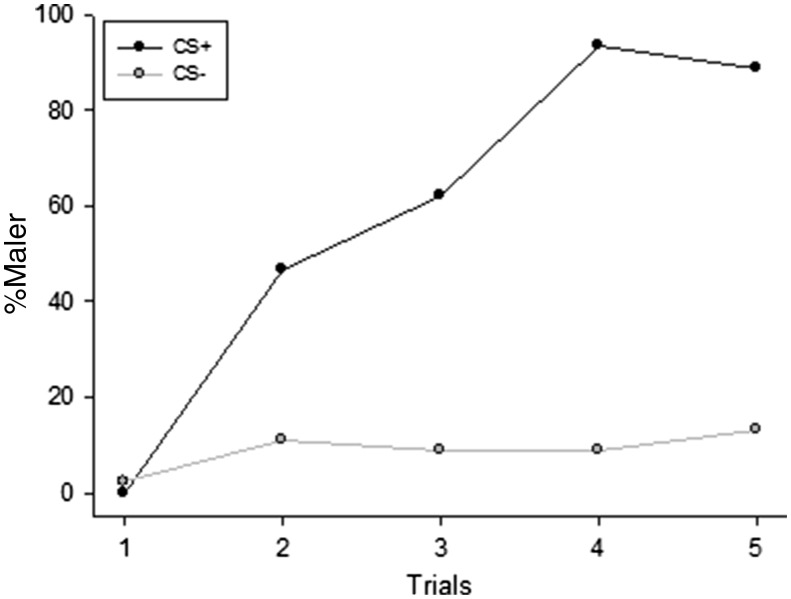
The acquisition curves for the CS+ and CS− were similar for the two odors used as CS+ (GLMM: χ2 = 2.68; df = 1; P = 0.10). Therefore, data were analyzed together (Figure 1). Ants discriminated the CS+ and the CS− along the successive trials (with a higher level of response for the CS+), as revealed by a significant stimulus * trials interaction (GLMM: χ2 = 26.01; df = 1; P < 0.001). The post-hoc analysis revealed that ants learned to respond to the CS+ along successive trials (GLMM: χ2 = 69.03; df = 1; P < 0.001), while the level of response to the CS− did not change significantly (GLMM: χ2 = 2.70; df = 1; P = 0.099). Overall, ants exhibited high learning performance at the last trial of conditioning with 89% of responses to CS+ and 13% of responses to the CS−. In differential conditioning, high percentage of responses to the CS+ paired with low percentage of responses to the CS− is clear indication of learning.
Figure 1.
Learning curves of conditioned stimuli (CS+, dark line and CS−, gray line) along trials, expressed in percentage of maxilla-labium extension responses (%MaLER). The two stimuli were discriminated along trials (GLMM: χ2 = 26.01; df = 1; P < 0.001). MaLER significantly increased during the CS+ trials (GLMM: χ2 = 69.03; df = 1; P < 0.001) but remained low during CS− trials (GLMM: χ2 = 2.71; df = 1; P = 0.10).
Personality and learning
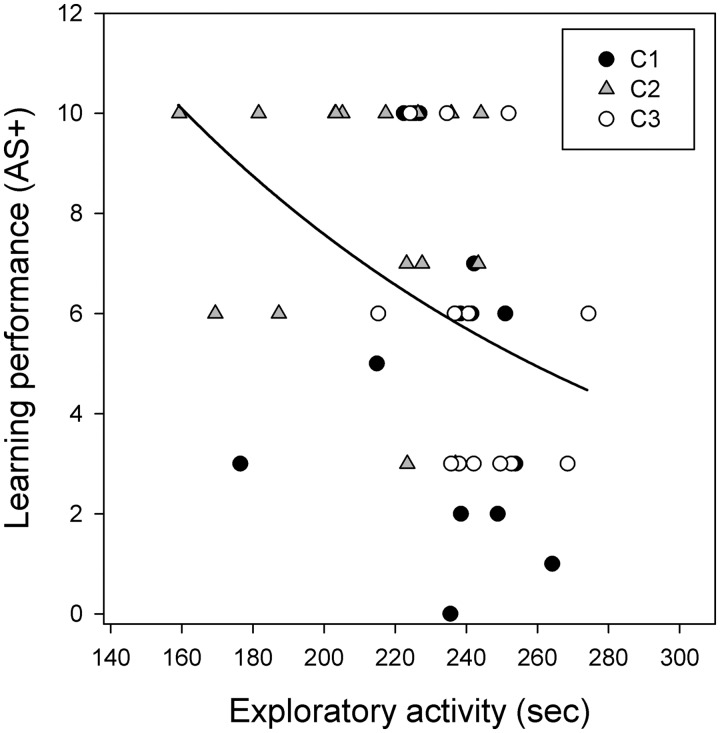
The generalized linear mixed-effects model revealed that exploratory activity significantly predicted learning performance (Figure 2): active-explorers were slow-learners (χ2 = 5.64, P = 0.017), meaning that these ants generally needed more conditioning trials than inactive-explorers to learn to respond to the rewarded odor (CS+); while neither sociability (χ2 = 0.22, P = 0.638) nor aggression (χ2 = 0.07, P = 0.784) was associated with learning (Supplementary Figure S3).
Figure 2.
Relationship between exploratory activity and learning performance (acquisition score, AS+; mean ± SD = 6.489 ± 3.195); colonies (C1, C2, C3) are depicted by different symbols. Exploratory activity is expressed as the average of the two repeats. The solid line represents the regression line.
Discussion
We found individual differences in ant behavior across different tests, with significant repeatability in one out of three behaviors (aggression) and an indication for an “exploration-aggression” syndrome, also described in other species (e.g. rabbits, Rödel et al. 2015). All our repeatability values fall within the most commonly found in animal behavior and they are similar to those observed in vertebrates (Bell et al. 2009). The differential olfactory conditioning experiment confirmed that carpenter ants can learn this task and that there is individual variability in learning performance (as already shown by Perez et al. 2013). However, only exploratory activity, the behavior showing the lowest repeatability in the present dataset, predicted learning performance: according to the expectations, the more active ants in the exploratory activity test (ants that spent more time in mobility in the open field, i.e. active-explorers) were slower learners in the classical conditioning task, which requires accurate cue perception and information storage.
Personality types have been recently described in social insects (Pinter-Wollman 2012; Jandt et al. 2013), but our data provide evidence of within-individual consistency in aggression over 3 weeks, a significant proportion of the lifespan for ant foragers (Hölldobler and Wilson 1990). In eusocial insects, repeatability has rarely been shown at the individual level (Kühbandner et al. 2014) and evidence of behavioral syndromes is still scarce. For instance, in Myrmica ants, boldness and aggression were correlated only in the patroller caste (Chapman et al. 2011), while in Temnothorax ants, aggression and exploration were correlated at the colony level (Modlmeier et al. 2012) and in the ant Aphaenogaster senilis a proactive-reactive behavioral syndrome was shown at the colony level (Blight et al. 2016). Inter-species differences could arise from the broad ecological diversification of ants (>14,000 described species) resulting in different life histories. In social insects, recent evidence of personality was mainly related to “colony” personality (Chapman et al. 2011; Wray et al. 2011; Blight et al. 2016), and the link between individual and collective phenotype remains to be elucidated (Pinter-Wollman 2012). For instance, some keystone individuals might exert disproportionate effects within a colony and play an important role in collective behaviors (Modlmeier et al. 2014a, 2014b).
Individual ants learned the valence of the conditioned stimuli, one rewarded (CS+) and one unrewarded (CS−), confirming their ability to solve this learning task (Guerrieri and d’Ettorre 2010; Perez et al. 2013), but ants that were less active in exploratory activity test (ants that spent less time in mobility during the open field, i.e. inactive-explorers) were also faster learners. Presumably, inactive-explorers/fast-learners explore more slowly than active-explorers/slow-learners, thus being eventually more successful in perception of cues, sampling, and information storage as they adopt a cognitive style that emphasizes accuracy over speed. A remarkable study on bumblebees foraging strategies (Burns and Dyer 2008), looking at speed-accuracy approach in a flower discrimination task, provides functional support to this hypothesis: so-called “slow-accurate” bees collected nectar more efficiently than “fast-inaccurate” bees. Such individual strategies within the colony would favor a mixed strategy over a single strategy in situations of heterogeneous food distribution. More generally, habitat variations across space or time may favor different personality types so that, for instance, individuals with different exploratory activity levels will have a fitness advantage in different local habitats, according to the habitat dependent selection hypothesis (see e.g. Guillette et al. 2011).
When learning involves a new activity-based task, such as operant conditioning, individuals with high exploratory activity (e.g. faster and/or highly mobile) are predicted to perform better, although individuals with lower exploratory activity are predicted to perform better in reversal learning, which involves information updating, for example, learning that environmental cues have changed in meaning (Sih and Del Giudice 2012). In vertebrates there is mounting evidence that personality affects learning, but the studies so far have used operant conditioning tasks, in which individuals must proactively perform an action to learn the relationship with its outcome. In fact, fast-explorers were better learners in an acoustic operant discrimination task, but slow-explorers had the best performance in reversing previously learned rules with the same task (Guillette et al. 2011). Such results were confirmed by a test of speed-accuracy trade-offs on the same species (Guillette et al. 2015) and also in cavies (Guenther et al. 2014), again with similar activity-based protocols. However, previous studies in great tits, although not testing learning, suggested that slow-individuals, which took longer to approach a novel object and to visit artificial trees, were more thorough explorer than fast-individuals, which had overall higher exploration speed but were more superficial explorers (Verbeek et al. 1994). This is in accordance with our results in ants showing that in a classical differential conditioning task, which involves cue perception and information storage, inactive-explorers outperformed active-explorers. In the natural environment, inactive-explorers would spend more time in sampling the local environment and would be more efficient in, for example, forming associations between a given plant odor and the nectar reward than active-explorers, which in turn would be more efficient in, for instance, discovering novel food sources. This leads to the relevance of testing the same individuals with different kind of learning tasks, as recommended by Griffin et al. (2015). In bumblebees that were allowed to fly for foraging, there is evidence that some individuals perform consistently better than others in a conditioning task across modalities (e.g. visual and olfactory, Muller and Chittka 2012), but the social insect literature on this topic is still very scarce. It would be interesting to test the ants in an operant conditioning task, based on activity, where we would expect active-explorers outperforming inactive-explorers in learning.
Our conclusion concerning personality and cognition must be considered preliminary and indicative, since exploratory activity, the putative personality trait significantly associated with learning, was not significantly repeatable for the 45 individuals involved in these experiments, thus not fulfilling an important criterion for personality. We have not a cogent explanation for this low repeatability, but it might be simply due to sample size. In fact, in another experiment on the same species and caste using a higher sample size (N = 125, five colonies), exploratory activity measured with the same protocol showed a high and significant repeatability over time (R = 0.38, P = 0.001, d’Ettorre et al. 2016). Another relevant point for future studies is that repeatability of learning and individual consistency across different learning tasks should be assessed as well (Griffin et al. 2015), which would allow demonstrating that a given personality type is indeed associated with a given “cognitive style” encompassing multiple cognitive abilities as hypothesized by Pavlov (see Introduction).
The study of the interactions between animal personality and cognition is still in its infancy and presents several challenges (Carere and Locurto 2011; Sih and Del Giudice 2012; Griffin et al. 2015). Our data highlight a previously unreported link between a commonly assessed personality trait and associative learning performance in an insect. Future work should expand the type of cognitive tasks, test speed accuracy trade-offs, and investigate the actual relevance of this association on the interplay between individual and collective personality. We predict that individuals that consistently perform better in certain cognitive tasks (e.g. activity based) may be keystone individuals (Modlmeier et al. 2014b) playing a significant role at the group level when, for example, a change in the local environment asks for a change in foraging strategy.
Supplementary Material
Acknowledgments
Supported by European Commission (FP7-MC-ERG-2009-256524 and H2020-MSCA-IF-2014-659106). We thank Heiko Rödel and M. Cristina Lorenzi for statistical advice and three anonymous referees for insightful suggestions.
Supplementary Material
Supplementary material can be found at http://www.cz.oxfordjournals.org/
References
- Bates D, Maechler M, Bolker B, 2013. Lme4: Linear Mixed Effects Models Using S4 Classes R package version 0.999999-2. Available from: http://CRAN.R-project.org/package = lme4.
- Bell AM, Hankison SJ, Lawskowski KL, 2009. The repeatability of behavior: a meta-analysis. Anim Behav 77:771–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatkar A, Whitcomb WH, 1970. Artificial diet for rearing various species of ants. Flo Entomol 53:229–232. [Google Scholar]
- Blight O, Díaz-Mariblanca GA, Cerdá X, Boulay R, 2016. A proactive-reactive syndrome affects group success in an ant species. Behav Ecol 27:118–125. [Google Scholar]
- Bos N, Dreier S, Jørgensen CG, Nielsen J, Guerrieri FJ. et al. , 2012. Learning and perceptual similarity among cuticular hydrocarbons in ants. J Insect Physiol 58:138–146. [DOI] [PubMed] [Google Scholar]
- Brandstaetter AS, Endler A, Kleineidam CJ, 2008. Nestmate recognition in ants is possible without tactile interaction. Naturwissenschaften 95:601–608. [DOI] [PubMed] [Google Scholar]
- Burns JG, Dyer AG, 2008. Diversity of speed-accuracy strategies benefits social insects. Curr Biol 18: R953–R954. [DOI] [PubMed] [Google Scholar]
- Carere C, Locurto C, 2011. Interaction between animal personality and animal cognition. Curr Zool 57:491–498. [Google Scholar]
- Carere C, Maestripieri D, 2013. Animal Personalities: Behavior, Physiology, and Evolution. Chicago University Press. [Google Scholar]
- Chapman BB, Thain H, Coughlin J, Hughes WHO, 2011. Behavioural syndromes at multiple scales in Myrmica ants. Anim Behav 82:391–397. [Google Scholar]
- d’Ettorre P, 2013. Learning and recognition of identity in ants In: Menzel R, Benjamin PR, editors. Invertebrate Learning and Memory. Elsevier, 501–513. [Google Scholar]
- d’Ettorre P, Carere C, Demora L, Le Quinquis P, Signorotti L. et al. , 2016. Individual differences in exploratory activity relate to cognitive judgement bias in carpenter ants. Behav Proc S0376-6357:30263–30267. doi: 10.1016/j.beproc.2016.09.008. [DOI] [PubMed] [Google Scholar]
- Dukas R, 2004. Evolutionary biology of animal cognition. Annu Rev Ecol Syst 35:347–374. [Google Scholar]
- Errard C, Hefetz A, 1997. Label familiarity and discriminatory ability of ants reared in mixed groups. Insectes Soc 44:189–198. [Google Scholar]
- Giurfa M, 2013. Cognition with few neurons: higher-order learning in insects. Trends Neurosci 36:285–294. [DOI] [PubMed] [Google Scholar]
- Griffin A, Healy SD, Guillette LM, 2015. Cognition and personality: an analysis of an emerging field. Trends Ecol Evol 30:207–214. [DOI] [PubMed] [Google Scholar]
- Guenther A, Brust V, Dersen M, Trillmich F, 2014. Learning and personality types are related in cavies Cavia aperea. J Comp Psychol 128:74–81. [DOI] [PubMed] [Google Scholar]
- Guillette LM, Hahn AH, Hoeschele M, Przyslupski A-M, Sturdy CB, 2015. Individual differences in learning speed, performance accuracy and exploratory behaviour in black-capped chickadees. Anim Cogn 18:165–178. [DOI] [PubMed] [Google Scholar]
- Guillette LM, Reddon AR, Hurd PL, Sturdy CB, 2009. Exploration of a novel space is associated with individual differences in learning speed in black-capped chickadees Poecile atricapillus. Behav Proc 82:265–270. [DOI] [PubMed] [Google Scholar]
- Guillette LM, Reddon AR, Hoeschele M, Sturdy CB, 2011. Sometimes slower is better: slow-exploring birds are more sensitive to changes in a vocal discrimination task. Proc R Soc Lond B 278:767–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrieri FJ, e’Ettorre P, 2010. Associative learning in ants: conditioning of the maxilla-labium extension response in Camponotus aethiops. J Insect Physiol 56:88–92. [DOI] [PubMed] [Google Scholar]
- Hölldobler B, Wilson EO, 1990. The Ants. Cambridge: Harvard University Press. [Google Scholar]
- Jandt JM, Bengston S, Pinter-Wollman N, Pruitt JN, Raine NE. et al. , 2013. Behavioural syndromes in social insects: personality at multiple levels. Biol Rev 89:48–67. [DOI] [PubMed] [Google Scholar]
- Jeanson R, Weidenmüller A, 2014. Interindividual variability in social insects: proximate causes and ultimate consequences. Biol Rev 89:671–687. [DOI] [PubMed] [Google Scholar]
- Kühbandner S, Modlmeier A, Foitzik S, 2014. Age and ovarian development are related to worker personality and task allocation in the ant Leptothorax acervorum. Curr Zool 60:392–400. [Google Scholar]
- Modlmeier AP, Liebmann JE, Foitzik S, 2012. Diverse societies are more productive: a lesson from ants. Proc R Soc Lond B 279:2142–2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modlmeier AP, Keiser CN, Shearer TA, Pruitt JN, 2014a. Species-specific influence of group composition on collective behaviors in ants. Behav Ecol Sociobiol 68:1929–1937. [Google Scholar]
- Modlmeier AP, Keiser CN, Watters JV, Sih A, Pruitt JN, 2014b. The keystone individual concept: an ecological and evolutionary overview. Anim Behav 89:53–62. [Google Scholar]
- Muller H, Chittka L, 2012. Consistent interindividual differences in discrimination performance by bumblebees in colour, shape and odour learning tasks (Hymenoptera: Apidae: Bombus terrestris). Entomol Gen 34:1–8. [Google Scholar]
- Nagakawa K, Schielzeth H, 2010. Repeatability for Gaussian and non-Gaussian data: a practical guide for biologists. Biol Rev Camb Philos Soc 85:935–956. [DOI] [PubMed] [Google Scholar]
- Ottoni EB, 2000. EthoLog 2.2: a tool for the transcription and timing of behavior observation sessions. Behav Res Meth Instrum Comput 32:446–449. [DOI] [PubMed] [Google Scholar]
- Perez M, Giurfa M, d’Ettorre P, 2015. The scent of mixtures: rules of odour processing in ants. Scientific Reports 5:8659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez M, Rolland U, Giurfa M, d’Ettorre P, 2013. Sucrose responsiveness, learning success, and task specialization in ants. Learn Mem 20:417–420. [DOI] [PubMed] [Google Scholar]
- Pinter-Wollman N, 2012. Personality in social insects: how does worker personality determine colony personality? Curr Zool 58:579–587. [Google Scholar]
- Raine NE, Chittka L, 2008. The correlation of learning speed and natural foraging success in bumble-bees. Proc R Soc Lond B 275:803–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Réale D, Reader SM, Sol D, McDougall PD, Dingemanse NJ, 2007. Integrating animal temperament within ecology and evolution. Biol Rev 82:291–318. [DOI] [PubMed] [Google Scholar]
- Réale D, Dingemanse NJ, 2012. Animal Personality In: eLS. Chichester: John Wiley & Sons, LTD. [Google Scholar]
- Rödel HG, Zapka M, Talke S, Kornatz T, Bruchner B. et al. , 2015. Survival costs of fast exploration during juvenile life in a small mammal. Behav Ecol Sociobiol 69:205–217. [Google Scholar]
- Scheiner R, Page RE, Erber J, 2001. Responsiveness to sucrose affects tactile and olfactory learning in preforaging honey bees of two genetic strains. Behav Brain Res 120:67–73. [DOI] [PubMed] [Google Scholar]
- Shettleworth SJ, 2010. Cognition, Evolution, and Behavior. 2ndedn.Oxford University Press. [Google Scholar]
- Sih A, Bell A, Johnson JC, 2004. Behavioral syndromes: an ecological and evolutionary overview. Trends Ecol Evol 19:372–378. [DOI] [PubMed] [Google Scholar]
- Sih A, Del Giudice M, 2012. Linking behavioural syndromes and cognition: a behavioural ecology perspective. Phil Trans R Soc 367:2762–2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroeymeyt N, Guerrieri FJ, van Zweden JS, D’ettorre P, 2010. Rapid decision-making with side-specific perceptual discrimination in ants. PLoS ONE 5:e12377.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trompf L, Brown C, 2014. Personality affects learning and trade-offs between private and social information in guppies, Poecilia reticulata. Anim Behav 88:99–106. [Google Scholar]
- Verbeek MEM, Drent PJ, Wiepkema PR, 1994. Consistent individual differences in early exploratory behaviour of male great tits. Anim Behav 48:1113–1121. [Google Scholar]
- Wray MK, Mattila HR, Seeley TD, 2011. Collective personalities in honeybee colonies are linked to colony fitness. Anim Behav 81:559–568. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.