Abstract
Most habitats in nature are heterogeneous, incorporating favorable and unfavorable microhabitats for different animals, based on their ecological niche. Unsuitable microhabitats have negative consequences for individual growth and survival. Animals, therefore, should fine-tune their location within the habitat by dispersing away from such microhabitats. We studied the suitability of different constant microhabitat conditions for wormlion larvae, a trap-building predator, tested in groups under laboratory conditions. Wormlions construct pit-traps in loose soil and capture small arthropod prey. As wormlions occur in high densities in nature, testing in groups is thus more indicative of their natural behavior than testing individuals. Wormlions responded strongly to biotic conditions—high conspecific density, starvation, and large body mass of conspecifics—by either increasing pit-relocation events or moving away from the microhabitat center to the periphery of the arena, probably opting for a way out. In other instances, individuals increased their distance to the nearest neighbor, thereby changing the spatial pattern toward a more regular pattern, potentially indicating interference competition. The only abiotic condition apparently perceived by wormlions as unsuitable was shallow sand, which led to frequent relocations. The two other abiotic factors—illumination and sand particle size—had no observable effect on behavior, although wormlions in nature always occur under shade in fine sand, and prefer both shade and fine sand particle size under laboratory conditions when given a choice. Under the fine spatial scale of the present experiment, biotic factors appear to be more influential than abiotic ones.
Keywords: antlions, dispersal, relocation, spatial pattern, wormlions
Dispersal and habitat selection are important processes for population and meta-population dynamics (Clobert et al. 2009). A species’ ability to disperse or to distinguish between different habitats will impact its capacity to respond to environmental changes, colonize new habitats, and increase genetic diversity. Therefore, these processes have a great influence on individual survival and reproduction (Harrison 1989; Clobert et al. 2009; Price 2010). Habitat evaluation and choice is influenced by many biotic and abiotic factors, to name but a few: intra-specific density and competition, predation and parasitism risk, inbreeding avoidance, energetic/searching costs, and optimal temperature (Belovsky 1981; Martin 2001; Bowler and Benton 2005; Ronce 2007; Benard and McCauley 2008). Ideally, animals should choose the habitat providing the best chance of survival and reproduction.
The relative importance and interaction between biotic and abiotic factors in their effect on habitat choice is complex, and often species-specific (Rosenberg 1987). For instance, warbler habitat choice in Arizona is strongly affected by precipitation and also by interactions between co-occurring, ecologically similar species (Martin 2001). Juvenile corals select where to settle based on abiotic cues, such as light, but are also attracted to certain cues produced by algae, indicating that the site is suitable for settlement (Price 2010). However, even within the same species, the relative importance of various factors may fluctuate. The relative importance of biotic and abiotic factors also often depends on the spatial scale considered, with smaller-local scales being more sensitive to biotic factors and larger scales to abiotic factors (Jackson et al. 2001; Price 2010).
Once a habitat has been chosen, individuals need either to coexist with conspecifics or to further disperse. The spatial pattern reflects whether individuals are clumped together in space or scattered around. In other words, it is a spectrum bounded in its two edges by individuals much closer to each other than expected and individuals maximizing as much as possible the distances among them. The spatial pattern reflects both biotic and abiotic factors. For example, a regular spatial pattern could result from competition over limited resources or other negative interactions among individuals. A clumped spatial pattern could stem from aggregation in prey-rich small areas, or other benefits, such as dilution the risk of predation (Henschel and Lubin 1997; Perfecto and Vandermeer 2008; Scharf et al. 2008a; Hirsch 2011; Santini et al. 2011). Clumping together is also a product of abiotic conditions that enable lower expenditure on maintenance, fast growth or better reproduction and survival (e.g., available water or suitable temperature: Watson et al. 2007; Scharf et al. 2011; Halliday and Blouin-Demers 2014).
The dispersion of conspecifics is influenced by between-individual interactions, such as attraction or repulsion, and by dispersal tendency and ability (Svendsen 1974; Fangliang et al. 1997). Attraction and repulsion are related to various factors, such as sociability level of the species and individuals (Febrer et al. 2006), or the best way to defend against predation [either grouping or spacing out; Scharf et al. (2012) and references therein].
Abiotic factors are not only important for initial habitat choice but also crucial for dispersal decisions. Individuals tend to disperse away when preferred local abiotic conditions deteriorate, as typical in ephemeral habitats (Ronce 2007). The spatial pattern type of individuals in a certain habitat (i.e., regular, random, or clumped patterns) can fluctuate through time with changing conditions, such as density and food availability (Cushman et al. 1988; Matsura and Takano 1989; Orians and Wittenberger 1991; Juell et al. 1994). Individual position in the habitat center or periphery is also important, because it often results from the trade-off between hunger and safety (outer positions are more dangerous but richer in food; Okamura 1986; Krause and Ruxton 2002). However, central/peripheral positions may also reflect the individual’s arrival time at the habitat. New arrivals are expected to settle in the periphery of the previous residents, which now occupy the highest quality zones in the interior (Bertness and Grosholz 1985; Orians and Wittenberger 1991).
Unlike mobile organisms, more sessile organisms cannot easily abandon an unsatisfying or deteriorating habitat; thus, strong selection operates on their initial habitat choice and stress tolerance (Orians and Wittenberger 1991; Huey et al. 2002; Scharf and Ovadia 2006). Trap-building predators—web-building spiders and pit-building antlions and wormlions—construct traps to capture arthropod prey and serve as an interesting case study of convergent evolution (Devetak 2008; Dor et al. 2014; Scharf et al. 2016). Their sedentary lifestyle and dependence on specific habitat characteristics for the construction of their trap, without which they cannot catch prey, makes them an ideal model system for the study of habitat choice. They depend on both biotic and abiotic habitat conditions, such as habitat structure, conspecific density, temperature, and light (Lubin et al. 1993; Herberstein and Fleisch 2003; Pruitt et al. 2011; Adar et al. 2016). It has been suggested that antlions are more affected by abiotic factors than spiders and that the former are susceptible to shifts in their physical environment (Scharf and Ovadia 2006). Previous studies on wormlions have shown that they prefer shade, and deep, obstacle-free, and small-particle-sized sand (Devetak and Arnett 2015; Adar et al. 2016).
We examined here the extent to which favorable and unfavorable factors influence the spatial pattern, movement, and trap relocation of a trap-building predator, the wormlion larva (Diptera: Vermileonidae). Wormlions are sedentary insects, found in high clustered densities in different spatial patterns (Dor et al. 2014). The motivation for using wormlions in this study is their strong dependence on the abiotic environment, owing to their usage of the substrate to build a pit-trap and due to their preference for shaded, sheltered habitats. Another reason is that wormlions are not social in any common way, but often occur in high densities, with pits often leaned one against another. Both suggest that wormlions are on the one hand dependent on their immediate microhabitat, while on the other hand are very tolerant to conspecifics, and therefore should be more sensitive to abiotic factors than biotic ones. Finally, more is known on the ecologically similar antlions, which are taxonomically unrelated but construct pit-traps in sand and loose soils too. The latter heavily rely on abiotic factors for the construction of their pit, and therefore, for successful foraging (Heinrich and Heinrich 1984; Scharf and Ovadia 2006; Scharf et al. 2009; Devetak and Arnett 2015). However, differences between wormlions and antlions also exist, such as the intensity of cannibalism being high in antlions and not existing in wormlions (Scharf et al. 2016). The lack of cannibalism in wormlions may increase their sensitivity to abiotic factors and may limit their need to consider other conspecifics. Previous studies have either focused on individual responses to different biotic and abiotic factors (e.g., Scharf et al. 2009) or tested only a limited set of factors in their effect on group dynamics (e.g., Rosenberg 1987). Evaluating concomitantly multiple factors in focal systems is important because it adds a necessary layer of complexity and ecological realism to our understanding of a biological system.
We examined the effect of three biotic factors (density, hunger, and body mass) and three abiotic factors (sand depth, sand particle size, and light) on the spatial pattern, pit relocation events, and distance from the area center of wormlion groups. (1) We predicted that wormlions would be more affected by abiotic than biotic factors. Wormlions have been previously shown to strongly respond to sand particle size, sand depth, and light (Devetak 2008; Adar et al. 2016; Katz et al. 2016). (2) We also predicted that under unfavorable conditions, including shallow sand, coarse particle size, strong illumination, high density and starvation, the spatial pattern would become more regular and relocation events more frequent than under more favorable conditions. Under such unfavorable conditions the ability to construct a pit is limited, construction costs are higher, and tolerance of conspecifics becomes lower.
Materials and Methods
Studied organism, collection, and general procedure
The studied wormlion species (Vermileo sp.) is still undescribed but there is probably a single wormlion species in Israel (Freidberg A, personal communication). The fly larvae (Diptera: Vermileonidae) construct cone-shaped pits in loose soil in order to capture small arthropod prey (Wheeler 1930). This species is abundant in urban areas, found at high densities under shelters protecting it from direct sun and rain. The fly undergoes five to six instar stages in about a year. The adult emerging is short-lived. While antlion species have been the subject of previous work on behavior and life history, wormlions have been much less studied (Dor et al. 2014).
Wormlions were collected at Tel Aviv University, north-west Tel Aviv, between December 2015 and July 2016. Larvae were kept in individual cups (4.5 cm diameter) filled with 2 cm-deep fine homogenized sand (< 106 mm) for 48 h. In order to standardize wormlions’ hunger level, after 48 h and once pits were built, each larva received one similar-sized flour beetle Tribolium castaneum larva, except for the feeding treatments (described below). Larvae were weighed using an analytical balance (accuracy of 0.01 mg) prior to each experiment. During all experiments except the illumination experiment, the room was illuminated by fluorescent ceiling lights in a 14:10 light/dark cycle (maximum of 104 ± 21.2 LUX; Sper Scientific 840020 Dual-Scale Light Meter). This regime was used to mimic natural illumination conditions. The trays were placed beneath shelves to imitate natural shaded conditions. In the illumination versus dark experiment, we either blocked the room illumination from reaching the shelves by placing wooden panels in front of them or placing fluorescent bulbs directly above each tray, resulting in constant illuminated or constant dark conditions. Experiments were conducted in 16 circular aluminum trays (r = 12.5 cm, area = 490.6 cm2), filled with 2 cm-deep fine homogenized sand. In the sand depth experiment, we either filled the trays with 2 cm sand (deep) or 0.5 cm sand (shallow). For each experiment ∼224 individual larvae were collected and allocated to trays, 14 individuals per tray (density was similar to natural field density; Dor et al. 2014), with the exception of the density experiment, in which density was varied. At the start of each experiment wormlion larvae were placed simultaneously in the center of a restricted circular area within the arena (r = 5 cm, area = 78.5 cm2), located at the center of the larger circular tray. Since larval movement depends on sand presence, movement there was restricted to the sub-circle sand area. Larvae were then left undisturbed for 48 h to allow pit construction, and the trays were then photographed using a digital camera and sand was added to fill the entire tray (Figure 1). By adding this additional sand, we changed the density (from 17.83 × 10 − 2 to 2.85 × 10 − 2 individuals per cm2) and effectively opened up an unoccupied habitat to which the wormlion larvae could relocate (the area inhabitable was increased by five times). On each of the next 4 days, trays were photographed and changes in the number of constructed pits were noted. We measured three traits on the last day of each experiment: average number of pits constructed per individual, average distance from the tray center for each pit, and Nearest-Neighbor Index (hereafter, NNI, calculated as the ratio between the observed and expected distances to the nearest neighbors; Krebs 1999, chap. 4). NNI of one (i.e., identity between the observed and expected distances between individuals) represents a random spatial pattern, while lower or higher values than one indicate clumped or regular spatial patterns, respectively. In other words, in clumped spatial patterns the observed inter-individual distances are smaller than expected, while in regular spatial patterns the opposite holds true. Since wormlions may abandon an existing pit and construct a new one, we counted together both occupied and unoccupied pits and divided this by the number of larvae per tray (occupied pits can be recognized by visually identifying the larva at the end of the pit as a small black dot). Average number of pits constructed per individual and average distance from the center represent aspects of dispersal tendency. NNI reflects the level of intra-specific competition and perhaps other forces of attraction and repulsion. Measurements were based on photos using ImageJ (Abramoff et al. 2004). Following the experiments, all wormlions were released back to their original habitat.
Figure 1.
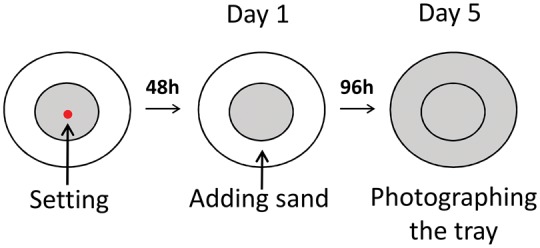
Experimental design illustrating the basic common model of all six manipulations. Red dot represents the center of the tray, where the wormlion larvae were simultaneously released 48 h prior to the manipulation. The gray coloration represents the area where sand was present, and the white represents areas clear of sand at this stage.
Biotic and abiotic factors
We experimentally manipulated three biotic and three abiotic factors and examined their effect on the spatial pattern, average number of pits constructed per individual, and distance from the arena center for each pit. Density and body mass had several levels (e.g., four density levels) with fewer replications per treatment group, while for feeding regime and abiotic factors there were two levels (e.g., starved vs. fed). Newly collected wormlions were used for each experiment.
Density: 240 wormlions were allocated to four density levels: 6, 12, 18, and 24 individuals per tray (four replicates for each level; mean body mass: 7.7 ± 4.0 mg; mean ± 1 SD; collected in December 2015).
Body mass: 224 wormlions were grouped according to body mass (mean body mass: 7.5 ± 4.4 mg; mean ± 1 SD; collected in January 2016), by clustering the heaviest 14 individuals of the collected wormlions in a tray, then the next heaviest 14 and so on until the lightest 14 individuals were clustered in a tray. This procedure formed trays with different average body mass and low variance of body mass within each tray (see Supplementary Material, Table S1).
Feeding regime: 224 wormlions were either starved for 2 weeks, or fed with a total of four flour beetle larvae per individual for the same period (eight replicates each; 14 larvae/tray; mean body mass: 8.1 ± 4.2 mg; mean ± 1 SD; see Supplementary Material Table S2; collected in July 2016).
Sand depth: 224 wormlions were placed in groups either in deep or shallow sand, 2 versus 0.5 cm, respectively (eight replicates each, 14 larvae/tray; mean body mass: 6.9 ± 3.6 mg; mean ± 1 SD; collected in April 2016).
Sand particle size: 224 wormlions were placed either in fine or coarse sand, with particle size smaller and larger than 106 mm, respectively (eight replicates each; 14 larvae/tray; mean body mass: 8.8 ± 4.1 mg; mean ± 1 SD; collected in March 2016).
Illumination: 224 wormlion larvae were placed in trays under illumination or dark conditions (eight replicates each; 14 larvae/tray; mean body mass: 7.4 ± 3.6 mg; mean ± 1 SD; collected in April 2016).
Statistical analyses
To determine whether the different levels of each examined factor influenced the spatial pattern and relocation tendency, we compared NNI values, average distance from the tray center, and average number of pits constructed per individual, calculated for the last day of the experiment (day 5) between the different levels, using linear regression analysis for density and body mass experiments, and t-test for the other four experiments. We present in the Supplementary Material an alternative analysis of the data using PCA on the three measured behavioral traits. The results are very similar (Supplementary Table S9 and Supplementary Figures S1–S3).
Results
All statistical results are summarized in Table 1. See Supplementary Material, Tables S3–S8, for descriptive statistics for body mass and all behavioral traits in each experiment.
Table 1.
The effect of density, body mass, feeding regime, sand depth, sand particle size and illumination on NNI, average distance from the center, and average number of pits constructed per individual
| Experiment | NNI | Distance from the center | Pits per individual |
|---|---|---|---|
| Density | t = 5.284, R2 = 0.666, | t = 4.711, R2 = 0.613, | t = -0.180, R2 = 0.002, |
| P < 0.001 | P < 0.001 | P = 0.860 | |
| Body mass | t = 2.670, R2 = 0.337, | t = 3.268, R2 = 0.433, | t = 4.199, R2 = 0.557, |
| P = 0.018 | P = 0.006 | P = 0.001 | |
| Feeding regime | t = 1.530, df = 14, | t = 1.085, df = 14, | t = 2.166, df = 14, |
| P = 0.148 | P = 0.296 | P = 0.048 | |
| Sand depth | t = −0.280, df = 14, | t = −3.248, df = 14, | t = −5.661, df = 14, |
| P = 0.784 | P = 0.006 | P < 0.001 | |
| Sand particle size | t = −1.217, df = 14, | t = 0.3931, df = 14, | t = −1.674, df = 14, |
| P = 0.244 | P = 0.700 | P = 0.116 | |
| Illumination | t = 0.773, df = 14, | t = 0.408, df = 14, | t = −0.134, df = 14, |
| P = 0.452 | P = 0.690 | P = 0.896 |
Notes: Results of linear regression are presented for density and body mass factors and t-test for all other factors. Significant results are in bold.
Biotic factors
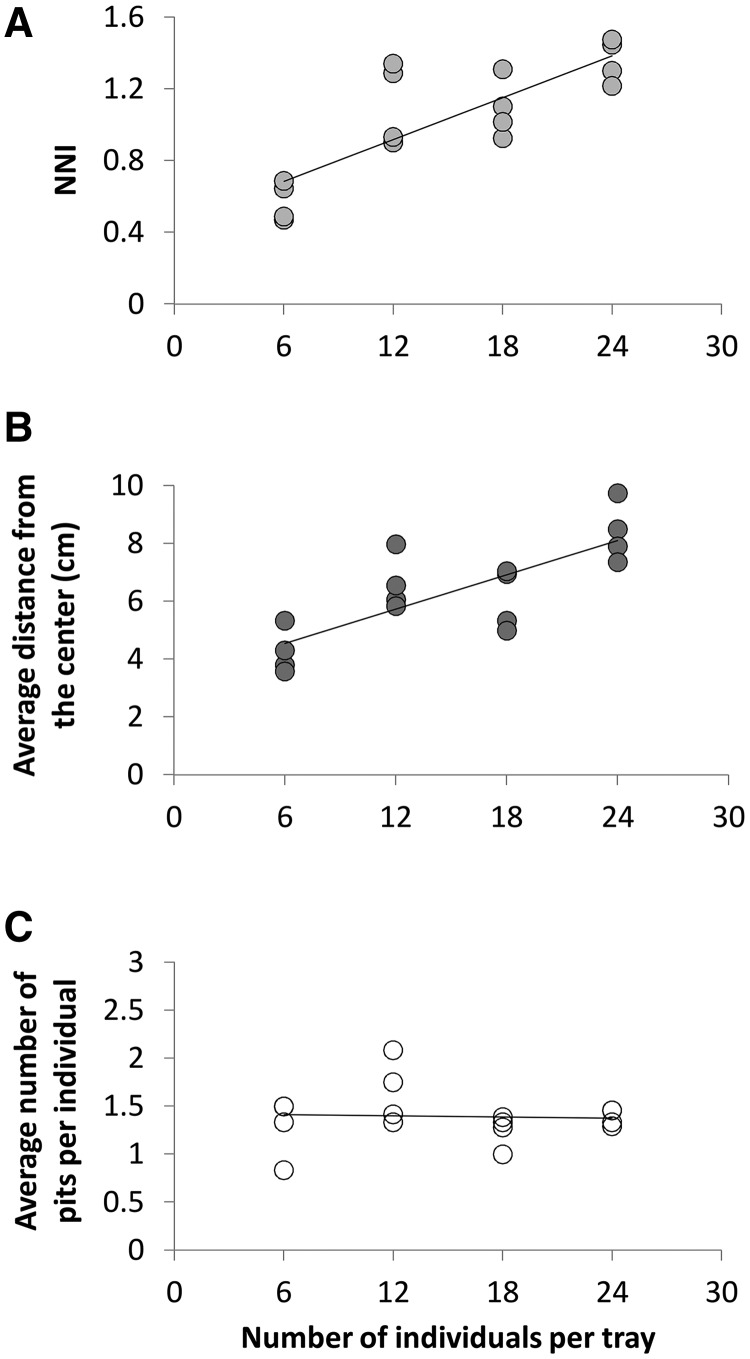
Density: Increased density led to larger NNI values and a more regular spatial pattern, reflected in larger distances between neighbors, as well as larger average distance from the tray center. The number of constructed pits per individual, however, was not affected by density (Figure 2).
Figure 2.
The positive effect of wormlion density (number of individuals/490.6 cm2) on (A) the NNI, and (B) the average distance from the tray center. (C) Wormlion density had no effect on the average number of pits constructed per individual.
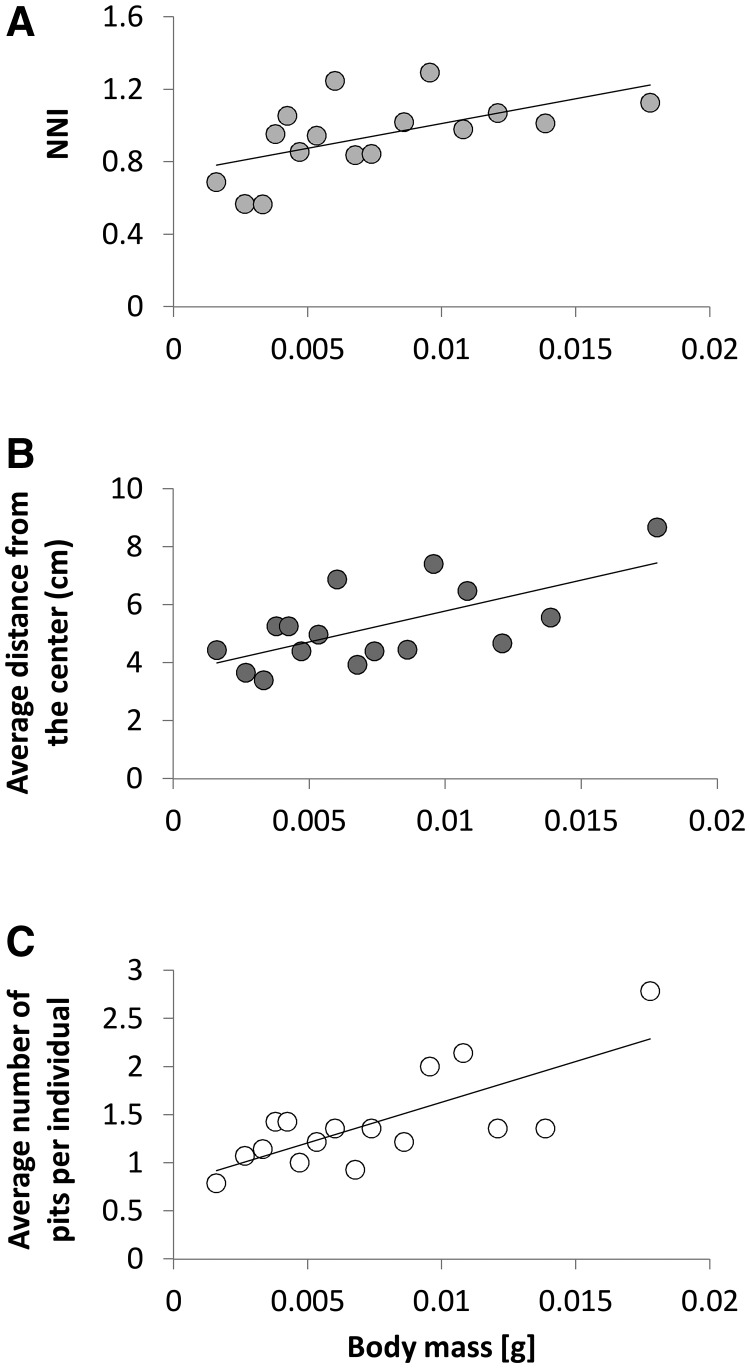
Body mass: With increasing average body mass in the tray, the spatial pattern became more regular, wormlions were positioned closer to the tray edge, and more pits were constructed per individual (Figure 3).
Figure 3.
The positive effect of body mass on (A) NNI, (B) average distance from the tray center, and (C) the average number of pits constructed per individual.
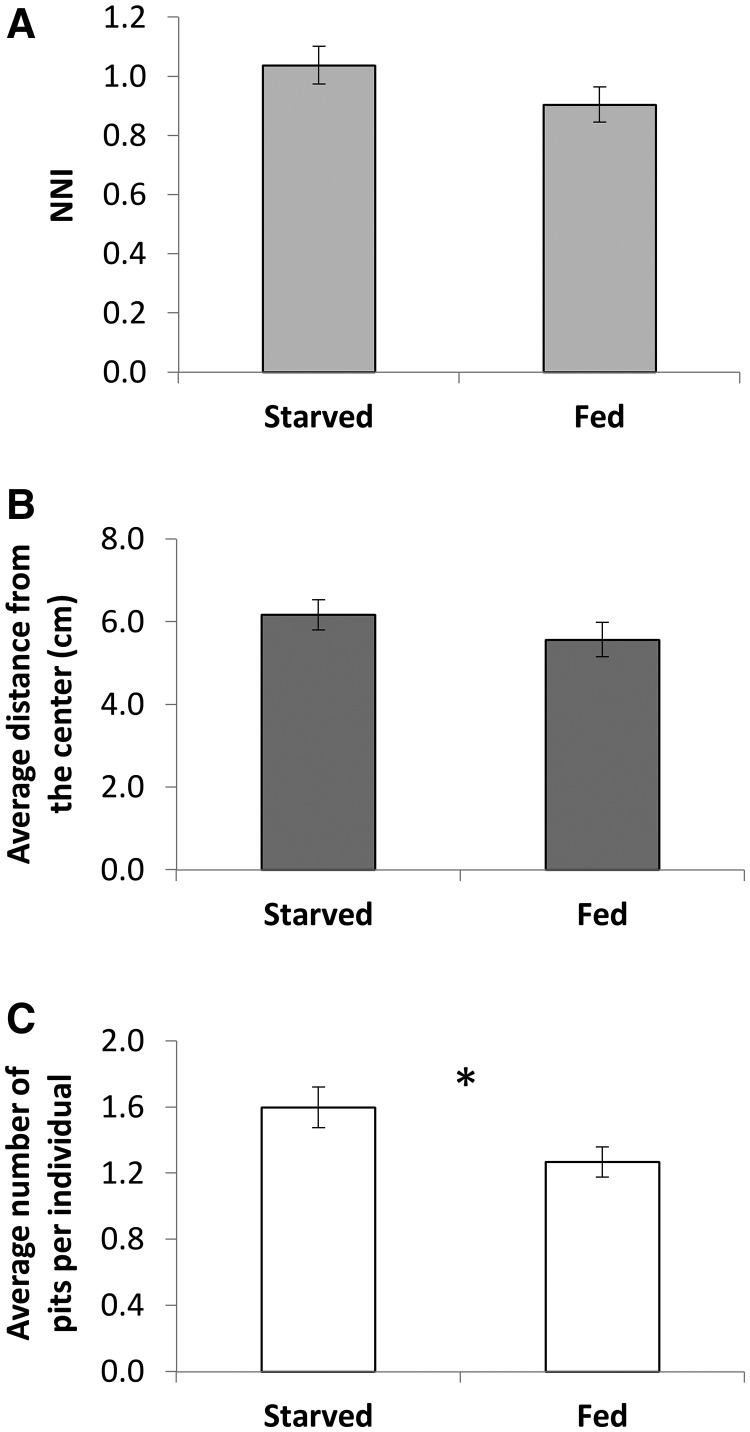
Feeding regime: Starved individuals constructed more pits on average than well-fed individuals. There was nevertheless no effect of feeding regime on NNI or the average distance from the center (Figure 4).
Figure 4.
There was no effect of feeding regime (fed vs. starved) on (A) the NNI, and (B) the average distance from the tray center. (C) Starved individuals constructed on average more pits than fed ones.
Abiotic factors
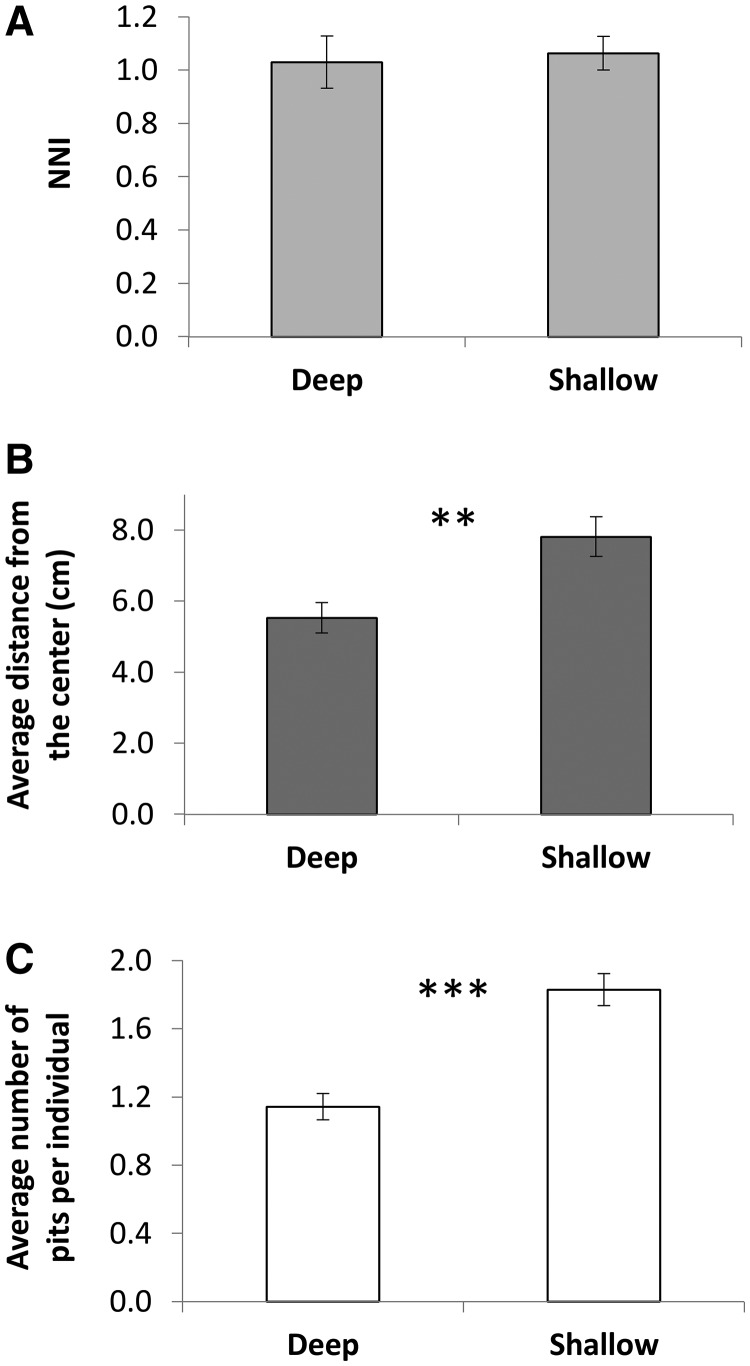
Sand depth: In shallow sand compared with deep sand, wormlions were more frequently positioned close to the tray edge and constructed more pits. However, there was no difference between shallow and deep sand in NNI values (Figure 5).
Figure 5.
Sand depth had (A) no effect on the NNI. Shallow sand led to (B) larger average distances from the center, and (C) more pits constructed per individual.
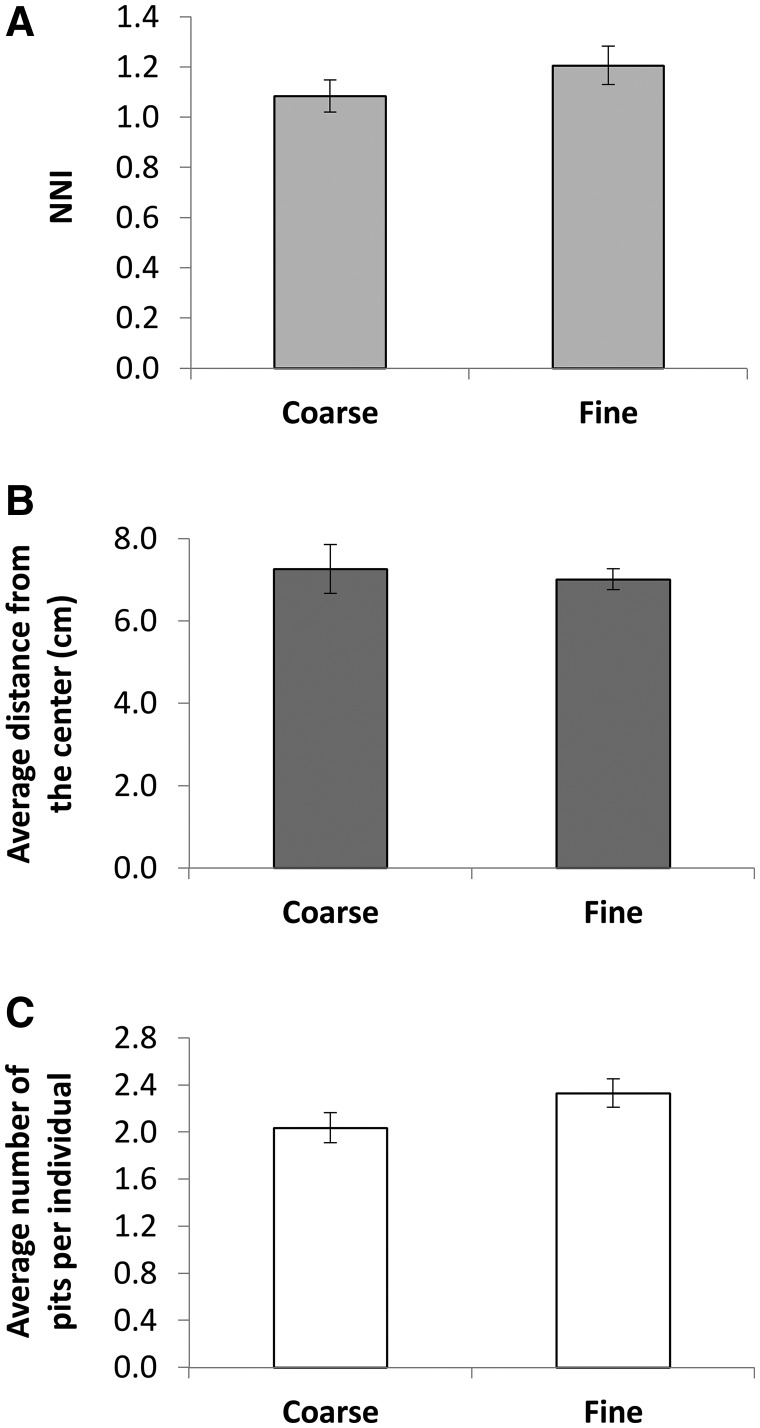
Sand particle size: Sand particle size had no effect on any of the three traits measured (NNI, distance from the tray center, and average number of pits constructed per individual; Figure 6).
Figure 6.
Sand particle size had no effect on any of the measured behavioral traits.
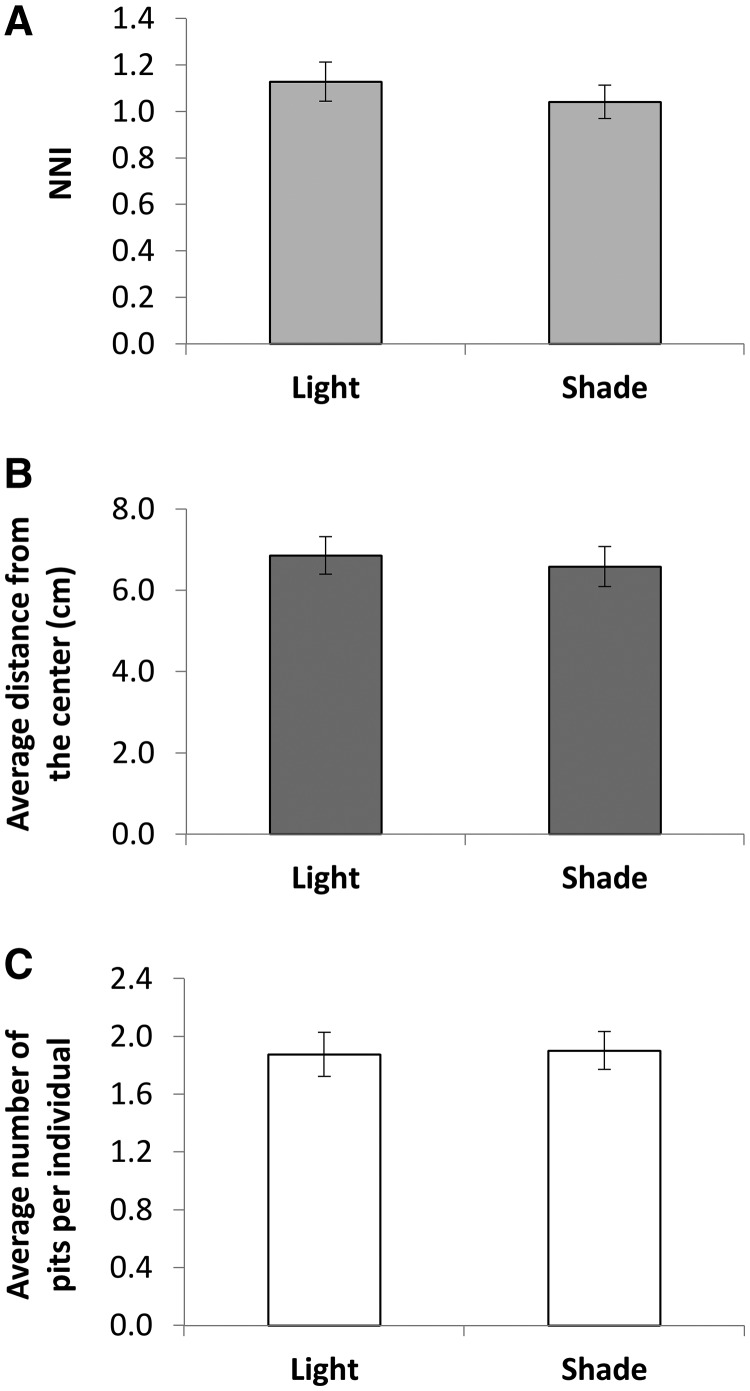
Illumination: There was no effect of illumination on any of the three traits measured (Figure 7).
Figure 7.
Illumination had no effect on any of the measured behavioral traits.
Discussion
According to our prediction, the spatial pattern (clumped, random, or regular), distance from the center of the arena, and relocation tendency were all impacted by a variety of biotic and abiotic factors: poor conditions led to more relocations and a more regular spatial pattern. Only partially supporting our prediction, the spatial pattern was solely affected by biotic factors while the distance from center and relocation tendency were affected by both biotic and abiotic factors. Under the experimental conditions, when an effect was detected, unfavorable conditions led to more regular spatial patterns, increased distance from the center of the arena (or movement), and a higher average number of pits constructed per individual. Together, these results suggest that intraspecific competition and dispersal tendency intensify under unfavorable conditions. Other animals, such as a sweat bee species, a golden silk spider, and white-footed mice, all showed a similar trend, with favorable habitat conditions promoting higher tolerance of intraspecific neighbors, resulting in higher local density (Rypstra 1985; Potts and Willmer 1997; Anderson et al. 2003). In the present study, biotic factors were more often significant in their effect on the measured traits, opposing our prediction. For example, NNI, representing the level of intra-specific competition, was solely influenced by biotic factors (density and body mass). This suggests that wormlions are more dependent on such biotic factors than are the ecologically similar antlions.
Biotic factors
The spatial pattern often reflects the nature of between-individual interactions and dispersal ability (Svendsen 1974; Fangliang et al. 1997; Henschel and Lubin 1997; Day and Zalucki 2000). Clumped spatial patterns stem from environmental heterogeneity and attraction to favorable conditions or clumped resources, positive social interactions, and dilution of predation risk (e.g., Turchin and Kareiva 1989; Jeanson et al. 2005; Van Wilgenburg and Elgar 2007). In contrast, a shift to more regular dispersion patterns is expected when the intraspecific competition or mutual disturbance are strong (e.g., Phillips and MacMahon 1981; Matsura and Takano 1989; Birkhofer et al. 2006). Body mass and conspecific density affected the spatial pattern here, suggesting that negative social interactions are responsible for the wormlions demonstrating a more regular dispersion pattern. In other words, neighbors are tolerated if they are small or not adjacent. Such negative interactions could be mediated through behaviors like sand-throwing by neighbors, which requires more frequent maintenance of the pits, or perceived intraspecific competition for food, as shown in other trap-building predators, antlions, and spiders, respectively (Lubin et al. 2001; Scharf and Ovadia 2006). Indeed, smaller antlions could be more easily tolerated because they construct smaller pits and consequently perhaps throw less sand and cause lower disturbance to neighbors (Griffiths 1991; Scharf et al. 2009).
The average number of pits constructed per individual and average distance from center of the arena both reflect wormlion tendency to disperse from the current habitat and can serve as proxies of the adequacy of local conditions (Bertness and Grosholz 1985). All biotic factors affected dispersal. High density is known in many other animals to affect dispersal tendency (Bowler and Benton 2005). As noted, large body mass induced dispersal in the present system. Although smaller, less competitive individuals are probably more strongly affected by competition, it could be that larger individuals have a higher chance than smaller ones to reach a more suitable habitat, or that dispersal for them is less risky, costly, or triggered by aggressiveness, that is expressed more often by larger individuals (Janson 1985; Bowler and Benton 2005). This could be supported by marking and following individuals of different body size and test whether there is a link between position in the microhabitat and body size. Larger neighbors may construct larger pits and consume more prey, resulting in more frequent disturbance by sand-throwing (Griffiths 1991). Interestingly, the opposite trend has been shown to hold true for antlions, with larger larvae relocating less frequently than smaller ones (Griffiths 1993; Scharf et al. 2008). Larger or more competitive individuals are generally those that hold high-quality sites and disperse less frequently than smaller or less competitive individuals. This has been shown, for example, in the red flour beetle with dispersers being of lower competitive ability than non-dispersers (Zirkle et al. 1988).
Starvation often reduces the perceived site quality and leads to dispersal in general (e.g., Torres et al. 2002; Oku 2010), and relocation in trap-building predators (Heinrich and Heinrich 1984; Rosenberg 1987; Eltz 1997). For individual antlions, relocation takes place more quickly when the cessation of prey arrival is abrupt rather than gradual (Jenkins 1994), but it remains to be tested as to whether group dynamics are similar across species. Similar to our experiments, Rosenberg (1987) demonstrated that in a pit-building antlion species, starvation led to more relocations than abiotic conditions (illumination conditions and sand particle size). Most trap-building predators relocate their traps when prey is scarce (Riechert 1992; Scharf and Ovadia 2006) and wormlions are not exceptional.
Abiotic factors
Abiotic factors had little effect on all measured traits. Nevertheless, shallow sand had a clear effect in inducing dispersal, measured as both number of pits constructed and the distance from the center. Antlions, similarly, construct smaller pits in shallow sand and try to relocate away of this unfavorable microhabitat (Loria et al. 2008; Scharf et al. 2008). Sand particle size and illumination had no effect on any of the measured traits. The lack of effect of these two latter factors is surprising, as wormlion larvae are known to prefer fine sand particle size and shady habitats when given a simple choice (Devetak and Arnett 2015; Adar et al. 2016; Katz et al. 2016). We posit two explanations for these outcomes. First, wormlions were not given a choice of different conditions in this experiment. Here, we merely provided individuals with more area for dispersal and exploration, while in other experiments a choice was given prior to pit construction and initial habitat choice. Thus, wormlions could decide there between two different microhabitats when placed exactly in the middle of an arena (cf. our design vs. Devetak 2008; Adar et al. 2016). It is also possible that sensory limitations of external cues compared with the more immediate effect of hunger and body mass might explain some of the difference between the effects of biotic versus abiotic factors. For example, illumination in wormlions is perceived via their very small eyes (relative to other larvae insects, as antlions; Wheeler 1930, chap. 5), while conspecifics and sand traits are sensed by vibrations in sand, similar to antlions (Devetak et al. 2007). Second, it is possible that sand particle size has an effect only at a finer temporal scale, with the successive construction stages lasting for different periods of time under fine and coarse sand (Klokočovnik et al. 2012).
Wormlions should take into account the possible costs and benefits of relocating their pits. In trap-building predators, the cost of relocating the trap after its construction as well as the danger involved in movement should be traded-off against the likelihood of settling in a more favorable location (e.g., Lubin et al. 1993). Because we have no reason to assume that the likelihood of detecting a better location or predation risk was differently perceived among the experiments, we suggest that the costs for the wormlions to disperse in response to different factors might have varied, potentially leading to relocations only under specific conditions.
We suggest two explanations for the lack of abiotic effects. First, the unfavorable abiotic conditions applied here might not have been stressful enough. Second, testing spatial pattern on different spatial scales might have generated different outcomes. For example, spiders and forest trees are regularly distributed on a local scale due to local competition (e.g., negative density dependence mechanisms), while the same species exhibited a clumped spatial pattern due to abiotic constraints on larger spatial scales (Szwagrzyk and Czerwczak 1993; Birkhofer et al. 2007). The same could hold true for wormlions. Furthermore, testing wormlions on different temporal scales might lead to different results, as they could either acclimate to unfavorable conditions or alternatively increase dispersal rate, as they accumulate more information on the unsuitability of the current habitat. Future studies should test possible interactions among the studied factors, as multiple stressors can have a non-additive and surprising effect on behavior. It is also important to repeat our experiments under different spatial and temporal scales.
Wormlions versus antlions
Both antlions and wormlions depend on the substrate in their habitat to construct traps. Therefore, we expected that similar to antlions, the effect of abiotic factors on individual position and relocation would be strong (Scharf and Ovadia 2006; Scharf et al. 2008; Devetak and Arnett 2015). Both taxa demonstrate a striking example of convergent evolution due to their similar foraging strategy (hunting small arthropods using pit-traps constructed in loose soil). To a casual observer, their lifestyles appear indistinguishable. The study here, however, highlights an upper limit to their apparent similarities. Based on the already known differences between the two groups, that is, the propensity to cannibalize (antlions are highly cannibalistic while wormlions are not), and the effect of body mass on pit size (much stronger in antlions; cf. Scharf et al. 2008; Dor et al. 2014), we expected wormlions to respond more to abiotic factors than to biotic ones. We were therefore surprised that our prediction was not supported. We suggest that further studies should examine the extent to which behavioral rules vary among apparently similar species or species with similar foraging strategies. Only by doing so will we begin to construct a more cohesive understanding of how species’ ecology, behavior, and evolutionary history combine to mold patterns in habitat decision-making rules.
Funding
The authors are grateful to the US-Israel Binational Science Foundation [No. 2013086 to I.S. and J.N.P.] and to the People Programme (Marie Curie Actions) of the European Union’s Seventh Framework Programme (FP7/2007-2013) under REA grant agreement [No. 333442] for funding this study. This manuscript is part of N.K.’s PhD thesis.
Supplementary material
Supplementary material can be found at http://www.cz.oxfordjournals.org/.
Supplementary Material
References
- Abramoff MD, Magalhaes PJ, Ram SJ, 2004. Image processing with Image. J. Biophotonics Int 11:36–42. [Google Scholar]
- Adar S, Dor R, Scharf I, 2016. Habitat choice and complex decision making in a trap-building predator. Behav Ecol 27:1491–1498. [Google Scholar]
- Anderson CS, Cady AB, Meikle DB, 2003. Effects of vegetation structure and edge habitat on the density and distribution of white-footed mice Peromyscus leucopus in small and large forest patches. Can J Zool 81:897–904. [Google Scholar]
- Belovsky GE, 1981. Optimal activity times and habitat choice of moose. Oecologia 48:22–30. [DOI] [PubMed] [Google Scholar]
- Benard MF, McCauley SJ, 2008. Integrating across life‐history stages: consequences of natal habitat effects on dispersal. Am Nat 171:553–567. [DOI] [PubMed] [Google Scholar]
- Bertness MD, Grosholz E, 1985. Population dynamics of the ribbed mussel Geukensia demissa: the costs and benefits of an aggregated distribution. Oecologia 67:192–204. [DOI] [PubMed] [Google Scholar]
- Birkhofer K, Henschel JR, Scheu S, 2006. Spatial-pattern analysis in a territorial spider: evidence for multi-scale effects. Ecography 29:641e648. [Google Scholar]
- Birkhofer K, Scheu S, Wise DH, 2007. Small-scale spatial pattern of web-building spiders (Araneae) in alfalfa: relationship to disturbance from cutting, prey availability, and intraguild interactions. Environ Entomol 36:801–810. [DOI] [PubMed] [Google Scholar]
- Bowler DE, Benton TG, 2005. Causes and consequences of animal dispersal strategies: relating individual behaviour to spatial dynamics. Biol Rev 80:205–225. [DOI] [PubMed] [Google Scholar]
- Clobert J, Galliard L, Cote J, Meylan S, Massot M, 2009. Informed dispersal, heterogeneity in animal dispersal syndromes and the dynamics of spatially structured populations. Ecol Lett 12:197–209. [DOI] [PubMed] [Google Scholar]
- Cushman JH, Martinsen GD, Mazeroll AI, 1988. Density- and size-dependent spacing of ant nests: evidence for intraspecific competition. Oecologia 77:522–525. [DOI] [PubMed] [Google Scholar]
- Day MD, Zalucki MP, 2000. Effect of density on spatial distribution, pit formation and pit diameter of Myrmeleon acer Walker (Neuroptera: Myrmeleontidae): patterns and processes. Austral Ecol 25:58–64. [Google Scholar]
- Devetak D, Mencinger-Vračko B, Devetak M, Marhl M, Špernjak A, 2007. Sand as a medium for transmission of vibratory signals of prey in antlions Euroleon nostras (Neuroptera: Myrmeleontidae). Physiol Entomol 32:268–274. [Google Scholar]
- Devetak D, 2008. Substrate particle size-preference of wormlion Vermileo vermileo (Diptera: Vermileonidae) larvae and their interaction with antlions. Eur J Entomol 105:631–635. [Google Scholar]
- Devetak D, Arnett AE, 2015. Preference of antlion and wormlion larvae (Neuroptera: Myrmeleontidae; Diptera: Vermileonidae) for substrates according to substrate particle sizes. Eur J Entomol 112:500–509. [Google Scholar]
- Dor R, Rosenstein S, Scharf I, 2014. Foraging behaviour of a neglected pit-building predator: the wormlion. Anim Behav 93:69–76. [Google Scholar]
- Eltz T, 1997. Foraging in the ant-lion Myrmeleon mobilis hagen 1888 (neuroptera: Myrmeleontidae): behavioral flexibility of a sit-and-wait predator. J Insect Behav 10:1–11. [Google Scholar]
- Fangliang H, Legendre P, LaFrankie JV, 1997. Distribution patterns of tree species in a Malaysian tropical rain forest. J Veg Sci 8:105–114. [Google Scholar]
- Febrer K, Jones TA, Donnelly CA, Dawkins MS, 2006. Forced to crowd or choosing to cluster? Spatial distribution indicates social attraction in broiler chickens. Anim Behav 72:1291–1300. [Google Scholar]
- Griffiths D, 1991. Intraspecific competition in larvae of the ant‐lion Morter sp. and interspecific interactions with Macroleon quinquemaculatus. Ecol Entomol 16:193–201. [Google Scholar]
- Griffiths D, 1993. Intraspecific competition in ant-lion Macroleon quinquemaculatus larvae in the field. Oecologia 93:531–537. [DOI] [PubMed] [Google Scholar]
- Halliday WD, Blouin-Demers G, 2014. Red flour beetles balance thermoregulation and food acquisition via density-dependent habitat selection. J Zool 294:198–205. [Google Scholar]
- Harrison S, 1989. Long‐distance dispersal and colonization in the bay checkerspot butterfly Euphydryas editha Bayensis. Ecology 70:1236–1243. [Google Scholar]
- Heinrich B, Heinrich MJE, 1984. The pit-trapping foraging strategy of the antlion Myrmeleon immaculatus DeGeer (Neuroptera: Myrmeleontidae). Behav Ecol Sociobiol 14:151–160. [Google Scholar]
- Henschel JR, Lubin YD, 1997. A test of habitat selection at two spatial scales in a sit-and-wait predator: a web spider in the Namib desert dunes. J Anim Ecol 66:401–413. [Google Scholar]
- Herberstein ME, Fleisch AF, 2003. Effect of abiotic factors on the foraging strategy of the orb‐web spider Argiope keyserlingi (Araneae: Araneidae). Austral Ecol 28:622–628. [Google Scholar]
- Hirsch BT, 2011. Within-group spatial position in ring-tailed coatis: balancing predation, feeding competition, and social competition. Behav Ecol Sociobiol 65:391–399. [Google Scholar]
- Huey RB, Carlson M, Crozier L, Frazier M, Hamilton H. et al. , 2002. Plants versus animals: do they deal with stress in different ways? Integr Comp Biol 42:415–423. [DOI] [PubMed] [Google Scholar]
- Jackson DA, Peres-Neto PR, Olden JD, 2001. What controls who is where in freshwater fish communities the roles of biotic, abiotic, and spatial factors. Can J Fish Aquat Sci 58:157–170. [Google Scholar]
- Janson C, 1985. Aggressive competition and individual food consumption in wild brown capuchin monkeys Cebus apella. Behav Ecol Sociobiol 18:125–138. [Google Scholar]
- Jeanson R, Rivault C, Deneubourg JL, Blanco S, Fournier R. et al. , 2005. Self-organized aggregation in cockroaches. Anim Behav 69:169–180. [Google Scholar]
- Jenkins BA, 1994. The behavioural response of the antlion Myrmeleon pictifrons to a sudden change in prey capture rate. Acta Oecol 15:231–240. [Google Scholar]
- Juell JE, Fernö A, Furevik D, Huse I, 1994. Influence of hunger level and food availability on the spatial distribution of Atlantic salmon Salmo salar L. in sea cages. Aquac Res 25:439–451. [Google Scholar]
- Katz N, Subach A, Pruitt J, Scharf I, 2016. Habitat preference of wormlions and their behavioral repeatability under illumination/shade conditions. Ecol Entomol 41:716–726. [Google Scholar]
- Klokočovnik V, Devetak D, Orlačnik M, 2012. Behavioral plasticity and variation in pit construction of antlion larvae in substrates with different particle sizes. Ethology 118:1102–1110. [Google Scholar]
- Krause J, Ruxton GD, 2002. Living in Groups. Oxford: Oxford University Press. [Google Scholar]
- Krebs CJ, 1999. Ecological Methodology. 2nd edn. New York: Addison-Wesley Educational Publishers. [Google Scholar]
- Loria R, Scharf I, Subach A, Ovadia O, 2008. The interplay between foraging mode, habitat structure, and predator presence in antlions. Behav Ecol Sociobiol 62:1185–1192. [Google Scholar]
- Lubin Y, Ellner S, Kotzman M, 1993. Web relocation and habitat selection in desert widow spider. Ecology 74:1915–1928. [Google Scholar]
- Lubin Y, Henschel JR, Baker MB, 2001. Costs of aggregation: shadow competition in a sit‐and‐wait predator. Oikos 95:59–68. [Google Scholar]
- Martin TE, 2001. Abiotic vs. biotic influences on habitat selection of coexisting species: climate change impacts?. Ecology 82:175–188. [Google Scholar]
- Matsura T, Takano H, 1989. Pit-relocation of antlion larvae in relation to their density. Res Popul Ecol 31:225–234. [Google Scholar]
- Okamura B, 1986. Group living and the effects of spatial position in aggregations of Mytilus edulis. Oecologia 69:341–347. [DOI] [PubMed] [Google Scholar]
- Oku K, 2010. Males of the two-spotted spider mite attempt to copulate with mated females: effects of double mating on fitness of either sex. Exp Appl Acarol 50:107–113. [DOI] [PubMed] [Google Scholar]
- Orians GH, Wittenberger JF, 1991. Spatial and temporal scales in habitat selection. Am Nat 137:S29–S49. [Google Scholar]
- Perfecto I, Vandermeer J, 2008. Spatial pattern and ecological process in the coffee agroforestry system. Ecology 89:915–920. [DOI] [PubMed] [Google Scholar]
- Phillips DL, MacMahon JA, 1981. Competition and spacing patterns in desert shrubs. J Ecol 69:97–115. [Google Scholar]
- Potts S, Willmer PAT, 1997. Abiotic and biotic factors influencing nest‐site selection by Halictus rubicundus, a ground‐nesting halictine bee. Ecol Entomol 22:319–328. [Google Scholar]
- Price N, 2010. Habitat selection, facilitation, and biotic settlement cues affect distribution and performance of coral recruits in French Polynesia. Oecologia 163:747–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruitt JN, DiRienzo N, Kralj-Fišer S, Johnson JC, Sih A, 2011. Individual- and condition-dependent effects on habitat choice and choosiness. Behav Ecol Sociobiol 65:1987–1995. [Google Scholar]
- Riechert SE, 1992. Spiders as representative ‘sit-and-wait’ predators In: Crowley MJ, editor. Natural Enemies: The Population Biology of Predators, Parasites and Diseases. Oxford: Blackwell Scientific Publications, 313–328. [Google Scholar]
- Ronce O, 2007. How does it feel to be like a rolling stone? Ten questions about dispersal evolution. Annu Rev Ecol Evol Syst 38:231–253. [Google Scholar]
- Rosenberg RH, 1987. Pit dispersion in antlion larvae (Neuroptera: Myrmeleontidae): is competition important? FlaEntomol 70:175–178. [Google Scholar]
- Rypstra A, 1985. Aggregations of Nephila clavipes (L.) (Araneae, Araneidae) in relation to prey availability. J Arachnol 13:71–78. [Google Scholar]
- Santini G, Ramsay PM, Tucci L, Ottonetti L, Frizzi F, 2011. Spatial patterns of the ant Crematogaster scutellaris in a model ecosystem. Ecol Entomol 36:625–634. [Google Scholar]
- Scharf I, Ovadia O, 2006. Factors influencing site abandonment and site selection in a sit-and-wait predator: a review of pit-building antlion larvae. J Insect Behav 19:197–218. [Google Scholar]
- Scharf I, Hollender Y, Subach A, Ovadia O, 2008a. Effect of spatial pattern and microhabitat on pit construction and relocation in Myrmeleon hyalinus (Neuroptera: Myrmeleontidae) larvae. Ecol Entomol 33:337–345. [Google Scholar]
- Scharf I, Subach A, Ovadia O, 2008b. Foraging behaviour and habitat selection in pit-building antlion larvae in constant light or dark conditions. Anim Behav 76:2049–2057. [Google Scholar]
- Scharf I, Golan B, Ovadia O, 2009. The effect of sand depth, feeding regime, density, and body mass on the foraging behaviour of a pit‐building antlion. Ecol Entomol 34:26–33. [Google Scholar]
- Scharf I, Fischer-Blass B, Foitzik S, 2011. Spatial structure and nest demography reveal the influence of competition, parasitism and habitat quality on slavemaking ants and their hosts. BMC Ecol 11:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharf I, Ovadia O, Fotzik S, 2012. The advantage of alternative tactics of prey and predators depends on the spatial pattern of prey and social interactions among predators. Popul Ecol 54:187–196. [Google Scholar]
- Scharf I, Daniel A, MacMillan HA, Katz N, 2016. The effect of fasting and body reserves on cold tolerance in 2 pit-building insect predators. Curr Zool 63:287–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svendsen GE, 1974. Behavioral and environmental factors in the spatial distribution and population dynamics of a yellow‐bellied marmot population. Ecology 55:760–771. [Google Scholar]
- Szwagrzyk J, Czerwczak M, 1993. Spatial patterns of trees in natural forests of East‐Central Europe. J Veg Sci 4:469–476. [Google Scholar]
- Torres JB, Evangelista WS, Barras R, Guedes RNC, 2002. Dispersal of Podisus nigrispinus (Het., Pentatomidae) nymphs preying on tomato leafminer: effect of predator release time, density and satiation level. J Appl Entomol 126:326–332. [Google Scholar]
- Turchin P, Kareiva P, 1989. Aggregation in Aphis varians: an effective strategy for reducing predation risk. Ecology 70:1008–1016. [Google Scholar]
- Van Wilgenburg E, Elgar MA, 2007. Colony structure and spatial distribution of food resources in the polydomous meat ant Iridomyrmex purpureus. Insect Soc 54:5–10. [Google Scholar]
- Watson DM, Roshier DA, Wiegand T, 2007. Spatial ecology of a root parasite: from pattern to process. Austral Ecol 32:359–369. [Google Scholar]
- Wheeler W, 1930. Demons of the Dust. New York: Norton and Company Inc. Publishers. [Google Scholar]
- Zirkle DF, Dawson PS, Lavie B, 1988. An experimental analysis of the genetic relationships among life-history traits and emigration behavior in Tribolium castaneum. Oikos 53:391–397. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.