Abstract
Patient: Female, 65
Final Diagnosis: Non uremic calciphylaxis
Symptoms: Skin lesions
Medication: —
Clinical Procedure: —
Specialty: Nephrology
Objective:
Rare disease
Background:
Calciphylaxis results from abnormal calcification of small to medium sized vessels, resulting in painful ischemic necrosis of the surrounding tissues. It is most commonly seen in patients with end stage renal disease on dialysis, but has also been reported in patients with preserved renal function.
Case Report:
We report a case of non uremic calciphylaxis in a 65-year-old female who presented with painful skin lesions and ulcerations involving both thighs one month after receiving a liver transplantation. She was treated with sodium thiosulfate along with wound care and hyperbaric oxygen with complete resolution of the lesions, but with residual scarring.
Conclusions:
Non uremic calciphylaxis is a rare phenomenon that is poorly understood. It should be in the differential of unexplained skin lesions even in the absence of renal insufficiency. Sodium thiosulfate plays a role in treatment, but wound care remains the main focus of treatment.
MeSH Keywords: Calciphylaxis, Hyperbaric Oxygenation, Sodium Thiosulfate
Background
The term calciphylaxis refers to abnormal calcification of small to medium sized vessels, resulting in painful ischemic necrosis of the surrounding tissues. Histologically, it involves dermal arteriolar calcification, subintimal fibrosis, and thrombotic occlusion [1]. It is most commonly seen in patients with end stage renal disease (ESRD) on dialysis due to abnormal calcium phosphorus metabolism and disruption of parathyroid hormone regulation, and is referred to as calcific uremic arteriopathy (CUA). However, there are few cases reported in the literature in the setting of preserved renal function. The term non uremic calciphylaxis (NUC) is used to refer to these cases [2]. We report a rare case of NUC after liver transplantation in a patient with hepatocellular carcinoma (HCC).
Case Report
A 65-year-old Caucasian female developed painful skin lesions and ulcerations involving both thighs one month after receiving a liver transplant. Her post-transplantation course was uneventful except for deconditioning. She initially noticed subcutaneous nodules a few days after receiving a liver transplant. These nodules progressively increased in size and eventually became painful. The pain was severe enough to limit her ability to ambulate. She also described a subjective feeling of weakness. There was no associated fever, chills, chest pain, or dyspnea. Her past medical history was significant for end stage liver disease secondary to hepatitis C diagnosed 10 years ago during the surgical repair of perforated gastric ulcer. Her liver disease was complicated with HCC, recurrent ascites requiring paracentesis, and acute kidney injury with creatinine (Cr) up to 2.9 mg/dL, six weeks prior to transplantation. Her renal function eventually improved with Cr down to the range of 0.8–1.1 mg/dL at the time of transplantation. There is no family history of autoimmune diseases, liver disease, or renal disease. Her social history is significant for smoking and alcohol drinking, but she quit both 10 years ago when she was diagnosed with her liver disease. Physical examination was remarkable for muscle wasting along with multiple tender subcutaneous nodules involving bilateral medial thighs. Some of those nodules showed overlying violaceous reticular patches with central necrotic ulcerations (Figure 1). Her admission medications included mycophenolate 360 mg twice daily, tacrolimus 3 mg twice daily, prednisone 2.5 mg daily, aspirin 81 mg daily, sulfamethoxazole-trimethoprim 800–160 mg three times weekly, gabapentin 100 mg twice daily, oxycodone 5 mg as needed, and acyclovir 400 mg twice daily. There was no history of calcium supplementation or warfarin use but she was on weekly ergocalciferol 50,000 units for almost six months prior to her liver transplantation. Laboratory values are shown in Table 1. They were significant for normal corrected calcium 9.2 mg/dL, slightly high phosphorus 4.7 mg/dL, normal parathyroid hormone 11 pg/mL, and high vitamin D 25 84.2 mg/mL. Her Cr level was 0.96 mg/dL with an eGFR of 60 mL/minute and blood urea nitrogen of 11 mg/dL. Immune serologies were unremarkable except for positive rheumatoid factor but negative for anti-CCP. Testing for cryoglobulin was negative with normal C3 and C4. Protein C activity and protein S screen were both within normal limits. A biopsy of these lesions was eventually done with findings of necrotic leukocytic infiltrate with thrombosis of subcutaneous small caliber subcutaneous blood vessels along with vascular and adipocytic calcification, compatible with calciphylaxis. She was started on intravenous sodium thiosulfate infusions 12.5 mg three times a week. She received wound care along with hyperbaric oxygen for a total of five months after which her lesions healed with complete resolution of pain but with significant residual scarring (Figure 2).
Figure 1.
Subcutaneous nodules with ulceration and eschar formation of overlying skin involving the medial side of the thighs bilaterally. Black arrows: necrotic lesions.
Table 1.
Laboratory values.
| Laboratory | Value | Reference range |
|---|---|---|
| Hemoglobin (g/dl) | 7.2 | 12.0–15.0 |
| White blood count (k/ul) | 4.9 | 4.4–11.0 |
| Platelet count (k/ul) | 228 | 150–400 |
| Creatinine (mg/dl) | 0.96 | 0.4–1.0 |
| Blood urea nitrogen (mg/dl) | 11 | 7.0–25.0 |
| Potassium (mEq/L) | 4.5 | 3.5–5.1 |
| Chloride (mEq/L) | 101 | 98.0–110.0 |
| Bicarbonate (mEq/L) | 24 | 21.0–30.0 |
| Sodium (mMol/L) | 137 | 135.0–147.0 |
| Calcium (mg/dl) | 8.4 | 8.5–10.6 |
| Phosphorus (mg/dl) | 4.7 | 2.0–4.0 |
| Albumin (g/dl) | 3.0 | 3.5–5.0 |
| Vitamin D 25 (ng/ml) | 84.2 | 30.0–80.0 |
| Vitamin D 1,25 (pg/mL) | 36.1 | 19.9–79.3 |
| Parathyroid hormone (Pg/ml) | 11 | 10.0–65.0 |
| C4 (mg/dl) | 47 | 10–49 |
| C3 (mg/dl) | 159 | 88–200 |
| Anti-SSA | Negative | Negative |
| Anti-SSB | Negative | Negative |
| Anti CCP IgG (IU/ml) | <0.5 | <5.0 |
| Rheumatoid factor (IU/mL) | 31 | <24 |
| Cryoglobulin (%ppt) | Negative | Negative |
| SCL70Ab (Units) | <0.2 | <1.0 |
| Cardiolipin IgM (gpl/ml) | 6.6 | <12.5 |
| Cardiolipin IgG (gpl/ml) | 3.2 | <15.0 |
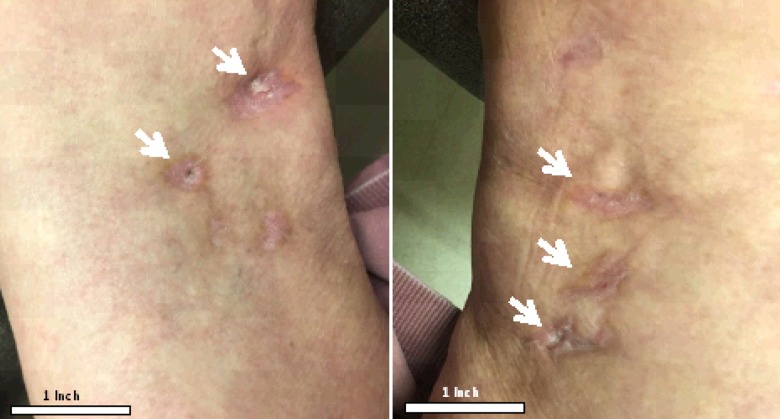
Figure 2.
Healed lesions with residual scarring after five months of treatment with sodium thiosulfate, wound care, and hyperbaric oxygen. White arrows: healed lesions with scarring of both right and left thighs.
Discussion
Calciphylaxis is a rare vascular disease caused by calcium deposition within the lumen of small and medium sized blood vessels. It was first described in animal models in 1961 by Hans Selye, but was later reported in humans as a syndrome primarily seen in uremic patients [3]. The etiology is not well understood but is presumed to be related to abnormalities in calcium phosphorus metabolism that precipitate calcification in the uremic environment, and decrease in the levels of inhibitors of calcification like fetuin-A and matrix Gla protein [4]. However, there are cases reported in the absence of renal insufficiency termed NUC. A systemic review published by Nigwegar et al., identified common risk factors including hyperparathyroidism, connective tissue diseases, alcoholic liver disease, and malignancies. Diabetes, preceding corticosteroid use, chemotherapy (cyclophosphamide, Adriamycin, and fluorouracil), albumin infusion, blood transfusion, protein C and S deficiency, Crohn’s disease, POEMS syndrome, vitamin D deficiency, weight loss, chronic kidney disease (not ESRD), and osteomalacia treated with nadroparin calcium have also been reported as potential risk factors [5,6].
Pathogenesis of NUC is not well understood. Some of the factors like parathyroid hormone, corticosteroids and liver disease are known to increase the expression of RANK ligand and decrease the expression of osteoprotegerin, thus activating NFκB or degrading the inhibitory protein of NFκB (or a combination of these) resulting in dysregulation of extraskeletal mineralization [5]. Warfarin has also been linked to the development of NUC by inhibiting vitamin K-dependent carboxylation of matrix Gla protein (MGP). MGP is a mineral-binding extracellular matrix protein that is synthesized by vascular smooth muscle, endothelium, and chondrocytes. It inhibits calcification of arteries and cartilage in animal models [7]. Another hypothesis links the increased expression of osteopontin to the development of NUC. Osteopontin is secreted by smooth muscle cells that dedifferentiate into osteoblast like cells. It is a chemoattractant that is highly expressed in skin biopsies of patients with calciphylaxis [8].
Tissue biopsy remains the gold standard to diagnose calciphylaxis. Histopathological findings are small to medium vessel calcification with intimal hyperplasia, inflammatory responses, endovascular fibrosis, associated panniculitis, extravascular calcium deposition, thrombosis, and tissue necrosis [7].
Wound care is the main stay of calciphylaxis treatment given the extensive tissue necrosis. Sodium thiosulfate has been an essential part of treatment despite the absence of studies that show its effectiveness. Sodium thiosulfate is believed to facilitate the mobilization of calcium from the affected vessels by enhancing the solubility of calcium deposits in aqueous solution. The beneficial effects of sodium thiosulfate are thought to be partially due to enhanced solubility of calcium deposits in aqueous solution. Initial experiments by Yatzidis demonstrated that calcium thiosulfate is 250, 1,000, 3,600, and 100,000 times more soluble in aqueous solution than calcium sulfate, citrate, phosphate, and oxalate respectively. Sodium thiosulfate is also believed to have antioxidant properties that are capable of restoring endothelial function and enhancing endothelial nitric oxide production, promoting vasodilation and reducing pain [9]. Hyperbaric oxygen is also used adjunctive to sodium thiosulfate. Hyperbaric oxygen therapy involves breathing 100% oxygen at a pressure of 2–3 times atmospheric pressure with many demonstrable effects in wound healing including reversal of wound hypoxia, upwards to level of at least 400 mm Hg. Chronic, non-healing wounds such as calciphylaxis, are often hypoxic with tissue partial pressures of oxygen (pO2) less than 40 mm Hg. Tissue hypoxia contributes to poor wound healing as the formation of collagen matrix and angiogenesis are directly related to adequate oxygen tension. In addition to counteracting local wound hypoxia, hyperbaric oxygen also promotes vasoconstriction resulting in decreased edema, inhibiting neutrophil adhesion and subsequent inflammatory cascade, increasing neutrophil bactericidal activity and augmenting the bacteriostatic or bactericidal effects of certain antibiotics against anaerobic micro-organisms resulting in better control of wound infections [10,11]. Treatment of the NUC also includes discontinuation of warfarin and the use of alternative anticoagulants to alleviate the effect on the inhibitors of calciphylaxis like MGA Protein. Despite these treatments, there is a high mortality rate associated with calciphylaxis.
The risk factors identified in our case were mainly liver disease and the steroid received with transplantation. Vitamin D deficiency has been reported as a risk factor for NUC, but the role of hypervitaminosis D in our patient was not clear. In theory, disruption of calcium phosphorus metabolism is seen with both high and low vitamin D levels. Though the presence of liver disease has been identified as a common risk factor, the development of NUC post liver transplant is unusual. Bohorquez et al. reported four cases of CUA post simultaneous liver and kidney transplantation [12] but this is the first case, to our knowledge, of NUC to develop post liver transplantation. Multidisciplinary approach of treatment is essential. Nephrologists are the most experienced specialist to manage calciphylaxis given its strong association with renal disease. They play an important role in diagnosing, managing and monitoring response to treatment in NUC. Our case showed that successful treatment of NUC is possible with early intervention and aggressive approach despite its well-known poor prognosis.
Conclusions
The development of calciphylaxis in non uremic patients is a rare phenomenon that is poorly understood. NUC should be in the differential of skin lesions in the setting of liver disease and should be still considered even after liver transplantation. Skin biopsy should be done to confirm the diagnosis as clinical presentation is variable. Traditional management approach with sodium thiosulfate along with wound care and hyperbaric oxygen remain the first line of treatment. Nephrologists are the most qualified in managing these patients despite the absence of renal disease given their experience with CUA. While large controlled prospective studies might not be feasible, retrospective studies in large medical centers can potentially be done to look for common precipitating factors.
Footnotes
Conflicts of interest
None.
References:
- 1.Reiter N, El-shabrawi L, Leinweber B, et al. Calcinosis cutis: Part I. Diagnostic pathway. J Am Acad Dermatol. 2011;65(1):1–12. doi: 10.1016/j.jaad.2010.08.038. [DOI] [PubMed] [Google Scholar]
- 2.Fergie B, Valecha N, Miller A. A case of nonuremic calciphylaxis in a caucasian woman. Case Rep Dermatol Med. 2017;2017:6831703. doi: 10.1155/2017/6831703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Selye H, Gentile G, Jean P. An experimental model of “dermatomyositis” induced by calciphylaxis. Can Med Assoc J. 1961;85:770–76. [PMC free article] [PubMed] [Google Scholar]
- 4.Sowers KM, Hayden MR. Calcific uremic arteriolopathy: Pathophysiology, reactive oxygen species and therapeutic approaches. Oxid Med Cell Longev. 2010;3(2):109–21. doi: 10.4161/oxim.3.2.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nigwekar SU, Wolf M, Sterns RH, Hix JK. Calciphylaxis from nonuremic causes: A systematic review. Clin J Am Soc Nephrol. 2008;3(4):1139–43. doi: 10.2215/CJN.00530108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Somorin AO, Al harbi A, Subaity Y, Zaman AU. Calciphylaxis: Case report and literature review. Afr J Med Med Sci. 2002;31(2):175–78. [PubMed] [Google Scholar]
- 7.Schafer C, Heiss A, Schwarz A, et al. The serum protein alpha 2-Heremans-Schmid glycoprotein/fetuin-A is a systemically acting inhibitor of ectopic calcification. J Clin Invest. 2003;112(3):357–66. doi: 10.1172/JCI17202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kyttaris VC, Timbil S, Kalliabakos D, et al. Calciphylaxis: A pseudo-vasculitis syndrome. Semin Arthritis Rheum. 2007;36(4):264–67. doi: 10.1016/j.semarthrit.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 9.Araya CE, Fennell RS, Neiberger RE, Dharnidharka VR. Sodium thiosulfate treatment for calcific uremic arteriolopathy in children and young adults. Clin J Am Soc Nephrol. 2006;1(6):1161–66. doi: 10.2215/CJN.01520506. [DOI] [PubMed] [Google Scholar]
- 10.Maroz N, Mohandes S, Field H, et al. Calciphylaxis in patients with preserved kidney function. J Am Coll Clin Wound Spec. 2014;6(1–2):24–28. doi: 10.1016/j.jccw.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Basile C, Montanaro A, Masi M, et al. Hyperbaric oxygen therapy for calcific uremic arteriolopathy: A case series. J Nephrol. 2002;15(6):676–80. [PubMed] [Google Scholar]
- 12.Bohorquez HE, Chamorro N, Garces J, et al. Calciphylaxis in simultaneous liver-kidney transplantation. Am J Transplant. 2015;15(4):1105–9. doi: 10.1111/ajt.13082. [DOI] [PubMed] [Google Scholar]