Abstract
Physical activity can enhance cognitive function and increase resistance against deleterious effects of stress on mental health. Enhanced cognitive function and stress resistance produced by exercise are conserved among vertebrates, suggesting that ubiquitous mechanisms may underlie beneficial effects of exercise. In the current review, we summarize the beneficial effects of exercise on cognitive function and stress resistance and discuss central and peripheral signaling factors that may be critical for conferring the effects of physical activity to brain circuits involved in cognitive function and stress. Additionally, it is suggested that norepinephrine and serotonin, highly conserved monoamines that are sensitive to exercise and able to modulate behavior in multiple species, could represent a convergence between peripheral and central exercise signals that mediate the beneficial effects of exercise. Finally, we offer the novel hypothesis that thermoregulation during exercise could contribute to the emotional effects of exercise by activating a subset of temperature-sensitive serotonergic neurons in the dorsal raphe nucleus that convey anxiolytic and stress-protective signals to forebrain regions. Throughout the review, we discuss limitations to current approaches and offer strategies for future research in exercise neuroscience.
Keywords: anxiety, norepinephrine, physical activity, serotonin, wheel running
Introduction
It is now well established that the physical activity status of the organism impacts the structure and function of the nervous system, resulting in changes in associated behaviors (Cotman et al. 2007; Kramer & Erickson 2007; Gomez-Pinilla & Hillman 2013; Morgan et al. 2015). Physical activity status refers to the degree to which an organism engages in regular physical activity behaviors, whether these are for survival, fitness, or fun. Risk factors for a sedentary lifestyle are well documented, as are beneficial effects of regular physical activity. Taking center stage among the beneficial effects of physical activity are an enhancement of cognitive functions and an increase in stress resistance. In the current review, we will draw from data obtained from a variety of vertebrates to briefly summarize the benefits of physical activity on cognitive function and stress resistance. Next, we will discuss signaling factors potentially involved in communicating the effects of exercise to the nervous system. Signaling factors considered will be both those initiated from the periphery during exercise, as well those originating from within the brain itself. Finally, we will summarize data consistent with the hypothesis that the monoamines norepinephrine (NE) and serotonin (5-HT) are a key point of convergence of peripheral and central signaling factors and are thus intimately involved in the mechanisms by which exercise enhances cognitive function and increases stress resistance.
Exercise Enhances Cognitive Function: Role of Growth Factors
The beneficial effects of physical activity on cognitive function have been extensively reviewed (Kramer & Erickson 2007; Gomez-Pinilla & Hillman 2013; Cassilhas et al. 2015; Fischer 2015; Hamilton & Rhodes 2015; Prakash et al. 2015). Physical activity can impact a wide variety of cognitive and learning processes including executive control (Hillman et al.2003; Hillman et al. 2014), attentional processing (Budde et al. 2008; Medina et al. 2010), and spatial memory (Cassilhas et al. 2015). Although exercise is able to impact cognitive function in healthy individuals, physical activity is most beneficial in clinical populations. For example, physical activity can slow the cognitive decline associated with age (Kramer et al. 2006; Scherder et al. 2014), Alzheimer’s disease (Yang & Coote 1998), Parkinson’s disease (Murray et al. 2014; David et al. 2015), and after traumatic brain injury (Szabo 1994; Griesbach et al. 2009). Cognitive benefits of physical activity appear to be conserved in vertebrates and, in addition to humans, have been reported in nonhuman primates (Rhyu et al. 2010), rodents (van Praag et al. 1999a; Griffin et al. 2009), and zebra fish (Luchiari & Chacon 2013).
Exercise elicits structural plasticity in a wide variety of brain regions related to cognitive functions (Morgan et al. 2015). In the hippocampus, for example, exercise increases angiogenesis (Van der Borght et al. 2009), dendritic complexity (Redila & Christie 2006; Shih et al. 2013), spine density (Eadie et al. 2005), volume (Thomas et al. 2015), and adult neurogenesis (van Praag et al. 1999b; van Praag 2008). Growth factors seem to be especially important for mediating the cognitive and structural plasticity after exercise. Exercise increases circulating insulin-like growth factor-1 (IGF-1; Llorens-Martin et al. 2010), vascular endothelial growth factor (VEGF; Schobersberger et al. 2000), and brain-derived neurotropic factor (BDNF; Coelho et al. 2013; but see Maass et al. 2015). These circulating growth factors can influence the brain directly (Carro et al. 2000). Indeed, several studies report that circulating BDNF (Trejo et al. 2001; Trejo et al. 2008) or IGF-1 (Duman et al. 2009) are important for the increase in neurogenesis and the antidepressant effects of exercise in animal models. Exercise also increases the synthesis of growth factors within the brain, including the hippocampus, cortex, and amygdala (Neeper et al. 1995; Neeper et al. 1996; Gomez-Pinilla et al. 1997; Ding et al. 2006; Greenwood et al. 2009). Blocking the function of BDNF with intra-hippocampal administration of an immunoadhesin chimera (TrkB-IgG) that mimics the BDNF receptor, TrkB, to selectively bind BDNF has been reported to prevent the enhancement of cognitive function produced by exercise in rats (Vaynman et al. 2004; Gomez-Pinilla et al. 2008). BDNF could also contribute to the increase in synaptic plasticity and neurogenesis noted after exercise (Poo 2001; Gomez-Pinilla et al. 2008; Ding et al. 2011; Park & Poo 2013), which together could contribute to beneficial effects of exercise (Bjornebekk et al. 2005; Clark et al. 2008; Duman et al. 2008; Marais et al. 2009; Fuss et al. 2010; Voss et al. 2013). Consistent with the rodent data are reports that humans with a prominent single-nucleotide polymorphism on the BDNF gene (BDNF Val66Met) display impaired learning and memory (Brooks et al. 2014; Montag et al. 2014). One particularly interesting potential role of adult hippocampal neurogenesis is the clearance of old memories in order to make way for new learning (Frankland et al. 2013). Akers et al. (2014) reported that mice allowed voluntary access to running wheels for 6 weeks after learning of contextual fear conditioning showed markedly weaker memory of the conditioning context compared with sedentary mice, and this forgetting was dependent on the formation of new cells in the hippocampus (Akers et al. 2014). However, exercise-induced neurogenesis might elicit forgetting only under specific learning conditions. Greenwood et al. (2009) observed no evidence of exercise-induced loss of fear memories using a contextual fear conditioning protocol that resulted in high levels of fear in adult rats (Greenwood et al. 2009). Moreover, Mustroph et al. (2015) found that adult hippocampal neurogenesis was not necessary for wheel running to abolish the memory for cocaine conditioned place preference in mice (Mustroph et al. 2015).
Importantly, facilitation of adult neurogenesis and memory benefits from exercise seems to be conserved across vertebrate taxa. Zebra fish exercise trained by swimming against a current, for example, demonstrates enhanced learning of appetitive Pavlovian conditioning (Luchiari & Chacon 2013). European Starling bird exercise trained in a wind tunnel that mimics the birds’ natural flight were reported to display enhanced neuronal recruitment in the telencephalon, which houses the birds’ cerebral cortex, amygdala, and hippocampus, during flight compared with another group of birds that were not trained. The enhanced neuronal recruitment in the trained group was paralleled by an increase in neurogenesis in the telencephalon, as revealed by doublecortin immunohistochemistry (Hall et al. 2014). These data suggest that the increase in adult neurogenesis and associated memory benefits may be a conserved effect of exercise in vertebrates. To our knowledge, the effects of exercise on neural models of learning and memory in invertebrates have not been investigated. The characterization of an effective exercise training paradigm in Drosophila (Tinkerhess et al. 2012a) could provide convenient means with which to investigate the genetic and molecular mechanisms underlying the effects of exercise on learning. In fact, several weeks of exercise training slowed the age-related decline in climbing performance in Drosphila, an effect that was dependent on the presence of spargel, the invertebrate homolog of the vertebrate transcriptional co-activator PPAR-γ co-activator-1α (PGC-1α; Tinkerhess et al. 2012b). This is especially interesting considering that muscle PGC-1α drives the expression of a newly identified myokine, FNDC5 (Irisin; Bostrom et al. 2012), which has been reported to mediate the increase in hippocampal BDNF after exercise in mice (Wrann et al. 2013).
Exercise Increases Stress Resistance
In addition to enhancing cognitive function and learning and memory processes, physical activity is also well known to confer protection against deleterious effects of stress. The stress-buffering effects of exercise can be observed in a wide variety of stress responsive systems, including oxidative stress (Ozbeyli et al. 2015), attenuation of mild stress-induced increases in hypothalamic–pituitary–adrenal (HPA) axis activity (Droste et al. 2006; Greenwood et al. 2008; Campeau et al. 2010), facilitation of habituation of the sympathetic nervous system (Masini et al. 2005) and HPA axis (Sasse et al. 2008; Sasse et al. 2013) response to chronic stress, modulation of stress-induced monoaminergic signaling (Dishman 1997a; Dishman et al. 1997; Greenwood et al. 2003a; Greenwood et al. 2003b; Clark et al. 2015), and reduction in incidence and severity of stress-related psychiatric disorders such as depression (Babyak et al. 2000; Blumenthal et al. 2012), anxiety (Herring et al. 2010; Asmundson et al. 2013; Stonerock et al. 2015), and post-traumatic stress disorder (Newman & Motta 2007; Powers et al. 2015).
Interestingly, like the enhancement of cognitive function, exercise-induced stress resistance is conserved in vertebrates. For example, rats allowed voluntary access to running wheels for 6 weeks are protected against stress-induced elevations in HPA axis activity (Droste et al. 2006; Greenwood et al. 2008; Campeau et al. 2010) and depression- and anxiety-like behavioral effects of exposure to a variety of laboratory stressors (reviewed in Greenwood & Fleshner 2011). Chronically trained rainbow trout displayed an attenuation of the stress hormone cortisol in response to an acute bout of forced exercise compared with their untrained counterparts (Hernandez et al. 2002). Additionally, interval-trained Atlantic salmon displayed enhanced survival and attenuated cardiac pro-inflammatory cytokine response to a viral challenge (Castro et al. 2011). There is also evidence that the anxiolytic effect of exercise extends to fish. Mosquito fish exercise trained for 28 days by placement in a water tank with a constant current demonstrated behavioral changes consistent with an anxiolytic effect. Compared with untrained fish, trained fish displayed greater exploratory behavior in a novel context and had a reduced latency to leave a familiar refuge (Sinclair 2014). These data suggest that increasing physical activity status of the organism ubiquitously reduces consequences of stress across vertebrate taxa, although more work needs to be done to characterize the different species that can benefit from exercise.
One issue that arises when investigating the effects of exercise on brain and behavior is the ability to differentiate exercise effects from those of environmental enrichment. Including a wheel in a rat’s cage, for example, provides an object on which the rat can climb and explore, and complex enriched housing environments engage cognitive processes and can initiate structural plasticity, enhance cognitive function, and increase stress resilience (Comery et al. 1995; Fan et al. 2007; Markham et al. 2009; Veena et al. 2009a, 2009b; Fischer 2015; Grimberg-Henrici et al. 2015; Hullinger et al. 2015; Ji et al. 2015; Novkovic et al. 2015). Many of the studies reporting beneficial effects of enriched environments, however, have included running wheels as part of the enriched housing. Recent work attempting to differentiate the effects of running from complex environmental enrichment have shown that at least some of the effects of long-term exercise in rodents are above and beyond those produced by environmental enrichment alone (Nyhuis et al. 2010; Kobilo et al. 2011; Mustroph et al. 2012). Thus, although cognitive engagement is certainly part of the exercise stimulus, and enriched environments that engage cognitive processes can certainly benefit brain plasticity and behavior, at least some of the effects of exercise are independent from these enrichment factors. An important goal of future studies should be to determine the role of cognitive engagement in the beneficial effects of exercise.
Potential Signals Responsible for Conveying the Effects of Exercise to Neural Circuits Involved in Cognitive Function and Stress
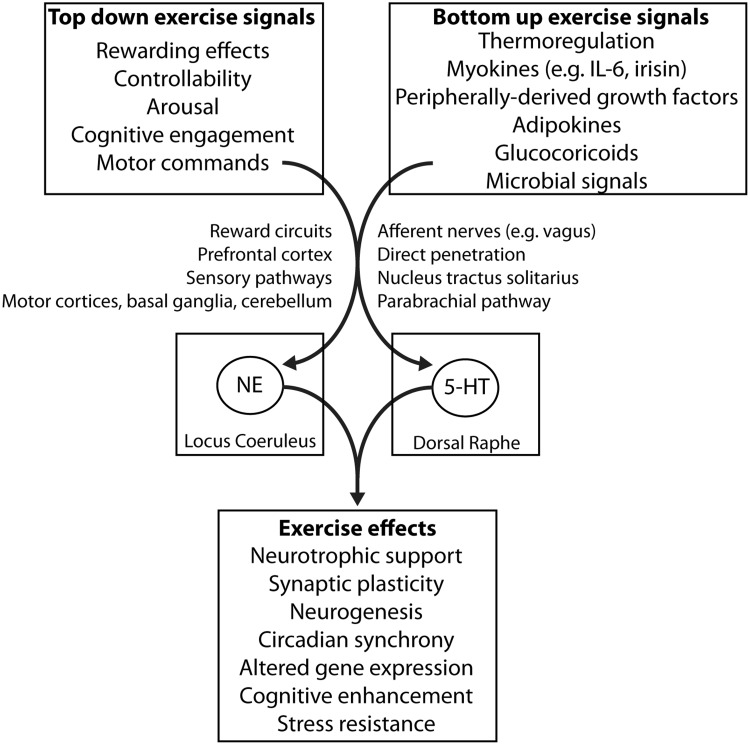
Data elucidating the intracellular signaling pathways, neurotransmitter systems, and means of synaptic plasticity that are sensitive to physical activity are rapidly emerging. However, a critical unanswered question is how the experience of exercise is communicated to the brain to result in altered neural activity, gene expression, or synaptic plasticity required for the observed behavioral effects of exercise. Indeed, the National Institutes of Health recently implemented a Common Fund with the goal of identifying these signals in exercising humans (http://commonfund.nih.gov/MolecularTransducers). We will refer to these potential means of exercise-to-brain communication simply as exercise signals. Exercise signals could originate from 2 sources: from the periphery or from within the central nervous system. Signals derived from the periphery could include activity of afferent nerves or factors released from working muscle, among other factors (Figure 1). These signals would communicate with the brain in a “bottom-up” fashion, whereas central factors, such as psychological processes recruited during physical activity including learning exercise routines, choosing to exercise, exercise reward, or an increase in central arousal (Figure 1), would signal in a “top-down” manner. Exercise signals that could be involved in the effects of physical activity on adult neurogenesis in the hippocampus have been recently reviewed (Bolijn 2015). Some of these signals, as well as others that could be important for conferring the broad effects of physical activity on cognitive function and stress resistance, are shown in Figure 1.
Figure 1.
NE and 5-HT neurons of the locus coeruleus and dorsal raphe nucleus are a site of convergence of central (top-down) and peripheral (bottom-up) signals conveying the experience of exercise to brain circuits involved in cognition, stress, and emotional behavior.
Monoamines as Signaling Factors
Considering the array of behaviors and brain areas impacted by physical activity, and that many effects of physical activity seem to be conserved across the animal kingdom, it is possible that exercise signals derived from various sources could converge on a common target within the central nervous system that could then at least partly contribute to the wide variety of neural and behavioral changes elicited by exercise. Of these signals, monoamines such as dopamine (DA), NE, and 5-HT represent likely candidates. The current review focuses on NE and 5-HT because the majority of work focuses on these monoamines. Discussions on the potential role of DA can be found elsewhere (Knab & Lightfoot 2010; Greenwood et al. 2011; Monteiro-Junior et al. 2015). Consistent with a role for monoamines as exercise signals are the facts that monoamines: 1) are highly conserved, 2) innervate brain regions involved in cognitive function and stress from their metencephalic and mesencephalic origins, 3) have long been implicated in memory modulation and behavioral responses to stress, 4) are known to be sensitive to the physical activity status of the organism, and 5) respond to many of the exercise signals listed in Figure 1. The following sections will consider the involvement of NE and 5-HT in the behavioral effects of physical activity. Central to this theme is the idea that NE and 5-HT could represent key points of convergence between bottom-up and top-down signaling factors involved in communicating the experience of exercise to the brain circuits involved in cognitive processes and stress (Figure 1).
Role of NE in Cognitive Benefits of Exercise
Rodent models provide a convenient tool with which to study the mechanisms underlying the beneficial effects of exercise on behavior. Rodent studies investigating the effects of exercise on cognitive function have been hyperfocused on learning processes supported by the hippocampus. This attention to the hippocampus makes sense considering that hippocampus-dependent memory is readily assessed in rats and mice, and some of the most commonly observed effects of exercise are an increase in growth factors and birth of new neurons in the adult hippocampus. The hippocampus is thought to be important for learning and memory of contextual and spatial information (Rudy et al. 2002), as well as declarative memory. Interestingly, exercise-induced improvements in hippocampal function seem to be independent of exercise controllability: both forced treadmill training (Albeck et al. 2006; Griffin et al. 2009; Li et al. 2013; Inoue et al. 2015) or swimming (Chae et al. 2012), as well as voluntary access to running wheels (Merrill 1993; van Praag et al. 1999a; Vaynman et al. 2004; Greenwood et al. 2009), have been reported to enhance some types of memory. For this reason, exercise signals responsible for the effects of exercise on hippocampal function could be insensitive to the top-down signaling factor of exercise controllability. Along with peripheral signaling factors released from muscle, central NE would likely be insensitive to the controllability of exercise, as NE neurons in the locus coeruleus (LC) are thought to be important for arousal, attention, and mood (Sara & Bouret 2012); states likely impacted by voluntary as well as forced exercise. This does not imply that all effects of exercise are insensitive to the type or controllability of exercise employed. Treadmill training, for example, fails to prevent stress-induced deficits in goal-directed learning thought to depend on the striatum (Greenwood et al. 2013).
Increases in neurogenesis and plasticity factors such as BDNF in the hippocampus are thought to be critical to the cognitive benefits of exercise (Cotman et al. 2007). Interestingly, similar to the benefits to hippocampal function, increases in BDNF and neurogenesis in the hippocampus are insensitive to exercise controllability. Both voluntary wheel running (Neeper et al. 1995; Oliff et al. 1998; Greenwood et al. 2009), forced wheel running (Leasure & Jones 2008), and forced treadmill training (Griffin et al. 2009; Kim et al. 2015) increase plasticity factors including BDNF in the hippocampus. Increases in circulating factors could be important for these hippocampal changes after exercise, and this possibility has been recently reviewed (Bolijn 2015). Often overlooked, however, is the contribution of NE to the hippocampal and cognitive effects of exercise (discussed previously in Greenwood & Fleshner 2013).
It is possible that the increase in plasticity factors could influence neural and behavioral effects of exercise by eliciting plastic changes in NE systems. BDNF, for example, has been shown to contribute to LC noradrenergic neuronal plasticity during development (Holm et al. 2003), aging (Matsunaga et al. 2006; Nakai et al. 2006), and in response to specific signals such as opioids that could be recruited during exercise (Akbarian et al. 2002). Repeated activation of NE systems during exercise could also directly influence plasticity factors. Prior work indicates that central NE systems (Dunn & Dishman 1991; Dunn 1996a; Dunn 1996b) including LC NE neurons (Greenwood & Fleshner 2013) are activated by exercise, and there are a variety of neuroplastic changes that occur in the NE system with chronic exercise that are consistent with repeated NE activation (Brown & Van Huss 1973; Ostman & Nyback 1976; Brown et al. 1979; Dishman 1997b; a; Dishman et al. 1997; Dishman et al. 2000). The LC receives input from autonomic regions of the medullary reticular formation and the nucleus of the solitary tract (NTS; Aston-Jones et al. 1991), as well as a recently identified dense projection from the cerebellum (Schwarz et al. 2015). These inputs suggest that the LC could be a site of integration between afferent information regarding medullary autonomic reflexes, including from the sensory vagus nerve relayed from the NTS, with motor information from the cerebellum. Thus, several bottom-up or top-down exercise signaling mechanisms could converge on LC NE activity, which would then function to facilitate sensory gating, focus attention, or improve learning during exercise.
Of particular note is that activation of the LC via the vagus nerve could be a pathway through which circulating cytokines (Goehler et al. 2000; Guyon et al. 2008; Pedersen & Febbraio 2008) or products from optimized gut microbiota (Mika & Fleshner 2015; Mika et al. 2015b) signal the brain during exercise. Consistent with a role for gut microbiota are recent data reporting that wheel running can increase the abundance of beneficial microbial species in the rat gut, especially during early life (Mika & Fleshner 2015; Mika et al. 2015b). These microbes include Lactobacillus and Anaerostipes species that can release a variety of products including cytokines, neurotransmitters, and single chain fatty acids, which could communicate with the brain through vagal afferent signaling. Central 5-HT systems could be an additional central target of vagal nerve-mediated NE activity through a mechanism involving α1b-adrenergic receptor (ADR) located on raphe 5-HT neurons (Day et al. 2004). Indeed, repeated stimulation of the vagus nerve activates 5-HT neurons in the dorsal raphe nucleus (DRN; Manta et al. 2009) and elicits increases in extracellular levels of both NE and 5-HT in the hippocampus and cortex (Manta et al. 2013).
Increases in NE release could influence learning and cognitive function by initiating plasticity in target regions of the LC. For example, β-ADRs are linked to the transcription of BDNF through adenosine 3', 5'-monophosphate (cAMP)-dependent protein kinase (PKA)-induced phosphorylation of cAMP response element-binding protein (CREB; (Conti et al. 2002)). Most importantly, the increase in hippocampal BDNF after exercise has been shown to be dependent on central noradrenergic signaling. Blockade of either CREB (Chen & Russo-Neustadt 2009) or β-ADRs (Ivy et al. 2003) prevents the exercise-induced increase in BDNF and the improved contextual memory typically observed after 6 weeks of wheel running (Van Hoomissen et al. 2004). Additionally, Garcia et al. (2003) reported that N-(2-chloroethyl)-N-ethyl-2-bromobenzylamine (DSP-4) lesions of the LC reduces BDNF mRNA in the hippocampus of exercised rats to a level equivalent to sedentary rats, which are unaffected by DSP-4 lesions (Garcia et al. 2003). This potential role of NE in exercise-induced plasticity and cognitive enhancement is similar to the suggested contribution of noradrenergic signaling to the increase in BDNF after administration of chronic antidepressants (Duman & Monteggia 2006).
NE could contribute to other benefits of exercise in addition to initiating neural plasticity in the hippocampus. LC NE neurons also project to the prefrontal cortex and amygdala. NE in these regions could contribute to modulation of attention and executive function (Berridge & Spencer 2015), as well as emotional behavior. For example, acute wheel running in close proximity to the acquisition phase of fear extinction enhances fear extinction memory (Siette et al. 2014) and reduces the relapse of conditioned fear responding after extinction (Mika et al. 2015a). NE has similar effects. Infusion of the mixed β-ADR agonist isoproterenol directly into the medial prefrontal cortex (PFC), including the infralimbic region, immediately before acquisition of contextual fear extinction enhances the learning and retention of contextual fear extinction (Do-Monte et al. 2010). Microinfusions of NE into the basolateral amygdala immediately after extinction of contextual fear enhance the consolidation of fear extinction memory (Berlau & McGaugh 2006). Given that exercise activates LC NE neurons, these data suggest that NE could contribute to the facilitation of fear extinction produced by acute exercise. However, it is unlikely that NE is solely responsible, as NE agonists seem to only enhance the recall of fear extinction memory if memory testing occurs in the context in which fear extinction learning occurred (Morris & Bouton 2007). In contrast, exercise during the acquisition of fear extinction prevents the relapse of fear in novel contexts (Mika et al. 2015a). Nevertheless, LC NE systems could contribute to a variety of neuroplastic, cognitive, and emotional effects of exercise. Related to this idea are data demonstrating that galanin, a neuropeptide co-expressed with NE in LC neurons, contributes to anxiolytic and stress-protective effects of exercise (Sciolino & Holmes 2012; Sciolino et al. 2015).
Central 5-HT Systems and Exercise-Induced Stress Resistance
In addition to NE, 5-HT has been implicated in contributing to the beneficial effects of exercise on brain function and behavior. Research has focused on changes in central 5-HT systems after exercise, and how these changes could contribute to antidepressant, anxiolytic, and stress-buffering effects of exercise (reviewed in Dishman 1997a; Greenwood & Fleshner 2011). The 5-HT system displays alterations in gene expression and functional activity after an increase in physical activity in animals as diverse as humans and lizards. The changes in 5-HT systems after exercise include adaptations both within cell body regions in raphe nuclei (Greenwood et al. 2003a, 2005b; Loughridge et al. 2013) as well as adaptations in raphe target regions involved in regulating specific behaviors (Chaouloff 1994; Dishman et al. 1997; Greenwood et al. 2012; Christianson & Greenwood 2014). It should be mentioned that prior work investigating the effects of forced exercise such as treadmill training on central 5-HT systems should be interpreted with caution. Specific 5-HT systems are quite sensitive to stress (described below), and treadmill training can be a potent stressor (Brown et al. 2007) that produces inconsistent results in animal models of stress-related disorders (Burghardt et al. 2004; Greenwood et al. 2013; Hong et al. 2015; Kim et al. 2015). There is recent evidence that the stressor intensity of the treadmill training protocol could contribute to the divergent effects of treadmill training on stress-resistance and emotional behavior. Otsuka et al. (2015) ran rats on a treadmill for 30 min at varying intensities. Only low-intensity training reduced anxiety- and depression-like behavior in the elevated plus maze and forced swim test, respectively. High-intensity treadmill running activated the HPA axis as indicated by cFos expression in corticotropin releasing factor-containing neurons in the hypothalamus, and did not alter behavior (Otsuka et al. 2015). Another recent study reported that although both high and low intensity forced treadmill training increased dentate gyrus volume and cell proliferation, high-intensity exercise increased circulating corticosterone, whereas only low-intensity treadmill training increased the survival of the newly born neurons (Inoue et al. 2015).
In the remainder of the current review, we briefly summarize prior work on the effects of exercise on 5-HT systems, add discussion of new data, and consider the signals by which exercise could communicate with central 5-HT systems. In particular, we discuss data suggesting that thermoregulation could be an important factor signaling the experience of exercise to the central 5-HT system.
Populations of 5-HT Neurons Differ in Their Roles in Stress and Emotional Behavior
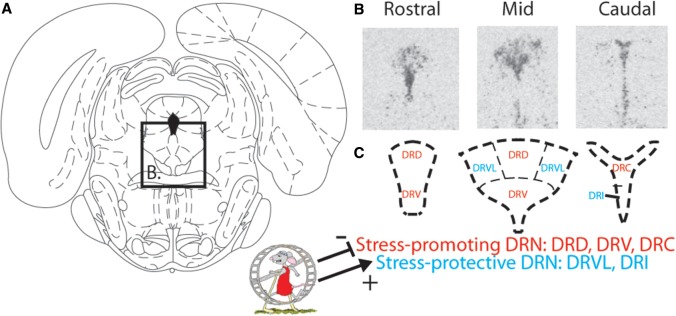
The majority of 5-HT innervating the forebrain in vertebrates originate in the midbrain DRN. The role of DRN 5-HT neurons in behavioral responses to stress and emotional behavior has been extensively investigated (Graeff et al. 1996; Clark & Neumaier 2001; Lowry et al. 2005; Maier & Watkins 2005; Richardson-Jones et al. 2010; Paul & Lowry 2013; Paul et al. 2014). One theme that emerges from this work is that 5-HT neurons in the DRN are heterogeneous in terms of receptor expression, projection sites, and roles in emotional behavior (Figure 2). For example, subsets of DRN 5-HT neurons located in the rodent dorsal and ventral aspects of the mid-DRN, and in the dorsal aspect of the caudal DRN, seem to contribute to the development of anxiety and depression after stress (Lowry et al. 2008). Within this stress-promoting region of the DRN are 5-HT neurons projecting to the basolateral amygdala and dorsal striatum (Commons et al. 2003; Hale et al. 2008, 2012), structures critical for processing fear- and anxiety-related stimuli (LeDoux 2003) and goal-directed learning (Yin et al. 2005), respectively; and in which 5-HT can elicit anxiety and deficits in goal-directed learning through a mechanism involving 5-HT2CR (Christianson et al. 2010; Strong et al. 2011; Greenwood et al. 2012; Christianson & Greenwood 2014). Consistent with a role for subsets of DRN 5-HT neurons promoting stress and anxiety are the observations that exposure to diverse anxiety-eliciting drugs (Abrams et al. 2005; Lawther et al. 2015), social defeat (Gardner et al. 2005), an anxiogenic open-field (Hale et al. 2008), or uncontrollable stress (Grahn 1999; Greenwood et al. 2003a), all potently and selectively activate 5-HT neurons in these stress-promoting DRN subregions. Moreover, controllable stress, which does not produce anxiety and in fact leads to protection against future uncontrollable stressors, inhibits activity of these stress-promoting DRN 5-HT subsets (Amat et al. 2005, 2010).
Figure 2.
Dual role of DRN serotonergic (5-HT) neurons in stress and emotional behavior. (A) Cartoon of the rat midbrain modified from the Rat Brain Atlas in Stereotaxic Coordinates (Paxinos, 1998) with the location of the DRN highlighted. (B) Coronal sections of the rat DRN showing expression of tryptophan hydroxylase 2 mRNA labeled with in situ hybridization at rostral, mid, and caudal DRN levels. (C) Cartoon depicting a simplified version of subpopulations of DRN 5-HT neurons involved, in general, in the promotion of stress and anxiety behaviors (stress-promoting DRN 5-HT neurons; red), or in stress coping and anxiolytic effects (stress-protective DRN 5-HT neurons; blue). Exercise constrains activation of stress-promoting DRN 5-HT neurons and activates stress-protective DRN 5-HT neurons. DRD, dorsal aspect of the DRN; DRV, ventral aspect of the DRN; DRVL, ventrolateral aspect of the DRN; DRC, caudal aspect of the DRN; DRI, intrafascicular region of the DRN.
In contrast to the stress-promoting DRN 5-HT neurons, 5-HT neurons located in the ventrolateral and interfascicular regions of the DRN are implicated in inhibition of panic, enhanced stress coping, and anxiolytic responses (Figure 2; Hale et al. 2012, 2013; Paul & Lowry 2013; Paul et al. 2014). These 5-HT neurons project heavily to the hippocampus, medial septum, and regions of the prefrontal cortex thought to be dysregulated in depressed subjects (Drevets 2000). The idea that distinct 5-HT systems modulate affective state, whereby one promotes anxiety and depression and the other stress coping, allows the possibility that disruption in either system (i.e., hyperactivity of the stress-promoting region or hypoactivity of the stress-protective region) could lead to stress-related disorders. Indeed, this dual role of 5-HT in emotional behavior could explain discrepancies in the literature regarding the role of 5-HT in depression.
Exercise Shifts Activity of Stress-Responsive 5-HT Systems from Stress-Promoting to Stress-Protective
Given the potential dual role of DRN 5-HT neurons in emotional and stress-related behaviors, it is possible that exercise could promote stress resistance by either attenuating activity of stress-promoting 5-HT systems and/or increasing activation of stress-protective 5-HT systems. There is evidence that exercise does both. Six weeks of voluntary wheel running attenuates the number of DRN 5-HT neurons expressing the neural activity marker c-Fos after acute exposure to an uncontrollable stressor (Greenwood et al. 2003a). The most robust effect of exercise was observed in the dorsal and ventral regions of the rostral—mid DRN; those same regions implicated in promoting anxiety and deleterious behavioral effects of stress. Exposure to the same acute stressor potentiates 5-HT efflux in the dorsal striatum in response to subsequent exposure to mild stress, which can interfere with goal-directed learning through a mechanism involving 5-HT2CR (Strong et al. 2011). Six weeks of voluntary wheel running also prevented the stress-induced exaggerated 5-HT efflux in the dorsal striatum (Clark et al. 2015) and the corresponding deficits in goal-directed escape learning (Greenwood et al. 2003a).
Interestingly, constraint over activation of stress-responsive 5-HT systems after exercise was also observed in male Anolis carolinensis lizards. Acute restraint stress increased the 5HIAA/5-HT ratio in the striatum and nucleus accumbens, indicative of high 5-HT activity in these regions. Acute exercise reversed this stress-induced 5-HT activity (Emerson et al. 2000). It makes sense that constraint over activity of striatum-projecting 5-HT neurons would be a conserved exercise adaptation. Serotonin in discrete brain regions including the striatum is thought to contribute to the initiation of central fatigue during exercise (Bailey et al. 1992; 1993b; Davis & Bailey 1997), perhaps by reducing DA efflux required to maintain motor activity (De Deurwaerdere et al. 2004). Thus, in addition to protection against deleterious emotional effects of stress, the development of constraint over activation of stress- and fatigue-promoting DRN 5-HT systems after chronic exercise could serve to delay the onset of fatigue during intense exercise bouts, a common training effect.
Exercise not only results in dynamic changes in serotonergic responses to stress, it also produces static changes in gene expression within cell body regions and terminal sites of central 5-HT circuits. Indeed, a microarray analysis indicated that voluntary wheel running dramatically alters gene expression within laser-captured neurons of the stress- and anxiety-promoting region of the DRN (Loughridge et al. 2013). One of the DRN genes that increases in expression after exercise is the gene coding for the 5-HT1A inhibitory autoreceptor (Loughridge et al. 2013). Notably, the increase in 5-HT1A mRNA occurs in the same region of the DRN showing the biggest attenuation in activity after stressor exposure in physically active rats (Greenwood et al. 2003a, 2005b), and in a time course consistent with the protective effect of exercise against stress-induced anxiety- and depression-like behaviors (Greenwood et al. 2005a). Because 5-HT1A mRNA is almost exclusively co-localized with 5-HT transporter mRNA in the DRN (Day et al. 2004), and wheel running attenuates activity of DRN 5-HT neurons in the stress-promoting DRN, it is a reasonable assumption that the difference in 5-HT1A mRNA in the DRN produced by exercise results in a functional increase in 5-HT1A autoreceptors. An increase in 5-HT1A-mediated autoinhibition is one mechanism by which exercise could lead to constraint over activity of stress-promoting DRN 5-HT neurons during exposure to stress.
Exercise also alters expression of 5-HT-related genes in terminal regions of the DRN. For example, 6 weeks of voluntary wheel running reduces 5-HT2CR mRNA in the dorsal striatum and amygdala (Greenwood et al. 2012). Adaptation in these regions could be particularly important for effects of exercise on emotional behavior. Thus, exercise seems to produce protection against the stress-promoting effects of 5-HT at multiple levels: one being within the DRN through constraint of neural activity, and another being resistance to the effects of 5-HT through downregulation of post-synaptic receptors. Consistent with this idea are the observations that wheel running produces resistance to the depression- and anxiety-like behaviors elicited by microinjection of a 5-HT2CR agonist directly into the dorsal striatum or amygdala (Greenwood et al. 2012), as well as by rapid increases in extracellular 5-HT elicited by acute systemic administration of a selective 5-HT reuptake-inhibitor (Greenwood et al. 2008).
Constraint over stress-induced 5-HT signaling, particularly at 5-HT2 receptors, could not only be an important contributor to the stress-buffering effects of exercise on emotional behavior, but could also contribute to memory-boosting effects of exercise in the face of stress. In addition to interfering with goal-directed learning through a serotonergic mechanism in the striatum (Strong et al. 2011), stress can damage hippocampal circuits leading to memory impairments (McEwen et al. 2015). Although whether beneficial effects of exercise extend to protection against stress-induced memory impairments is still an active area of inquiry, several observations suggest that it would. First, exercise can prevent the hippocampal-dependent memory impairments and amnesia produced by acute or chronic stress (Fleshner et al. 2014; Patki et al. 2014). Second, exercise prevents reductions in hippocampal BDNF mRNA and protein produced by exposure to a severe acute stressor (Adlard & Cotman 2004; Greenwood et al. 2007). Unlike the effect of NE on BDNF which is stimulatory, the effects of 5-HT on BDNF are mixed. Consistent with divergent behavioral effects of different subpopulations of 5-HT neurons is the observation that 5-HT seems to have opposing effects on BDNF mRNA in different brain regions. For example, although 5-HT increases BDNF in the cortex (Vaidya et al. 1997), activation of 5-HT2 receptors in the hippocampus is necessary (Vaidya et al. 1999) and sufficient (Vaidya et al. 1997; Fleshner et al. 2014) for stress-induced reductions in BDNF. Therefore, constraint over 5-HT neural activity could help preserve hippocampal-dependent memory even in the face of stress. Together, these data suggest that constraint over stress-promoting DRN 5-HT systems is a conserved effect of exercise that could contribute to resistance against both stress-induced anxiety- and depression-like behavior, as well as impaired cognitive function.
Exercise not only attenuates activity of stress and anxiety-promoting 5-HT systems, it also activates anxiolytic 5-HT systems. Recording from DRN 5-HT neurons during physical activity in cats and rats, Jacobs and Fornal demonstrated that 5-HT neuronal activity is acutely responsive to exercise (Jacobs & Fornal 1993; 1997; 1999). These data led Jacobs and Fornal to hypothesize that midbrain 5-HT neurons play a pivotal role in the control of movement. A plethora of additional data, including 1 report in Anolis carolinensis lizards (Emerson et al. 2000), are consistent with 5-HT responding to acute exercise (Chaouloff 1989; Chaouloff et al. 1989; Dey et al. 1992; Bailey et al. 1993a). The majority of this prior work, however, utilized forced running on a treadmill. Data suggesting increases in 5-HT activity after voluntary exercise are limited (Dishman 1997a). Voluntary wheel running elicits the biggest changes in gene expression in the rostral DRN (Greenwood et al. 2005b), the region of the DRN most intimately connected with the motor region of the basal ganglia (Steinbusch et al. 1981). Interestingly, axons of some 5-HT neurons in the rostral DRN have been reported to branch and project to both the basal ganglia and the amygdala (Imai et al. 1986); thus, providing a potential site of interplay between motor activity and emotional behavior within the 5-HT system. Although there is limited evidence that repeated activity of the 5-HT system during exercise is necessary for the stress-protective effects of exercise, one study reported that depleting 5-HT with PCPA prevented the antidepressant-like effects of voluntary wheel running in the forced swim and tail suspension tests, 2 rodent models of depression-like behavior (Cunha et al. 2012). Together, these data suggest that exercise recruits 5-HTsystems, but whether exercise-induced activation is independent of an aversive stress response and is selective to stress-protective populations of DRN 5-HT neurons remains to be determined.
Potential Signals Communicating the Effects of Exercise to the 5-HT System
It is not clear what signals drive the changes in the central 5-HT system after exercise, but several top-down and bottom-up signaling possibilities are provided in Figure 1. One possibility is that repeatedly choosing to exercise and exerting control over that exercise elicits plasticity in prefrontal-cortical-striatal circuits capable of inhibiting stress-promoting DRN 5-HT neurons during stress (Maier et al. 2006; Maier 2015). However, the observation that the protective effect of exercise occurs in rats with lesions of the prefrontal cortex does not support this mechanism (Greenwood et al. 2013; Christianson & Greenwood 2014). An additional scenario is communication between brain reward circuits and the serotonergic system. Indeed, both voluntary (Lett et al. 2000; Greenwood et al. 2011) and forced (Herrera et al. forthcoming) wheel running are rewarding and activate central reward circuitry. Neurons in the nucleus accumbens responding to rewarding stimuli could communicate with DRN 5-HT neurons either through direct projections (Zhang et al. 2013) or indirectly through the habenula (Hong & Hikosaka 2008). In addition to these top-down exercise signals, 5-HT neurons could simply respond to incoming motor-related sensory information and, as such, be a primary player in driving motor activity, as hypothesized by Jacobs and Fornal (1993). Repeated top-down recruitment of 5-HT systems during exercise could then lead to plastic changes within stress-promoting DRN 5-HT systems, which could contribute to the constraint over their activity during stressor exposure. Future studies are required to test this hypothesis.
In addition to responding to top-down exercise signals, DRN 5-HT neurons could be signaled by peripheral, bottom-up factors such as the vagus nerve, as well as growth factors. Similar to the sensitivity of LC NE neurons to growth factors, BDNF receptor TrkB is expressed on DRN 5-HT neurons (Madhav et al. 2001), and intra-DRN administration of BDNF altered firing patterns of DRN 5-HT neurons (Celada et al. 1996). It is also possible that input from the LC drives the changes in gene expression observed in the stress-promoting 5-HT system in response to exercise. Noradrenergic projections from the LC to the DRN target the rostral portion of the DRN most heavily (Peyron et al. 1996), and it is the rostral region of the DRN that shows the greatest response to wheel running (Greenwood et al. 2005b).
An additional bottom-up signaling factor not previously discussed is thermoregulation during exercise. Serotonergic neurons are part of the central circuitry underlying thermoregulatory cooling (Lowry et al. 2009; Madden & Morrison 2010). Increases in ambient heat, core, and skin temperature activate DRN 5-HT neurons via the spinal–parabrachial pathway (Hale et al. 2011). Notably, whereas stress-promoting populations of DRN 5-HT neurons respond minimally to warm temperature; the stress-protective regions of the DRN include the temperature-sensitive 5-HT neuron population (Hale et al. 2011, 2013). Activation of the stress-protective DRN by increases in body temperature is consistent with emerging data suggesting that increasing temperature is indeed anxiolytic and antidepressant (reviewed in Raison et al. 2014)). A small clinical trial in adults diagnosed with major depressive disorder, for example, demonstrated that whole-body hyperthermia can reduce depression symptoms (Hanusch et al. 2013). Moreover, human functional magnetic resonance imagery studies reveal that feelings of pleasantness elicited by warm (41 °C) cutaneous stimuli activates the mid-orbitofrontal cortex, pregenual cingulate cortex, and the ventral striatum (Rolls et al. 2008); areas that are dysregulated in depression (Drevets et al. 2008) and that receive 5-HT projections from the stress-protective DRN (Van Bockstaele et al. 1993). These data support the hypothesis proposed by Lowry & colleagues that function of the temperature-sensitive, stress-protective DRN is impaired in depressed patients, and successful antidepressant strategies restore function in this area (Lowry et al. 2009; Hale et al. 2013; Raison et al. 2014). Consistent with this idea are the observations that depressed patients have impaired thermoregulatory cooling (Ward & Doerr 1986), and a common “side effect” of antidepressant drugs is an increase in sweating (i.e., thermoregulatory cooling; Marcy & Britton 2005). These data allow the hypothesis that, similar to the acute antidepressant effect of increases in temperature, the transient increase in temperature and thermoregulatory cooling during exercise could be responsible for the mood-enhancing effects of exercise by activating the temperature-sensitive, stress-protective DRN. Indeed, data indicate that the temperature-sensitive DRN 5-HT neurons are responsive to exercise. In a preliminary study, rats with a history of 6 weeks of access to running wheels were sacrificed during the peak of their active cycle and immunohistochemistry was used to measure the effect of acute exercise on immediate early genes. Compared with rats housed with locked wheels, chronic runners responded with an increase in FosB/ΔFosB in 5-HT neurons of the interfascicular DRN (Arnold 2015), one of the regions responding most robustly to increases in temperature (Hale et al. 2011; Hale et al. 2013). In summary, it is possible that either top-down or bottom-up exercise signals including thermoregulation could lead to the changes in gene expression observed in the central 5-HT system after exercise, constraint over 5-HT neural activity, and stress resistance.
Summary
Physical activity enhances cognitive function and improves stress resistance and mental health. A variety of plastic changes contribute to the effects of exercise, including structural changes within brain circuits, enhanced neurotrophic support, neurogenesis, and modulation of gene expression. Many of the effects of exercise on brain and behavior are observed in a variety of species; suggesting ubiquitous exercise signaling mechanisms that are conserved among vertebrates. Longstanding and recent data are consistent with the idea that the conserved monoamines NE and 5-HT could mediate the effects of exercise on brain circuits involved in cognition and stress. NE and 5-HT systems are sensitive to both peripheral signals and central neural circuits recruited during exercise; thus, they represent important nodes of convergence between top-down and bottom-up exercise signals. Exercise signals potentially targeting central monoaminergic systems include those originating from the periphery, such as microbial products, myokines, adipokines, growth factors, glucocorticoids, and temperature; as well as those originating from within-brain circuits recruited during exercise such as primary motor regions (cortex, basal ganglia, cerebellum), prefrontal–striatal controllability circuits, reticular activating system-arousal centers, reward circuits, and sensory-motor circuits involved in the cognitive engagement occurring during exercise.
Considering the array of signals capable of recruiting NE and 5-HT systems during exercise, it is unlikely that any one of these signals is going to be solely necessary for the beneficial impact of exercise on brain function and behavior. However, it is possible that signals differ in their importance for mediating specific exercise effects. Information regarding which type, duration, and intensity of exercise optimally recruit specific exercise signals could therefore be potentially useful to tailor exercise programs to specific benefits. Finally, one of the primary uses of exercise neuroscience research could be as a tool to identify novel signals and targets that, although most likely not solely necessary for exercise effects, could be sufficient to act independently of exercise as cognitive enhancers or stress prophylactic strategies.
References
- Abrams JK, Johnson PL, Hay-Schmidt A, Mikkelsen JD, Shekhar A et al. , 2005. Serotonergic systems associated with arousal and vigilance behaviors following administration of anxiogenic drugs. Neuroscience 133:983–997. [DOI] [PubMed] [Google Scholar]
- Adlard PA, Cotman CW, 2004. Voluntary exercise protects against stress-induced decreases in brain-derived neurotrophic factor protein expression. Neuroscience 124:985–992. [DOI] [PubMed] [Google Scholar]
- Akbarian S, Rios M, Liu RJ, Gold SJ, Fong HF et al. , 2002. Brain-derived neurotrophic factor is essential for opiate-induced plasticity of noradrenergic neurons. J Neurosci 22:4153–4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akers KG, Martinez-Canabal A, Restivo L, Yiu AP, De Cristofaro A et al. , 2014. Hippocampal neurogenesis regulates forgetting during adulthood and infancy. Science 344:598–602. [DOI] [PubMed] [Google Scholar]
- Albeck DS, Sano K, Prewitt GE, Dalton L, 2006. Mild forced treadmill exercise enhances spatial learning in the aged rat. Behav Brain Res 168:345–348. [DOI] [PubMed] [Google Scholar]
- Amat J, Aleksejev RM, Paul E, Watkins LR, Maier SF, 2010. Behavioral control over shock blocks behavioral and neurochemical effects of later social defeat. Neuroscience 165:1031–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amat J, Baratta MV, Paul E, Bland ST, Watkins LR et al. , 2005. Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nat Neurosci 8:365–371. [DOI] [PubMed] [Google Scholar]
- Arnold M GB, McArthur JA, Clark PJ, Fleshner M, Lowry CA, 2015. Effects of repeated voluntary or forced exercise on rat brain serotonergic systems. Society for Neuroscience 2013 Abstract Viewer/Itinerary Planner [cited 2016 February 26]. Available from: http://www.abstractsonline.com/Plan/ViewAbstract.aspx?sKey= e58dbe80-9cf3-4b20-9770-dcd79b4f6acf&cKey = ce110a0d-8aa4-4bab-8e99-8cf5ab183edd&mKey=d0ff4555-8574-4fbb-b9d4-04eec8ba0c84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asmundson GJ, Fetzner MG, Deboer LB, Powers MB, Otto MW et al. , 2013. Let’s get physical: a contemporary review of the anxiolytic effects of exercise for anxiety and its disorders. Dep Anx 30:362–373. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Shipley MT, Chouvet G, Ennis M, van Bockstaele E et al. , 1991. Afferent regulation of locus coeruleus neurons: anatomy, physiology and pharmacology. Prog Brain Res 88:47–75. [DOI] [PubMed] [Google Scholar]
- Babyak M, Blumenthal JA, Herman S, Khatri P, Doraiswamy M et al. , 2000. Exercise treatment for major depression: maintenance of therapeutic benefit at 10 months. Psychosom Med 62:633–638. [DOI] [PubMed] [Google Scholar]
- Bailey SP, Davis JM, Ahlborn EN, 1992. Effect of increased brain serotonergic activity on endurance performance in the rat. Acta Physiol Scand 145:75–76. [DOI] [PubMed] [Google Scholar]
- Bailey SP, Davis JM, Ahlborn EN, 1993a. Neuroendocrine and substrate responses to altered brain 5-HT activity during prolonged exercise to fatigue. J Appl Physiol 74:3006–3012. [DOI] [PubMed] [Google Scholar]
- Bailey SP, Davis JM, Ahlborn EN, 1993b. Serotonergic agonists and antagonists affect endurance performance in the rat. Int J Sports Med 14:330–333. [DOI] [PubMed] [Google Scholar]
- Berlau DJ, McGaugh JL, 2006. Enhancement of extinction memory consolidation: the role of the noradrenergic and GABAergic systems within the basolateral amygdala. Neurobiol Learn Mem 86:123–132. [DOI] [PubMed] [Google Scholar]
- Berridge CW, Spencer RC, 2015. Differential cognitive actions of norepinephrine a2 and a1 receptor signaling in the prefrontal cortex. Brain Res . doi: 10.1016/j.brainres.2015.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjornebekk A, Mathe AA, Brene S, 2005. The antidepressant effect of running is associated with increased hippocampal cell proliferation. Int J Neuropsychopharmacol 8:357–368. [DOI] [PubMed] [Google Scholar]
- Blumenthal JA, Smith PJ, Hoffman BM, 2012. Is Exercise a viable treatment for depression? ACSMs Health Fit J 16:14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolijn S LP, 2015. How the body talks to the brain; peripheral mediators of physical activity-induced proliferation in the adult hippocampus. Brain Plast 1:5–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L et al. , 2012. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 481:463–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks SJ, Nilsson EK, Jacobsson JA, Stein DJ, Fredriksson R et al. , 2014. BDNF polymorphisms are linked to poorer working memory performance, reduced cerebellar and hippocampal volumes and differences in prefrontal cortex in a Swedish elderly population. PLoS ONE 9:e82707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown BS, Payne T, Kim C, Moore G, Krebs P et al. , 1979. Chronic response of rat brain norepinephrine and serotonin levels to endurance training. J Appl Physiol 46:19–23. [DOI] [PubMed] [Google Scholar]
- Brown BS, Van Huss W, 1973. Exercise and rat brain catecholamines. J Appl Physiol 34:664–669. [DOI] [PubMed] [Google Scholar]
- Brown DA, Johnson MS, Armstrong CJ, Lynch JM, Caruso NM et al. , 2007. Short-term treadmill running in the rat: what kind of stressor is it? J Appl Physiol 103:1979–1985. [DOI] [PubMed] [Google Scholar]
- Budde H, Voelcker-Rehage C, Pietrabyk-Kendziorra S, Ribeiro P, Tidow G, 2008. Acute coordinative exercise improves attentional performance in adolescents. Neurosci Lett 441:219–223. [DOI] [PubMed] [Google Scholar]
- Burghardt PR, Fulk LJ, Hand GA, Wilson MA, 2004. The effects of chronic treadmill and wheel running on behavior in rats. Brain Res 1019:84–96. [DOI] [PubMed] [Google Scholar]
- Campeau S, Nyhuis TJ, Sasse SK, Kryskow EM, Herlihy L et al. , 2010. Hypothalamic pituitary adrenal axis responses to low-intensity stressors are reduced after voluntary wheel running in rats. J Neuroendocrinol 22:872–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carro E, Nunez A, Busiguina S, Torres-Aleman I, 2000. Circulating insulin-like growth factor I mediates effects of exercise on the brain. J Neurosci 20:2926–2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassilhas RC, Tufik S, de Mello MT, 2015. Physical exercise, neuroplasticity, spatial learning and memory. Cell Mol Life Sci 73(5):975–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro V, Grisdale-Helland B, Helland SJ, Kristensen T, Jorgensen SM et al. , 2011. Aerobic training stimulates growth and promotes disease resistance in Atlantic salmon Salmo salar. Comp Biochem Physiol A Mol Integr Physiol 160:278–290. [DOI] [PubMed] [Google Scholar]
- Celada P, Siuciak JA, Tran TM, Altar CA, Tepper JM, 1996. Local infusion of brain-derived neurotrophic factor modifies the firing pattern of dorsal raphe serotonergic neurons. Brain Res 712:293–298. [DOI] [PubMed] [Google Scholar]
- Chae CH, Lee HC, Jung SL, Kim TW, Kim JH et al. , 2012. Swimming exercise increases the level of nerve growth factor and stimulates neurogenesis in adult rat hippocampus. Neuroscience 212:30–37. [DOI] [PubMed] [Google Scholar]
- Chaouloff F, 1989. Physical exercise and brain monoamines: a review. Acta Physiol Scand 137:1–13. [DOI] [PubMed] [Google Scholar]
- Chaouloff F, 1994. Influence of physical exercise on 5-HT1A receptor-and anxiety-related behaviours. Neurosci Lett 176:226–230. [DOI] [PubMed] [Google Scholar]
- Chaouloff F, Laude D, Elghozi JL, 1989. PHysical exercise: evidence for differential consequences of tryptophan on 5-HT synthesis and metabolism in central serotonergic cell bodies and terminals. J Neural Transm 78:121–130. [DOI] [PubMed] [Google Scholar]
- Chen MJ, Russo-Neustadt AA, 2009. Running exercise-induced up-regulation of hippocampal brain-derived neurotrophic factor is CREB-dependent. Hippocampus 19:962–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson JP, Greenwood BN, 2014. Stress-protective neural circuits: not all roads lead through the prefrontal cortex. Stress 17:1–12. [DOI] [PubMed] [Google Scholar]
- Christianson JP, Ragole T, Amat J, Greenwood BN, Strong PV et al. , 2010. 5-hydroxytryptamine 2C receptors in the basolateral amygdala are involved in the expression of anxiety after uncontrollable traumatic stress. Biol Psychiatry 67:339–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark MS, Neumaier JF, 2001. The 5-HT1B receptor: behavioral implications. Psychopharmacol Bull 35:170–185. [PubMed] [Google Scholar]
- Clark PJ, Amat J, McConnell SO, Ghasem PR, Greenwood BN et al. , 2015. Running reduces uncontrollable stress-evoked serotonin and potentiates stress-evoked dopamine concentrations in the rat dorsal striatum. PLoS ONE 10:e0141898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark PJ, Brzezinska WJ, Thomas MW, Ryzhenko NA, Toshkov SA et al. , 2008. Intact neurogenesis is required for benefits of exercise on spatial memory but not motor performance or contextual fear conditioning in C57BL/6J mice. Neuroscience 155:1048–1058. [DOI] [PubMed] [Google Scholar]
- Coelho FG, Gobbi S, Andreatto CA, Corazza DI, Pedroso RV et al. , 2013. Physical exercise modulates peripheral levels of brain-derived neurotrophic factor (BDNF): a systematic review of experimental studies in the elderly. Arch Gerontol Geriatrics 56:10–15. [DOI] [PubMed] [Google Scholar]
- Comery TA, Shah R, Greenough WT, 1995. Differential rearing alters spine density on medium-sized spiny neurons in the rat corpus striatum: evidence for association of morphological plasticity with early response gene expression. Neurobiol Learn Mem 63:217–219. [DOI] [PubMed] [Google Scholar]
- Commons KG, Connolley KR, Valentino RJ, 2003. A neurochemically distinct dorsal raphe-limbic circuit with a potential role in affective disorders. Neuropsychopharmacology 28:206–215. [DOI] [PubMed] [Google Scholar]
- Conti AC, Cryan JF, Dalvi A, Lucki I, Blendy JA, 2002. cAMP response element-binding protein is essential for the upregulation of brain-derived neurotrophic factor transcription, but not the behavioral or endocrine responses to antidepressant drugs. J Neurosci 22:3262–3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC, Christie LA, 2007. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci 30:464–472. [DOI] [PubMed] [Google Scholar]
- Cunha MP, Oliveira A, Pazini FL, Machado DG, Bettio LE et al. , 2012. The antidepressant-like effect of physical activity in the voluntary running wheel. Med Sci Sports Exerc 45(5):851–859. [DOI] [PubMed] [Google Scholar]
- David FJ, Robichaud JA, Leurgans SE, Poon C, Kohrt WM et al. , 2015. Exercise improves cognition in Parkinson’s disease: the PRET-PD randomized, clinical trial. Mov Disord 30:1657–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JM, Bailey SP, 1997. Possible mechanisms of central nervous system fatigue during exercise. Med Sci Sports Exerc 29:45–57. [DOI] [PubMed] [Google Scholar]
- Day HE, Greenwood BN, Hammack SE, Watkins LR, Fleshner M et al. , 2004. Differential expression of 5HT-1A, alpha1b adrenergic, CRF-R1, and CRF-R2 receptor mRNA in serotonergic, gamma-aminobutyric acidergic, and catecholaminergic cells of the rat dorsal raphe nucleus. J Comp Neurol 474:364–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Deurwaerdere P, Navailles S, Berg KA, Clarke WP, Spampinato U, 2004. Constitutive activity of the serotonin 2C receptor inhibits in vivo dopamine release in the rat striatum and nucleus accumbens. J Neurosci 24:3235–3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey S, Singh RH, Dey PK, 1992. Exercise training: significance of regional alterations in serotonin metabolism of rat brain in relation to antidepressant effect of exercise. Physiol Behav 52:1095–1099. [DOI] [PubMed] [Google Scholar]
- Ding Q, Vaynman S, Akhavan M, Ying Z, Gomez-Pinilla F, 2006. Insulin-like growth factor I interfaces with brain-derived neurotrophic factor-mediated synaptic plasticity to modulate aspects of exercise-induced cognitive function. Neuroscience 140:823–833. [DOI] [PubMed] [Google Scholar]
- Ding Q, Ying Z, Gomez-Pinilla F, 2011. Exercise influences hippocampal plasticity by modulating brain-derived neurotrophic factor processing. Neuroscience 192:773–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dishman RK, 1997a. Brain monoamines, exercise, and behavioral stress: animal models. Med Sci Sports Exerc 29: 63–74. [DOI] [PubMed] [Google Scholar]
- Dishman RK, 1997b. The Norepinephrine Hypothesis. Washington, DC: Taylor & Francis. [Google Scholar]
- Dishman RK, Renner KJ, White-Welkley JE, Burke KA, Bunnell BN, 2000. Treadmill exercise training augments brain norepinephrine response to familiar and novel stress. Brain Res Bull 52:337–342. [DOI] [PubMed] [Google Scholar]
- Dishman RK, Renner KJ, Youngstedt SD, Reigle TG, Bunnell BN et al. , 1997. Activity wheel running reduces escape latency and alters brain monoamine levels after footshock. Brain Res Bull 42:399–406. [DOI] [PubMed] [Google Scholar]
- Do-Monte FH, Kincheski GC, Pavesi E, Sordi R, Assreuy J et al. , 2010. Role of beta-adrenergic receptors in the ventromedial prefrontal cortex during contextual fear extinction in rats. Neurobiol Learn Mem 94:318–328. [DOI] [PubMed] [Google Scholar]
- Drevets WC, 2000. Functional anatomical abnormalities in limbic and prefrontal cortical structures in major depression. Prog Brain Res 126:413–431. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Furey ML, 2008. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struc Func 213:93–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droste SK, Schweizer MC, Ulbricht S, Reul JM, 2006. Long-term voluntary exercise and the mouse hypothalamic-pituitary-adrenocortical axis: impact of concurrent treatment with the antidepressant drug tianeptine. J Neuroendocrinol 18:915–925. [DOI] [PubMed] [Google Scholar]
- Duman CH, Schlesinger L, Russell DS, Duman RS, 2008. Voluntary exercise produces antidepressant and anxiolytic behavioral effects in mice. Brain Res 1199:148–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman CH, Schlesinger L, Terwilliger R, Russell DS, Newton SS et al. , 2009. Peripheral insulin-like growth factor-I produces antidepressant-like behavior and contributes to the effect of exercise. Behav Brain Res 198:366–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, Monteggia LM, 2006. A neurotrophic model for stress-related mood disorders. Biol Psychiatry 59:1116–1127. [DOI] [PubMed] [Google Scholar]
- Dunn A, Reigle T, Youngstedt S, Armstrong R, Dishman R, 1996a. Brain norepinephrine and metabolites after treadmill training and wheel running in rats. Med Sci Sports Exerc 28:204–209. [DOI] [PubMed] [Google Scholar]
- Dunn AL, Dishman RK, 1991. Exercise and the neurobiology of depression. Exerc Sport Sci Rev 19:41–98. [PubMed] [Google Scholar]
- Dunn AL, Reigle TG, Youngstedt SD, Armstrong RB, Dishman RK, 1996b. Brain monoamines and metabolites after treadmill training and wheel running in rats. Med Sci Sports Exerc 28:204–209. [DOI] [PubMed] [Google Scholar]
- Eadie BD, Redila VA, Christie BR, 2005. Voluntary exercise alters the cytoarchitecture of the adult dentate gyrus by increasing cellular proliferation, dendritic complexity, and spine density. J Comp Neurol 486:39–47. [DOI] [PubMed] [Google Scholar]
- Emerson AJ, Kappenman DP, Ronan PJ, Renner KJ, Summers CH, 2000. Stress induces rapid changes in serotonergic activity: restraint and exertion. Behav Brain Res 111:83–92. [DOI] [PubMed] [Google Scholar]
- Fan Y, Liu Z, Weinstein PR, Fike JR, Liu J, 2007. Environmental enrichment enhances neurogenesis and improves functional outcome after cranial irradiation. Eur J Neurosci 25:38–46. [DOI] [PubMed] [Google Scholar]
- Fischer A, 2015. Environmental enrichment as a method to improve cognitive function: what can we learn from animal models? Neuroimage. doi: 10.1016/j.neuroimage.2015.11.039. [DOI] [PubMed] [Google Scholar]
- Fleshner M, Greenwood BN, Yirmiya R, 2014. Neuronal-glial mechanisms of exercise-evoked stress robustness. Curr Top Behav Neurosci 18:1–12. [DOI] [PubMed] [Google Scholar]
- Frankland PW, Kohler S, Josselyn SA, 2013. Hippocampal neurogenesis and forgetting. Trends Neurosci 36:497–503. [DOI] [PubMed] [Google Scholar]
- Fuss J, Ben Abdallah NM, Hensley FW, Weber KJ, Hellweg R et al. , 2010. Deletion of running–induced hippocampal neurogenesis by irradiation prevents development of an anxious phenotype in mice. PLoS ONE 5(9):e12769 doi: 10.1371/journal.pone.0012769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia C, Chen MJ, Garza AA, Cotman CW, Russo-Neustadt A, 2003. The influence of specific noradrenergic and serotonergic lesions on the expression of hippocampal brain-derived neurotrophic factor transcripts following voluntary physical activity. Neuroscience 119:721–732. [DOI] [PubMed] [Google Scholar]
- Gardner KL, Thrivikraman KV, Lightman SL, Plotsky PM, Lowry CA, 2005. Early life experience alters behavior during social defeat: focus on serotonergic systems. Neuroscience 136:181–191. [DOI] [PubMed] [Google Scholar]
- Goehler LE, Gaykema RP, Hansen MK, Anderson K, Maier SF et al. , 2000. Vagal immune-to-brain communication: a visceral chemosensory pathway. Auton Neurosci 85:49–59. [DOI] [PubMed] [Google Scholar]
- Gomez-Pinilla F, Dao L, So V, 1997. Physical exercise induces FGF-2 and its mRNA in the hippocampus. Brain Res 764:1–8. [DOI] [PubMed] [Google Scholar]
- Gomez-Pinilla F, Hillman C, 2013. The influence of exercise on cognitive abilities. Compr Physiol 3:403–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Pinilla F, Vaynman S, Ying Z, 2008. Brain-derived neurotrophic factor functions as a metabotrophin to mediate the effects of exercise on cognition. Eur J Neurosci 28:2278–2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graeff FG, Guimaraes FS, De Andrade TG, Deakin JF, 1996. Role of 5-HT in stress, anxiety, and depression. Pharmacol Biochem Behav 54:129–141. [DOI] [PubMed] [Google Scholar]
- Grahn RE, Will MJ, Hammack SE, Maswood S, McQueen MB et al. , 1999. Activation of serotonin-immunoreactive cells in the dorsal raphe nucleus in rats exposed to an uncontrollable stressor. Brain Res 826:35–43. [DOI] [PubMed] [Google Scholar]
- Greenwood BN, Fleshner M, 2011. Exercise, stress resistance, and central serotonergic systems. Exerc Sport Sci Rev 39:140–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood BN, Fleshner M, 2013. Mechanisms Underlying the Relationship between Physical Activity and Anxiety: Animal Data. New York: Routledge. [Google Scholar]
- Greenwood BN, Foley TE, Burhans D, Maier SF, Fleshner M, 2005a. The consequences of uncontrollable stress are sensitive to duration of prior wheel running. Brain Res 1033:164–178. [DOI] [PubMed] [Google Scholar]
- Greenwood BN, Foley TE, Day HE, Burhans D, Brooks L et al. , 2005b. Wheel running alters serotonin (5-HT) transporter, 5-HT(1A), 5-HT(1B), and alpha(1b)-adrenergic receptor mRNA in the rat raphe nuclei. Biol Psychiatry 57:559–568. [DOI] [PubMed] [Google Scholar]
- Greenwood BN, Foley TE, Day HE, Campisi J, Hammack SH et al. , 2003a. Freewheel running prevents learned helplessness/behavioral depression: role of dorsal raphe serotonergic neurons. J Neurosci 23:2889–2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood BN, Foley TE, Le TV, Strong PV, Loughridge AB et al. , 2011. Long-term voluntary wheel running is rewarding and produces plasticity in the mesolimbic reward pathway. Behav Brain Res 217:354–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood BN, Kennedy S, Smith TP, Campeau S, Day HE et al. , 2003b. Voluntary freewheel running selectively modulates catecholamine content in peripheral tissue and c-Fos expression in the central sympathetic circuit following exposure to uncontrollable stress in rats. Neuroscience 120:269–281. [DOI] [PubMed] [Google Scholar]
- Greenwood BN, Spence KG, Crevling DM, Clark PJ, Craig WC et al. , 2013. Exercise-induced stress resistance is independent of exercise controllability and the medial prefrontal cortex. Eur J Neurosci 37:469–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood BN, Strong PV, Brooks L, Fleshner M, 2008. Anxiety-like behaviors produced by acute fluoxetine administration in male Fischer 344 rats are prevented by prior exercise. Psychopharmacology 199:209–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood BN, Strong PV, Foley TE, Fleshner M, 2009. A behavioral analysis of the impact of voluntary physical activity on hippocampus-dependent contextual conditioning. Hippocampus 19:988–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood BN, Strong PV, Foley TE, Thompson RS, Fleshner M, 2007. Learned helplessness is independent of levels of brain-derived neurotrophic factor in the hippocampus. Neuroscience 144:1193–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood BN, Strong PV, Loughridge AB, Day HE, Clark PJ et al. , 2012. 5-HT(2C) receptors in the basolateral amygdala and dorsal striatum are a novel target for the anxiolytic and antidepressant effects of exercise. PloS ONE 7:e46118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griesbach GS, Hovda DA, Gomez-Pinilla F, 2009. Exercise-induced improvement in cognitive performance after traumatic brain injury in rats is dependent on BDNF activation. Brain Res 1288:105–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin EW, Bechara RG, Birch AM, Kelly AM, 2009. Exercise enhances hippocampal-dependent learning in the rat: evidence for a BDNF-related mechanism. Hippocampus 19:973–980. [DOI] [PubMed] [Google Scholar]
- Grimberg-Henrici CG, Vermaak P, Elizabeth Bolhuis J, Nordquist RE, van der Staay FJ, 2015. Effects of environmental enrichment on cognitive performance of pigs in a spatial holeboard discrimination task. Anim Cogn 19(2):271–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyon A, Massa F, Rovere C, Nahon JL, 2008. How cytokines can influence the brain: a role for chemokines? J Neuroimmunol 198:46–55. [DOI] [PubMed] [Google Scholar]
- Hale MW, Dady KF, Evans AK, Lowry CA, 2011. Evidence for in vivo thermosensitivity of serotonergic neurons in the rat dorsal raphe nucleus and raphe pallidus nucleus implicated in thermoregulatory cooling. Exp Neurol 227:264–278. [DOI] [PubMed] [Google Scholar]
- Hale MW, Hay-Schmidt A, Mikkelsen JD, Poulsen B, Bouwknecht JA et al. , 2008. Exposure to an open-field arena increases c-Fos expression in a subpopulation of neurons in the dorsal raphe nucleus, including neurons projecting to the basolateral amygdaloid complex. Neuroscience 157:733–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale MW, Raison CL, Lowry CA, 2013. Integrative physiology of depression and antidepressant drug action: implications for serotonergic mechanisms of action and novel therapeutic strategies for treatment of depression. Pharmacol Therapeut 137:108–118. [DOI] [PubMed] [Google Scholar]
- Hale MW, Shekhar A, Lowry CA, 2012. Stress-related serotonergic systems: implications for symptomatology of anxiety and affective disorders. Cellul Mol Neurobiol 32:695–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall ZJ, Bauchinger U, Gerson AR, Price ER, Langlois LA et al. , 2014. Site-specific regulation of adult neurogenesis by dietary fatty acid content, vitamin E and flight exercise in European starlings. Eur J Neurosci 39:875–882. [DOI] [PubMed] [Google Scholar]
- Hamilton GF, Rhodes JS, 2015. Exercise regulation of cognitive function and neuroplasticity in the healthy and diseased brain. Prog Mol Biol Transl Sci 135:381–406. [DOI] [PubMed] [Google Scholar]
- Hanusch KU, Janssen CH, Billheimer D, Jenkins I, Spurgeon E et al. , 2013. Whole-body hyperthermia for the treatment of major depression: associations with thermoregulatory cooling. Am J Psychiatry 170:802–804. [DOI] [PubMed] [Google Scholar]
- Hernandez MD, Mendiola P, de Costa J, Zamora S, 2002. Effects of intense exercise training on rainbow trout growth, body composition and metabolic responses. J Physiol Biochem 58:1–7. [DOI] [PubMed] [Google Scholar]
- Herrera JJ, Fedynska S, Ghasem PR, Wieman T, Clark PJ et al. . Forthcoming. Neurochemical and behavioral indices of exercise reward are independent of exercise controllability. Eur J Neurosci. doi:10.1111/ejn.13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring MP, O'Connor PJ, Dishman RK, 2010. The effect of exercise training on anxiety symptoms among patients: a systematic review. Arch Inter Med 170:321–331. [DOI] [PubMed] [Google Scholar]
- Hillman CH, Pontifex MB, Castelli DM, Khan NA, Raine LB et al. , 2014. Effects of the FITKids randomized controlled trial on executive control and brain function. Pediatrics 134:e1063–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman CH, Snook EM, Jerome GJ, 2003. Acute cardiovascular exercise and executive control function. Int J Psychophysiol 48:307–314. [DOI] [PubMed] [Google Scholar]
- Holm PC, Rodriguez FJ, Kresse A, Canals JM, Silos-Santiago I et al. , 2003. Crucial role of TrkB ligands in the survival and phenotypic differentiation of developing locus coeruleus noradrenergic neurons. Development. 130:3535–3545. [DOI] [PubMed] [Google Scholar]
- Hong S, Hikosaka O, 2008. The globus pallidus sends reward-related signals to the lateral habenula. Neuron 60:720–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong YP, Lee HC, Kim HT, 2015. Treadmill exercise after social isolation increases the levels of NGF, BDNF, and synapsin I to induce survival of neurons in the hippocampus, and improves depression-like behavior. J Exerc Nutrition Biochem 19:11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hullinger R, O'Riordan K, Burger C, 2015. Environmental enrichment improves learning and memory and long-term potentiation in young adult rats through a mechanism requiring mGluR5 signaling and sustained activation of p70s6k. Neurobiol Learn Mem 125:126–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai H, Steindler DA, Kitai ST, 1986. The organization of divergent axonal projections from the midbrain raphe nuclei in the rat. J Comp Neurol 243:363–380. [DOI] [PubMed] [Google Scholar]
- Inoue K, Okamoto M, Shibato J, Lee MC, Matsui T et al. , 2015. Long-term mild, rather than intense, exercise enhances adult hippocampal neurogenesis and greatly changes the transcriptomic profile of the hippocampus. PLoS ONE 10:e0128720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivy AS, Rodriguez FG, Garcia C, Chen MJ, Russo-Neustadt AA, 2003. Noradrenergic and serotonergic blockade inhibits BDNF mRNA activation following exercise and antidepressant. Pharmacol Biochem Behav 75:81–88. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Fornal CA, 1993. 5-HT and motor control: a hypothesis. Trends Neurosci 16:346–352. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Fornal CA, 1997. Serotonin and motor activity. Curr Opin Neurobiol 7:820–825. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Fornal CA, 1999. Activity of serotonergic neurons in behaving animals. Neuropsychopharmacology 21:9S–15S. [DOI] [PubMed] [Google Scholar]
- Ji MH, Wang XM, Sun XR, Zhang H, Ju LS et al. , 2015. Environmental enrichment ameliorates neonatal sevoflurane exposure-induced cognitive and synaptic plasticity impairments. J Mol Neurosci 57:358–365. [DOI] [PubMed] [Google Scholar]
- Kim TW, Lim BV, Kim K, Seo JH, Kim CJ, 2015. Treadmill exercise alleviates stress-induced impairment of social interaction through 5-hydroxytryptamine 1A receptor activation in rats. J Exerc Rehabil 11:192–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knab AM, Lightfoot JT, 2010. Does the difference between physically active and couch potato lie in the dopamine system? Int J Biol Sci 6: 133–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobilo T, Liu QR, Gandhi K, Mughal M, Shaham Y et al. , 2011. Running is the neurogenic and neurotrophic stimulus in environmental enrichment. Learn Mem 18:605–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer AF, Erickson KI, 2007. Capitalizing on cortical plasticity: influence of physical activity on cognition and brain function. Trends Cogn Sci 11:342–348. [DOI] [PubMed] [Google Scholar]
- Kramer AF, Erickson KI, Colcombe SJ, 2006. Exercise, cognition, and the aging brain. J Appl Physiol 101:1237–1242. [DOI] [PubMed] [Google Scholar]
- Lawther AJ, Clissold ML, Ma S, Kent S, Lowry CA et al. , 2015. Anxiogenic drug administration and elevated plus-maze exposure in rats activate populations of relaxin-3 neurons in the nucleus incertus and serotonergic neurons in the dorsal raphe nucleus. Neuroscience 303:270–284. [DOI] [PubMed] [Google Scholar]
- Leasure JL, Jones M, 2008. Forced and voluntary exercise differentially affect brain and behavior. Neuroscience 156:456–465. [DOI] [PubMed] [Google Scholar]
- LeDoux J, 2003. The emotional brain, fear, and the amygdala. Cell Mol Neurobiol 23:727–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lett BT, Grant VL, Byrne MJ, Koh MT, 2000. Pairings of a distinctive chamber with the aftereffect of wheel running produce conditioned place preference. Appetite 34:87–94. [DOI] [PubMed] [Google Scholar]
- Li H, Liang A, Guan F, Fan R, Chi L et al. , 2013. Regular treadmill running improves spatial learning and memory performance in young mice through increased hippocampal neurogenesis and decreased stress. Brain Res 1531:1–8. [DOI] [PubMed] [Google Scholar]
- Llorens-Martin M, Torres-Aleman I, Trejo JL, 2010. Exercise modulates insulin-like growth factor 1-dependent and -independent effects on adult hippocampal neurogenesis and behaviour. Mol Cell Neurosci 44:109–117. [DOI] [PubMed] [Google Scholar]
- Loughridge AB, Greenwood BN, Day HE, McQueen MB, Fleshner M, 2013. Microarray analyses reveal novel targets of exercise-induced stress resistance in the dorsal raphe nucleus. Front Behav Neurosci 7:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry CA, Hale MW, Evans AK, Heerkens J, Staub DR et al. , 2008. Serotonergic systems, anxiety, and affective disorder: focus on the dorsomedial part of the dorsal raphe nucleus. Ann NY Acad Sci 1148:86–94. [DOI] [PubMed] [Google Scholar]
- Lowry CA, Johnson PL, Hay-Schmidt A, Mikkelsen J, Shekhar A, 2005. Modulation of anxiety circuits by serotonergic systems. Stress 8:233–246. [DOI] [PubMed] [Google Scholar]
- Lowry CA, Lightman SL, Nutt DJ, 2009. That warm fuzzy feeling: brain serotonergic neurons and the regulation of emotion. J Psychopharmacol 23:392–400. [DOI] [PubMed] [Google Scholar]
- Luchiari AC, Chacon DM, 2013. Physical exercise improves learning in zebrafish, Danio rerio. Behav Processes 100:44–47. [DOI] [PubMed] [Google Scholar]
- Maass A, Duzel S, Brigadski T, Goerke M, Becke A et al. , 2015. Relationships of peripheral IGF-1, VEGF and BDNF levels to exercise-related changes in memory, hippocampal perfusion and volumes in older adults. Neuroimage. doi: 10.1016/j.neuroimage.2015.10.084. [DOI] [PubMed] [Google Scholar]
- Madden CJ, Morrison SF, 2010. Endogenous activation of spinal 5-hydroxytryptamine (5-HT) receptors contributes to the thermoregulatory activation of brown adipose tissue. Am J Physiol Regul Integr Comp Physiol 298:R776–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhav TR, Pei Q, Zetterstrom TS, 2001. Serotonergic cells of the rat raphe nuclei express mRNA of tyrosine kinase B (trkB), the high-affinity receptor for brain derived neurotrophic factor (BDNF). Brain Res Mol Brain Res 93:56–63. [DOI] [PubMed] [Google Scholar]
- Maier SF, 2015. Behavioral control blunts reactions to contemporaneous and future adverse events: medial prefrontal cortex plasticity and a corticostriatal network. Neurobiol Stress 1:12–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier SF, Amat J, Baratta MV, Paul E, Watkins LR, 2006. Behavioral control, the medial prefrontal cortex, and resilience. Dialogues Clin Neurosci 8:397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier SF, Watkins LR, 2005. Stressor controllability and learned helplessness: the roles of the dorsal raphe nucleus, serotonin, and corticotropin-releasing factor. Neurosci Biobehav Rev 29:829–841. [DOI] [PubMed] [Google Scholar]
- Manta S, Dong J, Debonnel G, Blier P, 2009. Optimization of vagus nerve stimulation parameters using the firing activity of serotonin neurons in the rat dorsal raphe. Eur Neuropsychopharmacol 19:250–255. [DOI] [PubMed] [Google Scholar]
- Manta S, El Mansari M, Debonnel G, Blier P, 2013. Electrophysiological and neurochemical effects of long-term vagus nerve stimulation on the rat monoaminergic systems. Int J Neuropsychopharmacol 16:459–470. [DOI] [PubMed] [Google Scholar]
- Marais L, Stein DJ, Daniels WM, 2009. Exercise increases BDNF levels in the striatum and decreases depressive-like behavior in chronically stressed rats. Metab Brain Dis 24:587–597. [DOI] [PubMed] [Google Scholar]
- Marcy TR, Britton ML, 2005. Antidepressant-induced sweating. Ann Pharmacother 39:748–752. [DOI] [PubMed] [Google Scholar]
- Markham JA, Herting MM, Luszpak AE, Juraska JM, Greenough WT, 2009. Myelination of the corpus callosum in male and female rats following complex environment housing during adulthood. Brain Res 1288:9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masini CV, Sauer S, Campeau S, 2005. Ferret odor as a processive stress model in rats: neurochemical, behavioral, and endocrine evidence. Behav Neurosci 119:280–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsunaga W, Isobe K, Shirokawa T, 2006. Involvement of neurotrophic factors in aging of noradrenergic innervations in hippocampus and frontal cortex. Neurosci Res 54:313–318. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Bowles NP, Gray JD, Hill MN, Hunter RG et al. , 2015. Mechanisms of stress in the brain. Nat Neurosci 18:1353–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina JA, Netto TL, Muszkat M, Medina AC, Botter D et al. , 2010. Exercise impact on sustained attention of ADHD children, methylphenidate effects. Atten Defic Hyperact Disord 2:49–58. [DOI] [PubMed] [Google Scholar]
- Merrill AH, Wang E, Mullins R E, Grindeland R E, Popova IA, 1993. Analyses of plasma for metabolic and hormonal changes in rats flown aboard COSMOS 2044. J Appl Physiol 54:214–226. [DOI] [PubMed] [Google Scholar]
- Mika A, Bouchet CA, Bunker P, Hellwinkel JE, Spence KG et al. , 2015a. Voluntary exercise during extinction of auditory fear conditioning reduces the relapse of fear associated with potentiated activity of striatal direct pathway neurons. Neurobiol Learn Mem 125:224–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mika A, Fleshner M, 2015. Early-life exercise may promote lasting brain and metabolic health through gut bacterial metabolites. Immunol Cell Biol 94(2):151–157. [DOI] [PubMed] [Google Scholar]
- Mika A, Van Treuren W, Gonzalez A, Herrera JJ, Knight R et al. , 2015b. Exercise is more effective at altering gut microbial composition and producing stable changes in lean mass in juvenile versus adult male F344 rats. PLoS ONE 10:e0125889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montag C, Felten A, Markett S, Fischer L, Winkel K et al. , 2014. The role of the BDNF Val66Met polymorphism in individual differences in long-term memory capacity. J Mol Neurosci 54:796–802. [DOI] [PubMed] [Google Scholar]
- Monteiro-Junior RS, Cevada T, Oliveira BR, Lattari E, Portugal EM et al. , 2015. We need to move more: neurobiological hypotheses of physical exercise as a treatment for Parkinson’s disease. Med Hypotheses 85:537–541. [DOI] [PubMed] [Google Scholar]
- Morgan JA, Corrigan F, Baune BT, 2015. Effects of physical exercise on central nervous system functions: a review of brain region specific adaptations. J Mol Psychiatry 3:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RW, Bouton ME, 2007. The effect of yohimbine on the extinction of conditioned fear: a role for context. Behav Neurosci 121:501–514. [DOI] [PubMed] [Google Scholar]
- Murray DK, Sacheli MA, Eng JJ, Stoessl AJ, 2014. The effects of exercise on cognition in Parkinson’s disease: a systematic review. Transl Neurodegener 3:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustroph ML, Chen S, Desai SC, Cay EB, DeYoung EK et al. , 2012. Aerobic exercise is the critical variable in an enriched environment that increases hippocampal neurogenesis and water maze learning in male C57BL/6J mice. Neuroscience 219:62–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustroph ML, Merritt JR, Holloway AL, Pinardo H, Miller DS et al. , 2015. Increased adult hippocampal neurogenesis is not necessary for wheel running to abolish conditioned place preference for cocaine in mice. Eur J Neurosci 41:216–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai S, Matsunaga W, Ishida Y, Isobe K, Shirokawa T, 2006. Effects of BDNF infusion on the axon terminals of locus coeruleus neurons of aging rats. Neurosci Res 54:213–219. [DOI] [PubMed] [Google Scholar]
- Neeper SA, Gomez-Pinilla F, Choi J, Cotman C, 1995. Exercise and brain neurotrophins. Nature 373:109. [DOI] [PubMed] [Google Scholar]
- Neeper SA, Gomez-Pinilla F, Choi J, Cotman CW, 1996. Physical activity increases mRNA for brain-derived neurotrophic factor and nerve growth factor in rat brain. Brain Res 726:49–56. [PubMed] [Google Scholar]
- Newman CL, Motta RW, 2007. The effects of aerobic exercise on childhood PTSD, anxiety, and depression. Int J Emerg Ment Health 9:133–158. [PubMed] [Google Scholar]
- Novkovic T, Mittmann T, Manahan-Vaughan D, 2015. BDNF contributes to the facilitation of hippocampal synaptic plasticity and learning enabled by environmental enrichment. Hippocampus 25:1–15. [DOI] [PubMed] [Google Scholar]
- Nyhuis TJ, Masini CV, Sasse SK, Day HE, Campeau S, 2010. Physical activity, but not environmental complexity, facilitates HPA axis response habituation to repeated audiogenic stress despite neurotrophin mRNA regulation in both conditions. Brain Res 1362:68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliff HS, Berchtold NC, Isackson P, Cotman CW, 1998. Exercise-induced regulation of brain-derived neurotrophic factor (BDNF) transcripts in the rat hippocampus. Brain Res Mol Brain Res 61:147–153. [DOI] [PubMed] [Google Scholar]
- Ostman I, Nyback H, 1976. Adaptive changes in central and peripheral noradrenergic neurons in rats following chronic exercise. Neuroscience 1:41–47. [DOI] [PubMed] [Google Scholar]
- Otsuka T, Nishii A, Amemiya S, Kubota N, Nishijima T et al. , 2015. Effects of acute treadmill running at different intensities on activities of serotonin and corticotropin-releasing factor neurons, and anxiety- and depressive-like behaviors in rats. Behav Brain Res 298:44–51. [DOI] [PubMed] [Google Scholar]
- Ozbeyli D, Gokalp AG, Koral T, Ocal OY, Dogan B et al. , 2015. Protective effect of exercise and sildenafil on acute stress and cognitive function. Physiol Behav 151:230–237. [DOI] [PubMed] [Google Scholar]
- Park H, Poo MM, 2013. Neurotrophin regulation of neural circuit development and function. Nat Rev Neurosci 14: 7–23. [DOI] [PubMed] [Google Scholar]
- Patki G, Li L, Allam F, Solanki N, Dao AT et al. , 2014. Moderate treadmill exercise rescues anxiety and depression-like behavior as well as memory impairment in a rat model of posttraumatic stress disorder. Physiol Behav 130:47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul ED, Johnson PL, Shekhar A, Lowry CA, 2014. The Deakin/Graeff hypothesis: focus on serotonergic inhibition of panic. Neurosci Biobehav Rev [46 Pt] 3:379–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul ED, Lowry CA, 2013. Functional topography of serotonergic systems supports the Deakin/Graeff hypothesis of anxiety and affective disorders. J Psychopharmacol 27:1090–1106. [DOI] [PubMed] [Google Scholar]
- Pedersen BK, Febbraio MA, 2008. Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol Rev 88:1379–1406. [DOI] [PubMed] [Google Scholar]
- Peyron C, Luppi PH, Fort P, Rampon C, Jouvet M, 1996. Lower brainstem catecholamine afferents to the rat dorsal raphe nucleus. J Comp Neurol 364:402–413. [DOI] [PubMed] [Google Scholar]
- Poo MM, 2001. Neurotrophins as synaptic modulators. Nat Rev Neurosci 2:24–32. [DOI] [PubMed] [Google Scholar]
- Powers MB, Medina JL, Burns S, Kauffman BY, Monfils M et al. , 2015. Exercise augmentation of exposure therapy for PTSD: rationale and pilot efficacy data. Cogn Behav Ther 44:314–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash RS, Voss MW, Erickson KI, Kramer AF, 2015. Physical activity and cognitive vitality. Annu Rev Psychol 66:769–797. [DOI] [PubMed] [Google Scholar]
- Raison CL, Hale MW, Williams LE, Wager TD, Lowry CA, 2014. Somatic influences on subjective well-being and affective disorders: the convergence of thermosensory and central serotonergic systems. Front Psychol 5: 1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redila VA, Christie BR, 2006. Exercise-induced changes in dendritic structure and complexity in the adult hippocampal dentate gyrus. Neuroscience 137:1299–1307. [DOI] [PubMed] [Google Scholar]
- Rhyu IJ, Bytheway JA, Kohler SJ, Lange H, Lee KJ et al. , 2010. Effects of aerobic exercise training on cognitive function and cortical vascularity in monkeys. Neuroscience 167:1239–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson-Jones JW, Craige CP, Guiard BP, Stephen A, Metzger KL et al. , 2010. 5-HT1A autoreceptor levels determine vulnerability to stress and response to antidepressants. Neuron 65:40–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET, Grabenhorst F, Parris BA, 2008. Warm pleasant feelings in the brain. NeuroImage 41:1504–1513. [DOI] [PubMed] [Google Scholar]
- Rudy JW, Barrientos RM, O'Reilly RC, 2002. Hippocampal formation supports conditioning to memory of a context. Behav Neurosci 116:530–538. [DOI] [PubMed] [Google Scholar]
- Sara SJ, Bouret S, 2012. Orienting and reorienting: the locus coeruleus mediates cognition through arousal. Neuron 76:130–141. [DOI] [PubMed] [Google Scholar]
- Sasse SK, Greenwood BN, Masini CV, Nyhuis TJ, Fleshner M et al. , 2008. Chronic voluntary wheel running facilitates corticosterone response habituation to repeated audiogenic stress exposure in male rats. Stress 11:425–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasse SK, Nyhuis TJ, Masini CV, Day HE, Campeau S, 2013. Central gene expression changes associated with enhanced neuroendocrine and autonomic response habituation to repeated noise stress after voluntary wheel running in rats. Front Physiol 4:341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherder E, Scherder R, Verburgh L, Konigs M, Blom M et al. , 2014. Executive functions of sedentary elderly may benefit from walking: a systematic review and meta-analysis. Am J Geriatr Psychiatry 22:782–791. [DOI] [PubMed] [Google Scholar]
- Schobersberger W, Hobisch-Hagen P, Fries D, Wiedermann F, Rieder-Scharinger J et al. , 2000. Increase in immune activation, vascular endothelial growth factor and erythropoietin after an ultramarathon run at moderate altitude. Immunobiology 201:611–620. [DOI] [PubMed] [Google Scholar]
- Schwarz LA, Miyamichi K, Gao XJ, Beier KT, Weissbourd B et al. , 2015. Viral-genetic tracing of the input-output organization of a central noradrenaline circuit. Nature 524:88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciolino NR, Holmes PV, 2012. Exercise offers anxiolytic potential: a role for stress and brain noradrenergic-galaninergic mechanisms. Neurosci Biobehav Rev 36:1965–1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciolino NR, Smith JM, Stranahan AM, Freeman KG, Edwards GL et al. , 2015. Galanin mediates features of neural and behavioral stress resilience afforded by exercise. Neuropharmacology 89:255–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih PC, Yang YR, Wang RY, 2013. Effects of exercise intensity on spatial memory performance and hippocampal synaptic plasticity in transient brain ischemic rats. PLoS ONE 8:e78163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siette J, Reichelt AC, Westbrook RF, 2014. A bout of voluntary running enhances context conditioned fear, its extinction, and its reconsolidation. Learn Mem 21:73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair EL, NdSC, Ward AJW, Seebacher F, 2014. Exercise changes behavior. Functional Ecol 28:652–659. [Google Scholar]
- Steinbusch HW, Nieuwenhuys R, Verhofstad AA, Van der Kooy D, 1981. The nucleus raphe dorsalis of the rat and its projection upon the caudatoputamen: a combined cytoarchitectonic, immunohistochemical and retrograde transport study. J Physiol 77:157–174. [PubMed] [Google Scholar]
- Stonerock GL, Hoffman BM, Smith PJ, Blumenthal JA, 2015. Exercise as treatment for anxiety: systematic review and analysis. Ann Behav Med 49:542–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong PV, Christianson JP, Loughridge AB, Amat J, Maier SF et al. , 2011. 5-hydroxytryptamine 2C receptors in the dorsal striatum mediate stress-induced interference with negatively reinforced instrumental escape behavior. Neuroscience 197:132–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo C, Thiemermann C, We CC, Perretti M, Vane JR, 1994. Attenuation of the induction of nitric oxide synthase by endogeneous glucocorticoids accounts for endotoxin tolerance in vivo. Proc Natl Acad Sci USA 91: 271–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas AG, Dennis A, Rawlings NB, Stagg CJ, Matthews L et al. , 2015. Multi-modal characterization of rapid anterior hippocampal volume increase associated with aerobic exercise. Neuroimage. doi: 10.1016/j.neuroimage.2015.10.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinkerhess MJ, Ginzberg S, Piazza N, Wessells RJ, 2012a. Endurance training protocol and longitudinal performance assays for Drosophila melanogaster. J Vis Exp. doi: 10.3791/3786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinkerhess MJ, Healy L, Morgan M, Sujkowski A, Matthys E et al. , 2012b. The Drosophila PGC-1alpha homolog spargel modulates the physiological effects of endurance exercise. PLoS ONE 7:e31633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trejo JL, Carro E, Torres-Aleman I, 2001. Circulating insulin-like growth factor I mediates exercise-induced increases in the number of new neurons in the adult hippocampus. J Neurosci 21:1628–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trejo JL, Llorens-Martin MV, Torres-Aleman I, 2008. The effects of exercise on spatial learning and anxiety-like behavior are mediated by an IGF-I-dependent mechanism related to hippocampal neurogenesis. Mol Cell Neurosci 37:402–411. [DOI] [PubMed] [Google Scholar]
- Vaidya VA, Marek GJ, Aghajanian GK, Duman RS, 1997. 5-HT2A receptor-mediated regulation of brain-derived neurotrophic factor mRNA in the hippocampus and the neocortex. J Neurosci 17:2785–2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidya VA, Terwilliger RM, Duman RS, 1999. Role of 5-HT2A receptors in the stress-induced down-regulation of brain- derived neurotrophic factor expression in rat hippocampus. Neurosci Lett 262:1–4. [DOI] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Biswas A, Pickel VM, 1993. Topography of serotonin neurons in the dorsal raphe nucleus that send axon collaterals to the rat prefrontal cortex and nucleus accumbens. Brain Res 624:188–198. [DOI] [PubMed] [Google Scholar]
- Van der Borght K, Kobor-Nyakas DE, Klauke K, Eggen BJ, Nyakas C et al. , 2009. Physical exercise leads to rapid adaptations in hippocampal vasculature: temporal dynamics and relationship to cell proliferation and neurogenesis. Hippocampus 19:928–936. [DOI] [PubMed] [Google Scholar]
- Van Hoomissen JD, Holmes PV, Zellner AS, Poudevigne A, Dishman RK, 2004. Effects of beta-adrenoreceptor blockade during chronic exercise on contextual fear conditioning and mRNA for galanin and brain-derived neurotrophic factor. Behav Neurosci 118:1378–1390. [DOI] [PubMed] [Google Scholar]
- van Praag H, 2008. Neurogenesis and exercise: past and future directions. Neuromolecular Med 10:128–140. [DOI] [PubMed] [Google Scholar]
- van Praag H, Christie BR, Sejnowski TJ, Gage FH, 1999a. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci USA 96:13427–13431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH, 1999b. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci 2:266–270. [DOI] [PubMed] [Google Scholar]
- Vaynman S, Ying Z, Gomez-Pinilla F, 2004. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur J Neurosci 20:2580–2590. [DOI] [PubMed] [Google Scholar]
- Veena J, Srikumar BN, Mahati K, Bhagya V, Raju TR et al. , 2009a. Enriched environment restores hippocampal cell proliferation and ameliorates cognitive deficits in chronically stressed rats. J Neurosci Res 87:831–843. [DOI] [PubMed] [Google Scholar]
- Veena J, Srikumar BN, Raju TR, Shankaranarayana Rao BS, 2009b. Exposure to enriched environment restores the survival and differentiation of new born cells in the hippocampus and ameliorates depressive symptoms in chronically stressed rats. Neurosci Lett 455:178–182. [DOI] [PubMed] [Google Scholar]
- Voss MW, Erickson KI, Prakash RS, Chaddock L, Kim JS et al. , 2013. Neurobiological markers of exercise-related brain plasticity in older adults. Brain Behav Immun 28:90–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward NG, Doerr HO, 1986. Skin conductance. A potentially sensitive and specific marker for depression. J Nerv Ment Dis 174:553–559. [PubMed] [Google Scholar]
- Wrann CD, White JP, Salogiannnis J, Laznik-Bogoslavski D, Wu J et al. , 2013. Exercise induces hippocampal BDNF through a PGC-1alpha/FNDC5 pathway. Cell Metabol 18:649–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Coote JH, 1998. Influence of the hypothalamic paraventricular nucleus on cardiovascular neurones in the rostral ventrolateral medulla of the rat. J Physiol 513:521–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin HH, Ostlund SB, Knowlton BJ, Balleine BW, 2005. The role of the dorsomedial striatum in instrumental conditioning. Eur J Neurosci 22:513–523. [DOI] [PubMed] [Google Scholar]
- Zhang JP, Xu Q, Yuan XS, Cherasse Y, Schiffmann SN et al. , 2013. Projections of nucleus accumbens adenosine A2A receptor neurons in the mouse brain and their implications in mediating sleep-wake regulation. Front Neuroanat 7:43. [DOI] [PMC free article] [PubMed] [Google Scholar]