Abstract
Animal and human studies suggest an association between depression and aberrant immune response. Further, common inflammatory markers may change during the course of antidepressant treatment in patients. The objective of this study was to evaluate changes in inflammatory markers and clinical outcomes from subjects enrolled in the Combination of Medications to Enhance Depression Outcome (CO-MED) trial. At baseline and week 12 (treatment completion), plasma samples of 102 participants were analyzed via a multiplex assay comprised of inflammatory markers using a 27-plex standard assay panel plus a 4-plex human acute phase xMAP technology based platform. We carried out analyses in two steps. First, t-tests were used to identify inflammatory marker levels that changed between baseline and week 12. For markers that were altered, logistic regression models were then conducted to look for associated changes in remission at week 12. Among the 31 inflammatory markers analyzed, several cytokines (IL-5, IFN-γ, IL-13), two chemokines (Eotaxin-1/CCL11, RANTES) and an acute-phase reactant (serum amyloid P component) showed change from baseline to week 12. However, only two indicated differential remission responses. Interestingly, increased levels of Eotaxin-1/CCL11 correlated with remission at week 12, whereas decreased levels of IFN-γ correlated with non-remission at week 12. Results suggest that these inflammatory proteins may serve as predictors of treatment response.
Keywords: Antidepressants, Cytokines, COMED, Eotaxin-1/CCL11, IFN-γ, Multiplex
1. Introduction
A state of sub-threshold systemic inflammation has been implicated in the pathophysiology of Major Depressive Disorder (MDD) (Dantzer et al., 2008; Felger and Lotrich, 2013), while few studies show specific changes in inflammatory biomarkers with antidepressant treatment. Several reports demonstrate that MDD patients, as compared to healthy controls, have elevated levels of inflammatory cytokines during depressive episodes, including interleukin (IL) -1, IL-6, IL-8, IL-10, interferon-gamma (IFN-γ) and tumor necrosis factor-alpha (TNFα) (Kenis and Maes, 2002; Maes et al., 1999; Munzer et al., 2013). The association of MDD with inflammation is also supported by the fact that IFN treatment of hepatitis C and certain types of cancers frequently induces depressive symptoms (Hauser et al., 2002). Grassi-Oliveira and colleagues have also reported that levels of certain chemokines (monocyte chemoattractant protein-1 [MCP-1/CCL-2] and RANTES/CCL5) are lower in serum samples from adults with MDD and suicidal ideation as compared to controls, while higher levels of Eotaxin-1/CCL-11 (further referred to as Eotaxin-1) are seen in those with MDD and suicidal ideation (Grassi-Oliveira et al., 2012), although neither study assessed changes with treatment.
Inflammatory markers have also been examined as predictors and moderators of treatment response in depression. Brunoni and colleagues showed that IL-2, IL-4, and IL-17 (but not TNFα) decreased over 6 weeks of treatment with transcranial direct current stimulation (tDCS) and/or sertraline in MDD patients, independent of treatment response (Brunoni et al., 2014). Similarly, tricyclic antidepressants (TCAs) have been shown to decrease IFN-γ, IL-17, IL-22 (Himmerich et al., 2010a; Himmerich et al., 2010b). In the Genome-Based Therapeutic Drugs for Depression (GENDEP) study, baseline C-reactive protein (CRP), a biomarker for systemic inflammation, predicted differential response to two different treatments. Specifically, lower levels of CRP were associated with improved response with escitalopram, while higher levels of CRP were associated with improved response to nortriptyline (Uher et al., 2014). Similar results were seen in the Combining Medications to Enhance Depression Outcomes (CO-MED) trial, in which showed that higher baseline CRP levels were associated to better responsiveness to bupropion-SSRI combination, as compared to SSRI monotherapy (Jha et al., 2017). Further, combinations of antidepressants and anti-inflammatory drugs like fluoxetine, a selective serotonin reuptake inhibitor (SSRI), and celecoxib (Cyclooxygenase-2, COX-2 inhibitor) have also been associated with a greater anti-depressant effect than fluoxetine (Akhondzadeh et al., 2009).
While recent attention has focused on a systemic inflammatory state, there is evidence that some depressed participants have a suppressed immune response (Maes, 1995). Pertinent to our study, cytokines have been analyzed for depression but have not been measured concurrently with acute-phase reactants that serve as markers for a systemic inflammatory state. Recent advances in non-invasive multiplex proteomic xMAP technology platform can be utilized to quantitatively measure multiple (rather than single) inflammatory markers that may be associated with treatment outcomes (Bot et al., 2015; Stelzhammer et al., 2014).
Here, we used an unbiased and non-targeted exploratory approach to measure baseline to exit levels of a variety of inflammatory markers, including cytokines, chemokines, growth factors and acute-phase reactants in plasma samples from participants of the Combining Medications to Enhance Depression Outcomes (CO-MED) trial. CO-MED compared three treatment groups: monotherapy with selective serotonin reuptake inhibitor (SSRI) (escitalopram plus placebo) versus two combination therapies: bupropion plus escitalopram (bupropion-SSRI) and venlafaxine plus mirtazapine (venlafaxine-mirtazapine) (Rush et al., 2011). As the three treatment arms did not differ in rates of clinical improvement solely based on symptoms during the CO-MED trial, we combined the three treatment arms for this analysis. Considering the strong link between inflammation and depression and its effects on response to antidepressant treatment, we postulated that antidepressant medications may not only act by inhibiting the reuptake of serotonergic or norepinephrine neurotransmitters, but also by altering the inflammatory response as reflected by change in the levels of inflammatory biomarkers.
2. Methods
2.1. Study Overview
The CO-MED trial is a single-blind, randomized, placebo-controlled trial with 665 participants for first-step MDD treatment, including an acute (12 weeks) and long-term continuation (additional 4 months) phase. Randomization was stratified by clinical sites and participants were assigned to one of the three treatment group (SSRI monotherapy, bupropion-SSRI combination, and venlafaxine-mirtazapine combination) in a 1:1:1 ratio. Study visits were conducted at baseline and weeks 1, 2, 4, 6, 8, 10, 12 for the acute phase. At each visit, study physicians used measurement based care (MBC) for dosage adjustments based on the scores of Quick Inventory of Depressive Symptoms-Clinician Rated scale (QIDS-C) and Frequency, Intensity, and Burden of Side Effects Rating (FIBSER) scale. The QIDS-C items were extracted from Inventory of Depressive Symptomatology – Clinician-Rated (IDS-C) scale. Clinical outcomes were determined by the Quick Inventory of Depressive Symptoms Self-Report version (QIDS-SR). Plasma was collected at baseline at week 12 (exit) in a subset of patients.
2.2. Study participants
Broad inclusion and minimal exclusion criteria were used to ensure reasonably representative subject groups. Depressed outpatients seeking treatment at participating clinical sites and planning to continue living in the area of that clinical site for the duration of the study were eligible to enroll in the study. These sites included both primary and psychiatric care clinics, and were selected to ensure adequate minority representation. To be included in the study, participants had to meet clinical criteria for nonpsychotic, recurrent (greater than 1 previous episode) or chronic (current episode greater than 2 years) MDD as defined by a clinical interview and confirmed by the MINI International Neuropsychiatric Interview (MINI). Participants had to have at least 2 months duration of the current depressive episode and score 16 or greater on the 17-item Hamilton Rating Scale. Exclusion criteria for this clinical trial are fully listed on the clinicaltrials.gov website (https://clinicaltrials.gov/ct2/show/NCT00590863).
These studies were carried out in accordance with the latest version of the Declaration of Helsinki. The Institutional Review Boards at UT Southwestern Medical Center at Dallas, the University of Pittsburgh Data Coordinating Center, each participating regional center, and all relevant clinical sites reviewed and approved the study protocol, all consent documents, and study procedures. All participants were informed of the study in detail, including risks and benefits, and provided written informed consent prior to completing any study procedures. An independent data safety and monitoring board also monitored the study. Further details of CO-MED trial have been described by Rush et al. (2011).
A subgroup of 102 participants (out of the total 665 enrolled in the CO-MED trial) provided plasma samples at both baseline and week 12 and were used for these analyses.
Antidepressant medications
Participants in all three treatment groups received two types of pills. The first medication was known to both participants and study personnel, while the second medication was known only to study personnel. All dose adjustments were based on clinical response and tolerability according to the principles of MBC (Trivedi et al., 2006).
Participants in SSRI monotherapy treatment group were started on 10 mg/day dose of escitalopram with the option to increase the dose to 20 mg/day at week 4 visit or later if QIDS-C score was greater than 5. Pill placebo was added at week 2 in single-blind fashion with the option to increase the dose at week 4 visit or later if QIDS-C score was greater than 5. At the end of 12 weeks, mean escitalopram dose was 17.6 mg/day and mean placebo dose was 1.4 pills/day. Participants in Bupropion-SSRI treatment group were started on sustained release (SR) bupropion 150 mg/day and the dose was increased to 300 mg/day at week 1 visit. At week 2, escitalopram 10 mg/day was started in single-blind fashion. At week 4 visit, bupropion SR was increased to 400 mg/day and/or escitalopram was increased to 20 mg/day if QIDS-C score was greater than 5. For visits at week 6 and later, doses could be increased to 400 mg/day of bupropion SR and 20 mg/day of escitalopram if QIDS-C score was greater than 5. At the end of 12 weeks, mean bupropion SR dose was 324.0 mg/day and mean escitalopram dose was 14.0 mg/day. Participants in venlafaxine-mirtazapine combination treatment group were started on extended release (XR) venlafaxine 37.5 mg/day for 3 days and then increased to 75 mg/day. At week 1 visit, venlafaxine XR was increased to 150 mg/day. At week 2 visit, if the score on QIDS-C was greater than 5 then mirtazapine 15 mg/day was added in single-blind fashion. At week 4 visit, if QIDS-C was greater than 5 then venlafaxine XR dose was increased to 225 mg/day and/or mirtazapine was increased to 30 mg/day. At week 6, if QIDS-C was greater than 5 then mirtazapine could be raised to 45 mg/day. At week 8, if QIDS was greater than 5 then venlafaxine XR could be raised to 300 mg/day. At the end of 12 weeks, mean venlafaxine XR dose was 207.6 mg/day and mean mirtazapine dose was 25.3 mg/day.
2.3. Research Outcomes
At baseline, participants provided sociodemographic information. At baseline and all treatment visits, participants completed the 16-item Quick Inventory of Depressive Symptomatology – Self-Report (QIDS-SR) scale, and clinicians administered the Inventory of Depression Symptomatology – Clinician Rating (IDS-C). The primary outcome in the CO-MED trial was symptomatic remission based on the last two consecutive measurements of QIDS-SR obtained during the 12-week acute phase trial. Back-to-back measurements were taken into account to ensure that a single “good week” did not falsely identify remission. A participant was considered to be in remission at week 12 if the QIDS-SR total score at week 12 and the QIDS-SR total score at the last available visit before week 12 was 8 or less at one visit and 6 or less at the other visit. Those who exited before having two post-baseline measures were considered not remitted.
2.3.1 Quick Inventory of Depressive Symptomatology self-report (QIDS-SR16)
Of the 16 items (each item has 4 choices which are scored from 0–3) in QIDS-SR, nine items are used to calculate the total score which can range from 0–27. The Pearson moment correlations between QIDS-SR and HRSD-17 has been previously reported as 0.86 (Rush et al., 2003). The Cronbach’s α of QIDS-SR ranged from 0.86–0.87 in previous reports (Rush et al., 2003; Trivedi et al., 2004).
2.4. Blood Processing and Multiplex Inflammatory Markers Profiling
At a single site, peripheral venous samples were collected into EDTA tubes (purple top) from participants at baseline and week 12. Samples were shipped to the Biologic Core of the National Institute of Mental Health Repository and Genomics Resource (NIMH RGR) (RUCDR Infinite Biologics, Piscataway, NJ). Following receipt, blood was centrifuged at 2500 rpm for 10 minutes at room temperature. Extracted plasma was aliquoted (500 μl each) and stored at −80° C. When plasma was requested for experimental evaluation, samples were shipped from the NIMH RGR on dry ice. Thus, there were no freeze/thaw cycles. Quantification of inflammatory biomarkers was performed by the Microarray Core at UT Southwestern Medical Center. All samples were run simultaneously, and lab personnel were blinded to treatment allocation and outcomes. All available plasma samples at baseline (pre-treatment) and exit (post-treatment) were subjected to inflammatory marker profiling using multiplex immunoassay. As compared to other technologies, the multiplexed immunoassay profiling approach may help reduce experimental variability due to its standardized protocols, thereby making it an ideal platform for reliably and accurately identifying inflammatory factors in an unbiased manner. The concentrations of 31 inflammatory markers in baseline and week 12 plasma samples were analyzed by quantitative multiplex assays using the Bioplex Pro™ human cytokine standard 27-plex and 4-plex human acute phase, both based on xMAP technology (Bio-Rad Laboratory, Hercules, CA, USA) according to the pre-optimized protocol provided by the manufacturer. The list of the names of the inflammatory markers estimated in the Bio-Rad multiplex microbead assays and their assay characteristics are available at http://www.bio-rad.com/webroot/web/pdf/lsr/literature/Bulletin_6335.pdf. Data were collected and analyzed using a Bio-Rad Bioplex Magpix 200 instrument equipped with Bioplex Manager software version 6.0 (Bio-Rad Laboratory, Hercules, CA, USA). The precision based on both intra and inter-assays variations was <10%, within the detection limits provided by the manufacturer. The plasma samples and standards from the kits were diluted based on the manufacturer’s manual. The immunoassay data were expressed in terms of pg/ml (27 plex, after 1:4 dilution) and ng/ml (4-plex, after 1:10,000 fold dilution) protein estimated from Bradford protein concentration in respective plasma samples using the standards provided in the kit (Bio-Rad Laboratory, Hercules, CA, USA) and human serum albumin (Sigma Chemical, St. Louis, MO, USA) according to the procedure provided by the manufacturer (http://www.bio-rad.com/webroot/web/pdf/lsr/literature/4110065A.pdf).
2.5. Statistical Analysis
All 31 inflammatory markers, which included acute-phase reactants, cytokines, chemokines, and growth factors were quantified from participants’ plasma collected at baseline and week 12. After excluding markers with >30% of the values falling outside the detection threshold, 22 out of 31 markers remained for further analysis. Analyses of these 22 inflammatory markers were conducted using a two-step process (Bot et al., 2015). The first step was to determine if there was a change in the inflammatory marker levels between the baseline and week 12 using a two-tailed paired t-test comparing baseline to week 12 marker levels, setting an alpha at p < 0.10 (Saville, 1990) with no correction for multiple comparisons (Rothman, 1990). The second step was designed to determine if inflammatory markers were related to treatment outcome and included only those markers with p < 0.10 from the first step. Logistic regression models included remission at week 12 as the dependent variable and change in the inflammatory markers from baseline to week 12 as a main effect, a treatment group main effect, and interactions between change in the markers and treatment group. As the treatment groups did not differ in the outcome variables as previously reported (Rush et al., 2011), we did not expect a significant treatment group-by-marker interaction, although we included this interaction for confirmatory purposes. Additional covariates included baseline severity, gender, age, race, number of other medical conditions, and NSAID use. All covariates were included in the initial logistic regression model to identify change from baseline to week 12, but only significant covariates were retained for the final reported analyses.
3. Results
3.1. Demographic and Clinical Characteristics of the Study Participants
Demographic and clinical characteristics for the clinical trial (n=665) have been previously published (Rush et al., 2011). Among the 102 participants who provided baseline and week 12 blood, 72.5% (n=74) were female, the mean age was 42.4 ± 10.7 years, and the average BMI was 33.11 ± 9.04 kg/m2. 66.7% (n=68) used NSAIDs, and of these individuals, the percentage in each treatment arm was: 36.7% (n=25) in Bupropion/Escitalopram; 32.3% (n=22) in Escitalopram/Placebo; and 30.9% (n=21) in Venlafaxine/Mirtazapine treatment groups. The clinical characteristics of study participants across the three treatment groups are shown in Table 1.
Table 1.
Sociodemographic and clinical characteristics of depressed patients by treatment group.
| Baseline Variable | All | All | Bupropion/Escitalopram | Escitalopram/Placebo | Venlafaxine/Mirtazapine | |||
|---|---|---|---|---|---|---|---|---|
| N=102 | % | N | % | N | % | N | % | |
|
| ||||||||
| Gender | ||||||||
| Female | 74 | 72.5 | 27 | 75.0 | 22 | 75.9 | 25 | 67.6 |
|
|
||||||||
| Male | 28 | 27.5 | 9 | 25.0 | 7 | 24.1 | 12 | 32.4 |
|
| ||||||||
| Race | ||||||||
| White | 73 | 71.6 | 28 | 77.8 | 18 | 62.1 | 27 | 73.0 |
|
|
||||||||
| Black | 19 | 18.6 | 6 | 16.7 | 6 | 20.7 | 7 | 18.9 |
|
| ||||||||
| Other | 10 | 9.8 | 2 | 5.6 | 5 | 17.2 | 3 | 8.1 |
|
| ||||||||
| Ethnicity | ||||||||
| Hispanic | 21 | 20.6 | 7 | 19.4 | 5 | 17.2 | 9 | 24.3 |
|
| ||||||||
| Employed | 46 | 45.1 | 16 | 44.4 | 8 | 27.6 | 22 | 59.5 |
|
| ||||||||
| NSAID User | 68 | 66.7 | 25 | 69.4 | 22 | 75.9 | 21 | 56.8 |
|
| ||||||||
| Mean | STD | Mean | STD | Mean | STD | Mean | STD | |
|
| ||||||||
| Age (yrs) | 46.42 | 10.7 | 48.11 | 10.3 | 49.38 | 10.2 | 42.46 | 10.5 |
|
| ||||||||
| BMI (Kg/m2) | 33.11 | 9.0 | 33.73 | 6.9 | 33.10 | 11.6 | 32.52 | 8.8 |
|
| ||||||||
| Education (yrs) | 13.72 | 2.8 | 13.44 | 2.6 | 14.41 | 3.6 | 13.43 | 2.2 |
|
| ||||||||
| Income | 2429.68 | 2351.6 | 2302.63 | 2214.6 | 2934.00 | 3205.0 | 2171.54 | 1636.3 |
|
| ||||||||
| HRS-D | 23.71 | 4.8 | 23.14 | 4.1 | 23.86 | 5.5 | 24.14 | 4.8 |
|
| ||||||||
| IDS-C | 37.75 | 9.0 | 36.19 | 8.5 | 37.69 | 9.5 | 39.32 | 8.9 |
|
| ||||||||
| QIDS-C | 15.75 | 3.7 | 14.86 | 3.7 | 16.14 | 3.5 | 16.30 | 3.7 |
|
| ||||||||
| QIDS-SR | 14.90 | 4.1 | 13.86 | 4.7 | 15.17 | 3.5 | 15.70 | 3.9 |
|
| ||||||||
| Number GMCs | 2.08 | 1.3 | 1.92 | 1.5 | 2.14 | 1.4 | 2.19 | 1.2 |
3.2. Changes in levels of inflammatory markers over the 12 weeks of acute phase treatment
The first analysis screened 31 inflammatory cytokines, chemokines, and growth factors for change from baseline to week 12. Nine of the markers had 30% or more values which fell under the lower limit of detection (LLD) and thus were excluded from analysis (IL-15, IL-7, IL-2, GM-CSF, Haptoglobin, VEGF, MCP-1, IL-12, and IL-10). The mean level and standard deviation of the remaining 22 inflammatory markers at baseline, week 12, and change from baseline to week 12 are presented in Table 2. Six markers showed a significant difference (at p < .10) between baseline and week 12 levels: Serum Amyloid P component (t(101)= −5.77, p<0.0001), IL-5 (t(101)= −4.07 p<0.0001), IFN-γ (t(101)=−2.31, p=0.03), Eotaxin-1 (t(101)= 2.02, p < 0.05), RANTES (t(96)= −1.95, p <0.06) and IL-13 (t(101)=−1.71, p<0.10). These six inflammatory markers were then tested as predictors of treatment outcome at week 12.
Table 2.
Inflammatory Marker Levels Pre- and Post-Treatment 2. Inflammatory plasma concentrations at baseline and week 12.
| Baseline | Week 12 | ||||
|---|---|---|---|---|---|
| N | Mean | SD | Mean | SD | |
| A2m (ng/ml) | 102 | 27.19 | 44.87 | 32.51 | 44.33 |
| CRP (mg/L) | 102 | 5.0 | 0.6 | 4.4 | 0.57 |
| SerAmyP (ng/ml) | 102 | 3.38 | 1.29 | 2.51 | 1.22 |
| BASFGF (pg/ml) | 102 | 101.92 | 29.92 | 106.89 | 29.02 |
| EOTAXIN-1 (pg/ml) | 102 | 75.94 | 26.98 | 86.02 | 39.19 |
| G-CSF (pg/ml) | 102 | 95.07 | 28.86 | 93.83 | 24.75 |
| IFN-γ (pg/ml) | 102 | 252.17 | 105.34 | 221.99 | 73.21 |
| IL-13 (pg/ml) | 102 | 19.95 | 19.19 | 15.89 | 18.09 |
| IL-17A (pg/ml)* | 102 | 25.57 | 10.71 | 26.18 | 11.03 |
| IL-1β (pg/ml) | 102 | 11.09 | 7.07 | 11.33 | 5.49 |
| IL-1ra (pg/ml) | 102 | 672.54 | 969.82 | 618.24 | 423.24 |
| IL-4 (pg/ml) | 102 | 8.9 | 2.45 | 8.39 | 2.22 |
| IL-5 (pg/ml) | 102 | 63.57 | 18.32 | 55.05 | 13.12 |
| IL-6 (pg/ml) | 92 | 17.16 | 8.91 | 16.4 | 6.12 |
| IL-8 (pg/ml) | 100 | 47.08 | 90.21 | 37.44 | 45.96 |
| IL-9 (pg/ml) | 98 | 35.37 | 81.44 | 24.25 | 18.29 |
| IL-10 (pg/ml) | 102 | 486.53 | 289.3 | 580.62 | 658.12 |
| MIP-1α (pg/ml) | 102 | 9.37 | 5.66 | 9.92 | 6.53 |
| MIP-1β (pg/ml) | 102 | 90.9 | 44.88 | 86.77 | 41.48 |
| PDGF-B (pg/ml) | 102 | 1580.79 | 1217.01 | 1485.12 | 1164.2 |
| RANTES (pg/ml) | 97 | 9944.47 | 2417.93 | 9221.38 | 2056 |
| TNF-α (pg/ml) | 102 | 109.62 | 34.38 | 114.05 | 27.45 |
Immunoassay data are expressed in pg/ml (27 plex, after 1:4 dilution) and ng/ml (4-plex, after 1:10,000 fold dilution);
dilution factor.
3.3. Change in Eotaxin-1 and IFN-γ over 12 weeks of treatment as a predictor of remission
Of the 94 participants with outcome data included in the logistic regression analyses, 50% (n=47) were remitters and 50% (n=47) were non-remitters. The logistic regression model included baseline depression severity as a covariate, change in marker level as a main effect, type of treatment as a main effect, and the interaction between marker level change and type of treatment.
Using the logistic regression models, the majority of the six aforementioned inflammatory markers demonstrated no significant main effects or interactions, including Serum Amyloid P, IL-5, RANTES, and IL-13. We conclude that there is no associations between changes in these marker levels and remission in the CO-MED cohort.
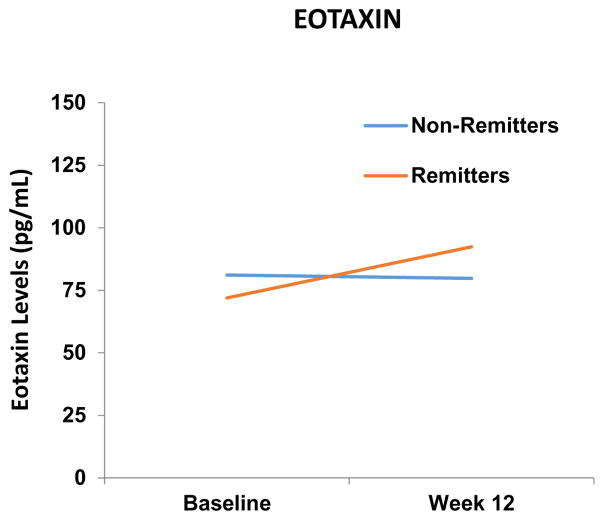
However, the logistic model testing for Eotaxin-1 level demonstrates a main effect for Eotaxin-1 level change (X2 (1) = 3.89, p < 0.05) and a main effect for the baseline depression severity covariate (X2 (1) < 5.97, p < 0.020), but no main effect for type of treatment (X2 (2) < 0.68, p < 0.72), and no interaction between type of treatment and change in Eotaxin-1 level (X2 (2) < 3.02, p < 0.23). For the remitters, the Eotaxin-1 levels increased from 75.94 (SD= 28.32) at baseline to 92.40 (SD= 48.36) at week 12, whereas for non-remitters there was no significant change in level from 81.11 (SD= d 24.83) at baseline to 79.75 (SD= 26.25) at week 12 (Figure 1). Thus, an increase in Eotaxin-1 levels is associated with remission.
Figure 1.
Eotaxin levels increased from baseline-to-week 12 in remitters.
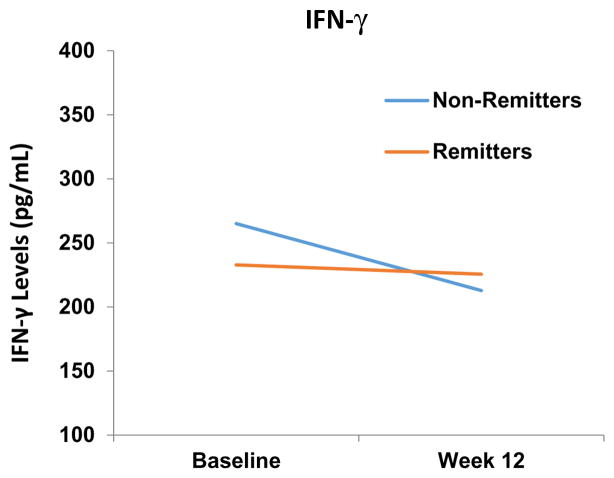
Additionally, the logistic model included a main effect for IFN-γ level changes (X2 (1) = 4.04, p < 0.05) and a main effect for the baseline depression severity covariate (X2 (1) =4.8, p < 0.03), but no main effect for type of treatment (X2 (2) =1.03, p < 0.60) or for the type of treatment by IFN-γ levels changes interaction (X2 (2) = 0.32, p < 0.86). The IFN-γ levels remained relatively unchanged for remitters from 232.97 (SD= 97.22) at baseline to 225.50 (SD= 82.09) at week 12, whereas there was a significant decline in IFN-γ level for non-remitters from 265.00 (SD= 106.69) at baseline to 212.83 (SD= 61.07) at week 12 (Figure 2). Thus, a decrease in IFN-γ levels is associated with non-remission.
Figure 2.
IFN-γ levels decreased from baseline-to-week 12 in non-remitters.
4. Discussion
Utilization of an unbiased non-targeted exploratory approach, coupled with multiplex immunoassay panels, may enable the assessment of a broad range of inflammatory markers such as cytokines, chemokines, growth factors and acute-phase reactants. In this report, we used commercially-available multiplex kits to quantify a broad spectrum of inflammatory marker levels at baseline and 12 weeks of treatment in participants with MDD. Of 22 markers analyzed, only 6 demonstrated a significant change from baseline to exit, and of these six, only two demonstrated clinically significant associations with treatment outcome. Participants who achieved remission had increased Eotaxin-1 levels. Alternatively, levels of IFN-γ were unchanged in remitters but significantly lower in non-remitters. To our knowledge, no previous report has evaluated the relationship of Eotaxin-1 or IFN-γ to treatment response with different antidepressant medications.
It is important to consider the mechanisms through which these inflammatory markers may be impacted by depression and subsequent antidepressant treatment. While a large amount of attention has recently been given to the state of increased systemic inflammation in depression, earlier evidence suggested a suppression of leukocytes, especially T helper lymphocytes (Maes, 1995). Hence, Eotaxin-1, an eosinophil-attracting chemokine that works through the CCR3 receptor (Melik-Parsadaniantz and Rostene, 2008), may demonstrate clinical significance by stabilizing the biological response to different antidepressant treatments. A putative biological mechanism underlying the association between increased Eotaxin-1 and remission is that it may reflect increased levels of mammalian targets of rapamycin (mTOR) protein. Inhibition of mTOR has been shown to reduce levels of Eotaxin-1 in animal models (Fredriksson et al., 2012; Mushaben et al., 2011). Additionally, rapid activation of the mTOR signaling pathway is reportedly related to synaptogenesis and the rapid-antidepressant effects of ketamine (Li et al., 2010). Hence, our findings suggest that Eotaxin-1 may be an easily detectable peripheral marker of synaptogenesis and/or effective antidepressant treatment. On the contrary, however, other studies have either found an association of Eotaxin-1 with more severe depression (as compared to healthy controls) (Maes et al., 1999) or a lack of association with MDD (Grassi-Oliveira et al., 2012; Magalhaes et al., 2014; Myung et al., 2016). The demographics of these studies largely differ from ours, however, as one is limited to young, unmedicated patients and another is limited to females, either of which may help explain the discrepancy in results. Nonetheless, our results may be considered preliminary in nature and will merit future studies with larger sample sizes to determine the agreement or discordance with previous studies.
Our finding that overall IFN-γ levels significantly decreased over the 12 week treatment course is consistent with prior studies showing reduction of IFN-γ with antidepressant treatment. Diamond et al. have shown that fluoxetine and desipramine suppress IFN-γ production, which is paralleled with the anti-proliferative properties of T-cell production (Diamond et al., 2006). There is also evidence from Maes et al. suggesting antidepressants such as trazodone, clomipramine, and sertraline suppress the release of IFN-γ (and others: IL-6, IL-1β, IL-2) (Maes et al., 1999). Lastly, Himmerich et al. also found that IFN-γ levels are reduced following administration of tricyclic antidepressants (Himmerich et al., 2010a). Interestingly, when separated into remission vs. non-remission groups, IFN-γ levels remained unchanged in patients with remission but were reduced in patients with non-remission. Mechanistically, IFN-γ is a potent activator of the kynurenine pathway, largely regarded as a detrimental pathway in tryptophan metabolism and oftentimes associated with MDD (Strasser et al., 2017). The observation that IFN-γ levels are higher at baseline in non-remitters than in remitters may indicate higher baseline kynurenine activity and thus more difficulty responding to antidepressant treatment, although kynurenine and/or its metabolites were not directly measured.
Recently, Schmidt and colleagues analyzed 9 inflammatory markers (IL-2, IL-4, GM-CSF, IL-5, IL-10, IL-12, IFN-γ, TNF-alpha and CRP) in n=30 MDD participants at baseline and 4 weeks after treatment (Schmidt et al., 2016). In many cases, our results were similar, in that significant changes were not observed before and after treatment, particularly in non-responders. Interestingly, their results showed that responders had decreased IFN-γ levels, which is contrary to our findings. Numerous variables may explain this discrepancy, including their use of serum versus plasma, a shorter treatment interval, and different prescribed medications. Overall, these differences stress the importance of replicating studies and standardizing procedures in order to increase the probability of finding a reliable biomarker(s) for future research and treatment.
There are several limitations to our report. We did not conduct a priori analyses to test for power, so the absence of change in some inflammatory markers in our study may be due simply to a small sample size. Additionally, our baseline-to-exit assessment time points were separated by 3 months. Because we did not collect blood samples throughout the study, it is not possible to test for change(s) in inflammatory marker levels at a time soon after treatment intervention. Thus, we may not have detected inflammatory markers that are altered early in treatment. Additional research utilizing more frequent collection of blood samples with various time points may provide more information on the interplay of antidepressants, treatment response, and changes in levels of inflammatory markers.
Acknowledgments
The authors thank the clinical staff at each clinical site, including site investigators and co-investigators for their assistance with this project; all of the study participants; and Jennifer Furman, Ph.D. for her extensive editorial and administrative contributions. This work was supported by the National Institute of Mental Health under contract N01 MH-90003 to the University of Texas Southwestern Medical Center at Dallas (principal investigators, M.H. Trivedi and A.J. Rush). Forest Pharmaceuticals, GlaxoSmithKline, Organon, and Wyeth Pharmaceuticals provided medications for this trial at no cost. This work was also supported in part through the Center for Depression Research and Clinical Care (Principal Investigator: Madhukar H. Trivedi, MD) and Hersh Foundation (MHT). The content of this publication does not necessarily reflect the views or policies of the U.S. Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. government. NIMH had no role in the drafting or review of the manuscript or in the collection or analysis of the data.
References
- 1.Akhondzadeh S, Jafari S, Raisi F, Nasehi AA, Ghoreishi A, Salehi B, Mohebbi-Rasa S, Raznahan M, Kamalipour A. Clinical trial of adjunctive celecoxib treatment in patients with major depression: a double blind and placebo controlled trial. Depress Anxiety. 2009;26(7):607–611. doi: 10.1002/da.20589. [DOI] [PubMed] [Google Scholar]
- 2.Bot M, Chan MK, Jansen R, Lamers F, Vogelzangs N, Steiner J, Leweke FM, Rothermundt M, Cooper J, Bahn S, Penninx BW. Serum proteomic profiling of major depressive disorder. Transl Psychiatry. 2015;5:e599. doi: 10.1038/tp.2015.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brunoni AR, Machado-Vieira R, Zarate CA, Valiengo L, Vieira EL, Bensenor IM, Lotufo PA, Gattaz WF, Teixeira AL. Cytokines plasma levels during antidepressant treatment with sertraline and transcranial direct current stimulation (tDCS): results from a factorial, randomized, controlled trial. Psychopharmacology (Berl) 2014;231(7):1315–1323. doi: 10.1007/s00213-013-3322-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9(1):46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diamond M, Kelly JP, Connor TJ. Antidepressants suppress production of the Th1 cytokine interferon-gamma, independent of monoamine transporter blockade. Eur Neuropsychopharmacol. 2006;16(7):481–490. doi: 10.1016/j.euroneuro.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 6.Felger JC, Lotrich FE. Inflammatory cytokines in depression: neurobiological mechanisms and therapeutic implications. Neuroscience. 2013;246:199–229. doi: 10.1016/j.neuroscience.2013.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fredriksson K, Fielhaber JA, Lam JK, Yao X, Meyer KS, Keeran KJ, Zywicke GJ, Qu X, Yu ZX, Moss J, Kristof AS, Levine SJ. Paradoxical effects of rapamycin on experimental house dust mite-induced asthma. PloS one. 2012;7(5):e33984. doi: 10.1371/journal.pone.0033984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grassi-Oliveira R, Brieztke E, Teixeira A, Pezzi JC, Zanini M, Lopes RP, Bauer ME. Peripheral chemokine levels in women with recurrent major depression with suicidal ideation. Rev Bras Psiquiatr. 2012;34(1):71–75. doi: 10.1590/s1516-44462012000100013. [DOI] [PubMed] [Google Scholar]
- 9.Hauser P, Khosla J, Aurora H, Laurin J, Kling MA, Hill J, Gulati M, Thornton AJ, Schultz RL, Valentine AD, Meyers CA, Howell CD. A prospective study of the incidence and open-label treatment of interferon-induced major depressive disorder in patients with hepatitis C. Mol Psychiatry. 2002;7(9):942–947. doi: 10.1038/sj.mp.4001119. [DOI] [PubMed] [Google Scholar]
- 10.Himmerich H, Fulda S, Sheldrick AJ, Plumakers B, Rink L. IFN-gamma reduction by tricyclic antidepressants. Int J Psychiatry Med. 2010a;40(4):413–424. doi: 10.2190/PM.40.4.e. [DOI] [PubMed] [Google Scholar]
- 11.Himmerich H, Milenovic S, Fulda S, Plumakers B, Sheldrick AJ, Michel TM, Kircher T, Rink L. Regulatory T cells increased while IL-1beta decreased during antidepressant therapy. J Psychiatr Res. 2010b;44(15):1052–1057. doi: 10.1016/j.jpsychires.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 12.Jha MK, Minhajuddin A, Gadad BS, Greer T, Grannemann B, Soyombo A, Mayes TL, Rush AJ, Trivedi MH. Can C-reactive protein inform antidepressant medication selection in depressed outpatients? Findings from the CO-MED trial. Psychoneuroendocrinology. 2017;78:105–113. doi: 10.1016/j.psyneuen.2017.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kenis G, Maes M. Effects of antidepressants on the production of cytokines. Int J Neuropsychopharmacol. 2002;5(4):401–412. doi: 10.1017/S1461145702003164. [DOI] [PubMed] [Google Scholar]
- 14.Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, Li XY, Aghajanian G, Duman RS. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329(5994):959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maes M. Evidence for an immune response in major depression: a review and hypothesis. Prog Neuropsychopharmacol Biol Psychiatry. 1995;19(1):11–38. doi: 10.1016/0278-5846(94)00101-m. [DOI] [PubMed] [Google Scholar]
- 16.Maes M, Song C, Lin AH, Bonaccorso S, Kenis G, De Jongh R, Bosmans E, Scharpe S. Negative immunoregulatory effects of antidepressants: inhibition of interferon-gamma and stimulation of interleukin-10 secretion. Neuropsychopharmacology. 1999;20(4):370–379. doi: 10.1016/S0893-133X(98)00088-8. [DOI] [PubMed] [Google Scholar]
- 17.Magalhaes PV, Jansen K, Stertz L, Ferrari P, Pinheiro RT, da Silva RA, Kapczinski F. Peripheral eotaxin-1 (CCL11) levels and mood disorder diagnosis in a population-based sample of young adults. J Psychiatr Res. 2014;48(1):13–15. doi: 10.1016/j.jpsychires.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 18.Melik-Parsadaniantz S, Rostene W. Chemokines and neuromodulation. J Neuroimmunol. 2008;198(1–2):62–68. doi: 10.1016/j.jneuroim.2008.04.022. [DOI] [PubMed] [Google Scholar]
- 19.Munzer A, Sack U, Mergl R, Schonherr J, Petersein C, Bartsch S, Kirkby KC, Bauer K, Himmerich H. Impact of antidepressants on cytokine production of depressed patients in vitro. Toxins (Basel) 2013;5(11):2227–2240. doi: 10.3390/toxins5112227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mushaben EM, Kramer EL, Brandt EB, Khurana Hershey GK, Le Cras TD. Rapamycin attenuates airway hyperreactivity, goblet cells, and IgE in experimental allergic asthma. J Immunol. 2011;187(11):5756–5763. doi: 10.4049/jimmunol.1102133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Myung W, Lim SW, Woo HI, Park JH, Shim S, Lee SY, Kim DK. Serum Cytokine Levels in Major Depressive Disorder and Its Role in Antidepressant Response. Psychiatry Investig. 2016;13(6):644–651. doi: 10.4306/pi.2016.13.6.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1(1):43–46. [PubMed] [Google Scholar]
- 23.Rush AJ, Trivedi MH, Ibrahim HM, Carmody TJ, Arnow B, Klein DN, Markowitz JC, Ninan PT, Kornstein S, Manber R, Thase ME, Kocsis JH, Keller MB. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry. 2003;54(5):573–583. doi: 10.1016/s0006-3223(02)01866-8. [DOI] [PubMed] [Google Scholar]
- 24.Rush AJ, Trivedi MH, Stewart JW, Nierenberg AA, Fava M, Kurian BT, Warden D, Morris DW, Luther JF, Husain MM, Cook IA, Shelton RC, Lesser IM, Kornstein SG, Wisniewski SR. Combining medications to enhance depression outcomes (CO-MED): acute and long-term outcomes of a single-blind randomized study. Am J Psychiatry. 2011;168(7):689–701. doi: 10.1176/appi.ajp.2011.10111645. [DOI] [PubMed] [Google Scholar]
- 25.Saville DJ. Multiple Comparison Procedures - the Practical Solution. Am Stat. 1990;44(2):174–180. [Google Scholar]
- 26.Schmidt FM, Schroder T, Kirkby KC, Sander C, Suslow T, Holdt LM, Teupser D, Hegerl U, Himmerich H. Pro- and anti-inflammatory cytokines, but not CRP, are inversely correlated with severity and symptoms of major depression. Psychiatry Res. 2016;239:85–91. doi: 10.1016/j.psychres.2016.02.052. [DOI] [PubMed] [Google Scholar]
- 27.Stelzhammer V, Haenisch F, Chan MK, Cooper JD, Steiner J, Steeb H, Martins-de-Souza D, Rahmoune H, Guest PC, Bahn S. Proteomic changes in serum of first onset, antidepressant drug-naive major depression patients. Int J Neuropsychopharmacol. 2014;17(10):1599–1608. doi: 10.1017/S1461145714000819. [DOI] [PubMed] [Google Scholar]
- 28.Strasser B, Becker K, Fuchs D, Gostner JM. Kynurenine pathway metabolism and immune activation: Peripheral measurements in psychiatric and co-morbid conditions. Neuropharmacology. 2017;112(Pt B):286–296. doi: 10.1016/j.neuropharm.2016.02.030. [DOI] [PubMed] [Google Scholar]
- 29.Trivedi MH, Rush AJ, Ibrahim HM, Carmody TJ, Biggs MM, Suppes T, Crismon ML, Shores-Wilson K, Toprac MG, Dennehy EB, Witte B, Kashner TM. The Inventory of Depressive Symptomatology, Clinician Rating (IDS-C) and Self-Report (IDS-SR), and the Quick Inventory of Depressive Symptomatology, Clinician Rating (QIDS-C) and Self-Report (QIDS-SR) in public sector patients with mood disorders: a psychometric evaluation. Psychol Med. 2004;34(1):73–82. doi: 10.1017/s0033291703001107. [DOI] [PubMed] [Google Scholar]
- 30.Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, Norquist G, Howland RH, Lebowitz B, McGrath PJ, Shores-Wilson K, Biggs MM, Balasubramani GK, Fava M, Team SDS. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163(1):28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]
- 31.Uher R, Tansey KE, Dew T, Maier W, Mors O, Hauser J, Dernovsek MZ, Henigsberg N, Souery D, Farmer A, McGuffin P. An inflammatory biomarker as a differential predictor of outcome of depression treatment with escitalopram and nortriptyline. Am J Psychiatry. 2014;171(12):1278–1286. doi: 10.1176/appi.ajp.2014.14010094. [DOI] [PubMed] [Google Scholar]