Abstract
Male animals with more conspicuous visual and acoustic signals increase their mating success, but also increase the risk of being attacked by eavesdropping predators. In rodents, males with richer sex pheromones often have higher attractiveness to females, but whether or not the males are also at higher predation risk is poorly known. Here, we used 2 laboratory inbred strains of the rat Rattus norvegicus, Brown Norway (BN) and Lewis (LEW), and wild-captured rats as odor donors to assess the relationship between the pheromone levels in male rats and attractiveness to domestic cats Felis catus. LEW rats had significantly higher levels of male pheromones (e.g., 4-heptanone, 2-heptanone, and 9-hydroxy-2-nonanone) than BN rats. Simultaneously, wild-captured male rats were selectively assigned to 2 groups (HIGH or LOW) based on pheromone content as determined by gas chromatography–mass spectrometry (GC-MS). Binary choice tests were carried out during the night in the test room. We found that cats spent more time investigating male bedding and urine of LEW rats than the counterpart of BN rats. Likewise, cats also preferred bedding and urine odor of the HIGH wild rats compared with the counterparts of LOW wild rats. Adding synthetic analogs of the 3 pheromone ketones into the urine of either BN rats or LOW wild rats significantly increased their attractiveness to cats. Our data suggest that the rats with exaggerated male pheromones more strongly attracted predators and thus as a consequence may suffer from elevated predation risk.
Keywords: olfactory preference, pheromone, predator risk, sexual ornament
A long-standing evolutionary hypothesis proposes that trade-offs between sexual selection and nature selection often shape the sexual signals in wild animals (Zuk et al.1993; Kotiaho 2001; Andersson and Simmons 2006; Hernandez-Jimenez and Rios-Cardenas 2012). Sexual selection tends to favor greater elaboration to benefit from attracting potential mates, whereas natural selection frequently favors less conspicuous traits for the bearer to avoid the cost of detection by predators and parasites (Zahavi 1975; Zuk et al.1993; Kotiaho 2001). The cost or handicap of sexually selected traits and the negative effects on fitness of the trait bearer are of central importance to sexual selection theory, where the costs are defined as having a negative influence on the fitness, and in particular on the survival, of the trait bearer (Zahavi 1975; Kotiaho 2001). Growing evidence supports such a “benefit-cost trade-off” hypothesis. For example, high-quality male field crickets Teleogryllus commodus invest more energy in calling during early adulthood but die while still young (Hunt et al. 2004); sexual attractiveness in male guppies Poecilia reticulata is positively correlated with both degree of sexual ornamentation and with mortality rate (Godin and Briggs 1996; Brooks 2000).
Predation has the potential to act as an evolutionarily significant cost on sexual traits (Kotiaho 2001). Predation-mediated natural selection tends to favor individuals with less conspicuousness to reduce the risk of being attacked by a predator; on the contrary, sexual selection promotes the evolution of conspicuous traits to maximize mating success (Andersson and Simmons 2006; Hernandez-Jimenez and Rios-Cardenas 2012). Some experiments have demonstrated that sexual selection favors the exaggeration of male traits including pheromones, whereas the links between differentiated male traits and predator attraction to them have been experimentally studied have focused mainly on visual and acoustic signaling (Zuk and Kolluru 1998; Godin and McDonough 2003; Roberts and Gosling 2003; Head et al. 2005; Andersson and Simmons 2006; Cordes et al. 2014; Zhang and Zhang 2014). For example, the more brightly colored individuals of paired size-matched male guppies are preferentially captured by the blue Acara cichlid fish Aequidens pulcher and more attractive males of lesser wax moths Achroia grisella experience greater predation risks than the less attractive males because ultrasonic calling for mates also attracts predatory bats (Godin and McDonough 2003; Cordes et al. 2014).
Scent signals of prey including deer mice Peromyscus maniculatus, house mice Mus musculus, brown rats Rattus norvegicus and several vole species and some reptiles have been demonstrated to attract eavesdropping predators such as snakes and small carnivores, whereas the costs of more conspicuous male pheromones versus less conspicuous pheromones are rarely examined empirically (Cushing 1984; Chiszar et al. 1997; Koivula and Korpimaki 2001; Wisenden and Thiel 2002; Ylönen et al. 2003; Amo et al. 2004; Svensson et al. 2004; Husak et al. 2006; Hughes et al. 2010, 2012). Rodents and their mammal predators such as small carnivores rely primarily on chemical signals to mediate intra-specific and inter-specific interactions and thus can be used as ideal models to examine the cost of male pheromones of rodents by predation pressure (Wyatt 2003). Like house mice, the pheromones of brown rats R. norvegicus have been chemically characterized and identified and thus each component of these pheromones can be quantified and manipulated (Novotny et al. 1999; Zhang et al. 2008; Zhang and Zhang 2011, 2014). Therefore, it has been well demonstrated that high-quality and sexier males usually produce stronger pheromones and thus attract more female mates by using mice and rats (Novotny et al. 1990; Roberts and Gosling 2003; Zhang and Zhang 2014). Recently, using 2 inbred laboratory strains of the brown rat, Lewis (LEW) and Brown Norway (BN), we found that LEW male urine has considerably higher levels of male pheromones (e.g., 4-heptanone, 2-heptanone, and 9-hydroxy-2-nonanone) than BN male urine and that exaggerated male pheromones serve as a “sexual chemical ornament” to attract female mates, independent of genetic compatibility (Bender et al. 1994; Zhang and Zhang 2011; Zhang 2012; Zhang and Zhang 2014). Based on this, it is reasonable to further use them as animal models to evaluate the possible costs of exaggerated male pheromones in rats.
Currently, we hypothesized here that the cost of the exaggerated male pheromones could be reflected by facilitation of the detection by predators according to the above-mentioned “benefit-cost trade-off hypothesis”. We first used LEW and BN rats as odor (bedding and urine) donors with respective rich and poor male pheromones to test olfactory responses of domestic cats Felis catus, and explored the possible relationship between male pheromone contents in rats and predation risk from domestic cats. Finally, we used wild-captured rats R. norvegicussocer to examine whether there were individual differences in male pheromone contents with possible effects on cat attraction in nature (Godin and McDonough 2003; Hughes et al. 2012; Cordes et al. 2014).
Material and Methods
Subjects
In Experiment 1, 7 BN and 7 LEW male rats at the age of 8 weeks were purchased from Vital River Laboratories, Beijing, China. The housing room had a reversed 14:10 h light:dark photoperiod (light on at 19:00) and was maintained at 25 ± 1°C (mean ± standard error [SE]). The rats were housed in groups of 3–4 in plastic cages, and they were singly housed for 2 weeks prior to use. Standard rat chow and tap water were provided ad libitum.
In Experiment 2, 25 male and 18 female wild rats were captured in the countryside around Chendu City, Sichuan Province, China, and then transported to our field station in the suburb of Beijing City in May 2013 (Wang 2003). The rats were housed in groups of 3–4 in plastic cages, and were singly housed for 2 weeks prior to use. Standard rat chow and tap water were provided ad libitum.
In total, 7 male and 2 female adult stray domestic cats at the age of 2–5 years were trapped and then raised in stainless steel pet cages (3 m × 2.5 m × 2.2 m). They were fed on thawed rat carcasses and commercial cat chaw with sufficient water supply. These cats were tested after 1-month acclimation. Each cat was used once every 3 days, and was fasting on the testing day.
The housing room of wild rats and domestic cats were exposed to natural photoperiods and kept at 25 ± 1°C. The behavioral tests were carried out from October to November 2014.
Rat bedding and urine collection
Pine shaving bedding in rat cages were left unchanged for 1 week and individually collected as rat odor materials. For urine collection, each rat was placed in a clean rat cage (37 cm × 26 cm × 17 cm) with a net bottom and underneath a plastic plate was placed. Urine was absorbed immediately after it was urinated and transferred to a vial in ice. All collected materials were kept at −20°C prior to use.
To prepare urine samples for chemical analysis, we first thawed the frozen urine and mixed 160 μL urine sample with 160 μL dichloromethane (purity > 99.5%; DIMA Technology, Inc., Beijing, China), stirred thoroughly, and stored it at 4°C for 12 h, and then used the bottom phase (the layer with dichloromethane).
Behavioral tests
An infrared camera was mounted on the ceiling of the testing room (5 m × 2.5 m × 2.2 m). Two plastic tubes (50 cm × 5 cm for each) with one end open and the other end closed were fixed to the wall in the corners, 150 cm apart. Either a disposable paper cup (300 mL) filled with bedding materials or a small vial with 20 μL of an aqueous odor sample was placed inside the tube to mimic a rat hole and avoid direct contact with the cats (Figure 1).
Figure 1.
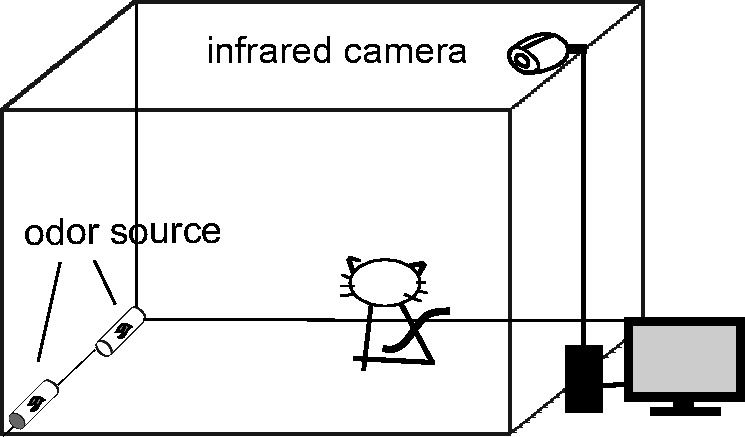
An infrared camera was installed on the ceiling of the testing room (5 × 2.5 × 2.2 m). Two plastic tubes (50 × 5 cm for each) with one end open and the other end closed were fixed in the corners and along the wall, 150 cm apart. A disposable paper cup (300 mL) filled with a bedding material or a small vial with 20μL of an aqueous odor sample was put inside the tube.
The tests were carried out from 21:00 to 6:00. We set up the odorants, and then introduced a cat into the test room. Each cat was allowed to freely investigate the room, and all its activities were recorded by an infrared video camera linked with a computer. An observer then watched the video replay to record the time when the test cat approached a plastic tube and orientated its nostrils close to the opening of the plastic tube within 10-cm distance. The positions of the 2 stimuli were exchanged between trails. The observer was blind to the odorants being presented. Between trials, the plastic tubes and the test room were cleaned thoroughly with 75% alcohol solution. Each cat had been placed in test rooms for 3-night habituation period before formal trials.
Wild rat grouping and odorant presentation
According to the 2-heptanone (major component) levels in the urine samples as measured by gas chromatography–mass spectrometry (GC-MS), 6 males with the richest 2-heptanone were selected as the HIGH group and another 6 males with the poorest 2-heptanone as the LOW group. Randomly paired urine samples from 6 LOW rats and 6 HIGH rats, as well as paired samples from 7 BN and 7 LEW strains were individually used as odor donors to test the olfactory preferences of the domestic cats.
We prepared 4 urine samples mixed equally from: (1) 7 BN males, (2) 7 LEW males, (3) 6 males of LOW wild rats, and (4) 6 males of HIGH wild rats. Second, according to the mean concentrations of 4-heptanone, 2-heptanone, and 9-hydroxy-2-nonanone in urine as measured with GC-MS, we added the synthetic analogs of the 3 ketones into mixed male urine of BN rats to produce authentic concentrations equal to those in LEW males to mimic LEW male urine. Likewise, the ketones were added into the urine mixture of LOW wild rats to simulate HIGH wild rats. Lastly, we paired the pure urine mixture with adjusted urine mixture and presented them to cats.
Chemical analysis
Chemical analysis was carried out with a 6890 Agilent gas chromatograph coupled to a 5973 mass spectrometer and the MS Library NIST 2002 (Agilent Technologies, Inc., Santa Clara, CA, USA). The GC had an HP5-MS column (30 m × 0.25 mm internal diameter × 0.25 μm film thickness, Agilent Technologies, Inc., Santa Clara, CA, USA), carrier gas helium at 1.0 mL/min, and injector set at 280°C. The oven was programmed at 5°C/min from 50 to 100°C, then ramped 10°C /min until 280°C, and held for 5 min. Finally, the temperature was increased to 300°C and held for 10 min post-run to clean the column. MS was in the electron impact mode 70 eV, and the transfer line was set at 280°C. Scanning mass ranged from 30–450 amu (Zhang et al. 2008; Zhang and Zhang 2011). In total, 3 μL of the extract of urine samples was injected in the splitless mode.
Tentative identifications were made by comparing the mass spectra of GC peaks with those in the MS library and in the published literature (Zhang et al. 2008; Zhang and Zhang 2011). Seven compounds, 4-heptanone, 2-heptanone, dimethyl sulfone, 4-methyl phenol, 4-ethyl phenol, 9-hydroxy-2-nonanone, and squalene (all purity > 98%; ACROS Organics, Morris Plains, NJ, USA) were further confirmed by matching retention times and mass spectra with the authentic analogs, then we quantified them by comparing their peak areas in the samples with the established standard curve of GC peak areas versus concentrations (Zhang et al. 2008; Zhang and Zhang 2011).
Statistical analysis
We used Kolmogorov–Smirnov test to examine the distribution of raw data, and either parametric or nonparametric test was applied to behavioral tests and chemical data. If data were normally distributed, paired-samples t-test and independent t-test were used for behavioral and GC data, respectively; otherwise, Wilcoxon signed-rank test and Mann–Whitney U test were used, respectively. All statistical analyses were conducted using SPSS (v15.0, SPSS Inc., Chicago, IL, USA). Alpha was set at P < 0.05.
Ethical notes
The procedures of animal care and use in this study fully complied with Chinese legal requirements and were approved by the Animal Use Committee of the Institute of Zoology, Chinese Academy of Sciences (approval number IOZ12017). The wild rats were caught using live traps, housed individually in plastic cages (37 × 26 × 17 cm), and then transported form Chengdu to Beijing. Bedding, food, and water were available during transportation. After the study, the cats were released and rats were still kept in animal houses for breeding.
Results
Male pheromone contents in laboratory rats of 2 inbred strains and wild rats
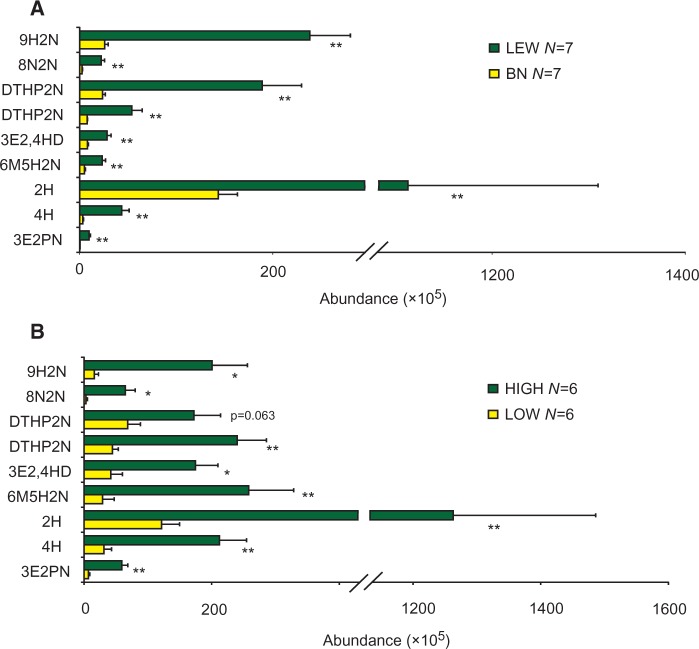
Using GC-MS, we chemically characterized 12 compounds from LEW and BN male urine, including 9 ketones, 2 phenols, and 1 sulfone (Figure 2).
Figure 2.
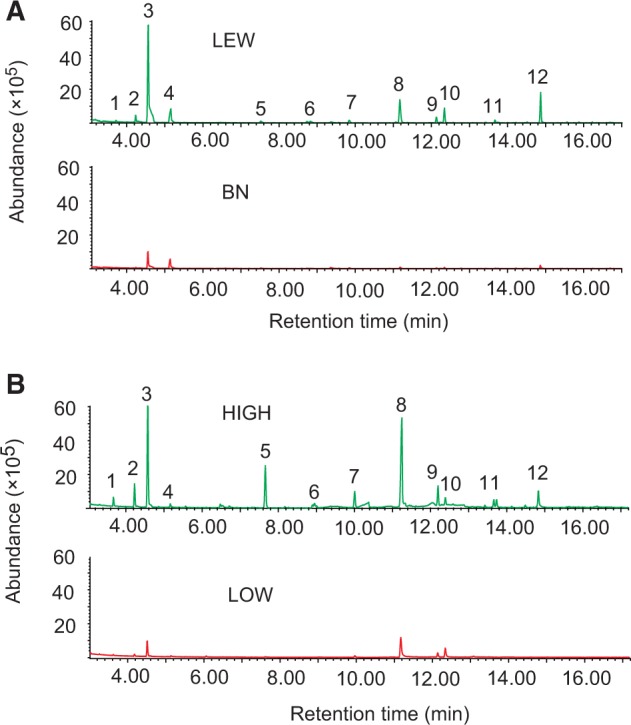
Representative GC profile of dichloromethane extract from LEW and BN urine (A), and wild rats of HIGH and LOW groups (B). GC conditions are described in Materials and methods section. Numbered GC peaks correspond to compounds in Table 1.
The 9 ketones and 2 phenols were richer in LEW rats than in BN rats (Table 1). The 9 ketones, previously known as potential male pheromones, roughly accounting for 81% (GC peak area percentage) of all varying compounds in LEW males and 53% in BN males (Figure 3A; Table 1). The authentic levels of 4-heptanone, 2-heptanone ketones, and 9-hydroxy-2-nonanone of these 9 ketones in LEW and BN male urine were roughly measured to be 2.01 ppm, 22.03 ppm, and 7.38 ppm and 0.06 ppm, 3.76 ppm, and 0.86 ppm, respectively.
Table 1.
Comparisons of abundances (= GC peak area) of urinary volatiles between the 2 laboratory strains of rats, and between the 2 wild groups ([mean ± standard deviation] × 105 , BN = Brown Norway rats, LEW = Lewis rats, N = sample size)
| No. | RT (min) | Compounds | Laboratory strains |
Wild groups |
||||
|---|---|---|---|---|---|---|---|---|
| BN (N = 7) | LEW(N = 7) | P (T/Z) | LOW (N = 5) | HIGH (N = 5) | P (T/Z) | |||
| 1 | 3.72 | 3-ethyl-2-pentanone | 0.46 ± 0.90 | 9.78 ± 3.77 | 0.001 (3.202) | 6.86 ± 5.79 | 59.12 ± 25.18 | 0.003 (4.954) |
| 2 | 4.24 | 4-heptanonea | 3.36 ± 1.90 | 43.58 ± 20.46 | 0.002 (5.178) | 31.25 ± 31.33 | 212.18 ± 112.44 | 0.010 (3.797) |
| 3 | 4.56 | 2-heptanonea | 143.73 ± 52.25 | 1,112.79 ± 520.88 | 0.003 (4.898) | 121.43 ± 74.63 | 1,262.79 ± 590.75 | 0.005 (4.695) |
| 4 | 5.14 | dimethyl sulfonea | 166.79 ± 58.91 | 221.43 ± 71.54 | 0.145 (1.560) | 35.78 ± 30.53 | 104.48 ± 103.85 | 0.055 (1.922) |
| 5 | 7.53 | 6-methyl-5-hepten-2-one | 4.99 ± 2.52 | 23.38 ± 9.13 | 0.001 (5.139) | 29.10 ± 48.17 | 257.92 ± 186.81 | 0.010 (2.562) |
| 6 | 8.84 | 4-methyl-phenola | 2.45 ± 2.34 | 32.97 ± 23.82 | 0.006 (3.373) | 120.30 ± 262.62 | 296.03 ± 436.96 | 0.229 (1.203) |
| 7 | 9.85 | 3-ethyl-2,4-heptanedione | 8.13 ± 2.47 | 28.34 ± 11.26 | 0.003 (4.639) | 41.98 ± 47.90 | 174.54 ± 93.06 | 0.025 (2.242) |
| 8 | 11.18 | 4-ethyl-phenola | 26.51 ± 26.19 | 148.69 ± 104.29 | 0.003 (3.003) | 740.54 ± 1,183.02 | 672.31 ± 683.00 | 0.873 (0.160) |
| 9 | 12.14 | a dialkyl tetrahydro-2 h-pyran-2-one | 7.75 ± 1.34 | 54.06 ± 28.43 | 0.005 (4.305) | 44.59 ± 23.91 | 239.95 ± 121.23 | 0.003 (3.873) |
| 10 | 12.34 | a dialkyl tetrahydro-2 h-pyran-2-one | 23.68 ± 7.78 | 189.09 ± 107.96 | 0.002 (4.043) | 68.42 ± 52.77 | 172.37 ± 109.99 | 0.063 (2.087) |
| 11 | 13.68 | 8-nonen-2-one | 2.54 ± 1.35 | 22.26 ± 9.45 | 0.001 (5.464) | 3.08 ± 5.66 | 64.68 ± 40.89 | 0.020 (2.325) |
| 12 | 14.86 | 9-hydroxy-2-nonanonea | 25.94 ± 9.66 | 238.38 ± 111.54 | 0.002 (5.020) | 16.00 ± 17.81 | 200.65 ± 146.60 | 0.027 (3.063) |
aThe compounds are definitively identified. RT = retention time, using independent t-test or Mann–Whitney U test. Values in parentheses are either T or Z values. Z values are given in italics.
Figure 3.
Comparison of the relative abundance of the urinary ketones between LEW and BN rats (A) and wild rats of HIGH and LOW groups (B) (mean ± SE; **P < 0.01, independent t-test or Mann–Whitney U test. 3E2PN: 3-ethyl-2-pentanone; 4H: 4-heptanone; 2H: 2-heptanone; 8N2N: 8-nonen-2-one; 6M5H2N: 6-methyl-5-hepten-2-one; 9H2N: 9-hydroxy-2-nonanone; 3E2,4HD: 3-ethyl-2,4-heptanedione; DTHP2N: a dialkyl tetrahydro-2 h-pyran-2-one; DS: dimethyl sulfone; 4MP: 4-methyl-phenol; 4EP: 4-ethyl-phenol).
Likewise, from 20 male and 11 female wild rats, these 12 volatiles were also detected; according to the ketone levels, we divided the 20 males into 3 groups, HIGH (N = 7), MEDIUM (N = 6), and LOW (N = 7) (Figure 3B; Table 1). All 9 ketones had higher levels in males than in females, indicative of sexually dimorphic traits (Figure 4).
Figure 4.
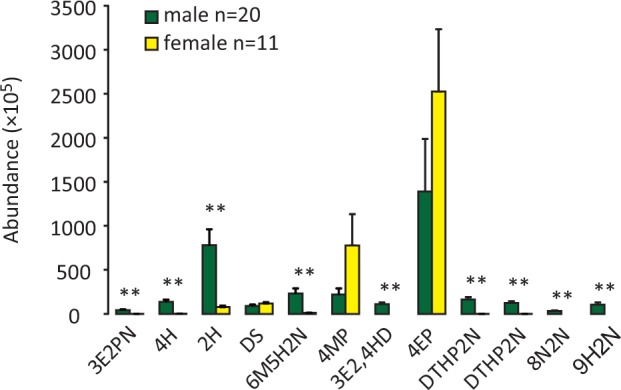
Comparisons of the abundances of urinary volatiles between male and female wild rats (mean ± SE, *P < 0.05, **P < 0.01, independent t-test or Mann–Whitney U test. 3E2PN: 3-ethyl-2-pentanone; 4H: 4-heptanone; 2H: 2-heptanone; 8N2N: 8-nonen-2-one; 6M5H2N: 6-methyl-5-hepten-2-one; 9H2N: 9-hydroxy-2-nonanone; 3E2,4HD: 3-ethyl-2,4-heptanedione; DTHP2N: a dialkyl tetrahydro-2 h-pyran-2-one; DS: dimethyl sulfone; 4MP: 4-methyl-phenol; 4EP: 4-ethyl-phenol).
GC-MS analysis further revealed that all 9 ketones were significantly or marginally significantly richer in the HIGH than in the LOW group (Figure 3B). The levels of 4-heptanone, 2-heptanone, and 9-hydroxy-2-nonanone in HIGH and LOW wild rats were roughly measured to be 9.2 ppm, 48.6 ppm, and 13.8 ppm and 1.4 ppm, 4.8 ppm, and 2.1 ppm, respectively.
Olfactory responses by cats to the rat odorants with different pheromone levels
Behavioral 2-choice tests revealed that cats spent more time sniffing LEW male bedding (t = 5.232, df = 7, P = 0.001) or urine (z = 2.668, df = 7, P = 0.008) as compared with the counterparts of BN male urine (Figure 5A). Similarly, cats showed an olfactory preference for the bedding (z = 2.666, df = 7, P = 0.008), or urine (z = 2.670, df = 7, P = 0.008) from the HIGH group over the counterparts from the LOW group in wild male rats (Figure 5B).
Figure 5.
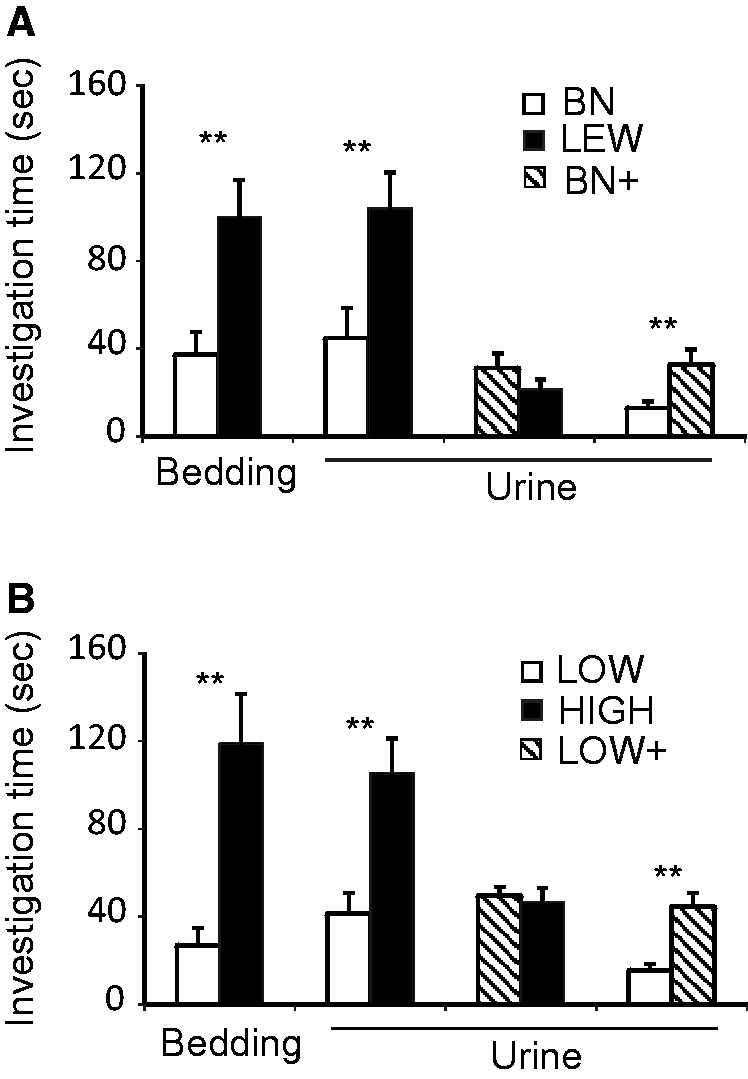
Duration of investigation (mean ± SE, sec) by cats (N = 9) of 2 different rat scents (different rat bedding or rat urine) during a choice test in (A) laboratory inbred rats, or (B) wild rats BN+ = BN urine supplemented with 3 ketones; LOW+ = LOW urine supplemented with 3 ketones; **P < 0.01, paired t-test or Wilcoxon signed-rank test).
Olfactory preferences by cats and male pheromone levels of rats
Two choice tests revealed that cats exhibited an olfactory preference for BN urine having been treated with the 3 ketones over raw BN urine (t = 3.665, df = 7, P = 0.006), but showed no choice between LEW urine and the BN urine added with the synthetic analogs of the 3 ketone pheromones (Figure 5A). Likewise, cats showed an overt preference for the LOW wild rat urine having been added with the 3 ketones to the raw LOW wild rat urine (t = 6.962, df = 7, P < 0.001), but they responded similarly to HIGH wild rat urine and LOW wild rat urine having been added with the 3 ketones (Figure 5B).
Discussion
Our experiments demonstrated that both laboratory and wild brown rats with exaggerated sex pheromones are more attractive to domestic cats and thus more likely to put themselves at an increased predation risk. Although it is well known that exaggerated male pheromones increased the benefits of mating and reproductive success, we provided the first evidence that exaggerated male pheromones may also increase attractiveness to predators and hence increase the costs due to predation (Zuk and Kolluru 1998; Kotiaho 2001; Godin and McDonough 2003; Hughes et al. 2012; Cordes et al. 2014).
BN and LEW are 2 inbred rat strains, which have differentiated genetic background and volatile profiles, and have been used as animal models in rat pheromone research (Zhang and Zhang 2011, 2014). Using these models, we recently found that when male pheromones reached a higher concentration than a certain threshold, they became exaggerated male signals that shaped female mating preferences (Zhang and Zhang 2014). We also observed that 11 urine-metabolized volatile compounds were more abundant in LEW males than in BN males (Zhang and Zhang 2011, 2014). Of these 11 compounds, the 9 ketones instead of the 2 phenols were proven to be potential male pheromones due to their male-biased expression and androgen dependency. Particularly, the 3 major ketone components 4-heptanone, 2-heptanone, and 9-hydroxy-2-nonanone have been verified by their abilities to restore sexual attractiveness of castrated male urine in laboratory rats (Zhang et al. 2008; Zhang and Zhang 2011, 2014). Here, adding these 3 ketones into BN male urine resulted in increased attractiveness to cats, suggesting that exaggerated male pheromones in rats might increase susceptibility to predation risk in natural selection.
In wild-captured rats, we further proved that domestic cats exhibited olfactory preferences for rats with high pheromone levels over those with low pheromone levels and that these 3 ketones of male pheromones could increase cat attraction. Additionally, because sex pheromones usually are conserved within species and these ketones were also male-biased in wild brown rats, this suggests that the ketones are male pheromone components of these wild rats (Zhang et al. 2008; Niehuis et al. 2013; Zhang and Zhang 2014). This implies that, in nature, wild male rats have remarkable individual differences in sex pheromone levels to produce exaggerated male signals, and thus are likely to be easily detected by predators thereby increasing predation risk on themselves (Hughes et al. 2010; Hughes et al. 2012).
Predation pressure is often regarded as a selective force of natural selection balancing sexual selection as for male signals (Godin and McDonough 2003; Head et al. 2005; Husak et al. 2006; Cordes et al. 2014). The olfactory preferences of domestic cats for exaggerated male pheromones in rat urine suggest that enhanced male pheromones, such as visual and acoustic signaling, are also linked with increased predator attraction and consequent predation risk (Godin and Briggs 1996; Godin and McDonough 2003; Head et al. 2005; Cordes et al. 2014). Because odorant signals, unlike acoustic and visual signals, are long-lasting even if the signalers are absent, both signalers with richer pheromones and their social partners thus may suffer from long-lasting predation risk by eavesdropping predators (Hughes et al. 2010, 2012).
As assumed by the handicap and the Fisherian hypotheses of sexual selection, selection for costly exaggerated male ornaments due to increased mating success appears to be balanced by natural selection (Zahavi 1975; Andersson and Simmons 2006; Prokop et al. 2012). Secondary sexual traits may represent a compromise between attractiveness to conspecific females and avoidance of detection by hetero-specific predators (Zuk and Kolluru 1998; Basolo and Wagner 2004). Several theoretical studies suggested that extravagant sexual traits are costly as did empirical studies (Brooks 2000; Jennions et al. 2001; Kotiaho 2001; Hunt et al. 2004). In natural populations of brown rats, the evolution of male pheromones might arise from the opposing effects of natural and sexual selection rather than sexual selection alone (Wedell and Tregenza 1999; Brooks 2000; Basolo and Wagner 2004; Taylor et al. 2007; Harari and Steinitz 2013). The predation cost that females experience when mating with more attractive males can be outweighed by the benefits of increased attractiveness in their sons (Qvarnström and Forsgren 1998; Hedrick 2000; Head et al. 2005).
In conclusion, our results showed that males with exaggerated pheromones strikingly attract eavesdropping predators, thus revealing the conflict between sexual attractiveness and predation risk (Cushing 1984; Chiszar et al. 1997; Wisenden and Thiel 2002; Ylönen et al. 2003; Amo et al. 2004; Svensson et al. 2004; Hughes et al. 2010; Hughes et al. 2012). In rodents, male pheromones might be a trade-off between mating benefits and predation costs (Basolo and Wagner 2004). Unlike acoustic and visual signals, pheromones are the oldest of all communicative signals. They are products of metabolism and are absolutely critical for exploring the evolution of sexual traits driven by the opposing forces of natural and sexual selection (Wyatt 2003).
Acknowledgments
We are grateful to Jin-Hua Zhang and You-Jin Ren for sample collection and animal care, and Jian-Guo Jiang and Gui-Jun Wen for capturing wild rats.
Funding
This work was supported by the Strategic Priority Research Program of the Chinese Academy of Sciences [XDB11010400], grants from China National Science Foundation [91231107 and 31301887], and the Foundation of State Key Laboratory of IPM [ChineseIPM1401].
References
- Amo L, Lopez P, Martin J, 2004. Chemosensory recognition of its lizard prey by the ambush smooth snake Coronella austriaca. J Herpetol 38:451–454. [Google Scholar]
- Andersson M, Simmons LW, 2006. Sexual selection and mate choice. Tren Ecol Evol 21:296–302. [DOI] [PubMed] [Google Scholar]
- Basolo AL, Wagner WE, 2004. Covariation between predation risk, body size and fin elaboration in the green swordtail Xiphophorus helleri. Biol J Linn Soc 83:87–100. [Google Scholar]
- Bender K, Balogh P, Bertrand M, Den Bieman M, Von Deimling O, et al. , 1994. Genetic characterization of inbred strains of the rat Rattus norvegicus. J Exp Anim Sci 36:151–165. [PubMed] [Google Scholar]
- Brooks R, 2000. Negative genetic correlation between male sexual attractiveness and survival. Nature 406:67–70. [DOI] [PubMed] [Google Scholar]
- Chiszar D, Lukas W, Smith HM, 1997. Response to rodent saliva by two species of rodentiophagous snakes. J Chem Ecol 23:829–836. [Google Scholar]
- Cordes N, Engqvist L, Schmoll T, Reinhold K, 2014. Sexual signaling under predation: attractive moths take the greater risks. Behav Ecol 25:409–414. [Google Scholar]
- Cushing BS, 1984. A selective preference by least weasels for estrous versus diestrus urine of prairie deer mice. Anim Behav 32:1263–1265. [Google Scholar]
- Godin JGJ, Briggs SE, 1996. Female mate choice under predation risk in the guppy. Anim Behav 51:117–130. [Google Scholar]
- Godin JGJ, McDonough HE, 2003. Predator preference for brightly colored males in the guppy: a viability cost for a sexually selected trait. Behav Ecol 14:194–200. [Google Scholar]
- Harari AR, Steinitz H, 2013. The evolution of female sex pheromones. Curr Zool 59:569–578. [Google Scholar]
- Head M, Hunt J, Jennions M, Brooks R, 2005. The indirect benefits of mating with attractive males outweigh the direct costs. PLoS Biol 3:e33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrick AV, 2000. Crickets with extravagant mating songs compensate for predation risk with extra caution. Proc R Soc B Biol Sci 267:671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Jimenez A, Rios-Cardenas O, 2012. Natural versus sexual selection: predation risk in relation to body size and sexual ornaments in the green swordtail. Anim Behav 84:1051–1059. [Google Scholar]
- Hughes NK, Kelley JL, Banks PB, 2012. Dangerous liaisons: the predation risks of receiving social signals . Ecol Lett 15:1326–1339. [DOI] [PubMed] [Google Scholar]
- Hughes NK, Price CJ, Banks PB, 2010. Predators are attracted to the olfactory signals of prey. PLoS ONE 5:e13114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt J, Brooks R, Jennions MD, Smith MJ, Bentsen CL, et al. , 2004. High-quality male field crickets invest heavily in sexual display but die young. Nature 432:1024–1027. [DOI] [PubMed] [Google Scholar]
- Husak JF, Macedonia JM, Fox SF, Sauceda RC, 2006. Predation cost of conspicuous male coloration in collared lizards Crotaphytus collaris: an experimental test using clay-covered model lizards. Ethology 112:572–580. [Google Scholar]
- Jennions MD, Moller AP, Petrie M, 2001. Sexually selected traits and adult survival: a meta-analysis. Quart Rev Biol 76:3–36. [DOI] [PubMed] [Google Scholar]
- Koivula M, Korpimaki E, 2001. Do scent marks increase predation risk of microtine rodents? Oikos 95:275–281. [Google Scholar]
- Kotiaho JS, 2001. Costs of sexual traits: a mismatch between theoretical considerations and empirical evidence. Biol Rev 76:365–376. [DOI] [PubMed] [Google Scholar]
- Niehuis O, Buellesbach J, Gibson JD, Pothmann D, Hanner C, et al. , 2013. Behavioural and genetic analyses of Nasonia shed light on the evolution of sex pheromones. Nature 494:345–348. [DOI] [PubMed] [Google Scholar]
- Novotny M, Harvey S, Jemiolo B, 1990. Chemistry of male dominance in the house mouse Mus domesticus. Experientia 46:109–113. [DOI] [PubMed] [Google Scholar]
- Novotny MV, Ma W, Zidek L, Daev E, 1999. Recent biochemical insights into puberty acceleration, estrus induction, and puberty delay in the house mouse. In: Johnston RE, Müller-Schwarze D, Sorensen PW, editors. Advances in Chemical Signals in Vertebrates. New York: Springer, 99–116. [Google Scholar]
- Prokop ZM, Michalczyk L, Drobniak SM, Herdegen M, Radwan J, 2012. Meta-analysis suggests choosy females get sexy sons more than “good genes”. Evolution 66:2665–2673. [DOI] [PubMed] [Google Scholar]
- Qvarnström A, Forsgren E, 1998. Should females prefer dominant males? Tren Ecol Evol 13:498–501. [DOI] [PubMed] [Google Scholar]
- Roberts SC, Gosling LM, 2003. Genetic similarity and quality interact in mate choice decisions by female mice. Nat Gen 35:103–106. [DOI] [PubMed] [Google Scholar]
- Svensson GP, Larsson MC, Hedin J, 2004. Attraction of the larval predator Elater ferrugineus to the sex pheromone of its prey Osmoderma eremita, and its implication for conservation biology. J Chem Ecol 30:353–363. [DOI] [PubMed] [Google Scholar]
- Taylor ML, Wedell N, Hosken DJ, 2007. The heritability of attractiveness. Curr Biol 17:R959–R960. [DOI] [PubMed] [Google Scholar]
- Wang YX, 2003. A Complete Checklists of Mammal Species and Subspecies in China. Beijing: China Forestry Publishing House. [Google Scholar]
- Wedell N, Tregenza T, 1999. Successful fathers sire successful sons. Evolution 53:620–625. [DOI] [PubMed] [Google Scholar]
- Wisenden BD, Thiel TA, 2002. Field verification of predator attraction to minnow alarm substance. J Chem Ecol 28:433–438. [DOI] [PubMed] [Google Scholar]
- Wyatt TD, 2003. Pheromones and Animal Behaviour: Communication by Smell and Taste. Cambridge: Cambridge University Press. [Google Scholar]
- Ylönen H, Sundell J, Tiilikainen R, Eccard JA, Horne T, 2003. Weasels’ Mustela nivalis nivalis preference for olfactory cues of the vole Clethrionomys glareolus. Ecology 84:1447–1452. [Google Scholar]
- Zahavi A, 1975. Mate selection: selection for a handicap. J Theor Biol 53:205–214. [DOI] [PubMed] [Google Scholar]
- Zhang JX, Sun LX, Zhang JH, Feng ZY, 2008. Sex- and gonad- affecting scent compounds and 3 male pheromones in the rat. Chem Sens 33:611–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YH, 2012. Chemical Basis and Behavioral Functions of Socio - Sexual Odor in Vertebrates [Doctor thesis]. [Beijing]: Graduate University of Chinese Academy of Sciences. [Google Scholar]
- Zhang YH, Zhang JX, 2011. Urine-derived key volatiles may signal genetic relatedness in male rats. Chem Sens 36:125–135. [DOI] [PubMed] [Google Scholar]
- Zhang YH, Zhang JX, 2014. A male pheromone-mediated trade-off between female preferences for genetic compatibility and sexual attractiveness in rats. Front Zool 11:73. [Google Scholar]
- Zuk M, Kolluru GR, 1998. Exploitation of sexual signals by predators and parasitoids. Quart Rev Biol 73:415–438. [Google Scholar]
- Zuk M, Simmons LW, Cupp L, 1993. Calling characteristics of parasitized and unparasitized populations of the field cricket Teleogryllus oceanicus. Behav Ecol Sociobiol 33:339–343. [Google Scholar]