Abstract
Background
The prognosis of pancreatic carcinoma (PC) remains poor and the American Joint Committee on Cancer (AJCC) 8th staging system for survival prediction in PC patients after curative resection is still limited. Thus, the aim of this study is to refine a valuable prognostic model and novel staging system for PC with curative resection.
Methods
The data of 3,458 patients used in this study were retrieved from the Surveillance, Epidemiology, and End Results database registry of National Cancer Institute. The prognostic value of lymph node ratio (LNR) was analyzed in the primary cohort and prognostic nomogram based on the LNR was established to create a novel staging system. Then, analyses were conducted to evaluate the application of the formulated nomogram staging system and the AJCC 8th staging system. The predictive performance of model was further validated in the internal validation cohort.
Results
Significant positive correlations were found between LNR and all factors except for surgical procedures. The results of univariate and multivariate analyses showed that LNR was identified as an independent prognostic indicator for overall survival (OS) in both primary and validation cohorts (all P < 0.001). A prognostic nomogram based on the LNR was formulated to obtain superior discriminatory abilities. Compared with the AJCC 8th staging system, the formulated nomogram staging system showed higher hazard ratios of stage II, III, and IV disease (reference to stage I disease) that were 1.637, 2.300, and 3.521, respectively, by univariate analyses in the primary cohort and the distinction between stage I, II, and III disease at the beginning or end of the survival curves was more apparent. All these results were further verified in the validation cohort.
Conclusion
LNR can be considered as a useful independent prognostic indicator for PC patients after curative resection regardless of the surgical procedures. Compared with the AJCC 8th staging system, the formulated nomogram showed superior predictive accuracy for OS and its novel staging system revealed better risk stratification.
Keywords: pancreatic head carcinoma, lymph node ratio, nomogram, prognosis, decision curve analysis, AJCC
Introduction
Pancreatic carcinoma (PC), ~90% malignantly originated from glandular epithelium of ductal adenocarcinoma, is regarded as the fourth leading cause of cancer-related deaths in the USA and ninth leading cause in China.1,2 Despite advances in multiple therapies, such as surgery, neoadjuvant chemoradiotherapy, immunotherapies, and so on, it is still a devastating disease, whose 5-year overall survival (OS) is limited to 8%.3 Nowadays, surgical resection remains to be considered as the only potentially curative treatment for PC patients. However, only 20% of candidates are suitable for successful resection owing to difficulty in early definite diagnosis.4
According to its dismally malignant behaviors, accurate staging system of PC is essential and needed to counsel patients regarding prognosis appropriately. The American Joint Committee on Cancer (AJCC) 8th staging system of PC is generally based on three factors: tumor size and extent (T), lymph node metastasis (N), and distant metastasis (M), which should be one of the most robust prognostic factors of cancer-specific survival. Reliable confirmation of stage after surgery or at diagnosis is a vital indicator in administrating the following therapeutic strategy.5 However, issues on whether non-tumor, node, metastasis (TNM) factors should be added to risk stratification of PC remain controversial. It also shows that the AJCC 8th staging system is deficiently formulated and cumbersome for the prognostic prediction after operative resection.6 In addition, the number of positive lymph node metastasis depends on the selection of surgical procedures and circumspective examination of pathologists, which may lead to giant bias and large error in assessing the ability of lymph node metastasis.
In 2004, Berger et al7 reported that only lymph node ratio (LNR), the number of positive lymph nodes divided by the total examined lymph nodes, had an impact on OS and disease-free survival in PC patients. In recent years, LNR has been considered as a robust predictor of survival in PC patients better than positive lymph nodes.8–11 In addition, non-TNM factors, such as age, grade, serum index, and so on, have also been reported as independent prognostic predictors for survival of PC patients after curative resection.12–14 Therefore, a novel staging system breaking through the traditional TNM staging system should be established to assist in risk stratification and survival predictor precisely.
The purpose of this study is to further assess the predictive value of LNR in postoperative PC patients and to formulate a new prognostic model through developing a nomogram. Then, according to the formulated nomogram, a novel nomogram-based staging system was refined and compared with the AJCC 8th staging system.
Methods
Patient population and data source
The data used in this study were retrieved from the Surveillance, Epidemiology, and End Results (SEER) database registry of National Cancer Institute. All the data accessed from the SEER database were freely available. The selection criteria were as follows: first, the PC patients were selected based on the column of site and morphology for tumor of pancreas (primary site – labeled): C25.0. Second, according to the International Classification of Diseases for Oncology (3rd edition) for tumor of histology/behavior, carcinoma (8010/3), adenocarcinoma (8140/3), and infiltrating duct carcinoma (8500/3) were all included. Another selection criterion was diagnosed from 2010 to 2013 with surgery procedure of pylorus preserving pancreatoduodenectomy (PPPD), Whipple procedure, total pancreatectomy, or extended pancreatoduodenectomy (PD).
The following data were received for each patient: gender, age, grade, liver metastasis, lung metastasis, scope of lymph node surgery, regional lymph node positive, regional lymph node examined, TNM stage, and survival information. The TNM stage of the AJCC 8th edition was evaluated based on the following codes: collaborative stage (CS) tumor size 2004, CS extension 2004, CS lymph nodes 2004, CS metastases at DX 2004, and derived AJCC stage group (7th edition; 2010+). However, patients with unknown characteristics or lack of survival information were excluded in our study. After the inclusion and exclusion criteria, the whole enrolled cohort was considered as the primary cohort. Next, we further randomly select a validation cohort by 1 to 1 ratio as an internal verification. The LNR was calculated with regional lymph node positive divided by regional lymph node examined. This study was approved by the ethics committee of Zhongshan Hospital, Fudan University.
Statistical analysis
All statistical analyses and random allocation were performed by SPSS 21.0 statistical package (IBM Corporation, Armonk, NY, USA) and R project version 3.3.3 (http://www.r-project.org/) for Windows. The cutoff value of LNR was determined by the receiver operating characteristic (ROC) curve. The OS was compared by Kaplan–Meier curves and analyzed using the log-rank test via GraphPad Prism 6 Software (GraphPad Software Inc., San Diego, CA, USA). The univariate and multivariate analyses and hazard ratios (HRs) were used by Cox proportional hazards regression model to find its independent prognostic risks, and P < 0.05 was considered as statistically significant difference.
A novel prognostic nomogram based on LNR for OS was formulated by the rms package in R project (Bell Laboratories, Murray Hill, NJ, USA). Its predictive performance was measured by concordance index (C-index), calibration curve, and decision curve analysis (DCA) as previously described.6 The prognostic prediction was more precise with larger C-index, superior consistency, and wider threshold probability or net benefit. Bootstraps with 1,200 resample in primary cohort or 600 resample in validation cohort were used for such activities. The cutoff value of formulated nomogram staging system was determined by X-tile software. (Yale University, New Haven, CT, USA)
Results
Clinicopathological characteristics
From the criteria above, 3,458 patients with histologically confirmed pancreatic head carcinoma from the SEER database were finally included, and the detailed baseline characteristics were displayed in Table 1. In total, there were 1,760 male and 1,698 female patients with a median age of 67 years (range, 29–95 years) in primary cohort. Approximately 10%, 50%, and 40% of patients suffered from well, moderate, and poor pathological differentiations, respectively. Of the total patients, 68 patients suffered from liver metastasis and 21 patients suffered from lung metastasis. Three hundred sixty-nine, 2,430, 453, and 206 patients underwent PPPD, Whipple procedure, total pancreatectomy, and extended PD, respectively. The median OS was 20 months (range, 1–59 months). In addition, the 1- and 3-year OS rates were 69.6% and 28.5%, respectively. According to the TNM staging system of the AJCC 8th edition, the separate stage of patients was recorded. The clinicopathological characteristics of internal validation cohort randomly selected from primary cohort were displayed in Table S1.
Table 1.
Correlations between LNR and clinicopathological characteristics of patients with resected pancreatic head carcinoma in the primary cohort
| Variables | SEER cohort
|
||
|---|---|---|---|
| LNR £ 0.092 (No. of patients = 1565) | LNR > 0.092 (No. of patients = 1893) | P-value | |
| Age (years) | 0.024 | ||
| <70 | 898 | 1158 | |
| ≥70 | 667 | 735 | |
| Gender | <0.001 | ||
| Male | 737 | 1023 | |
| Female | 828 | 870 | |
| Grade | <0.001 | ||
| Well differentiation | 195 | 173 | |
| Moderate differentiation | 855 | 921 | |
| Poor differentiation | 515 | 799 | |
| Liver metastasis | 0.016 | ||
| Yes | 21 | 47 | |
| No | 1544 | 1846 | |
| Lung metastasis | 0.048 | ||
| Yes | 5 | 16 | |
| No | 1560 | 1877 | |
| T classification | <0.001 | ||
| T1 | 317 | 198 | |
| T2 | 791 | 1004 | |
| T3 | 198 | 347 | |
| T4 | 259 | 344 | |
| N classification | <0.001 | ||
| N0 | 988 | 0 | |
| N1 | 566 | 891 | |
| N2 | 11 | 1002 | |
| M classification | <0.001 | ||
| M0 | 1529 | 1788 | |
| M1 | 36 | 105 | |
| TNM staging system | <0.001 | ||
| I | 717 | 0 | |
| II | 553 | 690 | |
| III | 259 | 1098 | |
| IV | 36 | 105 | |
| Regional lymph nodes surgery | <0.001 | ||
| None | 32 | 27 | |
| 1–3 | 79 | 43 | |
| ≥4 | 1454 | 1823 | |
| Surgery | 0.441 | ||
| PPPD | 181 | 188 | |
| Whipple | 1092 | 1338 | |
| Total pancreatectomy | 198 | 255 | |
| Extended PD | 94 | 112 | |
Note: Bold figures indicate statistical significant P<0.05.
Abbreviations: LNR, lymph node ratio; PD, pancreatoduodenectomy; PPPD, pylorus preserving pancreatoduodenectomy; SEER, Surveillance, Epidemiology, and End Results; TNM, tumor, node, metastasis.
Correlations between LNR and clinicopathologic characteristics
The median LNR of all enrolled patients was 0.115 (range, 0–1). With the ROC analysis, the optimal cutoff value for LNR was 0.092. The area below the curve was 0.627 (P < 0.001; Figure S1).
The low-risk cohort (LNR £0.092) and high-risk cohort (LNR > 0.092) were determined within the primary cohort. High-risk cohort consisted of 54.7% (1,893 of 3,458) in the primary cohort and 54.8% (922 of 1684) in the internal validation cohort, which is displayed in Tables 1 and S1. Intriguingly, it was statistically significant that LNR was associated with all characteristics except for surgical procedures in the primary cohort. However, in the validation cohort, gender (P = 0.001), grade (P < 0.001), lung metastasis (P = 0.016), T, N, M classification (all P < 0.001), TNM staging system (P < 0.001), and regional lymph nodes surgery (P = 0.023) were confirmed its correlations with LNR. All these analyses indicated no relationships between LNR and the selection of surgical procedures.
Prognostic significance of LNR
As univariate analysis showed, older age (P < 0.001), advanced grade (P < 0.001), liver metastasis (P < 0.001), lung metastasis (P = 0.001), higher LNR (P < 0.001), advanced T, N, M classification, and TNM staging system (all P < 0.001) were significantly considered as risk factors in the primary cohort (Table 2), which was the same as the validation cohort (Table S2). In multivariate analysis of primary or validation cohort for OS, older age (P < 0.001; HR, 1.331; 95% CI, 1.223–1.448 and P < 0.001; HR, 1.338; 95% CI, 1.184–1.511, respectively), advanced grade (P < 0.001; HR, 1.356; 95% CI, 1.269–1.450 and P < 0.001; HR, 1.408; 95% CI, 1.279–1.550, respectively), advanced T classification (P < 0.001; HR, 1.152; 95% CI, 1.086–1.222 and P = 0.001; HR, 1.161; 95% CI, 1.065–1.266, respectively), and elevated LNR level (P < 0.001; HR, 1.563; 95% CI, 1.376–1.776 and P < 0.001; HR, 1.478; 95% CI, 1.237–1.766) remained as independent prognostic indicators. Furthermore, advanced M classification (P = 0.003; HR, 2.085; 95% CI, 1.274–3.412) was also verified as an independent survival predictor in the validation cohort (Table S2).
Table 2.
Univariate and multivariate analyses of prognostic factors associated with overall survival of patients with resected pancreatic head carcinoma in the primary cohort
| Variables | Overall survival
|
|||
|---|---|---|---|---|
| No. of patients (N = 3458) | Univariate (P-value) | Multivariate (P-value) | Hazard ratio (95% CI) | |
| Age (years) | <0.001 | <0.001 | 1.331 (1.223–1.448) | |
| <70 | 2056 | |||
| ≥70 | 1402 | |||
| Gender | 0.088 | |||
| Male | 1760 | |||
| Female | 1698 | |||
| Grade | <0.001 | <0.001 | 1.356 (1.269–1.450) | |
| Well differentiation | 368 | |||
| Moderate differentiation | 1776 | |||
| Poor differentiation | 1314 | |||
| Liver metastasis | <0.001 | 0.097 | 1.387 (0.943–2.042) | |
| Yes | 68 | |||
| No | 3390 | |||
| Lung metastasis | 0.001 | 0.701 | 1.111 (0.650–1.900) | |
| Yes | 21 | |||
| No | 3437 | |||
| LNR | <0.001 | <0.001 | 1.563 (1.376–1.776) | |
| ≤0.092 | 1565 | |||
| >0.092 | 1893 | |||
| T classification | <0.001 | <0.001 | 1.152 (1.086–1.222) | |
| T1 | 515 | |||
| T2 | 1795 | |||
| T3 | 545 | |||
| T4 | 603 | |||
| N classification | <0.001 | 0.545 | 1.037 (0.922–1.166) | |
| N0 | 988 | |||
| N1 | 1457 | |||
| N2 | 1013 | |||
| M classification | <0.001 | 0.097 | 1.343 (0.948–1.902) | |
| M0 | 3317 | |||
| M1 | 141 | |||
| TNM staging system | <0.001 | 0.396 | 1.052 (0.935–1.184) | |
| I | 717 | |||
| II | 1243 | |||
| III | 1357 | |||
| IV | 141 | |||
| Regional lymph nodes surgery | 0.891 | |||
| None | 59 | |||
| 1–3 | 122 | |||
| ≥4 | 3277 | |||
| Surgery | 0.134 | |||
| PPPD | 369 | |||
| Whipple | 2430 | |||
| Total pancreatectomy | 453 | |||
| Extended PD | 206 | |||
Abbreviations: LNR, lymph node ratio; PD, pancreatoduodenectomy; PPPD, pylorus preserving pancreatoduodenectomy; TNM; tumor, node, metastasis.
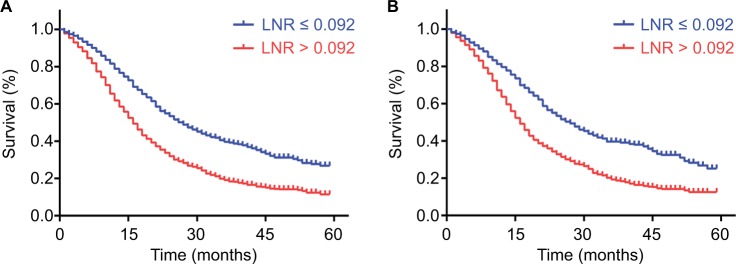
Through the analysis of Kaplan–Meier curves, LNR larger than 0.092 was significantly associated with poorer OS than low-risk cohort in both primary and validation cohorts (Figure 1A and B). In the primary cohort, the 1-, 2-, and 3-year OS rates were 61.8%, 32.0%, and 19.2% in higher LNR cohort and 81.6%, 53.5%, and 39.7% in lower LNR cohort, respectively. The same distinction was exhibited in the validation cohort with 63.7%, 32.9%, and 19.4% in higher LNR cohort and 79.7%, 53.5%, and 39.4% in lower LNR cohort, respectively.
Figure 1.
Kaplan–Meier survival curves for patients according to LNR.
Notes: Patients with LNR larger than 0.092 were inclined to significantly poorer OS in the primary cohort (A) and validation cohort (B). P values were determined by the log-rank test.
Abbreviations: LNR, lymph node ratio; OS, overall survival.
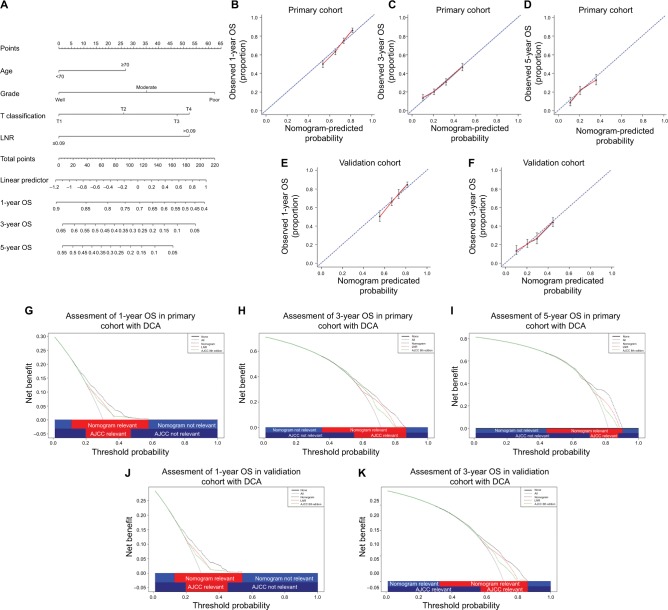
Novel prognostic nomogram for OS prediction
One more accurate prognostic nomogram that integrated age, grade, T classification, and LNR was proposed by multivariate Cox regression models (Figure 2A). The C-index for OS prediction with the formulated nomogram was 0.633 (95% CI, 0.6326–0.6334), which was higher than the C-index of TNM staging system (0.583; 95% CI, 0.5826–0.5834) in the primary cohort. The higher C-index, the better predictive accuracy for OS the system achieved. Therefore, the nomogram containing LNR was formulated to predict the survival with superior performance. According to the formulated nomogram, we examined its performance in the validation cohort with C-index of the formulated nomogram (0.630; 95% CI, 0.6291–0.6309) compared to TNM staging system (0.579; 95% CI, 0.5781–0.5799).
Figure 2.
Prognostic nomogram, calibration curves, and DCA.
Notes: The nomogram predicts 1-, 3-, and 5-year OS in patients with pancreatic head carcinoma (A). The calibration curves predict OS at 1 year (B), 3 years (C), and 5 years (D) in the primary cohort and at 1 year (E) and 3 years (F) in the validation cohort. The nomogram-predicted OS is plotted on the x axis, and the actually observed OS is plotted on the y axis. DCA depicts the clinical net benefit in pairwise comparisons across the different models. The formulated nomogram is compared with the AJCC 8th staging system in terms of 1- (G), 3- (H), and 5-year (I) OS in the primary cohort and 1- (J) and 3-year (K) OS in the validation cohort. On DCA, the nomogram showed superior net benefit with a wider range of threshold probabilities compared with AJCC 8th staging system.
Abbreviations: AJCC, American Joint Committee on Cancer; DCA, decision curve analysis; LNR, lymph node ratio; OS, overall survival.
As shown in the calibration plot, the observed probability of 1-, 3-, and 5-year OS in the primary cohort and 1- and 3-year OS in the validation cohort showed optimal consistency with the nomogram-predicted OS (Figure 2B–2F). In DCA, the formulated nomogram yielded preferable net benefit along with a wider field of threshold probability compared to the TNM staging system of the AJCC 8th edition (Figure 2G–K), which indicated more robust predictive power for predicting OS at 1, 3, and 5 years. Meanwhile, higher threshold probability represented superior estimations of decision outcomes.
AJCC 8th staging system and survival
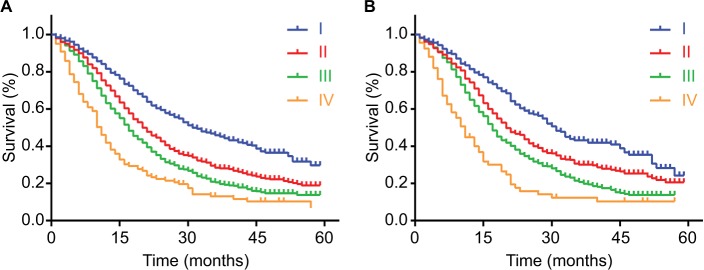
In the primary cohort, 4.08% (141 of 3,458) of patients had stage IV tumors based on the AJCC 8th staging system (Table 2). The discrimination among stage I, II, and III diseases was not obvious at the beginning of the survival curves, and all these survival curves had the trend in convergence in the end (Figure 3A). There were the same shortcomings of the survival curves in the validation cohort (Figure 3B). In addition, compared with stage I tumor, the HRs of stage II, III, and IV tumors by univariate analyses according to the AJCC 8th staging system were 1.556, 1.997, and 3.099 in the primary cohort and 1.46, 1.915, and 3.136 in the validation cohort, respectively.
Figure 3.
Kaplan–Meier survival curves for patients according to the AJCC 8th staging system.
Notes: The discrimination between stage I, II, III, and IV diseases was distributed by the AJCC 8th staging system in the primary cohort (A) and validation cohort (B). P values were determined by the log-rank test.
Abbreviation: AJCC, American Joint Committee on Cancer.
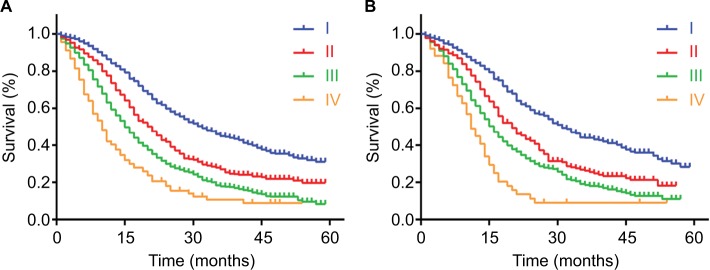
Formulated nomogram staging system and survival
According to the formulated nomogram, we defined total points not larger than 88 points as stage I disease, larger than 88 points but not larger than 114 points as stage II disease, larger than 114 points but not larger than 169 points as stage III disease, and larger than 169 points as stage IV disease. Through these changes, there were 1,141, 634, 1,569, and 114 patients with stage I, II, III, and IV disease, respectively. On the basis of the new classification, the distinction among stage I, II, and III disease at the beginning or end of the survival curves was larger than that of the AJCC 8th staging system in the primary cohort, so did it in the validation cohort (Figure 4A and B). According to the formulated nomogram staging system, survival curves were also separated as better as that in the AJCC 8th staging system between stages, and an obvious increase in HRs was observed with statistical significance. Compared with stage I disease, the HRs of stage II, III, and IV disease by univariate analyses was 1.637, 2.300, and 3.521 in the primary cohort and 1.626, 2.188, and 3.605 in the validation cohort, respectively.
Figure 4.
Kaplan–Meier survival curves for patients according to the formulated nomogram staging system.
Notes: The discrimination between stage I, II, III, and IV diseases was distributed by the formulated nomogram staging system in the primary cohort (A) and validation cohort (B). P values were determined by the log-rank test.
Discussion
In this study, 3,458 total patients with resected pancreatic head carcinoma were finally enrolled and analyzed. Through the analysis of the clinicopathologies, LNR had significant correlations with age, gender, grade, liver or lung metastasis, TNM stage, and regional lymph nodes surgery. However, there was no relationship between LNR and surgery procedures. LNR was confirmed as an independent prognostic risk factor in the univariate and multivariate analyses, so did age, grade, and T classification. Finally, nomogram based on the LNR, age, grade, and T classification was formulated and manifested superior predictive value compared to the AJCC 8th staging system alone. In addition, the formulated nomogram staging system revealed better performance in risk stratification for prognosis of patients with resected pancreatic head carcinoma than the AJCC 8th staging system. All these results were verified in the internal validation cohort.
As we knew, lymph node involvement appeared to be one of the most important risks for predicating OS of resected PC patients.10,15,16 Nevertheless, the total number of examined positive lymph nodes was still imperfect as a pivotal predictor owing to its influence on surgical procedures. We found that LNR did not appear to be associated with surgical procedures, because no matter how expansive of lymph nodes surgery was, LNR reflected its ability in involvement and metastasis, while absolute positive lymph node counts was severely affected by the scope.17 In addition, LNR showed excellent discrimination between OS prediction.10,18–20 Thus, extended lymphadenectomy may not be necessary for PC patients, because it could increase the postoperative complications, morbidities, and mortalities, and even influence the quality of life.21,22 In general, the assessment of LNR could make patients utmostly benefit from the surgery and still be evaluated accurately on their survival risks, which may guide the following therapies.
As large amounts of factors turned up as prognostic indicators for resected PC patients,23–25 the AJCC 8th staging system seemed to lose its powerful efficiency in the evaluation of prognosis. Smith and Mezhir3,20 established a predictive model of pancreatic cancer patients in 2014, but it was applied in few guidelines or consensuses owing to complexity. Nomogram, a quantitative rating predictive model, had revealed its mighty power in survival prediction, which may have the chance to replace the TNM staging system.26 According to this study, age, grade, and T classification (all P < 0.001) besides LNR were also considered as independent prognostic factors. Compared to the AJCC 8th staging system, the concept of tumor size and lymph nodes status were still involving in the formulated nomogram. Besides this, age and differentiation grade were further incorporated into the novel model. Asano et al27 had reported the role of age in the survival of resected PC patients. Surgery was considered as a giant damage to patients’ physical functions and immune system, which may lead to serious comorbidities and mortalities. In addition, tumor differentiation reflected the biological behaviors of PC, which was highlighted in several studies for its vital role in survival.14 Thus, the formulated nomogram merged T, N status and other significant factors together to obtain the much more precise model specially with the validation of superior consistent calibration curves and wider ranges of DCA.
According to the formulated nomogram staging system, the median OS of each stage was 32, 21, 15, and 10 months in the primary cohort compared to 31, 21, 17, and 11 months evaluated by the AJCC 8th staging system. In addition, with the comparison between the nomogram staging system and the AJCC 8th staging system in the primary cohort, we could directly discover that the change in HR for patients in each stage with the nomogram staging system (HRs for stage II, III, and IV, 1.637, 2.300, and 3.521, respectively, with stage I as the reference) was larger than that with the AJCC 8th staging system, so did the discrimination ability in the survival curves (HRs for stage II, III, and IV, 1.556, 1.997, and 3.099, respectively, with stage I as the reference). The same results were verified in the validation cohort. Multiple weaknesses in the AJCC 8th staging system were exposed,5 whereas the novel nomogram staging system showed perfect discrimination among each stage.
A limitation of this study was retrospective essentially, so a large-scale and multicenter prospective study should be launched to prove our results and eliminate the selective bias. Next, the cutoff value of LNR used in our study may not be appropriate to other studies, and a meta-analysis containing various LNR validation studies may be required to determine the most suitable cutoff value.
Conclusion
LNR could be a robust prognostic predictor for PC patients with curative resection. The proposed nomogram containing T classification, LNR, age, and grade reveals a superior prognostic model. In addition, the formulated nomogram staging system confirmed its excellent discrimination and risk stratification compared to the AJCC 8th staging system.
Supplementary materials
ROC curves for OS.
Note: The area under the curve for LNR was 0.627.
Abbreviations: LNR, lymph node ratio; OS, overall survival; ROC, receiver operating characteristic.
Table S1.
Correlations between LNR and clinicopathological characteristics of patients with resected pancreatic head carcinoma in the validation cohort
| Variables | SEER cohort
|
||
|---|---|---|---|
| LNR £ 0.092 (no. of patients = 762) | LNR > 0.092 (no. of patients = 922) | P-value | |
| Age (years) | 0.145 | ||
| <70 | 452 | 579 | |
| ≥70 | 310 | 343 | |
| Gender | 0.001 | ||
| Male | 360 | 509 | |
| Female | 402 | 413 | |
| Grade | <0.001 | ||
| Well differentiation | 94 | 88 | |
| Moderate differentiation | 432 | 456 | |
| Poor differentiation | 236 | 378 | |
| Liver metastasis | 0.127 | ||
| Yes | 11 | 23 | |
| No | 751 | 899 | |
| Lung metastasis | 0.016 | ||
| Yes | 0 | 9 | |
| No | 762 | 913 | |
| T classification | <0.001 | ||
| T1 | 134 | 102 | |
| T2 | 382 | 491 | |
| T3 | 96 | 167 | |
| T4 | 150 | 162 | |
| N classification | <0.001 | ||
| N0 | 473 | 0 | |
| N1 | 284 | 468 | |
| N2 | 5 | 454 | |
| M classification | <0.001 | ||
| M0 | 747 | 870 | |
| M1 | 15 | 52 | |
| TNM staging system | <0.001 | ||
| I | 335 | 0 | |
| II | 262 | 362 | |
| III | 150 | 508 | |
| IV | 15 | 52 | |
| Regional lymph nodes surgery | 0.023 | ||
| None | 16 | 15 | |
| 1–3 | 35 | 21 | |
| ≥4 | 711 | 886 | |
| Surgery | 0.616 | ||
| PPPD | 85 | 86 | |
| Whipple | 533 | 657 | |
| Total pancreatectomy | 100 | 129 | |
| Extended PD | 44 | 50 | |
Note: Bold figures indicate statistical significant P<0.05.
Abbreviations: LNR, lymph node ratio; PD, pancreatoduodenectomy; PPPD, pylorus preserving pancreatoduodenectomy; SEER, Surveillance, Epidemiology, and End Results; TNM, tumor, node, metastasis.
Table S2.
Univariate and multivariate analyses of prognostic factors associated with overall survival of patients with resected pancreatic head carcinoma in the validation cohort
| Variables | Overall survival
|
|||
|---|---|---|---|---|
| No. of patients (N = 1684) | Univariate (P-value) | Multivariate (P-value) | Hazard ratio (95% CI) | |
| Age (years) | <0.001 | <0.001 | 1.338 (1.184–1.511) | |
| <70 | 1031 | |||
| ≥70 | 653 | |||
| Gender | 0.257 | |||
| Male | 869 | |||
| Female | 815 | |||
| Grade | <0.001 | <0.001 | 1.408 (1.279–1.550) | |
| Well differentiation | 182 | |||
| Moderate differentiation | 888 | |||
| Poor differentiation | 614 | |||
| Liver metastasis | 0.001 | 0.987 | 1.005 (0.585–1.724) | |
| Yes | 34 | |||
| No | 1650 | |||
| Lung metastasis | 0.024 | 0.800 | 0.904 (0.416–1.967) | |
| Yes | 9 | |||
| No | 1675 | |||
| LNR | <0.001 | <0.001 | 1.478 (1.237–1.766) | |
| ≤0.092 | 762 | |||
| >0.092 | 922 | |||
| T classification | <0.001 | 0.001 | 1.161 (1.065–1.266) | |
| T1 | 236 | |||
| T2 | 873 | |||
| T3 | 263 | |||
| T4 | 312 | |||
| N classification | <0.001 | 0.128 | 1.139 (0.963–1.348) | |
| N0 | 473 | |||
| N1 | 752 | |||
| N2 | 459 | |||
| M classification | <0.001 | 0.003 | 2.085 (1.274–3.412) | |
| M0 | 1617 | |||
| M1 | 67 | |||
| TNM staging system | <0.001 | 0.942 | 0.994 (0.838–1.178) | |
| I | 335 | |||
| II | 624 | |||
| III | 658 | |||
| IV | 67 | |||
| Regional lymph nodes surgery | 0.817 | |||
| None | 31 | |||
| 1–3 | 56 | |||
| ≥4 | 1597 | |||
| Surgery | 0.150 | |||
| PPPD | 171 | |||
| Whipple | 1190 | |||
| Total pancreatectomy | 229 | |||
| Extended PD | 94 | |||
Abbreviations: LNR, lymph node ratio; PD, pancreatoduodenectomy; PPPD, pylorus preserving pancreatoduodenectomy; TNM, tumor, node, metastasis.
Acknowledgments
This work was supported by the following grants: the National Natural Science Foundation of China (81401923, 81572294, 81702304, 81773068, 81702964, and 81272731), the Shanghai Pujiang Program (16PJD013), and the Science and Technology Commission of Shanghai Municipality (11JC1402502).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 3.Pu N, Lv Y, Zhao G, et al. Survival prediction in pancreatic cancer patients with no distant metastasis: a large-scale population-based estimate. Future Oncol. 2017;14(2):165–175. doi: 10.2217/fon-2017-0380. [DOI] [PubMed] [Google Scholar]
- 4.Kamarajah SK, Burns WR, Frankel TL, Cho CS, Nathan H. Validation of the American Joint Commission on Cancer (AJCC) 8th edition staging system for patients with pancreatic adenocarcinoma: a Surveillance, Epidemiology and End Results (SEER) analysis. Ann Surg Oncol. 2017;24(7):2023–2030. doi: 10.1245/s10434-017-5810-x. [DOI] [PubMed] [Google Scholar]
- 5.Allen PJ, Kuk D, Castillo CF, et al. Multi-institutional validation study of the American Joint Commission on Cancer (8th Edition) changes for T and N staging in patients with pancreatic adenocarcinoma. Ann Surg. 2017;265(1):185–191. doi: 10.1097/SLA.0000000000001763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pu N, Gao S, Xu Y, et al. Alkaline phosphatase-to-albumin ratio as a prognostic indicator in pancreatic ductal adenocarcinoma after curative resection. J Cancer. 2017;8(16):3362–3370. doi: 10.7150/jca.20917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berger AC, Watson JC, Ross EA, Hoffman JP. The metastatic/examined lymph node ratio is an important prognostic factor after pancreaticoduodenectomy for pancreatic adenocarcinoma. Am Surg. 2004;70(3):235–240. Discussion 240. [PubMed] [Google Scholar]
- 8.Tol JA, Brosens LA, van Dieren S, et al. Impact of lymph node ratio on survival in patients with pancreatic and periampullary cancer. Br J Surg. 2015;102(3):237–245. doi: 10.1002/bjs.9709. [DOI] [PubMed] [Google Scholar]
- 9.Elshaer M, Gravante G, Kosmin M, Riaz A, Al-Bahrani A. A systematic review of the prognostic value of lymph node ratio, number of positive nodes and total nodes examined in pancreatic ductal adenocarcinoma. Ann R Coll Surg Engl. 2017;99(2):101–106. doi: 10.1308/rcsann.2016.0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhan HX, Xu JW, Wang L, Zhang GY, Hu SY. Lymph node ratio is an independent prognostic factor for patients after resection of pancreatic cancer. World J Surg Oncol. 2015;13:105. doi: 10.1186/s12957-015-0510-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamamoto Y, Ikoma H, Morimura R, et al. The clinical impact of the lymph node ratio as a prognostic factor after resection of pancreatic cancer. Anticancer Res. 2014;34(5):2389–2394. [PubMed] [Google Scholar]
- 12.Ji F, Fu SJ, Guo ZY, et al. Prognostic value of combined preoperative lactate dehydrogenase and alkaline phosphatase levels in patients with resectable pancreatic ductal adenocarcinoma. Medicine (Baltimore) 2016;95(27):e4065. doi: 10.1097/MD.0000000000004065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hayman TJ, Strom T, Springett GM, et al. Outcomes of resected pancreatic cancer in patients age ≥70. J Gastrointest Oncol. 2015;6(5):498–504. doi: 10.3978/j.issn.2078-6891.2015.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rochefort MM, Ankeny JS, Kadera BE, et al. Impact of tumor grade on pancreatic cancer prognosis: validation of a novel TNMG staging system. Ann Surg Oncol. 2013;20(13):4322–4329. doi: 10.1245/s10434-013-3159-3. [DOI] [PubMed] [Google Scholar]
- 15.Liu ZQ, Xiao ZW, Luo GP, et al. Effect of the number of positive lymph nodes and lymph node ratio on prognosis of patients after resection of pancreatic adenocarcinoma. Hepatobiliary Pancreat Dis Int. 2014;13(6):634–641. doi: 10.1016/s1499-3872(14)60264-2. [DOI] [PubMed] [Google Scholar]
- 16.Valsangkar NP, Bush DM, Michaelson JS, et al. N0/N1, PNL, or LNR? The effect of lymph node number on accurate survival prediction in pancreatic ductal adenocarcinoma. J Gastrointest Surg. 2013;17(2):257–266. doi: 10.1007/s11605-012-1974-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jang JY, Kang MJ, Heo JS, et al. A prospective randomized controlled study comparing outcomes of standard resection and extended resection, including dissection of the nerve plexus and various lymph nodes, in patients with pancreatic head cancer. Ann Surg. 2014;259(4):656–664. doi: 10.1097/SLA.0000000000000384. [DOI] [PubMed] [Google Scholar]
- 18.Ashfaq A, Pockaj BA, Gray RJ, Halfdanarson TR, Wasif N. Nodal counts and lymph node ratio impact survival after distal pancreatectomy for pancreatic adenocarcinoma. J Gastrointest Surg. 2014;18(11):1929–1935. doi: 10.1007/s11605-014-2566-5. [DOI] [PubMed] [Google Scholar]
- 19.Wentz SC, Zhao ZG, Shyr Y, et al. Lymph node ratio and preoperative CA 19–9 levels predict overall survival and recurrence-free survival in patients with resected pancreatic adenocarcinoma. World J Gastrointest Oncol. 2012;4(10):207–215. doi: 10.4251/wjgo.v4.i10.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith BJ, Mezhir JJ. An interactive Bayesian model for prediction of lymph node ratio and survival in pancreatic cancer patients. J Am Med Inform Assoc. 2014;21(e2):e203–e211. doi: 10.1136/amiajnl-2013-002171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Orci LA, Meyer J, Combescure C, et al. A meta-analysis of extended versus standard lymphadenectomy in patients undergoing pancre-atoduodenectomy for pancreatic adenocarcinoma. HPB (Oxford) 2015;17(7):565–572. doi: 10.1111/hpb.12407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ignjatovic I, Knezevic S, Knezevic D, et al. Standard versus extended lymphadenectomy in radical surgical treatment for pancreatic head carcinoma. J BUON. 2017;22(1):232–238. [PubMed] [Google Scholar]
- 23.Lee YS, Kim H, Kim HW, et al. High expression of MicroRNA-196a indicates poor prognosis in resected pancreatic neuroendocrine tumor. Medicine (Baltimore) 2015;94(50):e2224. doi: 10.1097/MD.0000000000002224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shirai Y, Shiba H, Haruki K, et al. Preoperative platelet-to-albumin ratio predicts prognosis of patients with pancreatic ductal adenocarcinoma after pancreatic resection. Anticancer Res. 2017;37(2):787–793. doi: 10.21873/anticanres.11378. [DOI] [PubMed] [Google Scholar]
- 25.Martinez-Useros J, Georgiev-Hristov T, Fernandez-Acenero MJ, et al. UNR/CDSE1 expression as prognosis biomarker in resectable pancreatic ductal adenocarcinoma patients: a proof-of-concept. PLoS One. 2017;12(8):e0182044. doi: 10.1371/journal.pone.0182044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jing CY, Fu YP, Shen HJ, et al. Albumin to gamma-glutamyltransferase ratio as a prognostic indicator in intrahepatic cholangiocarcinoma after curative resection. Oncotarget. 2017;8(8):13293–13303. doi: 10.18632/oncotarget.14530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Asano T, Yamada S, Fujii T, et al. The Charlson age comorbidity index predicts prognosis in patients with resected pancreatic cancer. Int J Surg. 2017;39:169–175. doi: 10.1016/j.ijsu.2017.01.115. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ROC curves for OS.
Note: The area under the curve for LNR was 0.627.
Abbreviations: LNR, lymph node ratio; OS, overall survival; ROC, receiver operating characteristic.
Table S1.
Correlations between LNR and clinicopathological characteristics of patients with resected pancreatic head carcinoma in the validation cohort
| Variables | SEER cohort
|
||
|---|---|---|---|
| LNR £ 0.092 (no. of patients = 762) | LNR > 0.092 (no. of patients = 922) | P-value | |
| Age (years) | 0.145 | ||
| <70 | 452 | 579 | |
| ≥70 | 310 | 343 | |
| Gender | 0.001 | ||
| Male | 360 | 509 | |
| Female | 402 | 413 | |
| Grade | <0.001 | ||
| Well differentiation | 94 | 88 | |
| Moderate differentiation | 432 | 456 | |
| Poor differentiation | 236 | 378 | |
| Liver metastasis | 0.127 | ||
| Yes | 11 | 23 | |
| No | 751 | 899 | |
| Lung metastasis | 0.016 | ||
| Yes | 0 | 9 | |
| No | 762 | 913 | |
| T classification | <0.001 | ||
| T1 | 134 | 102 | |
| T2 | 382 | 491 | |
| T3 | 96 | 167 | |
| T4 | 150 | 162 | |
| N classification | <0.001 | ||
| N0 | 473 | 0 | |
| N1 | 284 | 468 | |
| N2 | 5 | 454 | |
| M classification | <0.001 | ||
| M0 | 747 | 870 | |
| M1 | 15 | 52 | |
| TNM staging system | <0.001 | ||
| I | 335 | 0 | |
| II | 262 | 362 | |
| III | 150 | 508 | |
| IV | 15 | 52 | |
| Regional lymph nodes surgery | 0.023 | ||
| None | 16 | 15 | |
| 1–3 | 35 | 21 | |
| ≥4 | 711 | 886 | |
| Surgery | 0.616 | ||
| PPPD | 85 | 86 | |
| Whipple | 533 | 657 | |
| Total pancreatectomy | 100 | 129 | |
| Extended PD | 44 | 50 | |
Note: Bold figures indicate statistical significant P<0.05.
Abbreviations: LNR, lymph node ratio; PD, pancreatoduodenectomy; PPPD, pylorus preserving pancreatoduodenectomy; SEER, Surveillance, Epidemiology, and End Results; TNM, tumor, node, metastasis.
Table S2.
Univariate and multivariate analyses of prognostic factors associated with overall survival of patients with resected pancreatic head carcinoma in the validation cohort
| Variables | Overall survival
|
|||
|---|---|---|---|---|
| No. of patients (N = 1684) | Univariate (P-value) | Multivariate (P-value) | Hazard ratio (95% CI) | |
| Age (years) | <0.001 | <0.001 | 1.338 (1.184–1.511) | |
| <70 | 1031 | |||
| ≥70 | 653 | |||
| Gender | 0.257 | |||
| Male | 869 | |||
| Female | 815 | |||
| Grade | <0.001 | <0.001 | 1.408 (1.279–1.550) | |
| Well differentiation | 182 | |||
| Moderate differentiation | 888 | |||
| Poor differentiation | 614 | |||
| Liver metastasis | 0.001 | 0.987 | 1.005 (0.585–1.724) | |
| Yes | 34 | |||
| No | 1650 | |||
| Lung metastasis | 0.024 | 0.800 | 0.904 (0.416–1.967) | |
| Yes | 9 | |||
| No | 1675 | |||
| LNR | <0.001 | <0.001 | 1.478 (1.237–1.766) | |
| ≤0.092 | 762 | |||
| >0.092 | 922 | |||
| T classification | <0.001 | 0.001 | 1.161 (1.065–1.266) | |
| T1 | 236 | |||
| T2 | 873 | |||
| T3 | 263 | |||
| T4 | 312 | |||
| N classification | <0.001 | 0.128 | 1.139 (0.963–1.348) | |
| N0 | 473 | |||
| N1 | 752 | |||
| N2 | 459 | |||
| M classification | <0.001 | 0.003 | 2.085 (1.274–3.412) | |
| M0 | 1617 | |||
| M1 | 67 | |||
| TNM staging system | <0.001 | 0.942 | 0.994 (0.838–1.178) | |
| I | 335 | |||
| II | 624 | |||
| III | 658 | |||
| IV | 67 | |||
| Regional lymph nodes surgery | 0.817 | |||
| None | 31 | |||
| 1–3 | 56 | |||
| ≥4 | 1597 | |||
| Surgery | 0.150 | |||
| PPPD | 171 | |||
| Whipple | 1190 | |||
| Total pancreatectomy | 229 | |||
| Extended PD | 94 | |||
Abbreviations: LNR, lymph node ratio; PD, pancreatoduodenectomy; PPPD, pylorus preserving pancreatoduodenectomy; TNM, tumor, node, metastasis.