Abstract
Ectotherms generally demonstrate nonlinear changes in performance (e.g., movement speed, individual growth, population growth) as a function of temperature that are characterized by thermal performance curves (TPC). Predation risk elicits phenotypic and behavioral changes that likewise impact performance measures. We tested whether exposure to predation Orthocyclops modestus impacts the maximum population growth rate (rmax) TPC of the protist Paramecium aurelia. We fit predator and non-predator exposed P. aurelia population growth rates to a function previously shown to best describe Paramecium population growth rate TPC’s (Lactin-2) and compared subsequent parameter estimates between curves. For Paramecium exposed to predation risk, maximum population growth increased more rapidly as temperatures rose and decreased more rapidly as temperatures fell compared to the initial temperature. The area under each TPC curve remained approximately the same, consistent with the idea of a trade-off in performance across temperatures. Our results indicate TPCs are flexible given variation in food web context and that trophic interactions may play an important role in shaping TPCs. Furthermore, this and other studies illustrate the need for a mechanistic model of TPCs with parameters tied to biologically meaningful properties.
Keywords: paramecium, phenotypic plasticity, predation, reaction norm, temperature, thermal performance curve.
Ectotherm biology is dominated by the effects of temperature-dependent processes (Brown et al. 2004; Kingsolver 2009). Likewise, predation risk has a strong influence on organism physiology (e.g., Bernard 2004; Handelsman et al. 2013), behavior (Lima and Dill 1990; Sih 1994), and life history (Riessen 1999). Behavioral and phenotypic plasticity enables organisms to optimize fitness by matching traits to environmental pressures such as temperature and predation risk (Lima and Dill 1990; Stearns 1992; Sih 1994; Bernard 2004; Handelsman et al. 2013). How species respond to changing trophic interactions and temperature regimes influence their ability to persist through environmental perturbations such as those caused by invasive species, habitat loss, and a warming and more variable climate (Vasseur et al. 2014; Walsh et al. 2015). Organisms attend to and allocate resources among traits in order to optimize fitness in response to several simultaneously varying environmental pressures (Stearns 1992). However, most studies investigate plasticity in response to a single varying type of environmental pressure (Miner et al. 2015).
Temperature-dependent performance, particularly of population growth rate, is often used to predict the effects of climate change (mainly changing temperature means and variation) on the persistence of species (Deutsch et al. 2008; Bozinovic et al. 2011; Clusella-Trullas et al. 2011; Kingsolver et al. 2013; Vasseur et al. 2014). Temperature-dependent performance can be characterized by thermal performance curves (TPCs) that describe variation in a performance metric across a range of temperatures (Scheiner 2002; Angilletta 2009; Kingsolver 2009). These curves generally adhere to a shape characterized by an exponential increase at cooler temperatures and a peak at an optimal temperature (Topt) where performance is maximized. Above the Topt there is a steep decrease in performance until it reaches some critical upper temperature (CTmax) (Scheiner 2002; Angilletta 2009; Kingsolver 2009). While this general shape is repeated across taxa, there is considerable variation in TPC shape within and among species attributable to genetic, latitudinal, or acclimation effects (Izem and Kingsolver 2005; Angilletta 2009; Kingsolver 2009; Clusella-Trullas et al. 2011). Predation risk also shapes the TPCs of individual-level performance metrics (e.g., burst speed, relative growth rate; Culler et al. 2014; Katzenberger et al. 2014), but whether and how TPCs of population-level metrics (e.g., population growth rate) change in response to predation risk is unknown. If population-level TPCs do change shape in response to predation, then TPCs that are measured without predation risk (the majority of those reported to date) will be less useful for predicting the consequences of future temperature change than currently thought.
At any given temperature, mortality risk (e.g., predation) can alter individual-level behavior or physiology in ways that cause a concomitant change in rm (Cole 1954; Lima 1998; Riessen 1999). Furthermore, the effects of these pressures on rm differ with respect to size-dependent selection on body size and death rate. Earlier age at first reproduction reduces the likelihood of dying prior to first reproduction when mortality is size-independent and constant (Brown and Sibly 2006) and size does not confer additional benefits (e.g., Peters 1983; Kingsolver and Pfennig 2004; Luhring and Holdo 2015). This is seen in size-dependent predation risk in Daphnia where cues from gape-limited predators induced a larger body size through delayed age of first reproduction (which would decrease rm), but cues from predators with a preference for larger prey items induced a smaller size at maturity and earlier age of first reproduction (which would increase rm) (Riessen 1999). Since TPCs connect performance at one temperature to performance at another temperature, predation-induced changes in performance at one temperature may come with a cost to performance at another temperature, generating a mechanism by which overall TPC shape might change under predation risk.
To test whether predation pressure can change the performance of temperature-dependent population-level metrics, we investigated the effects of exposure to predation on rm TPCs in Paramecium aurelia. Cyclopoid copepods are generalist predators that apply substantial predation pressure on protists and other zooplankton in freshwater ponds (Kalinoski and DeLong 2015). Orthocyclops modestus co-occurs with P. aurelia in freshwater ponds and forages at a high rate on many similar-sized protists (Novich et al. 2014). Because all size classes of P. aurelia are susceptible to predation by copepods, we expect that their TPCs will emphasize accelerated growth rates at temperatures closest to their natal temperature. However, it is unclear whether such increases, if detected, would occur to the same degree across all temperatures, or whether increase in growth rate at some temperatures would be accompanied by decreases in growth rate at other temperatures (e.g., a trade-off). Because changing physiology and behavior in response to predation risk are generally governed by trade-offs that incur costs (Lima and Dill 1990; Sih 1994), we predict that although predation will change the shape of rm TPCs, the area under the TPC curves will be constrained. Here we test directly whether predation risk alters the temperature dependence of growth rates in P. aurelia by estimating the growth rate TPCs for populations with and without exposure to predation risk.
Materials and Methods
Study species rearing and maintenance
We collected the ciliate P. aurelia and the copepod O. modestus (hereafter cyclops) from a freshwater pond at the Spring Creek Prairie Audubon Center in southeastern Nebraska (Novich et al. 2014). Paramecium aurelia was isolated from pond water samples and grown in media made from filtered and autoclaved local pond water and liquid protozoa medium from Carolina Biological Supply (Burlington, NC, USA) at a ratio of 1:9 liquid protozoa medium to water. The media was autoclaved and then bacterized with a mix of unidentified bacteria from the local source by passing local water through a 5-μm syringe filter onto an agar plate, allowing the bacterial community to grow for a few days, and then inoculating the protozoan medium with these bacteria. We kept cyclops in filtered pond water in Petri dishes and fed them with a range of protist prey. We kept both cultures at room temperature, ∼24°C.
Predation trials
We initiated 6 replicate P. aurelia populations in 60-mm Petri dishes. Three of these were predation treatments and 3 were controls without predation. Population densities were ∼740 (± 98 SD) cells mL−1 to start, estimated by counts of 3 separate 0.2-mL samples from the stock culture. We rinsed cyclops in sterile pond water, added 3 cyclops to each of the 3 predation dishes, and allowed them to predate the P. aurelia for 4 days. We added the equivalent amount of rinse water to the no-predation dishes to control for any potential differences generated by rare bacteria or other microorganisms that might have entered the cultures through the rinse water. We allowed the P. aurelia in the control dishes to grow without predation risk over this same 4-day period. After 4 days, mean P. aurelia densities had doubled in the control dishes (1,856 ± 718 cells mL−1) but remained approximately constant in the predation dishes (790 ± 217 cells mL−1), indicating substantial predation on P. aurelia by cyclops.
Postpredation growth rates
We measured maximum population growth rates by allowing 4 cells to grow overnight (20–23 h) in 35-mm Petri dishes with 2 mL of bacterized protozoan medium. No predators were used in these trials—we were measuring the changes in population growth rate induced by ∼8 generations of growth under predation risk. We collected individual cells with pipettes, and while watching through a stereoscopic microscope, placed 4 cells into each dish. We visually confirmed that all 4 cells made it into each dish alive. By starting these trials with very low densities and high resource levels, we ensured that the observed growth rates were close to maximal. Three replicate growth trials for the predation and no-predation treatments were conducted at each of 9 temperatures (12°C, 16°C, 20°C, 24°C, 27°C, 30°C, 33°C, 36°C, and 40°C), for a total of 54 trials. The following day, counts were made of the total number of cells in each dish. Growth rates r were calculated using the formula for exponential growth: , where Nt is population size at time t, and t is the actual time elapsed during each trial.
Curve-fitting and statistical analyses
We used the Lactin-2 function (eq. 1) to fit temperature-dependent r across temperature treatments because it allows for negative values at both cold and hot extremes and provides a relatively good fit for rm TPCs (Lactin et al. 1995; Krenek et al. 2011):
| (1) |
In this function, Tmax changes the temperature at which the TPC begins to decelerate and approach the x axis at the higher end, ΔT is a reference temperature, and λ and ρ are constants that determine the steepness of the rising portion of the curve and the height of the overall curve, respectively (see Supplementary Figure 1 for effect of changes to each fit parameter). Because the shape and x-intercept of the fit is dependent on multiple parameters, Tmax is not the same as CTmax (critical upper temperature where rm is 0). We fit the Lactin-2 curve to the rm values across temperatures (constrained to the temperature range used in the experiment) for each treatment (predator or no predator) using the curve fitting tool in MatLab (R2015b) to produce estimates and 95% confidence estimates of the 4 parameters comprising the curve. Differences in parameter values between treatments were determined by comparing 95% confidence bounds. We calculated the area under the curve for the mean, upper and lower 95% confidence limits of each fitted curve to compare curve areas between treatments.
Results
TPC parameters and area under the curve
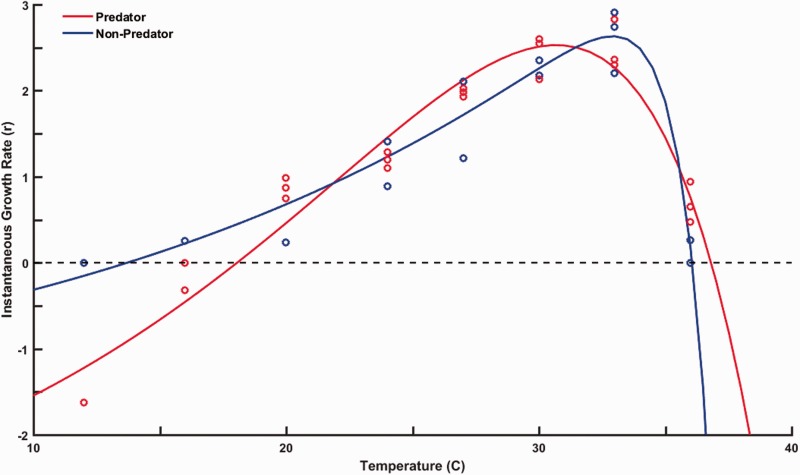
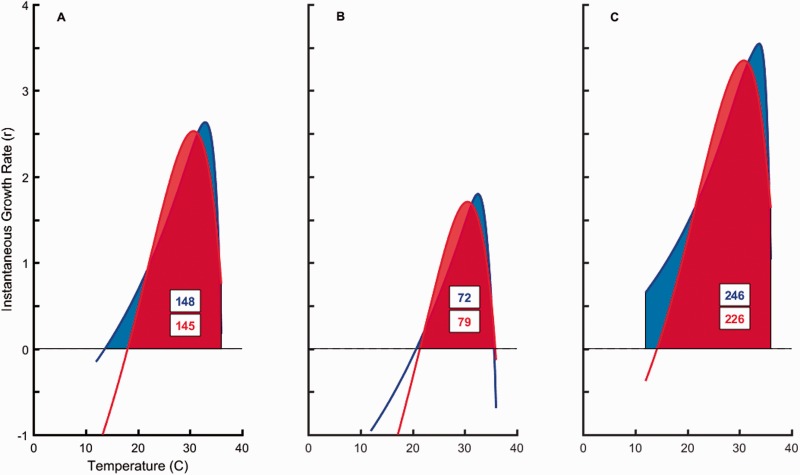
Both treatments demonstrated negative rm at extreme temperatures (12 °C and 40 °C) with no survival at 40 °C (Figure 1). Data from 40 °C treatments were dropped from analysis because rm cannot be calculated when abundances are zero. The Lactin-2 function fit the shape of predator and non-predator treatment TPCs well and captured the negative values at both temperature extremes (non-predator adjusted R2 = 0.90; predator adjusted R2 = 0.92). All 4 parameter estimates differed in either variance (ρ and ΔT) or means (Tmax and λ) between the predator and non-predator treatments (Table 1). The areas under the non-predator and predator TPCs were nearly identical for each mean, upper and lower prediction bound (Figure 2). The parameter means that differed between the treatments were associated with vertical (λ) and horizontal shift (Tmax) of the curve. Paramecium aurelia exposed to predators demonstrated a subsequently higher rm across a span of 9.5 °C (21.9–31.4 °C) compared to non-predator treatments. However, predator exposed P. aurelia had poorer performance at cooler temperatures (<21.9 °C), a 3.6 °C narrower thermal breadth (18.7 °C vs. 22.4 °C), and a 2.2 °C cooler Topt (30.7 °C) than the non-predator control (32.9 °C) (Table 2).
Figure 1.
Paramecium aurelia TPC’s for instantaneous growth rate (rmax). P. aurelia were raised at 24 oC in the absence (blue) or presence of a predator (red) before being raised at a series of temperatures. Curves were fitted to a Lactin-2 function.
Table 1.
Curve fit parameters for temperature-dependent growth rate (rm) in P. aurelia raised with or without predators
| Parameter | Non-predator (µ ± 95% CB) | Predator (µ ± 95% CB) |
|---|---|---|
| ρ | 0.048 (0.006) | 0.084 (0.092) |
| ΔT | 1.22 (1.15) | 6.37 (8.57) |
| T max | 36.61 (0.61) | 39.25 (1.39) |
| λ | −1.93 (0.40) | −3.59 (0.46) |
Confidence bounds (CB) are presented from fitting a Lactin-2 function to the data for predator and non-predator treatments. Adjusted R2 for non-predator (0.90) and predator (0.92) treatments indicated a good fit for both curves.
Figure 2.
Areas under mean, lower and upper 95% prediction limits of predator and non-predator treatment TPCs. Lactin-2 fitted TPC curves for P. aurelia TPC’s after being raised in the absence (blue) and presence of a predator (red). Areas under the curve are denoted (within boxes) for fitted model means (A), lower 95% (B), and upper 95% prediction bounds (C).
Table 2.
TPC parameters for temperature-dependent growth rate (rm) in P. aurelia raised with or without predators
| Parameter | Non-predator | Predator | Effect of predation |
|---|---|---|---|
| CTmin | 13.7 | 18.1 | +4.4 |
| CTmax | 36.1 | 36.8 | +0.7 |
| T opt | 32.9 | 30.7 | −2.2 |
| P max | 2.6 | 2.5 | −0.1 |
| TPB | 22.4 | 18.7 | −3.7 |
| TSM | 8.9 | 6.7 | −2.2 |
Parameters were solved from the equation of the TPC fitted to the predator and non-predator treatments. CTmin, lower critical temperature; CTmax, upper critical temperature; Topt, optimal temperature; maximum rm at Topt – Pmax; TPB, thermal performance breadth; TSM, thermal safety margin. Effect of predation shown as difference between non-predator and predator treatments.
Discussion
Our study demonstrated a multi-generational effect of predation risk on maximum population growth rate (rm) TPCs in P. aurelia. The overall shape of P. aurelia TPCs changed in response to predation (Figure 1) indicating that the effect of predation on subsequent performance is temperature dependent. Furthermore, the lack of difference in total TPC area between treatments supports the idea that improving performance at one area of the TPC comes at the cost of performance in another (i.e., trade-off) (Kingsolver 2009).
Paramecium aurelia appear to increase rm in response to predation risk from cyclops, as would be consistent with animals reproducing earlier (in this case, dividing at a smaller size) in response to increased size-independent mortality risk. However, the ability to increase rm was temperature-dependent with predator-exposed P. aurelia showing an increased rm across a 9.5 °C range of temperatures close to the rearing temperature and a slightly elevated CTmax (Table 2). This enhanced performance at temperatures close to the rearing temperature was accompanied by a 2.2 °C cooler Topt and 3.7 °C narrower breadth of temperatures over which rm was positive in postpredation trials (Table 2). These changes are consistent with a trade-off between performance at a current temperature and ability to adjust to a broader range of temperatures (e.g., generalist–specialist trade-off; Izem and Kingsolver 2005; Kingsolver 2009). Furthermore, as predicted by the “hotter is better” rule (Kingsolver and Huey 2008) the decreased Topt seen in the predator-exposed population, was accompanied by a decreased overall maximum performance Pmax (Table 2). Paramecium experiencing elevated mortality risk from predation can increase their rm at current temperatures; however, this appears to come at the cost of a decreased ability to survive cooler temperatures and a decreased maximum population growth rate (Pmax).
The shape and bounds of TPCs are important for predicting the effects of climate change on population persistence (Deutsch et al. 2008; Martin and Huey 2008; Vasseur et al. 2014). For example, the location of an organism’s Topt relative to the current environmental temperature, known as the thermal safety margin (TSM; Deutsch et al. 2008), is thought to determine the impact of warming on performance. Animals with larger TSM’s are predicted to benefit more from increased warming than animals with smaller TSM’s because of their closer proximity to the rapid decline in performance above the Topt, assuming that the decline is equally precipitous in both cases. Here, predation pressure reduced Topt by 2.2 °C and thus reduced the calculated TSM of our study species when compared to the non-predator control. However, the predator-exposed population TPC showed a less precipitous decline in performance above the Topt compared to that of the non-predator treatment. This predator-induced change in TPC shape beyond the Topt complicates the use TSM’s for predicting vulnerability to climate change.
Projected increases in both climate variability and the occurrence of extreme weather events (Katz et al. 1992; Easterling et al. 2000; Overpeck and Udall 2010) could pose problems for organisms with narrow thermal tolerance breadth. In our study, predation narrowed the breadth of temperatures over which positive population growth occurred by 3.7 °C, mostly through an increased vulnerability to colder temperatures (decreased cold tolerance of 4.4 °C). Models using TPCs to predict population vulnerability to climate change generally focus on changes to the upper limits of TPCs and in some cases, do not account for negative population growth at colder temperatures (e.g., Vasseur et al. 2014). In our study, predation risk induced an apparent trade-off between increased performance at current or warmer temperatures and a decreased survivability at colder temperatures. TPCs derived for P. aurelia populations raised without predation pressures would thus potentially overestimate the ability of this species to tolerate colder temperatures in natural food webs.
The temperature-dependent nature of biological processes makes TPCs important and widely used tools for predicting effects of climate change on species persistence (Deutsch et al. 2008; Kingsolver et al. 2013; Vasseur et al. 2014). However, an assumption of using TPCs characterized outside of the context of a functioning food web is that recent trophic interactions do not influence their shape. Our results suggest that such assumptions may be violated in systems where predation pressure may induce trade-offs that reshape TPCs. This effect may be weaker in performance measures such as burst speed in field-collected, long-lived animals, which are not far removed from the context of their environment. In contrast, measuring rm as a response to temperature generally requires the use of several replicate populations of animals with short generation times raised at different controlled temperatures in a laboratory setting. The controlled nature of the lab may be necessary to get measurements of fitness that relate strongly to population persistence such as rm, but may also increase the chance of creating a TPC that does not necessarily match that of animals in situ.
Supplementary Material
Supplementary material can be found at http://www.cz.oxfordjournals.org/.
Funding
TML was supported by a University of Nebraska Population Biology Program of Excellence Postdoctoral Fellowship.
Supplementary Material
Supplementary Data
Acknowledgment
We thank Rachel Novich for help with data collection.
References
- Angilletta MJ, Jr., 2009. Thermal Adaptation: A Theoretical and Empirical Synthesis. Oxford: Oxford University Press. [Google Scholar]
- Bernard MF, 2004. Predator-induced phenotypic plasticity in organisms with complex life histories. Annu Rev Ecol Evol Syst 35:651–673. [Google Scholar]
- Bozinovic F, Bastias DA, Boher F, Clavijo-Baquet S, Estay SA, et al. , 2011. The mean and variance of environmental temperature interact to determine physiological tolerance and fitness. Physiol Biochem Zool Ecol Evol Approach 84:543–552. [DOI] [PubMed] [Google Scholar]
- Brown JH, Gillooly JF, Allen AP, Savage VM, West GB, 2004. Toward a metabolic theory of ecology. Ecology 85:1771–1789. [Google Scholar]
- Brown JH, Sibly RM, 2006. Life-history evolution under a production constraint. Proc Natl Acad Sci USA 103:17595–17599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clusella-Trullas S, Blackburn TM, Chown SL, 2011. Climate predictors of temperature performance curve parameters in ectotherms imply complex responses to climate change. Am Nat 177:738–751. [DOI] [PubMed] [Google Scholar]
- Cole LC, 1954. The population consequences of life history phenomena. Q Rev Biol 29:103–137. [DOI] [PubMed] [Google Scholar]
- Culler LE, McPeek MA, Ayres MP, 2014. Predation risk shapes thermal physiology of a predaceous damselfly. Oecologia 176:653–660. [DOI] [PubMed] [Google Scholar]
- Deutsch CA, Tewksbury JJ, Heuy RB, Sheldon KS, Ghalambor CK, et al. , 2008. Impacts of climate warming on terrestrial ectotherms across latitude. Proc Natl Acad Sci USA 105:6668–6672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easterling DR, Meehl GA, Parmesan C, Changnon SA, Karl TR, et al. , 2000. Climate extremes: observations, modeling, and impacts. Science 289:2068–2074. [DOI] [PubMed] [Google Scholar]
- Handelsman CA, Broder ED, Dalton CM, Ruell EW, Myrick CA, et al. , 2013. Predator-induced phenotypic plasticity in metabolism and rate of growth: rapid adaptation to a novel environment. Integr Comp Biol 53:975–988. [DOI] [PubMed] [Google Scholar]
- Izem R, Kingsolver JG, 2005. Variation in continuous reaction norms: quantifying directions of biological interest. Am Nat 166:277–289. [DOI] [PubMed] [Google Scholar]
- Kalinoski RM, DeLong JP, 2015. Beyond body mass: how prey traits improve predictions of functional response parameters. Oecologia 180:543–550. [DOI] [PubMed] [Google Scholar]
- Katz RW, Brown BG, 1992. Extreme events in a changing climate: variability is more important than averages. Clim Change 21:289–302. [Google Scholar]
- Katzenberger M, Hammond J, Duarte H, Tejedo M, Calabuig C, et al. , 2014. Swimming with predators and pesticides: how environmental stressors affect the thermal physiology of tadpoles. PLoS One 9:e98265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsolver JG, 2009. The well-tempered biologist. Am Nat 174:755–768. [DOI] [PubMed] [Google Scholar]
- Kingsolver JG, Diamond SE, Buckley LB, 2013. Heat stress and the fitness consequences of climate change for terrestrial ectotherms. Funct Ecol 27:1415–1423. [Google Scholar]
- Kingsolver JG, Huey RB, 2008. Size, temperature, and fitness: three rules. Evol Ecol Res 10:251–268. [Google Scholar]
- Kingsolver JG, Pfennig DW, 2004. Individual-level selection as a cause of Cope’s rule of phyletic size increase. Evolution 58:1608–1612. [DOI] [PubMed] [Google Scholar]
- Krenek S, Berendonk TU, Petzoldt T, 2011. Thermal performance curves of Paramecium caudatum: a model selection approach. Eur J Protistol 47:124–137. [DOI] [PubMed] [Google Scholar]
- Lactin DJ, Holliday NJ, Johnson DL, Craigen R, 1995. Improved rate model of temperature-dependent development by arthropods. Environ Entomol 24:68–75. [Google Scholar]
- Lima SL, 1998. Nonlethal effects in the ecology of predator-prey interactions. BioScience 48:25–34. [Google Scholar]
- Lima SL, Dill LM, 1990. Behavioral decisions made under the risk of predation: a review and prospectus. Can J Zool 68:619–640. [Google Scholar]
- Luhring TM, Holdo RM, 2015. Trade-offs between growth and maturation: the cost of reproduction for surviving environmental extremes. Oecologia 178:723–732. [DOI] [PubMed] [Google Scholar]
- Martin TL, Huey RB, 2008. Why suboptimal is optimal: Jensen’s inequality and ectotherm thermal preferences. Am Nat 171:E102–E118. [DOI] [PubMed] [Google Scholar]
- Miner BG, Sultan SE, Morgan SG, Padilla DK, Relyea RZ, 2015. Ecological consequences of phenotypic plasticity. Trends Ecol Evol 20:685–692. [DOI] [PubMed] [Google Scholar]
- Novich RA, Erickson EK, Kalinoski RM, DeLong JP, 2014. The temperature independence of interaction strength in a sit-and-wait predator. Ecosphere 5:137. [Google Scholar]
- Overpeck J, Udall B, 2010. Dry times ahead. Science 328:1642–1643. [DOI] [PubMed] [Google Scholar]
- Peters RH, 1983. The Ecological Implications of Body Size. Cambridge: Cambridge University Press. [Google Scholar]
- Riessen HP, 1999. Predator-induced life history shifts in Daphnia: a synthesis of studies using meta-analysis. Can J Fish Aquat Sci 56:2487–2494. [Google Scholar]
- Scheiner SM, 2002. Selection experiments and the study of phenotypic plasticity. J Evol Biol 15:889–898. [Google Scholar]
- Sih A, 1994. Predation risk and the evolutionary ecology of reproductive behavior. J Fish Biol 45:111–130. [Google Scholar]
- Stearns SC, 1992. The Evolution of Life Histories. Oxford: Oxford University Press. [Google Scholar]
- Vasseur DA, DeLong JP, Gilbert B, Greig HS, Harley DG, et al. , 2014. Increased temperature variation poses a greater risk to species than climate warming. Proc Roy Soc B 281:20132612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh MR, Cooley FC IV, Biles K, Munch SB, 2015. Predator-induced phenotypic plasticity within- and across- generations: a challenge for theory? Proc Roy Soc B 282:20142205. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Data