Abstract
Processes of adaptation to urban environments are well described for relatively few avian taxa, mainly passerines, but selective forces responsible for urban colonization in ecologically different groups of birds remain mostly unrecognized. The aim of this article is to identify drivers of recent urban colonization (Łódź, central Poland) by a reed-nesting waterbird, the Eurasian coot Fulica atra. Urban colonizers were found to adopt a distinct reproductive strategy by maximizing the number of offspring (carryover effects of higher clutch size), whereas suburban individuals invested more in the quality of the progeny (higher egg volume), which could reflect differences in predatory pressure between 2 habitats. In fact, reduced predation rate was strongly suggested by elevated hatching success in highly urbanized areas, where probability of hatching at least 1 chick was higher by 30% than in suburban natural-like habitats. Coots nesting in highly urbanized landscape had considerably higher annual reproductive success in comparison to suburban pairs, and the difference was 4-fold between the most and least urbanized areas. There was also a constant increase in size-adjusted body mass and hemoglobin concentration of breeding coots from the suburbs to the city centre. Urban colonization yielded no survival benefits for adult birds and urban individuals showed higher site fidelity than suburban conspecifics. The results suggest that the recent urban colonization by Eurasian coots was primary driven by considerable reproductive benefits which may be primarily attributed to: (1) reduced predation resulting from an exclusion of most native predators from highly urbanized zones; (2) increased condition of urban-dwelling birds resulting from enhanced food availability.
Keywords: adult survival, capture–recapture analysis, Eurasian coot, Fulica atra, hemoglobin concentration, reproductive success, urbanization
Rapid urbanization is a global phenomenon. Total urban area continues to expand, driven by urban population growth in developing nations and low population density of newly urbanized areas in the developed world (Liu et al. 2003). The recent projections show an increase in urban population to nearly 5 billion by 2030 and the global urban land is expected to triple by this time (Seto et al. 2012). Urban expansion and associated land-cover change is known to threaten biodiversity via habitat loss or fragmentation (McKinney 2008). Urbanization is currently considered one of the leading causes of species extinction (McKinney 2006) and is predicted to entail a serious biodiversity degradation and biotic homogenization in the nearest future (McKinney 2006; McDonald et al. 2008). Despite this, an increasing number of species adapt to human-dominated landscapes and become dependent on urban resources (Kark et al. 2007).
An adaptation to urban life may carry a wide spectrum of fitness-related benefits for birds and other animals. Due to decreased species diversity in urban environments, colonizers may experience relaxed interspecific competition, especially from specialist species which are often excluded from urban faunas (Filippi-Codaccioni et al. 2009). Intraspecific competition may also be reduced in urban populations of birds, for example, transition to nesting on anthropogenic structures may sometimes provide virtually unlimited source of nesting sites (Sumasgutner et al. 2014; Wang et al. 2015). Other benefits of urban life include increased temperature, which buffers seasonal variation and extends breeding season within urban heat islands (Tryjanowski et al. 2013). Human activities in urban landscape also elevate local productivity relative to surrounding wildland, while urban management strategies often make spatially and temporally patchy resources more continuously available to animals (Shochat et al. 2006). Finally, urban populations are hypothesized to experience reduced predatory pressure, following an observation that many natural predators avoid built-up areas or are persecuted by humans (Adams 1994; Gering and Blair 1999). However, the generality of all these patterns is under question, since a large body of research on the processes of urban colonization by birds is limited to very few taxonomic groups, mainly passerines (reviewed in Chamberlain et al. 2009; but see Mannan et al. 2008; Rutz 2008; Sumasgutner et al. 2014). It has also been argued that factors driving colonization events may vary greatly between sites, much depending on local conditions, for example, on the composition of predatory species (Tomiałojć 1978; Eden 1985; Sasvári et al. 1995; Gering and Blair 1999; Jokimäki and Huhta 2000; Thorington and Bowman 2003).
As predation is usually a major determinant of nesting success in ground- and reed-nesting waterfowl (Klett et al. 1988; Pieron and Rohwer 2010), it could be expected, that urban populations of waterbirds are likely to primarily benefit from reduced brood losses resulting from the scarcity of natural (mainly mammalian) predators. Second, much better access to artificial food resources (due to popular public feeding of waterbirds) can exert a positive impact on the nutritional condition of adults and young, thus increasing their survival and annual reproductive output. On the other hand, urban nesting sites of waterbirds are usually much less attractive in terms of habitat characteristics. Tall emergent vegetation is often scarce at artificial urban ponds (Traut and Hostetler 2003), thus limiting possibilities for effective nest concealment and increasing probability of egg predation by natural urban predators (e.g., corvids) or non-natural predators (e.g., dogs). As in other animals, urban colonization is also likely to increase levels of stress resulting from many novel anthropogenic perturbations, such as the permanent presence of humans or noise and light pollution (Partecke et al. 2006). This may cause deleterious effects on physiological and reproductive performance, at least unless colonizers adjust their stress response to the urban conditions.
The aim of this article was to identify selective forces that may drive the processes of urban colonization in reed-nesting waterbirds. For this purpose, I have chosen to study a recently established urban population of the Eurasian coot Fulica atra, a common waterbird that nests in emergent shore vegetation on a wide range of still or slow-flowing waters (Snow and Perrins 1998). The mechanisms which regulate processes of urban colonization are likely to be very complex and difficult to disentangle while focusing the analyses solely on basic reproductive traits. Thus, in this study, I collected data on a broad range of fitness-related components of Eurasian coots providing a thorough insight into the process of urban invasion. Except for assessing different measures of reproductive performance, I used capture–recapture models to analyse adult survival and evaluated how urbanization affected physiological condition of birds. The latter analysis was based on the measurements of the whole-blood hemoglobin concentration, which is the most important determinant of oxygen-carrying capacity of blood in vertebrates (Hawkey et al. 1991). Hemoglobin concentration is considered a relatively robust indicator of physiological state in birds (reviewed in Minias 2015a), reflecting not only nutritional condition and the quality of diet (Pryke and Rollins 2012; Pryke et al. 2012), but also the level of infestation with parasites (Norte et al. 2013) and air pollution (Herrera-Dueñas et al. 2014).
Materials and Methods
Process of urbanization and study area
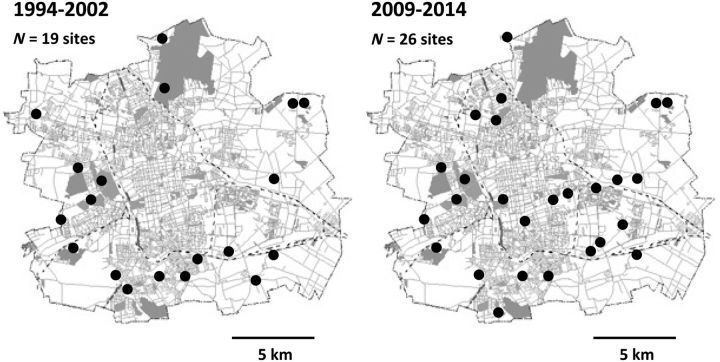
The process of urban colonization by the Eurasian coot was studied in the city of Łódź (51° 46′ N, 19° 28′ E; 293 km2, 708,500 inhabitants), central Poland. During the city-wide bird surveys made from 1994 to 2002 breeding attempts of Eurasian coots were recorded at 19 different waterbodies or pond complexes located in the outer zones of the city (Janiszewski et al. 2009) with the mean distance of 6.8 ± 0.5 km (range: 3.3–10.9 km) to the city centre (Figure 1). All nesting sites were located in the areas of low or moderate urbanization level, with the mean share of built-environment area within 200 m from the shoreline of 22.3 ± 3.7% (range: 0–54.9%). The surveys conducted in 2009–2014 indicated that Eurasian coots have colonized 13 waterbodies located mostly in the city centre (mean distance of all colonized sites to the city centre: 4.4 ± 2.1 km, range: 1.4–7.0 km; Figure 1). Consistently, large majority of the colonized sites were located in the areas of high or very high urbanization level (mean 42.5 ± 26.1% share of built-environment area, range 10.2–92.2%). At the same time, 6 suburban nesting sites (mean distance to the city centre: 7.5 ± 1.7 km) were abandoned. The number of available nesting sites did not change considerably throughout this period, neither in the centre nor in the peripheries of the city.
Figure 1.
Breeding sites of the Eurasian coot in the urban area of Łódź, central Poland, in 1994–2002 (after Janiszewski et al. 2009) and 2009–2014, indicating recent colonization of the city centre.
In 2009–2014, all potential breeding sites of coots (64 water bodies) located within the administrative borders of the city were monitored throughout each breeding season starting from early March, when ice cover usually melted and first birds started to arrive at their territories. The size of the breeding population varied between 25 and 51 pairs each year. Throughout the whole study period, coot territories were recorded on 42 water bodies, out of which 22 were clustered in 6 complexes of ponds, resulting in 26 different nesting locations (Figure 1). The size of the occupied water bodies ranged from 470 to 114,000 m2. Coot territories were established on a spectrum of water bodies starting from highly disturbed artificial ponds with no natural shoreline and lack of reed vegetation up to undisturbed natural-like habitats with large areas of reed vegetation.
Urbanization gradient
To describe urban gradient I used 3 habitat variables: (1) distance to the city centre (DC); (2) share of built-environment area within 200 m from the shoreline (BEA); and (3) share of farmland area within 200 m from the shoreline (FARM); all of which were measured using satellite maps. All 3 variables were inter-correlated (all P < 0.05; except for DC–BEA correlation, where P = 0.08), indicating a decrease in the share of built-environment area and increase in the share of farmland area proceeding from the city centre to the city suburbs. Using these variables, I clustered all breeding sites of coots (K-means clustering, 50 iterations) into 5 distinct groups characterized by different level of urbanization. The following urban zones were distinguished:
Highly urbanized area in the city centre (DC = 2,350 ± 404 m). Very high share of built-environment area (69.0% ± 9.2 %) and very high human disturbance (PM, pers. obs.; Janiszewski et al. 2009). No farmlands.
Parklands in the city centre (DC = 3,465 ± 200 m). Very low share of built-environment area (11.4% ± 2.4 %), but very high human disturbance (PM, pers. obs.; Janiszewski et al. 2009). No farmlands.
Medium urbanized area (DC = 5,528 ± 178 m). Medium share of built-environment area (42.4% ± 4.8 %) and medium human disturbance (PM, pers. obs.; Janiszewski et al. 2009). No farmlands.
Low urbanized area in the suburbs (DC = 6,327 ± 110 m). Low share of built-environment area (21.1% ± 2.2 %) and low human disturbance (PM, pers. obs.; Janiszewski et al. 2009). Low share of farmland area (8.1% ± 3.7 %).
Natural-like habitats in the suburbs (DC = 9,663 ± 709 m). Very low share of built-environment area (10.6% ± 3.7 %) and very low human disturbance (PM, pers. obs.; Janiszewski et al. 2009). Large share of farmland area (51.6% ± 10.6 %).
Data collection
In total, 310 breeding attempts of coots were recorded throughout the study period. Since 2010, clutch size was recorded upon egg laying completion and eggs were measured with callipers to ± 0.1 mm (n = 140 complete clutches with 1,170 eggs). As conspecific brood parasitism frequently occurs in coots (Lyon 1993; Jamieson et al. 2000), 2 abnormally large clutches (>11 eggs) were excluded from the analysis of clutch size and egg size as they were very likely to contain parasitic eggs. The criterion of >11 eggs was set on the basis of clutch size distribution, with 11 egg clutches being still relatively common (4.3% of all complete clutches, n = 140). Egg volume (V) was calculated in cm3 according to the formula of Coulson (1968): V = 0.4866 × 10-3 × L × B2, where L is egg length, B is egg breadth. Hatching success was assessed as a binary state (at least 1 egg hatched vs. all eggs unhatched) for 287 breeding attempts. Fledging success was assessed as the number of fledged offspring for 257 breeding attempts, including 35 repeated broods and 12 second broods. These data was used to calculate annual reproductive output of coot pairs (n = 210).
During the entire study period, 75 adult Eurasian coots from the studied urban population were captured. Birds were mostly caught on nests with noose traps made of monofilament nylon line or by hand. All birds were ringed and marked with plastic neck collars to enhance resighting probability. At capture, basic biometric measurements were collected (tarsus length, head length, and wing length) and body mass was recorded with an electronic balance to the nearest 1 g. I used body mass adjusted for structural size and for between-sex variation as the first body condition index. To derive this estimate, I first reduced all biometric measurements to the first principal component of the principal component analysis (PCA), because the multivariate metric is likely to express the overall structural size of birds more reliably than any single measurement (Freeman and Jackson 1990). All measurements had similar contributions (0.22–0.38) and PC1 accounted for 66.4% of variation in all reduced biometrical traits. Second, I extracted residuals from a second-order curve of body mass on PC1 (R2 = 0.52; F2,72 = 38.29, P < 0.001) and, then, removed variation in these residuals explained by sex (ANOVA: F1,73 = 3.85, P = 0.054), which followed Roulin et al. (2007). The latter residuals were used as a condition index, along with the whole-blood concentration of hemoglobin. Hemoglobin concentrations were measured in most of captured coots (n = 66, not measured in nine captured individuals for technical reasons). For this purpose, 20 μl of blood was collected from the tarsal vein of each bird and the concentration of hemoglobin was determined using a portable HemoCue Hb 201+ photometer (HemoCue Hb, Ängelholm, Sweden).
Molecular sexing
For the purpose of molecular sexing, an additional blood sample of ∼100 μl was collected from the tarsal vein of 55 birds and the amplification of the chromo-helicase-DNA-binding (CHD) region was performed with the primer pair P2 and P8 (Griffiths et al. 1998) according to the protocol described in Minias (2015b). Using this dataset, discriminant functions were developed to allow identification of adult coot sex with morphometric measurements. A set of 2 measurements with most discriminatory power (head length and wing length), which was shown to yield ∼95% classification success (Minias 2015b), was used for sexing of the remaining coots (n = 20 individuals).
Statistical analysis
Clutch size, mean egg volume, and annual reproductive success were analysed with general linear mixed models (GLMMs), where the identity of territory was entered as a random factor to avoid pseudoreplication resulting from repeated measures of the same pairs (often unmarked) breeding repeatedly in the same sites. The effects of year and urban zone were entered as fixed factors, the effect of first versus repeated/second clutch was entered as a binary fixed factor, and laying date (day of the year) was entered as a covariate (analyses of clutch size and egg volume only). Laying date and clutch size were only weakly correlated (r = -0.18, P = 0.030, n = 140 complete first clutches), and thus could be included into one model without violating the assumption of little/no multicollinearity in the data. In the analysis of egg volume, the effect of clutch size was also included. GLMMs with territory identity included as a random factor were also used to analyze hemoglobin concentrations and size-adjusted body mass of breeding coots. In these models, urban zone and sex (only the analysis of hemoglobin concentration) were entered as fixed factors, while date of measurement was included as a covariate. Due to the low sample sizes in some years, I did not test for between-seasonal variation in these traits. Stepwise procedures of backward removal were used to select for significant independent variables. Following recommendations of Ruxton and Beauchamp (2008), I used contrast analysis to test for 2 specific a priori hypotheses on how all the studied traits varied among the distinguished urban zones. First, I tested for a linear trend of the studied traits along the urbanization gradient, changing gradually from the most-urbanized zone (1) to the least urbanized zone (5). The second hypothesis assumed that the studied traits differed between highly urbanized areas (urban zones 1–3) and low urbanized areas (suburban zones 4–5). All GLMMs were analysed with JMP Pro 10 (SAS Institute Inc., Cary, NC, USA). Hatching success was analysed with GLMM for binomial distribution with a logit link implemented in SPSS 22.0 (IBM Corp., Armon, NY, USA); the effects of year and urban zone were entered as fixed factors and laying date was entered as a covariate. Under this model, pairwise contrasts were calculated to test for the differences in the response binary trait between urban zones. All values were presented as means ± SE.
Capture–recapture models
Survival rates were analyzed with a capture–recapture multistate model for live encounters (Hestbeck et al. 1991; Brownie et al. 1993) implemented in program MARK (White and Burnham 1999). Two states were defined: (A) breeding within the study plot; (B) breeding/non-breeding outside the study plot. Two basic assumptions on the resighting probability were considered: (1) since all potential nesting sites within the study plot were checked each year for breeding birds and all breeders were scanned for neck collars, resighting probability in this strata was fixed to pA = 1; (2) resighting probability in the second strata pB (outside the study plot) was different from pA and was constant between years. In the models, the transition from A to B was considered as emigration from the study plot, while transition from A to A was considered as site fidelity. Similarly, to the analyses of reproductive traits, I tested for 2 specific hypotheses on coot survival: (1) linear trend of survival along the urbanization gradient, where urban zones were included as a covariate; (2) differences in survival between highly urbanized areas (urban zones 1–3) and low urbanized areas (suburban zones 4–5). Goodness-of-fit was tested on a fully parameterized model with bootstrap routine provided by program MARK. 100 simulations were conducted and deviance of the fitted model was compared with randomly generated deviance values, resulting in the estimate of the variance inflation factor ĉ = 1.11. I used Akaike’s Information Criterion corrected for small sample size and adjusted for overdispersion (QAICC) to select the model of best fit. Likelihood-ratio (LR) test was used to compare selected pairs of reduced and general models.
Results
Clutch size and egg size
Clutch size did not differ between first and repeated/second clutches (8.32 ± 0.12 vs. 8.71 ± 0.34 eggs, F1,80 = 1.02, P = 0.31), but it decreased with egg-laying date (F1,81 = 6.68, P = 0.011, β = -0.09 ± 0.03) and showed significant yearly variation (annual means ranging from 7.19 ± 0.22 to 8.70 ± 0.26 eggs; F4,81 = 3.56, P = 0.009). After controlling for these effects, I found significant variation in clutch size between urban zones (F4,81 = 3.00, P = 0.038). Although the linear trend in clutch size over the urbanization gradient was non-significant (contrast analysis: F = 1.73, df = 1, P = 0.20), I found that coots nesting in 2 least urbanized zones laid smaller clutches in comparison to pairs nesting in more urbanized sites (contrast analysis: F = 5.97, df = 1, P = 0.022; Figure 2A).
Figure 2.
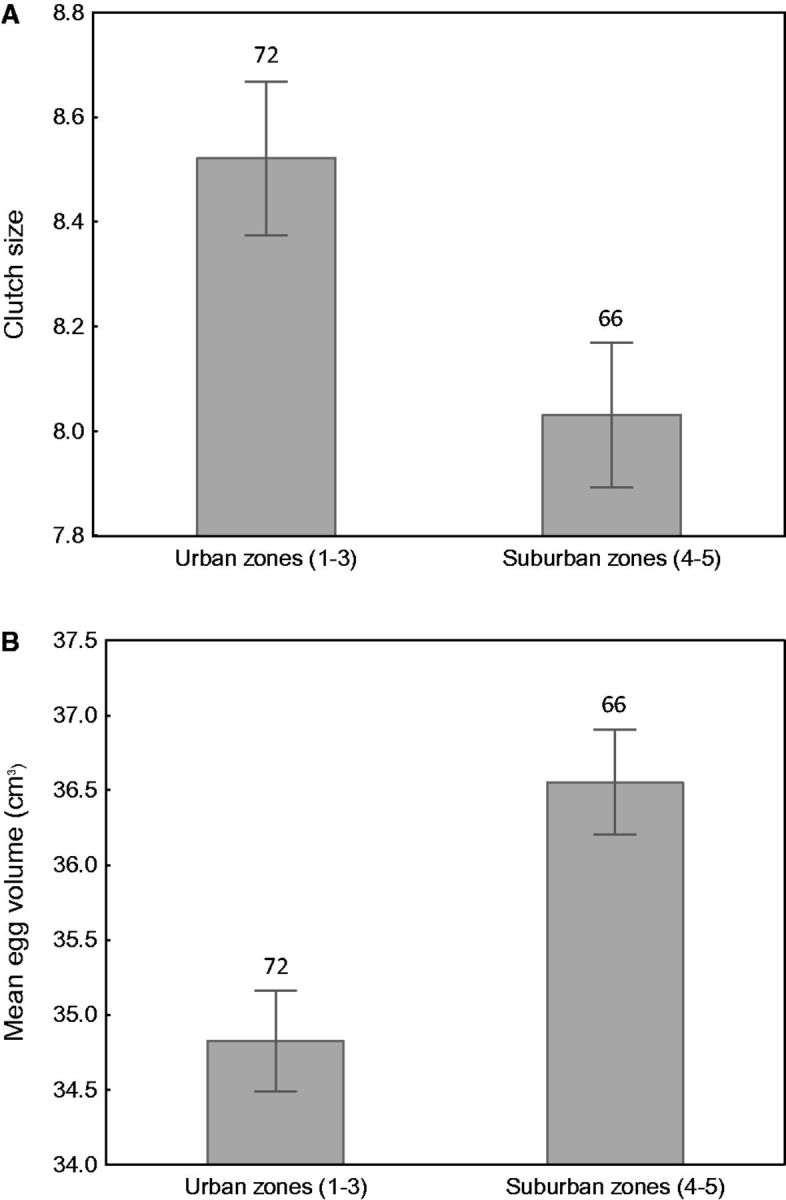
Differences in clutch size (A) and mean egg volume (B) of Eurasian Coots nesting in highly urbanized areas (zones 1–3) and low urbanized areas (zones 4–5) of Łódź, central Poland. Means ± SE are presented, sample sizes are shown above each bar.
Mean egg volume did not depend on clutch size (F1,86 = 0.68, P = 0.41), indicating no trade off between these traits within individuals. It also did not vary with egg-laying date (F1,85 = 0.32, P = 0.57), between years (F4,80 = 0.58, P = 0.67), nor between first and repeated/second clutches (35.46 ± 0.32 vs. 35.53 ± 0.78 cm3, F1,84 = 0.02, P = 0.89). Urban zones were found to be the only significant predictor of mean egg volume in the studied coot population (F1,87 = 2.62, P = 0.047). The linear trend in the mean egg volume over the urbanization gradient was not significant (contrast analysis: F = 2.45, df = 1, P = 0.12), but coots nesting in 2 least urbanized zones laid eggs of larger volume than pairs nesting in more urbanized sites (contrast analysis: F = 7.14, df = 1, P = 0.010; Figure 2B).
Body condition
Hemoglobin concentrations of breeding coots differed between sexes (155.4 ± 3.7 g/dl vs. 141.8 ± 2.7 g/dl for males [n = 32] and females [n = 43], respectively; F1,25 = 8.12, P = 0.006) and decreased with date (F1,25 = 10.09, P = 0.003, β = -0.26 ± 0.08). After controlling for these effects, I found that mean hemoglobin concentrations differed significantly between urban zones (F1,25 = 3.23, P = 0.036), showing a decreasing trend from the most urbanized areas to the natural-like habitats in the suburbs (contrast analysis: F = 13.00, df = 1, P = 0.001; Figure 3A).
Figure 3.
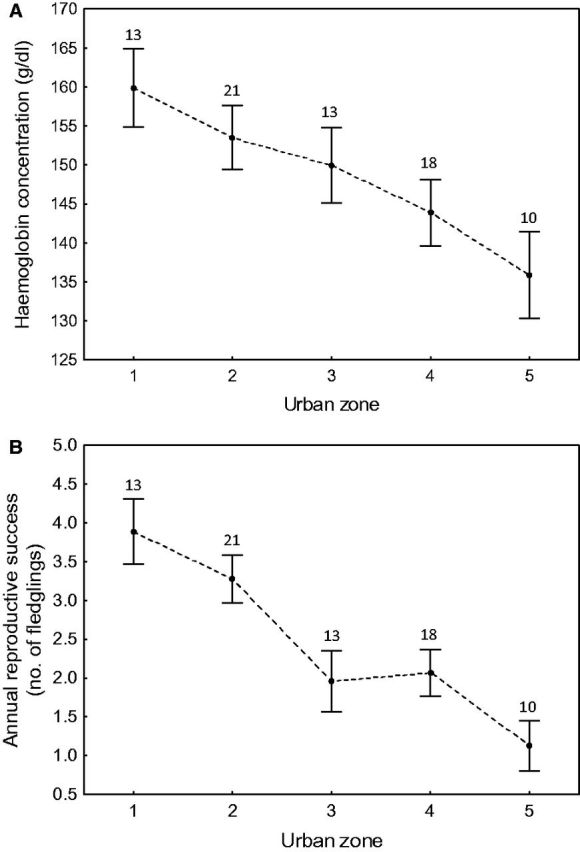
Linear trends of condition measured with the whole-blood hemoglobin concentration (A) and annual reproductive success (B) of Eurasian Coots nesting in Łódź, central Poland. Urban zones reflect urbanization gradient starting from highly urbanized areas in the city centre (zone 1) to natural-like habitats in the city suburbs (zone 5). Means ± SE are presented, sample sizes are shown for each zone.
Body mass residuals were also found to differ between urban zones (F1,33 = 3.55, P = 0.014) with a significant linear decrease from the city centre to the periphery (contrast analysis: F = 5.52, df = 1, P = 0.023). The effect of date was non-significant and excluded from the model (F1,32 = 3.29, P = 0.07).
Hatching success and annual reproductive output
The probability of hatching did not changed with date (F1,192 = 0.58, P = 0.45), showed no variation between years (F5,187 = 0.90, P = 0.48), and did not differ between first and repeated/second broods (0.75 ± 0.03 vs. 0.80 ± 0.06, F1,193 = 0.37, P = 0.54). Urban zones were the only significant predictor of hatching success in the studied population (F4,194 = 2.72, P = 0.030) and the probability of hatching was found to be significantly lower in the least urbanized zone (0.56 ± 0.08) in comparison to all other zones (from 0.79 ± 0.06 to 0.84 ± 0.07; contrast analysis: all P < 0.05).
There were significant differences in annual reproductive success between urban zones (F1,130 = 9.71, P < 0.001), decreasing linearly from highly urbanized areas in the city centre to natural-like habitats in the suburbs (contrast analysis: F = 34.81, df = 1, P < 0.001; Figure 3b). The effect of year was non-significant and excluded from the model (F5,125 = 1.68, P = 0.14). The number of fledglings raised in the breeding attempts with successful hatching also varied between urban zones (F4,61 = 8.44, P = 0.004), showing similar decreasing trend from the city centre to the periphery (contrast analysis: F = 11.04, df = 1, P = 0.004). This pattern remained significant (F1,60 = 8.90, P < 0.001; contrast analysis: F = 9.26, df = 1, P = 0.005) after accounting for clutch size, which had a positive carryover effect on fledging success (F1,60 = 4.53, P = 0.036, β = 0.23 ± 0.11)
Survival rate
There was no evidence for the linear trend in survival of coots along the urbanization gradient (Model 4, Table 1). The hypothesis of differences in survival between highly urbanized (zones 1–3) and low urbanized areas (zones 4–5) was also not supported by the data (Model 3, Table 1), although there was a tendency for lower survival of coots nesting in less urbanized zones (0.49 ± 0.10 vs. 0.56 ± 0.06). The annual survival rate of adult coots estimated by the most parsimonious model was 0.54 ± 0.05. I found support for differences in site fidelity of coots nesting in highly urbanized and low urbanized areas (LR test for Models 1 vs. 5, χ2 = 4.38, P = 0.036) with higher fidelity recorded in highly urbanized zones (0.82 ± 0.07 vs. 0.53 ± 0.13). There was no evidence for differences in survival of birds breeding within and outside the studied urban area (Model 2, Table 1). There was also no support for annual variation in survival rate (Model 10, Table 1) or site fidelity rate (Model 11, Table 1).
Table 1.
Evaluation of capture–recapture multistate models for Eurasian coots nesting in the urban area of Łódź, central Poland
| Model | QAICC | ΔQAICC | QAICC weight | Np | QDeviance |
|---|---|---|---|---|---|
| (1) φ (.) p (s) ψ (urban zones) | 209.96 | 0.00 | 0.30 | 4 | 201.59 |
| (2) φ (s) p (s) ψ (urban zones) | 211.56 | 1.60 | 0.13 | 5 | 201.01 |
| (3) φ (urban zones) p (s) ψ (urban zones) | 211.66 | 1.70 | 0.13 | 5 | 201.11 |
| (4) φ (urban gradient) p (s) ψ (urban zones) | 211.80 | 1.84 | 0.12 | 5 | 201.24 |
| (5) φ (.) p (s) ψ (.) | 212.19 | 2.23 | 0.10 | 3 | 205.98 |
| (6) φ (.) p (s) ψ (urban gradient) | 212.77 | 2.81 | 0.07 | 4 | 204.40 |
| (7) φ (urban zones) p (s) ψ (.) | 213.69 | 3.73 | 0.05 | 4 | 205.32 |
| (8) φ (.) p (s) ψ (s) | 213.72 | 3.76 | 0.05 | 4 | 205.35 |
| (9) φ (urban gradient) p (s) ψ (.) | 213.87 | 3.91 | 0.04 | 4 | 205.51 |
| (10) φ (t) p (s) ψ (urban zones) | 216.75 | 6.79 | 0.01 | 8 | 199.38 |
| (11) φ (.) p (s) ψ (t) | 218.11 | 8.15 | 0.01 | 7 | 203.06 |
where φ – survival probability, p – resighting probability, ψ – probability of transition between the states. Model subscripts: (urban zones) – differences between highly and low urbanized zones, (urban gradient) – linear trend along the urban zones, (s) – differences between states, (t) – time dependence (year-to-year variation), (.) – constant.
Discussion
In this study, I provided evidence for high reproductive benefits associated with recent urban colonization by a reed-nesting waterbird, the Eurasian coot. I found that coots nesting in highly urbanized landscape had considerably higher annual reproductive success in comparison to pairs breeding in the suburban natural-like habitats, and the difference was as much as 4-fold between the most and least urbanized zones. Urban colonizers were also in better physiological condition and had higher size-adjusted body mass during the breeding season, while adult survival did not varied significantly among the urban zones.
It seems highly probable that an increased reproductive output of urban-breeding coots may be the net effect of several different ecological factors and mechanisms of adaptation to city life. First, urban breeding individuals had higher clutch size in comparison to suburban individuals, which could be due to an increased food availability in the pre-laying period. Urban areas act as heat islands and ice cover usually melts earlier than in the adjacent wildland, which accelerates productivity of urban waters early in spring (Wilby and Perry 2006). Urban waterbirds have also an access to artificial food resources due to popular public feeding (Chapman and Jones 2009), which may be crucial for efficient accumulation of nutrient reserves required for egg production. While no data on the effects of pre-laying conditions on clutch size is available for the Eurasian coot, a slight but significant increase in clutch size was observed in supplementally fed American coots Fulica americana (Arnold 1994; but see Arnold and Ankney 1997). There is also a large body of experimental evidence for more taxonomically distant birds, for example, raptors and corvids, that availability of food resources during the pre-laying period can be an important determinant of clutch size (Newton and Marquiss 1981; Aparicio 1994; Soler and Soler 1996).
On the other hand, urban females produced eggs of smaller size in comparison to suburban females, despite the fact that there was no trade off between clutch size and egg size within individuals. This may suggest that coots adopted different reproductive strategies dependently on the level of urbanization, with urban birds aiming to maximize the number of offspring and suburban individuals investing more in the quality of the progeny. Such patterns could be possibly explained by predation pressure decreasing with increasing urbanization level, as birds are known to reduce clutch size when subject to high brood losses (Julliard et al. 1997; Doligez and Clobert 2003). This kind of plastic response could follow at least 2 different mechanism in such precocial species as Eurasian coot: (1) larger clutches take longer to produce and are thus vulnerable to predation (Perrins 1977); (2) loss of smaller clutch allows parents to invest more in the replacement clutch or to survive better to the next breeding season (Cody 1966). Although both mechanisms may explain variation in clutch size along predation gradient, only the first one would be consistent with increased investment in egg size by suburban birds.
Decreased predation rates in the studied urban landscape were suggested by low hatching success of coots breeding in the least urbanized zones, where the probability of hatching at least 1 chick was reduced by 29–33% in comparison to more urbanized areas. Although causes of some brood losses remained unknown, there was strong evidence for predation being a major cause of entire brood losses in the studied population, as inferred from the presence of broken eggs or large pieces of eggshells with the rest of the yolk and/or blood in the nests. This result implies that reduced predation may be a strong driver of urban colonization in waterbirds. In fact, predatory relaxation in urban environments has been proposed as the major explanation for increased productivity of many avian urban adapters (Eden 1985; Gering and Blair 1999). There is also empirical evidence from other Central European population of the Eurasian coot that urbanization level increases nesting success by reducing predation pressure. It has been reported that coots nesting in the vicinity of built-up areas in the region of Mazurian Lakes, NE Poland, had brood losses reduced by ∼60%, as human settlements provided a refuge from predators, mainly from an alien mustelid species, the American Mink Neovison vison (Brzeziński et al. 2012). The other predators which significantly contributed to the coot brood losses in the rural landscape of NE Poland were fox Vulpes vulpes, raccoon dog Nyctereutes procyonoides, otter Lutra lutra, marsh harrier Circus aeruginosus, and hooded crow Corvus cornix (Brzeziński et al. 2012). Similarly to the American Mink, most of these predators usually avoid highly urbanized areas, except for the Hooded Crow, which may exploit the advantages of the urban environment and which have thriving urban populations established across a range of Central European cities (Kövér et al. 2015). However, hooded crows were apparently absent from the highly urbanized zones of Łódź, nesting only in small numbers in the suburbs of the city (Janiszewski et al. 2009). While the peripheral distribution of hooded crows in Łódź could possibly facilitate high nesting success of urban coots, it has to be kept in mind that other patterns might be found in the cities holding large populations of this corvid. On the other hand, it should be also acknowledged that decreased predation rates in urban environments may not only result from exclusion of native predators, but also from the fact that many urban predators are effectively “subsidized” in terms of food and consequently reduce the frequency of their predatory behaviors (Chamberlain et al. 2009).
Finally, it seems likely that differences in reproductive output of coots recorded among the urban zones could be partially maintained by parallel variation in body condition. There was a constant decline from the city centre to the suburbs in size-adjusted body mass and whole-blood hemoglobin concentration of breeding coots and this pattern strongly suggests better feeding conditions in the more urbanized zones. While body mass is primarily expected to reflect food availability, hemoglobin concentrations in birds may strongly respond to other ecological factors such as parasitic rates or urban pollution (Minias 2015b). Thus, high hemoglobin concentrations of coots nesting in highly urbanized zones are surprising, considering the fact that urban pollutants, such as lead or cadmium, are known to cause anemia (Nyholm 1998). Lead may also directly affect hemoglobin production through inhibition of the enzyme δ-aminolevuline acid dehydratase (ALAD), which catalyzes porphyrin and heme biosynthesis (Papanikolaou et al. 2005). Some air pollutants, like CO, NOX, SOX, and other fuel combustion products, also induce excessive hemoglobin oxidation and may lead to methemoglobinemia (Sicolo et al. 2009). Consistently, with all these mechanisms, urban dwelling birds have usually been reported to have reduced hemoglobin concentrations in comparisons to rural conspecifics (Geens et al. 2010; Powell et al. 2013; Herrera-Dueñas et al. 2014). Although detailed information on air pollution in the studied urban area of Łódź are lacking, it seems that the influence of other ecological factors, such as the higher availability of food resources in urban landscape, may overcome negative effects of urban air pollutants on hemoglobin concentration in at least some city-dwelling populations of birds. It must be remembered, however, that in this study data on body condition was collected for adult birds only. While it could be expected that similar mechanisms should drive higher condition of coot offspring raised in more urbanized areas, opposite spatial patterns in offspring condition, although less likely, cannot be ruled out. For example, Brewer’s Sparrows Spizella breweri nesting in low quality habitats with higher nest predation fledged larger young than pairs nesting in more suitable habitats (Chalfoun and Martin 2007). Assuming a hypothetical scenario where higher condition of suburban offspring increases their post-fledging survival prospects, the difference in the overall number of individuals that survive to breed in habitats of different urbanization level could theoretically be less apparent than indicated by the number of fledglings.
Urban colonization seemed to yield no survival benefits in the studied coot population, as no difference was found in the annual survival rate between urban and suburban birds. So far, there is little consistency on how urbanization affect survival rate patterns in birds (Chamberlain et al. 2009). While higher survival of urban-dwelling individuals has been reported for several avian species, mainly due to enhanced food availability (Hõrak and Lebreton 1998; Stracey and Robinson 2012), some other studies indicated negative effects of urbanization on survival (Rollinson and Jones 2002; Whittaker and Marzluff 2009) or no significant differences in survival between urban and non-urban populations (Beck and Heinsohn 2006; Leston and Rodewald 2006; Ausprey and Rodewald 2011). Although no evidence for the effects of urbanization on coot survival was found in this study, there was relatively strong support for higher breeding site fidelity in urban birds, which can be likely explained by 2 non-exclusive mechanisms: (1) higher rate of nesting failure in suburban birds may facilitate breeding dispersal decisions, as site fidelity is known to be dependent on prior reproductive experience (Haas 1998); (2) habitat continuity may facilitate short-distance dispersal between suburban zones and adjacent wildland, while dispersal between fragmented highly urbanized landscapes can be hampered by low connectivity of patches of such habitat.
In conclusion, the recent urban colonization by a reed-nesting waterbird species, the Eurasian coot, was primarily driven by considerable reproductive benefits associated with breeding in urban environment. The results suggest that these benefits may be primarily attributed to: (1) reduced predation resulting from an exclusion of most native predators from highly urbanized zones; (2) increased condition of urban-dwelling birds resulting from enhanced food availability. Although no direct costs of urban colonization were found in the studied population, it cannot be excluded that an adaptation to novel anthropogenic conditions could yield some indirect long-term costs, for example, via increased stress response.
Funding
The study was financially supported by the research grant of the National Science Centre in Poland (2012/05/N/NZ8/00889).
Acknowledgments
I am thankful to all colleagues who helped in the fieldwork, especially Tomasz Janiszewski. I also thank 2 anonymous reviewers for constructive comments on the earlier draft of the manuscript. The study was performed by the permissions of the Local Bioethical Commission in Łodz (40/LB 620/2012) and the General Environmental Protection Directorate in Poland (DOP-OZGIZ.6401.03.199.2011.km).
References
- Adams LW, 1994. Urban Wildlife Habitats: A Landscape Perspective. Minneapolis: University of Minnesota Press. [Google Scholar]
- Aparicio JM, 1994. The seasonal decline in clutch size: an experiment with supplementary food in the kestrel Falco tinnunculus. Oikos 71: 451–458. [Google Scholar]
- Arnold TW, 1994. Effects of supplemental food on egg production in American Coots. Auk 111: 337–350. [Google Scholar]
- Arnold TW, Ankney CD, 1997. The adaptive significance of nutrient reserves to breeding American Coots: a reassessment. Condor 99: 91–103. [Google Scholar]
- Ausprey IJ, Rodewald AD, 2011. Postfledging survivorship and habitat selection across a rural-to-urban landscape gradient. Auk 128: 293–302. [Google Scholar]
- Beck NR, Heinsohn R, 2006. Group composition and reproductive success of cooperatively breeding white-winged choughs Corcorax melanorhamphos in urban and non-urban habitat. Austral Ecol 31: 588–596. [Google Scholar]
- Brownie C, Hines JE, Nichols JD, Pollock KH, Hestbeck JB, 1993. Capture-recapture studies for multiple strata including non-Markovian transitions. Biometrics 49: 1173–1187. [Google Scholar]
- Brzeziński M, Natorff M, Zalewski A, Żmihorski M, 2012. Numerical and behavioral responses of waterfowl to the invasive American mink: a conservation paradox. Biol Conserv 147: 68–78. [Google Scholar]
- Chalfoun AD, Martin TE, 2007. Assessments of habitat preferences and quality depend on spatial scale and metrics of fitness. J Appl Ecol 44: 983–992. [Google Scholar]
- Chamberlain DE, Cannon AR, Toms MP, Leech DI, Hatchwell BJ, et al. , 2009. Avian productivity in urban landscapes: a review and meta-analysis. Ibis 151: 1–18. [Google Scholar]
- Chapman R, Jones DN, 2009. Just feeding the ducks: quantifying a common wildlife-human interaction. Sunbird 39: 19–28. [Google Scholar]
- Cody ML, 1966. A general theory of clutch size. Evolution 20: 174–184. [DOI] [PubMed] [Google Scholar]
- Coulson JC, 1968. Egg size and shape in kittiwake Rissa tridactyla and their use in estimating age composition of populations. Proc Zool Soc Lond 140: 211–227. [Google Scholar]
- Doligez B, Clobert J, 2003. Clutch size reduction as a response to increased nest predation rate in the collared flycatcher. Ecology 84: 2582–2588. [Google Scholar]
- Eden SF, 1985. The comparative breeding biology of magpies Pica pica in an urban and a rural habitat (Aves: Corvidae). J Zool 205: 325–334. [Google Scholar]
- Filippi-Codaccioni O, Clobert J, Julliard R, 2009. Urbanisation effects on the functional diversity of avian agricultural communities. Acta Oecol 35: 705–710. [Google Scholar]
- Freeman S, Jackson WM, 1990. Univariate metrics are not adequate to measure avian body size. Auk 107: 69–74. [Google Scholar]
- Geens A, Dauwe T, Bervoets L, Blust R, Eens M, 2010. Haematological status of wintering great tits Parus major along a metal pollution gradient. Sci Total Environ 408: 1174–1179. [DOI] [PubMed] [Google Scholar]
- Gering JC, Blair RB, 1999. Predation on artificial bird nests along an urban gradient: predatory risk or relaxation in urban environments? Ecography 22: 532–541. [Google Scholar]
- Griffiths R, Double MC, Orr K, Dawson RJ, 1998. A DNA test to sex most birds. Mol Ecol 7: 1071–1075. [DOI] [PubMed] [Google Scholar]
- Haas CA, 1998. Effects of prior nesting success on site fidelity and breeding dispersal: an experimental approach. Auk 115: 929–936. [Google Scholar]
- Hawkey CM, Bennett PM, Gascoyne SC, Hart MG, Kirkwood JK, 1991. Erythrocyte size, number and haemoglobin content in vertebrates. Brit J Haematol 77: 392–397. [DOI] [PubMed] [Google Scholar]
- Herrera-Dueñas A, Pineda J, Antonio MT, Aguirre JI, 2014. Oxidative stress of house sparrow as bioindicator of urban pollution. Ecol Indic 42: 6–9. [Google Scholar]
- Hestbeck JB, Nichols JD, Malecki RA, 1991Estimates of movement and site fidelity using mark-resight data of wintering Canada geese. Ecology 72: 523–533. [Google Scholar]
- Hõrak P, Lebreton JD, 1998. Survival of adult great tits Parus major in relation to sex and habitat: a comparison of urban and rural populations. Ibis 140: 205–209. [Google Scholar]
- Jamieson IG, McRae SB, Simmons RE, Trewby M, 2000. High rates of conspecific brood parasitism and egg rejection in coots and moorhens in ephemeral wetlands in Namibia. Auk 117: 250–255. [Google Scholar]
- Janiszewski T, Wojciechowski Z, Markowski J, 2009. The atlas of breeding birds in Łódź. Wydawnictwo Uniwersytetu Łódzkiego (In Polish with English summary). [Google Scholar]
- Jokimäki J, Huhta E, 2000. Artificial nest predation and abundance of birds along an urban gradient. Condor 102: 838–847. [Google Scholar]
- Julliard R, McCleery RH, Clobert J, Perrins CM, 1997. Phenotypic adjustment of clutch size due to nest predation in the great tit. Ecology 78: 394–404. [Google Scholar]
- Kark S, Iwaniuk A, Schalimtzek A, Banker E. 2007. Living in the city: can anyone become an ‘urban exploiter'? J Biogeogr 34: 638–651. [Google Scholar]
- Klett AT, Shaffer TL, Johnson DH, 1988. Duck nest success in the prairie pothole region. J Wildl Manage 52: 431–440. [Google Scholar]
- Kövér L, Gyüre P, Balogh P, Huettmann F, Lengyel S, et al. , 2015. Recent colonization and nest site selection of the hooded crow (Corvus corone cornix L.) in an urban environment. Landsc Urban Plan 133: 78–86. [Google Scholar]
- Leston LF, Rodewald AD, 2006. Are urban forests ecological traps for understory birds? An examination using Northern cardinals. Biol Conserv 131: 566–574. [Google Scholar]
- Liu J, Daily GC, Ehrlich PR, Luck GW, 2003. Effects of household dynamics on resource consumption and biodiversity. Nature 421: 530–533. [DOI] [PubMed] [Google Scholar]
- Lyon BE, 1993. Conspecific brood parasitism as a flexible female reproductive tactic in American coots. Anim Behav 46: 911–928. [Google Scholar]
- Mannan RW, Steidl RJ, Boal CW, 2008. Identifying habitat sinks: a case study of Cooper’s hawks in an urban environment. Urban Ecosyst 11: 141–148. [Google Scholar]
- McDonald RI, Kareiva P, Forman RT, 2008. The implications of current and future urbanization for global protected areas and biodiversity conservation. Biol Conserv 141: 1695–1703. [Google Scholar]
- McKinney ML, 2006. Urbanization as a major cause of biotic homogenization. Biol Conserv 127: 247–260. [Google Scholar]
- McKinney ML, 2008. Effects of urbanization on species richness: a review of plants and animals. Urban Ecosyst 11: 161–176. [Google Scholar]
- Minias P, 2015a. The use of hemoglobin concentrations to assess physiological condition in birds: a review. Conserv Physiol 3: cov007. doi: 10.1093/conphys/cov007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minias P, 2015b. Sex determination of adult Eurasian coot Fulica atra by morphometric measurements. Waterbirds 38:191–194. [Google Scholar]
- Newton I, Marquiss M, 1981. Effect of additional food on laying dates and clutch sizes of sparrowhawks. Ornis Scand 12: 224–229. [Google Scholar]
- Norte AC, Lobato DNC, Braga EM, Antonini Y, Lacorte G, et al. , 2013. Do ticks and Borrelia burgdorferi s.l. constitute a burden to birds? Parasitol Res 115: 1903–1912. [DOI] [PubMed] [Google Scholar]
- Nyholm NEI, 1998. Influence of heavy metal exposure during different phases of the ontogeny on the development of pied flycatchers Ficedula hypoleuca in natural populations. Arch Environ Contam Toxicol 35: 632–637. [DOI] [PubMed] [Google Scholar]
- Papanikolaou NC, Hatzidaki EG, Belivanis S, Tzanakakis GN, Tsatsakis AM, 2005. Lead toxicity update: a brief review. Med Sci Monit 11: RA329–RA336. [PubMed] [Google Scholar]
- Partecke J, Schwabl I, Gwinner E, 2006. Stress and the city: urbanization and its effects on the stress physiology in European blackbirds. Ecology 87: 1945–1952. [DOI] [PubMed] [Google Scholar]
- Perrins CM, 1977. The role of predation in the evolution of clutch size. In: Stonehouse B, Perrins CM, editors. Evolutionary Ecology. London: Macmillan; 181–191. [Google Scholar]
- Pieron MR, Rohwer FC, 2010. Effects of large-scale predator reduction on nest success of upland nesting ducks. J Wildl Manage 74: 124–132. [Google Scholar]
- Powell C, Lill A, Johnstone CP, 2013. Body condition and chronic stress in urban and rural Noisy Miners. Open Ornithol J 6: 25–31. [Google Scholar]
- Pryke SR, Astheimer LB, Griffith SC, Buttemer WA, 2012. Covariation in life-history traits: differential effects of diet on condition, hormones, behavior, and reproduction in genetic finch morphs. Am Nat 179: 375–390. [DOI] [PubMed] [Google Scholar]
- Pryke SR, Rollins LA, 2012. Mothers adjust offspring sex to match the quality of the rearing environment. Proc R Soc B 279: 4051–4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollinson DJ, Jones DN, 2002. Variation in breeding parameters of the Australian magpie Gymnorhina tibicen in suburban and rural environments. Urban Ecosyst 6: 257–269. [Google Scholar]
- Roulin A, Christe P, Dijkstra C, Ducrest AL, Jungi TW, 2007. Origin-related, environmental, sex, and age determinants of immunocompetence, susceptibility to ectoparasites, and disease symptoms in the barn owl. Biol J Linn Soc 90: 703–718. [Google Scholar]
- Ruxton GD, Beauchamp G, 2008. Time for some a priori thinking about post hoc testing. Behav Ecol 19: 690–693. [Google Scholar]
- Rutz C, 2008. The establishment of an urban bird population. J Anim Ecol 77: 1008–1019. [DOI] [PubMed] [Google Scholar]
- Sasvári L, Csörgo T, Hahn I, 1995. Bird nest predation and breeding density in primordial and man-made habitats. Folia Zool 44: 305–314. [Google Scholar]
- Seto KC, Güneralp B, Hutyra LR, 2012. Global forecasts of urban expansion to 2030 and direct impacts on biodiversity and carbon pools. Proc Natl Acad Sci USA 109: 16083–16088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shochat E, Warren PS, Faeth SH, McIntyre NE, Hope D, 2006. From patterns to emerging processes in mechanistic urban ecology. Trends Ecol Evol 21: 186–191. [DOI] [PubMed] [Google Scholar]
- Sicolo M, Tringali M, Orsi F, Santagostino A, 2009. Porphyrin pattern and methemoglobin levels in Columba livia applied to assess toxicological risk by air pollution in urban areas. Arch Environ Contam Toxicol 57: 732–740. [DOI] [PubMed] [Google Scholar]
- Snow DW, Perrins CM, 1998. The Birds of the Western Palearctic. Oxford, UK:Oxford University Press. [Google Scholar]
- Soler M, Soler JJ, 1996. Effects of experimental food provisioning on reproduction in the jackdaw Corvus monedula, a semi-colonial species. Ibis 138: 377–383. [Google Scholar]
- Stracey CM, Robinson SK, 2012. Are urban habitats ecological traps for a native songbird? Season-long productivity, apparent survival, and site fidelity in urban and rural habitats. J Avian Biol 43: 50–60. [Google Scholar]
- Sumasgutner P, Nemeth E, Tebb G, Krenn HW, Gamauf A, 2014. Hard times in the city-–attractive nest sites but insufficient food supply lead to low reproduction rates in a bird of prey. Front Zool 11: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorington KK, Bowman R, 2003. Predation rate on artificial nests increases with human housing density in suburban habitats. Ecography 26: 188–196. [Google Scholar]
- Tomiałojć L, 1978. The influence of predators on breeding Woodpigeons in London parks. Bird Stud 25: 2–10. [Google Scholar]
- Traut AH, Hostetler ME, 2003. Urban lakes and waterbirds: effects of development on avian behavior. Waterbirds 26: 290–302. [Google Scholar]
- Tryjanowski P, Sparks TH, Kuźniak S, Czechowski P, Jerzak L, 2013. Bird migration advances more strongly in urban environments. PLoS ONE 8: e63482 doi:10.1371/journal.pone.0063482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Huang Q, Lan S, Zhang Q, Chen S, 2015. Common blackbirds Turdus merula use anthropogenic structures as nesting sites in an urbanized landscape. Curr Zool 61: 435–443. [Google Scholar]
- White GC, Burnham KP, 1999. Program MARK: survival estimation from populations of marked animals. Bird Stud (Suppl.) 46: S120–S139. [Google Scholar]
- Whittaker KA, Marzluff JM, 2009. Species-specific survival and relative habitat use in an urban landscape during the postfledging period. Auk 126: 288–299. [Google Scholar]
- Wilby RL, Perry GL, 2006. Climate change, biodiversity and the urban environment: a critical review based on London, UK. Prog Phys Geogr 30: 73–98. [Google Scholar]