Abstract
Meiotic drivers distort transmission to the next generation in their favor, with detrimental effects on the fitness of their homologues and the rest of the genome. Male carriers of meiotic drivers commonly inflict costs on their mates through genetic incompatibility, reduced fecundity, or biased brood sex ratios. Given these costs, evidence for female discrimination against male carriers is surprisingly rare. One of few examples is the t haplotype in house mice, a meiotic driver that shows strong transmission distortion in males and is typically homozygote lethal. As a consequence, mating between 2 t heterozygous (+/t) mice leads to high embryo mortality. Previous experiments showing that +/t females avoid this incompatibility cost by preferring +/+ versus +/t males have inferred preference based on olfactory cues or brief social interactions. Evidence from mating contexts in laboratory settings and semi-natural populations has been inconclusive. Here, we investigated female choice from a large number of no-choice mating trials. We found no evidence for discrimination against +/t males based on mating, remating, and copulatory behavior. Further, we found no evidence for avoidance of incompatibility through selective interactions between gametes. The likelihood of mating showed significant effects of female weight and genotype, suggesting that our test paradigm enabled females to exhibit mate choice. We discuss the strengths and limitations of our approach. By explicitly considering selection at both the individual and gene level, we argue why precopulatory female discrimination by +/t females may be less evolutionarily stable than discrimination by all females based on postcopulatory mechanisms.
Keywords: cryptic female choice, female preference, indirect benefits, mate choice, segregation distortion, t haplotype.
Female mate choice for genetic benefits is a much-debated topic in evolutionary biology (Kokko et al. 2006; Kuijper et al. 2012; Hughes 2015). Several hypotheses regarding the evolution of female preferences for heritable male traits have been formulated, some of which posit that choosy females benefit through producing attractive offspring (Fisherian runaway selection), whereas others propose that offspring inherit “good genes” from males that display preferred secondary sexual traits (Andersson and Simmons 2006). A further potential genetic (indirect) benefit of female preference is producing offspring with compatible alleles, where the genetic quality of the offspring depends on the genetic interactions of the parents’ alleles (Kempenaers 2007; Puurtinen et al. 2009). The different kinds of genetic benefits and direct non-genetic benefits are not mutually exclusive (Kokko et al. 2006), and the distinction between “good alleles” and “compatible alleles” (Kempenaers 2007) might not even be useful, because the frequency of an allele in a population partly determines its additive and non-additive components of genetic variance (Puurtinen et al. 2009). One of the challenges with regards to explaining the evolution of mate choice for genetic benefits is that directional preference should—but empirically does not—lead to the depletion of the genetic variation in the target of the preference (the “lek paradox”; Kirkpatrick and Ryan 1991). Several solutions to the lek paradox have been proposed, some of which rely on continuous generation of variation in genetic quality through deleterious mutations (Iwasa et al. 1991). Preference for “good” genes may thus be seen as discrimination against “bad” genes (Hughes 2015), where females avoid mating with males carrying deleterious alleles.
Genomic conflict is a potentially ubiquitous source of variation in genetic quality and compatibility (Burt and Trivers 2006). Selfish genetic elements undermine otherwise fair inheritance and promote their own success at the cost of the rest of the genome (Burt and Trivers 2006). Segregation distorters are selfish genetic elements that manipulate meiosis or postmeiotic stages of gamete production, thus exhibiting “meiotic drive”(Lindholm et al. 2016). Meiotic drive frequently targets male gametogenesis (Taylor and Ingvarsson 2003), presumably because male gametes are produced in excess and fast cell proliferation in spermatogenesis is under less control than oogenesis (Price and Wedell 2008). Mating with male carriers of such meiotic drivers can incur a variety of costs to females, either through reduced fertility owing to the elimination of a large proportion of the gametes (Price and Wedell 2008), to the production of sex-biased broods in the context of sex ratio distorters (Jaenike 2001), or due to genetic incompatibility between deleterious mutations located on the driver (Zeh and Zeh 1996). Female preference for males that do not carry meiotic drivers can be expected to evolve in order to avoid these fitness costs (Lande and Wilkinson 1999; Manser 2015). There are well-known empirical examples for female discrimination against drive-bearing males (Lenington et al. 1992; Wilkinson et al. 1998), but the evidence available so far indicates that precopulatory female discrimination is not a common strategy for avoiding the costs imposed by selfish genetic elements (Jaenike 2001; Price and Wedell 2008; Price et al. 2012; Wedell 2013). One issue is that any male trait used by females to detect drive males needs to be tightly genetically linked to the drive locus to prevent recombination from breaking up the association between trait and driver (Lande and Wilkinson 1999; Manser 2015). In one of the prominent examples of female preference for driver-free males, sex ratio drive in stalk-eyed flies (Wilkinson et al. 1998), male eye span has been identified as the target of female preference (Wilkinson et al. 1998; Cotton et al. 2014), and is influenced by a locus in the genomic region of the driver where recombination is strongly reduced (Johns et al. 2005). Thus, eye span represents an honest trait that females can use to avoid fertilization by males with a costly sex ratio distorter. Here, we focus on the second prominent example for precopulatory discrimination against driver males, the t haplotype in house mice, where the evidence is less conclusive than in the stalk-eyed flies example.
The t haplotype is an autosomal meiotic drive element that shows strong drive in males and normal transmission in females (Ardlie and Silver 1996; Lindholm et al. 2013). Drive occurs through an elaborate “poison–antidote” mechanism that impairs the motility of sperm not carrying the t haplotype within a +/t male’s ejaculate and thus gives t-bearing sperm an advantage in intra-ejaculate sperm competition (reviewed in Herrmann and Bauer 2012). Several major chromosome inversions provide tight genetic linkage of the t haplotype and strongly reduce recombination (Figueroa et al. 1985). Probably as a direct consequence of a build-up of mutations, many t haplotypes carry homozygote embryonic lethal alleles (Bennett 1975). The combination of strong male drive and homozygote embryo lethality makes 2 +/t individuals genetically incompatible partners: litter size of +/t females mated to +/t males is much smaller than in other crosses (Lindholm et al. 2013), giving +/t females a strong evolutionary incentive to avoid fertilization by +/t males. Females heterozygous for the t haplotype (+/t females) have been repeatedly shown to prefer the odor of wild-type males (+/+) over +/t males (Lenington 1991), though all studies were performed in a single laboratory that used wild-derived mice from a mixture of populations, some of which harbored t haplotype variants (different t haplotypes fall into 16 different complementation groups; Klein et al. 1984). The mechanistic basis for olfactory discrimination has not been identified, although the responsible locus was mapped to the t haplotype (Lenington et al. 1988). The major histocompatibility complex (MHC) was thought to offer a promising candidate for olfactory discrimination because several loci are located on the t haplotype (individual t haplotypes thus carry unique MHC alleles; Figueroa et al. 1985; Lindholm et al. 2013). However, it was empirically excluded as the target of female discrimination through the use of recombinant females that showed olfactory discrimination despite carrying a t haplotype with a wild-type MHC haplotype (Lenington et al. 1988). Thus, it remains unknown what exact signal females use to smell the difference between +/t and +/+ males.
Importantly, female preference for wild-type males has never been shown in an actual mating context (Lenington 1991). There is some evidence that female social preference has adaptive functions in house mice (Drickamer et al. 2000; Raveh et al. 2014), but 3 recent studies showed that the correlation between social preference and paternity share is at best moderate (Thonhauser et al. 2013; Manser et al. 2015; Zala et al. 2015). Instead, females appear to actively mate with multiple males when given the choice (Rolland et al. 2003; Thonhauser et al. 2013; Manser et al. 2015; Zala et al. 2015). Multiple mating (polyandry) offers a more parsimonious mechanism than precopulatory mate choice because it does not require the presence of a male phenotype that is tightly linked to the drive locus. Instead, postcopulatory processes such as sperm competition (Parker 1970) or cryptic female choice (Eberhard 1996) could simply exploit the fact that male meiotic drive is by default associated with ejaculate features (Haig and Bergstrom 1995). Strong evidence supports the notion that male meiotic drive reduces the sperm competitiveness of its carriers (Price and Wedell 2008; Price et al. 2008a; Wedell 2013; Sutter and Lindholm 2015), making polyandry a potentially powerful mechanism to avoid fertilization by male carriers of drive elements (Haig and Bergstrom 1995; Zeh and Zeh 1996).
If +/t males are indeed discriminated against by +/t females through pre- or postcopulatory processes, fertilization by a +/t male may be costly for +/+ females, too, because of investment into sons that are unattractive at least to part of the population or disadvantaged in postcopulatory competition. A meta-analysis suggested that benefits through sexy sons are more important for driving female preference than benefits through good genes effects (Prokop et al. 2012). Whenever discrimination by +/t females is not fully efficient, +/+ females mating with +/t males may also have fewer grandchildren due to genetic incompatibility caused by imprecision of their daughter’s mating decision. Whereas both good genes and sexy sons benefits may be important, the fitness benefits for the different female genotypes relative to the costs of pre- and postcopulatory mate choice are currently unknown but are crucial for assessing the net fitness of different behavioral strategies (Manser et al. 2015). Evidence for olfactory preference by +/+ females was found in some (Lenington 1983; Lenington and Egid 1985) but not in other studies (Coopersmith and Lenington 1992; Williams and Lenington 1993). Experiments involving actual mating contexts in conditions ranging from laboratory settings to natural conditions have found some indications for differences between +/t and +/+ females (Carroll et al. 2004; Lindholm et al. 2013; Manser et al. 2015), but may have been subject to biases through prenatal or early postnatal mortality. Moreover, these studies and an earlier one (Levine et al. 1980) showed paternity disadvantages for +/t males, but were unable to distinguish between pre- and postcopulatory processes. In natural populations, male dominance adds a further confounding factor that influences both male–male competition and female preference (Coopersmith and Lenington 1992), and the evidence for an effect of the t haplotype on male dominance is mixed (Franks and Lenington 1986; Lenington et al. 1996; Carroll et al. 2004).
While thus far there is evidence for olfactory discrimination against +/t males, it remains unclear how olfactory preference translates into precopulatory mate choice, and whether +/t females consistently differ from +/+ females. Here, we test female mate choice with respect to the t haplotype in an actual mating context. First, we test for female choice of +/t and +/+ males in a no-choice test paradigm where females are presented with only one male at a time, and ask whether female genotype at the t locus influences the outcome. We use the occurrence of mating and subtler measures of copulatory behavior to infer female preferences. In a second stage, we ask whether a female’s remating is influenced by the genotype of her first mate. Females may be able to recognize a male’s genotype by his ejaculate features (Angelard et al. 2008) and may thus show differential remating behavior dependent on the genetic quality of their first mate (the “trade-up” hypothesis; Pitcher et al. 2003). Finally, analyzing the distribution of embryo genotypes enables us to address the possibility that compatibility choice occurs between gametes (i.e., that t-bearing ova choose wild-type sperm).
Material and Methods
For this study, we investigated previously unreported aspects of 3 laboratory experiments that all followed a similar mating protocol. The first 2 experiments involved sperm competition trials to assess the effect of the t haplotype (Sutter and Lindholm 2015) and of the copulatory plug (Sutter and Lindholm 2016) on the outcome of postcopulatory competition between 2 males. In the third experiment, monogamous matings were conducted to validate copulatory plug size variation (Sutter and Lindholm 2016). For this study, we expanded our analyses to address questions related to precopulatory female choice and cryptic female choice.
Experimental animals
We used 259 female (mean age ± standard deviation (SD): 103 ± 28 days) and 162 male (79 ± 27 days) wild house mice Mus musculus domesticus. Subjects were sexually mature but initially sexually naïve laboratory-born F1–F3 descendants from a free-living population in Switzerland (König and Lindholm 2012), from which we introduce individuals into our breeding colony every generation. Mice were kept under standard laboratory conditions at a temperature of 22–24 °C under a 14:10 light:dark regime. The breeding colony was kept under a normal light cycle (lights on at 05:30 CET), with food (laboratory animal diet for mice and rats, no. 3430, Kliba) and water provided ad libitum. Paper towels and cardboard served as enrichment and nest building material. Experimental subjects were descendants of 62 breeding pairs, of which 31 consisted of at least one individual (typically the male) that had been caught in the free living population from which all breeding individuals descended from (König and Lindholm 2012). Breeding pairs consisted of monogamous pairs of non-sibling +/+ males and either +/+ or +/t females, the latter producing on average 50% +/t offspring. At the age of 23–28 days, we weaned offspring and kept them in same sex sibling groups in Makrolon Type III cages (23.5 × 39 × 15 cm). We separated male mice at latest when aggression started between brothers and kept them individually in Makrolon Type II cages (18 × 24 × 14 cm). Mating trials were conducted under a reversed 14:10 light:dark regime (lights on at 17:30 CET) in a room separated from the breeding colony. Animals were moved at least 2 weeks prior to being used in the experiment. We used +/t and +/+ males and females and diagnosed their t haplotype status before they entered the experiment. An ear punch tissue sample taken at weaning was used for genotyping and individual identification. We extracted DNA by salt-chloroform extraction (Müllenbach et al. 1989) and diagnosed t haplotype status as described below (section “Postcopulatory aspects”). The experimenter was blind with respect to genotype during all procedures, including mating trials, video observations, dissections and genotyping. All procedures received ethics approval by the Veterinary Office Kanton Zurich, Switzerland (license no. 110/2013) and were conducted in accordance with Swiss law.
Mating trials
The protocol for our mating trials has been described previously (Sutter and Lindholm 2015, 2016), and was similar in all 3 pooled experiments. We chose sexually receptive females in pro-oestrus or oestrus based on visual appearance of the vagina and/or on a quick microscopic inspection of vaginal smears that were taken with plastic inoculation loops (modified after Byers et al. 2012). Oestrus stage may affect the likelihood of mating and male copulatory behavior (Preston and Stockley 2006) and was thus included our analyses we included a categorical account of oestrus stage (“early,” “medium,” or “late” oestrus; Byers et al. 2012). Males and females were weighed to the nearest 0.1g immediately before the start of the trials, which was 1.8 h ± 0.8 (mean ± SD) after the beginning of the 10-h dark phase of the reversed light cycle (lights off at 07:30 CET). Females were paired with a male in his cage under a red light spot after having removed nesting material to facilitate video observation for the quantification of copulatory behavior. Females were checked every 1–1.5 h for the presence of a copulatory plug, indicating ejaculation by the male (McGill 1962). We released the pair into a handling bin and briefly restrained the female to check her vagina for a plug under dim white light, before reintroducing the pair into the cage. Thus, mice were out of their cage for approximately 1 min during a check. For the trials of one of the experiments (N = 45), females were sacrificed after their first mating as part of validation of copulatory plug removal methodology (Sutter and Lindholm 2016). For the remaining mated females (N = 170), the plug was then either removed or left intact (Sutter and Lindholm 2015,2016), after which the female was paired with the second male and checked every 30–60 min until either a second copulatory plug was observed or until the beginning of the next dark phase. After the second mating, the plug was again either removed or left intact. Thus, females either had both or neither of their mates’ plugs removed. Mated females were kept in isolation with nesting material and ad libitum food and water. Trials in which no plug by the first male was detected were stopped at the end of the dark phase and females were re-tested on a later occasion. Males were sexually rested for a minimum of 3 days after a trial with mating to allow sperm and seminal fluid replenishment (Sutter et al. 2016). Whenever possible, we used full brothers from the same litter (65/70 male pairs) for sperm competition trials to minimize the influence of genetic background and potential maternal effects on mating behavior and sperm competitiveness.
Copulatory behavior
Copulatory behavior in house mice is characterized by initial mounts, a variable number of mounts with intromission (during which the male inserts his penis and performs pelvic thrusts), and ejaculation including the deposition of the copulatory plug (McGill 1962). One copulatory series includes all mounts and intromissions and ends with ejaculation. Here, we recorded (1) the latency from introduction of the pair into the cage to the first mount (mount latency), (2) the latency from the first copulatory mount to ejaculation (ejaculation latency), and (3) the in copula duration at ejaculation as potential indicators of a female’s willingness to mate. We also used video recordings to confirm ejaculation by the second male.
Postcopulatory aspects
We sacrificed females 9 days (± 1 day) post coitum using gradual CO2 filling in their home cage and dissected females to retrieve implanted embryos. By doing so we avoided potential biases in the distribution of t genotypes due to early postimplantation embryonic mortality associated with the t haplotype (t/t embryos are resorbed in utero; Lindholm et al. 2013; Sutter and Lindholm 2015). Embryo viability and paternity results are described elsewhere (Sutter and Lindholm 2015, 2016). Here, we further genotyped the Hba-ps4 locus that is located in the genomic region of the t haplotype (Schimenti and Hammer 1990; Lindholm et al. 2013) to obtain data on embryo genotype frequencies (+/+, +/t and t/t) for t haplotype drive estimates and questions related to cryptic female choice with respect to gamete genotype.
Statistical analyses
An overview of the sample sizes available for the different analyses is given in Table 1. Data will be made available on Dryad on acceptance of the manuscript.
Table 1.
Overview of sample sizes available for the different analyses (mating, copulatory behavior, remating, and embryo genotype analyses)
| +/+ Females | +/t Females | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|
| First mate | +/+ | +/t | +/+ | +/t | |||||
| Paired with male | 151 | 145 | 107 | 85 | 488 | ||||
| Mated | 71 | 69 | 40 | 35 | 215 | ||||
| Copulatory behavior | 46 | 35 | 24 | 19 | 124 | ||||
| Second mate | +/+ | +/t | +/+ | +/t | +/+ | +/t | +/+ | +/t | |
| Paired with male | 27 | 30 | 39 | 16 | 14 | 18 | 17 | 9 | 170 |
| Remated | 19 | 21 | 29 | 14 | 12 | 12 | 13 | 7 | 127 |
| Sire genotype | +/+ | +/t | +/+ | +/t | |||||
| Sire mating order | 1st | 2nd | 1st | 2nd | 1st | 2nd | 1st | 2nd | |
| Embryo genotypes | 263 | 201 | 87 | 84 | 149 | 95 | 38 | 39 | 956 |
Using the functions lmer and glmer in lme4 (Bates et al. 2014) in R version 3.1.3 (R Core Team 2015), we analyzed data on mating and remating, copulatory behavior, and offspring genotypes with generalized linear mixed models (GLMMs) and linear mixed models (LMMs), depending on the response variable. We compared full models to null models using likelihood ratio tests (LRTs) to test the global null hypothesis that none of the predictors has a significant effect on the response variable, and extracted effect sizes from full models to avoid biasing effect sizes through removal of non-significant terms (Forstmeier and Schielzeth 2011). Continuous input variables were standardized to a mean of 0 and a SD of 1 to improve interpretability (Schielzeth 2010). Because many females were re-tested if they did not mate and because all males were used in multiple trials, we included the identity of the individuals as random effects in all models to account for multiple testing and avoid pseudoreplication. To account for the family structure inherent in our breeding design, we also included female and male parental origin as random effects. We obtained approximate 95% confidence intervals (c.i.) for fixed effects by multiplying Student’s t values for our sample sizes by the standard errors of the predicted values (Crawley 2007).
Controlling for relatedness
We generally controlled for relatedness between females and males by mating females to 2 males that were full brothers but not closely related to the female. However, in 5/488, trials females were accidentally paired with a full sibling from a different litter. Moreover, due to our within-population breeding design with a limited number of breeding pairs with overlapping generations, mating trials would by chance be staged between second-degree relatives (such as cousins). To include relatedness in our analyses, we included information from our breeding pedigree, where individuals not sharing any relatives in the 2 previous generations were assumed to be unrelated. Relatedness estimates thus ranged between 0 (no shared grandparents) and 0.5 (full siblings).
Mating trials
First, we analyzed mating success (whether or not a plug was detected in a mating trial) with binomial GLMMs. The full model included the following fixed effects: male and female genotype at the t locus and their interaction, male and female body weight and their interaction, female age, oestrus stage (categorical variable with 3 levels), and the pedigree-based relatedness between the 2 individuals (see above).
Second, we asked whether the genotype of a female’s first mate influenced her remating likelihood. We analyzed female remating similarly to mating success, here based on video observations. We included the following variables as fixed effects in a binomial GLMM: the genotypes of a female and her first mate as well as their interaction, female and male weight and their interaction, and relatedness between the female and the male. Because in one of the experiments, some of the first males’ copulatory plugs had been removed (Sutter and Lindholm 2016), we included plug removal as a categorical fixed effect with 2 levels.
Copulatory behavior
We analyzed 3 components of copulatory behavior (mount latency, ejaculation latency, and in copula duration at ejaculation) individually using LMMs. Full models contained female and male genotype and their interaction, female and male body weight and their interaction, oestrus stage and relatedness as fixed effects.
Postcopulatory aspects
Paternity outcomes have been published elsewhere and showed no evidence for an influence of female genotype on the sperm competition disadvantage of +/t males (Sutter and Lindholm 2015). Here, we investigated potential within-ejaculate discrimination at the gamete level, that is, whether penetration of t-bearing ova was non-random with respect to sperm genotype. The proportions of different genotypes of a female’s embryos were analyzed using binomial GLMMs. In these models, we only included female and male identity as random effects, because family-associated variances showed to be negligible. Significance of genotypic frequency estimates was assessed by comparing approximate 95% c.i. to null hypotheses based on previous estimates of transmission in males and females for this population (Lindholm et al. 2013) and on random gamete interactions. Mating order of the sire was included as a covariate to test for a change in the strength of drive with mating order (i.e., timing of ejaculation relative to ovulation) as suggested from work on delayed matings (Braden 1958) and postpartum oestrus matings (Lenington and Heisler 1991).
Results
Mating trials
The 3 experiments were conducted over the course of almost 2 years from January 2013 to December 2014, but initial inspection showed that mating success was not significantly different between the 3 experiments and they were subsequently pooled. In 488 mating trials, 215 females mated as indicated by the deposition of a copulatory plug. Individual females that mated did so after 1.8 ± 1.3 trials (mean ± SD; range 1–8). Females that never mated before the end of the experiments were tested 2.1 ± 1.4 times (range 1–9). In successful trials that led to ejaculation by the male, pairs were separated after 5.7 ± 1.6 h (range 1.5–9.5 h). Pairs that had not mated were separated after 8.5 ± 0.7 h (range 7–11 h).
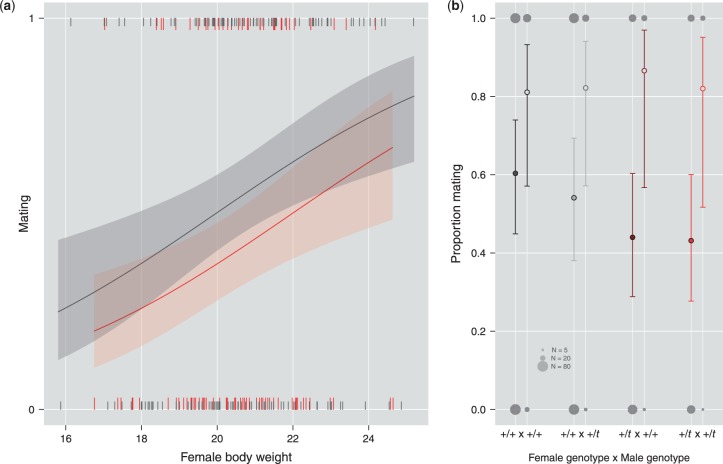
We analyzed mating as a binary outcome in a full model including 389 trials with all information available. Due to our full model approach, trials with missing information regarding any of the predictor variables—most commonly oestrus stage, male body weight, and relatedness—had to be excluded. Inspection of the full model showed significant effects of female genotype and female weight (Table 2 and Figure 1A). Thus, +/t females had a lower likelihood of mating (GLMM: 389 trials, 226 females, 117 males; b [95% c.i.] = 0.55 [1.05, 0.05], z = 2.17, P = 0.030), and heavier females were more likely to mate (0.45 [0.19, 0.72], z = 3.36, P < 0.001). There was neither a significant main effect of male genotype (P = 0.550), nor was the interaction with female genotype significant (P = 0.645; Figure 1B). These results were robust to a more conservative controlling for multiple testing of individual females, as a model including only each female’s first mating trial (GLMM on 207 trials including 104 males) showed very similar results. Thus, the positive effect of female weight on mating likelihood was not driven simply by re-testing females that had not mated at a younger age and had gained weight as time progressed.
Table 2.
Model summary from a full model on mating success
| Model | Response variable | Random effects | Fixed effects | Mean (SD) | Fixed effect centered/ standardized? | Estimate [approx. 95% c.i.] | z value/ F value | P |
|---|---|---|---|---|---|---|---|---|
| GLMM | Mating success | 1|Family/Male ID | Intercept (genotypes centered) | – | – | 0.02 [0.47, 0.51] | 0.07 | 0.941 |
| 1|Family/Female ID | Female t haplotype | – | y/n | 0.55 [1.05, 0.05] | 2.17 | 0.030 | ||
| Male t haplotype | – | y/n | 0.15 [0.63, 0.34] | 0.59 | 0.558 | |||
| Female weight [g] | 20.7 (1.7) | y/y | 0.45 [0.19, 0.72] | 3.36 | < 0.001 | |||
| Male weight [g] | 25.2 (2.0) | y/y | 0.10 [0.14, 0.35] | 0.83 | 0.404 | |||
| Female age [d] | 108 (29) | y/y | 0.09 [0.18, 0.36] | 0.65 | 0.515 | |||
| Relatedness | 0.02 (0.08) | n/n | 0.09 [3.06, 2.88] | 0.06 | 0.954 | |||
| Early oestrus | – | n/n | 0.31 [0.88, 0.26] | 1.07 | 0.285 | |||
| Late oestrus | – | n/n | 0.03 [0.57, 0.64] | 0.11 | 0.913 | |||
| Female × male t haplotype | – | – | 0.22 [0.72, 1.17] | 0.46 | 0.645 | |||
| Female × male weight | – | – | 0.05 [0.20, 0.30] | 0.41 | 0.683 |
GLMM = generalized linear mixed model. The intercept was centered for female and for male genotype by assigning values of 0.5 and +0.5 to +/+ and +/t individuals, respectively. Thus, the intercept corresponds to an average between +/+ and +/t individuals for unrelated individuals with average body weights, with females of average age at an intermediate oestrus stage. t haplotype shows the change for +/t relative to +/+ individuals. Centered and standardized fixed effects have a mean of 0 and a standard deviation of 1 (Schielzeth 2010). Approximate 95% c.i. were obtained by multiplying Student’s t values for our sample sizes by standard errors of the predicted values (Crawley 2007). 95% c.i. not overlapping 0 and P values < 0.05 are highlighted in bold.
Figure 1.
(a) Mating likelihood as a function of female weight and genotype. Mating likelihood of females increased with their weight and was higher for +/+ than for +/t females (Table 2). Ticks correspond to individual mating trials (only every female’s first trial is shown here, N = 247), lines and shaded areas show predictions and approximate 95% c.i. from a full GLMM on 389 trials (Table 2). +/t females are shown in red, +/+ females in gray. (b) No evidence for discrimination against +/t males by +/+ and +/t females. Circles and error bars depict mean and approximate 95% c.i. from full GLMMs on mating likelihood for first matings (solid circles) and rematings (open circles), dependent on female genotype and the genotype of first mates. Raw data are shown as background gray circles, with surface area proportional to sample size. Neither mating nor remating likelihood was significantly affected by male genotype or its interaction with female genotype (see main text and Tables 2 and 3).
Whether or not a mated female remated with her second mate was not significantly affected by any of the variables investigated, including female and male genotype and its interaction. Thus, the null hypothesis for the full model could not be rejected (GLMM: 145 trials, 84 males; P = 0.194; Table 3).
Table 3.
Model summaries on full model tests for remating and copulatory behavior
| Fixed effects |
LRT |
||||||
|---|---|---|---|---|---|---|---|
| Model | Response variable | Random effects | Full model | Null model | χ2 | df | P |
| GLMM | Remating | 1|Family/Male ID | Intercept | Intercept | 11.15 | 8 | 0.194 |
| 1| Female family | Female t haplotype | ||||||
| Male t haplotype | |||||||
| Female weight [g] | |||||||
| Male weight [g] | |||||||
| Relatedness | |||||||
| Plug removal | |||||||
| Female × male t haplotype | |||||||
| Female × male weight | |||||||
| LMM | Mount latency | 1|Family/Male ID | Intercept (genotypes centered) | Intercept | 2.42 | 9 | 0.983 |
| 1|Female family | Female t haplotype | ||||||
| Male t haplotype | |||||||
| LMM | Sqrt(Ejaculation latency) | 1|Family/Male ID | Female weight [g] | Intercept | 6.24 | 9 | 0.716 |
| 1|Female family | Male weight [g] | ||||||
| Relatedness | |||||||
| LMM | In copula at ejaculation | 1|Family/Male ID | Early oestrus | Intercept | 3.78 | 9 | 0.925 |
| Late oestrus | |||||||
| 1|Female family | Female × male t haplotype | ||||||
| Female × male weight | |||||||
GLMM = generalized linear mixed model, LMM = linear mixed model. Fixed effects were centered and standardized as indicated in Table 2 and were the same for all 3 models of copulatory behavior. Shown are the results from LRTs on the full versus the null model (including only the intercept and random effects).
Copulatory behavior
We analyzed mount latency, ejaculation latency and in copula duration at ejaculation to look for more cryptic signs of female mate choice. The null hypotheses for the full models on each of the 3 aspects of copulatory behavior could not be rejected (LMMs: 108 trials, 61 males; P = 0.983, P = 0.716 and P = 0.925). Thus, copulatory behavior was not significantly influenced by any of the variables investigated (Figure 2 and Table 3).
Figure 2.
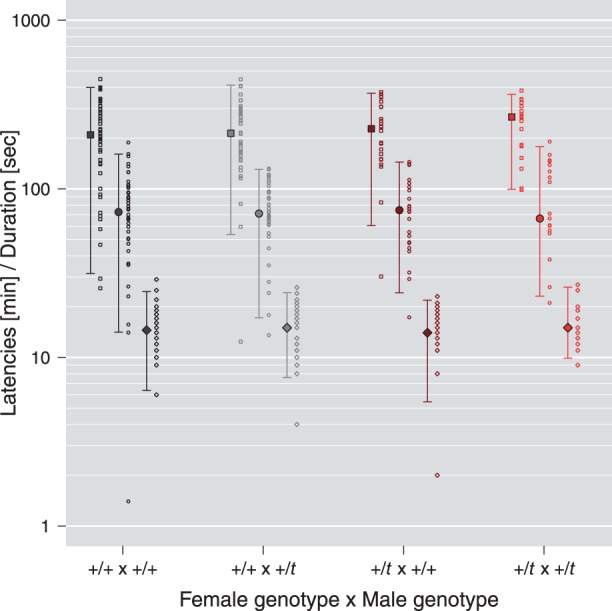
Three aspects of copulatory behavior of first-to-mate males as a function of female and male genotype. Mount latency [minutes; squares], ejaculation latency [minutes; circles] and in copula duration at ejaculation [seconds; diamonds] are shown on a log10-transformed scale for all 4 possible female x male genotype combinations. Copulatory behavior was not significantly affected by any of the variables investigated (Table 3). Small symbols represent raw data. Large symbols and error bars show median and 95% quantiles of the raw data. Ejaculation latencies of less than 1 min were treated as outliers and thus excluded.
Postcopulatory effects
Figure 3 depicts the predicted and empirical genotypic frequencies for the different crosses. Our estimate for male drive from matings between +/t males and +/+ females was 0.94 [0.87, 0.97], not significantly different from a previous estimate on this population (0.9; Lindholm et al. 2013). There was no evidence for an influence of mating order on male drive (GLMM: 171 embryos, 31 females, 32 males; z = 0.998, P = 0.318), meaning that male drive did not differ between males that were first versus second-to-mate (0.90 vs. 0.97). Transmission of the t haplotype from +/t females did not deviate from Mendelian segregation (0.53 [0.47, 0.60]). Based on 0.9 drive in males and 0.5 transmission in females, the expected distribution of embryo genotypes from +/t x +/t matings was 0.45 t/t, 0.5 +/t and 0.05 +/+. Our empirical estimates matched this prediction well: 0.44 t/t [0.29, 0.61], 0.56 +/t [0.43, 0.67] and 0 +/+. Again, order had no significant effect on this distribution (GLMM: 77 embryos, 15 females, 11 males; z = 0.202, P = 0.840). Overall, we found no evidence for a reduced transmission of the t haplotype in matings between genetically incompatible partners, and thus no influence of female genotype at the t locus on drive (cf. Lindholm et al. 2013).
Figure 3.
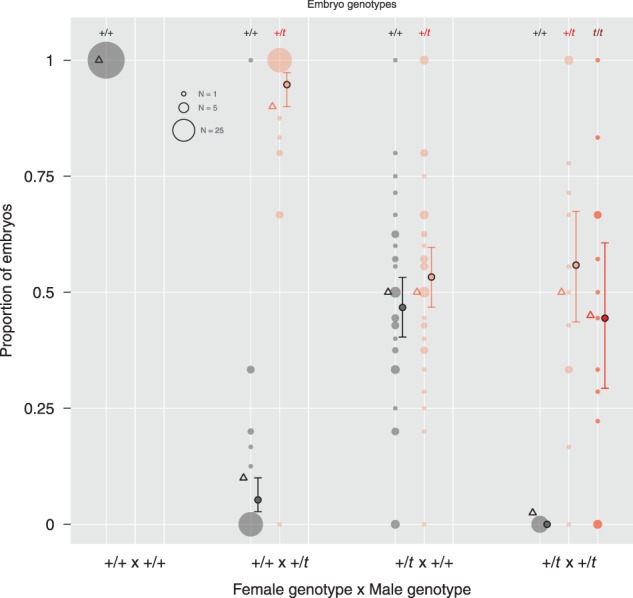
Distribution of embryo genotypes and estimates of male and female t transmission for different parental genotype combinations. Circles and error bars show predicted mean and approximate 95% c.i. for embryo genotype frequencies from GLMMs. The parental genotypes are indicated on the X-axis, embryo genotypes are indicated by colors, and by symbols at the top of the figure. Triangles show the predicted embryo genotype frequencies based on transmission of the t from +/t males to 0.9 of their offspring and Mendelian segregation in females as estimated for this laboratory population elsewhere (Lindholm et al. 2013). There was no evidence for drive reduction or for non-random fusion of sperm and ova in crossings of incompatible genotypes (see main text).
Discussion
In a large number of controlled mating trials, we found no evidence for female discrimination against male carriers of the t haplotype. This was true for precopulatory mate choice, copulatory behavior and remating. Moreover, we found no evidence for cryptic reduction of drive based on genotypes of embryos retrieved during gestation. These results highlight that precopulatory discrimination against t haplotype bearing males may not be a common female strategy to avoid fitness costs associated with this meiotic driver. Female multiple mating offers a more parsimonious and potentially more powerful mechanism.
Precopulatory female preference?
We found no evidence for precopulatory discrimination against +/t males, neither by genetically incompatible +/t females nor by +/+ females. Our findings contrast with previous studies that have reported consistent preferences by +/t females for the airborne scent of +/+ males over that of +/t males (Lenington 1991). Although urine from +/t males has been suggested to differ in volatile chemical profile from wild-type males (Jemiolo et al. 1991), female house mice appear to require information from non-volatile components of urine to develop preferences for individual males (Ramm et al. 2008; Roberts et al. 2010). Furthermore, some recent studies have suggested that the correlation between social preference and sexual preference (as measured by parentage of offspring) may be weak in wild-derived house mice (Thonhauser et al. 2013; Manser et al. 2015; Zala et al. 2015). Arena settings have also been used to investigate discrimination against +/t males, with mixed results (Lenington 1983; Franks and Lenington 1986). The only study so far that allowed females to choose between +/t and +/+ males while preventing male–male interactions found no support for precopulatory choice (Manser et al. 2015). The paternity disadvantage of +/t males was consistent with purely postcopulatory processes, but the experimental design did not allow for a conclusive distinction between pre- and postcopulatory mechanisms and did not control for female oestrus cycle (Manser et al. 2015). The findings reported here are consistent with females using a strategy that relies on the strong sperm competition disadvantage to +/t males (Sutter and Lindholm 2015).
Arguably, our assessment of female preference suffers from some limitations that merit discussion. We tested female choice in a laboratory setting, where choice was the outcome of a mating trial that was subject to interactions between female preference and environmental and male effects (Wagner 1998). Being introduced into and confined in a male’s cage, females might have had little chance to resist male coercion and exhibit choice according to their preferences. If male physical coercion influences mating, one might expect a significant positive effect of male weight, either as a main effect or in the interaction with female weight. However, we did not find any influence of male weight on mating outcome (Table 2). Moreover, the significant positive effect of female weight on mating was opposite to that predicted if light females were less able to resist male coercion (Figure 1A). We can only speculate on why heavier females were more likely to mate. First, heavier females may have a better ability to carry a pregnancy to full term. Second, females were kept in small same-sex groups where competition between females might have led to dominance interactions and reproductive suppression of subordinate females by heavier dominant females (Stockley et al. 2013). Third, if female fecundity increases with female body weight (Singleton et al. 2001), increased mating by heavier females may have been a product of male choice for heavier females (Dewsbury 1982). We also found a significant difference in mating likelihood between +/+ and +/t females, which may have been caused by male choice for +/+ over +/t females, or by a more reactive personality in +/t females (activity and exploration: Auclair et al. 2013; trappability: Lenington and Franks 1985; Drickamer and Lenington 1995; social rank and pregnancy likelihood: Franks and Lenington 1986). Additionally, our observations of copulatory behavior did not reveal evidence for more subtle expression of female preference, because more resistance against +/t males should have increased mount latency, ejaculation latency and/or decreased in copula duration at ejaculation. Although all females were presumably in oestrus, the incidence of mating was moderate, but comparable to a recent study that used females from a laboratory strain that is likely to have experienced positive selection on female mating propensity (Ramm and Stockley 2014). Trials in which mating did not occur could either indicate female and/or male mate choice, or inaccuracy in oestrus detection. Here, in the majority (82%) of the mating trials we detected oestrus using vaginal smears, a method that is well established for house mice (Byers et al. 2012), making it unlikely that oestrus detection was wrong in more than half of the mating trials and that there would have been an oestrus detection bias towards heavier females and +/+ females. Collectively, our findings suggest that females actively chose to mate rather than simply being forcefully mated, but did not discriminate against +/t males.
Sequential stimulus presentation in no-choice test paradigms has been proposed as a more powerful test of female preference than simultaneous stimulus presentation (Wagner 1998), and latency to copulation has been shown to be a reliable predictor of male mating success in field crickets (Shackleton et al. 2005). Studies in invertebrates and vertebrates (e.g., MacLaren and Rowland 2006; Rutstein et al. 2007) have established that no-choice tests enable females to exhibit mate preference, but have also highlighted that results and effect sizes can depend on the test paradigm used (for a meta-analysis see Dougherty and Shuker 2015). Our no-choice test paradigm offered the advantage of removing male–male competition, and the use of full brothers in the vast majority of trials ensured that +/t males did not systematically differ from +/+ males in genetic background. However, our mating design did not allow females to simultaneously compare males. Experiments with female brown lemmings Lemmus trimucronatus provided some evidence for female discrimination between dominant and defeated males in a no-choice setting, as did a simultaneous choice setting (Huck and Banks 1982). In house mice, no-choice tests have demonstrated cryptic male choice regarding mating likelihood (Ramm and Stockley 2014), copulatory behavior (Preston and Stockley 2006), and ejaculate allocation (Ramm and Stockley 2007). In the only study to date that directly compared preferences of female house mice between simultaneous stimulus presentation and no-choice trials, the authors found that females discriminated against hetero-subspecific males only when allowed to compare males directly, and appeared to mate indiscriminately in no-choice trials (Zinck and Lima 2013). However, this negative result from no-choice trials was based on a total of 12 trials, of which only 4 resulted in ejaculation. No-choice tests are associated with smaller effect sizes than simultaneous choice tests (Dougherty and Shuker 2015), thus Zinck and Lima’s (2013) study may have lacked the statistical power to detect more subtle discrimination during no-choice trials. Our large sample size makes it unlikely that our negative result is due to a lack of statistical power. Nevertheless, we cannot rule out that preference in female house is relative and may only be exhibited when more than one potential mate is available.
Is discrimination by +/t females plausible?
Expecting female discrimination against genetically incompatible males in the context of the t haplotype is intuitively appealing: genetic incompatibility has strong immediate fitness consequences, and the restriction of compatibility effects to few loci should facilitate the evolution of compatibility mate choice (Puurtinen et al. 2009). Disassortative mating should lead to negative linkage disequilibrium between the preference locus and the drive locus if there is no physical linkage of the preference locus to the t haplotype (Manser 2015). However, the strong linkage between the male signal and the drive locus that is required for stability of female preference (Lande and Wilkinson 1999; Manser 2015) is facilitated by major chromosomal inversions that encompass many potential candidate loci (e.g., MHC loci; Lindholm et al. 2013; but see Lenington et al. 1992). On the other hand, there are also good reasons to expect that t-specific female preference is not evolutionarily stable. First, the importance of MHC for mate choice in mice remains controversial (Roberts and Gosling 2003; Sherborne et al. 2007), and may be overridden by the influence of major urinary proteins (MUPs) that are not linked to the t haplotype (Krauter et al. 1982). Although choosing males with MHC alleles different from self could lead to discrimination of +/t males by +/t females, it might also result in potentially maladaptive preference for +/t males by +/+ females because they could on average share fewer alleles than with +/+ males. Second, discrimination against the t haplotype that is controlled by a locus located on the t haplotype may not be expected to evolve or remain evolutionarily stable. Suppression of selfish genetic elements to resolve genomic conflict is expected to evolve in unlinked genomic regions (Burt and Trivers 2006). In turn, selection acting on the driving element will favor escaping suppression. Thus, selection will favor driving elements that evade detection by females (Price et al. 2012). Even in females, the situation may not be as clear as stated previously. Lenington and colleagues stated that “… given the deleterious effects of t haplotypes when homozygous, it is possible that more copies of t chromosomes will be transmitted to the next generation if +/t females avoid mating with +/t males” (Lenington et al. 1988). We argue that, from the view of the t haplotype, even selection acting on the t haplotype in females will not favor discrimination against a copy of the same t haplotype unless the probability of +/t males inseminating +/+ females was reduced by mating with +/t females, for example, in a strictly monogamous population. In individual litters, the absolute copy number of lethal t haplotypes that are transmitted to the next generation is not decreased when females accept incompatible mates. The offspring carrying t haplotype may even benefit from homozygote lethality, if reduced sibling competition increases their individual fitness (Charlesworth 1994).
Although we cannot rule out that avoidance of +/t males can arise on the t haplotype (possibly as a by-product of pre-existing female preference loci being located in the genomic region of the t haplotype), such a preference is unlikely to be evolutionarily stable.
Alternative ways to avoid meiotic drivers
If precopulatory discrimination against male carriers of selfish genetic elements is indeed rare (Price and Wedell 2008), how else might females avoid the associated fitness costs? Postcopulatory female choice offers a possibility to select directly on the haploid genotype at the gamete level (Birkhead and Pizzari 2002). Importantly, unlike other phenotypic correlates of drive that may not reliably indicate the presence of a driver, changes in ejaculate features such as ejaculate size or the number of functional sperm are a direct and inevitable consequence of drive in males (Haig and Bergstrom 1995). X-linked sex ratio distortion reduces ejaculate size by killing virtually all of the Y-bearing sperm, offering a plausible mechanism for how females may detect driver males after insemination. Indeed, Drosophila simulans females use fewer of their stored sperm for fertilization and remate more quickly after mating with males carrying a sex ratio distorter than after mating with wild-type males (Angelard et al. 2008). The t haplotype does not affect ejaculate size but instead more subtly influences sperm motility features (reviewed in Olds-Clarke 1997), possibly making it more difficult for females to detect +/t males. Here, we found no evidence that remating was affected by a female’s first mate, either because females are unable to detect the t genotype, or because polyandry is a successful female strategy for avoiding fertilization by +/t males that is employed equally by +/t and +/+ females (Sutter and Lindholm 2015; see below). Nevertheless, there is some evidence from experimentally delayed matings (Braden 1958) and a comparison between matings during naturally cycling oestrus versus postpartum oestrus (Lenington and Heisler 1991), indicating that the timing of mating can affect drive, although this tends not to be the case for t haplotypes with strong male drive (Yanagisawa et al. 1961). Two previous studies have investigated the distribution of offspring genotypes in crosses between 2 +/t individuals and have found evidence for selective penetration that resulted in a reduction of drive (Bateman 1960; Lindholm et al. 2013). Here, we genotyped embryos that we retrieved at an early stage of gestation, thus including t/t embryos before resorption. Although we did not directly control the timing of mating and we did not know the timing of ovulation, first-to-mate males on average inseminated females earlier relative to ovulation than second-to-mate males. Our finding that drive was not affected by mating order is in line with previous work that found no effect of insemination relative to the timing of ovulation for t haplotypes with strong male drive (Yanagisawa et al. 1961). Further, we found no evidence for discrimination against t-bearing sperm by t-bearing ova, as the genotype distribution in embryos from +/t females that were sired by +/t males matched the expected distribution based on strong male drive and Mendelian inheritance in females. These effects suggest that if females do exhibit active postcopulatory discrimination against +/t males or against t-bearing sperm, +/+ and +/t females do so to the same extent (Sutter and Lindholm 2015; but see Lindholm et al. 2013). Here, our rather small sample size for fertilization of +/t females’ ova by +/t males prevents us from drawing firm conclusions. The small sample size was mainly caused by the +/t males’ strong disadvantage in sperm competition against +/+ males (Sutter and Lindholm 2015).
Because of the negative effects of male meiotic drive on male fertility and sperm competitiveness (Price and Wedell 2008; Price et al. 2008a; Sutter and Lindholm 2015), inciting sperm competition by mating with multiple males (Parker 1970) may offer a simple general mechanism for protection from the harmful effects of drive in males (Price et al. 2008b; Manser et al. 2011; Wedell 2013; Holman et al. 2015). Available evidence shows that female house mice are actively polyandrous (Rolland et al. 2003; Thonhauser et al. 2013; Manser et al. 2015) and that multiple mating is considerable in wild populations (Dean et al. 2006). Males carrying the t haplotype are strongly disadvantaged in sperm competition (Sutter and Lindholm 2015), particularly when first-to-mate (Sutter and Lindholm, 2016), suggesting that polyandry is only ineffective when all of a female’s mates are t heterozygous. Kempenaers (2007) suggested 3 questions to address when investigating mate choice for good versus compatible genes. The questions focus on (1) whether the optimal mate is different for individual females, (2) whether there is evidence that females chose accordingly, and (3) the mechanistic basis for the choice. In the context of the t haplotype, whereas (2) and (3) have received some empirical support, we argue that (1) has been somewhat neglected. When considering long-term fitness consequences, fertilization by +/t males appears costly to both +/t and +/+ females. Polyandry provides a very effective possibility for avoiding fertilization by costly +/t males, both for +/+ and +/t females, although the costs of polyandry (e.g., enhanced predation risk, sexually transmitted pathogens; Jennions and Petrie 2000) will influence the net fitness of this strategy. Importantly, polyandry offers a parsimonious explanation for a mechanism of discrimination that is inherently linked to the locus that inflicts the costs. More research in wild populations is needed to assess the importance of pre- and postcopulatory sexual selection on ecological dynamics of meiotic drive (Lindholm et al. 2016).
Acknowledgments
We thank Jari Garbely for DNA extraction and genotyping, Gabi Stichel and Sally Steinert for assistance with animal husbandry, Kerstin Musolf for advice on oestrus stage determination, and Barbara König for support. We also thank Andri Manser for helpful discussions and Laura Travers and 2 anonymous reviewers for comments on earlier versions of the manuscript.
Funding
This study was supported by the Swiss National Science Foundation Grant 138389.
References
- Andersson M, Simmons LW, 2006. Sexual selection and mate choice. Trends Ecol Evol 21:296–302. [DOI] [PubMed] [Google Scholar]
- Angelard C, Montchamp-Moreau C, Joly D, 2008. Female-driven mechanisms, ejaculate size and quality contribute to the lower fertility of sex-ratio distorter males in Drosophila simulans. BMC Evol Biol 8:326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardlie KG, Silver LM, 1996. Low frequency of mouse t haplotypes in wild populations is not explained by modifiers of meiotic drive. Genetics 144:1787–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auclair Y, König B, Lindholm AK, 2013. A selfish genetic element influencing longevity correlates with reactive behavioural traits in female house mice Mus domesticus. PLoS ONE 8:e67130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman N, 1960. Selective fertilization at the T-locus of the mouse. Genet Res 1:226–238. [Google Scholar]
- Bates D, Maechler M, Bolker B, Walker S, 2014. lme4: Linear mixed-effects models using Eigen and S4. R package version 1.0-6. Available form: http://CRAN.R-project.org/package=lme4.
- Bennett D, 1975. The T-locus of the mouse. Cell 6:441–454. [Google Scholar]
- Birkhead TR, Pizzari T, 2002. Postcopulatory sexual selection. Nat Rev Genet 3:262–273. [DOI] [PubMed] [Google Scholar]
- Braden A, 1958. Influence of time of mating and the segregation ratio of alleles at the T locus in the house mouse. Nature 181:786–787. [DOI] [PubMed] [Google Scholar]
- Burt A, Trivers R, 2006. Genes in Conflict: The Biology of Selfish Genetic Elements. Cambridge: Harvard University Press. [Google Scholar]
- Byers SL, Wiles MV, Dunn SL, Taft RA, 2012. Mouse estrous cycle identification tool and images. PLoS ONE 7:e35538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll LS, Meagher S, Morrison L, Penn DJ, Potts WK, 2004. Fitness effects of a selfish gene (the Mus t complex) are revealed in an ecological context. Evolution 58:1318–1328. [DOI] [PubMed] [Google Scholar]
- Charlesworth B, 1994. The evolution of lethals in the t-haplotype system of the mouse. Proc R Soc B Biol Sci 258:101–107. [DOI] [PubMed] [Google Scholar]
- Coopersmith CB, Lenington S, 1992. Female preferences based on male quality in house mice: interaction between male dominance rank and t-complex genotype. Ethology 90:1–16. [Google Scholar]
- Cotton A, Földvári M, Cotton S, Pomiankowski A, 2014. Male eyespan size is associated with meiotic drive in wild stalk-eyed flies Teleopsis dalmanni. Heredity (Edinb) 112:363–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley MJ, 2007. The R Book. Chichester: John Wiley & Sons Ltd. [Google Scholar]
- Dean MD, Ardlie KG, Nachman MW, 2006. The frequency of multiple paternity suggests that sperm competition is common in house mice Mus domesticus. Mol Ecol 15:4141–4151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewsbury D, 1982. Ejaculate cost and male choice. Am Nat 119:601–610. [Google Scholar]
- Dougherty LR, Shuker DM, 2015. The effect of experimental design on the measurement of mate choice: a meta-analysis. Behav Ecol 26:311–319. [Google Scholar]
- Drickamer L, Gowaty P, Holmes C, 2000. Free female mate choice in house mice affects reproductive success and offspring viability and performance. Anim Behav 59:371–378. [DOI] [PubMed] [Google Scholar]
- Drickamer L, Lenington S, 1995. Trappability of wild house mice Mus domesticus in large outdoor pens: implication for models of t–complex gene frequency. Am Midl Nat 133:283–289. [Google Scholar]
- Eberhard WG, 1996. Female Control: Sexual Selection by Cryptic Female Choice. Princeton: Princeton University Press. [Google Scholar]
- Figueroa F, Golubić M, Nizetić D, Klein J, 1985. Evolution of mouse major histocompatibility complex genes borne by t chromosomes. Proc Natl Acad Sci USA 82:2819–2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forstmeier W, Schielzeth H, 2011. Cryptic multiple hypotheses testing in linear models: overestimated effect sizes and the winner’s curse. Behav Ecol Sociobiol 65:47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks P, Lenington S, 1986. Dominance and reproductive behavior of wild house mice in a seminatural environment correlated with T-locus genotype. Behav Ecol Sociobiol 18:395–404. [Google Scholar]
- Haig D, Bergstrom C, 1995. Multiple mating, sperm competition and meiotic drive. J Evol Biol 8:265–282. [Google Scholar]
- Herrmann BG, Bauer H, 2012. The mouse t-haplotype: a selfish chromosome - genetics, molecular mechanism, and evolution In: Macholán M, Baird SJE, Munclinger P, Piálek J, editors. Evolution of the House Mouse. Cambridge: Cambridge University Press, 297–314. [Google Scholar]
- Holman L, Price TAR, Wedell N, Kokko H, 2015. Coevolutionary dynamics of polyandry and sex-linked meiotic drive. Evolution 69:709–720. [DOI] [PubMed] [Google Scholar]
- Huck UW, Banks EM, 1982. Differential attraction of females to dominant males: olfactory discrimination and mating preference in the brown lemming Lemmus trimucronatus. Behav Ecol Sociobiol 11:217–222. [Google Scholar]
- Hughes AL, 2015. Sexual selection and mate choice: Insights from neutralist perspectives. Evol Biol 42:366–378. [Google Scholar]
- Iwasa Y, Pomiankowski A, Nee S, 1991. The evolution of costly mate preferences II. The “Handicap” principle. Evolution 45:1431–1442. [DOI] [PubMed] [Google Scholar]
- Jaenike J, 2001. Sex chromosome meiotic drive. Annu Rev Ecol Syst 32:25–49. [Google Scholar]
- Jemiolo B, Xie T, Andreolini F, Baker AEM, Novotny M, 1991. The t complex of the house mouse: chemical characterization by urinary volatile profiles. J Chem Ecol 17:353–367. [DOI] [PubMed] [Google Scholar]
- Jennions MD, Petrie M, 2000. Why do females mate multiply? A review of the genetic benefits. Biol Rev Camb Philos Soc 75:21–64. [DOI] [PubMed] [Google Scholar]
- Johns PM, Wolfenbarger LL, Wilkinson GS, 2005. Genetic linkage between a sexually selected trait and X chromosome meiotic drive. Proc R Soc B Biol Sci 272:2097–2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempenaers B, 2007. Mate choice and genetic quality: a review of the heterozygosity theory. Adv Study Behav 37:189–278. [Google Scholar]
- Kirkpatrick M, Ryan M, 1991. The evolution of mating preferences and the paradox of the lek. Nature 350:33–38. [Google Scholar]
- Klein J, Sipos P, Figueroa F, 1984. Polymorphism of t-complex genes in European wild mice. Genet Res 44:39–46. [Google Scholar]
- Kokko H, Jennions MD, Brooks R, 2006. Unifying and testing models of sexual selection. Annu Rev Ecol Evol Syst 37:43–66. [Google Scholar]
- König B, Lindholm AK, 2012. The complex social environment of female house mice Mus domesticus In: Macholán M, Baird SJE, Munclinger P, Piálek J, editors. Evolution of the House Mouse. Cambridge: Cambridge University Press, 114–134. [Google Scholar]
- Krauter K, Leinwand L, D’eustachio P, Ruddle F, Darnell J, 1982. Structural genes of the mouse major urinary protein are on chromosome 4. J Cell Biol 94:414–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuijper B, Pen I, Weissing FJ, 2012. A guide to sexual selection theory. Annu Rev Ecol Evol Syst 43:287–311. [Google Scholar]
- Lande R, Wilkinson G, 1999. Models of sex-ratio meiotic drive and sexual selection in stalk-eyed flies. Genet Res 74:245–253. [Google Scholar]
- Lenington S, 1983. Social preferences for partners carrying “good genes” in wild house mice. Anim Behav 31:325–333. [Google Scholar]
- Lenington S, 1991. The t complex: a story of genes, behavior, and populations. Adv Study Behav 20:51–86. [Google Scholar]
- Lenington S, Coopersmith C, Williams J, 1992. Genetic basis of mating preferences in wild house mice. Am Zool 32:40–47. [Google Scholar]
- Lenington S, Drickamer LC, Robinson AS, Erhart M, 1996. Genetic basis for male aggression and survivorship in wild house mice Mus domesticus. Aggress Behav 22:135–145. [Google Scholar]
- Lenington S, Egid K, 1985. Female discrimination of male odors correlated with male genotype at the T locus: a response to T-locus or H-2-locus variability? Behav Genet 15:53–67. [DOI] [PubMed] [Google Scholar]
- Lenington S, Egid K, Williams J, 1988. Analysis of a genetic recognition system in wild house mice. Behav Genet 18:549–564. [DOI] [PubMed] [Google Scholar]
- Lenington S, Franks P, 1985. Trappability of wild house mice under conditions of confinement in relation to T-Locus genotype and other variables. J Mammal 66:145–148. [Google Scholar]
- Lenington S, Heisler I, 1991. Behavioral reduction in the transmission of deleterious t haplotypes by wild house mice. Am Nat 137:366–378. [Google Scholar]
- Levine L, Rockwell R, Grossfield J, 1980. Sexual selection in mice. V. Reproductive competition between +/+ and +/tw5 males. Am Nat 116:150–156. [Google Scholar]
- Lindholm AK, Dyer KA, Firman RC, Fishman L, Forstmeier W. et al. , 2016. The ecology and evolutionary dynamics of meiotic drive. Trends Ecol Evol 31:315–326. [DOI] [PubMed] [Google Scholar]
- Lindholm AK, Musolf K, Weidt A, König B, 2013. Mate choice for genetic compatibility in the house mouse. Ecol Evol 3:1231–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLaren RD, Rowland WJ, 2006. Differences in female preference for male body size in Poecilia latipinna using simultaneous versus sequential stimulus presentation designs. Behaviour 143:273–292. [Google Scholar]
- Manser A, 2015. Gene Drive and Sexual Selection in House Mice [Doctoral Dissertation]. Zurich, Switzerland: University of Zurich.
- Manser A, König B, Lindholm AK, 2015. Female house mice avoid fertilization by t haplotype incompatible males in a mate choice experiment. J Evol Biol 28:54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manser A, Lindholm AK, König B, Bagheri HC, 2011. Polyandry and the decrease of a selfish genetic element in a wild house mouse population. Evolution 65:2435–2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill TE, 1962. Sexual behavior in three inbred strains of mice. Behaviour 19:341–350. [Google Scholar]
- Müllenbach R, Lagoda P, Welter C, 1989. An efficient salt-chloroform extraction of DNA from blood and tissues. Trends Genet 5:391. [PubMed] [Google Scholar]
- Olds-Clarke P, 1997. Models for male infertility: the t haplotypes. Rev Reprod 2:157–164. [DOI] [PubMed] [Google Scholar]
- Parker GA, 1970. Sperm competition and its evolutionary consequences in the insects. Biol Rev 45:525–567. [Google Scholar]
- Pitcher TE, Neff BD, Rodd FH, Rowe L, 2003. Multiple mating and sequential mate choice in guppies: females trade up. Proc R Soc B Biol Sci 270:1623–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston BT, Stockley P, 2006. The prospect of sexual competition stimulates premature and repeated ejaculation in a mammal. Curr Biol 16:R239–R241. [DOI] [PubMed] [Google Scholar]
- Price TAR, Bretman AJ, Avent TD, Snook RR, Hurst GD. et al. , 2008a. Sex ratio distorter reduces sperm competitive ability in an insect. Evolution 62:1644–1652. [DOI] [PubMed] [Google Scholar]
- Price TAR, Hodgson DJ, Lewis Z, Hurst GDD, Wedell N, 2008b. Selfish genetic elements promote polyandry in a fly. Science 322:1241–1243. [DOI] [PubMed] [Google Scholar]
- Price TAR, Lewis Z, Smith DT, Hurst GDD, Wedell N, 2012. No evidence of mate discrimination against males carrying a sex ratio distorter in Drosophila pseudoobscura. Behav Ecol Sociobiol 66:561–568. [Google Scholar]
- Price TAR, Wedell N, 2008. Selfish genetic elements and sexual selection: their impact on male fertility. Genetica 132:295–307. [DOI] [PubMed] [Google Scholar]
- Prokop ZM, Michalczyk Ł, Drobniak SM, Herdegen M, Radwan J, 2012. Meta-analysis suggests choosy females get sexy sons more than “good genes”. Evolution 66:2665–2673. [DOI] [PubMed] [Google Scholar]
- Puurtinen M, Ketola T, Kotiaho JS, 2009. The good-genes and compatible-genes benefits of mate choice. Am Nat 174:741–752. [DOI] [PubMed] [Google Scholar]
- R Core Team, 2015. R: A language and environment for statistical computing. Vienna (Austria: ): R Foundation for Statistical Computing; Available from: http://www.R–project.org/. [Google Scholar]
- Ramm SA, Cheetham SA, Hurst JL, 2008. Encoding choosiness: female attraction requires prior physical contact with individual male scents in mice. Proc R Soc B Biol Sci 275:1727–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramm SA, Stockley P, 2007. Ejaculate allocation under varying sperm competition risk in the house mouse Mus musculus domesticus. Behav Ecol 18:491–495. [Google Scholar]
- Ramm SA, Stockley P, 2014. Sequential male mate choice under sperm competition risk. Behav Ecol 25:660–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raveh S, Sutalo S, Thonhauser KE, Thoß M, Hettyey A. et al. , 2014. Female partner preferences enhance offspring ability to survive an infection. BMC Evol Biol 14:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts SA, Simpson DM, Armstrong SD, Davidson AJ, Robertson DH. et al. , 2010. Darcin: a male pheromone that stimulates female memory and sexual attraction to an individual male’s odour. BMC Biol 8:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts SC, Gosling LM, 2003. Genetic similarity and quality interact in mate choice decisions by female mice. Nat Genet 35:103–106. [DOI] [PubMed] [Google Scholar]
- Rolland C, MacDonald D, de Fraipont M, Berdoy M, 2003. Free female choice in house mice: leaving best for last. Behaviour 140:1371–1388. [Google Scholar]
- Rutstein AN, Brazill-Boast J, Griffith SC, 2007. Evaluating mate choice in the zebra finch. Anim Behav 74:1277–1284. [Google Scholar]
- Schielzeth H, 2010. Simple means to improve the interpretability of regression coefficients. Methods Ecol Evol 1:103–113. [Google Scholar]
- Schimenti J, Hammer M, 1990. Rapid identification of mouse t haplotypes by PCR polymorphism (PCRP). Mouse Genome 87:108. [Google Scholar]
- Shackleton MA, Jennions MD, Hunt J, 2005. Fighting success and attractiveness as predictors of male mating success in the black field cricket Teleogryllus commodus: the effectiveness of no-choice tests. Behav Ecol Sociobiol 58:1–8. [Google Scholar]
- Sherborne AL, Thom MD, Paterson S, Jury F, Ollier WE. et al. , 2007. The genetic basis of inbreeding avoidance in house mice. Curr Biol 17:2061–2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton G, Krebs CJ, Davis S, Chambers L, Brown P, 2001. Reproductive changes in fluctuating house mouse populations in southeastern Australia. Proc R Soc B Biol Sci 268:1741–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockley P, Bottell L, Hurst JL, 2013. Wake up and smell the conflict: odour signals in female competition. Philos Trans R Soc Lond B Biol Sci 368:20130082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutter A, Lindholm AK, 2015. Detrimental effects of an autosomal selfish genetic element on sperm competitiveness in house mice. Proc R Soc B Biol Sci 282:20150974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutter A, Lindholm AK, 2016. The copulatory plug delays ejaculation by rival males and affects sperm competition outcome in house mice. J Evol Biol doi:10.1111/jeb.12898. [DOI] [PubMed] [Google Scholar]
- Sutter A, Lindholm AK, 2016. Meiotic drive changes sperm precedence patterns in house mice: potential for male alternative mating tactics? BMC Evol Biol 16:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutter A, Simmons LW, Lindholm AK, Firman RC, 2016. Function of copulatory plugs in house mice: mating behavior and paternity outcomes of rival males. Behav Ecol 27:185–195. [Google Scholar]
- Taylor DR, Ingvarsson PK, 2003. Common features of segregation distortion in plants and animals. Genetica 117:27–35. [DOI] [PubMed] [Google Scholar]
- Thonhauser KE, Raveh S, Hettyey A, Beissmann H, Penn DJ, 2013. Scent marking increases male reproductive success in wild house mice. Anim Behav 86:1013–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner W, 1998. Measuring female mating preferences. Anim Behav 55:1029–1042. [DOI] [PubMed] [Google Scholar]
- Wedell N, 2013. The dynamic relationship between polyandry and selfish genetic elements. Philos Trans R Soc Lond B Biol Sci 368:20120049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson G, Presgraves D, Crymes L, 1998. Male eye span in stalk-eyed flies indicates genetic quality by meiotic drive suppression. Nature 391:276–279. [Google Scholar]
- Williams J, Lenington S, 1993. Factors modulating preferences of female house mice for males differing in t-complex genotype: role of t-complex genotype, genetic background, and estrous condition of females. Behav Genet 23:51–58. [DOI] [PubMed] [Google Scholar]
- Yanagisawa K, Dunn L, Bennett D, 1961. On the mechanism of abnormal transmission ratios at t locus in the house mouse. Genetics 46:1635–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zala SM, Bilak A, Perkins M, Potts WK, Penn DJ, 2015. Female house mice initially shun infected males, but do not avoid mating with them. Behav Ecol Sociobiol 69:715–722. [Google Scholar]
- Zeh JA, Zeh DW, 1996. The evolution of polyandry I: intragenomic conflict and genetic incompatibility. Proc R Soc B Biol Sci 263:1711–1717. [Google Scholar]
- Zinck L, Lima SQ, 2013. Mate choice in Mus musculus is relative and dependent on the estrous state. PLoS ONE 8:e66064. [DOI] [PMC free article] [PubMed] [Google Scholar]