Abstract
Prey have evolved anti-predator defences that reduce or eliminate the risk of predation. Predators often reproduce at specific sites over many years causing permanent threats to local prey species. Such prey may respond by moving elsewhere thereby reducing local population abundance, or they may stay put and adjust their behavior to the presence of predators. We tested these predictions by analyzing population abundance and anti-predator behavior within 100 m of and 500 m away from nests of sparrowhawks Accipiter nisus and goshawks A. gentilis for 80 species of birds. Population abundance of prey was reduced by 11% near goshawk nests and by 15% near sparrowhawk nests when compared with nearby control sites in similar habitats. Flight initiation distance (FID) of prey, estimated as the distance at which birds took flight when approached by a human, increased by 50% in the presence of hawk nests, providing evidence of adjustment of anti-predator behavior to prevailing risks of predation. Susceptibility to predation was estimated as log transformed abundance of the observed number of prey items obtained from prey remains collected around nests minus log transformed expected number of prey according to point counts of breeding birds. FID increased from 10 to 46 m with increasing susceptibility of prey species to predation by the goshawk and from 12 to 15 m with increasing susceptibility of prey species to predation by the sparrowhawk. These findings suggest that prey adjust their distribution and anti-predator behavior to the risk of predation.
Keywords: accipiter hawks, FID, flight initiation distance, goshawk, population abundance, prey preference, sparrowhawk
Predation causes non-negligible risks for prey by increasing the probability of mortality and also non-lethal effects, and for predators by increasing the risk of injury (review in Caro 2005; Cooper and Blumstein 2015). Predators can influence fitness of prey by inducing costly defensive behavior. A number of studies have investigated the relationship between anti-predator behavior and the presence of raptors (e.g. Rytkönen and Soppela 1995; Forsman and Mönkkönen 2001; Thomson et al. 2011). Flight initiation distance (FID) is a measure of the risk that potential prey individuals take when approached by a human (Ydenberg and Dill 1986), and thus human disturbance can be considered a form of predation risk (Frid and Dill 2002). Although there are hundreds of studies of FID (review in Cooper and Blumstein 2015), there are only a handful of studies investigating FID in relation to predation and proximity of predators, which supposedly is the context in which this behavior evolved (Møller et al. 2006, 2010, 2011; Møller 2014; Møller and Erritzøe 2014; Samia et al. 2015). These studies have explicitly shown that individuals and species with shorter FID are exactly the same individuals and species that run an elevated risk of predation. In other words, there is a negative relationship between FID and the risk of predation. Economic escape theory suggests that prey should counterbalance predation risk and the cost of fleeing (such as loss of foraging opportunities) when deciding to escape from a potential predator (Ydenberg and Dill 1986; Cooper and Frederick 2007). If prey stay in their once chosen territory, they may adjust to the elevated risk of predation by changing their behavior to longer FID near nests of predators. This theory is supported by the observation that FID adjusted for body size is strongly negatively correlated with risk of predation by sparrowhawks Accipiter nisus (Møller et al. 2006). FID also predicts risk of predation by domestic cats Felis catus (Møller et al. 2010) and susceptibility to traffic (Møller et al. 2011), implying that animal responses to humans also reflect risk-taking behavior in other contexts. Prey may also reduce FID in the absence of predators, as is the case on islands (Cooper et al. 2014). Longer FID may arise due to phenotypic plasticity (if there is a raptor present during the breeding season, adopt a long FID; if not, use a short FID), or longer FID may be due to differential mortality of individuals with short FID (such individuals with short FID may already have died) thereby increasing mean local FID of potential prey, but not of non-prey (Møller 2015).
The distribution of predators can affect the choice of location for reproduction by prey (e.g. Geer 1978). Numerous studies have investigated how the distribution and the relative abundance of prey vary across the landscape (review in Caro 2005). Prey may avoid breeding near nests of predators thereby reducing population abundance in the proximity of predators (Suhonen et al. 1994; Norrdahl and Korpimäki 1998; Thomson et al. 2006a, b; Mönkkönen et al. 2007; Tornberg et al. 2015), or there may be few prey near predators because prey have already been consumed. Prey may choose to breed near predators thereby gaining protection against other species of predators, but running an elevated risk of predation relative to control sites without predators (Wiklund 1982; Paine et al. 1990; Blanco and Tella 1997; Tryjanowski 2001; Quinn et al. 2003; Quinn and Ueta 2008). Alternatively, prey may avoid predation by construction of more concealed nests near predators (Collias and Collias 1984; Hansell 2000). If 1 species of predator excludes competitors, this should result in fewer predators. Either way such a reduction in abundance of prey due to the presence of predators should everything else being equal increase FID.
Here, we first assess how prey of different species are distributed with respect to nests of raptors as a context for addressing how anti-predator behavior is linked directly to the risk of being killed by a raptor (Møller et al. 2006, 2010, 2011; Møller and Erritzøe 2014). This approach is novel despite the fact that hundreds of papers have investigated anti-predator behavior in general and flight-initiation distance in particular. FID in response to human approach is a reliable indicator of risk of predation by several predators (Møller et al. 2006, 2010, 2011; Møller and Erritzøe 2014). Likewise, individual prey that are captured by hawks had shorter FID than survivors (Møller 2014). There is only little evidence of habituation affecting FID, and FID is highly repeatable between measurements (review in Møller 2015).
The objectives of this study were (1) to test for differences in population abundance near and away from predators in a forest community of prey and (2) to test for differences in anti-predator behavior measured as FID near and away from predator nests. We used nests of 2 common Accipiter hawks and their prey as a model system because of extensive information available for these 2 species (Newton 1986; Kenward 2007). Prey size generally increases with predator size across species (Newton 1989). However, the relationship between risk of predation and body size of prey is generally hump-shaped, with small and large prey species being under-represented, and prey species of intermediate size being over-represented as prey relative to their abundance (Møller and Nielsen 2006, 2007; Götmark and Post 1996). Thus, we predicted that at nests of the small sparrowhawk large prey species would be relatively safe from predation because they are too large for successful predation, while at nests of the large goshawk both small and very large prey species would be relatively safe.
The Eurasian sparrowhawk is the most common avian predator in forested and partly open landscapes of the Palaearctic, and it has large sexual size dimorphism with males weighing on average 150 g and females 325 g (Newton 1986). Nests are placed in tall trees, and usually a new nest is constructed annually. The main prey are tits, thrushes, finches, buntings, and sparrows (Newton 1986). Males bring all food for the female during egg laying, incubation, and brooding until the nestlings reach an age of 3 weeks, and they also provide most food for older nestlings (Newton 1986; Bujoczek and Ciach 2009). Prey is usually plucked at traditional sites close to the nest (Møller and Nielsen 2006). The northern goshawk is the second-most common avian predator in the Palaearctic, with males weighing on average 768 g and females 1164 g (Møller et al. 2012). The large nests are usually reused for many years resulting in large nest structures that can weigh several hundred kilograms. The main prey are gulls, pheasants, partridges, pigeons, corvids, thrushes, and starlings (Kenward 2007). Male goshawks bring all food for the female during egg laying, incubation, and brooding, and they also bring most food for the nestlings (Kenward 2007). Again, prey is usually plucked at traditional sites near the nest (Møller and Nielsen 2006).
Materials and Methods
Point counts
We located 13 sparrowhawk A. nisus and 10 goshawk A. gentilis nests in Wielkopolska province, western Poland (52oN, 16oE). All hawk nests were located in trees, mainly pines and the majority of territories were known for at least 15 years (Kwieciński and Mizera 2006; Wylegała 2012). The study forests were only infrequently visited by humans, implying that there was only little human disturbance of birds near or 500 m away from hawk nests. In fact, we never observed any humans during our field work.
We recorded population abundance of potential prey using standardized point counts of breeding birds (Blondel et al. 1970; Bibby et al. 1992; Voříšek et al. 2010). Data on abundance of birds were collected using 3 point counts at a distance of 100 m from raptor nests and 3 point counts in a similar habitat at a distance of 500 m from raptor nests, carried out twice during May and June 2015, the former to account for residents and the latter to account for late arriving tropical migrants. We chose the distances of 100 m and 500 m to ensure that hunting activity by raptors would be higher at the former compared with the latter distance. Indeed, distances between neighboring sparrowhawk nests may be as small as 0.2 km and commonly reach a distance of 0.5 km (Newton et al. 1986). The sets of 3 point counts at and away from hawk nests were chosen explicitly so that the habitat was similar in terms of tree species and age of tree stands, thereby avoiding any difference due to factors other than presence or absence of raptors. All points were visited once between 06:00 and 10:00 for 5 min, only during favorable weather conditions without rain or strong wind. All diurnal bird species detected visually and acoustically within a distance of 100 m were recorded. Point counts provide highly reliable estimates of relative population abundance, and they constitute a standardized practical method for comparing bird communities among habitats and across temporal scales (Blondel et al. 1970; Bibby et al. 1992; Voříšek et al. 2010). We see no reason to expect bias from this method for the data collected within 100 m and at 500 m from hawk nests because the method was exactly the same as were the observers, the time, and the date of surveys.
Flight initiation distance
We estimated FID within a distance of 100 m of a hawk nest and at a control site with similar habitat 500 m away from this focal hawk nest. When an individual bird had been located with a pair of binoculars or with eyesight, P.T. and Z.K. moved at a normal walking speed toward the individual, while recording the number of steps, which approximately equals the number of meters (Møller et al. 2008a).
Approximately half of all individuals were recorded by sight and the other half from vocalizations within a distance of 100 m from a hawk nest and 500 m away from hawk nests. Thus, there was no reason to expect bias due to a different fraction of birds being recorded visually at the 2 types of sites. The distance at which the individual took flight was recorded as the FID, while the starting distance (SD; i.e. the experimenter–subject distance when approach began) was likewise recorded. SDs were on average 8.9 m (SE = 0.1), range 2–38 m, N = 982. SD was slightly shorter at 100 m compared with 500 m [log10-transformed data: 1.20 m (0.14) vs. 1.35 m (0.11)]. There was only a weak correlation between FID and SD in a General Linear Mixed Model (GLMM) with species as a random effect and territory as a fixed effect and SD as a covariate (F = 34.18, df = 1, 933.7, P < 0.0001; effect size r = 0.006). Thus, we did not consider SD in the subsequent analyses. If the individual was positioned in the vegetation, the height above ground was recorded to the nearest meter. P.T. only recorded FID on days with fine weather conditions and little or no wind or precipitation. FID was estimated as the Euclidian distance, which equals the square-root of the sum of the squared horizontal distance and the squared height above ground level (Blumstein 2006). In total, we recorded 428 FID for 38 species in the goshawk study and 552 FID for 42 species in the sparrowhawk study. However, we only used 416 FID for 30 species for goshawks to ensure that we had matching data for the area surrounding raptor nests and control areas, and likewise we used 531 FID for 32 species for sparrowhawks to ensure that we had information for both sites near raptor nests and control sites. We obtained FID data from areas less than 100 m and 500 m from raptor nests, and there was no indication that there was differential bias between the 2 distances. Furthermore, there was no difference in the rate of vocalizations per capita between these 2 distances.
All recordings were made during breeding in May–June 2015, when most individuals are sedentary, thus preventing the same individual from being recorded in different sites. P.T. avoided any effects of pseudo-replication by recording a single individual of a given sex, age, and species at a given site. However, a modest degree of resampling subjects has been shown not to influence the results of studies of this nature (Runyan and Blumstein 2004).
Estimates of FID have previously been shown to be consistent across a number of different contexts including across studies (Møller 2008a,b,c; Blumstein 2006), observers (Møller 2008a,b,c), countries (Møller 2008c), and seasons (Møller 2008c).
Prey abundance and prey susceptibility
We estimated susceptibility of prey to sparrowhawks and goshawks from estimates of the risk of predation based on a total of 125 and 82 prey items, respectively. Prey remains were systematically collected near nests as in other studies of the same species of hawks (Nielsen 2004; Møller and Nielsen 2006; Bujoczek and Ciach 2009), with only prey remains less than 1-month old as judged from decay of feathers and fading of colors being included. All nest sites were visited repeatedly during the breeding season, and sampling effort can therefore be considered to be similar across sites. We calculated the expected number of prey by using information on abundance based on the abundance of breeding birds in exactly the same study areas. We relied on our own systematic point counts of breeding birds (see above) to obtain an estimate of the mean breeding abundance of prey species in the study areas.
We estimated a logarithmic index of susceptibility of prey as the observed log10-transformed relative frequency of prey items found at nests minus the log10-transformed expected number of prey according to the bird survey data (Møller and Nielsen 2006). We used a logarithmic index to achieve a normally distributed variable. The expected number of prey according to abundance was estimated as the proportion of prey individuals of each species from the abundance based on point counts multiplied by the total number of prey individuals. Thus, if prey of a given species constituted 1% and available prey also constituted 1%, then the prey susceptibility index was 0. If prey of another species constituted 10% of all prey but available prey in the breeding bird community constituted 1%, then the prey susceptibility index was 1.0. Indeed, this prey susceptibility index is correlated with a number of ecological variables beyond the study sites (Møller and Nielsen 2007, 2010; Møller et al. 2008, 2010). Previous research has shown that prey susceptibility to sparrowhawk and goshawk predation in Finland was significantly positively correlated with susceptibility of the same species in Denmark (Møller et al. 2012). Thus, there is evidence of consistency in prey susceptibility by sparrowhawks and goshawks across large spatial scales.
Body mass
Body mass was recorded as the mean mass of males and females from the breeding season, as reported by Cramp and Perrins (1977–1994). If more than a single estimate was reported in that source, we used the one with the largest sample size.
Statistical analyses
We compared the population abundance and the FID of different bird species at hawk nests and 500 m away using a 1-sample t-test weighted by sample size under the null hypothesis that the difference in population abundance between the surroundings of raptor nests and control areas should be zero. Likewise, we used a 1-sample t-test for FID weighted by sample size under the null hypothesis that the difference in FID between the surroundings of raptor nests and control areas should be zero.
We used GLMM to analyze effects of raptors on abundance and FID. In a first GLMM, we investigated the relationship between abundance of breeding birds from point counts and raptor territory identity (random effect), prey species (fixed effect), raptor species (fixed effect), and whether the observation was made within 100 m from or more than 500 m away from the nest (fixed effect). In a second GLMM, we investigated the relationship between FID and raptor territory identity (random effect), susceptibility of prey to predation (covariate) and whether the observation was made within 100 m from or more than 500 m away from the nest (fixed effect). Variables were log10-transformed to achieve approximately normal distributions. We used Kendall rank order correlation to analyze the relationship between the difference in population abundance at 100 m and 500 m from hawk nests and the difference in FID at 100 m and 500 m from hawk nests. We did not use phylogenetically adjusted analyses for these statistical tests as we have previously shown that a phylogenetic signal is absent for analyses of FID and susceptibility to predation (Møller and Nielsen 2006; Møller et al. 2015). All analyses were made with JMP (SAS 2012).
Results
Population abundance of breeding birds and presence of hawks
We predicted that the abundance of prey was reduced near hawk nests. Nine out of 64 prey species had significantly lower abundance near than away from goshawk nests, while none showed the opposite pattern, differing significantly from the expected value of 3.2 species with a significant reduction (G = 7.59, df = 1, P = 0.006). Five out of 63 prey species had significantly lower abundance near than away from sparrowhawk nests (these species were greater spotted woodpecker Dendrocopos major, robin Erithacus rubecula, chaffinch Fringilla coelebs, great tit Paus major, willow tit Parus montanus), while none showed the opposite pattern, not significantly different from the expected value of 3.15 species with a significant reduction (G = 0.98, df = 1, P = 0.23). There was no significant difference in abundance of bird species between the 2 hawk species (Table 1). There was a significant difference in abundance between survey points within 100 m and 500 m away from hawk nests (Table 1). There was no significant main effect of susceptibility on abundance, nor was there any main effect of body mass (Table 1). The interaction between susceptibility and body mass was statistically significant, as was the interaction between proximity of hawk nests and body mass (Table 1). The interaction between susceptibility and distance was not statistically significant (Table 1).
Table 1.
GLMM of the relationship between abundance and territory identity (random effect), prey species (random effect), hawk species (fixed effect), whether the count was made within 100 m from or more than 500 m away from the nest (distance to nest; fixed effect), susceptibility of prey to predation (covariate), body mass (covariate), the interaction between susceptibility of prey to predation and body mass and between whether the count was made within 100 m from or more than 500 m away from the nest and body mass
| Source | F | df | P | Estimate | SE |
|---|---|---|---|---|---|
| Accipiter species | 0.55 | 1, 55.7 | 0.55 | −0.011 | 0.015 |
| Hawk nest proximity | 10.81 | 1, 6826 | 0.0010 | 0.021 | 0.007 |
| Susceptibility | 4.40 | 1, 6742 | 0.036 | −0.036 | 0.017 |
| Body mass | 1.09 | 1, 70.2 | 0.30 | −0.079 | 0.075 |
| Susceptibility × body mass | 6.56 | 1, 6515 | 0.011 | 0.068 | 0.027 |
| Hawk nest proximity × body mass | 15.29 | 1, 6809 | < 0.0001 | 0.038 | 0.010 |
| Susceptibility × Hawk nest proximity | 0.58 | 1, 6809 | 0.58 | 0.006 | 0.010 |
Notes: The random effect of territory had a variance of 0.0058 (SE = 0.0022), 95% CI 0.0014, 0.0101, accounting for 1.2% of the variance while the random effect of species had a variance of 0.201 (SE = 0.035), 95% CI 0.1332, 0.2692, accounting for 40.3% of the variance.
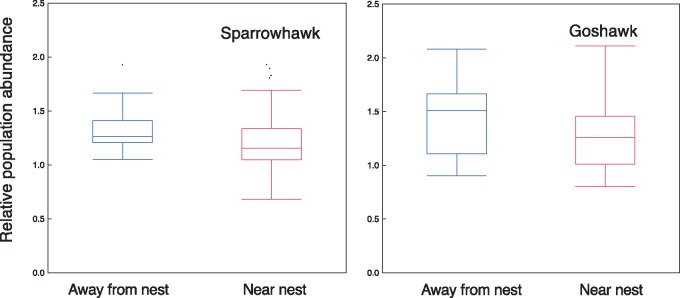
The difference in mean abundance of breeding birds between point counts at sparrowhawk nests and 500 m away was on average 0.169 individuals per 5-min point count (SE = 0.018), N = 26 species, differing significantly from the null expectation of zero difference between the 2 areas if population abundance of prey had remained unaffected (Figure 1; t = 9.23, df = 25, P < 0.0001). This amounted to a mean difference of 15%. Similarly, the mean difference in abundance of breeding birds between point counts at goshawk nests and 500 m away was on average 0.163 (SE = 0.031), N = 21 species, differing significantly from the null expectation of zero (t = 5.18, df = 20, P < 0.0001). This amounted to a difference of 11%. Thus, the abundance of breeding birds was reduced near nests of hawks.
Figure 1.
Box plots of relative population abundance (number of individuals recorded during 5 min of observations) for different species of birds within 100 m of nests (nests) and more than 500 m away (controls) from nests of goshawk and sparrowhawk. Box plots show medians, quartiles, 5- and 95-percentiles, and extreme values.
FID and presence of raptors
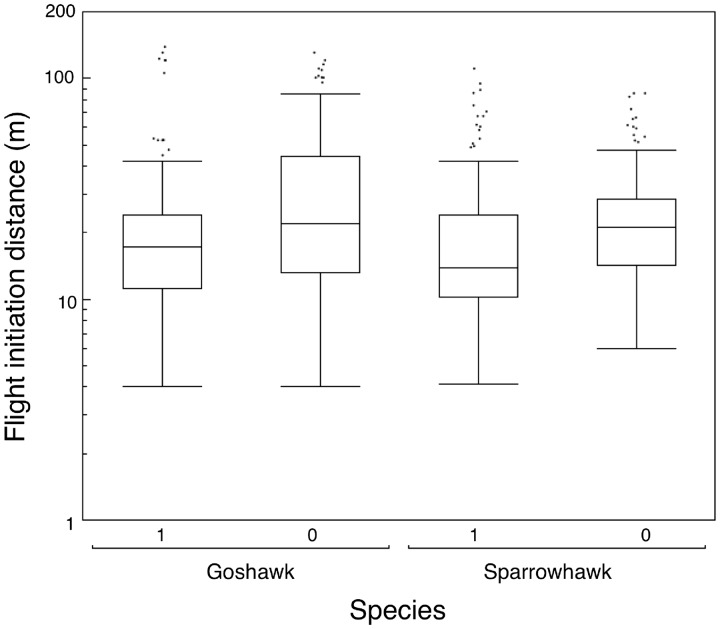
We predicted that prey took smaller risks in the presence of hawk nests. Eight out of 19 prey species had significantly longer FID near than away from goshawk nests, and none showed the opposite effect, differing significantly from the expected value of 0.95 species with a significant increase (G = 23.20, df = 1, P < 0.0001). Similarly, 11 out of 24 species had significantly longer FID at than away from sparrowhawk nests, and none showed the opposite effect, differing significantly from the expected value of 1.2 species with a significant reduction (G = 34.14, df = 1, P < 0.0001). Mean FID for different species at goshawk nests was 24.2 m (SE = 1.1), which was almost 50% longer than mean FID for the same species in similar habitat 500 m away from goshawk nests [Figure 2; 16.6 m (SE = 1.1), t = 5.18, df = 20, P < 0.0001]. The distribution of mean FIDs for different species of birds is shown in Figure 3. Mean FID at sparrowhawk nests was on average 21.0 m (SE = 1.1) which was almost 50% longer than mean FID for the same species in similar habitat 500 m from sparrowhawk nests (Figure 2; 14.2 m, SE = 1.1, t = 9.23, df = 25, P < 0.0001).
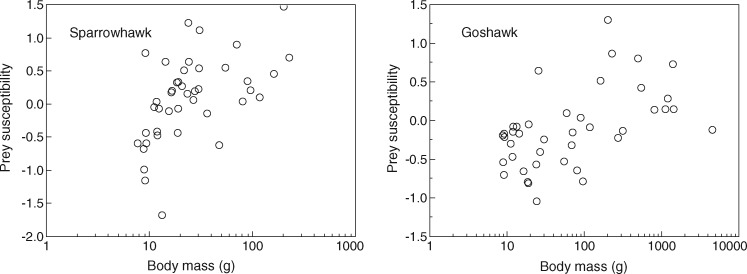
Figure 2.
Susceptibility of prey to sparrowhawk and goshawk predation for different bird species in relation to their body mass (g). Susceptibility was estimated as log observed abundance of prey minus log expected abundance of prey based on point counts of breeding birds.
Figure 3.
Box plots of FID (m) for different bird species within 100 m of nests (1) and more than 500 m away (0) from nests of goshawk and sparrowhawk. Box plots show medians, quartiles, 5- and 95-percentiles, and extreme values.
Mean FID for different prey species increased with the susceptibility of a given bird species to predation (Table 2). Independently, mean FID was longer near than away from nests of goshawks (Table 2; Figure 3). Similarly, mean FID for different prey species increased with body mass (Table 2). There was no significant difference in FID between hawk species (Table 1). The susceptibility of a species to predation depended on the distance to hawk nests as shown by the significant interaction between susceptibility and distance (Table 2). There was also a significant susceptibility by body mass interaction implying that FID decreased with increasing susceptibility within 100 m from hawk nests, but increased with increasing susceptibility 500 m away from hawk nests.
Table 2.
GLMM of the relationship between FID and territory identity (random effect), susceptibility of prey to predation (covariate), whether the observation was made within 100 m from or more than 500 m away from the nest (distance to nest; fixed effect), body mass (covariate), the interaction between susceptibility and distance to nest and hawk species
| Source | F | df | P | Estimate | SE |
|---|---|---|---|---|---|
| Susceptibility | 4.66 | 1, 670.1 | 0.031 | 0.051 | 0.023 |
| Distance to nest | 188.39 | 1, 954.4 | < 0.0001 | −0.086 | 0.006 |
| Body mass | 567.34 | 1, 963.2 | < 0.0001 | 0.358 | 0.015 |
| Hawk species | 3.60 | 1, 28.2 | 0.07 | 0.025 | 0.013 |
| Susceptibility × distance to nest | 9.75 | 1, 926.9 | 0.0019 | −0.042 | 0.014 |
| Susceptibility × body mass | 4.72 | 1, 457.5 | 0.030 | −0.100 | 0.046 |
Notes: The random effect of territory size had a variance of 0.0028 (SE = 0.0011), 95% CI 0.00059, 0.00503, accounting for 6.3% of the variance while the random effect of species had a variance of 0.0079 (SE = 0.0032), 95% CI 0.0016, 0.0143, accounting for 17.9% of the variance.
We tested whether the difference in FID between distances of 100 m and 500 m was correlated with the difference in relative population abundance between 100 m and 500 m for different species of birds. The positive correlation was highly significant for goshawk (Kendall τ = 0.46, N = 21 species, P = 0.0057), but the correlation was not significant for sparrowhawk (Kendall τ = −0.10, N = 26 species, P = 0.49). Thus, species of prey that had a large reduction in population abundance at hawk nests also had a larger increase in FID near nests.
Discussion
We provided evidence for prey being able to adjust their anti-predator behavior to the presence of predators by reducing their population abundance near hawk nests, mainly for prey species that were particularly susceptible to predation by Accipiter hawks. Furthermore, we documented a novel increase in FID within a distance of 100 m from hawk nests, but not at a distance of 500 m, and there were statistically independent effects of susceptibility on FID. In other words, there was evidence of phenotypic adjustment in FID near raptor nests but not away from such nests as shown by the significant interaction. These novel behavioral results for FID document an ability of prey to phenotypically adjust to the proximity of predators.
Predators may affect the population abundance of prey by changing the distribution of prey relative to the location of predators. Here, we have shown a reduction in population abundance of birds near nests of 2 raptors relative to control areas 500 m away from raptor nests. Such effects have previously been documented for a number of prey species (Suhonen et al. 1994; Forsman et al. 2001; Mönkkönen et al. 2007; Duncan and Bednekoff 2008; Morosinotto et al. 2010; Burgas et al. 2014; Tornberg et al. 2015) including prey of foxes Vulpes vulpes (Tryjanowski et al. 2002). Here, we have extended these findings in 2 important ways. First, we have shown that there is a consistent difference in body mass between prey at a distance of 100 m and 500 m from hawk nests. This suggests that Accipiter hawks differentially suppressed the abundance of potential prey species with small body mass. Second, we have shown here that the reduction in population abundance was correlated with susceptibility to predation.
We documented 2 responses to the proximity of hawk nests: A decrease in population abundance and an increase in FID near hawk nests. We found a significant positive correlation between the 2 responses, but only for the goshawk. However, there was an overall stronger effect on FID than on reduction in population abundance. It remains unclear why there is such a difference between raptor species, and also why the effect was stronger for increase in FID than for reduction in population abundance. One possibility is that goshawks use the same nests year after year while sparrowhawks build new nests every year and move around more than goshawks (Newton 1986; Kenward 2007). Another possibility is that the reduction in abundance may apply to several years if a larger proportion of prey near hawk nests are eaten, while all prey are able to respond behaviorally to the presence of a predator.
Here, we have shown that FID increased 50% when comparing estimates within a distance of less than 100 m from raptor nests when compared with more than 500 m away in the same habitat. This is as expected from economic escape theory. This 50% increase in FID probably implies an increase in the level of disturbance in the presence of Accipiter hawks. Such a level of disturbance probably implies that individual birds near raptor nests suffer a significant reduction in their energy balance, because repeated short distance movement such as those involved in FID is energetically very expensive as shown by studies of doubly labeled water (Tatner and Bryant 1986). Breeding birds living in environments with high predation risk produce fewer and smaller nestlings (Thomson et al. 2006a,b, 2012) and adults showed increased physiological stress (Thomson et al. 2010). These effects may be attributed to the energetic costs of disturbance documented by Tatner and Bryant (1986) or by removing prey from the population. Interestingly, these predictions concerning the energetic costs of avoiding predation are the same as those arising from indirect effects of predation due to disturbance of prey by predators (Abrams 1984; Lima and Dill 1990; Lima 1998; Balbontín and Møller 2015). We should expect that FID decreases in the absence of predators such as the reduction in FID observed in cities (Samia et al. 2015) near refuges at human habitation (Møller 2012). Here, we have documented a novel increase in FID near raptor nests as expected from economic escape theory (Ydenberg and Dill 1986; Cooper and Frederick 2007).
Several mechanisms may account for the observed differences in FID between birds living in the proximity of hawk nests and in similar habitat more than 500 m away. This could either be avoidance of areas near hawk nests or selective removal of prey by predators giving rise to habituation, phenotypic sorting, or micro-evolutionary change (Møller 2015). Habituation may be an unlikely explanation because any slight error might result in the prey individual falling prey to the predator. A more likely explanation is phenotypic sorting with individuals in poor condition more often being associated with raptor nests than individuals in prime condition (Thomson et al. 2006a, b; Morosinotto et al. 2010). Micro-evolutionary change implies change that can be attributed to directional selection, for which there is evidence, and heritability for which there is also empirical evidence (Møller 2014). Two examples of reduction in FID may be attributed to micro-evolutionary change. Animals on islands have lost or greatly reduced their fear of humans after divergence from mainland ancestors (Darwin 1868; Whittaker 1998; Blumstein and Daniel 2005; Cooper et al. 2014), and domesticated animals have likewise reduced their fear of humans in the process of domestication (Darwin 1868; Clutton-Brock 1987; Brubaker and Coss 2015).
The present study raises a question about the characteristics of the individual birds that breed close to raptors. We envisage 2 possibilities. They could be young individuals of poor phenotypic quality that have not been subject to viability selection. Alternatively, they could be older high-quality individuals with superior flight abilities that enjoy a fitness benefit in terms of protection at refuges next to raptor nests. We consider the first scenario possible given the reduction in population abundance by 11–15% between the vicinity of hawk nests and further away as reported in the present study, although we cannot exclude the second scenario either.
In conclusion, we have shown that birds breeding close to raptors have reduced population abundance and greatly increased FIDs compared with individuals located just a few hundred meters away. These differences in abundance were negligible or reversed for small species of potential prey, increasing to large differences in abundance for large-sized prey species.
Dan Blumstein, David Swanson, and 2 anonymous reviewers provided constructive and helpful suggestions.
References
- Abrams PA, 1984. Foraging time optimization and interactions in food webs. Am Nat 124:80–96. [Google Scholar]
- Balbontín J, Møller AP, 2015. Environmental conditions during early life affect aging in a short-lived passerine bird. Ecology 96:948–959. [DOI] [PubMed] [Google Scholar]
- Blanco G, Tella JL, 1997. Protective association and breeding advantages of choughs nesting in lesser kestrel colonies. Anim Behav 54:335–342. [DOI] [PubMed] [Google Scholar]
- Bibby CJ, Burgess ND, Hill DA, 1992. Bird Census Techniques. London: Academic Press. [Google Scholar]
- Blondel J, Ferry C, Frochot B, 1970. La méthode des indices ponctuels d’abondance (I.P.A.) au des relevés d’avifaune par ‘stations d’ecoute’. Alauda 38:55–71. [Google Scholar]
- Blumstein DT, 2006. Developing an evolutionary ecology of fear: how life history and natural history traits affect disturbance tolerance in birds. Anim Behav 71:389–399. [Google Scholar]
- Blumstein DT, Daniel JC, 2005. The loss of anti-predator behaviour following isolation on islands. Proc R Soc B 272:1663–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brubaker AS, Coss RG, 2015. Evolutionary constraints on equid domestication: comparison of flight initiation distances of wild horses Equus caballus ferus and plains zebras Equus quagga. J Comp Psychol 129:366–376. [DOI] [PubMed] [Google Scholar]
- Bujoczek M, Ciach M, 2009. Seasonal changes in the avian diet of breeding Sparrowhawks Accipiter nisus: how to fulfill the offspring’s food demands. Zool Stud 48:215–222. [Google Scholar]
- Burgas D, Byholm P, Parkkima T, 2014. Raptors as surrogates of biodiversity along a landscape gradient. J Appl Ecol 51:786–794. [Google Scholar]
- Caro T, 2005. Antipredator Defenses in Birds and Mammals. Chicago (IL: ): University of Chicago Press. [Google Scholar]
- Clutton-Brock J, 1987. A Natural History of Domesticated Mammals. Austin: University of Texas Press. [Google Scholar]
- Collias NE, Collias EC, 1984. Nest Building and Bird Behaviour. Princeton (NJ: ): Princeton University Press. [Google Scholar]
- Cooper WE Jr, Blumstein DT, 2015. Escaping from Predators: An Integrative View of Escape Decisions and Refuge Use. Cambridge: Cambridge University Press. [Google Scholar]
- Cooper WE Jr, Frederick WG, 2007. Optimal flight initiation distance. J Theor Biol 244:59–67. [DOI] [PubMed] [Google Scholar]
- Cooper RA Jr, Pyron WE, Garland T, 2014. Island tameness: living on islands reduces flight initiation distance. Proc R Soc B 281:20133019.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramp S, Perrins CM (eds), 1977. –1994. The Birds of the Western Palearctic. Vols 1–9 Oxford: Oxford University Press. [Google Scholar]
- Darwin C, 1868. The Variation of Animals and Plants under Domestication. London: John Murray. [Google Scholar]
- Duncan WJ, Bednekoff PA, 2008. Nesting with an enemy: the abundance of preferred and secondary prey near nesting Cooper’s hawks. Ethol Ecol Evol 20:51–59. [Google Scholar]
- Forsman JT, Mönkkönen M, 2001. Responses by breeding birds to heterospecific song and mobbing call playbacks under varying predation risk. Anim Behav 62:1067–1073. [Google Scholar]
- Forsman J, Mönkkönen M, Hukkanen M, 2001. Effects of predation on community assembly and spatial dispersion of breeding forest birds. Ecology 82:232–244. [Google Scholar]
- Frid A, Dill L, 2002. Human-caused disturbance stimuli as a form of predation risk. Conserv Ecol 6:1–11. [Google Scholar]
- Geer TA, 1978. Effects of sparrowhawks on nesting tits. Condor 80:419–423. [Google Scholar]
- Götmark F, Post P, 1996. Prey selection by sparrowhawks, Accipiter nisus: relative predation risk for breeding passerine birds in relation to their size, ecology and behaviour. Phil Trans R Soc Lond 351:1559–1577. [Google Scholar]
- Hansell MH, 2000. Bird Nests and Construction Behaviour. Cambridge: Cambridge University Press. [Google Scholar]
- Kwieciński Z, Mizera T, 2006. Liczebność i efekty lęgów ptaków szponiastych Falconiformes Kotliny Śremskiej w latach 2001–2002. Not Orn 47:230–240. [Google Scholar]
- Kenward RE, 2007. The Goshawk. London: T & AD Poyser. [Google Scholar]
- Lima SL, 1998. Nonlethal effects in the ecology of predator–prey interactions: what are the ecological effects of anti-predator decision-making?. BioScience 48:25–34. [Google Scholar]
- Lima SL, Dill LM, 1990. Behavioural decisions made under the risk of predation: a review and prospectus. Can J Zool 68:619–640. [Google Scholar]
- Møller AP, 2008a. Flight distance and blood parasites in birds. Behav Ecol 19:1305–1313. [Google Scholar]
- Møller AP, 2008b. Flight distance of urban birds, predation and selection for urban life. Behav Ecol Sociobiol 63:63–75. [Google Scholar]
- Møller AP, 2008c. Flight distance and population trends in European breeding birds. Behav Ecol 19:1095–1102. [Google Scholar]
- Møller AP, 2012. Urban areas as refuges from predators and flight distance of prey. Behav Ecol 23:1030–1035. [Google Scholar]
- Møller AP, 2014. Life history, predation and flight initiation distance in a migratory bird. J Evol Biol 27:1105–1113. [DOI] [PubMed] [Google Scholar]
- Møller AP, 2015. Birds In: Cooper WE Jr, Blumstein DT, editors. Escaping from Predators: An Integrative View of Escape Decisions and Refuge Use. Cambridge: Cambridge University Press, 88–112. [Google Scholar]
- Møller AP, Erritzøe J, 2014. Predator-prey interactions, flight initiation distance and brain size. J Evol Biol 27:34–42. [DOI] [PubMed] [Google Scholar]
- Møller AP, Erritzøe H, Erritzøe J, 2011. A behavioral ecology approach to traffic accidents: interspecific variation in causes of traffic casualties among birds. Zool Res 32:115–127. [DOI] [PubMed] [Google Scholar]
- Møller AP, Erritzøe J, Nielsen JT, 2010. Causes of interspecific variation in susceptibility to cat predation on birds. Chinese Birds 1:97–111. [Google Scholar]
- Møller AP, Nielsen JT, 2006. Prey vulnerability in relation to sexual coloration. Behav Ecol Sociobiol 60:227–233. [Google Scholar]
- Møller AP, Nielsen JT, 2007. Malaria and risk of predation: a comparative study of birds. Ecology 88:871–881. [DOI] [PubMed] [Google Scholar]
- Møller AP, Nielsen JT, 2010. Fear screams and adaptation to avoid imminent death: effects of genetic variation and predation. Ethol Ecol Evol 22:1–20. [Google Scholar]
- Møller AP, Nielsen JT, Garamszegi LZ, 2006. Song post exposure, song features and predation risk. Behav Ecol 17:155–163. [Google Scholar]
- Møller AP, Nielsen JT, Garamszegi LZ, 2008. Risk taking by singing males. Behav Ecol 19:41–53. [Google Scholar]
- Møller AP, Solonen T, Byholm P, Huhta E, Nielsen JT. et al. , 2012. Spatial consistency in susceptibility of prey species to predation by two Accipiter hawks. J Avian Biol 43:390–396. [Google Scholar]
- Møller AP, Tryjanowski P, Díaz M, Kwiecinski Z, Indykiewicz P. et al. , 2015. Urban habitats and feeders both contribute to flight initiation distance reduction in birds. Behav Ecol 26:861–865. [Google Scholar]
- Mönkkönen M, Husby M, Tornberg R, Helle P, Thomson RL, 2007. Predation as a landscape effect: the trade off by prey species between predation risks and protection benefits. J Anim Ecol 76:619–629. [DOI] [PubMed] [Google Scholar]
- Morosinotto C, Thomson RL, Korpimäki E, 2010. Habitat selection as an antipredator behaviour in a multi-predator landscape: all enemies are not equal. J Anim Ecol 79:327–333. [DOI] [PubMed] [Google Scholar]
- Newton I, 1986. The Sparrowhawk. London: T & AD Poyser. [Google Scholar]
- Newton I, 1989. Population Ecology of Raptors. London: T. & A. D. Poyser. [Google Scholar]
- Newton I, Wyllie I, Mearns R, 1986. Spacing of sparrowhawks in relation to food supply. J Anim Ecol 55:361–370. [Google Scholar]
- Nielsen JT, 2004. Prey selection of sparrowhawks in Vendsyssel, Denmark [Danish, with English summary]. Dansk Ornitol Foren Tidsskr 98:164–173. [Google Scholar]
- Norrdahl K, Korpimäki E, 1998. Fear in farmlands: how much does predator avoidance affect bird community structure? J Avian Biol 29:79–85. [Google Scholar]
- Paine RT, Wootton JT, Boersma PD, 1990. Direct and indirect effects of peregrine falcon predation on seabird abundance. Auk 107:1–9. [Google Scholar]
- Quinn J, Ueta M, 2008. Protective nesting associations in birds. Ibis 150:146–167. [Google Scholar]
- Quinn JL, Prop J, Kokorev Y, Black JM, 2003. Predator protection or similar habitat selection in red-breasted goose nesting associations: extremes along a continuum. Anim Behav 65:297–307. [Google Scholar]
- Runyan AM, Blumstein DT, 2004. Do individual differences influence flight initiation distance? J Wildl Manage 68:1124–1129. [Google Scholar]
- Rytkönen S, Soppela M, 1995. Vicinity of sparrowhawk nest affects willow tit nest defense. Condor 97:1074–1078. [Google Scholar]
- Samia DSM, Nakagawa S, Nomura F, Rangel TF, Blumstein DT, 2015. Increased tolerance to humans among disturbed wildlife. Nat Commun 6:8877.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samia DSM, Blumstein DT, Stankowich T, Cooper WE Jr, 2015. Fifty years of chasing lizards: new insights advance optimal escape theory. Biol Rev 91:349–366. [DOI] [PubMed] [Google Scholar]
- SAS, 2012. JMP Version 10.0. Cary: SAS Institute Inc. [Google Scholar]
- Suhonen J, Norrdahl K, Korpimäki E, 1994. Avian predation risk modifies breeding bird community on a large farmland area. Ecology 75:1626–1634. [Google Scholar]
- Tatner P, Bryant DM, 1986. Flight cost of a small passerine measured using doubly labeled water: implications for energetic studies. Auk 103:169–180. [Google Scholar]
- Thomson RL, Forsman JT, Sardà-Palomera F, Mönkkönen M, 2006a. Fear factor: prey habitat selection and its consequences in a predation risk landscape. Ecography 29:507–514. [Google Scholar]
- Thomson RL, Forsman JT, Mönkkönen M, Hukkanen M, Koivula K. et al. , 2006b. Predation risk effects on fitness related measures in the resident bird. Oikos 113:325–333. [Google Scholar]
- Thomson RL, Tomás G, Forsman JT, Broggi J, Mönkkönen M, 2010. Predator proximity as a stressor in breeding flycatchers: mass loss, stress protein induction, and elevated provisioning. Ecology 91:1832–1840. [DOI] [PubMed] [Google Scholar]
- Thomson RL, Forsman JT, Mönkkönen M, 2011. Risk taking in natural predation risk gradients: support for risk allocation from breeding pied flycatchers. Anim Behav 82:1443–1447. [Google Scholar]
- Thomson RL, Tomas G, Forsman JT, Mönkkönen M, 2012. Manipulating individual decisions and environmental conditions reveal individual quality in decision-making and non-lethal costs of predation risk. PLoS ONE 7:e52226.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornberg R, Rytkönen S, Välimäki P, Valkama J, Helle P, 2015. Northern goshawk Accipiter gentilis may improve black grouse success. J Ornithol 157:363–370. [Google Scholar]
- Tryjanowski P, 2001. Proximity of raven Corvus corax nest modifies breeding bird community in an intensively used farmland. Ann Zool Fenn 38:131–138. [Google Scholar]
- Tryjanowski P, Gołdyn B, Surmacki A, 2002. Influence of the red fox Vulpes vulpes on distribution and number of breeding birds in an intensively used farmland. Ecol Res 17:395–399. [Google Scholar]
- Voříšek P, Klvaňová A, Wotton S, Gregory RD, 2010. A Best Practice Guide for Wild Bird Monitoring Schemes. Bruxelles: European Union. [Google Scholar]
- Whittaker RJ, 1998. Island Biogeography. Oxford: Oxford University Press. [Google Scholar]
- Wiklund CG, 1982. Fieldfare Turdus pilars breeding success in relation to colony size nest position and association with merlins Falco columbarius. Behav Ecol Sociobiol 11:165–172. [Google Scholar]
- Wylegała P, 2012. Abundance changes of birds of prey Falconiformes and Common Raven Corvus corax on the Szamotuły Plain in 1999–2010. Ptaki Wielkopolski 1:159–163. [Google Scholar]
- Ydenberg RC, Dill LM, 1986. The economics of fleeing from predators. Adv Study Behav 16:229–249. [Google Scholar]