Abstract
Background
Ischemia-reperfusion injury is associated with vascular dysfunction. The aim of this study was to investigate the role of emodin, a Chinese herbal medicine, in hypoxia-reoxygenation injury in cultured human aortic endothelial cells (HAECs) and its effects on the expression of the peroxisome proliferator-activated receptor-γ (PPAR-γ) and endothelial nitric oxide synthase (eNOS) signaling pathway.
Material/Methods
An in vitro hypoxia-reoxygenation model used cultured human aortic endothelial cells (HAECs). A colorimetric method evaluated the activity of peroxisome proliferator-activated receptor-γ (PPAR-γ). Phosphorylation of PPAR-γ and endothelial nitric oxide synthase (eNOS) were measured by Western blotting. Expression of inflammatory cytokines, tumor necrosis factor (TNF)-α, interleukin (IL)-6 and IL-8 were evaluated by enzyme-linked immunosorbent assay (ELISA) and Western blotting. Nitric oxide (NO) production was detected by diaminofluorescein-FM diacetate (DAF-FM DA) fluorescence. Immunoprecipitation was used to evaluate the molecular coupling of heat shock protein (HSP)90 and eNOS.
Results
Hypoxia-reoxygenation injury of HAECs reduced the activity and phosphorylation of PPAR-γ, and eNOS, NO production, and HSP90/eNOS molecular coupling in a time-dependent manner. Hypoxia-reoxygenation increased the levels of inflammatory cytokines TNF-α, IL-6, and IL-8 in a time-dependent manner. Emodin treatment recovered PPAR-γ activity and phosphorylation, eNOS phosphorylation, and HSP90/eNOS coupling in HAECS in a concentration-dependent manner, which was reversed by the PPAR-γ inhibitor GW9662, and the eNOS inhibitor, L-NAME. The recovery of HSP90/eNOS coupling by emodin was impaired by GW9662 treatment.
Conclusions
An in vitro hypoxia-reoxygenation (ischemia-reperfusion injury) model of induction of endothelial cell inflammatory mediators showed that emodin recovered the PPAR-γ and eNOS pathway activity.
MeSH Keywords: Emodin, Endothelial Cells, Inflammation, PPAR gamma
Background
Ischemia-reperfusion injury is sustained and irreversible cell damage resulting from restoration of blood flow following ischemia due to vascular compromise and reperfusion [1]. Multiple events are initiated by reperfusion within a very short period after the restoration of blood flow, which involves complex intracellular signaling transduction and expression of inflammatory mediators. Previous studies have shown that vascular endothelial cells are sensitive and vulnerable to ischemia-reperfusion injury [2]. Also, ischemia-reperfusion injury can induce and enhance the inflammatory responses in many cell types, including endothelial cells [3]. The inflammation associated with ischemia-reperfusion injury is associated with the deterioration endothelial integrity and recruitment of lymphocytes and platelets, which are the initiators of the arterial thrombosis [4]. Therefore, it is accepted that the expression of endothelial inflammatory cytokines and mediators is one of the critical pathological changes in conditions such as acute coronary syndrome (ACS), which leads to myocardial ischemia-reperfusion injury [5].
Peroxisome proliferator-activated receptor-γ (PPAR-γ) is recognized as a factor exerting cardio-protective effects [6]. Several previous studies indicated that ischemia-reperfusion injury could inhibit the activation of the PPAR-γ signaling pathway by reducing the phosphorylation of PPAR-γ [7]. Also, some specific PPAR-γ agonists have been shown to attenuate ischemia-reperfusion cell injury in multiple organs such as brain, liver, heart, and lung, and that the PPAR-γ could further activate endothelial nitric oxide synthase (eNOS), which plays a critical role in maintaining and improving endothelial function in response to harmful stimuli [8]. Furthermore, eNOS was reported to suppress generation of inflammatory cytokines via nitric oxide (NO)-dependent mechanisms [9]. However, the involvement of PPAR-γ in ischemia-reperfusion vascular injury is poorly understood.
Emodin is one of the bioactive components extracted from Rheum palmatum L., which has been used as a medicinal herb in traditional Chinese medicine and has been used in the treatment of both ischemic disease and inflammatory disease for many decades [10]. The effects of emodin on PPAR-γ have been previously described [11,12]. Also, emodin has been found to suppress inflammation in a PPAR-γ-dependent manner [13].
The aim of this study was to investigate the role of emodin, a Chinese herbal medicine, in hypoxia-reoxygenation injury in cultured human aortic endothelial cells (HAECs) and its effects on the PPAR-γ and eNOS signaling pathway. In the current study, the in vitro hypoxia-reoxygenation model was used to simulate ischemia-reperfusion injury, and HAECs were exposed to hypoxia-reoxygenation injury. The expression of endothelial inflammatory cytokines and mediators was examined after emodin was administrated to hypoxia-reoxygenation exposed HAECs. To examine the involvement of the PPAR-γ and eNOS pathway, the effects of specific PPAR-γ and eNOS inhibitors were also studied.
Material and Methods
Cell culture, hypoxia-reoxygenation exposure, and treatments
Human aortic endothelial cells (HAECs) were obtained from the Type Culture Collection of the Chinese Academy of Sciences. Cells were cultured with endothelial basal medium (EBM-2) supplemented with 5% fetal bovine serum (FBS) (Invitrogen) and an antibiotic mixture, in a humidified environment with 5% CO2 and 95% normal air at 37°C.
Cells at a confluence of 80–90% were used for the subsequent experiments. The protocol of hypoxia-reoxygenation exposure was in accordance with previous studies [14]. Briefly, cultured HAECs were washed three times in phosphate buffered saline (PBS). The original medium was changed with a modified ischemia-mimetic solution (135 mM NaCl; 0.33 mM NaH2PO4; 8 mM KCl; 0.5 mM HEPES; 5 mM CaCl2; 20 mM lactate; pH, 6.8). Cells were then transferred to a hypoxic atmosphere of 5% CO2, 1% O2, 94% N2 and incubated for eight hours. After that, the medium was changed to the original medium and the cells were incubated under normoxia condition for 2 hours. Cells were treated with emodin (Sigma-Aldrich) at different concentrations (0, 5, 10, and 15 μmol/l) for 24 hours. Cells were also co-treated with the peroxisome proliferator-activated receptor-γ (PPAR-γ) inhibitor GW9662 (Sigma-Aldrich) at 5 μmol/l or endothelial nitric oxide synthase (eNOS) inhibitor Nω-Nitro-L-arginine methyl ester hydrochloride (L-NAME) (Sigma-Aldrich) at 100 μmol/l for 24 hours.
Nitric oxide (NO) production
The NO production in HAECs was determined using the NO-sensitive fluorescence probe diaminofluorescein-FM diacetate (DAF-FM DA) kit (Beyotime) according to the protocol provided by the manufacturer. HAECs were incubated with DAF-FM DA at a final concentration of 5 μmol/l at 37°C in a dark chamber for 20 minutes. Then, the cells were washed three times in PBS. Then the cells were excited at 495 nm and observed at 515 nm with an inverted fluorescence microscope.
Inflammatory cytokine detection
The concentrations inflammatory cytokines including tumor necrosis factor (TNF)-α, interleukin (IL)-6 and IL-8 in cell culture medium supernatant were determined by enzyme-linked immunosorbent assay (ELISA) with commercially available detection kits. Cell culture medium was acquired after centrifugation. Specifically, tumor necrosis factor (TNF)-alpha human uncoated ELISA kit (Invitrogen), IL-6 human ELISA kit (Invitrogen) and IL-8 human ELISA kit (Invitrogen) were used. All protocols were carried out in accordance with the instructions provided by the manufacturer.
Peroxisome proliferator-activated receptor-γ (PPAR-γ) activity assay
Cultured HAECs were lysed in a lysis buffer (pH, 7.4) 10 μmol/l Tris-HCl, 0.5 mmol/l NaCl in 1μmol/l ethylenediaminetetraacetic acid (EDTA), 0.05% SDS, 0.5% Triton X-100, supplemented with 1 μmol/l phenylmethanesulfonyl fluoride (PMSF). The pellets were collected after centrifugation at 15,000 g for 10 minutes at 4°C. A PPAR-γ transcription factor assay kit (Abcam, Cambridge, MA, USA) was used to determine the PPAR-γ activity by measuring the absorbance at 450 nm.
Western blotting
Cultured HAECs were lysed by RIPA lysis buffer system (Santa Cruz) and PSMF (Santa Cruz). A total protein extraction kit (Beyotime) was used to extract the protein. The concentration of protein in the samples was determined using a BCA protein assay kit (Pierce). Proteins were subjected to SDS-PAGE and were then transferred to PVDF/NC membranes. Non-specific binding was eliminated by incubating with blocking buffer. Primary antibodies against PPAR-γ (Abcam), eNOS (Cell Signaling Technology), phospho-eNOS (Cell Signaling Technology), TNF-α (Abcam), IL-6 (Abcam), IL-8 (Abcam) and GAPDH (Abcam) were used to incubate the membranes for 8 hours at 4°C. After washing in Tris-buffered saline with Tween 20 (TBST), membranes were incubated with secondary antibodies conjugated with horseradish peroxidase (HRP) (Abcam) for 30 minutes at room temperature. Signal West Pico reagent (Pierce ECL) was used to develop the membranes. The immunobands were visualized on X-ray films and further analyzed by using ImageJ software.
Immunoprecipitation
The binding of eNOS and heat shock protein (HSP)90 was evaluated by immunoprecipitation. The extracted proteins were incubated with protein G plus/protein A agarose beads (Calbiochem, CA, USA) and then incubated with primary antibodies against eNOS and HSP90. After centrifugation, the precipitates were subjected to immunoblotting procedures, as described above.
Statistical analysis
Data collected in this study were presented as the mean ± standard deviation (SD). Differences between groups were analyzed by the Student’s t-tests and ANOVA. The posthoc analysis was carried out by NSK tests. The statistical analysis was performed by SPSS version 16.0 software. Statistical significance was considered to be p<0.05.
Results
Hypoxia-reoxygenation exposure increased the levels of inflammatory cytokines in human aortic endothelial cells (HAECs) and cell culture medium supernatant
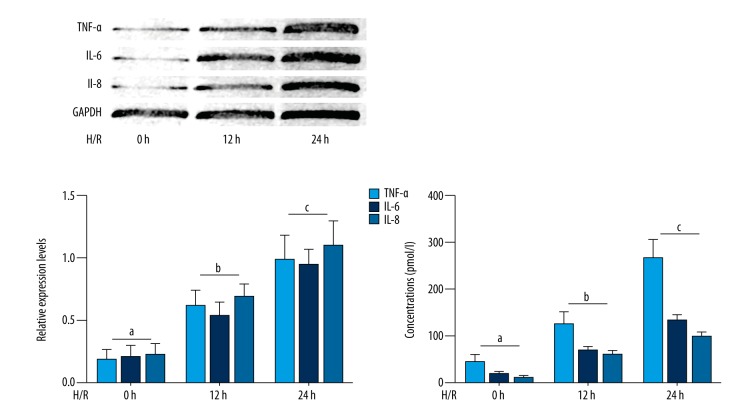
The results are shown in Figure 1. The hypoxia-reoxygenation exposure significantly increased expression levels of inflammatory cytokines including tumor necrosis factor (TNF)-α, interleukin (IL)-6 and IL-8 in cultured human aortic endothelial cells (HAECs) in a time-dependent manner. Also, the concentrations of these inflammatory cytokines were increased by hypoxia-reoxygenation exposure in the supernatant of the culture medium of HAECs in a time-dependent manner.
Figure 1.
Cultured human aortic endothelial cells (HAECs) exposed to hypoxia-reoxygenation and time-dependent inflammatory cytokine expression The upper part of left panel shows the immunoblots of tumor necrosis factor (TNF)-α, interleukin (IL)-6 and IL-8 in cultured HAECs exposed to hypoxia-reoxygenation for 0, 12, and 24 hours. Columns on the lower part of left panel indicate the relative expression levels (normalized to GAPDH) of TNF-α (light blue columns), IL-6 (deep blue columns) and IL-8 (blue columns) in cultured HAECs exposed to hypoxia-reoxygenation for 0, 12 and 24 hours respectively. Columns on the right of this figure indicate the detected concentrations of TNF-α (light blue columns), IL-6 (deep blue columns) and IL-8 (blue columns) in culture medium supernatant of cultured HAECs exposed to hypoxia-reoxygenation for 0, 12 and 24 hours respectively. Differences are significant (p<0.05) between a. and b.; and were significant (p<0.05) between b. and c.
Hypoxia-reoxygenation exposure inhibited the activation of peroxisome proliferator-activated receptor-γ (PPAR-γ), endothelial nitric oxide synthase (eNOS), nitric oxide (NO) signaling as well as the PPAR-γ-heat shock protein (HSP)90 interaction in cultured HAECs
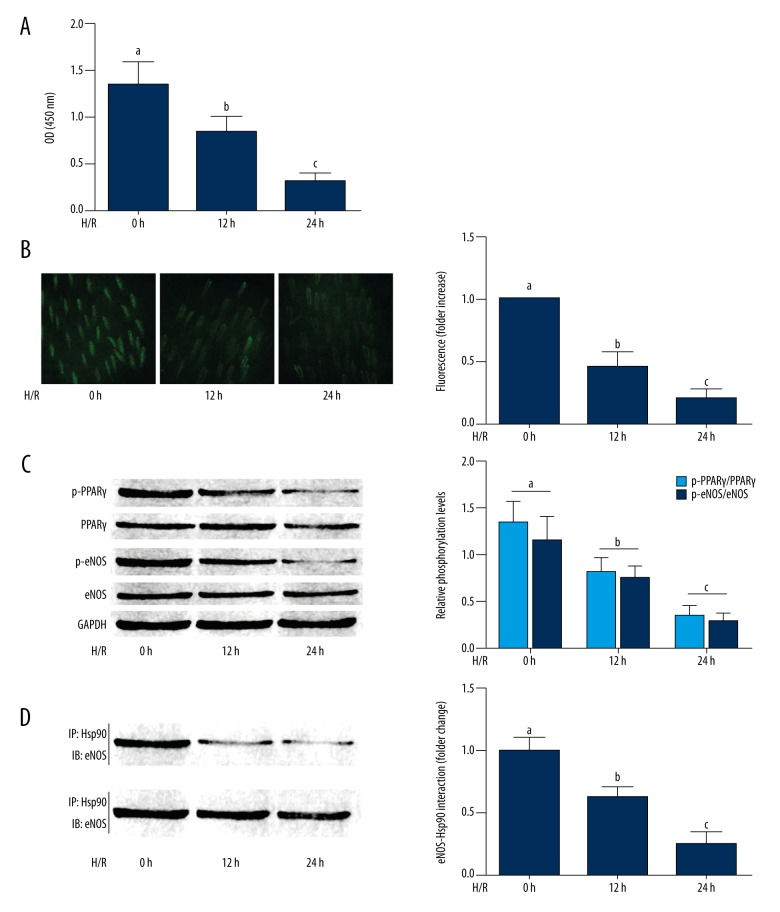
The inhibitory effect of hypoxia-reoxygenation exposure on the PPAR-γ/eNOS/NO pathway is shown in Figure 2. The PPAR-γ activity was reduced by hypoxia-reoxygenation exposure in a time-dependent manner. Also, the phosphorylation levels of PPAR-γ and eNOS were also decreased by hypoxia-reoxygenation exposure in a time-dependent manner. The association between HSP90 and eNOS were impaired by hypoxia-reoxygenation exposure. As a result, the NO production in HAECs was reduced by hypoxia-reoxygenation exposure in a time-dependent manner.
Figure 2.
Cultured human aortic endothelial cells (HAECs) exposed to hypoxia-reoxygenation and expression of peroxisome proliferator-activated receptor-γ (PPAR-γ) and endothelial nitric oxide synthase (eNOS). (A) Columns indicate the detected optical density (O.D) at 450nm indicating peroxisome proliferator-activated receptor-γ (PPAR-γ) activities in cultured HAECS exposed to hypoxia-reoxygenation for 0, 12, and 24 hours, respectively. (B) The left side shows the captured images of DAF-FM DA fluorescent stain of HAECs. Columns on the right part indicated the fluorescent intensities of DAG-FM DA stain of HAECS exposed to hypoxia-reoxygenation for 0, 12, and 24 hours, respectively. (C) The immunoblots of pPPAR-γ, PPAR-γ, p-eNOS, eNOS and GAPDH in HAECs are shown on the left. Columns on the right show the relative phosphorylation levels of PPAR-γ (white columns) and eNOS (black columns) in cultured HAECS exposed to hypoxia-reoxygenation for 0, 12, and 24 hours, respectively. (D) The immunoblots on the right show the immunoprecipitation analysis of heat shock protein (HSP)90/eNOS interaction in HAECs. Columns on the right indicate the eNOS/HSP90 association (normalized to HSP90) in HAECS exposed to hypoxia-reoxygenation for 0, 12, and 24 hours, respectively. Differences were significant (p<0.05) between a. and b. Differences were significant (p<0.05) between b. and c.
Emodin significantly reduced the expression of inflammatory cytokines in HAECS exposed to hypoxia-reoxygenation and culture medium supernatant which was impaired by inhibitors of PPAR-γ and eNOS
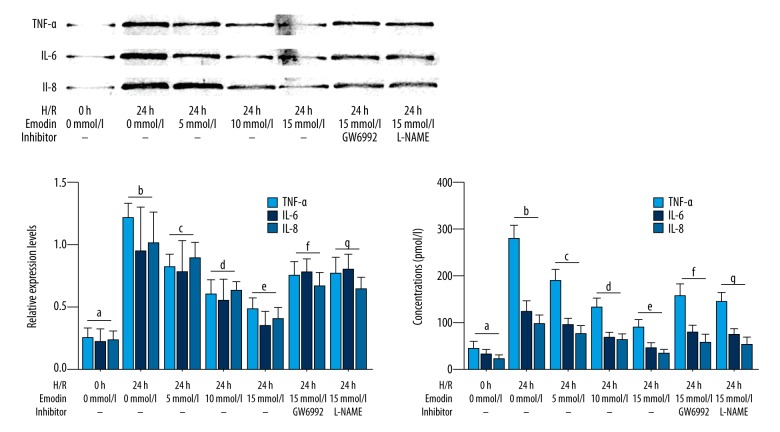
The effects of emodin on inflammatory cytokine levels in HAECS exposed to hypoxia-reoxygenation are shown in Figure 3. Emodin decreased the levels of TNF-α, IL-6, and IL-8 in HAECs exposed to hypoxia-reoxygenation and culture medium supernatant in a concentration-dependent manner. However, co-treatment with both the PPAR-γ inhibitor GW6992, and the eNOS inhibitor L-NAME, significantly impaired the inhibitory effects of emodin on inflammatory cytokines production in HAECS exposed to hypoxia-reoxygenation.
Figure 3.
Cultured human aortic endothelial cells (HAECs) exposed to hypoxia-reoxygenation, expression of inflammatory cytokines, and treatment with emodin and inhibitors of peroxisome proliferator-activated receptor-γ (PPAR-γ) and endothelial nitric oxide synthase (eNOS). The upper panel of left part of this figure shows the immunoblots of tumor necrosis factor (TNF)-α, interleukin (IL)-6 and IL-8 in cultured human aortic endothelial cells (HAECs) exposed to hypoxia-reoxygenation. Columns in the lower panel of the left part of this figure indicate the relative expression levels (normalized to GAPDH) of TNF-α (light blue columns), IL-6 (deep blue columns) and IL-8 (bleu columns) in HAECS exposed to hypoxia-reoxygenation treated with emodin and inhibitors of PPARγ/eNOS respectively. Columns on the right part of this figure indicated the detected concentrations of TNF-α (light blue columns), IL-6 (deep blue columns) and IL-8 (blue columns) in culture medium supernatant of HAECS exposed to hypoxia-reoxygenation co-treated with emodin and inhibitors of PPARγ/eNOS respectively. Differences were significant (p<0.05) between a. and b. Differences were significant (p<0.05) between b. and c. Differences were significant (p<0.05) between d. and c. Differences were significant (p<0.05) between e. and d. Differences were significant (p<0.05) between f. and e. Differences were significant (p<0.05) between g. and e.
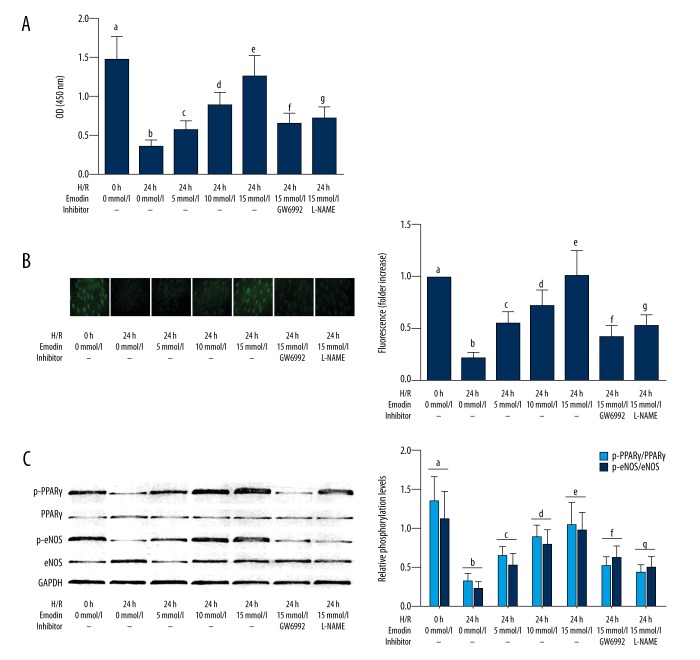
Emodin recovered the activation of PPARγ/eNOS/NO signaling in HAECS exposed to hypoxia-reoxygenation, which was impaired by inhibitors of PPARγ and eNOS. As shown in Figure 4A, emodin incubation significantly recovered the PPARγ activity in HAECS exposed to hypoxia-reoxygenation in a concentration-dependent manner, which was impaired by both the PPAR-γ inhibitor GW6992, and the eNOS inhibitor L-NAME. As shown in Figure 4B, emodin incubation significantly recovered the NO production in HAECS exposed to hypoxia-reoxygenation, in a concentration-dependent manner, which was reversed by PPAR-γ and eNOS inhibitors. As demonstrated in Figure 4C, emodin treatment increased phosphorylation levels of PPAR-γ and eNOS in HAECS exposed to hypoxia-reoxygenation.in a concentration-dependent manner, which was also impaired by PPAR-γ and eNOS inhibitors.
Figure 4.
Cultured human aortic endothelial cells (HAECs) exposed to hypoxia-reoxygenation, expression of inflammatory cytokines, and treatment with emodin and inhibitors of peroxisome proliferator-activated receptor-γ (PPAR-γ) and endothelial nitric oxide synthase (eNOS). (A) Columns show the detected O.D. at 450nm indicating peroxisome proliferator-activated receptor-γ (PPAR-γ) activities in cultured human aortic endothelial cells (HAECs) exposed to hypoxia-reoxygenation co-treated with emodin and inhibitors of PPARγ/endothelial nitric oxide synthase (eNOS), respectively. (B) The left part of this panel demonstrates the captured fluorescent images of diaminofluorescein-FM diacetate (DAF-FM DA) fluorescence staining of HAECs. Columns on the right part of this panel indicated the fluorescent intensities of DAG-FM DA stain of HAECS exposed to hypoxia-reoxygenation co-treated with emodin and inhibitors of PPARγ/eNOS, respectively. (C) The immunoblots of p-PPAR, PPAR, p-eNOS, eNOS and GAPDH are demonstrated on the right part of this panel. Columns on the right part indicate the relative phosphorylation levels of PPAR-γ (light blue columns) and eNOS (deep blue columns) in HAECS exposed to hypoxia-reoxygenation co-treated with emodin and inhibitors of PPARγ/eNOS, respectively. Differences were significant (p<0.05) between a. and b. Differences were significant (p<0.05) between b. and c. Differences were significant (p<0.05) between d. and c. Differences were significant (p<0.05) between e. and d. Differences were significant (p<0.05) between f. and e. Differences were significant (p<0.05) between g. and e.
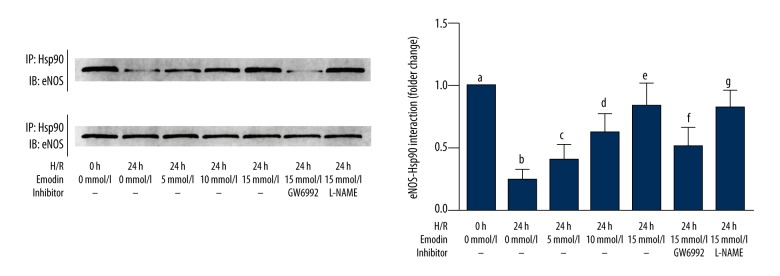
Emodin restored PPAR-γ – HSP90 interaction in HAECS exposed to hypoxia-reoxygenation that was impaired by inhibitors of PPAR-γ
The results were shown in Figure 5. Emodin treatment increased the association between HSP90 and eNOS in a concentration-dependent manner, which was impaired by the PPAR-γ inhibitor GW6992, rather than eNOS inhibitor L-NAME.
Figure 5.
Cultured human aortic endothelial cells (HAECs) exposed to hypoxia-reoxygenation and analysis of heat shock protein (HSP)90 and endothelial nitric oxide synthase (eNOS). The upper part of this figure demonstrates the immunoprecipitation analysis of HSP90/eNOS in HAECs. Columns on the lower part indicated the eNOS/Hsp90 association (normalized to HSP90) in HAECS exposed to hypoxia-reoxygenation for 0, 12, and 24 hours respectively. Differences were significant (p<0.05) between a. and b. Differences were significant (p<0.05) between b. and c. Differences were significant (p<0.05) between d. and c. Differences were significant (p<0.05) between e. and d. Differences were significant (p<0.05) between f. and e.
Discussion
Ischemia-reperfusion injury is accepted as one of the contributors to many cardiovascular diseases by bringing irreversible damage to the endothelium by inducing cell apoptosis and inflammation [15]. The inflammatory cytokines generated in vivo by ischemia-reperfusion injury would recruit immune cells, which would further participate in exacerbating blood vessel barrier dysfunction by direct inflammatory cell infiltration and promoting thrombosis by activating circulating platelets [16]. Ischemia-reperfusion injury has been defined as the restoration of blood flow after total occlusion of blood vessels, and hypoxia-reoxygenation is accepted as a model that mimics ischemia-reperfusion injury in vitro [17,18].
Inflammatory cytokines, including tumor necrosis factor (TNF)-α, interleukin (IL)-6 and IL-8 are considered to be typical inflammatory factors in vascular inflammation. TNF-α causes oxidative stress in vascular endothelial cells and activates several pro-inflammatory nuclear factors such as NF-κB, which further induces synthesis of more inflammatory cytokines [19]. IL-6 is involved in increasing vascular permeability under clinical situations that include myocardial infarction and stroke [20]. IL-8 plays a role in platelets aggregation and activation contributing to the formation of thrombosis [21]. In this study, hypoxia-reoxygenation.was performed to treat cultured human aortic endothelial cells (HAECs). The results showed that the levels of inflammatory cytokines, including TNF-α, IL-6, and IL-8 in cell culture supernatants of HAECs, and in HAECS exposed to hypoxia-reoxygenation occurred in a time-dependent manner. These results suggested that the vascular inflammatory response was stimulated during ischemia-reperfusion injury.
Nitric oxide (NO) is an important effective small molecule in the cardiovascular system participating in proliferation, apoptosis, and oxidative stress of the vascular endothelium [22]. NO plays a critical role in the development, maintenance, and regulation of the vascular inflammatory status in cardiovascular diseases, including atherosclerosis, stroke, and ischemia-reperfusion injury [23]. The formation of NO is catalyzed by endothelial nitric oxide synthase (eNOS), which is activated by phosphorylation. PPAR-γ has been considered as a nuclear factor exerting anti-inflammatory effects when activated [24]. Loss of PPAR-γ activity might result in elevation of levels of inflammatory factors in ischemic diseases such as cerebral ischemia [25]. Also, previous studies have shown that PPAR-γ activation stimulated the synthesis and release of NO, and so eNOS was identified as a downstream molecular target of PPAR-γ [26].
Heat shock protein (HSP)90 has been reported to be involved in the regulation of ischemia-reperfusions as HSP90 binds to eNOS to maintain the catalytic activity of eNOS. This coupling between HSP90 and eNOS being largely dependent on the activity of PPAR-γ [27]. In this study, the hypoxia-reoxygenation exposure was shown to decrease the activity and phosphorylation of PPAR-γ in cultured HAECs in a time-dependent manner, leading to the interruption of molecular coupling between HSP90 and eNOS. The phosphorylation level of eNOS was impaired, which resulted in the reduced production of NO and increased levels of inflammatory cytokines in HAECs. These results showed that hypoxia-reoxygenation induced the inflammatory response in HAECs. Loss of activation of PPAR-γ was critically involved in the process that leads to the impairment of eNOS activity by disrupting the molecular interaction between eNOS and HSP90.
Emodin is one of the bioactive components extracted from Rheum palmatum L., which has been used as a traditional Chinese herbal medicine for decades in Eastern Asia [28]. Emodin was reported in previous studies to exert endothelial protective effects in various diseases involving vascular endothelium injuries brought by hyperglycemia, irradiation, and cytotoxic drugs [29,30]. Emodin has also been considered to be an anti-inflammatory agent in infectious diseases, autoimmune diseases and allotransplantation [31,32]. Emodin was also described as an activator of PPAR-γ in previous investigations [13]. In the present study, emodin incubation recovered the activity and phosphorylation level of PPAR-γ in HAECS exposed to hypoxia-reoxygenation in a concentration-dependent manner. Also, the phosphorylation of eNOS was also increased in HAECS exposed to hypoxia-reoxygenation after incubation of emodin. We also found that the emodin incubation increased the molecular interaction between HSP90 and eNOS. As a result, the synthesis and secretion of NO were elevated when treated with emodin, whereas the inflammatory cytokines in HAECs were suppressed in HAECS exposed to hypoxia-reoxygenation. The treatment of inhibitors of PPAR-γ and eNOS impaired the anti-inflammatory effects of emodin on HAECS exposed to hypoxia-reoxygenation. Also, PPAR-γ inhibitor treatment impaired the molecular HSP90/eNOS association rather than eNOS inhibitor treatment. These data indicated that emodin inhibited the expression of endothelial inflammatory cytokines and mediators in a concentration-dependent manner. Recovery of PPAR-γ activity, which reinforced the coupling of HSP90 and eNOS was one of the molecular mechanisms of the anti-inflammatory effects of emodin in ischemia-reperfusion-induced endothelial injury.
This study had several limitations. Firstly, the current study was conducted in vitro. Though the in vitro hypoxia-reoxygenation model could simulate the in vivo ischemia-reperfusion injury model, the physiological and pathological changes were not the same. Thus, it would be more persuasive if an in vivo investigation was conducted. Second, the molecular mechanisms of the effects of eNOS on inflammation were not thoroughly investigated, and it is still unclear whether this effect was NO-dependent or NO-independent. Further studies using NO inhibition methods would be helpful.
Conclusions
The findings of this study showed that emodin, a Chinese herbal medicine had a protective effect on hypoxia-reoxygenation damage in cultured human aortic endothelial cells (HAECs) in vitro, by deactivating the peroxisome proliferator-activated receptor-γ (PPAR-γ) and endothelial nitric oxide synthase (eNOS) signaling pathway. This study investigated the possible molecular mechanism of endothelial cell injury due to ischemia-reperfusion and the expression of endothelial cell inflammatory mediators, and the therapeutic effect of emodin. The findings suggest that deactivation of the PPAR-γ and eNOS signaling pathway was critical to the expression of endothelial cell inflammatory cytokines due to hypoxia-reoxygenation in cultured HAECs. Emodin was shown to recover the PPAR-γ activity in HAECS exposed to hypoxia-reoxygenation, by the molecular interaction of HSP90 and eNOS. As a result, the NO production was increased and the inflammation was suppressed. We believe that our data would not only deepen our current understanding of endothelial injury induced by ischemia-reperfusion injury, but also provide a novel theoretical basis for potential clinical application of emodin in vascular diseases in the future.
Footnotes
Source of support: The Public Welfare Technology Application Research Program of Zhejiang Provincial Department (No. 2016C33123). The Science and Technology Program of Traditional Chinese Medicine of Zhejiang Province (No. 2013ZB015). The Medical and Health Science and Technology Program of Zhejiang Province (No. 2012KYA010). The Medical and Health Science and Technology Program of Zhejiang Province (No. 2012KYA001). The Medical and Health Science and Technology Program of Zhejiang Province (No. 2014KYA005)
References
- 1.Wang XC, Sun WT, Fu J, et al. Impairment of coronary endothelial function by hypoxia-reoxygenation involves trpc3 inhibition-mediated K-Ca channel dysfunction: Implication in ischemia-reperfusion injury. Sci Rep. 2017;7:5895. doi: 10.1038/s41598-017-06247-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cui H, Li N, Li X, et al. Tongxinluo modulates cytokine secretion by cardiac microvascular endothelial cells in ischemia/reperfusion injury. Am J Transl Res. 2016;8:4370–81. [PMC free article] [PubMed] [Google Scholar]
- 3.Wei W, Xie Y, Lai SC, et al. Benefits of anti-inflammatory therapy in the treatment of ischemia/reperfusion injury in the renal microvascular endothelium of rats with return of spontaneous circulation. Molec Med Rep. 2017;15:4231–38. doi: 10.3892/mmr.2017.6548. [DOI] [PubMed] [Google Scholar]
- 4.Kim SJ, Davis RP, Jenne CN. Platelets as modulators of inflammation. Sem Thromb Hemost. 2017 doi: 10.1055/s-0037-1607432. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 5.Sager HB, Nahrendorf M. Inflammation: A trigger for acute coronary syndrome. Quart J Nucl Med Molec Imag. 2016;60:185–93. [PubMed] [Google Scholar]
- 6.Agrawal YO, Sharma PK, Shrivastava B, et al. Hesperidin produces cardioprotective activity via ppar-gamma pathway in ischemic heart disease model in diabetic rats. PLoS One. 2014;9:e111212. doi: 10.1371/journal.pone.0111212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xiong D, Deng Y, Huang B, et al. Icariin attenuates cerebral ischemia-reperfusion injury through inhibition of inflammatory response mediated by NF-Kappa-beta, PPAR-alpha and PPAR-gamma in rats. Int Immunopharmacol. 2016;30:157–62. doi: 10.1016/j.intimp.2015.11.035. [DOI] [PubMed] [Google Scholar]
- 8.Kobayashi N, Ohno T, Yoshida K, et al. Cardioprotective mechanism of telmisartan via PPAR-gamma-eNOS pathway in Dahl salt-sensitive hypertensive rats. Am J Hypertens. 2008;21:576–81. doi: 10.1038/ajh.2008.27. [DOI] [PubMed] [Google Scholar]
- 9.Wang Y, Cui L, Xu H, et al. Trpv1 agonism inhibits endothelial cell inflammation via activation of eNOS/NO pathway. Atherosclerosis. 2017;260:13–19. doi: 10.1016/j.atherosclerosis.2017.03.016. [DOI] [PubMed] [Google Scholar]
- 10.Ka SO, Hwang HP, Jang JH, et al. The protein kinase 2 inhibitor tetrabromobenzotriazole protects against renal ischemia reperfusion injury. Sci Rep. 2015;5:14816. doi: 10.1038/srep14816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pang X, Liu J, Li Y, et al. Emodin inhibits homocysteine-induced C-reactive protein generation in vascular smooth muscle cells by regulating PPAR-gamma expression and ROS-ERK1/2/p38 signal pathway. PLoS One. 2015;10:e0131295. doi: 10.1371/journal.pone.0131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu Y, Chen X, Qiu M, et al. Emodin ameliorates ethanol-induced fatty liver injury in mice. Pharmacology. 2014;94:71–77. doi: 10.1159/000363413. [DOI] [PubMed] [Google Scholar]
- 13.Zhu T, Zhang W, Feng SJ, Yu HP. Emodin suppresses LPS-induced inflammation in RAW264.7 cells through a PPAR-gamma-dependent pathway. Int immunopharmacol. 2016;34:16–24. doi: 10.1016/j.intimp.2016.02.014. [DOI] [PubMed] [Google Scholar]
- 14.Jiang J, Chen DY, Liu ZT, et al. Effect of n-perfluorooctane on hypoxia/reoxygenation injury in human umbilical vein endothelial cells. Acta Cardiologica Sinica. 2016;32:716–22. doi: 10.6515/ACS20151228D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yao BJ, He XQ, Lin YH, Dai WJ. Cardioprotective effects of anisodamine against myocardial ischemia/reperfusion injury through the inhibition of oxidative stress, inflammation and apoptosis. Mol Med Rep. 2017;17(1):1253–60. doi: 10.3892/mmr.2017.8009. [DOI] [PubMed] [Google Scholar]
- 16.Begandt D, Thome S, Sperandio M, Walzog B. How neutrophils resist shear stress at blood vessel walls: Molecular mechanisms, subcellular structures, and cell-cell interactions. J Leukocyte Biol. 2017;102:699–709. doi: 10.1189/jlb.3MR0117-026RR. [DOI] [PubMed] [Google Scholar]
- 17.Xu Y, Gu Q, Tang J, et al. Substance P attenuates hypoxia/reoxygenation-induced apoptosis via the AKT signaling pathway and the NK1-receptor in H9C2 cells. Heart Lung Circ. 2017 doi: 10.1016/j.hlc.2017.09.013. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 18.He F, Wu Q, Xu B, et al. Suppression of stim1 reduced intracellular calcium concentration and attenuated hypoxia/reoxygenation induced apoptosis in H9C2 cells. Biosci Rep. 2017;37(6) doi: 10.1042/BSR20171249. pii: BSR20171249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fan R, Han Y, Han H, et al. DT-13 ameliorates TNF-alpha-induced nitric oxide production in the endothelium in vivo and in vitro. Biochem Biophys Res Commun. 2017;495(1):1175–81. doi: 10.1016/j.bbrc.2017.11.009. [DOI] [PubMed] [Google Scholar]
- 20.Recoquillon S, Gomez-Guzman M. Non-muscular myosin light chain kinase triggers intermittent hypoxia-induced interleukin-6 release, endothelial dysfunction and permeability. Sci Rep. 2017;7:13664. doi: 10.1038/s41598-017-13268-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nair J, Kakkar VV, Shanker J. Comparative analysis of inflammatory gene expression levels in metabolic syndrome and coronary artery disease. Indian J Med Res. 2017;145:777–85. doi: 10.4103/ijmr.IJMR_1678_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ashigai H, Ikeshima E, Koizumi K, et al. 2-ethylpyrazine induces vasodilatation by releasing nitric oxide in the endothelium. Biol Pharm Bull. 2017;40(12):2153–57. doi: 10.1248/bpb.b17-00551. [DOI] [PubMed] [Google Scholar]
- 23.Prieto D. Nitric oxide-mediated negative regulation of cyclooxygenase-2 induction in vascular inflammation. Am J Physiol Heart Circ Physiol. 2010;299:H600–1. doi: 10.1152/ajpheart.00593.2010. [DOI] [PubMed] [Google Scholar]
- 24.Yasuda S, Kobayashi H, Iwasa M, et al. Antidiabetic drug pioglitazone protects the heart via activation of PPAR-gamma receptors, Pi3-kinase, AKT, and eNOS pathway in a rabbit model of myocardial infarction. Am J Physiol Heart Circ Physiol. 2009;296:H1558–65. doi: 10.1152/ajpheart.00712.2008. [DOI] [PubMed] [Google Scholar]
- 25.Wang X, Li R, Wang X, et al. Umbelliferone ameliorates cerebral ischemia-reperfusion injury via upregulating the ppar gamma expression and suppressing txnip/nlrp3 inflammasome. Neurosci Lett. 2015;600:182–87. doi: 10.1016/j.neulet.2015.06.016. [DOI] [PubMed] [Google Scholar]
- 26.Li P, Zhang D, Wan M, Liu J. Ppargamma affects nitric oxide in human umbilical vein endothelial cells exposed to porphyromonas gingivalis. Arch Oral Biol. 2016;68:116–22. doi: 10.1016/j.archoralbio.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 27.Bi R, Bao C, Jiang L, et al. Microrna-27b plays a role in pulmonary arterial hypertension by modulating peroxisome proliferator-activated receptor gamma dependent HSP90-eNOS signaling and nitric oxide production. Biochem Biophys Res Commun. 2015;460:469–75. doi: 10.1016/j.bbrc.2015.03.057. [DOI] [PubMed] [Google Scholar]
- 28.Wu Y, Tu X, Lin G, et al. Emodin-mediated protection from acute myocardial infarction via inhibition of inflammation and apoptosis in local ischemic myocardium. Life Sci. 2007;81:1332–38. doi: 10.1016/j.lfs.2007.08.040. [DOI] [PubMed] [Google Scholar]
- 29.Gao Y, Zhang J, Li G, et al. Protection of vascular endothelial cells from high glucose-induced cytotoxicity by emodin. Biochem Pharmacol. 2015;94:39–45. doi: 10.1016/j.bcp.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 30.Wang J, Zhang Y, Zhu Q, et al. Emodin protects mice against radiation-induced mortality and intestinal injury via inhibition of apoptosis and modulation of p53. Environ Toxicol Pharmacol. 2016;46:311–18. doi: 10.1016/j.etap.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 31.Song ZC, Wang ZS, Bai JH, et al. Emodin, a naturally occurring anthraquinone, ameliorates experimental autoimmune myocarditis in rats. Tohoku J Exp Med. 2012;227:225–30. doi: 10.1620/tjem.227.225. [DOI] [PubMed] [Google Scholar]
- 32.Tong H, Chen K, Chen H, et al. Emodin prolongs recipient survival time after orthotopic liver transplantation in rats by polarizing the Th1/Th2 paradigm to Th2. Anat Rec (Hoboken) 2011;294:445–52. doi: 10.1002/ar.21352. [DOI] [PubMed] [Google Scholar]