Abstract
It has previously been hypothesized that hyperactivity of central auditory neurons following exposure to intense noise is a consequence of synaptic alterations. Recent studies suggest the involvement of NMDA receptors in the induction of this hyperactive state. NMDA receptors can mediate long term changes in the excitability of neurons through their involvement in excitotoxic injury and long term potentiation and depression. In this study, we examined the effect of administering an NMDA receptor blocker on the induction of hyperactivity in the dorsal cochlear nucleus (DCN) following intense sound exposure. Our prediction was that if hyperactivity induced by intense sound exposure is dependent on NMDA receptors, then blocking these receptors by administering an NMDA receptor antagonist just before animals are exposed to intense sound should reduce the degree of hyperactivity that subsequently emerges. We compared the levels of hyperactivity that develop in the DCN after intense sound exposure to activity recorded in control animals that were not sound exposed. One group of animals to be sound exposed received intraperitoneal injection of MK-801 twenty minutes preceding the sound exposure, while the other group received injection of saline. Recordings performed in the DCN 26–28 days post-exposure revealed increased response thresholds and widespread increases in spontaneous activity in the saline-treated animals that had been sound exposed, consistent with earlier studies. The animals treated with MK-801 preceding sound exposure showed similarly elevated thresholds but an attenuation of hyperactivity in the DCN; the attenuation was most robust in the high frequency half of the DCN, but lower levels of hyperactivity were also found in the low frequency half. These findings suggest that NMDA receptors are an important component of the hyperactivity-inducing mechanism following intense sound exposure. They further suggest that blockade of NMDA receptors may offer a useful therapeutic approach to preventing induction of noise-induced hyperactivity-related hearing disorders, such as tinnitus and hyperacusis.
Keywords: Auditory, hearing, neuroprotection, excitotoxicity, long term potentiation, tinnitus
Introduction
Exposure to intense sound causes neurons in the central auditory system to become hyperactive, whereby their levels of spontaneous neural activity are elevated. There is an abundance of evidence that increased spontaneous activity is an important neural correlate of tinnitus (see reviews of Roberts et al., 2010; Kaltenbach, 2011; Kalappa et al., 2014; Eggermont, 2015; Shore et al., 2016). Thus, an understanding of the mechanism underlying the induction of hyperactivity has considerable clinical relevance. Two general categories of mechanisms are under investigation to understand the emergence of hyperactivity following intense sound exposure. One category involves a shift in the balance of the relative strengths of excitatory and inhibitory synapses, such that the balance swings toward the side of increasing excitation (Willott and Lu, 1982; Salvi et al., 1990; Kaltenbach and McCaslin, 1996; Morest et al., 1997; Shore et al., 2008; Wang et al., 2009; Middleton et al., 2011). This category of changes could occur either pre- or post-synaptically and manifest as perturbations in the relative numbers and/or functional properties of excitatory and inhibitory synapses. The second category of mechanism that could underlie the hyperactive state includes alterations in ion conductance channels, the so-called intrinsic membrane properties of neurons (Holt et al., 2006; Finlayson and Kaltenbach, 2009; Li et al., 2013, 2015). This category could involve alterations in the relative strengths of channels that increase (Ca++ and Na+ conductances) or decrease (K+ and Cl− conductances) excitability.
Some receptors, most notably the ionotropic receptors, have both receptive and ion conductance functions. Among these, the N-methyl-D-aspartate receptors (NMDARs) figure prominently in the control of neural activity levels in the dorsal cochlear nucleus (DCN) by their role in the induction of spike-timing-dependent plasticity (Stefanescu and Shore, 2015; Shore et al., 2016). Long term potentiation (LTP) and long term depression (LTD) are two manifestations of spike-timing-dependent plasticity in which the synaptic connections between neurons are strengthened or weakened, respectively, by certain patterns of presynaptic stimulation, such as high frequency pulses, especially when combined with depolarization of the postsynaptic membrane. In the case of LTP, the result is a long lasting increase in the strength of synaptic transmission, which is observed as a sustained increase in post-synaptic excitatory currents. This can manifest as an enhanced level of resting activity and/or stimulus-evoked responsiveness. The mechanism of LTP has been reviewed and summarized recently (Henley and Wilkinson, 2016). It is initiated when NMDA receptors are activated by excess release of glutamate from presynaptic terminals. Unlike LTP, LTD is usually induced following low frequency pulses in the presence of postsynaptic membrane depolarization (Sweatt, 2016).
LTP and LTD have been observed in the DCN at the parallel fiber-fusiform cell synapse and the parallel fiber-cartwheel cell synapse when high frequency or low frequency stimuli are paired with depolarization of the postsynaptic membrane (Fujino and Oertel, 2003; Tzounopoulos, 2008). NMDA receptors are present on DCN fusiform and cartwheel cells (Petralia et al., 1994; Watanabe et al., 1994; Sato et al., 1998; Rubio et al., 2014) and are important for the induction of LTP in fusiform cells and LTD in cartwheel cells of the DCN (Manis and Molitor, 1996; Fujino and Oertel., 2003), as both processes can be blocked by NMDA receptor antagonists (Tzounopoulos et al., 2004, 2007; Fujino and Oertel, 2003). LTP has been hypothesized as a possible mechanism underlying induction of hyperactivity associated with tinnitus (Tzounopoulos, 2008; Mazurek et al., 2010), and recent evidence supports this hypothesis (Gao et al., 2011; Cakir et al., 2013). As mediators of LTP in fusiform cells and LTD in cartwheel cells, which are inhibitory to fusiform cells, NMDARs could serve as potential substrates for induction of tinnitus-related hyperactivity following excessive sound exposure, and thus NMDARs could make good therapeutic targets for its prevention (Brozoski et al., 2002; Shore et al., 2008; Finlayson and Kaltenbach, 2009; Manzoor et al, 2012).
Here, we compared the levels of hyperactivity that develop in intense sound exposed animals pretreated with either the NMDAR antagonist MK-801 or with saline. Our hypothesis was that if NMDARs are involved in the induction of hyperactivity through LTP or LTD type mechanisms, then pretreatment with MK-801 should reduce the induction of hyperactivity.
Results
Our results were obtained from the surface of the DCN of 24 animals (10 exposed-MK-801-treated, 10 exposed-saline-treated, and 4 control (unexposed)-saline-treated animals. Complete sets of spontaneous activity recordings were obtained from 150 sites in 10 exposed MK-801-treated animals, 150 sites in 10 exposed saline-treated animals, and 60 sites in 4 control saline treated animals. Frequency response areas were obtained from 9 of the exposed MK-801-treated animals, 9 of the exposed saline-treated animals, and all 4 of the control animals. Both sets of recordings were performed only at one time point at the end of the 26–28 day post-exposure recovery period (see Methods).
Fig. 2 compares neural response thresholds of the exposed-MK-801treated, exposed-saline-treated, and control groups. The data show that neural response thresholds were similarly elevated well above control levels in the two groups of exposed animals as a function of CF. Both exposed groups showed threshold elevations of between 25 and 45 dB above control levels. However, despite the finding that the difference between thresholds in control (unexposed) animals and both groups of exposed animals varied with CF, the thresholds in both groups of exposed animals were almost identical, indicating that MK-801 did not have any protective effect against the decrease in auditory sensitivity following the intense sound exposure.
Fig. 2.
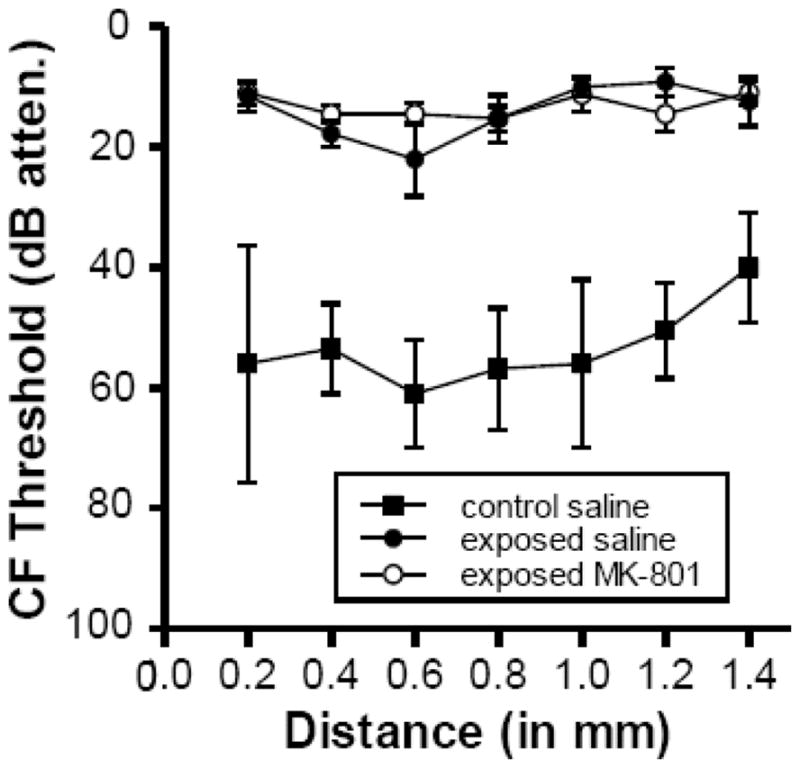
Comparison of neural response thresholds in control and the two groups of tone-exposed animals (MK-801-treated and saline-treated). Each point represents the mean CF threshold + SEM for all tuning curves that were recorded in a bin whose center is indicated on the abscissa. Tuning curve sample sizes ranged from 3–5 for control saline-treated animals (except for locus 0.55, which was based on a sample of 2 tuning curves), 5–8 for exposed saline treated (except for locus 1.4, which was based on a single tuning curve), and 7–12 for exposed MK-801 treated animals (except for loci 0.85 and 1.05, which were based on 4 and 3 tuning curves, respectively). Distance is relative to the lateral edge of the DCN. CF is defined as the frequency at which the response occurred at the lowest intensity.
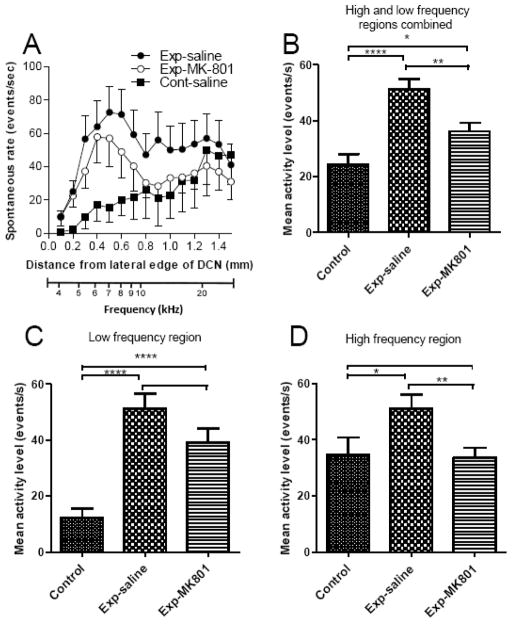
Figure 3A shows the spontaneous activity profiles for the three animal groups. Control animals (i.e., those not exposed to intense sound but treated with saline) showed activity of less than 20 events/s at all sites in the lateral 0.6 mm of the DCN. There was a trend toward higher values from the lateral to the medial direction, which continued into the medial half of the DCN. Except for the three most medial loci, activity remained at or below 35 events/s. The profile of activity in the exposed saline-treated animals was elevated well above that of controls. The activity profile reached a peak in the lateral half of the DCN, and is broader than that which has been observed in previous studies that used higher sound levels during exposure (125–130 dB SPL) (Kaltenbach et al., 2000). When the data were pooled across sites and across animals, this increase was found to be highly significant (p = 0.000001) (Fig. 3B).
Fig. 3.
A. Comparison of spontaneous activity profiles in control and intense-tone-exposed animals. B. Mean spontaneous rates in the three animal groups based on data pooled across all 15 medial-lateral locations and across all animals in each group. C,D. Similar comparisons as in B but for lateral (0.1–0.7 mm, representing frequencies below 10 kHz) and medial (0.8–1.5 mm, representing frequencies above about 10 kHz) halves of the DCN, respectively. Error bars show SEM in each case. Frequency equivalents of the topographic locations are indicated by the bottom scale. Statistical significances of differences are indicated by * p < 0.05, ** p <0.005, *** p < 0.0005, **** p < 0.00005.
As in the saline-treated exposed animals, the activity profile in the exposed animals pre-treated with MK-801 was also significantly increased above control levels (p = 0.016), although the significant increases were only in the lateral half of the DCN (0.1–0.7 mm loci) (p = 0.00002) (Fig. 3C); mean activity in the medial half (0.8–1.5 loci) was not significantly elevated (p = 0.90). Despite the presence of hyperactivity, the average degree of hyperactivity in the MK-801-treated exposed animals was lower than that observed in the saline-treated exposed animals at almost all locations along the medial-lateral axis; when the data from all 15 locations were pooled together across sites and across animals, the decrease in the level of hyperactivity in the MK-801-treated exposed group below that of the saline-treated exposed group was statistically significant (p = 0.0017) (Fig. 3B). The decrease in hyperactivity in the MK-801-treated group below that of the saline-treated group was more pronounced in the high frequency region (0.8–1.5 mm loci), where the decrease was highly significant (p = 0.0045), whereas activity in the low frequency region (0.1–0.7 mm loci) (the region representing frequencies below 10 kHz, the exposure frequency) in the MK-801 group was not as strongly reduced below levels observed in the saline-treated exposed animals (p = 0.10). Thus, although some protection was observed over much of the DCN with MK-801, the focus of this protective effect was in the high frequency region (the region above 10 kHz).
Discussion
Although MK-801 did not protect thresholds against the trauma of sound exposure, the drug did show a partial protective effect on spontaneous activity. The degree of hyperactivity that developed in the DCN following the exposure was significantly lower in the MK-801-treated exposed animals than in the saline-treated exposed animals. The amount by which the activity profile was downshifted in the MK-801-treated-animals was more or less uniform, ranging from 18–20 events/sec below the levels of activity in saline-treated exposed animals at 9/13 sites. However, because the curve for controls trended upward toward the medial direction, the downshifted curve in the MK-801-treated animals was enough to eliminate the differences from the points in the control curve in the medial half of the DCN, corresponding approximately to frequencies above the 10 kHz exposure frequency, normalizing activity in that region. In the lateral half of the DCN, corresponding to frequencies below 10 kHz, the averaged hyperactivity in the MK-801-treated exposed animals was also consistently reduced below that of the exposed animals treated with saline. However, the reduction in this region was not statistically significant, and the curve for the MK-801-treated exposed animals remained significantly higher than the control curve (Fig. 3C). Thus, MK-801 provided only partial protection against the hyperactivity-inducing effects of intense sound exposure.
One explanation for the lower levels of spontaneous activity in exposed animals treated with MK-801 might be that the sound exposure caused less cochlear damage in the MK-801-treated group than in the exposed animals treated with saline. Previous studies have reported excitotoxic injury induced by intense sound exposure in the cochlear hair cells (Puel et al., 1995, 1998; Wang et al., 2002) that can be prevented by injecting NMDAR antagonists, such as ifenprodil intracochlearly (Guitton and Dudai, 2007) or AM-101 intra-tympanically (Bing et al., 2015). Another study found that MK-801 did not prevent increases in auditory brainstem response thresholds after high level noise exposure in guinea pigs, even though it greatly decreased damage to peripheral processes of type I auditory nerve fibers (Jäger et al., 2000). Thus, the finding of similar threshold elevations in the MK-801-treated exposed animals as in the saline-treated exposed animals cannot rule out the possibility that our findings reflect an effect of MK-801 at the cochlear level.
Another explanation for the partial protective effect of MK-801 is that, by blocking NMDA receptors, it prevented induction of LTP in fusiform cells and/or LTD in cartwheel cells. Reducing LTD in cartwheel cells would strengthen their inhibitory effects on fusiform cells. Either change or a combination of both changes would reduce the amplitudes of excitatory postsynaptic currents in fusiform cells, thus limiting the degree to which fusiform cell spontaneous activity would be increased. This explanation would depend on activation of parallel fiber-fusiform cell synapses and/or parallel fiber-cartwheel cell synapses by the intense sound exposure. Since parallel fibers are the axons of granule cells, the intense sound exposure would have to activate inputs to granule cells either directly or indirectly. Most inputs to granule cells are non-auditory (Godfrey et al., 1997), coming from sources such as the cuneate nucleus, trigeminal ganglion and trigeminal nucleus (Shore et al., 2000; Zhou and Shore, 2004; Wright and Ryugo, 1996), the dorsal raphe nucleus and locus coeruleus (Klepper and Herbert, 1991, Thompson et al., 1995; Kromer and Moore, 1980), vestibular nerve and nucleus (Zhao et al., 1990; Barker et al., 2012) and dorsal root ganglion (Zhan et al., 2006). A small percentage of auditory nerve fibers, specifically the type II primary afferents, which originate from outer hair cells (Morgan et al., 1994; Brown and Ledwith, 1990; Berglund et al., 1994; Benson and Brown, 2004), as well as branches of the medial olivocochlear bundle (MOCB) (Brown et al., 1988; Benson and Brown, 1990; Benson et al., 1996) and descending projections from the auditory cortex (Weedman and Ryugo, 1996; Meltzer and Ryugo, 2006), also terminate in the granule cell domain. It is not yet known if high level sound exposure activates corticofugal pathways. In vitro studies of type II primary afferents suggest general insensitivity to low- to moderate-level sound, but likely responses to high intensity sound, although discharge rates of individual fibers are likely to be low (Weisz et al., 2009, 2014). MOCB fibers are more responsive to sound, although their maximal discharge rates in response to tones reach only about 60 spikes/s (Liberman and Brown, 1986; Brown et al., 1998). Summation of converging inputs from these many sources may produce the high frequency stimulus rate in the population of granule cells needed to induce LTP in fusiform cells. On the other hand, only low frequency stimulus rates, such as 1 Hz, are needed to induce LTD in cartwheel cells (Fujino and Oertel, 2003; Tzounopoulos et al., 2004).
A third possible explanation of MK-801’s effects is that it protected against excitotoxic injury that could have resulted from excessive release of glutamate from auditory nerve fibers and/or parallel fibers during the intense sound exposure. Besides excitotoxic injury induced by intense sound exposure in the cochlea, excess glutamate can also be toxic to neurons in the cochlear nucleus (Schweitzer et al., 1991). Intense sound exposure would have increased glutamatergic transmission throughout the auditory system, including the parallel fibers, since activation may also have been increased in the descending inputs to granule cells. The resultant increase in glutamate release from parallel fibers could have been sufficient to cause injury to cartwheel and fusiform cells. However, cartwheel cells might be more susceptible to such injury given their small size and the longer duration of their activation in response to parallel fiber stimulation (Waller et al., 1996; Davis et al., 1996). The induced injury to cartwheel cells could have disinhibited fusiform cells, to produce hyperactivity in exposed-saline-treated animals. Blocking NMDA receptors with MK-801 might have had a protective effect on cartwheel cells, thus reducing the degree of hyperactivity in fusiform cells.
While our results point to an NMDAR-mediated process in the induction of hyperactivity, they do not indicate that NMDA-receptor mediated processes are the only such mechanism. The fact that the blockade of NMDA receptors did not completely abolish the development of hyperactivity after intense sound exposure suggests that other mechanisms are also involved. This is reinforced by previous work in which exposures of animals while under anesthesia with an anesthetic that included another NMDAR antagonist, ketamine, did not prevent induction of hyperactivity (Kaltenbach and McCaslin, 1996; Kaltenbach and Afman, 2000; Kaltenbach et al., 1998, 2000). Lastly, in interpreting the effects of MK-801, it is important to consider its other effects that could influence neural activity as well as other roles of NMDAR activation. Other effects of MK-801 include antagonism of nicotinic acetylcholine receptors (Amador and Dani, 1991; Briggs and McKenna, 1991) and inhibition of serotonin and dopamine transporters (Iravani et al., 1999; Clarke and Reuben, 1995). All of these receptor types, as well as others, may occur in the circuitry of the DCN that is linked to fusiform cells (Godfrey et al., 1997). Thus, any of these ancillary effects of MK-801 could, either directly or indirectly affect synaptic inputs to fusiform cells and thereby sway the balance of their excitatory and inhibitory inputs toward the side that would favor less hyperactivity.
Methods
Animal subjects
Syrian golden hamsters (ages 2–3 months) were divided randomly into three groups, two of 10 each to be exposed in a soundproof room to intense sound and one group of 4 to be presented in a soundproof room with silence. One of the two groups to be exposed was treated with 4 mg/kg MK-801 in 0.9% (isotonic) saline, while the other was treated with 0.9% saline alone and served to control for any effects that might have been caused by fluid injection but not by MK-801. These treatments were administered intraperitoneally 30 minutes prior to exposure. The MK-801 dose was determined empirically to be the highest the animals could tolerate without showing signs of dyskinesia or anesthesia. These two groups of animals were subsequently exposed to an intense 10 kHz tone at a level of 115 dB SPL for a period of 4 hours. This level has previously been shown to be sufficient for the induction of a robust state of hyperactivity in the DCN (Finlayson and Kaltenbach, 2009; Manzoor et al., 2012; Salloum et al., 2014). Animals in a third group, which were subjected to the 4 hour exposure condition but without the sound turned on, were also pre-treated with isotonic saline and served to control for any effects of the exposure procedure that might not have been caused by the intense sound. All procedures used in these experiments were approved by the Institutional Animal Care and Use Committee of Wayne State University School of Medicine.
Sound exposure
The hamsters were exposed in groups of 3 or 4 inside a polycarbonate shoebox cage. Sound was delivered to the cage from a JBL2404H loudspeaker driven by a Harman Kardon 3370 amplifier whose input signal came from an HP3325A function generator. Before the animals were exposed, the sound level was calibrated using an Etymotic ER7C probe tube microphone placed inside the cage at the same height above the floor as the animals’ ears. The sound was turned on, and the amplifier output was adjusted until the level across the different locations within the cage was 115 (+ 6) dB SPL. The microphone was then removed, and the animals were placed back inside the cage. The sound was then turned on, initially at a moderate level (85 dB), then gradually increased in intensity every 2 minutes until the desired exposure level was reached. The tone was maintained continuously for the 4 hour period without interruption. Animals were monitored throughout the exposure period and showed no signs of distress. Control animals were similarly placed in a cage under identical conditions except that no sound was presented from the speaker during the 4 hour period. Following the exposure or control treatment, the animals were returned to the vivarium and retained for a post-exposure recovery period of 26–28 days.
Electrophysiology
After the recovery period, the animals were anesthetized with ketamine/xylazine [85/15 mg/kg] and surgically prepared for electrophysiological studies of neural activity in the DCN. The DCN was exposed by occipital craniotomy followed by removal of a small portion of the posterior cerebellum, medial and caudal to the left paraflocculus. To ensure that the DCN was not mechanically displaced during surgery, the cerebellar peduncle bordering the rostral margin of the DCN was kept completely undisturbed, and aspiration was performed only on cerebellar tissue that had been dissected into loose pieces with sharp forceps. The DCN thus exposed was judged to be healthy and fully functional if its surface had a smooth, shiny enamel-like appearance, with no sign of dilated blood vessels, and no sign that the aspirator contacted or caused drying of the DCN.
Two sets of measures were obtained in each animal: frequency response areas followed by recordings of spontaneous activity. These were collected from the DCN surface in each animal using a single micropipette electrode with an impedance of 0.4 Megohms. For the response areas, recordings were performed at 7 different sites, 200 μm apart along the medial-lateral axis, to sample and define the tonotopic coordinates needed for mapping spontaneous activity. For each locus, we tested the strength of the response to each of 800 frequency-intensity combinations (50 frequencies and 16 intensities) that fill the spectral and intensity stimulus space of DCN neurons. Frequency tuning of neural clusters at each site was apparent as the places within the stimulus space in which vertical tics were elevated above the general background representing spontaneous activity. From the tuning curves, we determined the frequency (characteristic frequency, CF) and threshold of the tip of the response area for each recording site.
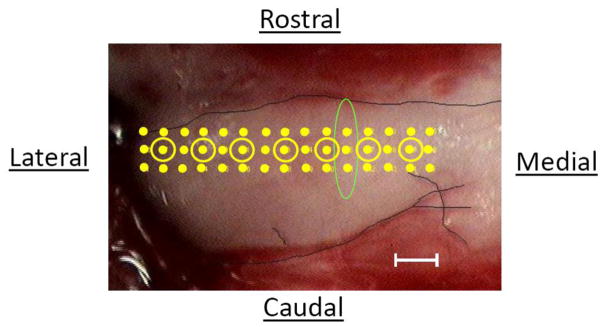
Within a few minutes of completing the measures of frequency tuning, we recorded spontaneous activity from the same row of recording sites from which tuning curves were obtained, except that the recordings were performed in a row of 15 sites spaced 100 μm apart and spanning the tonotopic axis (instead of 7 sites spaced 200 μm apart); spontaneous rates were also recorded in two additional rows, one 100 μm rostral and one 100 μm caudal to the first row (Fig. 1). At each recording site, we counted the total number of voltage events in the multiunit trace that dropped below −100 mV over a 90 second period. The counts summed over the 90 second period were converted to an activity rate, expressed in events/s. Based on previous findings, the tuning curve measurements should not affect the spontaneous activity measurements, since post-stimulus effects in the DCN are generally limited to the first few hundred milliseconds following tone offset (Kaltenbach et al., 1993)
Fig. 1.
Image of the left DCN of one of the animals in the control group, viewed from a dorsal perspective, showing the locations of the different recording sites used to map spontaneous activity. Points are spaced 100 μm apart both horizontally and vertically. Locations at which recordings of spontaneous activity were preceded by recordings of frequency tuning are shown by points surrounded by circles. The outline of the DCN is demarcated by the dark line. The oval highlights 3 points that were averaged to contribute to the point in Fig. 3A for the mean activity at 1.2 mm. Scale bar = 200 μm.
Data analysis
Response areas from the various recording sites were used to generate plots of CF threshold vs distance along the medial-lateral axis of the DCN. Both the exact locations of the recordings and the precise spacing between adjacent recording sites for collecting response areas varied somewhat from animal to animal, and as a result, it was necessary to pool points of approximately similar locations together. To accomplish this, we divided the medial-lateral length of the DCN into 7 bins of approximately equal width. These bins were used to pool response area measures in order to calculate a mean CF threshold for each of the 7 locations.
Spontaneous activity rates were averaged across the three loci recorded at each of the 15 medial-lateral sites (rostral, caudal, and middle) in each animal (Fig. 1). The average rates were then plotted as activity profiles, showing mean spontaneous rate as a function of lateral-to-medial distance along the tonotopic axis of the DCN for each animal. The mean activity profiles were then averaged across animals within each exposure/treatment group. Differences between the curves for each of the three animal groups (exposed MK-801-treated, exposed saline-treated, and unexposed saline-treated controls) were tested statistically using ANOVA followed by post hoc 2-tailed t-tests. Differences were considered significant if p < 0.05.
Neuronal hyperactivity develops in central auditory nuclei after noise exposure and is thought to contribute to the symptoms of tinnitus.
It has been hypothesized that the development of hyperactivity may involve NMDA receptors
This hypothesis was tested by pretreating animals with the NMDA receptor antagonist, MK-801
MK-801 reduced the level of hyperactivity that emerged in the dorsal cochlear nucleus after noise exposure.
This finding suggests that NMDA participate in the induction of hyperactivity after noise exposure.
Acknowledgments
We are grateful to the National Institute of Deafness and Other Communication Disorders (R01-DC009097) for providing the funding that supported this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amador M, Dani JA. MK-801 inhibition of nicotinic acetylcholine receptor channels. Synapse. 1991;7:207–215. doi: 10.1002/syn.890070305. [DOI] [PubMed] [Google Scholar]
- Barker M, Solinski HJ, Hashimoto H, Tagoe T, Pilati N, Hamann M. Acoustic overexposure increases the expression of VGLUT-2 mediated projections from the lateral vestibular nucleus to the dorsal cochlear nucleus. PLoS One. 2012;7(5):e35955. doi: 10.1371/journal.pone.0035955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bing D, Lee SC, Campanelli D, Xiong H, Matsumoto M, Panford-Walsh R, Wolpert S, Praetorius M, Zimmermann U, Chu H, Knipper M, Rüttiger L, Singer W. Cochlear NMDA receptors as a therapeutic target of noise-induced tinnitus. Cell Physiol Biochem. 2015;35:1905–1923. doi: 10.1159/000374000. [DOI] [PubMed] [Google Scholar]
- Benson TE, Brown MC. Postsynaptic targets of type II auditory nerve fibers in the cochlear nucleus. J Assoc Res Otolaryngol. 2004;5:111–125. doi: 10.1007/s10162-003-4012-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson TE, Brown MC. Synapses formed by olivocochlear axon branches in the mouse cochlear nucleus. J Comp Neurol. 1990;295:52–70. doi: 10.1002/cne.902950106. [DOI] [PubMed] [Google Scholar]
- Benson TE, Berglund AM, Brown MC. Synaptic input to cochlear nucleus dendrites that receive medial olivocochlear synapses. J Comp Neurol. 1996;365:27–41. doi: 10.1002/(SICI)1096-9861(19960129)365:1<27::AID-CNE3>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Berglund AM, Brown MC. Central trajectories of type II spiral ganglion cells from various cochlear regions in mice. Hear Res. 1994;75:121–130. doi: 10.1016/0378-5955(94)90063-9. [DOI] [PubMed] [Google Scholar]
- Briggs CA, McKenna DG. Effect of MK-801 at the human alpha 7 nicotinic acetylcholine receptor. Neuropharmacology. 1996;35:407–414. doi: 10.1016/0028-3908(96)00006-8. [DOI] [PubMed] [Google Scholar]
- Brown MC, Berglund AM, Kiang NY, Ryugo DK. Central trajectories of type II spiral ganglion neurons. J Comp Neurol. 1988;278:581–590. doi: 10.1002/cne.902780409. [DOI] [PubMed] [Google Scholar]
- Brown MC, Ledwith JV., 3rd Projections of thin (type-II) and thick (type-I) auditory-nerve fibers into the cochlear nucleus of the mouse. Hear Res. 1990;49:105–118. doi: 10.1016/0378-5955(90)90098-a. [DOI] [PubMed] [Google Scholar]
- Brown MC, Kujawa SG, Liberman MC. Single olivocochlear neurons in the guinea pig. II. Response plasticity due to noise conditioning. J Neurophysiol. 1998;79:3088–3097. doi: 10.1152/jn.1998.79.6.3088. [DOI] [PubMed] [Google Scholar]
- Brozoski TJ, Bauer CA, Caspary DM. Elevated fusiform cell activity in the dorsal cochlear nucleus of chinchillas with psychophysical evidence of tinnitus. J Neurosci. 2002;22:2383–2390. doi: 10.1523/JNEUROSCI.22-06-02383.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Çakır A, Ecevit MC, Bal R, Gürkan S, Alpay HC, Şerbetçioğlu MB. Assessment of Synaptic Plasticity via Long-Term Potentiation in Young Mice on the Day after Acoustic Trauma: Implications for Tinnitus. J Int Adv Otol. 2015;11:196–201. doi: 10.5152/iao.2015.1047. [DOI] [PubMed] [Google Scholar]
- Clarke PB, Reuben M. Inhibition by dizocilpine (MK-801) of striatal dopamine release induced by MPTP and MPP+: possible action at the dopamine transporter. British Journal of Pharmacology. 1995;114:315–322. doi: 10.1111/j.1476-5381.1995.tb13229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis KA, Miller RL, Young ED. Effects of somatosensory and parallel-fiber stimulation on neurons in dorsal cochlear nucleus. J Neurophysiol. 1996;76:3012–3024. doi: 10.1152/jn.1996.76.5.3012. [DOI] [PubMed] [Google Scholar]
- Eggermont JJ, Roberts LE. Tinnitus: animal models and findings in humans. Cell Tissue Res. 2015;361:311–36. doi: 10.1007/s00441-014-1992-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlayson PG, Kaltenbach JA. Alterations in the spontaneous discharge patterns of single units in the dorsal cochlear nucleus following intense sound exposure. Hear Res. 2009;256:104–117. doi: 10.1016/j.heares.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujino K, Oertel D. Bidirectional synaptic plasticity in the cerebellum-like mammalian dorsal cochlear nucleus. Proc Natl Acad Sci U S A. 2003;100:265–270. doi: 10.1073/pnas.0135345100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Manzoor N, Kaltenbach JA. Acute effect of noise exposure on spontaneous activity suggest the existence of activity-dependent plasticity (LTP and LTD) in the DCN, in vivo. ARO abstract 2012 [Google Scholar]
- Godfrey DA, Godfrey TG, Mikesell NL, Waller HJ, Yao W, Chen K, Kaltenbach JA. Chemistry of granular and closely related region of the cochlear nucleus. In: Syka J, editor. Acoustic Signal Processing in the Central Auditory System. Plenum Press; New York: 1997. [Google Scholar]
- Guitton MJ, Dudai Y. Blockade of cochlear NMDA receptors prevents long-term tinnitus during a brief consolidation window after acoustic trauma. Neural Plast. 2007;2007:80904. doi: 10.1155/2007/80904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henley JM, Wilkinson KA. Synaptic AMPA receptor composition in development, plasticity and disease. Nat Rev Neurosci. 2016 Apr 15; doi: 10.1038/nrn.2016.37. [DOI] [PubMed] [Google Scholar]
- Holt AG, Asako M, Duncan RK, Lomax CA, Juiz JM, Altschuler RA. Deafness associated changes in expression of two-pore domain potassium channels in the rat cochlear nucleus. Hear Res. 2006;216–217:146–53. doi: 10.1016/j.heares.2006.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iravani MM, Muscat R, Kruk ZL. MK-801 interaction with the 5-HT transporter: a real-time study in brain slices using fast cyclic voltammetry. Synapse. 1999;32:212–224. doi: 10.1002/(SICI)1098-2396(19990601)32:3<212::AID-SYN7>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Kalappa BI, Brozoski TJ, Turner JG, Caspary DM. Single unit hyperactivity and bursting in the auditory thalamus of awake rats directly correlates with behavioural evidence of tinnitus. J Physiol. 2014;592:5065–5078. doi: 10.1113/jphysiol.2014.278572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaltenbach JA. Tinnitus: Models and mechanisms. Hear Res. 2011;276:52–60. doi: 10.1016/j.heares.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaltenbach JA, McCaslin DL. Increases in Spontaneous Activity in the Dorsal Cochlear Nucleus Following Exposure to High Intensity Sound: A Possible Neural Correlate of Tinnitus. Aud Neurosci. 1996;3:57–78. [PMC free article] [PubMed] [Google Scholar]
- Kaltenbach JA, Afman CE. Hyperactivity in the dorsal cochlear nucleus after intense sound exposure and its resemblance to tone-evoked activity: a physiological model for tinnitus. Hear Res. 2000;140:165–172. doi: 10.1016/s0378-5955(99)00197-5. [DOI] [PubMed] [Google Scholar]
- Kaltenbach JA, Meleca RJ, Falzarano PR, Myers SF, Simpson TH. Forward masking properties of neurons in the dorsal cochlear nucleus: possible role in the process of echo suppression. Hear Res. 1993;67:35–44. doi: 10.1016/0378-5955(93)90229-t. [DOI] [PubMed] [Google Scholar]
- Kaltenbach JA, Zhang J, Afman CE. Plasticity of spontaneous neural activity in the dorsal cochlear nucleus after intense sound exposure. Hear Res. 2000;147:282–292. doi: 10.1016/s0378-5955(00)00138-6. [DOI] [PubMed] [Google Scholar]
- Klepper A, Herbert H. Distribution and origin of noradrenergic and serotonergic fibers in the cochlear nucleus and inferior colliculus of the rat. Brain Res. 1991;557:190–201. doi: 10.1016/0006-8993(91)90134-h. [DOI] [PubMed] [Google Scholar]
- Kromer LF, Moore RY. Norepinephrine innervation of the cochlear nuclei by locus coeruleus neurons in the rat. Anat Embryol (Berl) 1980;158:227–244. doi: 10.1007/BF00315908. [DOI] [PubMed] [Google Scholar]
- Li S, Choi V, Tzounopoulos T. Pathogenic plasticity of Kv7.2/3 channel activity is essential for the induction of tinnitus. Proc Natl Acad Sci U S A. 2013;110:9980–9985. doi: 10.1073/pnas.1302770110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Kalappa BI, Tzounopoulos T. Noise-induced plasticity of KCNQ2/3 and HCN channels underlies vulnerability and resilience to tinnitus. Elife. 2015;27:4. doi: 10.7554/eLife.07242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman MC, Brown MC. Physiology and anatomy of single olivocochlear neurons in the cat. Hear Res. 1986;24:17–36. doi: 10.1016/0378-5955(86)90003-1. [DOI] [PubMed] [Google Scholar]
- Manis PB, Molitor SC. N-methyl-D-aspartate receptors at parallel fiber synapses in the dorsal cochlear nucleus. J Neurophysiol. 1996;76:1639–1656. doi: 10.1152/jn.1996.76.3.1639. [DOI] [PubMed] [Google Scholar]
- Manzoor NF, Licari FG, Klapchar M, Elkin RL, Gao Y, Chen G, Kaltenbach JA. Noise-induced hyperactivity in the inferior colliculus: its relationship with hyperactivity in the dorsal cochlear nucleus. J Neurophysiol. 2012;108:976–988. doi: 10.1152/jn.00833.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazurek B, Olze H, Haupt H, Klapp BF, Adli M, Gross J, Szczepek AJ. Molecular biological aspects of neuroplasticity: approaches for treating tinnitus and hearing disorders. HNO. 2010;58:973–982. doi: 10.1007/s00106-010-2177-8. [DOI] [PubMed] [Google Scholar]
- Meltzer NE, Ryugo DK. Projections from auditory cortex to cochlear nucleus: A comparative analysis of rat and mouse. Anat Rec A Discov Mol Cell Evol Biol. 2006;288:397–408. doi: 10.1002/ar.a.20300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton JW, Kiritani T, Pedersen C, Turner JG, Shepherd GM, Tzounopoulos T. Mice with behavioral evidence of tinnitus exhibit dorsal cochlear nucleus hyperactivity because of decreased GABAergic inhibition. Proc Natl Acad Sci U S A. 2011;108:7601–7606. doi: 10.1073/pnas.1100223108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morest DK, Kim J, Bohne BA. Neuronal and transneuronal degeneration of auditory axons in the brainstem after cochlear lesions in the chinchilla: cochleotopic and non-cochleotopic patterns. Hear Res. 1997;103:151–168. doi: 10.1016/s0378-5955(96)00172-4. [DOI] [PubMed] [Google Scholar]
- Morgan YV, Ryugo DK, Brown MC. Central trajectories of type II (thin) fibers of the auditory nerve in cats. Hear Res. 1994;79:74–82. doi: 10.1016/0378-5955(94)90128-7. [DOI] [PubMed] [Google Scholar]
- Petralia RS, Yokotani N, Wenthold RJ. Light and electron microscope distribution of the NMDA receptor subunit NMDAR1 in the rat nervous system using a selective anti-peptide antibody. J Neurosci. 1994;14:667–696. doi: 10.1523/JNEUROSCI.14-02-00667.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puel JL, Pujol R, Tribillac F, Ladrech S, Eybalin M. Excitatory amino acid antagonists protect cochlear auditory neurons from excitotoxicity. J Comp Neurol. 1994;341:241–256. doi: 10.1002/cne.903410209. [DOI] [PubMed] [Google Scholar]
- Puel JL, Saffiedine S, Gervais d’Aldin C, Eybalin M, Pujol R. Synaptic regeneration and functional recovery after excitotoxic injury in the guinea pig cochlea. C R Acad Sci III. 1995;318:67–75. [PubMed] [Google Scholar]
- Puel JL, Ruel J, Gervais d’Aldin C, Pujol R. Excitotoxicity and repair of cochlear synapses after noise-trauma induced hearing loss. Neuroreport. 1998;9:2109–2114. doi: 10.1097/00001756-199806220-00037. [DOI] [PubMed] [Google Scholar]
- Roberts LE, Eggermont JJ, Caspary DM, Shore SE, Melcher JR, Kaltenbach JA. Ringing ears: the neuroscience of tinnitus. J Neurosci. 2010;30:14972–9. doi: 10.1523/JNEUROSCI.4028-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio ME, Fukazawa Y, Kamasawa N, Clarkson C, Molnár E, Shigemoto R. Target- and input-dependent organization of AMPA and NMDA receptors in synaptic connections of the cochlear nucleus. J Comp Neurol. 2014;522:4023–4042. doi: 10.1002/cne.23654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvi RJ, Saunders SS, Gratton MA, Arehole S, Powers N. Enhanced evoked response amplitudes in the inferior colliculus of the chinchilla following acoustic trauma. Hear Res. 1990;50:245–257. doi: 10.1016/0378-5955(90)90049-u. [DOI] [PubMed] [Google Scholar]
- Sato K, Kuriyama H, Altschuler RA. Differential distribution of NMDA receptor subunit mRNA in the rat cochlear nucleus. Microsc Res Tech. 1998;41:217–223. doi: 10.1002/(SICI)1097-0029(19980501)41:3<217::AID-JEMT5>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Schweitzer L, Jensen KF, Janssen R. Glutamate neurotoxicity in rat auditory system: cochlear nuclear complex. Neurotoxicol Teratol. 1991;13:189–193. doi: 10.1016/0892-0362(91)90010-t. [DOI] [PubMed] [Google Scholar]
- Shore SE, Vass Z, Wys NL, Altschuler RA. Trigeminal ganglion innervates the auditory brainstem. J Comp Neurol. 2000;419:271–285. doi: 10.1002/(sici)1096-9861(20000410)419:3<271::aid-cne1>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Shore SE, Koehler S, Oldakowski M, Hughes LF, Syed S. Dorsal cochlear nucleus responses to somatosensory stimulation are enhanced after noise-induced hearing loss. Eur J Neurosci. 2008;27:155–168. doi: 10.1111/j.1460-9568.2007.05983.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore SE, Roberts LE, Langguth B. Maladaptive plasticity in tinnitus - triggers, mechanisms and treatment. Nat Rev Neurol. 2016;12:150–60. doi: 10.1038/nrneurol.2016.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore SE, Roberts LE, Langguth B. Maladaptive plasticity in tinnitus - triggers, mechanisms and treatment. Nat Rev Neurol. 2016;12:150–160. doi: 10.1038/nrneurol.2016.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanescu RA, Shore SE. NMDA Receptors Mediate Stimulus-Timing-Dependent Plasticity and Neural Synchrony in the Dorsal Cochlear Nucleus. Front Neural Circuits. 2015;9:75. doi: 10.3389/fncir.2015.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweatt JD. Neural plasticity and behavior – sixty years of conceptual advances. J Neurochem. 2016;139:179–199. doi: 10.1111/jnc.13580. [DOI] [PubMed] [Google Scholar]
- Thompson AM, Moore KR, Thompson GC. Distribution and origin of serotoninergic afferents to guinea pig cochlear nucleus. J Comp Neurol. 1995;351:104–116. doi: 10.1002/cne.903510110. [DOI] [PubMed] [Google Scholar]
- Tzounopoulos T. Mechanisms of synaptic plasticity in the dorsal cochlear nucleus: plasticity-induced changes that could underlie tinnitus. Am J Audiol. 2008;17:S170–75. doi: 10.1044/1059-0889(2008/07-0030). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzounopoulos T, Kim Y, Oertel D, Trussell LO. Cell-specific, spike timing-dependent plasticities in the dorsal cochlear nucleus. Nat Neurosci. 2004;7:719–725. doi: 10.1038/nn1272. [DOI] [PubMed] [Google Scholar]
- Tzounopoulos T, Rubio ME, Keen JE, Trussell LO. Coactivation of pre- and postsynaptic signaling mechanisms determines cell-specific spike-timing-dependent plasticity. Neuron. 2007;54:291–301. doi: 10.1016/j.neuron.2007.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waller HJ, Godfrey DA, Chen K. Effects of parallel fiber stimulation on neurons of rat dorsal cochlear nucleus. Hear Res. 1998;98:169–179. doi: 10.1016/0378-5955(96)00090-1. [DOI] [PubMed] [Google Scholar]
- Wang H, Brozoski TJ, Turner JG, Ling L, Parrish JL, Hughes LF, Caspary DM. Plasticity at glycinergic synapses in dorsal cochlear nucleus of rats with behavioral evidence of tinnitus. Neuroscience. 2009;164:747–759. doi: 10.1016/j.neuroscience.2009.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M, Mishina M, Inoue Y. Distinct distributions of five NMDA receptor channel subunit mRNAs in the brainstem. J Comp Neurol. 1994;343:520–531. doi: 10.1002/cne.903430403. [DOI] [PubMed] [Google Scholar]
- Weedman DL, Ryugo DK. Projections from auditory cortex to the cochlear nucleus in rats: synapses on granule cell dendrites. J Comp Neurol. 1996;371:311–324. doi: 10.1002/(SICI)1096-9861(19960722)371:2<311::AID-CNE10>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Weisz C, Glowatzki E, Fuchs P. The postsynaptic function of type II cochlear afferents. Nature. 2009;461:1126–1129. doi: 10.1038/nature08487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisz CJ, Glowatzki E, Fuchs PA. Excitability of type II cochlear afferents. J Neurosci. 2014;34:2365–2373. doi: 10.1523/JNEUROSCI.3428-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willott JF, Lu SM. Noise-induced hearing loss can alter neural coding and increase excitability in the central nervous system. Science. 1982;216:1331–1334. doi: 10.1126/science.7079767. [DOI] [PubMed] [Google Scholar]
- Wright DD, Ryugo DK. Mossy fiber projections from the cuneate nucleus to the cochlear nucleus in the rat. J Comp Neurol. 1996;365:159–172. doi: 10.1002/(SICI)1096-9861(19960129)365:1<159::AID-CNE12>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Zhan X, Pongstaporn T, Ryugo DK. Projections of the second cervical dorsal root ganglion to the cochlear nucleus in rats. J Comp Neurol. 2006;496:335–348. doi: 10.1002/cne.20917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JS, Kaltenbach JA. Increases in spontaneous activity in the dorsal cochlear nucleus of the rat following exposure to high-intensity sound. Neurosci Lett. 1998;250:197–200. doi: 10.1016/s0304-3940(98)00482-0. [DOI] [PubMed] [Google Scholar]
- Zhou J, Shore S. Projections from the trigeminal nuclear complex to the cochlear nuclei: a retrograde and anterograde tracing study in the guinea pig. J Neurosci Res. 2004;78:901–907. doi: 10.1002/jnr.20343. [DOI] [PubMed] [Google Scholar]
- Zhao HB, Parham K, Ghoshal S, Kim DO. Small neurons in the vestibular nerve root project to the marginal shell of the anteroventral cochlear nucleus in the cat. Brain Res. 1995;700:295–298. doi: 10.1016/0006-8993(95)01078-a. [DOI] [PubMed] [Google Scholar]