Abstract
Aggression is a common behavioral strategy employed by animals to secure limited resources, but must be applied with restraint to limit potential costs including injury. How animals make the adaptive decision to fight or flee is barely known. Here, we review our work on crickets that reveals the roles of biogenic amines, primarily octopamine (the insect analog of noradrenaline) and nitric oxide (NO). Using aminergic drugs, we found that amines are not essential for actually initiating aggression. However, octopamine is necessary for mediating the aggression-promoting effects of potentially rewarding experiences including stimulation with a male antenna, physical exertion, winning, and resource possession. Hence, octopamine can be considered as the motivational component of aggression. Imposed handicaps that impede aggressive signaling revealed that the agonistic actions of an opponent perceived during fighting act to reduce aggression, and that crickets make the decision to flee the moment the accumulated sum of such aversive experiences exceeds some critical level. Treatment with nitridergic drugs revealed that the impact of the opponent’s aggressive actions is mediated by NO. NO acts to suppress aggression by promoting the tendency to flee and is primarily responsible for the depressed aggressiveness of subordinates after social defeat. Octopamine and dopamine can each restore aggression in subordinates, but only dopamine is necessary for normal recovery. The role of serotonin remains unclear, and is discussed. We conclude that octopamine and NO control the decision to fight or flee by mediating the effects of potentially rewarding and aversive experiences, respectively.
Keywords: agonistic behavior, agonistic signals, assessment, decision-making, experience-dependent plasticity, insects, neuromodulation, octopamine, social behavior
Introduction
Aggression between individuals of the same species is commonplace throughout the Animal Kingdom, largely due to the fact that they compete for the same territories, shelters, food, and sexual partners. Aggression is, however, dangerous and must be excised with restraint to ensure that the costs do not exceed the potential gains of the disputed resource. To limit costs, animals have evolved stereotyped, gradually escalating contests (Maynard Smith 1974; Parker 1974; Hardy and Briffa 2013). These are typified by the ritualized exchange of agonistic signals, which are thought to enable individuals to accrue information on the contestants’ abilities to secure the disputed resource, that is, resource holding potential (Hurd 2006; Arnott and Elwood 2009; Elwood and Arnott 2012). An animal's resource holding potential clearly depends on physique (size, strength, weaponry), but also on an animal’s tendency to invest energy in fighting, that is, its aggressive motivation, a factor determined by the presence and value of the disputed resource, as well as a wide variety of experiences including previous physical exertion, victories, and defeats (Archer 1988; Hsu et al. 2006, 2009; Nelson 2006). From these considerations (summarized in Figure 1), it seems clear that animals must in some way weigh up the impact of a wide variety of past and present experiences to assess the odds of whether it would be more opportune to fight or to flee. However, the proximate, underlying mechanisms are barely known.
Figure 1.
Experience-dependent plasticity of aggression (modified from Stevenson and Rillich 2012). An individual’s chances of winning an aggressive encounter is given by its “resource holding potential” (RHP) which depends on physical factors (size, strength, weight, weaponry) as well as on “aggressive motivation,” a factor determined by numerous experiences (physical exertion, winning, losing, and the presence and value of resources such as territory, food and potential mates). On confronting a competitor, agonistic signals exchanged during escalating ritualized fighting are evaluated to assess RHP and to decide when it would be more opportune to persist in fighting or to flee (references in text). Our work strives to understand the underlying mechanisms.
Aggression, as many behaviors, is known to be modulated by biogenic amines and the unconventional neuromodulator nitric oxide (NO), which each act in various ways to bias its expression (Nelson 2006). This contribution reviews recent insights from our work that reveal how adult male crickets employ the neuromodulatory power of biogenic amines and NO to control the decision to fight or flee. These insects represent an ideal model system for investigating experience-dependent plasticity of aggressive behavior. For one, their spectacular fighting behavior lasts only a few seconds and is characterized by a stereotyped sequence of increasingly aggressive motor performances or levels of aggression (Figure 2) that establish clear winners that become more aggressive, and losers that become submissive (Alexander 1961; Adamo and Hoy 1995; Hofmann and Stevenson 2000). Second, their fighting behavior is influenced by a wide variety of factors (Figure 1) including size (Brown et al. 2006), weaponry (Judge and Bonnano 2008), age and time of day (Dixon and Cade 1986), as well as by diverse experiences including physical exertion (Hofmann and Stevenson 2000; Stevenson et al. 2000, 2005), winning (Khazraie and Campan 1999; Iwasaki et al. 2006; Rillich and Stevenson 2011), losing (Iwasaki et al. 2006; Stevenson and Rillich 2013), the presence of shelters (Rillich et al. 2011), food (Nosil 2002), or females (Tachon et al. 1999), courtship and mating (Killian and Allen 2008; Judge et al. 2010), their song (Brown et al. 2007; Rillich et al. 2009; DiRienzo et al. 2012), social isolation, and crowding (Adamo and Hoy 1995; Iba et al. 1995; Stevenson and Rillich 2013). Third, their brains contain comparatively few neurones, but are nonetheless equipped with the capacity to generate sophisticated social interactions (Huber et al. 1989; Giurfa 2012) and make adaptive decisions without necessitating rational, conscious emotions, or reason as also illustrated here by our work.
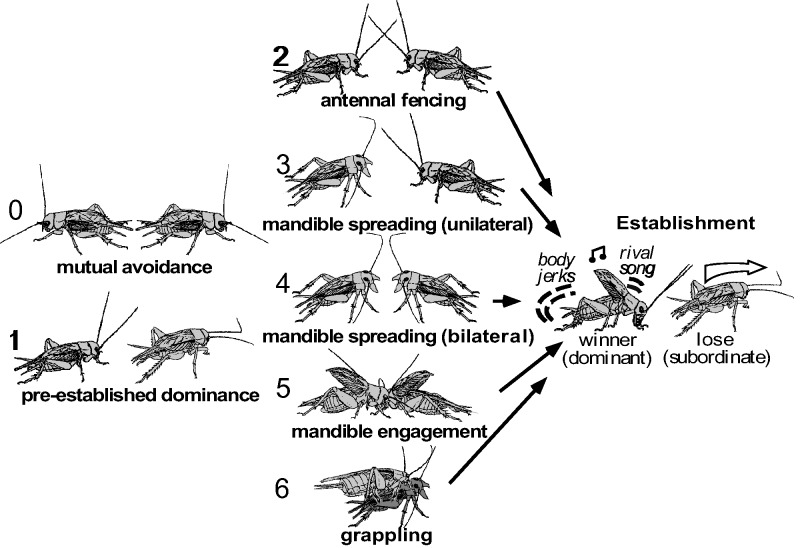
Figure 2.
Escalating levels of aggression for adult male crickets. Level 0 mutual avoidance: no aggressive interaction. Level 1 pre-established dominance: one cricket attacks, the other retreats. Level 2 antennal fencing. Level 3 mandible spreading (by one): one cricket displays spread mandibles. Level 4 mandible spreading (both): both crickets display spread mandibles. Level 5 mandible engagement: the mandibles interlock and the animals push against each other. Level 6 grappling: an all out fight, the animals may disengage and re-engage to bite other body parts. Fights are concluded at any level by 1 opponent retreating (the loser). The winner typically produces the rival song and body jerking movements, and is subsequently hyper-aggressive. The loser avoids contact to all conspecific males and exhibits reduced aggression for 3 or more hours after social defeat. Modified from Stevenson et al.(2005) in part redrawn from Huber et al. (1989).
The Decision to Fight and the Role of the Antennae
When 2 crickets meet, they are first faced with the choice of whether to fight or court. Work from various laboratories including our own has shown that the decision is influenced largely by mechanical and pheromonal cues that are exchanged during characteristic antennal fencing behavior on first contacting a conspecific (Adamo and Hoy 1995; Hofmann and Schildberger 2001; Nagamoto et al. 2005; Iwasaki and Katagiri 2008; Sakura and Aonuma 2013; Rillich and Stevenson 2015; see also Fernandez et al. 2010 on Drosophila). Hence, male courtship behavior (song production) can be readily evoked by simply lashing a male cricket’s antennae with the antenna from a female, whereas lashing a male’s antennae with either a bristle, a washed female antennae, or mere contact with male pheromone specifically releases agonistic behavior such as mandible spreading, a characteristic threat display (Alexander 1961; Iwasaki and Katagiri 2008; Rillich and Stevenson 2015). The exact nature of the pheromones involved is not known to date in crickets, but have been identified in fruit flies (Wang and Anderson 2010).
The Decision to Fight and the Role of Biogenic Amines
Aggressive behavior is correlated with changes in the levels of biogenic amines and aminergic function in numerous animals (mammals: Miczek and Fish 2006; Miczek et al. 2011; invertebrates: Kravitz and Huber 2003). In general terms, the adrenergic/noradrenergic system of mammals is viewed as preparing the animals for fight or flight as originally conceived by Cannon (1915). However, evidence for causal relationships between fluctuations in specific amines and the expression of aggressive behavior is surprisingly scarce and often inconsistent. Recent data nonetheless supports the idea in mammals that adrenaline/noradrenaline indeed promotes the expression of aggressive behavior (Nelson 2006; Nelson and Trainor 2007; Haden and Scarpa 2007).
Insects and other protostomes possess only trace amounts of noradrenaline/adrenaline, since in contrast to deuterostomes they convert the catecholamine substrate amino acid tyrosine first to tyramine and then octopamine, known only as trace amines in mammals (Evans 1985; Pflüger and Stevenson 2005). Similar to its catecholamine counterparts, octopamine is generally regarded to function as a neurotransmitter, neuromodulator, and neurohormone that also functions to prepare insects for dynamic actions, as exemplified by the fight or flight response (Libersat and Pflüger 2004; Verlinden et al. 2010). In crickets, increases in octopamine levels in the heamolymph or central nervous system are known to occur following a variety of experiences (Figure 3A) including flying, fighting, copulation, male antennal contact (Adamo et al. 1995), grouping (Iba et al. 1995), and even exposure to a mock predator (Adamo and Baker 2011). However, our work on crickets was the first to establish a causal relationship between octopamine and promotion of aggressive behavior (Stevenson et al. 2000; 2005), and this has since been verified in fruit flies (Hoyer et al. 2008; Zhou et al. 2008; Certel et al. 2010) and ants (Aonuma and Watanabe 2012).
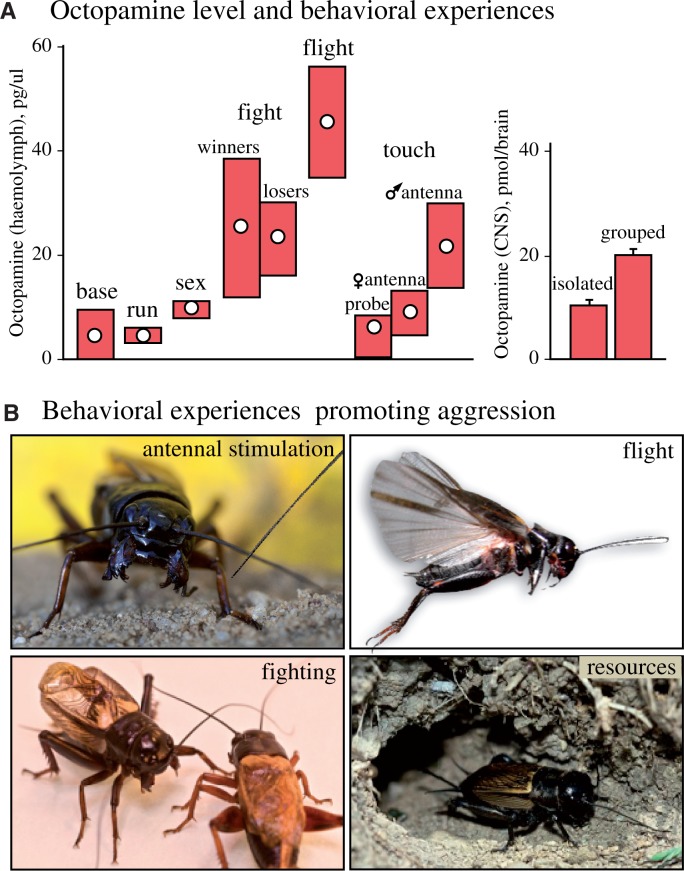
Figure 3.
Octopamine and aggression in crickets. (a) Bar chart giving changes in levels of octopamine following selected behavior. Left: hemolymph content of adult male crickets (pg/ul, circles median, bars interquartile range). Adopted from data generated by Adamo et al. 1995). Right: brain content (pmol/brain, bar mean, whisker standard error of mean) of adult crickets that were kept either in isolation or grouped. Adopted from data generated by Iba et al. 2005. (b) Behavioral experiences that influence aggression via the action of octopamine (references in text).
It is important to stress that amines are not necessary for actually initiating aggressive behavior. First, male crickets treated with alpha-methyl-tryrosine (AMT), a competitive inhibitor of octopamine and dopamine synthesis that effectively depletes these 2 amines from the cricket central nervous system (Sloley and Orikasa 1988), still interact aggressively and exhibit all basic components of normal fighting behavior, even though they are extremely lethargic, and reluctant to do so unless coaxed by antennal stimulation (Stevenson et al. 2000). Second, selective depletion of serotonin following treatment with its synthesis inhibitor alpha-methyl-tryptophan (AMTP) leads to general hyperactivity and enhanced responsiveness to escape-inducing stimuli, but again without noticeable influence on the expression of aggression (Stevenson et al. 2000, 2005). Confirming these observations, we more recently demonstrated (Rillich and Stevenson 2015) that the efficacy with which mechanical stimulation of a cricket’s antenna elicits the aggressive mandible threat response is unaffected by treatment with agents that selectively block insect octopamine receptors (epinastine), dopamine receptors (fluphenazine), or serotonin biosynthesis (AMTP).
Rather than acting as a releaser of aggressive behavior, amines modulate its expression depending on behavioral context and previous experiences. Specifically, octopamine functions to mediate the promoting effects of a wide variety of social and other experiences on aggressiveness (Figure 3B).
Antennal stimulation and the priming effect
Lashing a cricket’s antenna not only elicits the aggressive mandible threat display, but also leads to heightened expression of aggression of the stimulated animals in subsequently staged fights (Rillich and Stevenson 2015). This priming effect is particularly pronounced in subordinate crickets, which normally behave submissively. Interestingly, and in contrast to the aggression-releasing effect of antennal stimulation, the priming effect is only evident after prior stimulation with a male antenna, indicating that male pheromones are required for this. Moreover, while the aggression-releasing effect of antennal stimulation is unaffected by the octopamine-receptor antagonist epinastine, the subsequent priming effect is abolished by epinastine, but not, for example, by the insect dopamine-receptor blocker fluphenazin (Rillich and Stevenson 2015).
Physical exertion and the flight effect
In mammals, physical exertion can have wide and varied effects on the expression of aggression and related behaviors. In rodents, for example, moderate physical exercise can either subdue aggressive tendency (Hoffmann et al. 1987), or indirectly promote it by relieving behavioral depression and anxiety induced by social defeat (Patki et al. 2014). In crickets, physical exertion tends to increase aggressiveness in general, and also rescues from the depressing effects of social defeat (Hofmann and Stevenson 2000). Particularly, after an induced bout of flying, crickets nearly always escalate to the highest level of aggression and fight 2–3 times longer than usual. This flight effect is transient, and wanes over a period of approximately 15–45 min, which roughly corresponds to the time course of the increase in octopamine levels after flying (Adamo et al. 2005). Prior treatment with non-selective (reserpine) and semi-selective amine deleting agents (AMT, AMTP) as well as a variety of amine receptor blockers established that octopamine, rather than dopamine or serotonin, is necessary for mediating the flight effect in crickets (Stevenson et al. 2000, 2005). The flight effect also highlights the impact motor activity can have on seemingly unrelated behaviors. Whether the flight effect has any adaptive function remains to be established. Crickets can fly considerable distances (Walker and Masaki 1989), so that energetically costly flights might payoff by increasing the chances of securing key resources from rivals on arrival at a new habitat.
Victory and the winner effect
Winning an aggressive encounter with a conspecific is known to enhance an individual’s aggressiveness and likelihood to win a subsequent encounter in a wide variety of animals (Hsu et al. 2006, 2009; Rutte et al. 2006) including crickets (Khazraie and Campan 1999; Iwasaki et al. 2006; Rillich and Stevenson 2011). Insights into the proximate causes have only just begun to emerge from recent work that implicates essential roles for androgens in cichlid fish (Oliveira et al. 2009) and octopamine in insects (Rillich and Stevenson 2011).
In knockout tournaments between weight-matched crickets, the fights between winners of preceding contests become progressively more severe and longer (Rillich and Stevenson 2011). This winner effect was found to be transient and last less than 20 min, which is far shorter than in rodents (Fuxjager et al. 2010). Again, by investigating the effects of a pallet of aminergic drugs it was established that the winner effect in crickets is mediated specifically by octopamine rather than dopamine or tyramine (Rillich and Stevenson 2011). By repeatedly interrupting fights before either contestant scored a win, we also found that the physical exertion of fighting could alone enhance an individual’s aggressiveness. However, fights staged between crickets that previously had each only experienced an opponent retreating prior to any form of physical interaction, established that the mere sight of a submissive cricket is also sufficient to induce an equal if not greater winner effect. A similar phenomenon is also known in ants, where exposure to an opponent without physical fighting, increases the probability that it will display aggression in later encounters (Van Wilgenburg et al. 2010). Similarly, in humans, merely watching a previous victory elevates levels of the aggression promoting hormone testosterone (Carre and Putnam 2010). Crickets that won a previous fight are also significantly more likely to respond to antennal stimulation with the aggressive threat display (Rillich and Stevenson 2015). To our surprise, this aspect of the winner effect was not blocked by the octopamine-receptor antagonist epinastine. Antennal stimulation, thus, appears to have a promoting influence on aggression independent of octopamine, or indeed other amines.
Resources and the residency effect
Animals in possession of a key resource, an essentially non-physical experience, are also more likely to win disputes against contenders, but it is largely unknown how this is controlled (reviews: Kemp and Wiklund 2004; Hsu et al. 2006, 2009). Our work has revealed that octopamine mediates the promoting effect of possessing a resource in crickets (Rillich et al. 2011). In these insects, burrows are valuable assets offering shelter from predators and an aid to attract females that also prefer to mate with burrow owners, which are themselves inclined to fight more ferociously and ward off intruding males (Alexander 1961; Simmons 1986; Rodriguez-Munoz et al. 2011). The effect of owning a burrow is readily demonstrated in the laboratory by providing an initially submissive cricket with a dark, artificial burrow (e.g., a 35-mm film canister) into which it readily enters and remains. On removing the shelter, resident crickets are subsequently significantly more aggressive than control crickets without shelter and frequently win against aggressive intruders. As for the flight- and winner effects, this residency effect is transient, first becoming evident after at least 2 min of occupancy, maximal after 15 min, and wanes 15 min after removing the shelter. Thus, the residency effect does not depend on the initial sensory experience of shelter occupation per se. Increased aggressiveness with prolonged residency is known in many animal species (Cromarty et al. 1999) and is thought to reflect the perceived increase in resource value as the animal gathers more information on it and invests more time in it (Bradbury and Vehrencamp 1998). Our experiments using aminergic drugs, on the other hand, revealed the aggression-promoting experience of residency in crickets must somehow be mediated by octopamine, since it is prohibited in octopamine/dopamine depleted crickets and selectively blocked by treatment with octopamine antagonists, but unaffected by serotonin depletion (Rillich et al. 2011).
Social Isolation and Crowding
Although we found no direct involvement of octopamine or other amines, the influence of social isolation and crowding on aggression needs mention. Social isolation results in profound behavioral and physiological changes in mammals (Valzelli 1973; Fone and Porkess 2008; Cacioppo and Hawkley 2009) and insects (Lihoreau et al. 2009; Sokolowski 2010; Simpson and Stevenson 2015) that involve dramatic fluctuations in the levels of biogenic amines (insects: Iba et al. 1995; Rogers et al. 2004; Wada-Katsumata et al. 2011; mammals: Valzelli 1973; Jones et al. 1992; Hall et al. 1998). In general, isolation is also associated with increased aggression, whereas grouping reduces it in both vertebrates (Hsu et al. 2006, Ma et al. 2014) and insects including wasps (Pfennig and Reeve 1989), fruit flies (Zhou et al. 2008; Johnson et al. 2009), and crickets (Alexander 1961; Adamo and Hoy 1995; Iba et al. 1995). However, we found that crickets grouped in immediate proximity within individual mesh cages that permitting visual, and olfactory-mechanical antennal contact, were as aggressive as long-term isolates. Furthermore, when isolated crickets were grouped, they initially fought vigorous, but aggression then declined to the level of life-long grouped crickets within only 10 min. Conversely, grouped crickets regained the same level of aggressiveness of isolates after separation for only 3 h. Taken together our experiments revealed that social isolation in crickets simply allows the majority of animals taken from a grouped population to escape from social subjugation by a few dominant individuals in the group and thus recover their default state of aggressiveness as discussed below for the loser effect (Stevenson and Rillich 2013).
The Decision to Flee—Adding up the Odds
It is largely unknown how animals decide when it would be more opportune to flee. While prevailing assessment hypotheses (Payne 1998; Hurd 2006; Rutte et al. 2006; Arnott and Elwood 2009) agree that the decision is based on information gained from agonistic signals exchanged during fighting it is hotly debated who evaluates these signals (sender, receiver, or both), how they act on aggression (promote or suppress), and whether complex cognitive capacities are required (Arnott and Elwood 2009; Elwood and Arnott 2012; 2013; Fawcett and Mowles 2013).
By evaluating the effects of imposed handicaps that impede agonistic signalling, we found that crickets employ a simple algorithm for timing the decision to flee (Rillich et al. 2007). In the first set of experiments, we noted that pairs of crickets which both had either lamed mandibles or were both blinded by blackening their eyes would still fight each other with unabated ferocity. Surprisingly, the greater the number of handicaps the longer the animals fought. For example, pairs of crickets having lamed mandibles, blackened eyes, and clipped foreleg claws (used to flip an opponent) fought each other for over a minute rather than a few seconds as usual. This shows that crickets are unlikely to evaluate their own agonistic signals, and also that agonistic signals in effect must act to suppress aggression. Similarly, we also found that crickets with either blackened eyes or lamed mandibles also fought against untreated, equal sized opponents with almost unabated vigor and chance of winning. Surprisingly, however, “blinded” crickets won practically all fights against opponents with lamed mandibles (98% in an experiment where all animals were flown before to maximize aggressive motivation, Rillich et al. 2007; Figure 4 shows an example for non-flown crickets). The most parsimonious explanation for these novel findings is that rather than assessing their own, or comparing agonist signals (cf. Briffa 2008), crickets assess only their opponent’s agonistic signals for the decision to flee (for detailed accounts and arguments, see: Rillich et al. 2007; Stevenson and Rillich 2015). Accordingly, the “blinded” cricket persists longer in fighting and wins simply because it receives no visual and only limited physical input from an opponent with lamed mandibles, which is subjected to the full brunt of his adversary’s visual and physical actions and thus becomes the first to flee. Although we did not initially set out to test it, our results are fully conform with the core prediction of the Cumulative Assessment Model of Payne (1998) that an animal persists in fighting until the accumulated sum of the opponent's actions surpasses some critical threshold to flee. While it is also known for other behaviors, such as navigation and pathfinding (Chittka and Geiger 1995; Wittlinger et al. 2006) that insects have the capacity to sum up information for decision-making, it is not known how they do it.
Figure 4.
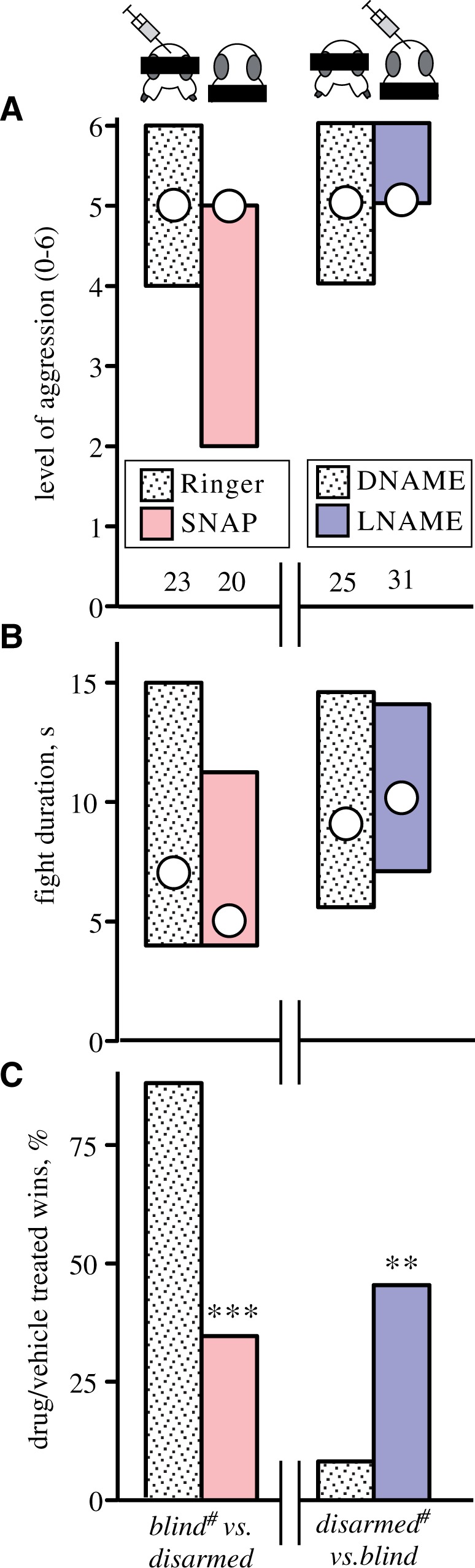
Effects of impaired agonistic signaling and nitridergic drugs on cricket aggression. (A) Level of aggression. (B) Fight duration (circles medians, bars interquartile ranges). (C) Win chances (%). In each case, male crickets deprived of visual information (blind) were matched against weight-matched males with disabled mandibles (disarmed), but 1 opponent (indicated by pictogram and # in the x-axis label) received either control solutions (gray bars, ringer, or DNAME the inactive enantiomer of LNAME) or the NO donor s-nitroso-n-acetyl-dl-penicillamine (SNAP, red bars), or the NO synthase inhibitor nitro-l-arginine methyl ester hydrochloride (LNAME blue bars). The number of fighting pairs is given above the top axis. Asterisks in (c) denote statistically significant differences (Chi-square test **, ***: p<, 0.01, 0.001, respectively). Adopted from Stevenson and Rillich (2015).
The Decision to Flee—the Role of NO
Our work on crickets revealed that the NO/cyclic guanosine 3,5-cyclic monophosphate (NO/cGMP) signaling pathway is a key component in the mechanism by which crickets add up the odds for the decision to flee (Stevenson and Rillich 2015). Once generated by the enzyme nitric oxide synthase (NOS), the unconventional gaseous neuromodulator NO is free to diffuse as a volume signal that can influence multiple targets by activating the intracellular second messenger cGMP via soluble guanylate cyclase (Müller 1997).
Corresponding to findings in mammals (Nelson et al. 1995;Trainor et al. 2007), treating crickets with a variety of established nitridergic drugs revealed that activators of the NO/cGMP pathway, such as the NO-donor SNAP (S-Nitroso-N-acetyl-DL-penicillamine), leads to less aggression, whereas treatment with inhibitors, such as LNAME (Nω-Nitro-L-arginine methyl ester hydrochloride), results in more aggressive and longer contests (Stevenson and Rillich 2015). While the behavioral role of NO/cGMP in mammalian aggression has not been specified, our experiments revealed that activation of this pathway mediates the impact of the opponent’s signaling efforts during fighting behavior. To show this, we evaluated the effects of nitridergic drugs on crickets that were handicapped to impede the exchange, and or, perception of agonistic signals during fighting (Figure 4). This first revealed that NO does not simply lower the tendency to escalate and invest time in fighting, that is, it does not reduce aggressive motivation. For example, blinded crickets treated with the NO-donor SNAP fought as harsh and as long as vehicle-treated blinded crickets against untreated opponents or against opponents with lamed mandibles. However, whereas the vehicle-treated, blinded crickets nearly always won against opponents with lamed mandibles, win chances of blind were less than 50% when pre-treated with SNAP. Conversely, instead of losing most fights, crickets with lamed mandibles won almost half the time when treated with the NOS synthesis inhibitor LNAME. These compensatory effects of nitridergic drugs on handicapped crickets reveal that NO must somehow be involved in the sensory processing of perceived agonistic actions of the opponent. Taken together, our data suggest that the sensory impact of these actions leads to activation of the NO signaling pathway in the central nervous system, which indirectly suppresses aggression by promoting the tendency to flee, rather than directly reducing the motivation to fight.
Verifying our proposal that fighting crickets add up each other’s agonistic actions during fighting, we made the novel observation that winners bear a short-term memory of the inflictions they experienced during the previous fight. Although winner-crickets are normally hyper-aggressive and defeat inexperienced opponents (RiIlich and Stevenson 2011), they lose the majority of fights against a fresh opponent when matched immediately after scoring a win (< 1 min). Similarly, delivery of a potentially aversive stimulus (2 short-wind puffs directed at the cerci) exclusively during the susceptible period just after winning is sufficient to transform winners to behave like losers and retreat on sighting an opponent. This implies that winners, having recently approached the verge of losing in a previous fight, need only a few more aversive experiences to become subordinate. The increased likelihood of winners to flee immediately after winning in response to an opponent’s actions or aversive stimuli was no longer evident when NO production was blocked with LNAME. This again illustrates that NO mediates the impact of aversive experiences on aggressive behavior.
Social Defeat and Maintenance of Submissive Behavior: The Loser Effect
Social defeat is followed by a prolonged period of reduced aggressiveness in practically all animals investigated (Hsu et al. 2006, 2009). The consequences of social defeat, which is accompanied by numerous changes in brain chemicals and gene expression are receiving increasing amounts of attention for understanding social behavior and psychiatric disorders such as depression and post-traumatic stress disorders (Huhman 2006; Hollis and Kabbaj 2014), but comparatively little is known about the proximate cause.
In crickets, losers of a previous fight retreat even from unfamiliar opponents (Alexander 1961; Adamo and Hoy 1995; Khazraie and Campan 1999; Hofmann and Stevenson 2000) and require 3 h on average to fully regain their aggressiveness (Rillich and Stevenson 2013). Confirming suggestions of other researchers (Iwasaki et al. 2007), our experiments show that the loser effect in defeated crickets (just as the much briefer susceptible period in winners), results largely from activation of the NO/cGMP signaling pathway. Treatment with activators (e.g., SNAP) greatly prolonged the duration of the loser effect, whereas treatment with inhibitors (e.g., LNAME) resulted in early recovery (Stevenson and Rillich 2015).
Importantly, submissive behavior of defeated crickets is not due to reduced aggressive motivation. For example, losers will often attack a reluctant opponent, such as another loser that retreat immediately on first contact (Stevenson and Rillich 2013), and losers also fight fiercely when deprived of seeing an opponent’s approach by blackening their eyes (Rillich et al. 2007). This suggests that the loser effect represents a period of increased susceptibility to aversive stimuli and probability to flee in response to an opponent’s agonist signals. This is exactly what we found for winners, only for them the susceptible period lasts less than a minute, possibly as a result of the ensuing winner effect, which is mediated by octopamine. Supporting this latter idea, the tissue-permeable octopamine-receptor agonist chlordimeform (CDM) rapidly restores aggression in losers (Stevenson et al. 2000, 2005). More recently, the insect dopamine-receptor agonist homovanillyl alcohol (HVA, Beggs and Mercer 2009) was also found to have this same effect as CDM, while depletion of octopamine and dopamine using AMT reversibly blocked loser recovery (Rillich and Stevenson 2014). Interestingly, loser recovery was also selectively prohibited by the insect dopamine-receptor antagonist fluphenazine, but not by the octopamine-receptor blocker epinastine, or by yohimbine, which blocks receptors for octopamine’s precursor tyramine. Hence, while octopamine and dopamine are each sufficient to restore aggression in subordinates, dopamine is necessary for losers to regain their aggressiveness after social defeat. This adds firm support to recent findings in fruit flies (Alekseyenko et al. 2013) and ants (Szczuka et al. 2013) suggesting a role for dopamine in insect aggression. Since interfering with dopaminergic signaling has not been found to influence aggression in socially inexperienced crickets (Rillich et al. 2011; Rillich and Stevenson 2011, 2014) dopamine may function only in subordinates to invoke the normal course of recovery after social defeat. Octopamine, on the other hand, can act as a mediator of experiences that promote aggressive motivation in both naive and subordinate crickets.
Serotonin and Aggression in Crickets
While the amine serotonin is renowned for its restraining effect on aggression in mammals (Nelson and Trainor 2007) it acts to promote aggression in crustaceans (Kravitz and Huber 2003) and stalk-eyed flies (Bubak et al. 2014), while work on fruit flies indicates it may have both promoting (Dierick and Greenspan 2007; Alekseyenko et al. 2010) and suppressing effects depending on the receptor subtype activated (Johnson et al. 2009).
Suggestions that reduced aggression after losing in crickets is due to lowered serotonin (Murakami and Itoh 2001, 2003) are not supported by our experiments using AMTP to deplete serotonin (Stevenson et al. 2000, 2005; Rillich and Stevenson 2015), although this treatment does appear to reduce win chances (Dyakonova et al. 1999), though possibly as a side effect of induced hyperactivity. It should also be borne in mind that in mammals, administered AMTP may be converted to alpha-methyl serotonin, which can substitue for serotonin in some behavioral tests (Sourkes 1991). Elevating serotonin levels by administrating the precursor 5-hydroxytryptophan (5HTP), on the other hand, had various and functional opposing effects on cricket aggression in that it induced an “aggressive-like” body posture, more frequent rival song production, and longer fights that did not resolve clear losers, while at the same time it reduced mandible threat display and attack behavior, and left win chances unchanged (Dyakonova and Krushinskii 2013).
These, in part functionally opposing, effects following 5HTP treatment could result from differential activation of different serotonin receptor subtypes (see Johnson et al. 2009 on Drosophila), or possibly a combination of their activation and desensitization (see Pranzatelli 1998 on rats). Clearly, more work is required, before any firm conclusions can be drawn regarding the role of serotonin in cricket aggression.
Conclusions and Outlook
Our work on crickets has provided some of the first insights into the proximal mechanisms of how animals make the adaptive decision of whether to flight or flee. Rather than invoking complex cognitive function, they appear to do this quite simply by exploiting the powers of neuromodulation to bias the balance between the activation thresholds of these opposing behaviors (Figure 5).
Figure 5.
A relative threshold model for the decision to fight or flee in crickets. Potentially positive, or rewarding experiences including antennal stimulation, physical exertion, winning, and resource possession, promote the tendency to fight via the action of octopamine. In this respect, octopamine can be regarded as representing the motivational component of aggression. Aversive experiences accumulated during fighting (opponent actions) promote the tendency to flee via the action of NO. The experience of social defeat may recruit dopamine, which is necessary for recovery. The role of serotonin is not clear, but it may be involved in suppressing aggression after social defeat. According to our model, a cricket will flee the moment the accumulated sum of opponent actions raises the tendency to flee, via the action of NO, above the tendency to fight, set by octopamine. Modified and updated from Stevenson and Rilllich (2012); Stevenson and Schildberger (2013).
Our experiments revealed that octopamine mediates the effects of experiences that promote aggression such as antennal stimulation, physical exertion, winning, and resource possession. These vastly different experiences, may have in common that crickets evaluate them as being in some way positive or rewarding, an idea which fits with the concept of octopamine’s role as a conveyer of reward, for example, in appetitive learning (Hammer and Menzel 1995; Perry and Barron 2013). In effect, octopamine drives the tendency to fight and accordingly raises the level of aggressive motivation, and with it the threshold to flee.
The timing of the decision to flee, in contrast, is determined largely by the actions of the opponent during fighting. In accord with the cumulative assessment hypothesis (Payne 1998), our behavioral experiments reveal that crickets persist in fighting until the sum of the opponent’s aversive actions endured during fighting in some way surpass the threshold to flee. Pharmacological data fully support the idea that such aversive sensory stimuli lead to activation of the NO/cGMP signaling pathway, and that this in effect promotes the tendency to flee, rather than reduce the motivation to fight. Interestingly, activation of NOS via calcium dependent calmodulin involves a shuttle mechanism in which the enzyme moves through a series of large-scale conformational changes (Salerno et al. 2013), which conceivably could form the molecular substrate for summating agonistic signals.
Once the decision to flee has been taken, NO appears to be largely responsible for the maintenance of submissive behavior that is exhibited by losers for at least 3 h. Octopamine and dopamine can each act independently to restore aggressiveness of subordinates, however, alone dopamine is necessary for recovery to occur naturally. It is nonetheless to be expected that other transmitter systems may also play some role. Given the similarities outlined here between the loser effect and the brief period of susceptibility in winners to aversive experiences, it will be interesting to discover whether the latter effect is also evident in mammals after scoring a win and whether this has any bearing on phenomena such as post-conflict trauma.
Serotonin’s role in cricket aggression remains unclear. Recent findings indicate that it can promote some aspects of dominant behavior while also being functionally important for controlling submissive behavior after social defeat (Dyakonova and Krushinskii 2013). These functionally opposing actions may be implemented by activating different specific receptor subtypes. However, genes encoding serotonin receptor subtypes in crickets have only recently been identified (Watanabe et al. 2011; Watanabe and Aonuma 2012) and we now need to know the distribution and specific pharmacology of serotonin receptor subtypes. We also need to discover how serotonin and other transmitter systems interact for the control of aggression.
While our relative threshold model (Figure 5) forms a neat conceptual basis that can explain how an individual’s experiences and an opponent’s aggressive potential can be integrated to determine when to flee, rather than persist, we still need to identify the underlying neuronal substrates that control aggression. Antennal afferent pathways in the cricket brain have been described in some detail (Staudacher et al. 2005; Yoritsune and Aonuma 2012) along with descending interneurones directly excited by mechanical antennal stimulation (Schöneich et al. 2011). These neurones respond to cricket song (Staudacher and Schildberger 1998) and can initiate walking or turning (Zorovic and Hedwig 2012), but it is not known whether they have any function whatsoever in aggressive behavior. In fact, apart from the original pioneering work of Franz Huber showing that focal stimulation in the region of the brain’s mushroom bodies evokes elements of aggressive behavior (Huber 1960), we have no firm knowledge of the role of the various brain centres in controlling cricket aggression. Elegant genetic techniques have identified candidate octopaminergic (Zhou et al. 2008) and serotonergic neurones (Alekseyenko and Kravitz 2014) that influence aggression in Drosophila, and similar techniques may hopefully be available for crickets in the near future. While, candidate octopaminergic (Stevenson and Spörhase-Eichmann 1995) and nitridergic cells (Ott and Burrows 1988) can also be readily identified by immunocytochemistry, their function in aggression will be difficult to resolve using classical electrophysiological techniques.
Finally, the roles of octopamine, serotonin, and NO in the control of cricket aggression appear similar to those emerging for their counterparts in the control of aggression in mammals. While we do not wish to imply that our findings are in any way directly applicable to “higher” animals, this review illustrates how insects with comparatively simple nervous systems and cognitive powers can make adaptive social decisions by simply modulating behavioral thresholds, and this we think is at least worth bearing in mind for endeavours to decipher the intricacies in the operation of our own brains.
Footnotes
† Dedicated to Franz Huber in recognition of his pioneering work on cricket neuroethology.
Funding
Funded by the Deutsche Forschungsgemeinschaft, Grant number STE 714/4-1.
References
- Adamo SA, Baker JL, 2011. Conserved features of chronic stress across phyla: the effects of long-term stress on behavior and the concentration of the neurohormone octopamine in the cricket Gryllus texensis. Horm Behav 60: 478–483. [DOI] [PubMed] [Google Scholar]
- Adamo SA, Hoy RR, 1995. Agonistic behavior in male and female field crickets Gryllus bimaculatus and how behavioral context influences its expression. Anim Behav 49: 1491–1501. [Google Scholar]
- Adamo SA, Linn CE, Hoy RR, 1995. The role of neurohormonal octopamine during ‘fight or flight' behavior in the field cricket Gryllus bimaculatus. J Exp Biol 198: 1691–1700. [DOI] [PubMed] [Google Scholar]
- Alekseyenko OV, Chan YB, Li R, Kravitz EA, 2013. Single dopaminergic neurones that modulate aggression in Drosophila. Proc Natl Acad Sci USA 110: 6151–6156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alekseyenko OV, Kravitz EA, 2014. Serotonin and the search for the anatomical substrate of aggression. Fly (Austin) 8:200–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alekseyenko OV, Lee C, Kravitz EA, 2010. Targeted manipulation of serotonergic neurotransmission affects the escalation of aggression in adult male Drosophila melanogaster. PLoS ONE 5: e10806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander RD, 1961. Aggressiveness, territoriality, and sexual behavior in field crickets (Orthoptera: Gryllidae). Behavior 17: 130–223. [Google Scholar]
- Aonuma H, Watanabe T, 2012. Octopaminergic system in the brain controls aggressive motivation in the ant Formica japonica. Acta Biol Hung 63(2 Suppl): 63–68. [DOI] [PubMed] [Google Scholar]
- Archer J, 1988. The Behavioral Biology of Aggression. Cambridge: Cambridge University Press. [Google Scholar]
- Arnott G, Elwood RW, 2009. Assessment of fighting ability in animal contests. Anim Behav 77: 991–1004. [Google Scholar]
- Beggs KT, Mercer AR, 2009. Dopamine receptor activation by honey bee queen pheromone. Curr Biol 19: 1206–1209. [DOI] [PubMed] [Google Scholar]
- Bradbury WJ, Vehrencamp L, 1998. Principles of Animal Communication. Sunderland (MA): Sinauer; 771–782. [Google Scholar]
- Briffa M, 2008. Decisions during fights in the house cricket Acheta domesticus: mutual or self assessment of energy, weapons and size? Anim Behav 75: 1053–1062. [Google Scholar]
- Brown WD, Chimenti AJ, Siebert JR, 2007. The payoff of fighting in house crickets: motivational asymmetry increases male aggression and mating success. Ethology 113: 457–465. [Google Scholar]
- Brown WD, Smith AT, Moskalik B, Gabriel J, 2006. Aggressive contests in house crickets: size, motivation and the information content of aggressive songs. Anim Behav 72: 225–233. [Google Scholar]
- Bubak AN, Grace JL, Watt MJ, Renner KJ, Swallow JG, 2014. Neurochemistry as a bridge between morphology and behavior: perspectives on aggression in insects. Curr Zool 60:778–790. [Google Scholar]
- Cacioppo JT, Hawkley LC, 2009. Perceived social isolation and cognition. Trends Cogn Sci 13: 447–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon WB, 1915. Bodily Changes in Pain, Hunger, Fear and Rage: An Account of Recent Researches into the Function of Emotional Excitement. NewYork and London: D. Appleton and Co. [Google Scholar]
- Carre JM, Putnam SK, 2010. Watching a previous victory produces an increase in testosterone among elite hockey players. Psychoneuro-endocrinology 35: 475–479. [DOI] [PubMed] [Google Scholar]
- Certel SJ, Leung A, Lin CY, Perez P, Chiang AS et al. , 2010. Octopamine neuromodulatory effects on a social behavior decision-making network in Drosophila males. PLoS ONE 5: e13248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chittka L, Geiger K, 1995. Can honey bees count landmarks? Anim Behav 49: 159–164. [Google Scholar]
- Cromarty SI, Mello J, Kass-Simon G, 1999. Time in residence affects escape and agonistic behavior in adult male American lobsters. Biol Bull 196: 105–112. [DOI] [PubMed] [Google Scholar]
- Dierick HA, Greenspan RJ, 2007. Serotonin and neuropeptide F have opposite modulatory effects on fly aggression. Nat Genet 39: 678–682. [DOI] [PubMed] [Google Scholar]
- DiRienzo N, Pruitt JN, Hedrick AV, 2012. Juvenile exposure to acoustic sexual signals from conspecifics alters growth trajectory and an adult personality trait. Anim Behav 84: 861–868. [Google Scholar]
- Dixon KA, Cade WH, 1986. Some factors influencing male male-aggression in the field cricket Gryllus integer (time of day, age, weight and sexual maturity. Anim Behav 34: 340–346. [Google Scholar]
- Dyakonova V, Krushinkskii AL, 2013. Serotonin precursor (5-hydroxytryptophan) causes substantial changes in the fighting behavior of male crickets Gryllus bimaculatus. J Comp Physiol A 199: 601–609. [DOI] [PubMed] [Google Scholar]
- Dyakonova VE, Schurmann F, Sakharov DA, 1999. Effects of serotonergic and opioidergic drugs on escape behaviors and social status of male crickets. Naturwissenschaften 86: 435–437. [DOI] [PubMed] [Google Scholar]
- Elwood RW, Arnott G, 2012. Understanding how animals fight with Lloyd Morgan's canon. Anim Behav 84: 1095–1102. [Google Scholar]
- Elwood RW, Arnott G, 2013. Assessments in contests are frequently assumed to be complex when simple explanations will suffice. Anim Behav 86: e8–e12. [Google Scholar]
- Evans PD, 1985. Octopamine. In: Kerkut GA, Gilbert LI, editors. Comprehensive Insect Physiology Biochemistry and Pharmacology. Oxford: Pergamon, 499–530. [Google Scholar]
- Fawcett TW, Mowles SL, 2013. Assessments of fighting ability need not be cognitively complex. Anim Behav 86: e1–e7. [Google Scholar]
- Fernandez MP, Chan YB, Yew JY, Billeter JC, Dreisewerd K et al. , 2010. Pheromonal and behavioral cues trigger male-to-female aggression in Drosophila. PLoS Biol 8: e1000541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fone KC, Porkess MV, 2008. Behavioral and neurochemical effects of post-weaning social isolation in rodents-relevance to developmental neuropsychiatric disorders. Neurosci Biobehav Rev 32: 1087–1102. [DOI] [PubMed] [Google Scholar]
- Fuxjager MJ, Forbes-Lorman RM, Coss DJ, Auger CJ, Auger AP et al. , 2010. Winning territorial disputes selectively enhances androgen sensitivity in neural pathways related to motivation and social aggression. Proc Natl Acad Sci USA 107: 12393–12398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giurfa M, 2012. Social learning in insects: a higher-order capacity? Front Behav Neurosci 6: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haden SC, Scarpa A, 2007. The noradrenergic system and its involvement in aggressive behaviors. Aggress Violent Behav 12:1–15. [Google Scholar]
- Hall FS, Wilkinson IS, Humby T, Inglis W, Kendall DA et al. , 1998. Isolation rearing in rats: pre- and postsynaptic changes in striatal dopaminergic systems. Pharmacol Biochem Behav 59: 859–872. [DOI] [PubMed] [Google Scholar]
- Hammer M, Menzel R, 1995. Learning and memory in the honey bee (Review). J Neurosci 15: 1617–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy ICW, Briffa M, 2013. Animal Contests . Cambridge: Cambridge University Press. [Google Scholar]
- Hoffmann P, Thorén P, Ely D, 1987. Effect of voluntary exercise on open-field behavior and on aggression in the spontaneously hypertensive rat (SHR). Behav Neural Biol 47: 346–355 [DOI] [PubMed] [Google Scholar]
- Hofmann HA, Schildberger K, 2001. Assessment of strength and willingness to fight during aggressive encounters in crickets. Anim Behav 62: 337–348. [Google Scholar]
- Hofmann HA, Stevenson PA, 2000. Flight restores fight in crickets. Nature 403: 613–613. [DOI] [PubMed] [Google Scholar]
- Hollis F, Kabbaj M, 2014. Social defeat as an animal model for depression. ILAR J 55: 221–232. [DOI] [PubMed] [Google Scholar]
- Hoyer SC, Eckart A, Herrel A, Zars T, Fischer SA et al. , 2008. Octopamine in male aggression of Drosophila. Curr Biol 18: 159–167. [DOI] [PubMed] [Google Scholar]
- Hsu Y, Earley RL, Wolf LL, 2006. Modulation of aggressive behavior by fighting experience: mechanisms and contest outcomes. Biol Rev Camb Philos Soc 81: 33–74. [DOI] [PubMed] [Google Scholar]
- Hsu YY, Lee IH, Lu CK, 2009. Prior contest information: mechanisms underlying winner and loser effects. Behav Ecol Sociobiol 63: 1247–1257. [Google Scholar]
- Huber F, 1960. Untersuchungen über die Funktion des Zentralnervensystems und insbesondere des Gehirnes bei der Fortbewegung und der Lauterzeugung der Grillen. Zeitschrift für vergleichende Tierphysiologie 44: 60–132. [Google Scholar]
- Huber F, Moore TE, Loher W, 1989. Cricket Behavior and Neurobiology. New York: Cornell University. [Google Scholar]
- Huhman K, 2006. Social conflict models: can they inform us about human psychopathology? Horm Behav 50: 640–646. [DOI] [PubMed] [Google Scholar]
- Hurd PL, 2006. Resource holding potential, subjective resource value, and game theoretical models of aggressiveness signalling. J Theor Biol 241: 639–648. [DOI] [PubMed] [Google Scholar]
- Iba M, Nagao T, Urano A, 1995. Effects of population density on growth, behavior and levels of biogenic amines in the cricket Gryllus bimaculatus. Zoolog Sci 12: 695–702. [Google Scholar]
- Iwasaki M, Delago A, Nishino H, Aonuma H, 2006. Effects of previous experience on the agonistic behavior of male crickets Gryllus bimaculatus. Zoolog Sci 23: 863–872. [DOI] [PubMed] [Google Scholar]
- Iwasaki M, Katagiri C, 2008. Cuticular lipids and odors induce sex-specific behaviors in the male cricket Gryllus bimaculatus. Comp Biochem Physiol A Mol Integr Physiol 149: 306–313. [DOI] [PubMed] [Google Scholar]
- Iwasaki M, Nishino H, Delago A, Aonuma H, 2007. Effects of NO/cGMP signaling on behavioral changes in subordinate male crickets Gryllus bimaculatus. Zoolog Sci 24: 860–868. [DOI] [PubMed] [Google Scholar]
- Jones GH, Hernandez TD, Kendall DA, Marsden DA, Robbins TW, 1992. Dopaminergic and serotonergic function following isolation rearing in rats: study of behavioral responses and postmortem and in vivo neurochemistry. Pharmacol Biochem Behav 43: 17–35. [DOI] [PubMed] [Google Scholar]
- Johnson O, Becnel J, Nichols CD, 2009. Serotonin 5-HT(2) and 5-HT(1A)-like receptors differentially modulate aggressive behaviors in Drosophila melanogaster. Neuroscience 158: 1292–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judge KA, Bonanno VL, 2008. Male weaponry in a fighting cricket. PLoS ONE 3: e3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judge KA, Ting JJ, Schneider J, Fitzpatrick MJ, 2010. A lover, not a fighter: mating causes male crickets to lose fights. Behav Ecol Sociobiol 64: 1971–1979. [Google Scholar]
- Kemp DJ, Wiklund C, 2004. Residency effects in animal contests. Proc Roy Soc B: Biol Sci 271: 1707–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khazraie K, Campan M, 1999. The role of prior agonistic experience in dominance relationships in male crickets Gryllus bimaculatus (Orthoptera: Gryllidae). Behav Proc 44: 341–348. [DOI] [PubMed] [Google Scholar]
- Killian KA, Allen JR, 2008. Mating resets male cricket aggression. J Insect Behav 21: 535–548. [Google Scholar]
- Kravitz EA, Huber R, 2003. Aggression in invertebrates. Curr Opin Neurobiol 13: 736–743. [DOI] [PubMed] [Google Scholar]
- Libersat F, Pflüger H-J, 2004. Monoamines and the orchestration of behavior. BioScience 54: 17–25. [Google Scholar]
- Lihoreau M, Brepson L, Rivault C, 2009. The weight of the clan: even in insects, social isolation can induce a behavioral syndrome. Behav Proc 82: 81–84. [DOI] [PubMed] [Google Scholar]
- Ma XC, Jiang D, Jiang WH, Wang F, Jia M et al. , 2011. Social isolation-induced aggression potentiates anxiety and depressive-like behavior in male mice subjected to unpredictable chronic mild stress. PLoS ONE 6: e20955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard Smith J, 1974. The theory of games and the evolution of animal conflicts. J Theor Biol 47: 209–221. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Fish EW, 2006. Monoamines, GABA, glutatamate and aggression. In: Nelson RJ, editor. Biology of Aggression. Oxford (NY): Oxford University Press, 114–149. [Google Scholar]
- Miczek KA, Nikulina EM, Takahashi A, Covington III HE, Yap JJ et al. , 2011. Gene expression in aminergic and peptidergic cells during aggression and defeat: relevance to violence, depression and drug abuse. Behav Genet 41: 787–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller U, 1997. The nitric oxide system in insects. Prog Neurobiol 51: 363–381. [DOI] [PubMed] [Google Scholar]
- Murakami S, Itoh MT, 2001. Effects of aggression and wing removal on brain serotonin levels in male crickets Gryllus bimaculatus. J Insect Physiol 47: 1309–1312. [DOI] [PubMed] [Google Scholar]
- Murakami S, Itoh MT, 2003. Removal of both antennae influences the courtship and aggressive behaviors in male crickets. J Neurobiol 57: 110–118. [DOI] [PubMed] [Google Scholar]
- Nagamoto J, Aonuma H, Hisada M, 2005. Discrimination of conspecific individuals via cuticular pheromones by males of the cricket Gryllus bimaculatus. Zoolog Sci 10: 1079–1088. [DOI] [PubMed] [Google Scholar]
- Nelson RJ, 2006. Biology of Aggression. Oxford: Oxford University Press. [Google Scholar]
- Nelson RJ, Demas GE, Huang PL, Fishman MC, Dawson VL et al. , 1995. Behavioral abnormalities in male mice lacking neuronal nitric oxide synthase. Nature 378: 383–386. [DOI] [PubMed] [Google Scholar]
- Nelson RJ, Trainor BC, 2007. Neural mechanisms of aggression. Nat Rev Neurosci 8: 536–546. [DOI] [PubMed] [Google Scholar]
- Nosil P, 2002. Food fights in house crickets Acheta domesticus and the effects of body size and hunger level. Can J Zool Revue Canadienne de Zoologie 80: 409–417. [Google Scholar]
- Oliveira RF, Silva A, Canario AVM, 2009. Why do winners keep winning? Androgen mediation of winner but not loser effects in cichlid fish. Proc Roy Soc B: Biol Sci 276: 2249–2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott SR, Burrows M, 1998. Nitric oxide synthase in the thoracic ganglia of the locust: distribution in the neuropiles and morphology of neurones. Journal of Comparative Neurology 395: 217–230. [DOI] [PubMed] [Google Scholar]
- Parker GA, 1974. Assessment strategy and the evolution of fighting behavior. J Theor Biol 47: 223–243. [DOI] [PubMed] [Google Scholar]
- Patki G, Solanki N, Atrooz F, Ansari A, Allam F et al. , 2014. Novel mechanistic insights into treadmill exercise based rescue of social defeat-induced anxiety-like behavior and memory impairment in rats. Physiol Behav 130: 135–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne RJH, 1998. Gradually escalating fights and displays: the cumulative assessment model. Anim Behav 56: 651–662. [DOI] [PubMed] [Google Scholar]
- Perry CJ, Barron AB, 2013Neural mechanisms of reward in insects. Ann Rev Entomol 58: 543–562 [DOI] [PubMed] [Google Scholar]
- Pfennig DW, Reeve HK, 1989. Neighbor recognition and context-dependent aggression in a solitary wasp Sphecius speciosus (Hymenoptera, Sphecidae). Ethology 80: 1–18. [Google Scholar]
- Pflüger H, Stevenson P, 2005. Evolutionary aspects of octopaminergic systems with emphasis on arthropods. Arthropod Struct Dev 34: 379–396. [Google Scholar]
- Pranzatelli MR, 1998. Effect of chronic treatment with 5-hydroxytryptophan on cortical serotonin. Clin Neuropharmacol 11: 257–262. [DOI] [PubMed] [Google Scholar]
- Rillich J, Buhl E, Schildberger K, Stevenson PA, 2009. Female crickets are driven to fight by the male courting and calling songs. Anim Behav 77: 737–742. [Google Scholar]
- Rillich J, Schildberger K, Stevenson PA, 2007. Assessment strategy of fighting crickets revealed by manipulating information exchange. Anim Behav 74: 823–836. [Google Scholar]
- Rillich J, Schildberger K, Stevenson PA, 2011. Octopamine and occupancy: an aminergic mechanism for intruder-resident aggression in crickets. Proc Roy Soc Lond B 278: 1873–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rillich J, Stevenson PA, 2011. Winning fights induces hyperaggression via the action of the biogenic amine octopamine in crickets. PloS ONE 6: e28891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rillich J, Stevenson PA, 2014. A fighter's comeback: dopamine is necessary for recovery of aggression after social defeat in crickets. Horm Behav 66: 696–704. [DOI] [PubMed] [Google Scholar]
- Rillich J, Stevenson PA, 2015. Releasing stimuli and aggression in crickets: octopamine promotes escalation and maintenance but not initiation. Front Behav Neurosci 9: 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Munoz R, Bretman A, Tregenza T, 2011. Guarding males protect females from predation in a wild insect. Curr Biol 21: 1716–1719. [DOI] [PubMed] [Google Scholar]
- Rogers SM, Matheson T, Sasaki K, Kendrick K, Simpson SJ et al. , 2004. Substantial changes in central nervous system neurotransmitters and neuromodulators accompany phase change in the locust. J Exp Biol 207: 3603–3617. [DOI] [PubMed] [Google Scholar]
- Rutte C, Taborsky M, Brinkhof MW, 2006. What sets the odds of winning and losing? Trends Ecol Evol 21:16–21. [DOI] [PubMed] [Google Scholar]
- Sakura M, Aonuma H, 2013. Aggressive behavior in the antennectomized male cricket Gryllus bimaculatus. J Exp Biol 216: 221–228. [DOI] [PubMed] [Google Scholar]
- Salerno JC, Ray K, Poulos T, Li H, Ghosh DK, 2013. Calmodulin activates neuronal nitric oxide synthase by enabling transitions between conformational states. FEBS Lett 587: 44–47. [DOI] [PubMed] [Google Scholar]
- Schöneich S, Schildberger K, Stevenson PA, 2011. Neuronal organization of a fast-mediating cephalo-thoracic pathway for antennal-tactile information in the cricket. J Comp Neurol 519: 1677–1690. [DOI] [PubMed] [Google Scholar]
- Simmons LW, 1986. Inter-male competition and mating success in the field cricket Gryllus bimaculatus (de Geer). Anim Behav 34: 567–579. [Google Scholar]
- Simpson S, Stevenson PA, 2015. Modulation of social behavior in insects. In Canli T, editor. The Oxford Handbook of Molecular Psychology. Oxford (NY): Oxford University Press. [Google Scholar]
- Sloley BD, Orikasa S, 1988. Selective depletion of dopamine, octopamine and 5-hydroxytryptamine in the nervous tissue of the cockroach Periplaneta americana. J Neurocytochem 51: 535–541. [DOI] [PubMed] [Google Scholar]
- Sokolowski MB, 2010. Social interactions in ‘‘simple’’ model systems (review). Neuron 65: 780–794. [DOI] [PubMed] [Google Scholar]
- Sourkes TL, 1991. Alpha-methyltryptophan as a therapeutic agent. Prog Neuropsychopharmacol Biol Psychiatry 15: 935–938. [DOI] [PubMed] [Google Scholar]
- Staudacher E, Schildberger K, 1998. Gating of sensory responses of descending brain neurones during walking in crickets. J Exp Biol 201: 559–572. [DOI] [PubMed] [Google Scholar]
- Staudacher EM, Gebhardt M, Dürr V, 2005. Antennal movements and mechanoreception: neurobiology of active tactile sensors. Adv Insect Physiol 32: 49–205. [Google Scholar]
- Stevenson PA, Dyakonova V, Rillich J, Schildberger K, 2005. Octopamine and experience-dependent modulation of aggression in crickets. J Neurosci 25: 1431–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson PA, Hofmann HA, Schoch K, Schildberger K, 2000. The fight and flight responses of crickets depleted of biogenic amines. J Neurobiol 43: 107–120. [PubMed] [Google Scholar]
- Stevenson PA, Rillich J, 2012. The decision to fight or flee: insights into underlying mechanism in crickets. Front Neurosci 6: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson PA, Rillich J, 2013. Isolation associated aggression: a consequence of recovery from defeat in a territorial animal. PLoS ONE 8: e74965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson PA, Rillich J, 2015. Adding up the odds: nitric oxide signalling underlies the decision to flee and post conflict depression of aggression. Sci Adv 1: e1500060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson PA, Schildberger K, 2013. Mechanisms of experience dependent control of aggression in crickets. Curr Opin Neurobiol 23: 318–323. [DOI] [PubMed] [Google Scholar]
- Stevenson PA, Sporhase-Eichmann U, 1995. Localization of octopaminergic neurons in insects. Comp Biochem Physiol B 11: 203–215. [DOI] [PubMed] [Google Scholar]
- Szczuka A, Korczynska J, Wnuk A, Symonowicz B, Gonzalez Szwacka A et al. , 2013. The effects of serotonin, dopamine, octopamine and tyramine on behavior of workers of the ant Formica polyctena during dyadic aggression tests. Acta Neurobiol Exp (Wars) 73: 495–520. [DOI] [PubMed] [Google Scholar]
- Tachon G, Murray AM, Gray DA, Cade WH, 1999. Agonistic displays and the benefits of fighting in the field cricket Gryllus bimaculatus. J Insect Behav 12: 533–543. [Google Scholar]
- Trainor BC, Workman JL, Jessen R, Nelson RJ, 2007. Impaired nitric oxide synthase signaling dissociates social investigation and aggression. Behav Neurosci 121: 362–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valzelli L, 1973. The “isolation syndrome” in mice. Psychopharmacologia 31: 305–320 [DOI] [PubMed] [Google Scholar]
- Van Wilgenburg E, Clemencet J, Tsutsui ND, 2010. Experience influences aggressive behavior in the Argentine ant. Biology Lett 6: 152–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verlinden H, Vleugels R, Marchal E, Badisco L, Pfluger HJ et al. , 2010. The role of octopamine in locusts and other arthropods. J Insect Physiol 56: 854–867. [DOI] [PubMed] [Google Scholar]
- Wada-Katsumata A, Yamaoka R, Aonuma H, 2011. Social interactions influence dopamine and octopamine homeostasis in the brain of the ant Formica japonica. J Exp Biol 214: 1707–1713. [DOI] [PubMed] [Google Scholar]
- Walker TJ, Masaki S, 1989. Natural history. In: Huber F, Moore TE, Loher W, editors. Cricket Behavior and Neurobiology. New York, London: Cornell University, 1–42. [Google Scholar]
- Wang L, Anderson DJ, 2010. Identification of an aggression-promoting pheromone and its receptor neurons in Drosophila. Nature 463: 227–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Aonuma H, 2012. Identification and expression analyses of a novel serotonin receptor gene, 5-HT2β, in the field cricket Gryllus bimaculatus. Acta Biol Hung 63:58–62. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Sadamoto H, Aonuma H, 2011. Identification and expression analysis of the genes involved in serotonin biosynthesis and transduction in the field cricket Gryllus bimaculatus. Insect Mol Biol 20: 619–635. [DOI] [PubMed] [Google Scholar]
- Wittlinger M, Wehner R, Wolf H, 2006. The ant odometer: stepping on stilts and stumps. Science 312: 1965–1967. [DOI] [PubMed] [Google Scholar]
- Yoritsune A, Aonuma H, 2012. The anatomical pathways for antennal sensory information in the central nervous system of the cricket Gryllus bimaculatus. Invert Neurosci. doi: 10.1007/s10158-012-0137-6. [DOI] [PubMed] [Google Scholar]
- Zhou C, Rao Y, Rao Y, 2008. A subset of octopaminergic neurons are important for Drosophila aggression. Nature Neurosci 11:1059–1067. [DOI] [PubMed] [Google Scholar]
- Zorovic M, Hedwig B, 2012. Descending brain neurons in the cricket Gryllus bimaculatus (de Geer): auditory responses and impact on walking. J Comp Physiol A 199(1):25–34. [DOI] [PubMed] [Google Scholar]