Abstract
Animal habitat-use patterns cannot be isolated from scale issues. Consequently, multi-scale studies provide a complete characterization of ecological patterns that can further explain the observed variation. Liolaemus constitutes the world’s second most speciose lizard genus. In this study, we assessed the relationships between home range size and environmental variables at 3 different spatial scales. The study at a local and regional scale was focused on the habitat specialist Liolaemus multimaculatus. The lizard’s home range was calculated using the minimum convex polygon method in populations from grassland sites of the coastal sand dunes of the Argentinean Pampas under 2 different conditions, with or without forestations of Acacia longifolia. On the other hand, at a geographical scale we considered the evolutionary implications of 20 species of Liolaemus. Home range size, phylogeny, ecological, environmental, and climatic data were obtained from the literature and remote sensing. L. multimaculatus home range varied from 12.66 to 570.00 m. Regionally, this species had smaller home ranges in forested habitats (: 94.02 m2) compared with the non-forested sites (: 219.78 m2). Habitat structure, vegetation types, and food availability would explain the space use at finer scales. When the 20 species of Liolaemus were considered, high mean air temperature and broad thermal amplitudes showed an inverse relationship with home range size. Neither net primary productivity nor phylogeny was good predictors for home range variation at geographical scale. This study highlights the scale dependence of the explicative capability of a set of environmental and intrinsic variables on home range patterns.
Keywords: environmental factors, home range, Liolaemus, lizard, scales
Ecological variability can be structured in a nested hierarchy of scales presenting patterns that may differ from level to level (McLoughlin and Ferguson 2000). The observation scale can have a profound influence on the pattern description because each individual, population, and clade is affected by the environment at different levels and thus responds to variability individualistically (Levin 1992). In this sense, the design of studies at multiple scales provides a complete characterization of ecological patterns and therefore explains more of the observed variation (Wiens 1989; Johnson et al. 2002; Mayor et al. 2009). Characteristics of ecological systems at fine scales differ significantly from those at broad scales (Schneider 1994). Therefore, some processes may be more prevalent than others, determining the range of patterns and processes that can be detected by using 1 scale or the other (Wiens 1989; Orians and Wittenberger 1991). For example, at a broad scale some physical processes such as climate can dominate or dissipate biological effects such as interspecific competition, which are more evident at finer scales (Wiens 1989; Pribil and Picman 1997). Intraspecific comparisons of ecological parameters amongst geographically close populations may reveal hidden processes and shed light on our knowledge on what factors play a role in the evolution at a broader scale, for example, at clade level (Zeng et al. 2013).
A recurring issue addressed by ecologists is to understand the forces constraining animal spatial distribution given the immensely integrative nature of movements and their multiple consequences (Smith and Ballinger 2001; Schick et al. 2008). Animals must move to search for resources such as food, mates, suitable thermal conditions, and shelter from predators (Swingland and Greenwood 1983). The space that individuals use to carry out their life cycles (birth, growth, mating, and death) is called home range (Burt 1943; Rose 1982), and it is a product of the interaction among a complex set of environmental variables acting at different scales, such as climate, habitat productivity, habitat complexity, and individuals’ intrinsic factors such as sex, age, and body size (Perry and Garland 2002). However, despite this variety of factors influencing individual movements, it has been suggested that home range size is largely a function of habitat productivity and resource distribution, as well as individual energy requirements (Harestad and Bunnell 1979; Ruby and Dunham 1987). Variability in habitat productivity and complexity induce changes in the location and size of lizards home ranges (Haenel et al. 2003; Wone and Beauchamp 2003; Wasiolka et al. 2009; Scoular et al. 2011). In general, lizards inhabiting highly structured and complex habitats have smaller home ranges than those from homogeneous environments with relatively scarce resources (Perry and Garland 2002). Diet type and foraging behavior are also crucial in setting a home range; with insectivore species and active foragers showing larger home ranges than sit-and-wait foragers, herbivore, and/or omnivore species (Christian and Waldschmidt 1984; Perry and Garland 2002; Verwaijen and Van Damme 2008).
With over 250 recognized species, Liolaemus constitutes the world’s second most speciose lizard genus (Lobo et al. 2010; Abdala and Quinteros 2014). Interestingly, this group of reptiles shows a wide distribution range, diverse dietary habits, and reproductive modes, whereas other traits are less variable, e.g., metabolic rate, preferred body temperature, and critical thermal maximum (Vidal-Maldonado and Labra-Lillo 2008; Cruz et al. 2011; Moreno-Azócar et al. 2012). Liolaemus multimaculatus is a small insectivorous sand lizard, and it is endemic of the coastal dunes of the Argentinean Pampas and northern Patagonia (Cei 1993; Vega 1997). Due to the relatively low abundance, habitat specialization, and restricted distribution, this species is categorized as vulnerable (Abdala et al. 2012). The native habitat of L. multimaculatus along the Pampas coastal dunes has been progressively modified due to urbanization and forestations (Vega et al. 2000; Isla 2013; Stellatelli et al. 2013a, b). In a previous study, Kacoliris et al. (2009) estimated the home range of L. multimaculatus in native grasslands of the Pampas; however, the environmental factors that are involved in the home range of this species remain unknown.
In this study, we evaluate the processes that are involved in the home range pattern variation of Liolaemus lizards at local (individual level) and regional (population level) scales, using the habitat specialist L. multimaculatus as a case study. At a wider geographical scale (species level), we studied a group of 20 species of Liolaemus, based on the available scientific bibliography. Our first particular aim was to identify patterns to assess the local and regional relationships of environmental variables, such as microhabitat structure, food abundance, and temperature, on the home range size of 2 populations of L. multimaculatus inhabiting different types of habitats (non-forested versus forested). We hypothesize that the presence of patches of exotic trees of Acacia longifolia in the forested habitat will reduce food abundance in grasslands (mainly insects) and, as a result, lizards will need to establish larger home ranges and move longer distances compared with lizards living in habitats resembling the original conditions (non-forested areas). Our second particular aim was to analyze the relationships between the observed home range patterns in different species of Liolaemus considering environmental variables such as climate, productivity, and altitude, and intrinsic factors, such as gender, habits, diet, and phylogeny, considering the evolutionary implications at a geographical scale. We predict that: (1) home range is inversely related to environmental temperature and elevation because lizards will have more thermal restrictions for moving, despite the homogeneity of the habitat structure in high elevation habitats (such as mountain environments); (2) home range is negatively related to habitat productivity because lizards living in zones with high net primary productivity will tend to move less in search of food resources; (3) according to the observed intersexual differences in home range size in many Liolaemus lizards, males will have larger home ranges than females; and (4) habits affect home range, since saxicolous or arboreal lizards may have relatively small home ranges because trees and rocky habitats are more structurally complex (i.e., richer) than open terrestrial ones.
Materials and Methods
Local and regional scales: L. multimaculatus as a case study
Home range and movement patterns
Fieldwork was conducted during February and March 2014 in 2 independent sites of approximately 25 ha of coastal dunes. One site is located at Monte Carlo village, and it has been forested with Acacia longifolia for 40 years (37°02°09.80″ S; 56°44°14.76″ W). The other site is located in the Mar Chiquita Reserve (37°44°13.35″S; 57°24°52.47″ W), which preserves the native plant community structure (Block 2014). Both sites are located on the coast of Buenos Aires province, Argentina. The home range of L. multimaculatus was obtained by radio-tracking 24 adult individuals (5 females and 5 males in the forested habitat; 7 females and 7 males in the non-forested habitat). We attached a 0.4 -g radio transmitter (TXB-001 G, TELENAX MR.) to each of the lizards’ back, glued with cyanoacrylate gel (Goodman et al. 2009). The device represented less than 10% of the lizards’ body mass (Knapp and Abarca 2009). Sex determination was based on the secondary sexual characters of the species (Cei 1993). We measured snout-vent length (SVL) with a digital caliper (SC111001, Schwyz MR., Buenos Aires, Argentina, 0.01 mm), and weighed lizards with a portable digital balance (CH02, Diamond Premium MR., China, 0.01 g).
Radio-tracking started 1 day after the device was attached to avoid any irregular behavior resulting from immediate capture (Wasiolka et al. 2009). Lizards were relocated from 09:00 to 18:00 local time (−3 GMT) using a LA12-Q portable receiver very high frequency (VHF) (AVM Instrument Company, Ltd.) with a 3-element Yagi antenna following the procedure of Stellatelli et al. (2016). We kept relocation intervals of 160 min according to Hansteen et al. (1997) and Wasiolka et al. (2009), who showed that these sampling intervals yielded uncorrelated data for area estimates. In each relocation point, we inserted a small numbered flag into the ground 2 m north of the original position of the lizard, to minimize disturbance. The position of each relocation point was calculated as x/y coordinates. During the sampling days (8 February–18 March 2014), the weather conditions were similar, with continuous sunshine and 21.08°C mean temperature (standard deviation [SD] = 1.77°C); 16.83 km/h mean wind speed (SD = 6.33 km/h), and the lizard activity was considered normal based on our experience.
Food resources
To analyze the spatial distribution of food resources, we compared the abundance of arthropods between forested and non-forested grasslands since L. multimaculatus is a genuine insectivore (Vega 1999). Food abundance in both sites was estimated by using pitfall traps (12 × 15 cm cylindrical cups, with eight 2 × 3 cm lateral holes in each one, 1 cm below the top). Cups were filled with a formalin-saturated NaCl solution and detergent as a tensioactive agent and covered with a plastic lid. Twenty traps were equally distributed in 4 transects separated 100 m from each other in each habitat type (Canepuccia et al. 2009). Thirty days later all traps were simultaneously removed, and the samples were taken to the laboratory for analysis. Arthropods were classified taxonomically to order level (sensu Morrone and Coscarón 1998). We only considered arthropods that belonged to Diptera, Coleoptera, Hymenoptera, and Araneae within the size range of 0.07–703 mm3, because they are the main components of the L. multimaculatus diet in the Pampas coastal sand dunes (Vega 1999).
Microhabitat use and environmental temperatures
To analyze the spatial distribution of lizards with respect to the structural features, we determined the substrate type (sand with or without leaf litter) and the physiognomic type of plants (trees, subshrubs, and herbs, following Cabrera and Zardini 1978). The microhabitat availability was visually estimated by the relative percentage of substrate and vegetation cover using 25-m2 grids within each habitat. We randomly set 30 grids in the forested habitat and 31 grids in the non-forested habitat. The number of replicates was calculated using species accumulation curves (Gyesel and Lyon 1987). Moreover, once the individuals’ home ranges were delimited, the total cover of each microhabitat type was visually estimated along each home range (Gyesel and Lyon 1987). The air temperature at 1 cm above ground was measured every time a lizard was spotted, using a thermocouple connected to a digital thermometer (SC133, Schwyz MR., Buenos Aires, Argentina). The mean air temperature (Ta) and the air temperature range (VarTa), the difference between the maximum and minimum temperature, were calculated for each lizard.
Geographical scale and evolutionary implications
Environmental variables
We considered the following variables: mean seasonal air temperature (Mtc), mean seasonal thermal amplitude (Var), net primary productivity (Npp), and altitudinal range (AlR, Appendix A). The Var was estimated as the average of the daily differences between maximum and minimum air temperatures. The parameters Mtc and Var were calculated on a seasonal basis, spring and summer periods: from October to March, considering that Liolaemus lizards are expected to hibernate, although some species may be active during winter (Fitzgerald et al. 1999). These variables were obtained from daily data from the NASA Web site (http://power.larc.nasa.gov/) for a 27-year period (1983–2010). The Npp index was based on the green scale of the terrain, and it was obtained from the NASA Earth Observations (NEO) Web site from NASA (http://neo.sci.gsfc.nasa.gov/) and analyzed using Geographic Information System (GIS) software (based on a grid of 0.1 degrees) for a seasonal window, corresponding to the period 2002–2013. Npp was used as an indicator of habitat quality, according to the resource rule of McNab (2010). Finally, we considered 3 altitudinal ranges; low (from sea level to 999 m above sea level—m.a.s.l.), medium (from 1,000 to 2,999 m.a.s.l.) and high elevation range (above 3,000 m.a.s.l.). Prior to performing the multiple interspecific comparisons, we ensured that the methodologies used by the cited studies were similar, as suggested by Perry and Garland (2002). These authors consider that the main sources of variation come from the calculation method and the minimum number of sightings per individual, but not from the study duration.
Intrinsic factors
We considered sex, mean SVL, type of diet, and habits of 20 species of Liolaemus (Appendix A). Since body size and allometry are often different for males and females (e.g., Fitch 1981; Abouheif and Fairbairn 1997), we analyzed each sex separately. Liolaemus lizards’ diets are categorized as herbivorous, insectivorous, or omnivorous (O’Grady et al. 2005; Vanhooydonck et al. 2010), although the diet of most species does not fully fit into only 1 category (Perry and Garland 2002). We classified the type of habits as saxicolous, arboreal, arenicolous, or terrestrial since these lizards are generally found in rocks, trees, sand, or ground floor (Tulli et al. 2012).
Data analysis
The home range of L. multimaculatus was calculated using the minimum convex polygon (MCP) method with the program Calhome (MS-DOS Version 1.0, 1994; Kie et al. 1994). To exclude outlier data points (e.g., when a lizard was frightened, and it ran away from its area), we used 95 % of the data points (Robles and Halloy 2009). Data area curves of the detection number and the cumulative home range area for the mean of all individuals were used to estimate the minimum number of detections that enhance the robustness of MCPs. Data were fitted with a curvilinear regression (y = b0 + b1 / x; P < 0.05). The curves showed that an average of 9 location points explained 85% of the estimated home range size. Thus, we considered a minimum of 9 sightings as the smallest sample size at which the number of sightings was found to be non-correlated with home range sizes (Rose 1982; Halloy and Robles 2002; Stellatelli et al. 2016). The number of sightings ( ± SD) was 11.10 ± 2.99 (n = 10) in the forested habitat and 16.85 ± 1.46 (n = 14) in the non-forested habitat. The mean distance per move, defined as the distance in straight line that a lizard moved between consecutive sightings, was calculated using the Calhome Program (MS-DOS Version 1.0, 1994; Kie et al. 1994).
When the assumptions of normality and homoskedasticity were met, we used a 2-way Analysis of Variance (ANOVA) (Zar 1984) to compare home range and mean distance per move, with habitat and sex as factors. Tukey’s post hoc comparison was used when appropriated (Zar 1984). We performed simple linear regression analyses to explore the relationship between home range, mean distance per move, body size, and temperature (Ta, VarTa) (Zar 1984). The food abundance and temperatures in forested and non-forested habitats were compared using Mann–Whitney U test (α = 0.05). We used discriminant-function analysis (DFA) to assess whether structural features inside home ranges differed from random grids. Functions created by DFA of the original variables maximize the separation between groups, indicating which variables contribute the most to the group separation (Zar 1984).
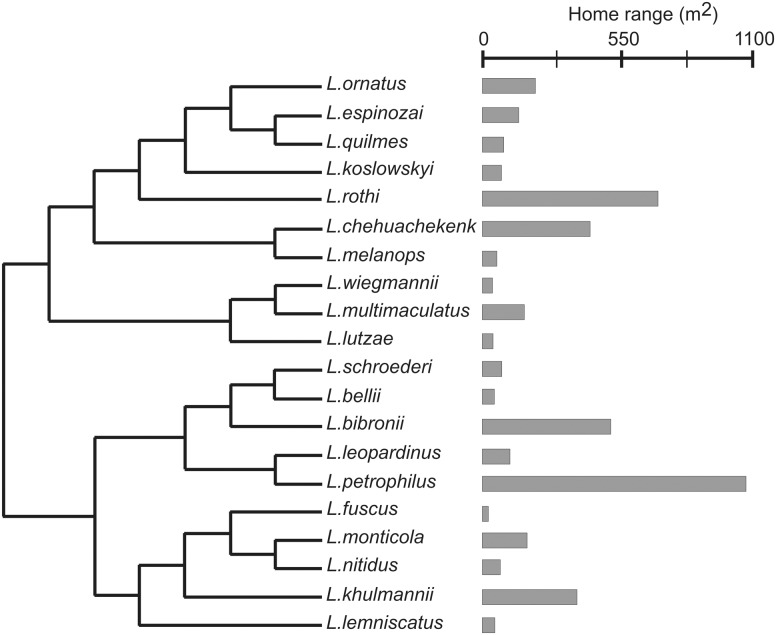
The multi-species comparative analysis raw data are provided in Appendix A, and it contains information regarding the environmental variables, intrinsic variables, and home ranges separated by gender, for the total adults of the twenty studied species. We only included data from those studies in which home ranges data were obtained by MCP methods. Data from studies that only showed mean values or that provided little methodological information were omitted. Given that species have a hierarchical relationship, they cannot be considered as independent data; therefore, phylogenetically based analyses should be performed (Harvey and Pagel 1991; Martins 1996). For this purpose, we used a composite tree (Figure 1) based on Pyron et al. (2013) and Lobo et al. (2010). Since branch lengths were not available, we used an arbitrary length (all branch lengths equal to 1).
Figure 1.
Mean home ranges (in m2) from the 20 Liolaemus species studied here (gray bars at the right of the tree) and their phylogenetic relationship. Topology is based on Pyron et al. (2013) and Lobo et al. (2010).
To test whether body size was related to the home range, we regressed the independent contrasts of home range with the independent contrasts of the SVL of each species using the PDAP PDTREE v1.15 module (Midford et al. 2003) in Mesquite v2.74 (Maddison and Maddison 2010). We found no significant relationship between independent contrasts of these variables (r2 = 0.004; slope = −2.003; P = 0.776); therefore, we used the original home range values from the selected bibliography and transformed it to log(10) in order to reach statistical assumptions of normality and homoskedasticity.
To analyze the geographic variation in home range size among Liolaemus lizards, we used phylogenetically based general linear models (PGLS), which include phylogenetic relatedness in the models and also estimate Pagel’s phylogenetic signal (λ) simultaneously to the regression parameters. PGLS analyses were done in “caper” (Orme et al. 2012) and “ape” (Paradis et al. 2004) packages, both developed in R (R Development Core Team 2011). This procedure is preferred as it has shown to outperform, or to be equivalent to, phylogenetic or non-phylogenetic methods depending on the obtained λ value (Revell 2010). Models were run using Npp, Mtc, and Var (and their combinations) as predictor variables, and home range as the dependent variable. We used the Akaike information criterion (AIC) to choose the regression models that provided the best fit among the candidate models (Angilletta 2006). We used Akaike weights (wi) as a measure of the strength of evidence for each model (Burnham and Anderson 2004). Finally, to test whether sex, type of habit (saxicolous, arboreal, arenicolous, or terrestrial), diet (herbivore, omnivore, or insectivore) or altitudinal range (low, medium, and high) affected home range size we performed a phylogenetically informed ANOVA (phylANOVA from Phytools), running 1,000 simulations for each of those variables (Revell 2012).
Results
Local and regional scales
The overall mean home range of L. multimaculatus was 167.38 m2 (SE = 31.35 m2; n = 24) and was not related to the SVL (r2 = 0.041, F1,22 = 1.98, P = 0.172). Home range size differed between habitats (2-way ANOVA: F1,20 = 4.48, P = 0.046), being 57% smaller in the forested habitat (94.02 m2) than in the non-forested one (219.78 m2; Table 1). The minimum home range of the species (12.66 m2) was observed in a male individual from the non-forested habitat, whereas the maximum (570 m2) belonged to a female from the same habitat type. We found no significant effect of sex on the home range size (F1,20 = 1.68, P = 0.209; Table 2) and no interactions between habitat and sex (F1,20 = 0.06, P = 0.816; Table 1). Also, we found no relationships between home range and mean air temperatures (r2 = 0.009, F1,22 = 0.22, P = 0.644), nor thermal range (r2 = 0.016, F1,22 = 0.37, P = 0.549). SVL was not related to mean distance per move (r2 = 0.031, F1, 22 = 0.71, P = 0.406). Mean distance per move was not affected by habitat type (2-way ANOVA: F1,20 = 2.91, P = 0.103) or sex (F1,20 = 2.91, P = 0.103), and no interactions between these factors were detected (F1,20 = 0.65, P = 0.430; Table 2). Mean distance per move was neither correlated with mean air temperature (r2 = 0.001, F1,22 = 0.03, P = 0.864) nor with thermal range (r2 = 0.052, F1,22 = 1.21, P = 0.284).
Table 1.
Home range size (m2) and movements of L. multimaculatus in forested and non-forested habitats
| Forested |
Non-forested |
|||||||
|---|---|---|---|---|---|---|---|---|
| N | ± SE | Minimum | Maximum | n | ± SE | Minimum | Maximum | |
| Home range (m2) | ||||||||
| Female | 5 | 125.55 ± 55.09 | 28.65 | 268.70 | 7 | 265.27 ± 60.68 | 71.68 | 570.00 |
| Male | 5 | 62.49 ± 28.82 | 22.15 | 90.44 | 7 | 174.28 ± 67.77 | 12.66 | 505.40 |
| Both sexes | 10 | 94.02 ± 28.66 | 22.15 | 268.70 | 14 | 219.78 ± 45.48 | 12.66 | 570.00 |
| Distance per move (m) | 10 | 5.69 ± 2.42 | 2.72 | 11.47 | 14 | 10.22 ± 7.37 | 2.57 | 28.48 |
n, number of individuals tracked; , mean; SE, standard error.
Table 2.
Mean (± standard deviation) of percentage of bare sand, leaf litter, vegetation cover of trees, subshrubs, and herbs in 2 Pampas dune habitats (forested versus non-forested) compared by Kruskal–Wallis (α = 0.05)
| Forested habitat (n = 30) | Non-forested habitat (n = 31) | Forested home range (n = 10) | Non-forested home range (n = 14) | H | df | P | |
|---|---|---|---|---|---|---|---|
| Trees | 24.37 ± 12.49 (a) | 0.00 ± 0.00 (b) | 2.92 ± 3.89 (b) | 0.00 ± 0.00 (b) | 68.41 | 3 | < 0.001 |
| Subshrubs | 15.63 ± 15.21 (a) | 8.11 ± 3.77 (b) | 0.40 ± 0.52 (c) | 11.65 9.42 (a) | 33.15 | 3 | < 0.001 |
| Herbs | 9.39 ± 8.14 (a) | 26.74 ± 14.56 (b) | 15.35 ± 6.83 (b) | 13.10 11.40 (b) | 26.67 | 3 | < 0.001 |
| Leaf litter | 16.93 ± 7.61 (a) | 0.00 ± 0.00 (b) | 4.83 ± 3.02 (c) | 1.55 0.92 (c) | 73.80 | 3 | <0.001 |
| Bare sand | 34.90 ± 8.37 (a) | 69.48 ± 12.17 (b) | 76.48 ± 8.71 (b) | 74.30 9.75 (b) | 59.74 | 3 | < 0.001 |
Different letters in brackets indicate significant differences (Dunn’s post hoc test, α = 0.05). H, Kruskal–Wallis statistic; n, number of grids; df, degrees of freedom; P, probability value.
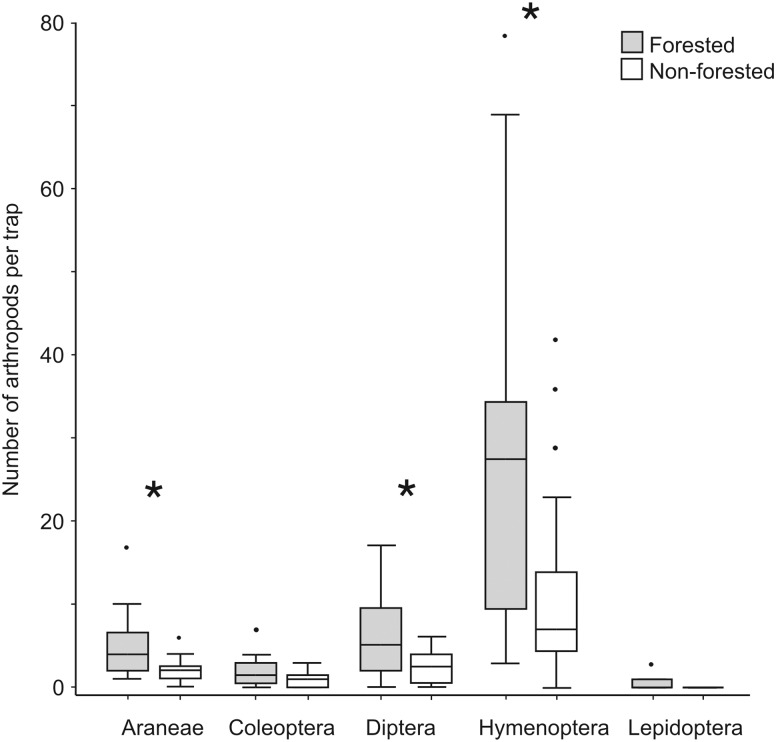
The abundance of some of the main arthropod preys consumed by L. multimaculatus was significantly greater in forested than in non-forested sites, suggesting a direct relationship with habitat complexity: Araneae (Mann–Whitney U tests: U = 91.50, n = 20, P < 0.05), Diptera (U = 110.00, n = 20, P < 0.05), and Hymenoptera (U = 105.00, n = 20, P < 0.05), except for Coleoptera (U = 133.50, n = 20, P = 0.064) and Lepidoptera (U = 168.00, n = 20, P = 0.273; Figure 2).
Figure 2.
Abundance comparisons of food items between forested (n = 20) and non-forested habitats (n = 20). Horizontal bar denotes median, vertical bar denotes range, upper–lower boundary of boxes denotes quartile, and point denotes outliers. Asterisks indicate significant differences between forested and non-forested habitats (Mann–Whitney U test, α = 0.05).
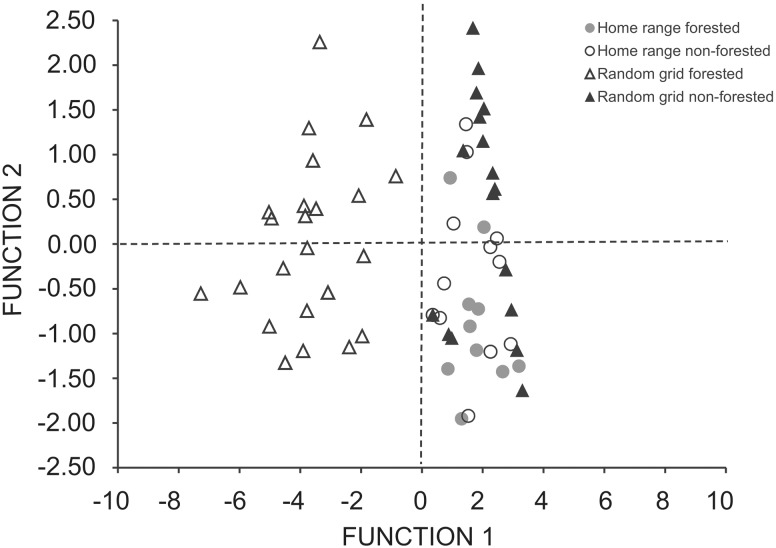
Microhabitat structure of each habitat type within lizards’ home range was significantly different compared with random grids (Table 2). The forested habitat showed a significantly higher coverage of A. longifolia, subshrubs and leaf litter, a significantly lower coverage of native herbs and less percentage of bare sand than the non-forested habitat (Table 2). The DFA indicated that the first 2 discriminant functions were statistically significant (P < 0.001 in both cases). The first one accounted for more than 95 % of the total variance (eigenvalue = 7.32; χ2 = 195.60; df = 12) and was negatively correlated with tree coverage (linear correlation = − 0.570) and positively related with bare sand (linear correlation = 0.650; Figure 3). The second discriminant function accounted for 3% of the total variance (eigenvalue = 0.23; χ2 = 23.94; df = 6) and was positively correlated with herbs coverage (linear correlation = 0.825) and negatively related with the percentage of bare sand (linear correlation = −0.724; Figure 3). Based on both discriminant functions, the variables trees, herbs, and bare sand contributed significantly to separate the 4 groups (Figure 3). The groups (i.e., home range from forested sites, home range from non-forested sites, random grids from forested sites and random grids from non-forested sites) were correctly classified with 81.39 % of accuracy. All random grids from the forested habitats (n = 30) were correctly classified by the model, whereas only 10% of the random grids from the total of non-forested sites (n = 31) were incorrectly classified. From the total home ranges, 50% of the forested sites (n = 10) and 57.14% of the non-forested sites (n = 14) were correctly classified. In both cases, the remaining percentage was classified as random grid from non-forested sites.
Figure 3.
Functions 1 and 2 from the DFA performed on the habitat-use data for L. multimaculatus, as well as on data from randomly chosen points.
Mean air temperatures inside L. multimaculatus home range were not different between forested and non-forested habitats (U = 49.00, n = 24, P = 0.230; Ta ± SD: forested = 32.39 ± 2.45°C, non-forested = 30.92 ± 0.88°C). On the other hand, the mean temperature range was significantly higher (U = 105.50, n = 24, P < 0.05) in non-forested (VarTa = 9.38 ± 1.81°C) than in forested sites (VarTa = 6.12 ± 4.06°C).
Geographical scale
In the interspecific comparative analysis, PGLS models showed a negative relationship between home range and mean air temperature for the overall data and for males and females. Thermal amplitude showed a significant negative correlation only when the overall data were considered (Table 3). No significant relationship was found between home range and net primary productivity. Additionally, PGLS models did not show phylogenetic signals of relationship residuals in any of the models (λ = 0 in all cases, Table 3). We did not observe significant differences in home range between species inhabiting different elevations (PhylANOVA F2,18 = 0.042; P = 0.976); neither among species with different type of habits (PhylANOVA F3,18 = 0.358; P = 0.891), nor species with different feeding habits (PhylANOVA F2,18 = 0.016; P = 0.979). On average, males had home ranges that were 34% larger than female home ranges (Phyl.pairedttest; tdbar = 5519.1, Pdbar < 0.001, n = 20; Table 3).
Table 3.
Best models after Akaike criterion
| Model | AIC | λ | Adjr2 | Intercept | TP | Npp | Mtc | Var | P |
|---|---|---|---|---|---|---|---|---|---|
| HRt∼Mtc+Var | 19.88 | 0.00 | 0.428 | 5.93 | 0.003 | −0.14 | <0.001 | ||
| −0.12 | 0.045 | ||||||||
| HRt∼Npp+Mtc+Var | 21.60 | 0.00 | 0.400 | 6.22 | 0.011 | −0.10 | 0.639 | ||
| −0.13 | 0.011 | ||||||||
| −0.14 | 0.077 | ||||||||
| HRml∼Mtc | 22.82 | 0.00 | 0.269 | 3.83 | 0.011 | −0.10 | 0.011 | ||
| HRml∼Mtc+Var | 21.46 | 0.00 | 0.345 | 5.54 | 0.011 | −0.13 | 0.002 | ||
| −0.09 | 0.096 | ||||||||
| HRfe∼Mtc | 27.66 | 0.00 | 0.303 | 4.07 | 0.008 | −0.13 | 0.008 | ||
| HRfe∼Mtc+Var | 25.21 | 0.00 | 0.414 | 6.38 | 0.005 | −0.17 | 0.001 | ||
| −0.13 | 0.056 |
Slopes of Mtc mean seasonal temperature (October–March), var seasonal thermal amplitude, and Npp net productivity index are shown as well as Intercept. TP is overall P value for the model. HR refers to home range of species and each sex (adults only); t for overall individuals per species, ml for males, and fe for females. Boldface denotes those significant P values for each partial correlation within the model. λ, phylogenetic signal (Pagel’s); AIC, Akaike information criterion.
Discussion
Our results support the conclusion of McLoughlin and Ferguson (2000), who mentioned that the ecological relationships are scale dependent, and consequently, the importance of factors affecting home range size is also relative to scale. In this work, we identified a set of factors influencing home range size in Liolaemus lizards that differed when this pattern was analyzed at the individual, population, or species level. These findings highlight new insights into the influence of processes underlying home range variations. At a local scale, L. multimaculatus home range showed a wide range of individual variation as previously noted in a study of a native grassland site of the Buenos Aires coastal dunes by Kacoliris et al. (2009) for the same species. The greatest variation between individual home ranges occurred in the population living in open areas of the non-forested dunes, probably as a result of the larger home range sizes achieved by some individuals compared with those of the forested sites. At the same time, the regional comparison of 2 populations of this species revealed a strong effect of exotic forestations of Acacia longifolia on the home range size, but not in the movement pattern within the home range. Contrary to our prediction, we identified that L. multimaculatus home range was 57% larger and that the abundance of some potentially consumable arthropods was lower in the non-forested habitat than in the forested one. Higher food availability together with a relatively higher patchy distribution of high-quality microhabitats could explain L. multimaculatus smaller home range in forested sites. In this sense, our results are in accordance with the optimal foraging theory, which suggests that organisms can adjust their home range in relation to local food resources. In this sense, the lizards should decrease the size of their home range with increasing food levels or predation risk (Simon 1975; Verwaijen and Van Damme 2008).
At a regional scale, differences in physiognomic habitat structure may also influence the home range among populations of the same vertebrate species (McLoughlin and Ferguson 2000). L. multimaculatus is a small animal (60–70 mm SVL at adult size) that may perceive forestations patches as barriers to movement, thus decreasing the use of space in forested habitats. A previous study showed that in forested habitats, the spatial distribution of L. multimaculatus is restricted to patches of native herbs mixed with bare sand without trees, which resemble the structural and thermal features of open dunes from non-disturbed habitats (Stellatelli et al. 2013a, 2014). The perceptual range (i.e., the distance from which animals can perceive a suitable habitat) plays a significant role in how lizards interact and move within the surrounding environment (Zuri and Bull 2000; Olden et al. 2004). Habitat specialists and small animals can perceive disturbed and low-quality patches as barriers to movements (Addicott et al. 1987; Laurance 1990; Grubb and Doherty 1999). The lack of differences in mean air temperatures between home ranges of individuals in both habitats indicates that lizards were able to select suitable thermal spots, although the availability of thermally suitable spots is diminished in forested patches of A. longifolia with respect to non-forested sites (Stellatelli et al. 2013a; Block et al. 2014). On the other hand, the higher predation risk for L. multimaculatus in areas with acacias may also explain the differences observed in the spatial ecology of this lizard (Stellatelli et al. 2015). A higher predator pressure may reduce home ranges of lizards (Salvador et al. 1995), minimizing their movements to decrease their exposition (Eifler et al. 2008). Despite the relatively higher abundance of prey in acacia zones, these areas are not suitable for the lizard populations. Hence, other factors, besides food availability may shape the spatial ecology of this lizard species at a population level.
Opposite to what was observed at local and regional scales, the seasonal mean temperatures and broad thermal amplitudes showed an inverse relationship with the home range of the 20 species of Liolaemus studied. As we expected, Liolaemus species living in sites with higher mean air temperatures had the smallest home ranges. We also observed an inverse pattern between environmental thermal amplitude and home range when considering both sexes together (however, the relationship for each sex was not significant). Contrary to our expectation, we found no effect of elevation in the home range of Liolaemus, possibly because latitude could offset the impact of altitude. Additionally, more than half of the studied species were distributed along the Andes Mountains, which may have caused some bias. Environmental temperatures were considered as a determinant factor of the time available for activity, influencing the capability of an individual to effectively patrol, monitor, or otherwise utilize an area (Sound and Veith 2000). Indeed, it was observed that lizards had smaller home ranges at sites with restrictive temperature regimes (Ruby and Dunham 1987; Grant 1990). Despite the fact that warm temperatures may be considered beneficial for ectothermic animals, very high environmental temperatures may lead them to shut down activity. For example, L. multimaculatus together with other species of Liolaemus, retreat during the warmer periods of the day (Vega 2001; Frutos et al. 2007; Frutos 2009; Belver et al. 2010).
The net primary productivity was not a significant predictor of the home range for the 20 Liolaemus species studied. Given that Npp is a measure of the green surface of the terrain, it is possible that the scale at which Npp was measured did not allow us to infer the food abundance adequately for Liolaemus, particularly for the insectivorous and/or omnivorous species that depend on insect abundance (Moreno-Azócar et al. 2015). We found that more than 80% of the studied species of Liolaemus were distributed in arid environments (low Npp) and that they mainly feed on ants and coleopterans that are abundant in those sites. This fact could also explain the lack of relationship between home range and Npp. It is likely that food resource data at a large scale may help clarify the pattern observed in relation to habitat variables. Unfortunately, this information is currently not available at a large scale, although it was explored in this study by our local and regional data.
Other studies on Liolaemus suggested that body size may explain intraspecific (intersexual) variations in the species home range (Simonetti and Ortiz 1980). However, in the 20 Liolaemus species that were analyzed, we only found an effect of sex. Supporting our prediction, in average, males tend to have larger home ranges than females. These intersexual differences may be related to social systems and differential behaviors (Perry and Garland 2002; Fox and Shipman 2003). Many Liolaemus are polygynous, and males have large territories to include more females and thus increase their reproductive success mainly during the breeding season. Contrary to this, females base their spatial distribution according to food resources, which may result in smaller home ranges (Robles and Halloy 2010). For similar-sized species, the trophic level may influence the home range size (McLoughlin and Ferguson 2000), but this was not observed in our results. Garland et al. (1993) warned that this type of analysis requires the consideration of phylogeny, because when the phylogenetic effect is controlled this tendency is diluted. Our findings suggest that the type of habitat does not affect home ranges; this reflects the methodological difficulty mentioned by Perry and Garland (2002) to accurately calculate home range size for a species using environments with complex vertical component, such as saxicolous or arboreal species.
We found that home range patterns of Liolaemus lizards might be affected by different processes operating at different scales. Home range size at a local scale, i.e., individual level, or at a regional scale, i.e., 2 populations of the same species, result from processes that may occur in a relatively short time period such as food availability, predation rates, or alterations in habitat physiognomy. At species level, the home range patterns were explained by climate and sex, both are processes that result from relatively slow evolutionary times (McLoughlin and Ferguson 2000). Although we analyzed the home range of less than 10% of the existing Liolaemus lizards species (what may be considered a species sampling problem), we found evidence of environmental and individual intrinsic factors affecting their spatial ecology. One limitation of this study is that it only included 20 out of 257 recognized species of the Liolaemus genus; therefore, the species that were studied are only distributed in 3 out of the 4 major clades within the phylogenetic diversity of the genus. At the moment, our results show that the ecological processes, rather than phylogeny, play a significant role in shaping the home range of these lizards.
Combining the results obtained at local, regional, and geographic scale, we conclude that the home range pattern in the 20 studied species of Liolaemus is the result of complex interactions between environmental factors, such as habitat structure, temperature (climate), and some intrinsic characteristics of the individuals (sex), where the phylogeny plays a secondary role. Although we agree with Perry and Garland (2002) regarding the methodological issues that could be affecting multi-source data studies, we have come up with consistent results. Our findings suggest that refined biological inferences and mechanistic understanding can be obtained from data by decomposing variance of home range size into components based on variation in spatial scale. Thus improving the comprehension of the effect of different factors acting at the level of population, species, or clade, and allowing major improvements in our understanding of the processes that determine the spatial ecology of ectothermic organisms.
Funding
Funding for the study was provided by Universidad Nacional de Mar del Plata (UNMdP), the Neotropical Grassland Conservancy (NGC) and the Conservation, Research, and Education Opportunities International (CREOi) nonprofit corporations. Additional support to this investigation was provided by CONICET graduate grants.
Acknowledements
The authors adhered to guidelines for the use of animals in research and to the legal requirements of our country (Scientific Purposes Permit 22230–21/2006–0) from the Buenos Aires province, Argentina.
Appendix A
List of species with data of their home range (HR), snout-vent length (SVL), biology (habits, diet), and environmental features. Data of HR and SVL are grouped in overall (t), by sex (males—ml, females—fe). The habits are discriminated in Sax: saxicolous, Arb: arboreal, Are: arenicolous, and Ter: terrestrial. Diet type is discriminated in Omn: omnivore or Ins: insectivore. Data corresponding to net primary productivity (Npp), mean seasonal environmental temperature (Mtc), seasonal thermal breath (Var), and altitudinal range (AlR: Low, ≤ 999 m.a.s.l.; Medium, 1000–2999 m.a.s.l.; High, ≥ 3000 m.a.s.l.) were obtained from the coordinates of localities according to the Author source.
| Intrinsic factors |
Extrinsic factors |
Home range |
Author | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Species | Habits | Diet | SVLml (mm) | SVLfe (mm) | SVLt (mm) | Npp | Mtc (°C) | Var (°C) | AlR | HRml (m2) | HRfe (m2) | HRt (m2) | |
| Liolaemus belli | Sax | Omn | 74.40 | 70.20 | 72.30 | 42.01 | 15.81 | 14.15 | Medium | 53.50 | 36.30 | 44.90 | Fox and Shipman 2003 |
| Liolaemus bibroni | Ter | Ins | 55.00 | — | 55.00 | 43.60 | 13.24 | 12.76 | Medium | 514.08 | —- | 514.08 | Frutos 2009 |
| Liolaemus cf. L. chehuachekenk | Ter | Omn | 75.50 | 75.00 | 75.25 | 43.60 | 13.24 | 12.76 | Low | 496.28 | 375.97 | 436.12 | Frutos 2009 |
| Liolaemus cf. L. rothi | Ter | Ins | 60.02 | 70.20 | 65.11 | 43.60 | 13.24 | 12.76 | Low | 759.14 | 665.27 | 712.20 | Frutos 2009 |
| Liolaemus espinozai | Are | Ins | 57.90 | 57.90 | 57.90 | 48.31 | 17.81 | 11.59 | Medium | 257.90 | 79.44 | 148.62 | Cabrera and Scrocchi 2012 |
| Liolaemus fuscus | Ter | Ins | 46.50 | 43.30 | 44.90 | 55.47 | 15.84 | 14.15 | Medium | 32.20 | 9.40 | 20.80 | Fox and Shipman 2003 |
| Liolaemus koslowskyi | Ter | Ins | 69.00 | 64.00 | 66.50 | 58.65 | 20.90 | 11.76 | Medium | 140.00 | 25.00 | 82.50 | Frutos and Belver 2007 |
| Liolaemus kuhlmanni | Are | Omn | 70.50 | 62.68 | 66.59 | 91.44 | 17.81 | 10.64 | Low | 775.9 | 157.70 | 382.50 | Simonetti and Ortiz 1980 |
| Liolaemus lemniscatus | Ter | Ins | 48.20 | 44.70 | 46.450 | 55.47 | 15.84 | 14.15 | Medium | 70.3 | 25.20 | 47.75 | Fox and Shipman 2003 |
| Liolaemus leopardinus | Sax | Omn | 85.80 | 81.00 | 83.40 | 42.01 | 15.81 | 14.15 | High | 112.4 | 106.80 | 109.60 | Fox and Shipman 2003 |
| Liolaemus lutzae | Are | Omn | 78.00 | 62.00 | 70.00 | 96.52 | 20.90 | 11.76 | Low | 59.8 | 22.30 | 41.05 | Rocha 1999 |
| Liolaemus melanops | Are | Ins | 76.40 | 71.80 | 74.10 | 46.17 | 17.87 | 13.02 | Low | 70.91 | 42.10 | 56.50 | Frutos et al. 2007 |
| Liolaemus monticola | Sax | Ins | 61.60 | 56.90 | 59.25 | 55.47 | 15.84 | 14.15 | Medium | 242.2 | 110.90 | 176.55 | Fox and Shipman 2003 |
| Liolaemus multimaculatus | Are | Ins | 60.45 | 54.84 | 57.64 | 101.37 | 18.61 | 9.14 | Low | 118.38 | 195.41 | 167.38 | This study |
| Liolaemus multimaculatus | Are | Ins | 60.45 | 54.84 | 57.64 | 107.49 | 18.61 | 9.14 | Low | 33.52 | 21.31 | 45.90 | Kacoliris et al. 2009 |
| Liolaemus nitidus | Sax | Ins | 86.90 | 82.10 | 84.50 | 55.47 | 15.84 | 14.15 | Medium | 74.90 | 70.30 | 72.60 | Fox and Shipman 2003 |
| Liolaemus ornatus | Ter | Omn | 65.80 | 66.10 | 65.95 | 51.94 | 11.82 | 11.85 | High | 283.80 | 145.50 | 214.65 | Guerra and Halloy 2008 |
| Liolaemus petrophilus | Sax | Ins | 81.60 | 71.40 | 76.5 | 43.60 | 13.24 | 12.76 | Medium | 979.6 | 1146.6 | 1063.1 | Frutos 2009 |
| Liolaemus quilmes | Ter | Ins | 61.30 | 57.20 | 59.25 | 61.87 | 17.81 | 10.64 | Medium | 132.20 | 29.20 | 80.70 | Halloy and Robles 2002 |
| Liolaemus schroederi | Arb | Ins | 52.80 | 59.90 | 56.35 | 55.47 | 15.84 | 14.15 | Medium | 88.10 | 68.10 | 78.10 | Fox and Shipman 2003 |
| Liolaemus wiegmannii | Are | Ins | 54.57 | 54.69 | 54.63 | 92.12 | 18.49 | 8.44 | Low | 48.82 | 29.88 | 39.35 | Stellatelli et al. 2016 |
References
- Abdala CS, Quinteros AS, 2014. Los últimos 30 años de estudios de la familia de lagartijas más diversa de Argentina. Actualización taxonómica y sistemática de Liolaemidae. Cuad Herpetol 28: 55–82. [Google Scholar]
- Abdala CS, Acosta JL, Acosta JC, Álvarez BB, et al. , 2012. Categorización del estado de conservación de las lagartijas y anfisbenas de la República Argentina. Cuad Herpetol 26: 215–248. [Google Scholar]
- Abouheif E, Fairbairn DJ, 1997. A Comparative analysis of allometry for sexual size dimorphism: Assessing Rensch’s Rule. Am Nat 149: 540–562. [Google Scholar]
- Addicott JF, Aho JM, Antolin MF, Padilla DP, Richardson JS, et al. , 1987. Ecological neighbourhoods: scaling environmental patterns. Oikos 49: 340–346. [Google Scholar]
- Angilletta MJ, 2006. Estimating and comparing thermal performance curves. J Therm Biol 31: 541–545. [Google Scholar]
- Belver LC, Kozykariski ML, Avila LJ, 2010. Diferencias sexuales y etarias en la actividad diaria y estacional de una población de Liolaemus koslowskyi (Liolaemini). Cuad Herpetol 24: 71–79. [Google Scholar]
- Block C, 2014. Selección de hábitat a escala de paisaje y microhábitat en lagartijas arenícolas: herramientas para el manejo sustentable del ecosistema dunícola costero de la Provincia de Buenos Aires [Ph.D. Thesis]. [Mar del Plata (Argentina)]: Universidad Nacional de Mar del Plata; 141 p. [Google Scholar]
- Block C, Stellatelli OA, Vega LE, García GO, Cruz FB, Isacch JP, 2014. Thermoregulation in coastal sand dunes: impacts of the habitat modification on the Wiegmann’s lizard. In: Kiernan MP, editor. Lizards: Thermal ecology, genetic diversity and functional role in ecosystems. New York: Nova Science Publisher Inc, 75–97. [Google Scholar]
- Burnham KP, Anderson DR, 2004. Multimodel inference: understanding AIC and BIC in model selection. Soc Meth Res 33: 261–304. [Google Scholar]
- Burt WH, 1943. Territoriality and home range concepts as applied to mammals. J Mam 24: 346–352. [Google Scholar]
- Cabrera MP, Scrocchi GJ, 2012. Áreas de acción en Liolaemus espinozai (Squamata: Liolaemidae) en Campo El Arenal, Catamarca, Argentina. Acta Zool Lilloana 56: 54–65. [Google Scholar]
- Cabrera A, Zardini E, 1978. Manual de la flora de los alrededores de Buenos Aires, segunda edición. Buenos Aires, Argentina: ACME. [Google Scholar]
- Canepuccia AD, Cicchino A, Escalante A, Novaro A, Isacch JP, 2009. Differential responses of marsh arthropods to rainfall-induced habitat loss. Zool Stud 48: 174–183. [Google Scholar]
- Cei JM, 1993. Reptiles del centro, centro oeste y sur de la Argentina. Monografia XIV. Torino, Italy: Museo Regionalie di Scienze Naturali Torino. [Google Scholar]
- Christian KA, Waldschmidt S, 1984. The relationship between lizard home range and body size: a reanalysis of the data. Herpetologica 40: 68–75. [Google Scholar]
- Cruz FB, Antenucci D, Luna F, Abdala CS, Vega LE, 2011. Energetics in Liolaemini lizards: implications of a small body size and ecological conservatism. J Comp Physiol B 181: 373–382. [DOI] [PubMed] [Google Scholar]
- Eifler DA, Eifler MA, Harris BR, 2008. Foraging under the risk of predation in desert grassland whiptail lizards Aspidoscelis uniparens. J Ethol 26: 219–223. [Google Scholar]
- Fitch HS, 1981. Sexual Size Differences in Reptiles. Kansas, USA: University of Kansas Museum Natural History Miscellaneous Publications; 70: 1–72. [Google Scholar]
- Fitzgerald LA, Cruz FB, Perotti G, 1999. Phenology of a lizard assemblage in the Dry Chaco of Argentina. Herpetologica 33: 526–535. [Google Scholar]
- Fox S, Shipman PA, 2003. Social behavior at high and low elevations. In: Fox SF, McCoy JK, Baird TL. editors. Lizard Social Behaviour. London, UK: Johns Hopkins University Press; 310–355. [Google Scholar]
- Frutos N, Belver LC, 2007. Dominio vital de Liolaemus koslowskyi Etheridge, (1993) (Iguania: Liolaemini) en el noroeste de la provincia de La Rioja, Argentina. Cuad Herpetol 21: 83–32. [Google Scholar]
- Frutos N, Camporro LA, Avila LJ, 2007. Ámbito de hogar de Liolaemus melanops Burmeister, 1888 (Squamata: Liolaemini) en el Centro de Chubut, Argentina. Gayana 71: 142–149. [Google Scholar]
- Frutos N, 2009. Dominio vital, movimiento y ritmo de actividad en una comunidad de saurios patagónicos del clado Liolaemini: un análisis evolutivo [Ph.D. Thesis]. [Argentina]: Facultad de Ciencias Exactas, Físicas y Naturales, Universidad Nacional de Córdoba. [Google Scholar]
- Garland T, Dickerman AW, Janis CM, Jones JA, 1993. Phylogenetic Analysis of Covariance by Computer Simulation. Sys Biol 42: 265–292. [Google Scholar]
- Goodman RM, Knapp CR, Bradley KA, Gerber GP, Alberts AC. 2009. Review of radio transmitter attachment methods for West Indian rock iguanas (genus Cyclura). Appl Herpetol 6: 151–170. [Google Scholar]
- Guerra I, Halloy M. 2008. Área de acción de Liolaemus ornatus (Iguania: Liolaemidae) en Abra Pampa, Jujuy: comparación con dos especies del complejo darwinii. Acta de Resúmenes del IX Congreso Argentino de Herpetología, 21. [Google Scholar]
- Grant BW, 1990. Trade-offs in activity time and physiological performance for thermoregulating desert lizards Sceloporus merriami. Ecology 71: 2323–2333. [Google Scholar]
- Grubb TC, Doherty PF, 1999. On home-range gap-crossing. Auk 116: 618–628. [Google Scholar]
- Gyesel LW, Lyon LJ, 1987. Análisis y evaluación del hábitat. In: Rodríguez-Tarrés R, editor. Manual de técnicas de gestión de vida silvestre. Washington DC, USA: The Wildlife Society; 331–334. [Google Scholar]
- Haenel GJ, Smith LC, John-Alder HB, 2003. Home range analysis in Sceloporus undulates: a test of spatial relationships and reproductive success. Copeia 1: 113–123. [Google Scholar]
- Halloy M, Robles C, 2002. Spatial distribution in a neotropical lizard Liolaemus quilmes (Liolaemidae): site fidelity and overlapping among males and females. Bull Md Herpetol Soc 38: 118–129. [Google Scholar]
- Hansteen TL, Andreassen HP, Ims RA, 1997. Effects of spatiotemporal scale on autocorrelation and home range estimations. J Wildl Manag 61: 280–290. [Google Scholar]
- Harestad AS, Bunnell FL, 1979. Home range and body weight: a reevaluation. Ecology 60: 389–402. [Google Scholar]
- Harvey PH, Pagel MD, 1991. The Comparative Method in Evolutionary Biology. New York, USA: Oxford University Press. [Google Scholar]
- Isla FI, 2013. From touristic villages to coastal cities: the costs of the big step in Buenos Aires. Ocean Coast. Manag 77: 59–65. [Google Scholar]
- Johnson CJ, Parker KL, Heard DC, Gillingham MP, 2002. Movement parameters of ungulates and scale-specific responses to the environment. J Anim Ecol 71: 225–235 [Google Scholar]
- Kacoliris FP, Williams JD, Ruiz de Arcaute C, Cassino C, 2009. Home range size and overlap in Liolaemus multimaculatus (Squamata: Liolaemidae) in Pampean coastal dunes of Argentina. South Am J Herpetol 4: 229–234. [Google Scholar]
- Kie JG, Badlwin JA, Evans CJ, 1994. Calhome Electronic User’s Manual. Fresno, USA: United States Forest Service. [Google Scholar]
- Knapp CR, Abarca JG, 2009. Effects of radio transmitter burdening on locomotor ability and survival of iguana hatchlings. Herpetologica 65: 363–372. [Google Scholar]
- Laurance WF, 1990. Comparative responses of five arboreal marsupials to tropical forest fragmentation. J Mam 71: 641–653. [Google Scholar]
- Levin SA, 1992. The problem of pattern and scale in ecology: the Robert H. MacArthur Award Lecture. Ecology 73: 1943–1967. [Google Scholar]
- Lobo F, Espinoza RE, Quinteros S, 2010. A critical review and systematic discussion of recent classification proposals for Liolaemid lizards. Zootaxa 2549: 1–30. [Google Scholar]
- Maddison WP, Maddison DR, 2010. Mesquite: a modular system for evolutionary analysis, Version 2.74. 2015 [cited 2015 July 12]. Available from: http://mesquiteproject.org. [Google Scholar]
- Mayor SJ, Schneider DC, Schaefer JA, Mahoney SP, 2009. Habitat selection at multiple scales. Écoscience 16: 238–247. [Google Scholar]
- Martins EP, 1996. Phylogenies and the Comparative Method in Animal Behavior. New York: Oxford University Press. [Google Scholar]
- McLoughlin PD, Ferguson SH, 2000. A hierarchical pattern of limiting factors helps explain variation in home range size. Écoscience 7: 123–130. [Google Scholar]
- McNab BK, 2010. Geographic and temporal correlations of mammalian size reconsidered: a resource rule. Oecologia 164: 13–23. [DOI] [PubMed] [Google Scholar]
- Midford PE, Garland Jr T, Maddison WP, 2003. PDAP Package, Version 1.15. 2015 [cited 2015 July 12]. Available from: http://mesquiteproject.org. [Google Scholar]
- Moreno-Azócar DL, Vanhooydonck B, Bonino MF, Perotti MG, Abdala CS, et al. , 2012. Chasing the Patagonian sun: comparative thermal biology of Liolaemus lizards. Oecologia 171: 773–788. [DOI] [PubMed] [Google Scholar]
- Moreno-Azócar DL, Perotti MG, Bonino MF, Schulte JA, Abdala CS, et al. , 2015. Variation in body size and degree of melanism within a lizards clade: is it driven by latitudinal and climatic gradients? J Zool 295: 243–253. [Google Scholar]
- Morrone JJ, Coscaron S, 1998. Biodiversidad de Artrópodos Argentinos. La Plata: Ediciones Sur. [Google Scholar]
- O’Grady SP, Morando M, Avila L, Dearing MD, 2005. Correlating diet and digestive tract specialization: examples from the lizard family Liolaemidae. Zoology 108:201–210. [DOI] [PubMed] [Google Scholar]
- Olden JD, Schooley RL, Monroe JB, Poff NL, 2004. Context dependent perceptual ranges and their relevance to animal movements in landscapes. J Anim Ecol 73:1190–1194. [Google Scholar]
- Orians GH, Wittenberger JF, 1991. Spatial and temporal scales in habitat selection. Am Nat 137: 29–49. [Google Scholar]
- Orme D, Freckleton R, Thomas G, Petzoldt T, Fritz S, et al. , 2012. Comparative analyses of phylogenetics and evolution in R, Version 0.5. 2015 [cited 2015 July 12]. Available from: http://caper.rforge.r-project.org/. [Google Scholar]
- Paradis E, Claude J, Strimmer K, 2004. APE: analyses of phylogenetics and evolution in R language. Bioinformatics 20: 289–290. [DOI] [PubMed] [Google Scholar]
- Perry G, Garland T, 2002. Lizard home ranges revisited: effects of sex, body size, diet, habitat, and phylogeny. Ecology 83: 1870–1885. [Google Scholar]
- Pribil S, Picman J, 1997. The importance of using the proper methodology and spatial scale in the study of habitat selection by birds. Can J Zool 75: 1835–1844. [Google Scholar]
- Pyron AR, Burbrink FT, Wiens JJ, 2013. A phylogeny and revised classification of Squamata, including 4161 species of lizards and snakes. BMC Evol Biol 13: 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team, 2013. R: a language and environment for statistical computing [Internet]. Vienna (Austria): R Foundation for Statistical Computing, 2015 [cited 2015 July 12]. Available form: http://www.R-project.org. [Google Scholar]
- Revell LJ, 2010. Phylogenetic signal and linear regression on species data. Method Ecol Evol 1: 319–329. [Google Scholar]
- Revell LJ, 2012. Phytools: an R package for phylogenetic comparative biology (and other things). Method Ecol Evol 3: 217–223.23467194 [Google Scholar]
- Robles C, Halloy M, 2010. Core area overlap in a neotropical lizard Liolaemus quilmes: relationship with territoriality and reproductive strategy. Herpetologica 20: 243–248. [Google Scholar]
- Robles C, Halloy M, 2009. Home Ranges and reproductive strategies in a neotropical lizard Liolaemus quilmes (Iguania: Liolaemidae). South Am J Herpetol 4: 253–258. [Google Scholar]
- Rocha CFD, 1999. Home range of the Tropidurid lizard Liolaemus lutzae: sexual and body size differences. Rev Bras Biol 59: 125–130. [Google Scholar]
- Rose B, 1982. Lizard home ranges: methodology and functions. Herpetologica 16: 253–269. [Google Scholar]
- Ruby DE, Dunham AE, 1987. Variation in home range size along an elevational gradient in the iguanid lizard Sceloporus merriami. Oecologia 71: 473–480. [DOI] [PubMed] [Google Scholar]
- Salvador A, Martin J, López P, 1995. Tail loss reduces home range size and access to females in male lizards Psammodromus algirus. Behav Ecol6: 382–387. [Google Scholar]
- Schick RS, Loarie SR, Colchero F, Best BD, Boustany A, et al. , 2008. Understanding movement data and movement processes: current and emerging directions. Ecol Lett 11: 1338–50. [DOI] [PubMed] [Google Scholar]
- Smith GR, Ballinger RE, 2001. The ecological consequences of habitat and microhabitat use in lizards: a review. Contem Herpet 3:1–1 [Google Scholar]
- Schneider DC, 1994. Quantitative Ecology: Spatial and Temporal Scaling. San Diego, USA: Academic Press. [Google Scholar]
- Scoular KM, Caffry WC, Tillman JL, Finan ES, Schwartz SK, et al. , 2011. Multiyear home-range ecology of common side blotched lizards in Eastern Oregon with additional analysis of geographic variation in home-range size. Herpetol Monogr 25: 52–75. [Google Scholar]
- Simon CA, 1975. The influence of food abundance on territory size in the iguanid lizard Sceloporus jarrovi. Ecology 56: 993–998. [Google Scholar]
- Simonetti J, Ortiz JC, 1980. Dominio en Liolaemus kuhlmanni (Reptilia: Iguanidae). An Mus Hist Nat Valparaiso 13: 167–172. [Google Scholar]
- Sound P, Veith M, 2000. Weather effects on intrahabitat movements of the western green lizard Lacerta bilineata (Daudin, 1802) at its northern distribution range border: a radio-tracking study. Can J Zool 78: 1831–1839. [Google Scholar]
- Stellatelli OA, Vega LE, Block C, Cruz FB, 2013a. Effects on the thermoregulatory efficiency of two native lizards as a consequence of the habitat modification by the introduction of the exotic tree Acacia longifolia. J Therm Biol 38: 135–142. [Google Scholar]
- Stellatelli OA, Vega LE, Block C, Cruz FB, 2013b. Effects of tree invasion on the habitat use of sand lizards. Herpetologica 69: 455–465. [Google Scholar]
- Stellatelli OA, Block C, Vega LE, Cruz FB, 2014. Responses of two sympatric sand lizards to exotic forestations in the coastal dunes of Argentina: some implications for conservation. Wild Res 41: 480–489. [Google Scholar]
- Stellatelli OA, Block C, Vega LE, Cruz FB, 2015. Non-native vegetation induces changes in predation pressure and escape behavior of two sand lizards (Liolaemidae: Liolaemus). Herpetologica 71: 136–142. [Google Scholar]
- Stellatelli OA, Block C, Vega LE, Isacch JP, Cruz FB, 2016. Factors affecting the spatial ecology of the lizard Liolaemus wiegmannii in the pampasic coastal dunes of Argentina. Herpetol J 26:11–19. [Google Scholar]
- Swingland IR, Greenwood PJ, 1983. The Ecology of Animal Movement. Oxford: Clarendon Press. [Google Scholar]
- Tulli MJ, Abdala V, Cruz FB, 2012. Effects of different substrates on the sprint performance of lizards. J Exp Biol 215: 774–84. [DOI] [PubMed] [Google Scholar]
- Vanhooydonck B, Cruz FB, Abdala CS, Moreno-Azócar DL, Bonino MF, et al. , 2010. Sex-specific evolution of bite performance in Liolaemus lizards (Iguania: Liolaemidae): the battle of the sexes. Biol J Linn Soc 101: 461–475. [Google Scholar]
- Vega L, 2001. Herpetofauna: diversidad, ecología e historia natural. In: Iribarne O, editor. Reserva de Biosfera Mar Chiquita: Características Físicas, Biológicas y Ecológicas. Mar del Plata, Argentina: Editorial Martín, 213–226. [Google Scholar]
- Vega LE, 1997. Reproductive activity and sexual dimorphism of Liolaemus multimaculatus (Sauria: Tropiduridae). Herpetologica 7: 49–53. [Google Scholar]
- Vega L, Bellagamba P, Fitzgerald L, 2000. Long-term effects of anthropogenic habitat disturbance on a lizard assemblage inhabiting coastal dunes of Argentina. Can J Zool 78:1–8. [Google Scholar]
- Vega LE, 1999. Ecología trófica de Liolaemus multimaculatus (Sauria:Tropiduridae). Boll Mus Reg Sci Nat Torino 16:27–38. [Google Scholar]
- Verwaijen D, Van Damme R, 2008. Wide home ranges for widely foraging lizards. Zoology 111:37–47. [DOI] [PubMed] [Google Scholar]
- Vidal-Maldonado MS, Labra-Lillo A, 2008. Herpetología de Chile. Santiago, Chile: Science Verlag. [Google Scholar]
- Wasiolka B, Jeltsch F, Henschel J, Blaum N, 2009. Space use of the spotted sand lizard Pedioplanis l. lineoocellata under different degradation states. Afr J Ecol 48: 96–104. [Google Scholar]
- Wiens JA, 1989. Spatial scaling in ecology. Func Ecol 3: 385–397. [Google Scholar]
- Wone B, Beauchamp B, 2003. Movement, home range and activity patterns of the horned lizard Phrynosoma mcallii. Herpetologica 37: 679–686. [Google Scholar]
- Zar JH, 1984. Biostatistical Analysis. New Jersey, USA: Prentice Hall. [Google Scholar]
- Zeng ZG, Zhao JM, Sun BJ, 2013. Life history variation among geographically close populations of the toad-headed lizard Phrynocephalus przewalskii: exploring environmental and physiological associations. Acta Oecol 51: 28–33. [Google Scholar]
- Zuri I, Bull CM, 2000. The use of visual cues for spatial orientation in the sleepy lizard Tiliqua rugosa. Can J Zool 78: 515–520. [Google Scholar]